Abstract
BACKGROUND & AIMS:
Gluten ingestion in celiac disease (CeD) patients can lead to gastrointestinal symptoms and small intestinal mucosal injury.
METHODS:
This gluten-challenge (GC) Phase 2 trial was double-blind, placebo-controlled, and assessed the efficacy and safety of a 1,200 mg dose of IMGX003 in CeD patients exposed to 2 g of gluten per day for 6 weeks. The change in the ratio of villus height to crypt depth (Vh:Cd) was the primary endpoint. Secondary endpoints included densities of intraepithelial lymphocytes (IEL) and symptom severity. These endpoints were evaluated by ANCOVA. Additional endpoints included serology and gluten-immunogenic peptides (GIP) in urine.
RESULTS:
Fifty (50) patients were randomized, and 43 patients completed (n=21 IMGX003, n=22 placebo). The mean ΔVh:Cd (primary endpoint) for IMGX003 vs. placebo was −0.04 vs. −0.35 (p = .057). The mean ΔIEL (secondary endpoint) for IMGX003 vs. placebo was 9.8 vs. 24.8 (p = .018). The mean change (worsening) in symptom severity (secondary endpoint) for IMGX003 vs. placebo was 0.22 vs. 1.63 (abdominal pain, p =.231), 0.96 vs. 3.29 (bloating, p = .204), and 0.02 vs. 3.20 (tiredness, p = .113). The 3 × 2-week trend-line significance for these symptoms, respectively, were p = .014, .030 and .002.
CONCLUSION:
IMGX003 reduced gluten-induced intestinal mucosal damage and symptom severity. ClinicalTrials.gov number NCT03585478.
Keywords: morphometry, inflammation, treatment
Lay Summary
Latiglutenase is given orally, protects the small intestine and reduces symptoms induced by gluten challenge without significant side effects in patients with celiac disease.
Graphical Abstract

Celiac disease (CeD), a genetically predisposed chronic small intestinal inflammatory disorder caused by exposure to gluten, affects about 1% of most populations.1,2,3 The only available treatment is a lifelong gluten-free diet (GFD). While a GFD can reduce symptoms and intestinal damage, the diet is neither easy nor readily achievable by many patients and further can be lacking in essential nutrients.4,5 The pathological lesion of villous atrophy in the proximal epithelium of the small intestine is due to an immune response to wheat, rye, or barley. Low levels of gluten exposure can lead to ongoing inflammation that can increase the risk of complications including lymphoma, bowel cancer, osteoporosis, anemia, and malnutrition.6,7 Nearly 50% of patients with celiac disease are moderately to severely symptomatic, and this is associated with significant financial and other burdens on them, as well as on their family, and friends.8
Latiglutenase (IMGX003, formerly ALV003) is an enzyme supplementation therapy comprised of two enzymes, designed to mitigate the impact of gluten exposure in patients attempting to adhere to a GFD. The treatment has shown success for attenuating gluten-induced mucosal injury in CeD patients in a gluten-challenge study (ALV003–1021)9 and in reducing multiple gluten-induced symptoms, particularly for serologically positive (tTG-IgA, DGP-IgG, DGP-IgG) patients in a “real-world” trial (ALV003–1221).10,11,12
Pathophysiologically relevant gluten peptides are generally highly resistant to proteolytic activity in the intestine.13 This resistance accounts for accumulation of gluten-derived intermediates in the small intestinal lumen, which drives a potent T-cell dependent response in CeD patients. This would suggest that proteases that target the gluten in ingested food should reduce the bioavailability of immunogenic peptides present after gluten exposure and perhaps provide a pharmacological basis for managing celiac disease.14 Latiglutenase (IMGX003) is delivered orally as a liquid that functions in the stomach during a meal and hence is a non- systemic treatment that works upstream of the CeD autoimmune response.
Patients and Methods
Study Design
This was a randomized, double-blind, placebo-controlled, gluten-challenge study in patients with treated celiac disease in remission to assess the efficacy and safety of a 1,200 mg dose of IMGX003 (formerly ALV003). The trial was conducted at the Mayo Clinic (Rochester, MN). The study protocol was approved by the Mayo Clinic Institutional Review Board. All authors had access to the study data and reviewed and approved the final manuscript.
Screening and Enrollment
Adult patients (18–80 years) were required to have physician-diagnosed, biopsy confirmed celiac disease, following a GFD for greater than one year and being histologically well-controlled as evidenced by measured Vh:Cd ≥ 2.0. A schematic summary and enrollment flow chart of the study is shown in Figure 1. Main inclusion criteria included biopsy-confirmed CeD diagnosis, >1 year on a GFD following diagnosis, tTG-IgA negative and Vh:Cd ≥ 2.0. Main exclusion criteria included active dermatitis herpetiformis, history of colitis, history of IgE-mediated reactions to wheat (i.e., wheat allergy) and Type 1 diabetes.
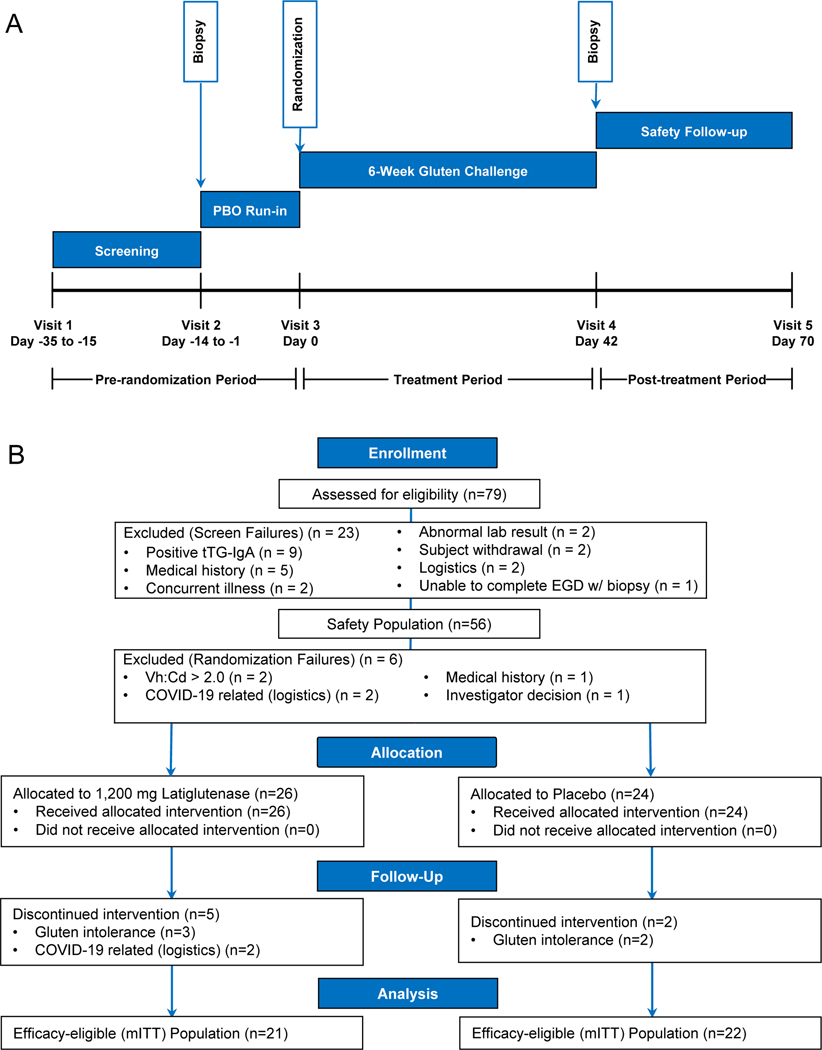
Figure 1.
