Abstract
Background and Objectives
Chronic inflammation of bone tissue often results in bone defects and hazards to tissue repair and regeneration. Cannabidiol (CBD) is a natural cannabinoid with multiple biological activities, including anti-inflammatory and osteogenic potential. This study aimed to investigate the efficacy and mechanisms of CBD in the promotion of bone marrow mesenchymal stem cells (BMSCs) osteogenic differentiation in the inflammatory microenvironment.
Methods and Results
BMSCs isolated from C57BL/6 mice, expressed stem cell characteristic surface markers and presented multidirectional differentiation potential. The CCK-8 assay was applied to evaluate the effects of CBD on BMSCs’ vitality, and demonstrating the safety of CBD on BMSCs. Then, BMSCs were stimulated with lipopolysaccharide (LPS) to induce inflammatory microenvironment. We found that CBD intervention down-regulated mRNA expression levels of inflammatory cytokines and promoted cells proliferation in LPS-treated BMSCs, also reversed the protein and mRNA levels downregulation of osteogenic markers caused by LPS treatment. Moreover, CBD intervention activated the cannabinoid receptor 2 (CB2) and the p38 mitogen-activated protein kinase (MAPK) signaling pathway. While AM630, a selective CB2 inhibitor, reduced phosphorylated (p)-p38 levels. In addition, AM630 and SB530689, a selective p38 MAPK inhibitor, attenuated the enhancement of osteogenic markers expression levels by CBD in inflammatory microenvironment, respectively.
Conclusions
CBD promoted osteogenic differentiation of BMSCs via the CB2/p38 MAPK signaling pathway in the inflammatory microenvironment.
Keywords: Cannabidiol (CBD), Osteogenic differentiation, Inflammatory microenvironment, Cannabinoid receptor 2 (CB2), p38 MAPK signaling pathway
Introduction
Long-term inflammatory states suppress bone reconstruction (1), controlling inflammation is of foremost importance to prevent inflammation-induced bone loss (2). During physiological bone healing, bone marrow mesenchymal stem cells (BMSCs) develop and differentiate into mature functional bone cells under the activation of cytokines (3) to maintain bone tissue homeostasis and repair (4). However, differentiation potential and functional status of stem cells were suppressed in exaggerated inflammatory microenvironment (5, 6). Therefore, agents that are characterized by anti-inflammatory property may promote osteogenic differentiation of BMSCs in the inflammatory microenvironment.
Cannabinoids have been used for thousands of years due to their multiple pharmacological functions, such as analgesic and anti-inflammatory effects. But psychoactivity makes them controversial to use (7). The isomers Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are two main cannabinoids (8). Unlike THC, non-psychoactive property of CBD avoids its abuse and public health problems. CBD possesses wide range of pharmacological functions including anti-epilepsy, anti-anxiety, anti-tumor, and of note anti-inflammation effects (9), such as gastrointestinal inflammation (10), non-alcoholic steatohepatitis (11), dermal inflammation (12) and osteoarthritis (13). Besides, some clinical studies have also shown that CBD promoted healing of non-infectious arthritis (14) and took beneficial effect on bone metabolism (15). Based on the anti-inflammatory effects of CBD, we hypothesized that CBD may present osteogenic potential in inflamma-tory conditions.
Lipopolysaccharide (LPS) is the main toxic factor generated by bacteria during infectious bone diseases (16). So, we applied LPS to induce inflammatory microenvironment, and investigated the osteogenic differentiation capacity of LPS-impaired BMSCs and related mechanisms by CBD treatment.
Materials and Methods
Cells isolation and culture
BMSCs were isolated from five-week-old male C57BL/6 mice (Animal Experimental Center of Chinese People’s Liberation Army General Hospital, Beijing). First, we separated the femur and washed the bone marrow cavity repeatedly to collect the cells. Then, cells were inoculated in α-minimum essential medium (α-MEM; Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin/streptomycin in a humidified incubator at 37℃ with 5% CO2. An optical microscope (Olympus, Japan) was used to daily observe the morphology and growth of the cells. Finally, the third to fifth passage cells (P3-5) were used for subsequent experiments.
Stem cells identification
P3 BMSCs were washed for three times, digested and collected for the following experiments. First, representative surface markers were analyzed by flow cytometry. After labeled with antibodies of CD11b, CD29, CD31, CD34, CD44, CD45, CD73, CD90, and CD105 (BD Biosciences, USA), the BMSCs were incubated at 4℃ for 30 min. After which, cells were washed with PBS, checked by flow cytometry (BD Celesta, USA) and finally analyzed using CytExpert 2.0 software.
Second, P3 BMSCs (2×104 per well) were incubated into 6-well plates. Osteogenesis differentiation was accessed using alizarin red staining (Sigma-Aldrich, USA) and adipogenesis induction was determined by oil red O staining (Sigma-Aldrich, USA) as previously described (17, 18) after incubation for 14 days.
Cell vitality assay
The effects of CBD on BMSCs’ vitality were assessed using CCK-8 assay. BMSCs (2×104 per well) were seeded in 24-well plates. After incubation overnight, supernatants were replaced with conditional medium containing different concentrations of CBD (0.1 μM, 0.5 μM, 2.5 μM, 5 μM, 10 μM; CATO, USA). Following with culture for 72 hrs, CCK-8 solution was added (Beyotime, Shanghai, China) and incubated for 4 hrs at 37℃. The absorbance was measured at 450 nm using microplate tester (Rayto RT-6000, USA).
