ABSTRACT
The cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthetase (cGAS)–stimulator of interferon genes (STING) pathway, comprising the DNA sensor cGAS, the second messenger cyclic GMP–AMP (cGAMP), and the endoplasmic reticulum (ER) adaptor protein STING, detects cytoplasmic double-stranded DNA (dsDNA) to trigger type I-interferon responses for host defense against pathogens. Previous studies defined a model for the allosteric activation of cGAS by DNA-binding, but recent work reveals other layers of mechanisms to regulate cGAS activation such as the phase condensation and metal ions, especially the discovery of Mn2+ as a cGAS activator. Activation of the 2′3′-cGAMP sensor STING requires translocating from the ER to the Golgi apparatus. The sulfated glycosaminoglycans at the Golgi are found to be the second STING ligand promoting STING oligomerization and activation in addition to 2′3′-cGAMP, while surpassed levels of 2′3′-cGAMP induce ER-located STING to form a highly organized ER membranous condensate named STING phase-separator to restrain STING activation. Here, we summarize recent advances in the regulation of cGAS–STING activation and their implications in physiological or pathological conditions, particularly focusing on the emerging complexity of the regulation.
Keywords: innate immunity, cGAS–STING, cGAMP, manganese (Mn2+), sulfated glycosaminoglycans (sGAGs), biomolecular condensate, STING phase-separator
Introduction
The innate immunity detects and defends against the invasion of a broad range of pathogens, including bacteria, viruses, parasites, and fungi. Recognition of pathogens is mediated by the germline-encoded pattern recognition receptors, which recognize cognate pathogen-associated molecular patterns or damage-associated molecular patterns (Murphy et al., 2014). The cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthetase (cGAS)–stimulator of interferon genes (STING) pathway recognizes double-stranded DNA (dsDNA) released by infected pathogens or self-DNA mislocalized in the cytoplasm (Motwani et al., 2019). cGAS binds to dsDNA and becomes activated to synthesize the second messenger 2′3′-cyclic GMP–AMP (2′3′-cGAMP). The endoplasmic reticulum (ER) protein STING, also named MITA or ERIS (Zhong et al., 2008; Sun et al., 2009), detects 2′3′-cGAMP and undergoes a translocation from the ER to the Golgi, where it becomes oligomerized to recruit and activate the downstream kinase TANK-binding kinase 1 (TBK1). The phosphorylated STING by TBK1 scaffolds the interactions between interferon regulatory factor 3 (IRF3) and TBK1, allowing for the phosphorylation and activation of IRF3 by TBK1 (Zhang et al., 2019). IRF3 then moves into the nucleus to initiate the expression of various cytokines including type I-interferons (IFNs), which eventually induce the production of hundreds of interferon-stimulated genes for host defense (Ishikawa and Barber, 2008).
cGAS is a nucleotidyltransferase that is allosterically activated by dsDNA-binding and catalyzes the formation of 2′3′-cGAMP from adenosine triphosphate and guanosine triphosphate (GTP) (Civril et al., 2013; Diner et al., 2013; Gao et al., 2013b; Kranzusch et al., 2013; Sun et al., 2013; Wu et al., 2013; Zhang et al., 2013). Besides cytosol dsDNA, cytoplasmic manganese (Mn2+) released from membrane-enclosed organelle binds to cGAS to enhance the sensitivity of cGAS to dsDNA by several orders of magnitude upon viral infection (Wang et al., 2018). Cytoplasmic Mn2+ also triggers cGAS to synthesize cGAMP independent of dsDNA by a novel catalytic mode, initiating type I-IFN response and cytokine production without any infection (Zhao et al., 2020). Therefore, Mn2+, as a powerful agonist of cGAS–STING, is now used in the antitumor treatments and vaccine adjuvants (Lv et al., 2020; Zhang et al., 2021).
STING (encoded by STING1), an ER-transmembrane protein, is the scaffold protein to recruit and activate downstream TBK1 and IRF3, leading to the expression of type I-IFNs and multiple pro-inflammatory cytokines (Ishikawa and Barber, 2008; Zhong et al., 2008; Sun et al., 2009). To be activated, STING undergoes a conformational change from ‘open’ to ‘close’, and then translocates to the Golgi apparatus to be oligomerized or polymerized (Shang et al., 2019; Zhang et al., 2019). Oligomerized STING recruits TBK1 to form STING–TBK1 oligomers to activate TBK1 by trans-phosphorylation, followed by TBK1 phosphorylation on STING and IRF3 to induce type I-IFNs and other cytokines (Shang et al., 2019; Zhang et al., 2019). cGAMP-bound STING exits ER via coat protein complex-II (COP-II) vesicles to translocate to the Golgi and trans-Golgi network (TGN). At the Golgi and TGN, STING oligomerizes with the help of sulfated glycosaminoglycans (sGAGs) and gets palmitoylated further (Fang et al., 2021; Taguchi et al., 2021). The coat protein complex-I (COP-I) vesicles regulate STING Golgi-to-ER retrograde traffic (Taguchi et al., 2021).
Biomolecular condensates are non-membrane-surrounded compartments in eukaryotic cells that allow molecules to be concentrated within condensates while still exchanging with the surroundings (Nott et al., 2015; Zeng et al., 2016; Banani et al., 2017; Boeynaems et al., 2018; Wang and Zhang, 2019). The dynamic of molecules and kinetics of biochemical reactions in the condensates can be regulated in a physical manner, which is different from classic membrane-surrounded organelles (Brangwynne et al., 2009; Li et al., 2012; Patel et al., 2015; Shin and Brangwynne, 2017). Recently discovered cGAS–DNA droplets and STING ER membranous biocondensates indicate that biomolecular condensation is a new mechanism modulating cGAS–STING signaling. Cytosol DNA-induced cGAS liquid-like droplets display enhanced cGAS activity (Du and Chen, 2018). On the contrary, continuously produced cGAMP by activated cGAS induces ER-resident STING to form an ER membranous condensate with a highly organized puzzle-like structure. Moreover, the STING condensate quickly undergoes a gel-like phase transition to isolate cGAMP-bound STING and TBK1 from IRF3, thus preventing STING from overreaction (Yu et al., 2021). The puzzle-like membranous structure of STING condensate highly resembles cubic membranes, a type of widely reported membrane structure that has been discovered for more than 60 years with only few clues about its initiation and functions (Yu et al., 2021).
In this review, we focus on the recent discoveries regarding the molecular mechanism of cGAS–STING activation and regulation, with a special focus on the cGAS activation by DNA and Mn2+, the STING ER–Golgi translocation and its activation by sGAGs, and the formation and function of STING membranous biomolecular condensates.
cGAS activation by dsDNA
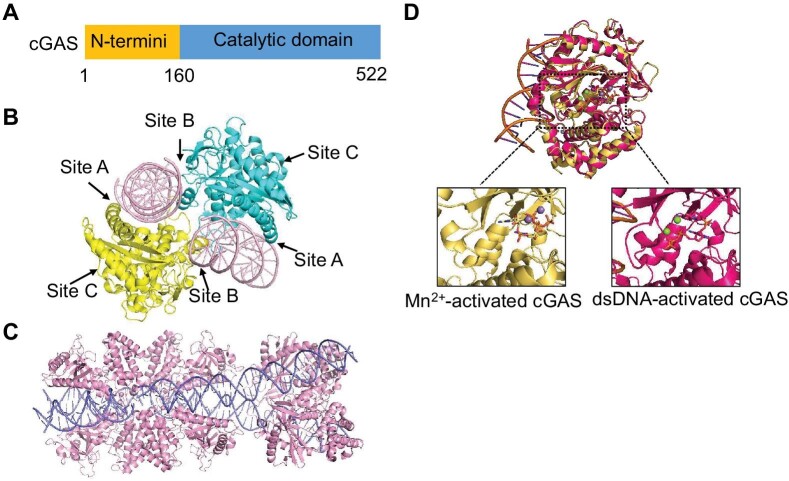
cGAS is constituted by a disordered amino-terminal stretch and a conserved Mab21 domain (residues aa 161–512 in humans) (Figure 1A). The Mab21 domain belongs to the nucleotidyltransferase superfamily (Kuchta et al., 2009), mediating dsDNA recognition and cGAMP synthesis (Civril et al., 2013; Gao et al., 2013c; Sun et al., 2013). The Mab21 domain consists of an N-lobe with a Rossman fold for substrate-binding and catalysis and a C-lobe bundle with a zinc-finger for dsDNA-binding and dimerization (Civril et al., 2013; Gao et al., 2013c; Sun et al., 2013). The catalytic domain of cGAS has three major DNA-binding sites (A, B, and C) (Figure 1B). DNA-binding site A recognizes dsDNA and induces the conformational switch to activate cGAS (Li et al., 2013; Zhang et al., 2014). The site B of cGAS binds to dsDNA and facilitates the formation of the 2:2 dimer complex (Li et al., 2013; Zhang et al., 2014). The 2:2 dimer complex is further stabilized by the interaction between zinc-fingers from each cGAS protomer, a process required for the enzymatic activation of cGAS (Civril et al., 2013). The site C promotes multivalent interaction among cGAS and DNA, thus promoting cGAS droplet formation and cGAMP production (Xie et al., 2019).