IMGX003-NCCIH-1721 CeliacShield study (A) schematic and (B) enrollment flow chart.
Patients who met Visit 1 (V1) protocol enrollment criteria were enrolled and began the screening period. Patients were evaluated for adherence to study criteria including testing negative for tTG-IgA serology and demonstrating compliance when completing the daily CDSD PRO instrument (≥85% compliance required). At Visit 2 (V2), patients underwent an esophagogastroduodenoscopy (EGD) with biopsy and those measuring Vh:Cd ≥ 2.0 proceeded to the placebo run-in period including continued daily CDSD PRO completion. Patients were dispensed run-in kits containing placebo (only) and instructed to ingest a single dose once daily with their evening meal (e.g., dinner) for 14 consecutive days. Reasons for placebo run-in period failure included noncompliance (≥85% compliance requirement for CDSD, gluten challenge, masked study medication, etc.). Patients who continued to meet eligibility requirements at Visit 3 (V3) began the randomized treatment period consistent with other descriptions above as well as with Figure 1A. Patients were randomized 1:1 to either placebo or 1,200 mg IMGX003. The details of the patients’ self-administered treatment are given in Supplemental Material.
At the end of the treatment period, Visit 4 (V4), patients were evaluated via EGD with biopsy, urinalysis, and serology. At the final visit (Visit 5, V5) a serology test was performed. Routine safety laboratory tests (chemistry, hematology, and urinalysis) and physical examination were performed at V1 and V5.
Study Medication, Gluten and Administration Description
IMGX003 consists of three components: two enzymes (IMGX001 and IMGX002) packaged in separate stickpacks and a sachet containing flavor (peach/mango) and buffer components. IMGX003, upon dissolution in 6–8 ounces of water, provides a fixed dose combination glutenase consisting of a 1:1 ratio by weight of the two gluten targeting enzymes which demonstrate complementary substrate sequence and chain length specificity. The properties of IMGX003 have been described previously9–12 and briefly presented along with a description of the gluten samples in Supplemental Material.
Masked study medication (MSM) (IMGX003 or placebo) and gluten were orally administered. The order of ingestion of MSM and gluten were as follows: (1) commence MSM ingestion after beginning the evening meal, (2) ingest gluten when approximately one-quarter of the way completed with the meal and (3) complete MSM ingestion by half-way through the meal.
Blinding
The randomization schedule was created by an independent statistician using a random number table with random block size. Randomization assignments were provided to the on-site pharmacy for execution. Only the independent biostatistician and the study pharmacist were unblinded to handle randomization codes and delivery of MSM.
Duodenal Biopsy
The primary endpoint was change in Vh:Cd and a secondary endpoint was change in IEL both measured before (V2) and after (V4) the gluten challenge period. EGD biopsies were obtained from the post bulbar duodenum, before (at V2) and after (at V4) the gluten challenge. Biopsy samples were individually formalin-fixed, paraffin embedded, oriented, cut and then: (1) stained with hematoxylin-eosin for villus height (Vh) and crypt depth (Cd) measurements and (2) stained with CD3+ for intraepithelial lymphocytes (IELs) measurements. At least 4 biopsy samples are taken, with 3–5 cuts per sample.15 Morphometric analysis of the samples was done using light microscopy by a reference gastrointestinal (GI) pathologist. Measurements included Vh:Cd and population of IELs. IELs are associated with inflammation within the mucosal lining of the GI tract. For reporting we obtained the ratio of the averages of Vh and Cd for endoscopy visit whereby the total of biopsy reads for Vh and Cd were converted to an average Vh:Cd by the operation of sum(Vh)/sum(Cd). A Vh:Cd value ≥ 2.0 served as a histologic inclusion criterion. V2 biopsies were re-read with the V4 biopsies in groups of at least 4 pairs of biopsy sets per subject to ensure minimal intra-reader time-lapsed variability and to enable masking of the pathologist to the pairing and sequence of biopsies.
Celiac Disease Symptom Diary (CDSD)
The CDSD is a daily diary administered to assess common CeD symptoms (abdominal pain, bloating, tiredness, nausea, diarrhea, and constipation). The presence or absence of each of the symptoms over the previous 24 hours was reported by the subject along with a severity score if a symptom was present.16 The scoring system for all symptoms is presented in Supplemental Material.
Weekly severity scores for abdominal pain, bloating, tiredness, and constipation were calculated for each week between V2 and V4 resulting in 8 weekly scores. The 2 weekly scores leading up to V3 (baseline) were averaged to form the baseline score for each subject. Each of the 6 individual post-baseline weekly scores was combined into 3 two-week average severity scores. A six-week average severity score was similarly calculated.
CeD Serology
Whole blood for CeD serology was collected and analyzed at V1, V4, and V5. Antibodies (tTG-IgA, DGP-IgA, DGP-IgG) were measured by an enzyme-linked immunosorbent assay (ELISA) (QUANTA Lite™ h-tTG IgA, QUANTA Lite™ Gliadin IgA II, QUANTA Lite™ Gliadin IgG II, INOVA Diagnostics, Inc., San Diego, CA). Although all three titers were measured the subject exclusion criterion for serology was based only on tTG-IgA positivity.
Gluten Immunogenic Peptides (GIP) in Urine
Urine samples were collected in-clinic at V3 and V4 (before and after the gluten challenge period) and frozen at −20C. Remote samples were collected and frozen by study patients once a week for 6 weeks during the gluten challenge (GC) period. Patients were instructed to collect their urine sample 6–12 hours following gluten ingestion with the evening meal. Lateral flow immunochromatographic assay (IVYCHECK GIP Urine) with the IVYCHECK Reader (IVYDAL In Vitro Diagnostics, Biomedal, Seville, Spain) was used to measure the concentration of GIP present in each urine sample.17
Safety Assessments
Safety assessments included adverse event monitoring, standard laboratory tests (blood chemistry, hematology, and urinalysis) and physical examination including vital signs. All adverse events were followed over the course of the study and presented in a by-subject listing. All treated patients were included in all summaries of adverse events for the safety population. All reported events were coded to the current Medical Dictionary for Regulatory Activities (MedDRA) adverse event dictionary. The frequency of each event for each system organ class and preferred term were summarized by severity (mild, moderate, severe) and by relatedness (related, not related) with the study medication, procedure, and gluten challenge. Since some patients may have reported the same event several times (headache, for example), the first occurrence of the worst reported case of the event was used for the purpose of analysis.