Alkaline phosphatase (ALP) activity
After incubated overnight, the culture medium of P3 BMSCs seeding in 24-well plates was replaced with osteogenic induction medium. Then, cells were randomly divided into the following groups: vehicle, LPS (10 μg/ml; Sigma-Aldrich, USA), LPS plus CBD (0.5 μM, 2.5 μM, 5 μM). Each group was provided with 3 secondary wells. After culturing for 1, 3, 5, and 7 days, ALP activity was measured following instructions of ALP detection kit (Sigma-Aldrich, USA). The absorbance at 520 nm was measured with microplate reader (Rayto RT-6000, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
P4 or P5 BMSCs were seeded in 6-well plates and cultured for 7 days under different culture conditions. Total RNA extraction, cDNA synthesis, and qRT-PCR procedures were performed with the following conditions: 95℃ for 3 min. 95℃ for 15 sec, 60℃ for 15 sec, 72℃ for 15 sec; 40 cycles (17). The 2-ΔΔCt method was adopted to analyze the relative gene expression and primers sequences are presented in Table 1.
Table 1.
Sequences of primers used for qRT-PCR
| Gene | Forward primer sequence (5’–3’) | Reverse primer sequence (5’–3’) |
|---|---|---|
| β-actin | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC |
| TNF-α | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| IL-6 | TCTGGAGCCCACCAAGAACGATAG | GTCACCAGCATCAGTCCCAAGAAG |
| Runx2 | CCTTCAAGGTTGTAGCCCTC | GGAGTAGTTCTCATCATTCCCG |
| ALP | AACCCAGACACAAGCATTCC | CCAGCAAGAAGAAGCCTTTG |
| OCN | GGACCATCTTTCTGCTCACTCTGC | TCCTGCTTGGACATGAAGGCTTT |
qRT-PCR: quantitative real-time polymerase chain reaction, TNF-α: tumor necrosis factor-α, IL-6: interleukin-6, Runx2: runt-related transcription factor 2, ALP: alkaline phosphatase, OCN: osteocalcin.
Western blot
Proteins of P4 or P5 BMSCs for osteogenic differentiation induction after 7 days were extracted. The detailed Western blotting routine was performed as previously described (18). The primary antibodies used were: cannabinoid receptor 1 (CB1; DF4918, dilution: 1:1,000, Affinity), cannabinoid receptor 2 (CB2; DF8646, dilution: 1:1,000, Affinity), phosphorylated p38 (p-p38; #4511, dilution: 1:1,000, Cell Signaling Technology), p38 (#8690, dilution: 1:1,000, Cell Signaling Technology), Runt-related transcription factor 2 (Runx2; AF5186, dilution: 1:1,000, Affinity), ALP (DF12525, dilution: 1:1,000, Affinity), osteocalcin (OCN; DF12303, dilution: 1:1,000, Affinity) and β-actin (BM0627, dilution: 1:200, Boster, China). Blots were quantified using ImageJ software.
Statistical analysis
Data are presented as means±standard deviations (SD) and were analyzed by GraphPad Prism Software (version 9.0, USA) and SPSS (version 23.0, USA). The Shapiro-Wilk test was used to evaluate the normality of data. Homogeneity of variance was tested using Levene’s test. One-way analysis of variance (ANOVA) test was used for multiple sample comparison. p<0.05 (two-sided) was considered statistically significant.
Results
Morphology, phenotypic characterization, and multidirectional differentiation capabilities of BMSCs
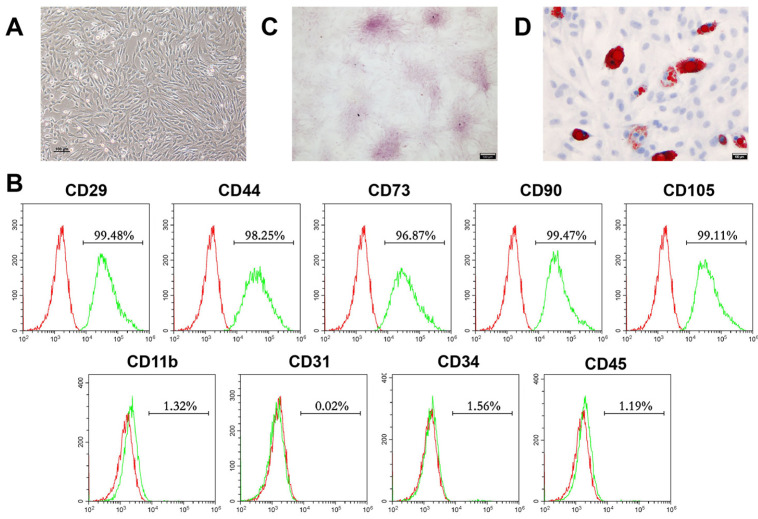
First, we identified the stem cells isolated. After culturing for 3 days, P2 BMSCs presented spindle-like morphology (Fig. 1A). The flow cytometry results (Fig. 1B) showed that BMSCs highly expressed mesenchymal stem cell surface markers: CD29 (99.48%), CD44 (98.25%), CD73 (96.87%), CD90 (99.47%) and CD105 (99.11%), but low expressed hematopoietic or endothelial stem cell markers: CD11b (1.32%), CD31 (0.02%), CD34 (1.56%) and CD45 (1.19%). Moreover, we performed alizarin red staining and Oil red O staining to verify the multidirectional differentiation capabilities of the isolated BMSCs, which are basis characteristic of stem cell (4). The presence of red calcium nodules after induction for 14 days demonstrated the osteogenic differentiation capacity of BMSCs. (Fig. 1C). In parallel, lipid droplets were detected after adipogenic induction for 14 days, which demonstrated the adipogenic potential of the isolated BMSCs (Fig. 1D). Taking together, these results indicated that we successfully isolated BMSCs.
