Abstract
Background
Excess visceral adiposity is associated with increased risk of cardiometabolic disorders. Short‐term well‐controlled clinical trials suggest that regular avocado consumption favorably affects body weight, visceral adiposity, and satiety.
Methods and Results
The HAT Trial (Habitual Diet and Avocado Trial) was a multicenter, randomized, controlled parallel‐arm trial designed to test whether consuming 1 large avocado per day for 6 months in a diverse group of free‐living individuals (N=1008) with an elevated waist circumference compared with a habitual diet would decrease visceral adiposity as measured by magnetic resonance imaging. Secondary and additional end points related to risk factors associated with cardiometabolic disorders were assessed. The primary outcome, change in visceral adipose tissue volume during the intervention period, was not significantly different between the Avocado Supplemented and Habitual Diet Groups (estimated mean difference (0.017 L [−0.024 L, 0.058 L], P=0.405). No significant group differences were observed for the secondary outcomes of hepatic fat fraction, hsCRP (high‐sensitivity C‐reactive protein), and components of the metabolic syndrome. Of the additional outcome measures, modest but nominally significant reductions in total and low‐density lipoprotein cholesterol were observed in the Avocado Supplemented compared with the Habitual Diet Group. Changes in the other additional and post hoc measures (body weight, body mass index, insulin, very low‐density lipoprotein concentrations, and total cholesterol:high‐density lipoprotein cholesterol ratio) were similar between the 2 groups.
Conclusions
Addition of 1 avocado per day to the habitual diet for 6 months in free‐living individuals with elevated waist circumference did not reduce visceral adipose tissue volume and had minimal effect on risk factors associated with cardiometabolic disorders.
Registration
URL: https://clinicaltrials.gov; Unique identifier: NCT03528031.
Keywords: avocado, habitual diet, randomized clinical trial, risk factors for cardiometabolic disorders, visceral fat
Subject Categories: Clinical Studies, Lipids and Cholesterol
Nonstandard Abbreviations and Acronyms
- HAT
Habitual Diet and Avocado Trial
- HEI
Healthy Eating Index
- VAT
visceral adipose tissue
Clinical Perspective.
What Is New?
In individuals with elevated waist circumference, consuming 1 large avocado per day (Avocado Supplemented Diet) for 6 months compared with habitual diets (Habitual Diet) had no significant effect on visceral adipose tissue volume.
At the end of the 6‐month intervention period, changes in hepatic fat fraction, high‐sensitivity C‐reactive protein, components of the metabolic syndrome, body weight, body mass index, insulin, very low‐density lipoprotein concentrations, and total cholesterol:high‐density lipoprotein cholesterol ratio were similar between the Avocado Supplemented and Habitual Diet groups.
What Are the Clinical Implications?
Consistent with prior observations, a change in dietary patterns rather than a single food or nutrient may be necessary to achieve clinically significant improvements in visceral adiposity and other cardiometabolic risk factors.
In the United States, ≈60% of adults have visceral adiposity, defined as a waist circumference of ≥40 inches for women and ≥35 inches for men. 1 , 2 Visceral adiposity is associated with elevated risk of type 2 diabetes, insulin resistance, systemic inflammation, cardiovascular disease, and all‐cause mortality. 3 , 4 , 5 , 6 To reduce the burden of these chronic disorders, strategies are needed to prevent the development of visceral adiposity.