A treatment-emergent adverse event (TEAE) is an adverse event that begins or worsens after the first dose of study treatment regardless of the suspected cause. Collection of TEAEs occurred during the randomized treatment period of the trial (V3 to V4).
All clinical laboratory data was provided as summary statistics for results and change from baseline values were calculated and presented by treatment and visit. Physical examination and vital sign results were listed for all individual patients in the safety population.
A futility analysis, unblinded to a third-party biostatistics firm, was performed when 20 randomized patients had completed the treatment period. In addition to monitoring subject safety, the Data Safety Monitoring Board (DSMB) evaluated the futility analysis results and made a recommendation for the trial to proceed.
Study Outcome Measures and Statistical Analysis
The study populations were defined as follows:
Intent-to-Treat (ITT): included all patients who were randomized to study treatment.
Modified ITT (mITT): included all patients who were treated and attended V4 after the gluten-challenge period.
Efficacy Eligible: Included all randomized patients who completed at least 6 weeks of treatment and had biopsies at V2 and V4. In this study efficacy eligible and mITT populations were the same, so we use the latter nomenclature henceforth.
Safety Population: Includes all patients who signed an informed consent at V1 (Screening) and received any amount of randomized MSM starting at V3.
Sample size numbers are presented in the enrollment flow chart in Figure 1B. A sample size calculation was performed utilizing pre-existing clinical study data. We anticipated the need to enroll 80 patients and finished with 79. We anticipated a 37.5% screening period attrition rate to yield 50 patients at randomization for an ITT analysis and finished with 50. We anticipated an additional 16% dropout rate post-randomization to yield 42 patients completing the study for the mITT analysis and finished with 43.
A sample size of 50 ITT patients (25 patients per treatment group) provided 86% power and two-sided 5% Type 1 error to detect a 0.40 difference between the treatment groups in change from baseline Vh:Cd at Week 6 assuming a standard deviation of 0.45. Using the same assumptions, the power dropped to 80% for the mITT analysis (43 patients).
Summary tables (descriptive statistics and/or frequency distributions) were developed for baseline variables, efficacy variables and safety variables. Continuous variables are described by descriptive statistics (n, mean, standard deviation, range and median). Frequency distributions (counts and percentage) of patients are provided for categorical data. All variables are summarized by treatment group for the efficacy-eligible population unless otherwise indicated.
The histology analysis for Vh:Cd and IEL was carried out on both the change from baseline and percent change from baseline using the mITT (same as efficacy-eligible) population, and comparisons were performed using two-sided tests at the 5% level of significance. A linear regression model was used for efficacy analysis to compare the distribution of changes and percent changes in Vh:Cd and IEL from V2 (baseline) to Visit 4 (post GC period) between the two treatment groups. The model included baseline Vh:Cd and treatment as covariates (ANCOVA). The change and percent change from baseline was also evaluated within each treatment group using a paired t-test.
To confirm results, a nonparametric Wilcoxon-Mann-Whitney (WMW) test was run for the sensitivity analyses for the between-group comparison. Similarly, the within-group change and percent change from baseline was evaluated using a Wilcoxon signed-rank test.
The primary analysis for Vh:Cd and analyses for IEL, symptoms and adverse events were performed using SAS Version 9.4. Other analyses, e.g., for serology, GIP in urine, and exploratory and sensitivity analyses were performed using GraphPad Prism 9.1.1 or XLSTAT 2021.1.1.
This clinical trial was registered (https://clinicaltrials.gov/ct2/show/NCT03585478) and all authors had access to the study data and reviewed and approved the final manuscript.
Results
Study Population
The demographics for this study are summarized in Table I and an enrollment flow chart presented in Figure 1B. Of the randomized ITT population of 50 patients (n=26 IMGX003, n=24 placebo), 43 patients completed the trial and constitute the mITT population (n=21 IMGX003, n=22 placebo). Age ranges were comparable for IMGX003 (42.7 mean) and placebo (45.0 mean). Most of the study population was female (74% total, 81% IMGX003, 67% placebo). The ethnicity and racial profile of the ITT population was predominantly white (96% IMGX003, 100% placebo). MSM and gluten compliance was measured during the placebo run-in and randomized treatment periods and was above 90% mean for each separate group and for all patients combined.
Table 1:
Demographics – All-Randomized Population
| Characteristic | Placebo (N = 24) | IMGX003 (N = 26) | All Patients (N = 50) |
|---|---|---|---|
|
| |||
| Age at Baseline (years) | |||
| n | 24 | 26 | 50 |
| Mean (SD) | 45.0 (13.88) | 42.7 (10.88) | 43.8 (12.34) |
| Median | 43.9 | 43.3 | 43.3 |
| Minimum, Maximum | 22.8, 66.4 | 22.2, 62.7 | 22.2, 66.4 |
| Gender | |||
| Male | 8 (33.3%) | 5 (19.2%) | 13 (26.0%) |
| Female | 16 (66.7%) | 21 (80.8%) | 37 (74.0%) |
| Ethnicity | |||
| Hispanic | 1 (4.2%) | 1 (3.8%) | 2 (4.0%) |
| Non-Hispanic | 23 (95.8%) | 25 (96.2%) | 48 (96.0%) |
| Race | |||
| American Indian or Alaska Native | 0 (0%) | 0 (0%) | 0 (0%) |
| Asian | 0 (0%) | 0 (0%) | 0 (0%) |
| Black or African American | 0 (0%) | 0 (0%) | 0 (0%) |
| Native Hawaiian or Other Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) |
| White | 24 (100.0%) | 25 (96.2%) | 49 (98.0%) |
| Other | 0 (0%) | 1 (3.8%) | 1 (2.0%) |
Histology
The summary results for histology (Vh:Cd and IEL) are tabulated for the mITT population in Table 2. The detailed mITT subject specific results are presented in Figures S1 and S2 (Supplemental Material). The mean histologic values for V2 (before GC) for IMGX003 and placebo, respectively, were 2.99 and 2.95 for Vh:Cd and 35.0 and 34.6 for IEL.