Fig. 1.
Morphology, phenotypic, and multidirectional differentiation characteristics of mouse BMSCs. (A) Re-presentative morphology image of passage 2 (P2) BMSCs was visual-ized by light microscopy (Scale bar, 100 μm). (B) P3 BMSCs surface markers were identified by flow cyto-metry. (C) Osteogenic differentiation capacities of isolated BMSCs were verified by alizarin red staining after incubation for 14 days (n=3; Scale bar, 100 μm). (D) Adipogenic differentiation capacities of isolated BMSCs were detected by oil red O staining after culture for 14 days (n=3; Scale bar, 100 μm). BMSCs: bone mesenchymal stem cells.
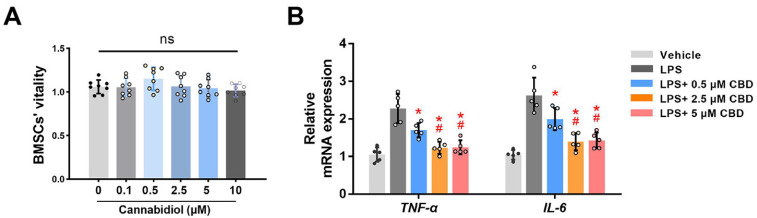
Next, we detected the effects of CBD on BMSCs’ viability. CCK-8 assay showed no statistical differences in absorbance values among different doses of CBD, after 72 hrs of administration (Fig. 2A), which indicated that CBD took no obviously toxic effects on BMSCs vitality in the tested concentrations.
Fig. 2.
CBD inhibits LPS-induced inflammatory response. (A) BMSCs’ viability was tested by CCK-8 assay after incubating with different concentrations of CBD for 72 hrs (n=8). (B) BMSCs were pretreated with LPS (10 μg/ml) for 12 hrs, and added different concentrations of CBD (0.5, 2.5, 5 μM) for 12 hrs. Then, BMSCs were harvested for detecting the mRNA expression levels of TNF-α and IL-6. β-actin was used as the internal control (n=5). CBD: cannabidiol, LPS: lipopolysaccharide, BMSCs: bone mesenchymal stem cells, CCK-8: cell counting kit-8, TNF-α: tumor necrosis factor-α, IL-6: interleukin-6, ns: no statistical significance. Data are presented as means±SD. *p<0.05 compared with the LPS group; #p<0.05 compared with the LPS plus 0.5 μM CBD group.
CBD downregulates LPS-induced inflammatory response
Previous study has demonstrated that CBD inhibits the inflammasome in human gingival mesenchymal stem cells (19). Here, LPS (10 μg/ml) (20) was applied on BMSCs to establish the inflammatory microenvironment, and we investigated whether CBD could downregulate LPS-induced inflammatory response of BMSCs. qRT-PCR results showed that mRNA expression levels of tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) were significantly increased in LPS-treated BMSCs. As expected, CBD pretreatment reversed this increase of aforementioned inflammatory cytokine expression levels (Fig. 2B). Also, the efficacy of 2.5 and 5 μM of CBD were more obvious than 0.5 μM CBD to decrease TNF-α and IL-6 mRNA expression levels (p<0.05), and no statistical difference was observed between 2.5 and 5 μM CBD groups. Overall, CBD could effectively decrease LPS-induced inflammatory response.