Figure 1.
Activation of cGAS by DNA and Mn2+. (A) cGAS contains a disordered N-terminus and a catalytic domain. (B) The crystal structure of cGAS–DNA complex with three DNA-binding sites labelled (PDB: 4LEZ). (C) The crystal structure of oligomeric cGAS bound to a long dsDNA, forming a ladder-like structure (PDB: 5N6I). (D) The crystal structure of dsDNA-activated cGAS (red, PDB: 4K98) and Mn2+-activated cGAS (yellow, PDB: 7BUJ). Conformation changes of Mn2+-activated cGAS are overall similar to that of dsDNA-activated cGAS but form a unique η1 helix at the activation loop to empty the catalytic pocket, thus rendering it catalytically competent.
However, dsDNA less than ∼30 base-pairs is unable to activate human cGAS in cells (Civril et al., 2013; Gao et al., 2013b; Li et al., 2013; Zhang et al., 2014), indicating that cGAS activation by dsDNA is length-dependent. The cGAS dimers can prearrange the flanking DNA to promote subsequent binding of more cGAS dimers to form a ladder-like structure and further promote cGAS activation by DNA (Andreeva et al., 2017; Figure 1C). Mutations in cGAS are rapidly accumulated during evolution. There are <60% of amino-acid identity between human and mouse cGAS. Interestingly, mouse cGAS is quite sensitive to shorter DNA species compared to human cGAS (Zhou et al., 2018). Structural and biochemical comparisons between human and mouse cGAS unveil two residue substitutions specific in human cGAS, K187 and L195, which are critical in determining the preference of human cGAS for longer DNA species (Zhou et al., 2018). Mouse cGAS with human-like N172K and R180L mutations are sufficient to gain DNA-length specificity and a restrained cGAMP production like human cGAS, while human cGAS with mouse-like L187N and L195R mutations is able to be activated by shorter DNA and has a higher enzyme activity as mouse cGAS. The results reveal a species-specific regulation of cGAS activities by several nucleic acids (Zhou et al., 2018). This mode of cGAS activation probably provides a safeguard mechanism in human cells to avoid spurious cGAS activation, as cGAS is triggered only when the long-length dsDNA exceeds a concentration threshold.
cGAS activation by Mn2+
Metal ions are critical components or regulators of nearly half of all enzymes and play pivotal roles in almost every aspect of biological processes. The important role of metal ions in immune responses and cancer therapies is receiving more and more attention and is one of the promising future directions in cancer immune. Therefore, metalloimmunology and metalloimmunotherapy have been termed recently to highlight the pivotal role of metal elements in immune responses as well as their cutting-edge applications in cancer immunotherapy (Li et al., 2019; Moon et al., 2021; Sun et al., 2021a). Particularly, divalent cations including Ca2+, Zn2+, and Mn2+ have been demonstrated to be involved in the regulation of the cGAS–STING pathway. Ca2+ is reported to regulate STING through the Ca2+ sensor stromal interaction molecule 1 (STIM1), which restrains STING to the ER (Srikanth et al., 2019). The STIM1 deficiency disrupts Ca2+ homeostasis and promotes the ER-to-Golgi translocation of STING, causing an enhanced STING activation (Srikanth et al., 2019). Recently, Du and Chen (2018) reported that Zn2+ increases cGAS activation by promoting the phase separation of cGAS–DNA complex. TPEN, a Zn2+ chelator, inhibits cGAS activation in cells, but it is noteworthy that TPEN is also able to chelate Fe2+, Mn2+, and some other divalent cations (Shumaker et al., 1998). Wang et al. (2018) found that viral infection induces the release of Mn2+ ions from membrane-enclosed organelles such as mitochondria and the Golgi into the cytosol. Accumulation of Mn2+ in cytosol tremendously enhances the sensitivity of cGAS to dsDNA and augments the binding affinity of STING to cGAMP, thus promoting the activation of the cGAS–STING pathway. Moreover, treatment of cells or mice with Mn2+ (such as MnCl2) can stimulate them into an antiviral state through the cGAS–STING-dependent type I-IFN responses. The dietary-induced Mn-insufficient mice produce less cytokines and are more vulnerable to DNA viruses as STING-deficient (Sting1 −/–) mice, reinforcing the critical role of Mn2+ in cGAS–STING activation upon DNA virus infection (Wang et al., 2018).
Importantly, physiological amount of Mn2+ activates cGAS mutants, which are defective in dsDNA/dimerization-dependent activation, both in vitro and in cells, indicating that Mn2+ activates monomeric cGAS (Hooy et al., 2020). Structural analysis reveals that the Mn2+-activated cGAS undergoes overall similar conformational changes to dsDNA-activated cGAS, but forms a unique η1 helix at the activation loop to empty the catalytic pocket, thus rendering it catalytically competent (Figure 1D). Moreover, in Mn2+-activated cGAS structures, the two linear intermediates pppG[2′-5′]pG and pG[2′-5′]pA bind to the active site in an inverted orientation compared to those in DNA-activated cGAS structures, suggesting a non-canonical cGAMP synthesis without substrate flip-over, which may explain the significantly accelerated activity of cGAS in the presence of Mn2+ (Wang et al., 2018; Hooy et al., 2020). Unlike the two Mg2+ in DNA-activated cGAS, two catalytic Mn2+ are found to bind to the triphosphate moiety of the inverted substrate, but without coordination by the acidic catalytic triad residues (Zhao et al., 2020). Therefore, changes in cytoplasmic concentration of Mn2+ may be a second messenger used by innate immune cells for their intracellular communication. Additionally, cGAS is found to preferentially utilize Mn2+ over Mg2+ for its catalytic activity, and Mg2+ facilitates Mn2+ utilization rather than competes against it (Hooy et al., 2020).
Mn2+-mediated STING activation is also important for the host defense against bacterial infections. A bacterial type VI secretion system (T6SS)-secreted 48-residue micropeptide crucial for the pathogenesis of Yersinia pseudotuberculosis is identified to inhibit c-di-GMP-triggered STING activation. The micropeptide inhibits STING signaling by chelating Mn2+ in host cells, mediating a previously unrecognized immune evasion strategy by bacteria (Zhu et al., 2021). Importantly, similar T6SS clusters containing putative Mn2+-binding effectors have been identified in diverse bacterial strains, suggesting that the Mn2+ sequestration mechanism might be employed by a broad range of pathogenic bacteria to suppress host innate immune responses.
Applications of Mn2+-activated cGAS–STING signaling
The ability of Mn2+ to activate cGAS-dependent signaling makes it applicable to develop Mn2+-based antimicrobial/antitumor drugs or immune adjuvants for vaccines (Van Herck et al., 2021). The nanoparticle and liposome-based Mn2+ delivery strategies are of particular interest (Hou et al., 2020; Wang et al., 2021c). In fact, as a natural micronutrient necessary for diverse biological activities, Mn2+ facilitates antigen uptake and presentation by the antigen-presenting cells (APCs), as well as germinal center formation in the adaptive immune responses (Zhang et al., 2021). A colloidal manganese salt (Mn jelly, MnJ) is formulated and proves to be an effective adjuvant for stimulating both humoral and cellular immune responses (Zhang et al., 2021). MnJ also functions as a mucosal adjuvant to promote secreted IgA production via intranasal immunization. The adjuvant effects of MnJ have been proved for various antigens, including proteins/peptides as T cell-dependent antigens and T cell-independent antigens such as the bacterial capsule polysaccharides, indicating that MnJ is a potent universal immune adjuvant (Zhang et al., 2021). Importantly, besides MnJ, various Mn2+-containing nanoparticles have been formulated and significantly improved the efficacy of vaccines against influenza virus (Cui et al., 2021), hepatitis B virus (Lin et al., 2021), rabies virus (Wang et al., 2021c), and novel coronaviruses (Sun et al., 2021b; He et al., 2021; Wang et al., 2021b) in different animal models.