Table 2:
Histologic Efficacy Analysis – mITT Population
| Vh:Cd Ratios | Placebo (N=22) | IMGX003 (N=21) |
|---|---|---|
|
| ||
| Visit 2 (Day −14; Baseline) | ||
| n | 22 | 21 |
| Mean (SD) | 2.95 (0.473) | 2.99 (0.326) |
| Median | 2.93 | 3.00 |
| Minimum, Maximum | 2.26, 3.89 | 2.13, 3.53 |
| Visit 4 (Day 42) | ||
| n | 22 | 21 |
| Mean (SD) | 2.61 (0.657) | 2.95 (0.504) |
| Median | 2.52 | 2.97 |
| Minimum, Maximum | 1.62, 3.73 | 1.99, 4.20 |
| Change from Baseline to Visit 4 (Day 42) | ||
| n | 22 | 21 |
| Mean (SD) | −0.35 (0.616) | −0.04 (0.466) |
| Median | −0.28 | −0.05 |
| Minimum, Maximum | −1.38, 1.01 | −1.04, 0.83 |
| Within-Group P-value [1] | .0148 | .6713 |
| Between-Group P-value [2] | .0570 | |
|
| ||
| IEL | Placebo (N=22) | IMGX003 (N=21) |
|
| ||
| Visit 2 (Day −14; Baseline) | ||
| n | 22 | 21 |
| Mean (SD) | 34.55 (13.588) | 35.05 (17.279) |
| Median | 38.00 | 33.00 |
| Minimum, Maximum | 12.00, 62.00 | 10.00, 82.50 |
| Visit 4 (Day 42) | ||
| n | 22 | 21 |
| Mean (SD) | 59.30 (23.697) | 44.83 (22.592) |
| Median | 54.75 | 37.50 |
| Minimum, Maximum | 17.50, 98.50 | 17.00, 98.50 |
| Change from Baseline to Visit 4 (Day 42) | ||
| n | 22 | 21 |
| Mean (SD) | 24.75 (22.941) | 9.79 (15.741) |
| Median | 24.75 | 7.00 |
| Minimum, Maximum | −12.50, 66.00 | −11.00, 54.00 |
| Within-Group P-value [1] | < .0001 | .0099 |
| Between-Group P-value [2] | .0181 | |
P-value was calculated using a paired t-test of Visit 4 (Day 42) compared to Visit 2 (Day −14) values within each treatment arm and indicates if the change at Visit 4 (Day 42) is statistically significantly different than 0.
P-value was calculated using a linear regression model including baseline Vh:Cd and treatment as covariates and indicates if the mean change/pct change from baseline at Visit 4 is different between the treatment arms.
The post GC treatment relative to baseline biopsies measured for placebo a mean ΔVh:Cd of −0.35 (median of −0.28); p = .015 and a mean ΔIEL of 24.75 (median of 24.8); p < .001. The corresponding values for latiglutenase (IMGX003) were a mean ΔVh:Cd of −0.04 (median of -0.05); p = .671 and a mean ΔIEL of 9.79 (median of 7.0); p < 0.01. The between group ANCOVA significance was p = .057 for ΔVh:Cd and p = .018 for ΔIEL (Supplemental Material, Figures S1 and S2). Based on the ratio of the means for both groups we computed a reduction of worsening for latiglutenase relative to placebo of 88% (83% for median) and 60% (72% for median), respectively, for ΔVh:Cd and ΔIEL.
Symptoms
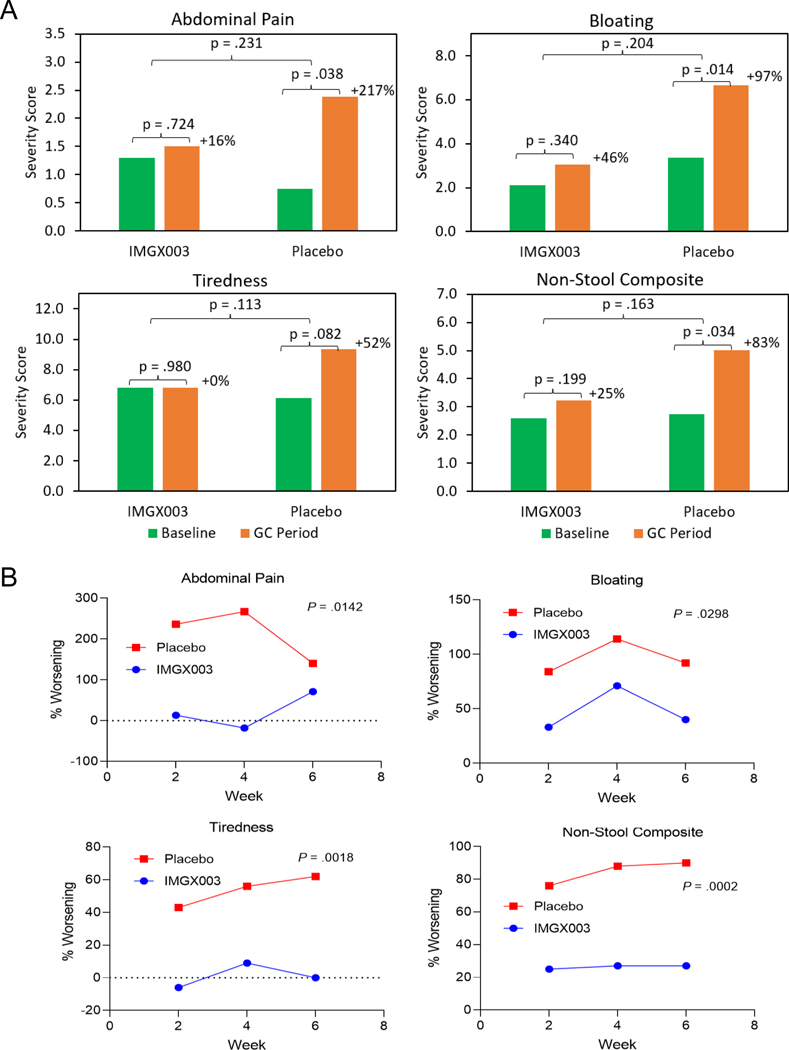
Results for the secondary endpoints of changes in symptom severity averaged over the 6-week GC treatment period relative to the 2-week baseline period are shown in Figure 2A (and reported in Table S1) for abdominal pain, bloating, tiredness, as well as the retrospective addition of non-stool composite, which consists of these symptoms and nausea. The mean change from baseline in symptom severity for IMGX003 vs. placebo, respectively, were 0.22 vs. 1.63 (abdominal pain, p = .231), 0.96 vs. 3.29 (bloating, p = .204), 0.02 vs. 3.20 (tiredness, p = .113), and 0.64 vs. 2.27 (non-stool composite, p = .163). Calculated symptom reduction based on the ratio of the measured means (Table S1) is 93% (abdominal pain), 53% (bloating), 99% (tiredness) and 70% (non-stool composite).
Figure 2.