CBD promotes osteogenic differentiation of BMSCs in the inflammatory microenvironment
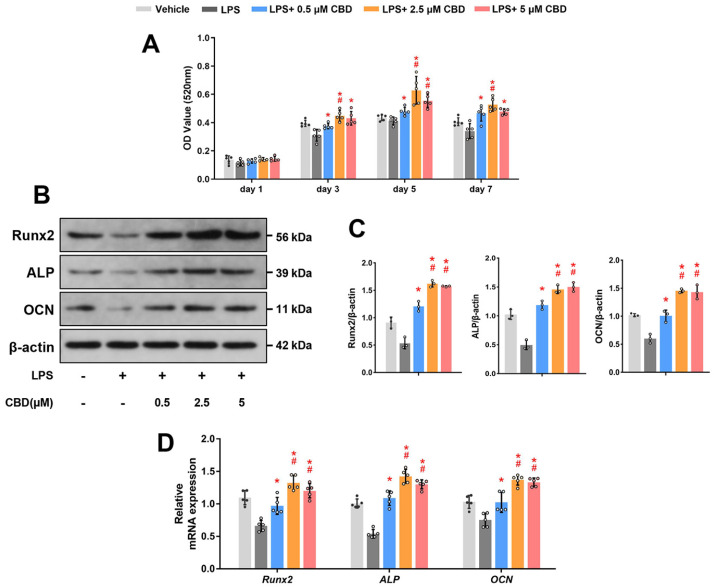
Subsequently, we wondered whether CBD could promote osteogenic differentiation of injured BMSCs in LPS-induced inflammatory environment. ALP is considered an important indicator in early osteogenesis (21). Thus, we detected the ALP activity of BMSCs after osteogenic induction and found that CBD significantly increased ALP activity in a dose-dependent and saturated manner. And 2.5 and 5 μM CBD groups were comparable at 3, 5 and 7 days (Fig. 3A), so 2.5 μM of CBD were selected as the intervention dose in the following experi-ments.
Fig. 3.
CBD promotes osteogenic differentiation of BMSCs in the inflammatory microenvironment. (A) BMSCs were co-treated with LPS (10 μg/ml) and different concentrations of CBD (0.5, 2.5, 5 μM), as indicated for 1, 3, 5, and 7 days, then ALP activity was detected (n=5). (B) BMSCs were co-treated with LPS and CBD (0.5, 2.5, 5 μM), as indicated for 7 days. Western blot was performed for detecting Runx2, ALP, and OCN. β-actin was used as the internal control (n=3). (C) Quantitative analysis of (B). (D) BMSCs were co-treated with LPS and CBD (0.5, 2.5, 5 μM), as indicated for 7 days. Then, BMSCs were harvested for detecting the mRNA expression levels of Runx2, ALP, and OCN by qRT-PCR. β-actin was used as the internal control (n=5). CBD: cannabidiol, BMSCs: bone mesenchymal stem cells, Runx2: runt-related transcription factor 2, ALP: alkaline phosphatase, OCN: osteocalcin, qRT-PCR: quantitative real-time polymerase chain reaction. Data are represented as means±SD. *p<0.05 compared with the LPS group; #p<0.05 compared with the LPS plus 0.5 μM CBD group.
Second, we determined osteogenesis-related protein expression levels in BMSCs using western blot. The expression levels of Runx2, an essential transcription factor in early osteogenesis (21), were declined in the LPS group, while CBD intervention reversed this decrease. Mean-while, the protein expression levels of ALP were consistent with the result of Runx2 protein. In addition, CBD pretreatment could also recover the OCN expression levels, a crucial marker expressed in late stage of bone formation (21) (Fig. 3B and 3C). The original blots are shown in Supplementary Fig. S1.
Last but not least, we detected the mRNA expression levels of Runx2, ALP and OCN, the results were consistent with the results of western blot (Fig. 3D). Collectively, these findings demonstrated that CBD reversed the attenuated osteogenic differentiation of BMSCs in the LPS-induced inflammatory microenvironment.
CBD activates the CB2-dependent p38 MAPK pathway
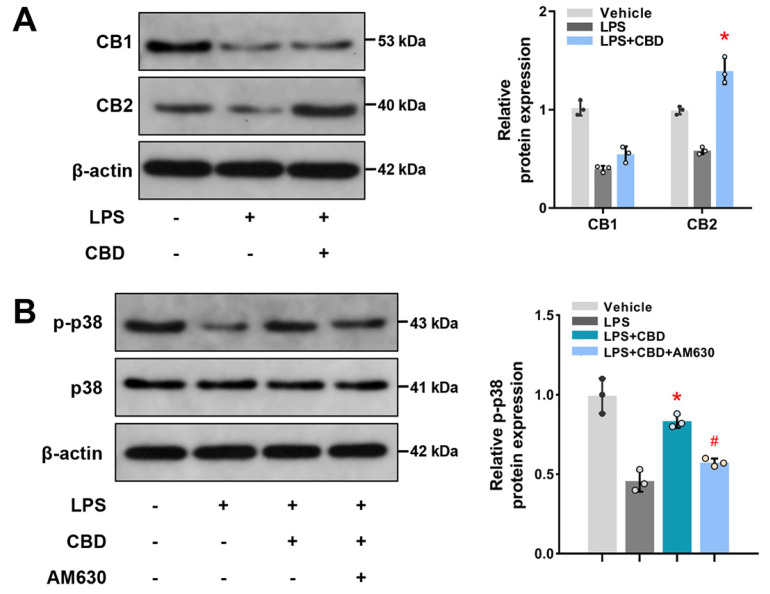
To explore the molecular mechanism of CBD administration on BMSCs osteogenic differentiation, we first detected whether CB1 and/or CB2, essential cannabinoid receptors expressed in osteocytes and related to bone metabolism (22, 23), participated in osteogenesis process of CBD administration. We found that both CB1 and CB2 restrained when co-treated with LPS (Fig. 4A). While, CBD significantly promoted CB2 expression in the inflamma-tory microenvironment, indicating that CBD might initiate intracellular effects via the activation of CB2. The original blots are shown in Supplementary Fig. S2.