Immune checkpoint molecules, such as the well-known PD-1 and PD-L1, are essential as brakes to prevent the overactivation of T cell-mediated immune responses. However, tumor cells harness these inhibitory mechanisms to escape the immune surveillance (Ishida et al., 1992; Freeman et al., 2000; Ahmadzadeh et al., 2009). The immune checkpoint inhibitors boost the immune response. Unfortunately, only ∼20% of tumor patients respond to the immunotherapies with high efficacies, presumably due to inefficient activation of the innate immune responses (Topalian et al., 2015; Ribas et al., 2016; Huang et al., 2017). The cGAS–STING pathway has gained enormous interests in the field of immuno-oncology. Activation of this pathway can drive both innate and adaptive immune responses by promoting APC cell activation and the cross-priming of T cells (Yum et al., 2019), especially among immunosuppressive tumor microenvironments. Lv et al. (2020) reported that Mn2+ is critical for the innate immune system to surveil the tumor cells, as Mn-insufficient mice exhibit less-controlled tumor growth and metastasis than normal mice, with a decreased level of tumor-infiltrating CD8+ T and NK cells. More importantly, when administrated intranasally, intravenously, or intratumorally, Mn2+ induces robust and systemic antitumor responses in several mouse models (lung metastatic melanoma B16F10, colon adenocarcinoma MC38, Lewis lung carcinoma LLC, and T lymphoma E.G7) (Lv et al., 2020). The antitumor responses are also exhibited in multidrug (immuno)-resistant cancer patients in a phase I clinical trial (Lv et al., 2020). In these mouse models, Mn2+ treatments effectively promote NK cell function, DC/macrophage maturation/activation, and CD8+ T cell differentiation/activation to suppress the tumor growth and metastasis (Lv et al., 2020). Mn2+ also exhibits a more efficient antitumor effect when combined with anti-PD-1 antibody therapy (Lv et al., 2020). Moreover, in patients with advanced metastatic solid tumors who failed in standard anticancer treatments such as chemotherapies, radiotherapy, and immunotherapies, Mn2+ administration exhibits a notably improved therapeutic efficacy with 45.5% objective response and 90.9% disease control (Lv et al., 2020). Significantly, similar enhanced antitumor effects by Mn2+-promoted cGAS–STING activation in various antitumor immunotherapies have been reported (Hou et al., 2020; Chen et al., 2021; Gao et al., 2021; Li et al., 2021a; Liu et al., 2021; Song et al., 2021; Sun et al., 2021a; Wang et al., 2021a; Yang et al., 2021; Yi et al., 2021). Considering the component simplicity and steadiness of Mn2+, Mn2+ treatment or combined antitumor therapies including immunotherapies may have promising clinical potential.
However, the actions of Mn2+ in physiological and pathological contexts, especially in immunity, remain to be fully understood. Unlike Mg2+, which is abundant in cytoplasm, cellular Mn2+ is mainly restrained in membrane-coated compartments like the Golgi apparatus and mitochondria (Martinez-Finley et al., 2013; Carmona et al., 2014). Viral or bacterial infection liberates Mn2+ from mitochondria and the Golgi to be accumulated in the cytoplasm, but the underlying mechanisms of Mn2+ release remain to be fully elucidated. Furthermore, there is no chemical tools available for the non-invasive and quantitative detection of Mn2+ in living cells or tissues now. Therefore, developing new tools and techniques to trace the location/distribution and dynamic changes of the labile Mn2+ pool in different cellular compartments is imperative. Moreover, the potential toxic effect of Mn2+ in human use including neurotoxicity should also be carefully evaluated.
Regulation of cGAS activation by biomolecular condensation
Moreover, the cytosolic dsDNA, especially long DNA molecules, substantially induce liquid–liquid phase separation of cGAS–DNA to form liquid-like droplets that promote cGAS activation and 2′3′-cGAMP production (Du and Chen, 2018). The disordered and positively charged N-terminus of cGAS binds to dsDNA and is critical for the cGAS–DNA phase separation (Wang et al., 2017; Du and Chen, 2018). Three-prime repair exonuclease 1 (TREX1) is a cytosolic exonuclease that digests DNA. Its deficiency causes the accumulation of self-DNA in the cytoplasm, which leads to severe autoimmune diseases mainly through aberrant cGAS–STING activation (Yan et al., 2010; Yan, 2017). The cGAS droplet is also shown to selectively restrain TREX1 to the external surface of the droplet, thus suppressing the DNA degradation by TREX1 and prolonging the activation of cGAS by DNA (Zhou et al., 2021). Interestingly, viral tegument factors, such as ORF52, VP22, and ORF9 proteins from herpes viruses, are demonstrated to interfere with the cGAS–DNA droplet formation by binding to dsDNA and replacing cGAS in the droplet, which effectively inhibit cGAS activation and subvert host defense (Xu et al., 2021; Figure 2).
Figure 2.
cGAS activation is regulated by biomolecular condensation. Cytosolic dsDNA substantially induces liquid–liquid phase separation of cGAS–DNA to form liquid-like droplets that promote cGAS activation and 2′3′-cGAMP production. TREX1, a cytosolic exonuclease that digests DNA, is selectively restrained to the external surface of the cGAS droplet, thus suppressing the DNA degradation within droplets and prolonging the activation of cGAS by DNA. Formation of cGAS–DNA droplets can be interfered by viral tegument factors, such as ORF52 and VP22 proteins from herpes viruses. Viral tegument factors bind to dsDNA and replace cGAS in the droplet, thus effectively inhibiting cGAS activation and subverting host defense.
STING activation by cyclic dinucleotides
The 2′3′-cGAMP produced by cGAS, as well as other cyclic dinucleotides (CDNs) from invaded bacteria, is detected by the ER-localized transmembrane protein STING. The C-terminal domain of STING containing the ligand-binding domain (LBD) and the cytosol-facing C-terminal tail (CTT) recognizes and binds to cGAMP. Cryo-electron structures (cryo-EM) of full-length STING proteins from human or chicken in the apo or 2′3′-cGAMP-bound states are recently resolved. The structures reveal a topology of the N-terminal transmembrane segment of STING, in which STING spans the ER membrane for four times. In the apo state, inactive STING molecules reside as dimers through interactions at the transmembrane regions as well as the LBD, with its LBD domains forming a V-shaped structure for ligand binding (Huang et al., 2012; Ouyang et al., 2012; Shang et al., 2012; Yin et al., 2012; Zhang et al., 2013). Upon binding to cGAMP, the lid of the LBD closes, leading to a 180° rotation of the LBD around the transmembrane domain. The rotation is coupled with conformational changes of the LBD to promote STING oligomerization in a side-by-side manner. The oligomerization of STING is required for its activation by CDNs.
Oligomerized STING recruits TBK1 to initiate downstream signaling. The CTT of STING contains a highly conserved TBK1-binding motif as well as a pLxIS motif, which is necessary for IRF3 recruitment and activation (Liu et al., 2015; Zhao et al., 2016). Recent cryo-EM structure of full-length chicken STING in complex with human TBK1 further unveils that the STING CTT has a β-strand-like conformation. The CTT is inserted into the groove between the kinase domain of one TBK1 and the scaffold and dimerization domain of another neighboring TBK1 (Zhang et al., 2019).
Interestingly, the phosphorylation site S366 of STING by TBK1 is far away from the active site of the directly associated TBK1, but can engage with TBK1 that binds to the adjacent STING (Zhang et al., 2019). In addition, there is minimal conformational change between the kinase domains of apo-TBK1 and TBK1 in complex with STING, suggesting that TBK1 is not activated by conformational changes in the kinase domain upon binding to STING (Tu et al., 2013; Zhang et al., 2019). Due to geometric constraints, the kinase domain of TBK1 normally cannot phosphorylate S172 in its activation loop to get activated, regardless of two TBK1 molecules are bound in close distance on one STING dimer (Larabi et al., 2013; Tu et al., 2013; Zhang et al., 2019). Besides, co-immunoprecipitation and imaging studies suggest that the inactive STING dimers on the ER have already recruited some TBK1 without TBK1 phosphorylation (Zhang et al., 2019). All these results suggest that STING phosphorylation by TBK1 requires the oligomerization of both STING and TBK1 molecules. The binding of TBK1 to STING oligomers induces the clustering of TBK1 and trans-autophosphorylation of TBK1. The TBK1 that is bound to the two CTT of one STING dimer can phosphorylate S366 of neighboring STING proteins that are not bound to TBK1 (Zhang et al., 2019). Activated STING and TBK1 then recruit and phosphorylate IRF3.
The Golgi translocation-dependent STING activation
The translocation of STING from the ER to the Golgi is required for STING activation by CDNs. Blocking STING translocation by Shigella effector IpaJ or Brefeldin A entirely inhibits the downstream signaling and cytokine production, regardless of the cGAMP is continuously produced by activated cGAS (Ishikawa et al., 2009; Konno et al., 2013; Dobbs et al., 2015). The COP-I and COP-II vesicles mediate the cargo trafficking between the ER and the Golgi and are thought to mediate the trafficking of STING as well. Particularly, COP-II is needed for vesicles sprouting from the ER to translocate to the Golgi (Barlowe and Helenius, 2016). The COP-II complex is composed of Sar1 (a small GTPase), Sec23, Sec24, Sec13, and Sec31. Knockdown of Sar1A and Sar1B (mammalian paralogs of Sar1), Sec13, Sec23, Sec24, or Sec31 impairs the translocation of STING from the ER to the Golgi and activation of STING, suggesting a critical role of COP-II vesicle-mediated translocation in STING activation (Ogawa et al., 2018; Sun et al., 2018; Gui et al., 2019; Ran et al., 2019). Additionally, ZDHHC1 (Zhou et al., 2014) and iRhom2 (Luo et al., 2016) have been reported to positively regulate STING translocation and activation. Eventually, the activated STING molecules are translocated to the lysosome for degradation (Gonugunta et al., 2017).