Symptom results for abdominal pain, bloating, tiredness, and non-stool composite which includes these symptoms and nausea. (A) Absolute severity for baseline and the 6-week GC treatment period for IMGX003 vs. placebo groups. Also shown are the magnitude of worsening for baseline vs. 6-week GC period. P-values are by ANCOVA for inter-group and paired t-test for intra-group results. (B) Mean change from baseline (% worsening) for three 2-week sequential GC treatment periods for IMGX003 vs. placebo. Also shown are the unpaired, 2-tailed, t-test p-values.
The mean change from baseline was evaluated over three 2-week periods and the percent changes (% worsening) show consistent reduction of symptom worsening for IMGX003 vs. placebo groups (Figure 2B). Based on effect size and trend significance, the p-values (unpaired 2-tailed t-test) for abdominal pain, bloating, tiredness, and non-stool composite were .014, .030, .002, and < .001, respectively.
Serology
The mean changes for all three serology titers (tTG-IgA, DGP-IgA, and DGP-IgG) before and after the GC treatment period were analyzed retrospectively and showed a trend towards worsening for placebo (3.45, 5.65 and 4.39, respectively) and improvement for latiglutenase (−0.83, −1.68 and −0.53, respectively), but none reached statistical significance (Figure S5 Supplemental Material). Despite the magnitude of the effect size, most patients did not show a change in serology and remained below the titer thresholds for CeD gluten-induced antibody activity (Figure S5).
Gluten Immunogenic Peptides (GIP) in Urine
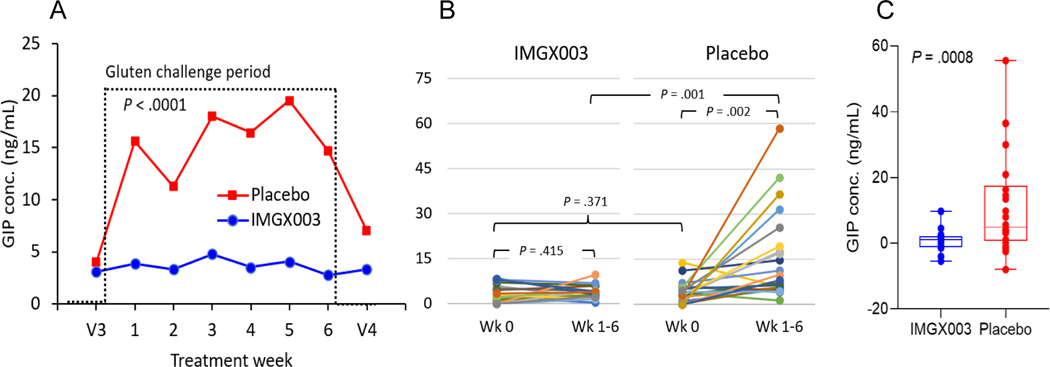
The exploratory (a priori) endpoint for baseline levels of GIP were measurable but at the lower limit of detection (3.4 ng/mL) indicating that both groups were generally compliant with a GFD prior to the GC treatment period (Figure 3A). The mean change in GIP in urine for the 6-week GC period relative to baseline for IMGX003 vs. placebo were 0.59 vs. 11.53; p = .001 (Figures 3B and 3C). Based on the ratio of mean changes we estimate an efficacy of gluten degradation in vivo of 95%. The individual patient responses pre and post GC treatment show wide variability for the placebo treated group.
Figure 3.
(A) Mean gluten immunogenic peptide (GIP) concentration in urine before (V3), during (6 weekly readings) and after (V4) the GC treatment period with unpaired, 2-tailed, t-test p-value. (B) Subject responses for GIP concentration in urine showing individual subject changes. (C) Box and whisker plot showing min and max, 1st and 3rd quartile and median and ANCOVA p-value.
Safety
There were no significant differences in adverse events between IMGX003 and placebo. Twenty (20) IMGX003 (76.9%) and 17 placebo (70.8%) patients experienced at least one treatment-emergent adverse event (TEAE); (Table 4, p = .751 by Fisher’s Exact Test). Twenty-six (26) patients (52%) (16 IMGX003 and 10 placebo) experienced at least one TEAE of mild severity. Moderate to severe TEAEs occurred in 4 IMGX003 and 7 placebo patients. No serious adverse events (SAEs) were observed. Twenty-eight (28) patients (56%) (16 IMGX003 and 12 placebo) experienced gastrointestinal (GI) disorders with nausea (18%), abdominal distension (16%) and diarrhea (16%) being the most common. The only other non-GI TEAE that exceeded 10% in all patients was fatigue (14%). There was no meaningful statistical differentiation between IMGX003 and placebo for any AEs.
Table 4:
Treatment-Emergent Adverse Events – Overall Summary in Safety Population
| Placebo (N=24) | IMGX003 (N=26) | Total (N=50) | ||
|---|---|---|---|---|
|
| ||||
| Number of Patients who Experienced at Least One | ||||
| Treatment-Emergent Adverse Event (TEAE) | 17 (70.8%) | 20 (76.9%) | 37 (74.0%) | |
| Related to Study Medication | 0 (0%) | 0 (0%) | 0 (0%) | |
| Related to Gluten | 14 (58.3%) | 19 (73.1%) | 33 (66.0%) | |
| Related to Procedure | 0 (0%) | 0 (0%) | 0 (0%) | |
| Patients with at Least One TEAE | Mild | 10 (41.7%) | 16 (61.5%) | 26 (52.0%) |
| Moderate | 6 (25.0%) | 4 (15.4%) | 10 (20.0%) | |
| Severe | 1 (4.2%) | 0 (0%) | 1 (2.0%) | |
| Serious TEAE | 0 (0%) | 0 (0%) | 0 (0%) | |
| Related to Study Medication | 0 (0%) | 0 (0%) | 0 (0%) | |
| Related to Gluten | 0 (0%) | 0 (0%) | 0 (0%) | |
| Related to Procedure | 0 (0%) | 0 (0%) | 0 (0%) | |
| Gastrointestinal disorders | 12 (50.0%) | 16 (61.5%) | 28 (56.0%) | |
| Nausea | 5 (20.8%) | 4 (15.4%) | 9 (18.0%) | |
| Abdominal distension | 3 (12.5%) | 5 (19.2%) | 8 (16.0%) | |
| Diarrhea | 4 (16.7%) | 4 (15.4%) | 8 (16.0%) | |
| Flatulence | 4 (16.7%) | 2 (7.7%) | 6 (12.0%) | |
| Vomiting | 2 (8.3%) | 3 (11.5%) | 5 (10.0%) | |
| General disorders and administration site conditions | 3 (12.5%) | 4 (15.4%) | 7 (14.0%) | |
| Fatigue | 3 (12.5%) | 4 (15.4%) | 7 (14.0%) | |
| Musculoskeletal and connective tissue disorders | 3 (12.5%) | 0 (0%) | 3 (6.0%) | |
| Arthralgia | 2 (8.3%) | 0 (0%) | 2 (4.0%) | |
| Myalgia | 1 (4.2%) | 0 (0%) | 1 (2.0%) | |
| Nervous system disorders | 0 (0%) | 3 (11.5%) | 3 (6.0%) | |
| Headache | 0 (0%) | 2 (7.7%) | 2 (4.0%) | |
| Migraine | 0 (0%) | 1 (3.8%) | 1 (2.0%) | |
Among patients that withdrew, three IMGX003 and two placebo were attributed to gluten intolerance. Two other IMGX003 patients were discontinued due to COVID-19 related logistical issues (Figure 1B).