Independent of total caloric intake, diet quality has been reported to affect visceral adiposity. 7 , 8 , 9 One food of recent interest is avocados. Avocados are a good source of fiber and oleic acid, a monounsaturated fatty acid (MUFA). Observational data have linked avocado intake with lower rates of metabolic syndrome. 10 , 11 A National Health and Nutrition Examination Survey analysis (2001–2012) reported that avocado consumers weighed less (−3.4 kg) and had smaller waist circumferences (−1.2 cm) than nonconsumers. 10 Data from a randomized clinical trial in overweight adults reported that consumption of half an avocado at the lunch meal was associated with higher postprandial satiety and lower desire to eat after 5‐hours. 12 However, in another study, 13 inclusion of 1 avocado a day, compared with no avocado, as part of a hypocaloric weight loss diet had no significant effect on weight loss, body mass index, total body fat, or visceral adipose tissue (VAT) despite higher reported levels of satiety. 13 A more recent intervention study also reported that in individuals with a body mass index ≥25 kg/m2, 1 avocado a day compared with an isocaloric diet with no avocados resulted in no significant difference in change in abdominal adiposity as measured by dual energy x‐ray absorptiometry (DEXA). 14 Interestingly, exploratory analyses suggested there might be a decrease in abdominal adiposity and ratio of VAT to subcutaneous abdominal adipose tissue in women but not men.
The aim of this study was to determine whether providing 1 avocado a day for 6 months compared with the habitual diet would alter VAT volume, hepatic fat fraction, systemic inflammation (hsCRP [high sensitivity C reactive protein]), and components of the metabolic syndrome in individuals with an elevated waist circumference. Our hypothesis was that addition of an avocado daily to participants' habitual diet would improve these outcomes.
METHODS
The data supporting this article are available from the Hass Avocado Board by request.
Study Design and Oversight
The HAT Trial (Habitual Diet and Avocado Trial) was a randomized, controlled, parallel‐arm, unblinded study conducted at 4 clinical centers in the United States. A trial coordinating center provided data management and statistical support. In addition, there was a central laboratory, magnetic resonance imaging (MRI) reading center, and 2 diet assessment centers (Data S1). A detailed description of the study design has been published. 15 The trial is registered at https://clinicaltrials.gov (NCT03528031).
The Hass Avocado Board sponsored the trial and provided avocados but did not have access to the data files at any time, blinded or unblinded. A steering committee consisting of principal investigators from the 4 clinical centers and the coordinating center provided oversight for the trial. There was no external monitoring board or analysis of interim results. The study was given initial approval by institutional review boards at each center and then administered by the coordinating center institutional review board.
Study Population
Inclusion criteria were ≥25 years, waist circumference ≥35 inches for women and ≥40 inches for men, and regular consumption of ≤2 avocados per month. Exclusion criteria were aversion to avocados, known avocado sensitivity, and unwillingness to undergo MRI scans. Additional criteria are included in Table S1. All participants provided written informed consent.
Randomization and Intervention
Eligible participants were randomly assigned to either a group who were given 1 avocado per day to consume (Avocado Supplemented Diet Group) or a group who continued their usual diet (Habitual Diet Group). Group assignment was determined using permuted block randomization with varying block sizes of 4 and 8 and stratification by clinical center. Allocation was concealed by using an interactive web response system for participant randomization. Neither participants nor clinical center staff were blinded to intervention assignment. The MRI reading center and central laboratory staff were blinded to intervention assignment.
Participants in the Avocado Supplemented Diet Group were instructed to follow their habitual diet and lifestyle, regularly provided with fresh Hass avocados to allow consumption of 1 per day for the 6‐month intervention period. Staff provided participants with written instructions describing how to ripen, cut, remove the pit of, and peel avocados, as well as serving ideas and recipes containing avocados. No additional dietary counseling or guidance was provided.
Participants in the Avocado Supplemented Diet Group picked up avocados from their study site every 2 weeks, during which interaction with study personnel was minimized. Participants in the Habitual Diet Group were instructed to follow their habitual diet and lifestyle and limit their avocado intake to ≤2 avocados/month.
Study Measurements and Outcomes
Apart from the biweekly avocado pickup of the Avocado Supplemented Diet Group, all participants were seen every 4 weeks through week 20, with a final visit at week 26. An extension of the 6‐month study period was needed toward the end of the trial because of the COVID‐19 pandemic. Avocados were delivered to participants still in the trial during the temporary research pause mandated at the clinical sites, which halted the collection of end point measurements until subsequent approval was granted to collect the final data.