Compared to the COP-II complex that mediates the protein translocation from the ER to the Golgi, COP-I complex mediates the retrograde Golgi-to-ER translocation (Letourneur et al., 1994; Scales et al., 1997). Recently, it has been reported that the mutation of COPA gene, which encodes COP-α of the COP-I complex, causes constitutive STING ER-exit and activation (Deng et al., 2020; Lepelley et al., 2020; Mukai et al., 2021; Steiner et al., 2022). Heterozygous missense mutation in WD40 domain of the COPA gene is related to the COPA syndrome, which is a Mendelian syndrome with autoimmune disorder featured with interstitial lung disease, joint inflammation, and elevated type I-IFN signaling (Watkin et al., 2015; Volpi et al., 2018). With the expression of autoimmune disease-related COP-α variants, STING cannot be retrieved back to the ER but accumulates on the Golgi. High concentration of STING molecules on the Golgi directly induces cGAS-independent but palmitoylation-dependent STING activation at the TGN (Mukai et al., 2021). SURF4, a protein that circulates between the ER/ER–Golgi intermediate compartment (ER/ERGIC), is an adaptor protein that facilitates COPA-mediated retrograde translocation (Deng et al., 2020; Mukai et al., 2021). Upon viral infection, cGAMP efficiently impairs the formation of STING/Sur4/COP-α complex, releasing STING from ER retrogradation. All these results support a notion that STING can be activated without cGAMP binding and factors in the Golgi drive STING activation, which is consistent with the role of sGAGs as the second STING ligand (see below).
Niemann–Pick type C1 (NPC1), a lysosomal membrane protein, is recently identified to recruit STING to the lysosome after STING activation at the Golgi. NPC1 deficiency enhances STING signaling by promoting STING accumulation at the Golgi via SREBP2–SCAP and impairing degradation of activated STING (Chu et al., 2021). In the steady state, TOLLIP is found to stabilize STING from degradation by direct interaction (Pokatayev et al., 2020).
STING activation by sGAGs in the Golgi
However, it remains unclear why STING activation requires its translocation from the ER to the Golgi. Recently, sGAGs synthesized in the Golgi apparatus are demonstrated to be critical for STING oligomerization and activation. Through a genome-wide CRISPR–Cas9 screen, genes involved in the GAG biosynthesis and sulfation are identified to be critical for STING activation (Fang et al., 2021).
Previous work demonstrated that negatively charged sulfate groups in sGAGs provide a great multivalent landing plug for proteins through electrostatic interactions. sGAGs are also known to interact with various proteins via binding to and neutralizing positively charged or polar residues of targeted proteins and to facilitate them to polymerize (Soares da Costa et al., 2017; Smock and Meijers, 2018). Similarly, STING binds to sulfated GAGs through its luminal, positively charged and polar residues. These residues are evolutionarily conserved, and selective mutation of specific residues inhibits STING activation (Fang et al., 2021). Furthermore, purified or chemically synthesized sGAGs can directly induce the oligomerization and activation of STING in vitro (Fang et al., 2021). Thus, sGAGs in the Golgi are necessary and sufficient to drive STING oligomerization and activation.
The chain-length and O-linked sulfation of sGAGs impact the level of STING oligomerization and, thereby, its activation (Fang et al., 2021). Deficiency of SLC35B1, which transports the sulfur donor into the Golgi for GAG sulfation, impairs STING activation and host defense to DNA viral infection. Interestingly, sGAGs are also important for the accumulation of STING at the Golgi after cGAMP stimulation (Fang et al., 2021). It was reported that STING becomes oligomerized and autoactivated when accumulated in the Golgi apparatus (Mukai et al., 2016; Deng et al., 2020; Lepelley et al., 2020). sGAGs, which are synthesized in the Golgi (Vigetti et al., 2014), may activate STING via promoting STING retention on the Golgi as well as STING oligomerization. The sGAGs-driven STING activation explains how STING oligomerization is regulated and provides a mechanistic understanding of the Golgi translocation-dependent STING activation.
In addition, previous reports demonstrate that STING palmitoylation at C88 and C91 also promotes STING oligomerization by allowing STING to cluster into lipid rafts at the TGN (Linder and Deschenes, 2007; Mukai et al., 2016; Taguchi et al., 2021). Mutation of C88 and C91 or treating cells with the palmitoylation inhibitor 2-bromopalmitate inhibits STING palmitoylation and IRF3 phosphorylation (Mukai et al., 2016). However, STING translocation from the ER to the Golgi is not affected in the STING C88/C91-mutant cells (Mukai et al., 2016). It is conceivable that STING palmitoylation facilitates its clustering into lipid rafts on the Golgi from STING cytosolic side whereas sGAGs induce STING oligomerization from STING luminal side, altogether leading to the full activation of STING and TBK1.
Notably, STING–sGAGs binding is probably regulated by levels of acidification. It has been well documented that after CDN binding, STING translocates from the ER to the perinuclear compartments that include the Golgi, endosomes, and autophagy-related compartments, while intracellular sGAGs are compartmentalized into organelles like the Golgi, secretory vesicles, and endosomes (Kolset et al., 2004). Interestingly, acidification develops along with the ER (pH = 7.0), the Golgi complex, and reaches its peak in the TGN (pH = 6.0) (Kim et al., 1998; Llopis et al., 1998). Since the imidazole group of histidine (H42 in human STING and H50 in mouse STING) is positively charged at lower pH, the high affinity of sGAGs and STING is only found at the acidic Golgi (Fang et al., 2021). The pH-regulated STING–sGAGs interaction may explain why STING oligomerization happens mainly on the TGN and demonstrate an elaborate regulation of STING activation along with the Golgi apparatus translocation.
Recently, cryo-EM structure of STING and a small-molecule compound C53 reveals a novel binding pocket of STING in its transmembrane domain. C53 promotes STING oligomerization by binding to an undiscovered pocket formed among two TM2 and two TM4 helices of two STING protomers (Lu et al., 2022). The residues H50 S53 in TM2 and Y106 M120 in TM4 are critical to cGAMP–C53-induced STING oligomerization (Lu et al., 2022). Notably, these luminal residues in the transmembrane domain binding pocket are also critical for sGAGs–STING interaction, and sGAGs have been proved to promote STING oligomerization upon viral infection, as the second STING ligand (Fang et al., 2021). Thus, it is quite possible that sGAGs are the natural ligand of the newly discovered STING binding pocket.
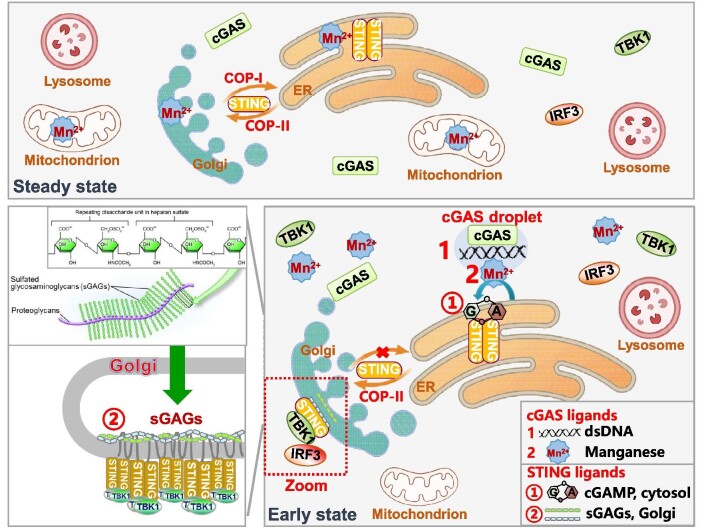
Collectively, it is likely that STING dimers are continuously transported to the Golgi and TGN from the ER by the COP-II complex. However, in a steady state, when the formation of STING/SURF4/COP-α axis is not impaired by cGAMP, STING proteins transported to the Golgi are immediately caught back by the COP-I complex, preventing STING from reaching the TGN and getting oligomerized. Upon cGAMP binding, STING released from Golgi-to-ER retrograde trafficking is able to translocate to the TGN, where the lower pH value enhances sGAGs–STING interaction and traps STING upon arrival. The dynamic equilibrium of STING ER–Golgi transportation regulates STING activation precisely (Figure 3).