No significant differences were detected between the IMGX003 and placebo cohorts for chemistry, hematology, and urinalysis measures as well as physical examination and vital signs collected at V1 and V5 (before and after the GC period).
Exploratory and Sensitivity Analysis
We also retrospectively applied other accepted methods of assessing histological change, e.g., conversion of quantitative histology to Marsh-Oberhuber (M-O) type scoring.18 Applying a conversion of measured Vh:Cd and IEL values to M-O like scoring19,20 gave a calculated reduction in histologic worsening of 75% (p = .035) for the change in scale for latiglutenase (IMGX003) vs. placebo (Table 3). Assessing the use of other M-O conversion scales21,22 produced similar results.
Table 3:
Summary of Histology for Various Treatment Populations and Subgroup Analysis
| Outcome Measure | Population, n | P-value | Reduction | |
|---|---|---|---|---|
| IMGX003 | Placebo | |||
|
| ||||
| Prospective: | ||||
| Vh:Cd (mITT)(1) | 21 | 22 | .057 | 88% |
| IEL (mITT) | 21 | 22 | .018 | 60% |
| Retrospective: | ||||
| VCIEL | 21 | 22 | .010 | 90% |
| Marsh-Oberhuber | 21 | 22 | .035 | 75% |
| Vh:Cd (Subgroup)(2) | 17 | 18 | .050 | 98% |
| Vh:Cd (Subgroup)(3) | 19 | 20 | .027 | 90% |
| Vh:Cd (Subgroup)(4) | 18 | 18 | <.001 | 100% |
Finnish method for average Vh:Cd is average of individual Sum(Vh)/Sum(Cd) and is the method used in the predecessor study ALV003–1021. Mayo Clinic method for average Vh:Cd is average of individual Vh:Cd ratios and gives p = .076 and reduction of 86%.
Subgroup analysis: Drop 4 patients (about 20%) in each group with lowest GIP concentration in urine.
Drop highest and lowest ΔVh:Cd values in each group.
Subgroup analysis: Exclude patients with VCIEL < 2.0 at baseline.
We combined Vh:Cd and IEL measurements into a blended histology score “VCIEL” that retains the general range of the Vh:Cd score, but includes IEL changes with equal weighting (Supplementary Material). This analysis led to a reduction of histologic damage of 90% (p = .010) for latiglutenase (IMGX003) vs. placebo (Table 3, Figure S3). Furthermore, when we applied this blended VCIEL threshold score <2.0 as an alternate exclusion criterion rather than Vh:Cd<2.0 we saw a 100% reduction for IMGX003 vs. placebo (p < .001).
A single placebo treated patient exhibited healing despite the gluten challenge (Figures S1 and S2). When we removed the high and low ΔVh:Cd for IMGX003 and placebo we saw an improved outcome of 90% reduction of histologic damage (p = .027).
Discussion
There are several therapeutic candidates for CeD currently in clinical trials.23 Enzyme directed therapy in concert with a meal9–12 and tight junction modulation in the small intestine have undergone post gluten-challenge studies.24,25 Other therapeutic candidates that have completed gluten-challenge studies include AMG 714 an interleukin 15 inhibitor,26 TAK-101 a nanoparticle encased gliadin protein,27 and ZED1227 a transglutaminase 2 inhibitor.28 It should be noted that although some of these trials, including the present study, have reported evidence of histologic protection to gluten challenge, no study yet has directly shown evidence of histologic healing in a real-world trial.
Despite strict adherence to a GFD, about half of CeD patients show evidence of persistent small intestinal mucosal injury (Marsh grade II-III).29 Small bowel mucosal recovery is essential for long-term health and highlights the need for an adjunct treatment to a GFD. This gluten-challenge study demonstrated the efficacy of a combination enzyme therapeutic candidate in breaking down purposefully ingested gluten-derived immunogenic peptides in the stomach, preventing their absorption in the small intestine and consequent histologic, symptomatic, and serologic effects. The amount of gluten consumed (2g for a single meal) would likely substantially exceed that accidently occurring while on a GFD,4 supporting such an approach for management for gluten triggered symptoms in treated patients.
Composite Histology Scale
The quantitative assessment of histology used in this study and other studies include the separate measures of villus crypt architecture and IELs; however, they have not been combined. Rostami, Marsh et al. presented arguments for attaching more significance to the range of IEL values for interpreting mucosal condition.30 We retrospectively combined both measures into a composite endpoint (VCIEL) of mucosal health that could be a useful assessment of interventions as well as for selection of patients for inclusion in trials that include gluten challenge. Patients who have too much disease activity at entry may show healing perhaps due to a Hawthorne effect despite the GC which was likely the cause of improvement in histology in a real-life study.10 Additionally, the sole reliance on Vh:Cd as a primary outcome measure may fail to capture disease relevant changes embodied in IEL measurements. Indeed, Marsh’s original concept of the spectrum of gluten sensitive enteropathy has incorporated change in IELs as an integral and indeed essential part of the scale.30
Gluten Challenge Compliance
The measured GIP concentrations in urine for placebo showed a wide variability despite the constant gluten challenge consumption prescribed for all patients (Figures 3B and 3C) suggesting that perhaps there was some non-compliance in consuming the prescribed gluten by some patients. However other explanations for individual variability need to be considered, such as due to differences in biological factors, size, environmental factors, fluid intake, etc.31 For example it has been reported that hydration and the timing of liquid consumption, not surprisingly, has a large effect on the measured GIP concentration in urine.32 Another explanation comes from a recent report where it was observed that GIP in urine values (measured by mass spectrometry) were lower in healthy non-CeD patients than for CeD patients.33 This suggests that permeability might be greater in CeD patients (e.g., due to a leaky gut) suggesting the possibility that permeability could vary for individual CeD patients based on their general histologic health.