Demographic data were collected at baseline. Weight, waist circumference, health‐related quality of life, and blood samples were collected at baseline and 12 and 26 weeks. Twenty‐four hour dietary intake data were collected using Nutrition Data System for Research (software versions 2017 and 2018) at baseline and 8, 16, and 26 weeks. These data were used to assess compliance, estimate energy and nutrient intake, and calculate the Healthy Eating Index (HEI) 2015. 16 The RAND 36‐Item Health survey was used to assess health‐related quality of life. 17
Safety data were collated by the coordinating center. Food sensitivity to avocado consumption, although uncommon, has been reported and staff were trained to refer participants to their primary care physician if they suspected an adverse reaction. Unexpected events involving a hospitalization were reported to the central institutional review board by the coordinating center.
The primary hypothesis was that providing 1 avocado per day for 6 months to participants in the Avocado Supplemented Diet Group would result in a reduction in visceral adiposity measured by MRI compared with the Habitual Diet Group. Secondary measures included hepatic lipid content, plasma hsCRP concentrations and components of the metabolic syndrome (plasma glucose, triglyceride and high‐density lipoprotein cholesterol [HDL‐C] concentrations, blood pressure, and waist circumference). Additional measures included plasma insulin, total cholesterol and low‐density lipoprotein cholesterol (LDL‐C) concentrations, fiber, HEI, and health‐related quality of life.
MRI Collection and Analysis
Participants underwent 2 abdominal MRI scans, 1 before randomization and 1 at the end of the nominal 6‐month intervention. MRI data were collected on 3T Skyra or Prisma scanners (Siemens Healthineers, Erlangen, Germany) using 7 different scanners at 5 imaging centers near the clinical sites. Two ex vivo preserved human livers, and a fat/water phantom were scanned at all sites to verify that consistent data were collected across all sites and over time. The ex vivo livers were formalin fixed, 1 chosen from an individual with no history of liver pathology and 1 with a history of nonalcoholic fatty liver disease. The fat/water phantom was an ≈4 L container that contained about 3.2 L of 5% agarose gel with 0.03% sodium azide, and 0.8 L of lard. VAT volume was assessed using a 2‐echo DIXON 3‐dimensional axial vibe sequence with field of view=400 mm, repetition time=5 ms, echo time=1.23, 2.46 ms, fractional anisotropy=9°, resolution=2.1×2.3×3.5 mm3, and acquisition time=17 seconds in a single breath hold. Slice coverage was enough to cover 4 cm above the dome of the liver to 7 cm below the top of the iliac crest. Hepatic fat fraction was assessed with point resolved spectroscopy sequence with repetition time=2000 ms, echo time=20 ms, Nx=8, and acquisition time=16 seconds in a single breath hold. This approach has a high level of precision 18 and provides detailed 3‐dimensional images of the abdomen that allow for quantification of even small (a few millimeters) collections of fat (Figure S1).
The MRI data collected at each site were anonymized and sent to the MRI reading center for centralized analysis. All analyses were performed blinded to treatment group. Dixon fat images for VAT volume were manually segmented to exclude subcutaneous fat using SliceOmatic and a watershed algorithm. Images were then thresholded using an implementation of the Otsu algorithm from ImageJ. Threshold results were manually reviewed and any nonvisceral fat (such as from the vertebral bodies) was manually removed. MR spectroscopy was processed with LCModel version 6.3 using lipid quantification settings, and T1 and T2 correction were applied. 19
Biochemical Measures