Figure 3.
The cGAS–STING pathway at steady state and early state of activation. A schematic illustrates the process of dsDNA-induced cGAS–STING activation. At a steady state, STING constantly translocates to the Golgi via COP-II vesicles but is immediately retrieved back to the ER by COP-I vesicles. Upon viral infection, cGAS activated by the cGAS ligand, the cytoplasmic dsDNA, starts to synthesize the STING ligand 2′3′-cGAMP. Multivalent interactions among cGAS and long DNA molecules induce liquid-like cGAS–DNA droplet formation to facilitate cGAS activation. Mn2+, as a second cGAS ligand, is released from membrane-enclosed organelles and accumulates in the cytoplasm upon infection. Mn2+ promotes cGAS activation by enhancing the sensitivity of cGAS to dsDNA. cGAMP interrupts the STING Golgi-to-ER retrogradation, so STING dimer is released to the Golgi. At the Golgi apparatus, the lower pH value enhances interaction between the second STING ligand sGAGs and STING by electrostatic interaction, promoting STING accumulation and oligomerization. Oligomerized STING recruits TBK1 and promotes TBK1 oligomerization and autophosphorylation, which in turn phosphorylates STING at S366. Activated STING–TBK1 oligomers then recruit and phosphorylate IRF3 to initiate the expression of various cytokines.
Negative regulation of STING by the STING phase-separator
Biomolecular condensation mediated by polyvalent interactions among molecules is another underlying mechanism modulating the cGAS–STING pathway. Biomolecular condensates are non-classic cellular compartments that concentrate molecules without a physical barrier, efficiently separating the interior components from surrounding material (Brangwynne et al., 2011; Banani et al., 2017; Boeynaems et al., 2018; Wang and Zhang, 2019). Usually, formation of biomolecular condensates is driven by weak multivalent intramolecular interactions provided by soluble proteins with modular domains or intrinsically disordered regions (IDR) (Li et al., 2012; Nott et al., 2015; Zeng et al., 2016). Biomolecular condensates often become viscoelastic, transforming from a liquid-like state into a gel-like state that stops exchanging molecules with the outside (Feng et al., 2014; Lin et al., 2015; Harmon et al., 2017). Biomolecular condensates affect biological reaction kinetics and specificity via regulating protein concentration within condensate or molecular motion via viscoelasticity (Brangwynne et al., 2009; Shin and Brangwynne, 2017).
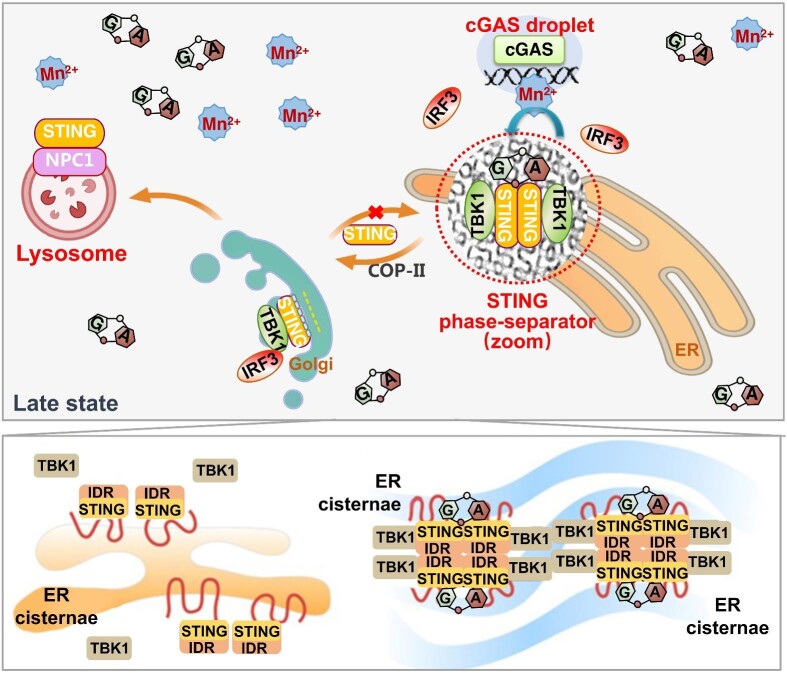
Apart from DNA-induced cGAS droplets (discussed above), cGAMP-induced ER membranous STING biomolecular condensate, which is named the STING phase-separator, is also reported recently (Yu et al., 2021). Upon infection of DNA virus, cGAMP produced by the activated cGAS first activates STING by promoting STING translocation from the ER to the Golgi. In the late stage of infection, however, continuously activated cGAS results in a constantly increased cGAMP concentration. When cGAMP levels exceed the threshold, ER-resident (untranslocated and unactivated) STING is induced to form a highly organized puzzle-like ER membranous biocondensate. The formation is supposed to be driven by both cGAMP-induced conformational change of STING dimers and weak intramolecular multivalent interactions among STING IDRs. It is hypothesized that such weak intramolecular interactions among STING proteins initially induce the formation of a highly fluidic membranous structure. The intramolecular interactions anneal or zipper ER cisternae inversely and transform the otherwise cytosolic side of cisternae into a restricted composition. With time, even higher levels of cGAMP further induce this membranous condensate to transit into a gel-like state, thus decreasing molecular dynamic within the condensate. In this way, the puzzle-like ER membranous STING phase-separator separates cGAMP-bound STING and TBK1 from their downstream protein IRF3, which prevents innate immunity from overactivation, presumably acting like a ‘STING–TBK1–cGAMP sponge’ (Figure 4).
Figure 4.
The cGAS–STING pathway at the late state of activation. A schematic illustrates the late state of cGAS–STING activation. At the end of STING activation, NPC1, a lysosome protein, recruits STING to the lysosome, where STING is degraded. Moreover, when continuously accumulated cytosol 2′3′-cGAMP exceeds a concentration threshold, it initiates ER-resident STING to form the STING phase-separator, a membranous biomolecular condensate with a highly organized puzzle-like membranous structure. The STING phase-separator is organized by multivalent interactions among STING IDR regions (aa 309–342, bottom). It functions to suppress the cGAS–STING pathway by trapping cGAMP-bound STING and TBK1 in gel-like condensates, thus buffering the concentration of free cytosolic cGAMP, STING, and TBK1.
2′3′-cGAMP activates STING potently. The affinity of 2′3′-cGAMP for human STING is very high, with a dissociation constant of 4.59 nM, which is further augmented by Mn2+. Theoretically, it should be degraded rapidly by their specific phosphodiesterases like other second messengers, such as cAMP/cGMP, c-di-GMP, c-di-AMP, and 3′3′-cGAMP, to ensure a well-controlled signaling. However, there is only little knowledge about how 2′3′-cGAMP concentrations are regulated within mammalian cells. In bacteria, there are three 3′3′-cGAMP-specific phosphodiesterases (V-cGAP1/2/3) in Vibrio cholera (Gao et al., 2015) and another one named PmxA in Myxococcus xanthus (Yadav et al., 2019). In mammals, only the extracellular matrix- and/or ER lumen-localized cGAMP hydrolase ENPP1 has been reported (Li et al., 2014), which indicates that 2′3′-cGAMP must be transported across the plasma membrane or the ER membrane for degradation. So far, no intracellular hydrolase degrading 2′3′-cGAMP has been discovered. It is likely that the ER membranous ‘STING–TBK1–cGAMP sponge’ may facilitate cGAMP ER-membrane transportation for ENPP1 degradation. Thus, this 2′3′-cGAMP-driven STING phase-separator may circumvent the requirement for the intracellular 2′3′-cGAMP hydrolase(s) to degrade the excessive 2′3′-cGAMP.
As the first reported highly organized membranous biocondensate formed by ER transmembrane protein, STING phase-separator is actually a discrete phase consisting of protein molecules and highly organized membranes. Multivalent interaction among STING molecules is only necessary but not sufficient for the formation of puzzle-like membranes. It is speculated that the highly condensed STING molecules may induce spontaneous membrane curvature (Stachowiak et al., 2012), together with weak antiparallel interaction among cytosolic domain (STING IDR), to induce the puzzle-like structure formation. Therefore, STING phase-separator is more like a ‘liquid crystal’ rather than a ‘liquid droplet’ referring to its physical properties.