As to the possibility that the large GIP variation for placebo might be due in part to non-compliance in taking the daily gluten, we correlated GIP to ΔVh:Cd and found a statistically significant trend (p = .012) toward decreased GIP with decreased ΔVh:Cd for the placebo group (Figure S6, Supplemental Material). We observed a non-statistically significant trend for the IMGX003 group, likely because the breakdown of gluten by IMGX003 in those patients made the GIP variability very small and giving less reason for non-compliance. To further test this hypothesis, we did a retrospective subgroup analysis on histology. The removal of the 20% of patients (4 each from IMGX003 and placebo) with the lowest measured GIP concentrations during the GC period led to a calculated reduction in Vh:Cd damage of 98% for IMGX003 vs. placebo and an improved p = .050, despite the subgroup populations decreasing to n=17 (IMGX003) and n=18 (placebo) (Table 3).
Symptoms
The current study evaluated the capability to measure clinically and statistically meaningful changes in symptom response due to the GC period and to detect meaningful reduction of the IMGX003 group relative to placebo. We did not expect the symptom endpoints to be well powered because of the low, and therefore noisy, baseline symptom scores for these relatively healthy patients. This may have accounted for the ANCOVA analysis not meeting statistical significance as the baseline scores are a weak co-variate (Table S1) whereas the ANOVA p-values were more significant in most cases. We did expect to see clinically significant trends, which was observed in Figure 2 with statistical significance observed for the 3 × 2-week trend analysis for abdominal pain, bloating, and tiredness as well as the non-stool composite of these symptoms and nausea (Figure 2B).
In summary, a gluten-targeting dual enzyme therapeutic candidate reduced the histologic, symptomatic, and serologic worsening resulting from a 6-week daily gluten challenge by breaking down the gluten in the stomach after consumption with a meal. Measurement of GIP in urine demonstrated IMGX003’s purported mechanism of action, namely degradation of gluten in the stomach thereby preventing the triggering of the immunogenic autoimmune response. Targeting gluten by degrading the immunogenic peptides before absorption minimizes or abrogates the cascading innate and adaptive immune responses that characterise the inflammatory responses to gluten in celiac disease.
Supplementary Material
What you Need to Know.
Background and Context
A gluten-free diet is the only available treatment for celiac disease. Latiglutenase (IMGX003) is an investigational dual-enzyme drug candidate that acts to degrade gluten in-vivo when consumed with a meal.
New Findings
Mucosal and symptom deterioration from gluten ingestion was reduced by latiglutenase relative to placebo. Measurements of gluten-immunogenic peptides (GIP) in urine indicated 95% gluten degradation in the stomach by latiglutenase.
Limitations
There was no evidence of safety issues relative to placebo; however, this limited duration Phase 2 trial was insufficient to assessing long-term effects.
Impact
The latiglutenase mechanism of action is degrading gluten in the stomach, as shown by GIP in urine measurements. Digestive elimination contributes to the favorable safety profile observed in studies to-date.
Acknowledgments
The authors thank the additional members of the IMGX003 CeliacShield study group: Chad Hinson (Mayo Clinic, Rochester, MN, USA), Vasiliy Loskutov (ImmunogenX, Inc., Newport Beach, CA, USA), and Anna Norum (ImmunogenX, Inc., Newport Beach, CA, USA). We also appreciate critical input and advice on clinical, regulatory, biostatistical, analytical, and recruiting support from Dr. Steven Linberg (Laytonsville, MD, USA), Dr. Lawrence Goldkind (Chevy Chase, MD, USA), Dr. Jorma Isola (Tampere, Finland), Dr. Robert Voyksner (LCMS Limited, Durham, NC, USA) and Pauline Luong (Creative Clinical R&D, Kirkland, WA, USA). Finally, we acknowledge the biostatistical services provided by the Matthew Baldwin and Jennifer Nezzer (Advance Research Associates, Santa Clara, CA, USA).
Grant Support:
This study was sponsored by ImmunogenX, Inc., Newport Beach, CA, USA and partially funded by the National Center for Complementary and Integrative Health (NCCIH) of the U.S. National Institutes of Health (NIH) in a grant to ImmunogenX (Grant No. R33AT009637). This project was further supported in part by the National Center for Advancing Translational Sciences (NCATS) of the NIH in a grant to Mayo Clinic (Grant No. UL1 TR002377) and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH in a grant to Stanford University (Grant No. R01 DK063158). The study contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures:
Dr. Joseph A. Murray (JAM): has received study grants from ImmusanT, National Institutes of Health, ImmunogenX, Johnson & Johnson, Kanyos/Anakion, Takeda Pharmaceutical, Allakos, Oberkotter, and Cour; consultancy fees from Bioniz, UKKO, Dren Bio, Dr. Schar USA, Chugai Pharma; GSK, holds patents licensed to Evelo Biosciences; and received royalties from Torax Medical.
Dr. Jack A. Syage: (JAS) has received a grant from National Center for Complementary and Integrative Health (NCCIH) of the U.S. National Institutes of Health (NIH) grant No. R33AT009637 for work reported here. He has received two other active NIH grants. JAS is a co-founder and stockholder in ImmunogenX, Inc.
Dr. Tseng-Teh Wu (TTW): has received grants from Cour Therapeutics and Johnson & Johnson. Matthew A. Dickason (MAD): is a stockholder in ImmunogenX, Inc.
Ana G. Ramos (AGR): is a stockholder in ImmunogenX, Inc.
Carol Van Dyke (CVD): nothing to disclose.
Irina Horwath (IH): nothing to disclose.
Dr. Philip T. Lavin (PTL): is a consultant to ImmunogenX.
Dr. Markku Mäki (MM): is owner and Chair of Board at Maki HealthTech Ltd (MHT). MHT has received Management/Advisory Affiliation fees from Dr. Falk Pharma, Actobio Therapeutics, Jilab, CO Consult, Ukko, Johnson & Johnson, Takeda Pharmaceutical Company Ltd, and Teva Pharmaceuticals; hold patent licensed to Labsystems Diagnostics from where MHT receives royalties via Tampere University Hospital and is on the Scientific Advisory Board of ImmunogenX.
Dr. Isabel Hujoel (IH): nothing to disclose.
Dr. Konstantinos A. Papadakis (KAP): is a stockholder in Abbott Laboratories, Amgen Inc, Johnson & Johnson, and Medtronic PLC.
Dr. Adam C. Bledsoe (ACB): nothing to disclose.
Dr. Chaitan Khosla (CK): has received grants from the National Institutes of Health, is a stockholder and member of the Board of Directors of ImmunogenX, Inc., and has received consulting fees from GlaxoSmithKline plc
Dr. Jennifer A. Sealey-Voyksner (JASV): is a co-founder and stockholder in ImmunogenX, Inc.