Fig. 4.
CBD activates the CB2-dependent p38 MAPK pathway. (A) BMSCs were pretreated with LPS (10 μg/ml) for 12 hrs, then CBD (2.5 μM) were added and cultured for another 12 hrs. Western blots and quantitative analysis were performed for determining CB1 and CB2. β-actin was used as the internal control (n=3). (B) BMSCs were pretreated with LPS for 12 hrs, then CBD and AM630 (10 μM) were added as indicated and incubated for another 12 hrs. Western blots and quantitative analysis for p-p38 and p38 were performed. β-actin was used as the internal control (n=3). CB1: cannabinoid receptor 1, CB2: cannabinoid receptor 2, p-p38: phosphorylated p38. Data are presented as means±SD. *p<0.05 compared with the LPS group; #p<0.05 compared with the LPS plus CBD group.
Mitogen-activated protein kinase (MAPK) family is a downstream signaling pathway of cannabinoid receptor, and p38 is an important MAPK isoform associated with inflammatory response (23). We found that p-p38 decreased in LPS-induced BMSCs, but CBD administration increased its expression (Fig. 4B). Furthermore, when inhibited CB2 with AM630, a selective antagonist of CB2, the effects of CBD activated p38 MAPK signaling pathway were blunted. The original blots are shown in Supplementary Fig. S3. Together, these results suggested CBD activates the CB2-dependent p38 MAPK pathway.
CB2-dependent p38 MAPK signaling pathway is required for CBD promoting BMSCs osteogenic differentiation
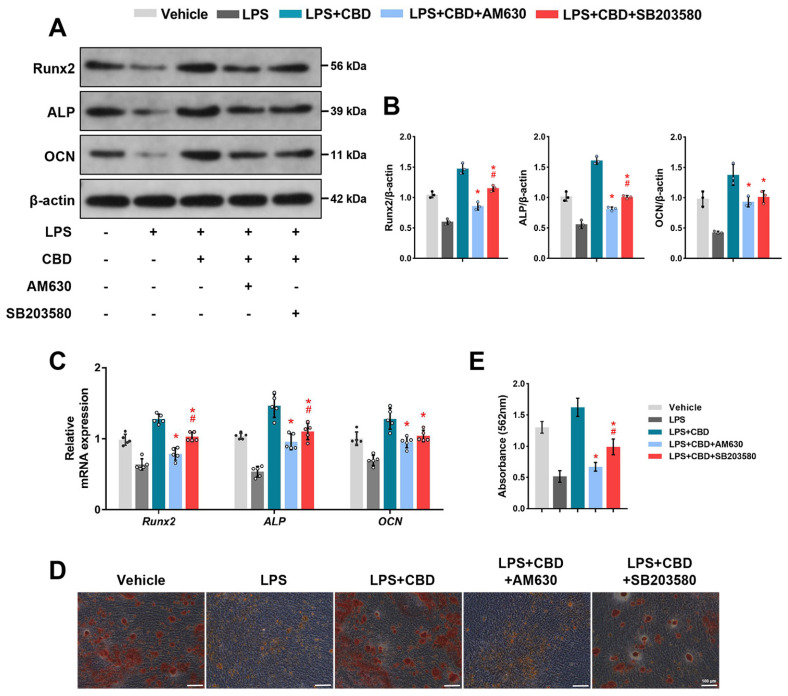
Next, to confirm the effects of the CB2-p38 MAPK signaling pathway on CBD promoting osteogenic differentiation of BMSCs, we used AM630 co-treated with CBD. Then we found that both the protein and mRNA levels of Runx2, ALP and OCN decreased compared with CBD group, indicating that CBD promoted osteogenic differentiation of BMSCs, at least partially, via CB2.
Additionally, SB203580, a selective p38 inhibitor, decreased both protein and mRNA levels of Runx2, ALP and OCN compared with CBD group, indicating that CBD enhanced osteogenic differentiation of BMSCs, at least partly, via p38 MAPK signaling pathway. Interestingly, we also found that the decreases of ALP and Runx2 protein and mRNA expression levels in the AM630 group were more obvious than the SB203580 group (Fig. 5A∼C). The original blots are shown in Supplementary Fig. S4. Moreover, alizarin red staining (Fig. 5D) and semi-quantitative analysis (Fig. 5E) showed that the application of CBD increased the deposits of calcium nodules compared with LPS-injured BMSCs, while co-treated with AM630 or SB203580 reduced the formation of calcium nodules, demonstrating that at the final stages of differentiation, CBD promotes osteogenic differentiation of BMSCs in the inflammatory microenvironment via the CB2-dependent p38 MAPK signaling pathway.
Fig. 5.
CB2-dependent p38 MAPK signaling pathway is required for CBD promoting osteogenic differentiation of BMSCs. BMSCs were pretreated with LPS (10 μg/ml) for 12 hrs. Then CBD (2.5 μM), AM630 (10 μM), and SB203580 (20 μM) were added as indicated and co-cultured with LPS for 7 days. (A) Western blots were performed for detecting the protein expression levels of Runx2, ALP, and OCN. β-actin was used as the internal control (n=3). (B) Quantitative analysis of (A). (C) BMSCs were harvested for measuring the mRNA expression levels of Runx2, ALP, and OCN using qRT-PCR. β-actin was used as the internal control (n=5). BMSCs were co-treated with these agents for 14 days and then alizarin red staining was performed. Representative images (D) and semiquantification (E) of alizarin red staining after incubation for 14 days (Scale bar, 100 μm). Data are presented as means±SD. *p<0.05 compared with the CBD plus LPS group. #p<0.05 compared with the CBD plus AM630 group.