Notably, the puzzle-like membrane structure in STING phase-separator looks like a type of membrane structure called cubic membranes. Cubic membranes (also termed honeycombed lamellae/cristae, puzzles tridimensionnels, organized smooth ER, tubuloreticular structures, cylindrical confronting cisternae, etc.) are highly curved, three-dimensional nanoperiodic membrane structures corresponding to mathematically well-defined triply periodic minimal surfaces (Chin et al., 1982; Yamamoto et al., 1996). Cubic membranes are possibly first described back in 1959 by Pappas and Brandt (1959) in the mitochondria of Pelomyxa carolinensis Wilson (Chaos chaos L.) and by Almsherqi et al. (2009) in the mitochondria of spermatids of Euscorpius Flavicaudis, followed by hundreds of papers demonstrating that they can actually emerge from almost any cytomembranes, including the ER, the nuclear envelope, mitochondrial membranes, and the Golgi complex. Actually, they have been observed in enormous types of cells across all kingdoms of life under different physiological or pathological conditions, especially in stressed, diseased, or virus-infected cells. However, knowledge about the formation and function of such structures in cells is very limited, and research so far remains descriptive (Almsherqi et al., 2009; Chong and Deng, 2012). Accordingly, these morphologies obtain >130 different nicknames (Almsherqi et al., 2009).
Importantly, cubic membranes have been frequently reported to occur in the pathogenesis of viral infection, neoplasia, muscular dystrophy, and many types of autoimmune diseases (Almsherqi et al., 2009). They are even regarded as an indicator of infection by HBV (Schaffner et al., 1977) and SIV/HIV (Grimley and Schaff, 1976; Kostianovsky et al., 1987; Lee et al., 1988; Kaup et al., 2005), viruses known to activate the cGAS–STING pathway (Sun et al., 2012; Gao et al., 2013a). RNA viruses, however, tend to induce mitochondrial cubic membranes (Schaffner et al., 1977) or form membranous cytoplasmic inclusions (Schaff et al., 1992). Strikingly, ER cubic membranes are found in lymph node tissues of almost all AIDS patients (Orenstein et al., 1983; Sidhu et al., 1983; Hammar et al., 1984), thus provoking much clinical and scientific interest. Nevertheless, the nature and pathogenesis of such structures remain obscure, although studies indicate a possible relation to the elevation of type I-IFNs (Luu et al., 1989), most likely via the activation of the cGAS–STING pathway. With the inspiration of STING phase-separator, more attention and efforts are needed to solve the long-lasting puzzles about the initiation and function of cubic membranes, especially those virus-related ones.
Positive regulation of STING by PC7A–STING condensates
Besides the cGAMP-induced STING phase-separator, it is reported that a synthetic polyvalent STING agonist PC7A also induces liquid–liquid phase separation of STING (Li et al., 2021b). PC7A is a pH-sensitive polymer composed of a seven-membered ring with a tertiary amine. It activates STING through the polymer-induced formation of STING–PC7A condensates. PC7A binds to a non-competitive STING surface site that is different from the cGAMP-binding pocket, therefore prolonging STING activation and the downstream signaling. Different from the gel-like ER-located STING phase-separator, PC7A–STING condensate remains liquid-like state and translocates to the ERGIC–Golgi similar to the cGAMP-induced STING translocation, thus promoting STING accumulation, oligomerization, and activation. PC7A with higher degrees of polymerization induces PC7A–STING droplets with lower dynamic, which lead to decreased STING activation compared to the liquid-like PC7A–STING droplets. The phenomenon of suppressed STING activation in PC7A–STING condensates with lower dynamics is consistent with that in STING phase-separator. Still, more efforts are needed to elucidate the details of PC7A–STING condensates, including the mechanisms of its translocation, the final destination of the condensates, and membrane structures within the PC7A–STING condensate, particularly those on the TGN.
Conclusions and perspectives
Our understanding of the regulation and activation of cGAS–STING signaling has advanced considerably over the past several years. The activity of cGAS is now revealed to be regulated by cytosol DNA, as well as metal ions including Zn2+ and Mn2+. As the cGAS–STING pathway is an important source of type I-IFNs, targeting cGAS by metal ions may offer tremendous therapeutic opportunities, such as immunotherapies and vaccine adjuvants (Figure 3). However, more works are still needed to fully understand the actions and side effects of Mn2+ in physiological and pathological conditions, as well as the process and mechanism of releasing Mn2+ from restrained cellular organelles during infection.
The activation and translocation of ER-membrane protein STING have remained one of the most enigmatic fields in innate immunity. Our review briefly introduces recent discoveries about structural advances in STING–TBK1 activation, COP-II/COP-I mediated STING ER–Golgi translocation, and sGAGs-mediated STING activation (Figure 3). The recently revealed agonist-binding pocket of STING at the transmembrane domain is quite adjacent to the binding sites of sGAGs. Since sGAGs are the second ligand of STING activation by facilitating STING oligomerization, it is quite possible that sGAGs are the natural ligand of STING transmembrane domain binding pocket. Structural studies of STING and sGAGs are in need to prove the hypothesis and elucidate the mechanisms of STING oligomerization in physiological conditions.
Cellular condensate formed by biologically regulated phase separation of biomolecules is now recognized as a fundamental mechanism for orchestrating physiological process, such as reaction kinetics or specificity by regulating molecular concentrations or sequestrating unwanted molecules. Recently discovered cGAS–DNA droplets, STING phase-separators, and PC7A–STING droplets indicate that the cGAS–STING pathway is also regulated in a manner of biomolecular condensation (Figure 4). Still, research on the regulation of innate immunity by biomolecular condensation has just begun and more attention is needed to elucidate the formation and regulation of cGAS droplets and STING phase-separators, as well as their function in physiological and pathological conditions. Also, due to the tight relationship between the STING phase-separator and cubic membranes, studies on the STING phase-separator indicate that previously discovered cubic membranes may also be a series of membranous biocondensate. Functional studies on the cubic membrane, especially the pathological ones, may need to focus on their physical properties to solve the long-lasting puzzles of their initiation and function.
Acknowledgements
The authors apologize for not including all the important discoveries in the area due to space limitation. The cartoon illustration is designed by Figdraw.
Contributor Information
Xiaoyu Yu, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, School of Life Sciences, Peking University, Beijing 100871, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Zhen Zhao, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, School of Life Sciences, Peking University, Beijing 100871, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Zhengfan Jiang, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, School of Life Sciences, Peking University, Beijing 100871, China; Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Funding
The work is supported by the National Natural Science Foundation of China (31830022 and 81621001), the Chinese Ministry of Science and Technology (2019YFA0508500 and 2020YFA0707800), and China Postdoctoral Science Foundation (2021M700242).
Conflict of interest: none declared.