Abbreviations Used:
- AE
Adverse events
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- Cd
Crypt depth
- CDSD
Celiac Disease Symptom Diary©
- CSBM
Continuous spontaneous bowel movement
- CeD
Celiac disease
- DGP
Deamidated gliadin peptide
- DH
Dermatitis herpetiformis
- DSMB
Data and Safety Monitoring Board
- EGD
Esophagogastroduodenoscopy
- GC
Gluten challenge
- GFD
Gluten-free diet
- GI
Gastrointestinal
- GIP
Gluten immunogenic peptides
- IEL
Intraepithelial lymphocytes
- IMGX001
Hordein vulgare endoprotease B2 proenzyme (proEP-B2) enzyme
- IMGX002
Sphingomonas capsulata prolyl endopeptidase (SC-PEP) enzyme
- IMGX003
IMGX001 + IMGX002
- mITT
Modified Intent-to-Treat
- MSM
Masked study medication
- PRO
Patient-reported outcome
- SAE
Serious adverse event
- tTG
Tissue transglutaminase
- TEAE
Treatment-Emergent Adverse Event
- VCIEL
A blended histology scale for Vh:Cd and IEL
- Vh
Villus height
- Vh:Cd
Villus height to crypt depth ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten- sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 2015;110:455–61. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009;137:1912–33. [DOI] [PubMed] [Google Scholar]
- 3.Lohi S, Mustalahti K, Kaukinen K et al. Increasing prevalence of celiac disease over time. Aliment Pharmacol Ther 2007;26:1217–25. [DOI] [PubMed] [Google Scholar]
- 4.Syage JA, Kelly CP, Dickason MA, Cebolla Ramirez A, Leon F, Dominguez R, Sealey-Voyksner JA. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am J Clin Nutr 2018;107:201–207. [DOI] [PubMed] [Google Scholar]
- 5.Lerner BA, Phan Vo LT, Yates S, et al. Detection of gluten in gluten-free labeled restaurant food: Analysis of crowd-sourced data. Am J Gastroenterol 2019;00:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CP, Bai JC, Liu E, Leffler DA. Celiac disease: clinical spectrum and management. Gastroenterol 2015;148:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green PHR, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol 2015;135:1099–1106. [DOI] [PubMed] [Google Scholar]
- 8.Guandalini S, Tundia N, Thakkar R, Macaulay D, Essenmacher K, Fuldcore M. Direct cost in patients with celiac disease in the U.S.A.: A retrospective claims analysis. Dig Dis Sci 2016;10:2823–2830. [DOI] [PubMed] [Google Scholar]
- 9.Lahdeaho M-L, Kaukinen K, Laurila K, et al. Glutenase ALV003 Attenuates Gluten-Induced Mucosal Injury in Patients With Celiac Disease. Gastroenterology 2014;146:1649–1658. [DOI] [PubMed] [Google Scholar]
- 10.Murray JA, Kelly CP, Green PH, Marcantonio A, Wu TT, Mäki M, Adelman DC. No Difference Between Latiglutenase and Placebo in Reducing Villous Atrophy or Improving Symptoms in Patients with Symptomatic Celiac Disease. Gastroenterology 2017;152:787–798. [DOI] [PubMed] [Google Scholar]
- 11.Syage JA, Murray JA, Green PHR, Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci 2017;62:2428–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syage JA, Green PHR, Khosla C, Adelman DC, Sealey-Voyksner JA, Murray JA. Latiglutenase Treatment for Celiac Disease: Symptom and Quality of Life Improvement for Seropositive Patients on a Gluten-Free Diet. GastroHep. 2019;1:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, and Khosla C. Structural basis for gluten intolerance in Celiac Sprue. Science 2002;297:2275–2279. [DOI] [PubMed] [Google Scholar]
- 14.Gass J, Bethune MT, Siegel M, Spencer A, and Khosla C.Combination enzyme therapy for gastric digestion of dietary gluten in celiac sprue patients. Gastroenterology 2007;133:472–480. [DOI] [PubMed] [Google Scholar]
- 15.Holm K, Mäki M, et al. Oats in the treatment of childhood coeliac disease: a 2-year controlled trial and a long-term clinical follow-up study. Alimentary Pharmacology & Therapeutics 2006;23:1463–1472. [DOI] [PubMed] [Google Scholar]
- 16.Clifford S, Taylor AJ, Gerber M, et al. Concepts and Instruments for Patient-Reported Outcome Assessment in Celiac Disease: Literature Review and Experts’ Perspectives. Value Health 2020;23:104–113. [DOI] [PubMed] [Google Scholar]
- 17.Moreno ML, Cebolla A, Munoz-Suano A, Carrillo-Carrion C, Comino I, Pizarro A, Leon F, Rodriguez-Herrera A, Sousa C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017;66:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberhuber G . Histopathology of celiac disease . Biomed Pharmacother 2000;54:368–372. [DOI] [PubMed] [Google Scholar]
- 19.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11(10):1185–1194. [DOI] [PubMed] [Google Scholar]
- 20.Bao F, Green PHR, Bhagat G. An update on celiac disease histopathology and the road ahead. Arch Pathol Lab Med. 2012;136:735–745. [DOI] [PubMed] [Google Scholar]
- 21.Adelman DC, Murray J, Wu T-T, Maki M, Green PH, Kelly CP. Measuring Change in Small Intestinal Histology in Patients with Celiac Disease. Am J Gastroenterol 2018;113:339–347. [DOI] [PubMed] [Google Scholar]
- 22.Daveson AJM, Popp A, Taavela J, et al. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep 2020;2:22–30. [Google Scholar]
- 23.Borrelli EP. Celiac Disease Pipeline: An Examination of New Treatment Options Currently under Investigation. Int J Celiac Dis 2018;6:33–36. [Google Scholar]
- 24.Leffler DA, Kelly CP, Abdallah HZ, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol 2012;107:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leffler DA, Kelly CP, Green PHR, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology 2015;148:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahdeaho M-L, Scheinin M, Vuotikka P. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol 2019;4:948–959. [DOI] [PubMed] [Google Scholar]
- 27.Kelly CP, Murray JA, Leffler D, et al. TAK-101 (TIMP-GLIA) prevents gluten challenge induced immune activation in adults with celiac disease. Gastroenterology 2020;58:S-135. [Google Scholar]
- 28.Schuppan D, Maki M, Lundin KEA, et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N Eng J Med 2021;385:35–45. [DOI] [PubMed] [Google Scholar]
- 29.Ilus T, Lähdeaho ML, Salmi T, et al. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am J Gastroenterol 2012;107:1563–1569. [DOI] [PubMed] [Google Scholar]
- 30.Rostami K, Marsh MN, Johnson MW, et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut 2017;66(12):2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose C, Parker A, Jefferson B, Cartmell E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit Rev Environ Sci Technol 2015;45:1827–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coto L, Sousa C, Cebolla A. Dynamics and considerations in the determination of the excretion of gluten immunogenic peptides in urine: individual variability at low gluten intake. Nutrients 2021;13:2624–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palanski BA, Weng N, Zhang L, et al. An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease. bioRxiv 2021; doi: 10.1101/2021.03.17.435829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.