Discussion
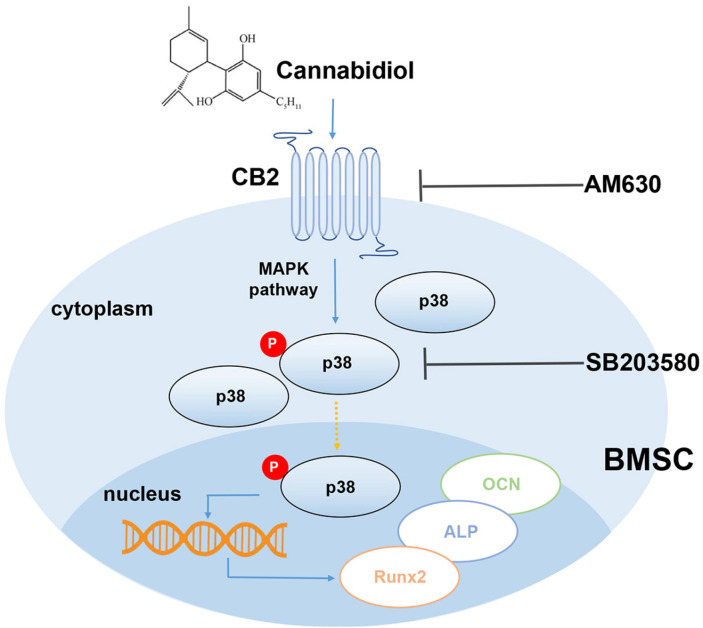
The result of our study, for the first time demonstrated that CBD could promote osteogenic differentiation of BMSCs in LPS-induced inflammatory microenviron-ments. The major findings are: (1) CBD intervention down-regulated TNF-α and IL-6 expression levels and up-regulated osteogenesis-related gene and protein levels, and enhanced calcium nodules formation in LPS-impaired BMSCs; (2) CBD intervention promoted osteogenesis of BMSCs via the CB2/p38 MAPK signaling pathway (Fig. 6).
Fig. 6.
Schematic diagram of CBD intervention.
Appropriate pro-inflammatory signaling is necessary in the early stage of fracture healing. However, chronic inflammation hazards to the osteogenic environment (24). Thus, inhibiting inflammation is essential to improve the osteogenic differentiation environment for BMSCs. Recent studies found that CBD, which is already approved by the FDA for refractory epilepsy treatment in children (25), could promote stem cells odontogenic and osteogenic differentiation (26, 27). Additionally, scaffolds combined CBD enhanced mesenchymal stem cell recruitment and regeneration of critical-sized bone defect (28) in non-inflammatory environment. Our study further demonstrated that CBD promoted BMSCs osteogenic differentiation in LPS-induced inflammatory environment based on the following results: 1) CBD intervention enhanced ALP activity; 2) up-regulated osteogenesis-related gene expression levels of BMSCs; 3) increased the expression levels of osteogenesis-related proteins.
It has been established that endogenous cannabinoid system is a key modulator of bone mass (29, 30). For example, CB1 or CB2 knockout mice developed osteoporosis and low bone mass phenotype (31). It was reported that inflammatory environments reduced cannabinoid receptors expression in stem cells (32), which might be associated with impaired cell function. Our results showed that LPS administration decreased both CB1 and CB2 expression, while CBD intervention reversed the down-regulation CB2, but took no effects on CB1 expression, thus excluding the possibility of CBD promoting osteogenic differentiation in inflammatory environment via CB1. However, research has implied that CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament cells (32). One possible explanation for this discrepancy is that we used the different type of stem cells. In fact, CB1 mainly expresses in central nervous systems, while CB2, mainly expressed in peripheral tissues, involves in regulating bone volume (15). Also, CB2 receptors are more densely distributed in osteocytes than CB1 (23, 29). Furthermore, CB2 has a higher affinity than CB1 to CBD and plays crucial effects in CBD promoted biomineralization (33). In fact, CB2 activation increases the expression levels of Runx2, ALP and bone sialoprotein (BSP) of osteocytes in non-inflammatory microenvironments (21). In our study, when applied AM630, a specific CB2 inhibitor, the effects of CBD on promoting osteogenic differentiation of BMSCs in LPS-induced inflammatory microenvironments were blunted. Collectively, these data indicated CBD promoted BMSCs osteogenic differentiation in inflammatory microenvironments via CB2.