Details regarding measurement of blood pressure, anthropometric measures, dietary assessment, and quality of life survey have been reported previously. 15 Briefly, blood sample (serum, plasma, red blood cells, and buffy coat) collection was standardized at all sites as per instructions in the Blood Processing Manual of Operation Procedures. Plasma total cholesterol, HDL‐C, triglyceride, and glucose concentrations were measured using an automated clinical chemistry analyzer (AU480, Beckman Coulter Inc., CA) with intra‐assay coefficients of variability of 2.0% to 3.0% and interassay coefficients of variability of 2.8% to 5%. 20 , 21 , 22 LDL‐cholesterol concentrations were calculated using the Friedewald formula, 23 except when triglyceride concentrations were over 400 mg/dL, in which case a direct LDL‐C 2 reagent colorimetric enzymatic assay was used (AU480, Beckman Coulter, Inc., CA) with intra‐ and interassay coefficients of variability of 2.4% and 3.6% respectively. very LDL‐C concentrations were calculated as triglycerides/2.2. Serum insulin and hsCRP concentrations were measured by solid‐phase, 2‐site chemiluminescent immunometric assays (IMMULITE 2000, Siemens Healthcare Diagnostics, Los Angeles, CA). 24 The intra‐ and interassay coefficients of variability were 3.0% and 5.0%, respectively for hsCRP and 3.5% and 5.0%, respectively, for insulin. Details regarding measurement of blood pressure, anthropometric measures, dietary assessment, and quality of life survey have been reported previously. 15
Statistical Analysis
The trial was designed to randomize 1000 participants over a 2‐year period and assumed a 10% lost to follow‐up rate. It was estimated that this sample size would provide 85% power to detect a 6‐month between‐group change in VAT volume of 0.5 L favoring the Avocado Supplemented Diet group. The prespecified primary analysis was an intent‐to‐treat comparison of the 2 randomized groups on the estimated 6‐month change in VAT volume, restricted to participants with complete data. The effect on change in VAT volume from baseline to follow‐up was estimated using a linear regression adjusted for randomized group and site, and primary hypothesis testing was 2 sided with α=0.05. Supporting analyses were done using all available VAT measurements at both baseline and follow‐up in a mixed effects model adjusting for randomized group and site to estimate the 6‐month effect on VAT. Prespecified subgroups of interest were defined using sex, ethnicity or race (non‐Hispanic White versus other [Native Hawaiian, Pacific Islander, or Other]), baseline VAT (median split), baseline HEI (median split), and baseline kilocalorie intake (median split). Subgroup hypotheses were tested by adding a categorical subgroup indicator to the linear regression model and an interaction term for intervention by subgroup. The significance of the interaction term adjusted for multiple comparisons was used to test for subgroup effects.
Effects on secondary and other measures were estimated with models on change from baseline similar to the primary analysis but using mixed effects when multiple postrandomization assessments were done. Effects averaged over the follow‐up period are reported when there is no significant time by intervention interaction. For these effects, reported P values are nominal; no adjustment for multiple comparisons was made. For the 24‐hour diet recall data, mixed effects models adjusted for randomized group and clinic for all available measurements were used to estimate visit‐specific means and the mean difference from baseline over follow‐up. Descriptive statistics are reported as means and SD except for highly skewed distributions, where median, first, and third quartiles are reported. Counts and categorical data are summarized as N and %.
RESULTS
Study Participants
From June 27, 2018 to March 4, 2020, 1008 participants were randomly assigned to either the Avocado Supplemented Diet Group or Habitual Diet Group (Figure 1). A total of 85 participants did not complete the follow‐up MRI, 50 in the Avocado Supplemented Diet Group and 35 in the Habitual Diet Group. At the time of randomization, participants mean age was 50 (14) (mean [SD]) years (Table 1) and 72% were women. The racial and ethnic distribution of the cohort was 68.8% White, 20.6% Hispanic, 14.8% Black, 5.8% Asian, and 10% either did not answer, were American Indian, other or multiple races or ethnicities. The mean waist circumference of women was 106 (12) cm and men 118 (12) cm. The mean VAT was 3.2 (1.4) liters and hepatic fat fraction 9.9 (11) %. Follow‐up measures were completed in October 2020. Of the 935 (93%) participants with final MRI scans, 12 were not readable, leaving 923 (92% of the randomized participants) with complete data.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) diagram.