References
- Ahmadzadeh M., Johnson L., Heemskerk B.et al. (2009). Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almsherqi Z.A., Landh T., Kohlwein S.D.et al. (2009). Chapter 6: cubic membranes the missing dimension of cell membrane organization. Int. Rev. Cell Mol. Biol. 274, 275–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva L., Hiller B., Kostrewa D.et al. (2017). cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549, 394–398. [DOI] [PubMed] [Google Scholar]
- Banani S.F., Lee H.O., Hyman A.A.et al. (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C., Helenius A. (2016). Cargo capture and bulk flow in the early secretory pathway. Annu. Rev. Cell Dev. Biol. 32, 197–222. [DOI] [PubMed] [Google Scholar]
- Boeynaems S., Alberti S., Fawzi N.L.et al. (2018). Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C.P., Eckmann C.R., Courson D.S.et al. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne C.P., Mitchison T.J., Hyman A.A. (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl Acad. Sci. USA 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona A., Roudeau S., Perrin L.et al. (2014). Environmental manganese compounds accumulate as Mn(II) within the Golgi apparatus of dopamine cells: relationship between speciation, subcellular distribution, and cytotoxicity. Metallomics 6, 822–832. [DOI] [PubMed] [Google Scholar]
- Chen C., Tong Y., Zheng Y.et al. (2021). Cytosolic delivery of thiolated Mn-cGAMP nanovaccine to enhance the antitumor immune responses. Small 17, 2006970. [DOI] [PubMed] [Google Scholar]
- Chin D.J., Luskey K.L., Anderson R.G.et al. (1982). Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc. Natl Acad. Sci. USA 79, 1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K., Deng Y. (2012). The three dimensionality of cell membranes: lamellar to cubic membrane transition as investigated by electron microscopy. Methods Cell Biol. 108, 319–343. [DOI] [PubMed] [Google Scholar]
- Chu T.T., Tu X., Yang K.et al. (2021). Tonic prime-boost of STING signalling mediates Niemann–Pick disease type C. Nature 596, 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civril F., Deimling T., de Oliveira Mann C.C.et al. (2013). Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Wang S., Lu W.et al. (2021). The adjuvanticity of manganese for microbial vaccines via activating the IRF5 signaling pathway. Biochem. Pharmacol. 192, 114720. [DOI] [PubMed] [Google Scholar]
- Dai J., Huang Y.J., He X.et al. (2019). Acetylation blocks cGAS activity and inhibits Self-DNA-induced autoimmunity. Cell 176, 1447–1460.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Chong Z., Law C.S.et al. (2020). A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J. Exp. Med. 217, e20201045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner E.J., Burdette D.L., Wilson S.C.et al. (2013). The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs N., Burnaevskiy N., Chen D.et al. (2015). STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Chen Z.J. (2018). DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Jiang Q., Guan Y.et al. (2021). Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity 54, 962–975.e8. [DOI] [PubMed] [Google Scholar]
- Feng Y., He D., Yao Z.et al. (2014). The machinery of macroautophagy. Cell Res. 24, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Long A.J., Iwai Y.et al. (2000). Engagement of the PD-1 immunoinhibitory receptor by a novel B7-family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Wu J., Wu Y.T.et al. (2013). Cyclic GMP–AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tao J., Liang W.et al. (2015). Identification and characterization of phosphodiesterases that specifically degrade 3′3′-cyclic GMP–AMP. Cell Res. 25, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Xie Y.Q., Lei K.W.et al. (2021). A manganese phosphate nanocluster activates the cGAS–STING pathway for enhanced cancer immunotherapy. Adv. Therap. 4, 2100065. [Google Scholar]
- Gao P., Ascano M., Wu Y.et al. (2013). Cyclic [G(2′,5′) pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP–AMP synthase. Cell 153, 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ascano M., Zillinger T.et al. (2013). Structure–function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154, 748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonugunta V.K., Sakai T., Pokatayev V.et al. (2017). Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 21, 3234–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P.M., Schaff Z. (1976). Significance of tubuloreticular inclusions in the pathobiology of human diseases. Pathobiol. Annu. 6, 221–257. [PubMed] [Google Scholar]
- Gui X., Yang H., Li T.et al. (2019). Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar S.P., Bockus D., Remington F.et al. (1984). More on ultrastructure of AIDS lymph nodes. N. Engl. J. Med. 310, 924. [DOI] [PubMed] [Google Scholar]
- Harmon T.S., Holehouse A.S., Rosen M.K.et al. (2017). Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6, e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Ding L., Cao K.et al. (2021). A human cell-based SARS-CoV-2 vaccine elicits potent neutralizing antibody responses and protects mice from SARS-CoV-2 challenge. Emerg. Microbes Infect. 10, 1555–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooy R.M., Massaccesi G., Rousseau K.E.et al. (2020). Allosteric coupling between Mn2+ and dsDNA controls the catalytic efficiency and fidelity of cGAS. Nucleic Acids Res. 48, 4435–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Tian C., Yan Y.et al. (2020). Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano 14, 3927–3940. [DOI] [PubMed] [Google Scholar]
- Hu M.M., Yang Q., Xie X.Q.et al. (2016). Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity 45, 555–569. [DOI] [PubMed] [Google Scholar]
- Huang A.C., Postow M.A., Orlowski R.J.et al. (2017). T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Liu X.Y., Du X.X.et al. (2012). The structural basis for the sensing and binding of cyclic di-GMP by STING. Nat. Struct. Mol. Biol. 19, 728–730. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K.et al. (1992). Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. (2009). STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup F.J., Bruno S.F., Matz-Rensing K.et al. (2005). Tubuloreticular structures in rectal biopsies of SIV-infected rhesus monkeys (Macaca mulatta). Ultrastruct. Pathol. 29, 357–366. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Johannes L., Goud B.et al. (1998). Non-invasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl Acad. Sci. USA 95, 2997–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S.O., Prydz K., Pejler G. (2004). Intracellular proteoglycans. Biochem. J. 379, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno H., Konno K., Barber G.N. (2013). Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 155, 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostianovsky M., Orenstein J.M., Schaff Z.et al. (1987). Cytomembranous inclusions observed in acquired immunodeficiency syndrome. Clinical and experimental review. Arch. Pathol. Lab. Med. 111, 218–223. [PubMed] [Google Scholar]
- Kranzusch P.J., Lee A.S., Berger J.M.et al. (2013). Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 3, 1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchta K., Knizewski L., Wyrwicz L.S.et al. (2009). Comprehensive classification of nucleotidyltransferase fold proteins: identification of novel families and their representatives in human. Nucleic Acids Res. 37, 7701–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabi A., Devos J.M., Ng S.L.et al. (2013). Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Rep. 3, 734–746. [DOI] [PubMed] [Google Scholar]
- Lee S., Harris C., Hirschfeld A.et al. (1988). Cytomembranous inclusions in the brain of a patient with the acquired immunodeficiency syndrome. Acta Neuropathol. 76, 101–106. [DOI] [PubMed] [Google Scholar]
- Lepelley A., Martin-Niclos M.J., Le Bihan M.et al. (2020). Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J. Exp. Med. 217, e20200600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E.C., Hennecke S.et al. (1994). Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Li J., Li S., Li Y.et al. (2021). A magnetic resonance nanoprobe with STING activation character collaborates with platinum-based drug for enhanced tumor immunochemotherapy. J. Nanobiotechnol. 19, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zheng P., Zhao J.et al. (2019). Metal-mediated immune regulations and interventions: prospects of the emerging field of metalloimmunology. Sci. Sin. Chim. 49, doi: 10.1360/SSC-2019-0040. [Google Scholar]
- Li L., Yin Q., Kuss P.et al. (2014). Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 10, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Banjade S., Cheng H.C.et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Luo M., Wang Z.et al. (2021). Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat. Biomed. Eng. 5, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Shu C., Yi G.H.et al. (2013). Cyclic GMP–AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39, 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H., Wei J., Zang R.et al. (2018). ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 9, 3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Guo R., Ma C.et al. (2021). Manganese breaks the immune tolerance of HBs-Ag. Open Forum Infect. Dis. 8, ofab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Protter D.S., Rosen M.K.et al. (2015). Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M.E., Deschenes R.J. (2007). Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84. [DOI] [PubMed] [Google Scholar]
- Liu S., Cai X., Wu J.et al. (2015). Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630. [DOI] [PubMed] [Google Scholar]
- Liu X., Xu Y., Yin L.et al. (2021). Chitosan-poly(acrylic acid) nanoparticles loaded with R848 and MnCl2 inhibit melanoma via regulating macrophage polarization and dendritic cell maturation. Int. J. Nanomedicine 16, 5675–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J., McCaffery J.M., Miyawaki A.et al. (1998). Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl Acad. Sci. USA 95, 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Shang G., Li J.et al. (2022). Activation of STING by targeting a pocket in the transmembrane domain. Nature 604, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W.W., Li S., Li C.et al. (2016). iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 17, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Luu J.Y., Bockus D., Remington F.et al. (1989). Tubuloreticular structures and cylindrical confronting cisternae: a review. Hum. Pathol. 20, 617–627. [DOI] [PubMed] [Google Scholar]
- Lv M., Chen M., Zhang R.et al. (2020). Manganese is critical for antitumor immune responses via cGAS–STING and improves the efficacy of clinical immunotherapy. Cell Res. 30, 966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley E.J., Gavin C.E., Aschner M.et al. (2013). Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 62, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon D.B., Lee S.G., Chung Y.K.et al. (2021). Over 500 liver transplants including more than 400 living-donor liver transplants in 2019 at Asan medical center. Transplant. Proc. 53, 83–91. [DOI] [PubMed] [Google Scholar]
- Motwani M., Pesiridis S., Fitzgerald K.A. (2019). DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 20, 657–674. [DOI] [PubMed] [Google Scholar]
- Mukai K., Konno H., Akiba T.et al. (2016). Activation of STING requires palmitoylation at the Golgi. Nat. Commun. 7, 11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K., Ogawa E., Uematsu R.et al. (2021). Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat. Commun. 12, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Travers P., Walport M. (2014). Janeway's Immunobiology (8th edn). New York and London: Garland Science.