The MAPK family includes three isoforms: extracellular signaling regulatory kinase (ERK), c-jun amino-terminal kinase (JNK), and p38 kinase (p38 MAPK), which are involved in inflammatory processes (32). In our findings, LPS inhibited the activation of p38 MAPK signaling pathway, while CBD activated p38 MAPK signaling pathway in an inflammatory environment, which was consistent with previous studies showed that CBD promoted MG-63 cells osteogenesis differentiation through p38 MAPK pathways in non-inflammatory microenvironment (34). Canna-binoids and their correspondent receptors could activate downstream MAPK isoforms (35). It was reported that overexpression of CB2 in osteoporosis increases p38 phosphorylation and mineralized extracellular matrix deposition (36). In contrast, when blocking CB2 with AM630 or siRNA targeted CB2, MAPK signaling pathway was suppressed (26, 31). We also found that p38 MAPK was restrained after CB2 inhibition using AM630, indicating that downstream p38 MAPK is effect-dependent on CB2. Further, p38 MAPK directly regulates downstream osteogenesis-related important target genes, such as Runx2 and osterix (37). Meanwhile, enhanced ALP activity and osteogenesis-related target genes expression of MSCs were also associated with MAPK activation in non-inflammatory environment (26, 27). We found that CBD intervention activated p38 and increased osteogenesis-related mRNA and protein expression levels in inflammatory environment. Moreover, when co-treated with p38 MAPK selective inhibitor, SB203580, the effects of CBD-promoted notably osteogenic differentiation of BMSCs diminished. Taking together, CBD promoted osteogenic differentiation of BMSCs in inflammatory microenvironment via the CB2-dependent p38 MAPK pathway.
This study also has some limitations. First, although we concluded that CB2 was the primary receptor in CBD-promoted osteogenesis, however, CB2 specific inhibitors did not completely inhibit CBD osteogenesis effects, indicating that CBD may also promote osteogenesis through other mechanisms. G protein-coupled receptor 55 (GPR55) is another important cannabinoid receptor, also participates in CBD-mediated bone mass regulation (38). So, other mechanisms, such as GPR55 signaling pathway, could not be elucidated. Second, the concrete experimental data linking osteogenesis differentiation of BMSCs with CB2 dependent p38 MAPK signaling activation remain unclear. Finally, we did not perform in vivo experiments. Further in vivo researches should be performed to clarify the osteogenesis potential of CBD in inflammatory micro-environments.
Overall, we verified the efficacy of CBD to promote osteogenic differentiation of BMSCs in the LPS-induced inflammatory microenvironment via the CB2-dependent p38 MAPK pathway. As a natural active drug with anti-inflammation property, CBD could be considered as a potential strategy for treating inflammatory bone diseases.
Supplementary Materials
Supplementary data including four figures can be found with this article online at https://doi.org/10.15283/ijsc21152.
Acknowledgments
This work was supported by grants from Project funded by China Postdoctoral Science Foundation (2019T120980, 2018M633722) and National Natural Science Foundation of China (NSFC51802350, NSFC51972339).
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149:393–404. doi: 10.1007/s00418-018-1643-3. [DOI] [PubMed] [Google Scholar]
- 2.Redlich K, Smolen J. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi M, Sun L, Blair HC. Special stem cells for bone. Cell Stem Cell. 2012;10:233–234. doi: 10.1016/j.stem.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Fierro FA, Nolta JA, Adamopoulos IE. Concise review: stem cells in osteoimmunology. Stem Cells. 2017;35:1461–1467. doi: 10.1002/stem.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D, Xu J, Liu O, Fan Z, Liu Y, Wang F, Ding G, Wei F, Zhang C, Wang S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immuno-modulation. J Clin Periodontol. 2012;39:1174–1182. doi: 10.1111/jcpe.12009. [DOI] [PubMed] [Google Scholar]
- 6.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 7.Izzo A, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. Erratum in: Trends Pharmacol Sci 2009; 30:609. [DOI] [PubMed] [Google Scholar]
- 8.Miranzadeh Mahabadi H, Bhatti H, Laprairie RB, Taghibiglou C. Cannabinoid receptors distribution in mouse cortical plasma membrane compartments. Mol Brain. 2021;14:89. doi: 10.1186/s13041-021-00801-x.1f1d3f61cff34f2aaf14b402ed126261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozela E, Juknat A, Gao F, Kaushansky N, Coppola G, Vogel Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13:136. doi: 10.1186/s12974-016-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couch D, Tasker C, Theophilidou E, Lund J, O'Sullivan S. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci (Lond) 2017;131:2611–2626. doi: 10.1042/CS20171288. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Wan T, Pang N, Zhou Y, Jiang X, Li B, Gu Y, Huang Y, Ye X, Lian H, Zhang Z, Yang L. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J Cell Physiol. 2019;234:21224–21234. doi: 10.1002/jcp.28728. [DOI] [PubMed] [Google Scholar]