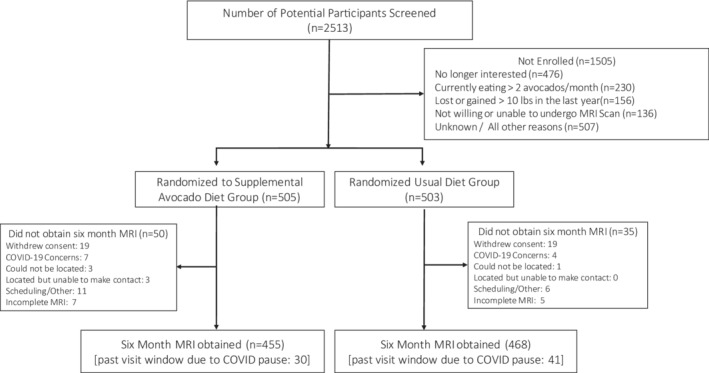
MRI indicates magnetic resonance imaging.
Table 1.
Baseline Characteristics of Study Participants
| Variables* | Overall (N=1008) | Study arms | |
|---|---|---|---|
| Avocado Supplemented Diet Group (n=505) | Habitual Diet Group (n=503) | ||
| Age, y | 50.3±14.0 | 50.1±14.3 | 50.4±13.8 |
| Women, n (%) | 730 (72.4) | 356 (70.5) | 374 (74.4) |
| Hispanic or Latino, n (%) | 207 (20.6) | 106 (21.0) | 101 (20.2) |
| Race, n (%) | |||
| Did not answer | 3 (0.3) | 1 (0.2) | 2 (0.4) |
| Black | 149 (14.8) | 68 (13.5) | 81 (16.1) |
| American Indian | 4 (0.4) | 2 (0.4) | 2 (0.4) |
| Asian | 58 (5.8) | 26 (5.1) | 32 (6.4) |
| White | 694 (68.8) | 361 (71.5) | 333 (66.2) |
| Other† | 72 (7.1) | 33 (6.5) | 39 (7.8) |
| Multiple races reported | 28 (2.8) | 14 (2.8) | 14 (2.8) |
| Anthropometric measures | |||
| Weight, kg | 93.2±19.0 | 93.2±19.0 | 93.1±18.9 |
| Body mass index, kg/m2 | 33.0±5.5 | 32.9±5.3 | 33.2±5.6 |
| Waist circumference, cm | 109±13 | 109±13 | 109±13 |
| Women | 106±12 | 106±12 | 107±12 |
| Men | 118±12 | 118±12 | 118±12 |
| Biochemical measures, mg/dL | |||
| Total cholesterol | 188±40 | 185±40 | 190±39 |
| LDL‐cholesterol | 112±34 | 110±34 | 114±34 |
| HDL‐cholesterol | |||
| Women | 55±13 | 55±13 | 56±13 |
| Men | 45±10 | 44±9 | 45±10 |
| Very LDL‐cholesterol | 21 (15, 29) | 21 (16, 29) | 21 (15, 29) |
| Triglyceride | 104 (76, 145) | 106 (79, 145) | 104 (73, 145) |
| Total cholesterol:HDL‐cholesterol | 3.7±1.0 | 3.8±1.0 | 3.7±1.0 |
| Glucose, mg/dL | 107±29 | 107±29 | 107±30.0 |
| Blood pressure, mm Hg | |||
| Diastolic | 77±10 | 77±10 | 76±11 |
| Systolic | 123±16 | 123±16 | 123±16 |
| Magnetic resonance imaging measures | |||
| Visceral adipose tissue, L | 3.2±1.4 | 3.2±1.4 | 3.2±1.4 |
| Hepatic fat fraction, % | 9.9±10.6 | 9.5±9.9 | 10.2±11.2 |
HDL indicates high‐density lipoprotein; and LDL, low‐density lipoprotein.
Continuous data are presented as mean±SD, or median (first quartile, third quartile). Categorical variables are presented as number and percent.
Other indicates Native Hawaiian, Pacific Islander, or Other.