- Nott T.J., Petsalaki E., Farber P.et al. (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Mukai K., Saito K.et al. (2018). The binding of TBK1 to STING requires exocytic membrane traffic from the ER. Biochem. Biophy. Res. Commun. 503, 138–145. [DOI] [PubMed] [Google Scholar]
- Orenstein J.M., Ewing JR E.P., Spira T.J.et al. (1983). Ultrastructural markers in AIDS. Lancet 322, 284–285. [Google Scholar]
- Ouyang S.Y., Song X.Q., Wang Y.Y.et al. (2012). Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity 36, 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G.D., Brandt P.W. (1959). Mitochondria. I. Fine structure of the complex patterns in the mitochondria of Pelomyxa carolinensis Wilson (Chaos chaos L.). J. Biophys. Biochem. Cytol. 6, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Lee H.O., Jawerth L.et al. (2015). A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Pokatayev V., Yang K., Tu X.T.et al. (2020). Homeostatic regulation of STING protein at the resting state by stabilizer TOLLIP. Nat. Immunol. 21, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran Y., Xiong M.G., Xu Z.S.et al. (2019). YIPF5 is essential for innate immunity to DNA virus and facilitates COPII-dependent STING trafficking. J. Immunol. 203, 1560–1570. [DOI] [PubMed] [Google Scholar]
- Ribas A., Hamid O., Daud A.et al. (2016). Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315, 1600–1609. [DOI] [PubMed] [Google Scholar]
- Scales S.J., Pepperkok R., Kreis T.E. (1997). Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Schaff Z., Eder G., Eder C.et al. (1992). Ultrastructure of normal and hepatitis virus infected human and chimpanzee liver: similarities and differences. Acta Morphol. Hung. 40, 203–214. [PubMed] [Google Scholar]
- Schaffner F., Dienstag J.L., Purcell R.H.et al. (1977). Chimpanzee livers after infection with human hepatitis viruses A and B: ultrastructural studies. Arch. Pathol. Lab. Med. 101, 113–117. [PubMed] [Google Scholar]
- Seo G.J., Yang A., Tan B.et al. (2015). Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 13, 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G., Zhang C., Chen Z.J.et al. (2019). Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP–AMP. Nature 567, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G.J., Zhu D.Y., Li N.et al. (2012). Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. Nat. Struct. Mol. Biol. 19, 725–727. [DOI] [PubMed] [Google Scholar]
- Shin Y., Brangwynne C.P. (2017). Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382. [DOI] [PubMed] [Google Scholar]
- Shumaker D.K., Vann L.R., Goldberg M.W.et al. (1998). TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium 23, 151–164. [DOI] [PubMed] [Google Scholar]
- Sidhu G.S., Stahl R.E., El-Sadr W.et al. (1983). Ultrastructural markers of AIDS. Lancet 321, 990–991. [DOI] [PubMed] [Google Scholar]
- Smock R.G., Meijers R. (2018). Roles of glycosaminoglycans as regulators of ligand/receptor complexes. Open Biol. 8, 180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares da Costa D., Reis R.L., Pashkuleva I. (2017). Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 19, 1–26. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu Y., Teo H.Y.et al. (2021). Manganese enhances the antitumor function of CD8+ T cells by inducing type I interferon production. Cell. Mol. Immunol. 18, 1571–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S., Woo J.S., Wu B.et al. (2019). The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat. Immunol. 20, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak J.C., Schmid E.M., Ryan C.J.et al. (2012). Membrane bending by protein–protein crowding. Nat. Cell Biol. 14, 944–949. [DOI] [PubMed] [Google Scholar]
- Steiner A., Hrovat-Schaale K., Prigione I.et al. (2022). Deficiency in coatomer complex I causes aberrant activation of STING signalling. Nat. Commun. 13, 2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F.et al. (2013). Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xing Y., Chen X.et al. (2012). Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One 7, e30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.S., Zhang J., Jiang L.Q.et al. (2018). TMED2 potentiates cellular IFN responses to DNA viruses by reinforcing MITA dimerization and facilitating its trafficking. Cell Rep. 25, 3086–3098. [DOI] [PubMed] [Google Scholar]
- Sun W., Li Y., Chen L.et al. (2009). ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl Acad. Sci. USA 106, 8653–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhang Y., Li J.et al. (2021a). Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yin Y., Gong L.et al. (2021b). Manganese nanodepot augments host immune response against coronavirus. Nano Res. 14, 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., Mukai K., Takaya E.et al. (2021). STING operation at the ER/Golgi interface. Front. Immunol. 12, 646304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Drake C.G., Pardoll D.M. (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu D., Zhu Z., Zhou A.Y.et al. (2013). Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell Rep. 3, 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herck S., Feng B., Tang L. (2021). Delivery of STING agonists for adjuvanting subunit vaccines. Adv. Drug Del. Rev. 179, 114020. [DOI] [PubMed] [Google Scholar]
- Vigetti D., Karousou E., Viola M.et al. (2014). Hyaluronan: biosynthesis and signaling. Biochim. Biophys. Acta 1840, 2452–2459. [DOI] [PubMed] [Google Scholar]
- Volpi S., Tsui J., Mariani M.et al. (2018). Type I interferon pathway activation in COPA syndrome. Clin. Immunol. 187, 33–36. [DOI] [PubMed] [Google Scholar]
- Wang C., Guan Y., Lv M.et al. (2018). Manganese increases the sensitivity of the cGAS–STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48, 675–687. [DOI] [PubMed] [Google Scholar]
- Wang C., Sun Z., Zhao C.et al. (2021). Maintaining manganese in tumor to activate cGAS–STING pathway evokes a robust abscopal anti-tumor effect. J. Control. Release 331, 480–490. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ning X., Gao P.et al. (2017). Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity 46, 393–404. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xie Y., Luo J.et al. (2021). Engineering a self-navigated MnARK nanovaccine for inducing potent protective immunity against novel coronavirus. Nano Today 38, 101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yuan Y., Chen C.et al. (2021). Colloidal manganese salt improves the efficacy of rabies vaccines in mice, cats, and dogs. J. Virol. 95, e0141421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang H. (2019). Phase separation, transition, and autophagic degradation of proteins in development and pathogenesis. Trends Cell Biol. 29, 417–427. [DOI] [PubMed] [Google Scholar]
- Watkin L.B., Jessen B., Wiszniewski W.et al. (2015). COPA mutations impair ER–Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X.et al. (2013). Cyclic GMP–AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P., Ye B., Wang S.et al. (2016). Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat. Immunol. 17, 369–378. [DOI] [PubMed] [Google Scholar]
- Xie W., Lama L., Adura C.et al. (2019). Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc. Natl Acad. Sci. USA 116, 11946–11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Liu C., Zhou S.et al. (2021). Viral tegument proteins restrict cGAS–DNA phase separation to mediate immune evasion. Mol. Cell 81, 2823–2837. [DOI] [PubMed] [Google Scholar]
- Yadav M., Pal K., Sen U. (2019). Structures of c-di-GMP/cGAMP degrading phosphodiesterase VcEAL: identification of a novel conformational switch and its implication. Biochem. J. 476, 3333–3353. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Masaki R., Tashiro Y. (1996). Formation of crystalloid endoplasmic reticulum in COS cells upon overexpression of microsomal aldehyde dehydrogenase by cDNA transfection. J. Cell Sci. 109, 1727–1738. [DOI] [PubMed] [Google Scholar]
- Yan N. (2017). Immune diseases associated with TREX1 and STING dysfunction. J. Interferon Cytokine Res. 37, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Regalado-Magdos A.D., Stiggelbout B.et al. (2010). The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11, 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yang Y., Bian J.Y.et al. (2021). Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today 38, 101109. [Google Scholar]
- Yi M., Niu M., Zhang J.et al. (2021). Combine and conquer: manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J. Hematol. Oncol. 14, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q., Tian Y., Kabaleeswaran V.et al. (2012). Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell 46, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhang L., Shen J.et al. (2021). The STING phase-separator suppresses innate immune signalling. Nat. Cell Biol. 23, 330–340. [DOI] [PubMed] [Google Scholar]
- Yum S., Li M.H., Frankel A.E.et al. (2019). Roles of the cGAS–STING pathway in cancer immunosurveillance and immunotherapy. Ann. Rev. Cancer Biol. 3, 323–344. [Google Scholar]
- Zeng M., Shang Y., Araki Y.et al. (2016). Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166, 1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shang G., Gui X.et al. (2019). Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang C.G., Guan Y.K.et al. (2021). Manganese salts function as potent adjuvants. Cell. Mol. Immunol. 18, 1222–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shi H.P., Wu J.X.et al. (2013). Cyclic GMP–AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wu J.X., Du F.H.et al. (2014). The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 6, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Shu C., Gao X.et al. (2016). Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Proc. Natl Acad. Sci. USA 113, E3403–E3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Ma Z., Wang B.et al. (2020). Mn2+ directly activates cGAS and structural analysis suggests Mn2+ induces a non-canonical catalytic synthesis of 2′3′-cGAMP. Cell Rep. 32, 108053. [DOI] [PubMed] [Google Scholar]
- Zhong B., Yang Y., Li S.et al. (2008). The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Lin H., Wang S.Y.et al. (2014). The ER-associated protein ZDHHC1 is a positive regulator of DNA virus-triggered, MITA/STING-dependent innate immune signaling. Cell Host Microbe 16, 450–461. [DOI] [PubMed] [Google Scholar]
- Zhou W., Mohr L., Maciejowski J.et al. (2021). cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol. Cell 81, 739–755.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Whiteley A.T., Mann C.C.D.et al. (2018). Structure of the human cGAS–DNA complex reveals enhanced control of immune surveillance. Cell 174, 300–311.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Xu L., Wang C.et al. (2021). T6SS translocates a micropeptide to suppress STING-mediated innate immunity by sequestering manganese. Proc. Natl Acad. Sci. USA 118, e2103526118. [DOI] [PMC free article] [PubMed] [Google Scholar]