- 12.Jastrząb A, Gęgotek A, Skrzydlewska E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells. 2019;8:827. doi: 10.3390/cells8080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philpott H, O'Brien M, McDougall J. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158:2442–2451. doi: 10.1097/j.pain.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrico C, Wesson S, Konduri V, Hofferek C, Vazquez-Perez J, Blair E, Dunner K, Jr, Salimpour P, Decker WK, Halpert MM. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain. 2020;161:2191–2202. doi: 10.1097/j.pain.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostu D, Lucaciu O, Mester A, Benea H, Oltean-Dan D, Onisor F, Baciut M, Bran S. Cannabinoids and bone regener-ation. Drug Metab Rev. 2019;51:65–75. doi: 10.1080/03602532.2019.1574303. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Pastou Terrier C, Gasque P. Bone responses in health and infectious diseases: a focus on osteoblasts. J Infect. 2017;75:281–292. doi: 10.1016/j.jinf.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Dong X, Ma W, Guan L, Wang YH, Huang XH, Chen JF, Zhao X. Proliferation, differentiation and immunoregulatory capacities of brown and white adipose-derived stem cells from young and aged mice. Int J Stem Cells. 2020;13:246–256. doi: 10.15283/ijsc20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Zhu B, Yin P, Zhao L, Wang Y, Fu Z, Dang R, Xu J, Zhang J, Wen N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6:1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]
- 19.Libro R, Scionti D, Diomede F, Marchisio M, Grassi G, Pollastro F, Piattelli A, Bramanti P, Mazzon E, Trubiani O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front Physiol. 2016;7:559. doi: 10.3389/fphys.2016.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Quan J, Long W, Chen H, Wang R, Guo J, Lin X, Mai S. LL-37 inhibits LPS-induced inflammation and sti-mulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 2018;372:178–187. doi: 10.1016/j.yexcr.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Qian H, Zhao Y, Peng Y, Han C, Li S, Huo N, Ding Y, Duan Y, Xiong L, Sang H. Activation of cannabinoid receptor CB2 regulates osteogenic and osteoclastogenic gene expression in human periodontal ligament cells. J Periodontal Res. 2010;45:504–511. doi: 10.1111/j.1600-0765.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Bab I, Zimmer A. Cannabinoid receptors and the regulation of bone mass. Br JPharmacol. 2008;153:182–188. doi: 10.1038/sj.bjp.0707593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idris A, Greig I, Ridge S, Baker D, Ross R, Ralston SH van't Hof R, author. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britch S, Babalonis S, Walsh S. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl) 2021;238:9–28. doi: 10.1007/s00213-020-05712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmuhl E, Ramer R, Salamon A, Peters K, Hinz B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem Pharmacol. 2014;87:489–501. doi: 10.1016/j.bcp.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Qi X, Liu C, Li G, Luan H, Li S, Yang D, Zhou Z. Investi-gation of in vitro odonto/osteogenic capacity of cannabidiol on human dental pulp cell. J Dent. 2021;109:103673. doi: 10.1016/j.jdent.2021.103673. [DOI] [PubMed] [Google Scholar]
- 28.Kamali A, Oryan A, Hosseini S, Ghanian MH, Alizadeh M, Baghaban Eslaminejad M, Baharvand H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regenera-tion of critical-sized bone defects. Mater Sci Eng C Mater Biol Appl. 2019;101:64–75. doi: 10.1016/j.msec.2021.112179. Erratum in: Mater Sci Eng C Mater Biol Appl 2021;126:112179. [DOI] [PubMed] [Google Scholar]
- 29.Idris A, Sophocleous A, Landao-Bassonga E, Ralston S van't Hof R, author. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619–5626. doi: 10.1210/en.2008-0150. [DOI] [PubMed] [Google Scholar]
- 30.Scutt A, Williamson EM. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif Tissue Int. 2007;80:50–59. doi: 10.1007/s00223-006-0171-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Lu HX, Wang J. Cannabinoid receptors in osteoporosis and osteoporotic pain: a narrative update of review. J Pharm Pharmacol. 2019;71:1469–1474. doi: 10.1111/jphp.13135. [DOI] [PubMed] [Google Scholar]
- 32.Yan W, Cao Y, Yang H, Han N, Zhu X, Fan Z, Du J, Zhang F. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Prolif. 2019;52:e12691. doi: 10.1111/cpr.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro G, Reyes-Resina I, Rivas-Santisteban R, Sánchez de Medina V, Morales P, Casano S, Ferreiro-Vera C, Lillo A, Aguinaga D, Jagerovic N, Nadal X, Franco R. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol. 2018;157:148–158. doi: 10.1016/j.bcp.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Kang M, Lee J, Park S. Cannabidiol induces osteoblast differentiation via angiopoietin1 and p38 MAPK. Environ Toxicol. 2020;35:1318–1325. doi: 10.1002/tox.22996. [DOI] [PubMed] [Google Scholar]
- 35.Rahaman O, Ganguly D. Endocannabinoids in immune regulation and immunopathologies. Immunology. 2021;164:242–252. doi: 10.1111/imm.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Li J, Mei G LianK, author. Restoration of osteogenic differentiation by overexpression of cannabinoid receptor 2 in bone marrow mesenchymal stem cells isolated from osteoporotic patients. Exp Ther Med. 2018;15:357–364. doi: 10.3892/etm.2017.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT. Interactions between extracellular signal-regulated kinase 1/2 and P38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res. 2021;36:2096–2097. doi: 10.1002/jbmr.561. Erratum in: J Bone Miner Res 2012;27:538-551. [DOI] [PubMed] [Google Scholar]
- 38.Whyte L, Ryberg E, Sims N, Ridge S, Mackie K, Greasley P, Ross RA, Rogers MJ. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.