Compliance With the Intervention
Participants from both intervention groups reported infrequent intake of avocado at baseline (Table 2). No significant in‐trial differences in avocado consumption were observed in the Habitual Diet Group, whereas the Avocado Supplemented Diet Group increased intake and maintained a high level of consumption. Based on self‐reported 24‐hour diet recalls during the follow‐up period, among the Avocado Supplemented Diet Group participants, 76% to 78% consumed at least 1 whole avocado and 12% to 17% consumed some avocado (total 88%–95%) each day. In contrast, 92% to 94% of the Habitual Diet Group participants reported consuming no avocado and the remainder only some avocado.
Table 2.
Avocado Intake by Intervention Group, Percentage*
| Avocado intake | Avocado Supplemented Group (n=504) | Habitual Diet Group (n=503) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |||||
| 8 wk | 16 wk | 26 wk | 8 wk | 16 wk | 26 wk | |||
| Percent participants* | ||||||||
| None | 90.9 | 4.9 | 8.1 | 11.9 | 91.7 | 94.2 | 92.8 | 91.5 |
| Some† | 8.1 | 16.7 | 15.7 | 12.1 | 8.1 | 5.6 | 7.2 | 8.3 |
| One+/d | 1.0 | 78.4 | 76.2 | 76.1 | 0.2 | 0.2 | 0.0 | 0.2 |
Percentage data from 24‐hour diet recalls collected for specified week by treatment arm.
Some defined as avocado consumption reported in the recall was <90% of the study‐provided amount of 168 g (151 g): total avocado <151 g; avocado reported but no amount given; avocado on toast, bread, salad, sandwich, etc but no amount given and no note for amount, and guacamole reported in the recall was less than the Nutrition Data System for Research equivalent to 168 g of avocado (1.5 cups); total guacamole <1.5 cups; and guacamole reported but no amount given.
Diet Composition Intake
The daily energy intake remained stable for the Habitual Diet Group and significantly increased at weeks 8, 16, and 26 for the Avocado Supplemented Diet Group. During the intervention, dietary fiber, fat (total fat and MUFA, both g per day and % energy), and HEI were significantly higher in the Avocado Supplemented Diet Group than the Habitual Diet Group (Table S2). Dietary carbohydrate and protein intake were significantly lower in the Avocado Supplemented Diet Group than the Habitual Diet Group.
Change in Primary, Secondary, and Additional Measures
The primary outcome, change in VAT volume from baseline to 6 months, was similar between diet groups; 0.074 L in the Avocado Supplemented Diet Group and 0.057 L in the Habitual Diet Group (P=0.405; Table 3, Table S3, Figure S2). The estimated mean difference was 0.017 L (−0.024 L, 0.058 L, mean and CIs). The effect of the intervention was consistent across prespecified participant subgroups (Figure 2, Table S4). Inclusion of all available MRI data, including participants who did not have final MRI measures, did not significantly affect this result (Data S1).
Table 3.
Model‐Based Estimates of Change in Prespecified Primary, Secondary, and Additional Outcome Measures by Intervention Group
| Outcomes | Supplemented Avocado Diet Group | Habitual Diet Group | Effect | 95% CI | P value |
|---|---|---|---|---|---|
| Primary* | |||||
| Visceral adipose tissue, L | 0.074 | 0.057 | 0.017 | −0.024 to 0.058 | 0.405 |
| Secondary* | |||||
| Hepatic fat fraction, % | 0.58 | 0.21 | 0.37 | −0.31 to 1.06 | 0.285 |
| High‐sensitivity C‐reactive protein, mg/L | 0.788 | 0.435 | 0.352 | −0.845 to 1.550 | 0.564 |
| Components of the metabolic syndrome | |||||
| Waist circumference, cm | 0.007 | −0.108 | 0.116 | −0.320 to 0.551 | 0.603 |
| Systolic BP, mm Hg | −1.286 | −0.034 | −1.251 | −2.566 to 0.064 | 0.062 |
| Diastolic BP, mm Hg | −1.012 | −0.324 | −0.688 | −1.601 to 0.225 | 0.139 |
| Triglyceride, mg/dL | 2.959 | 4.069 | −1.110 | −7.624 to 5.403 | 0.738 |
| Glucose, mg/dL | 0.629 | 1.577 | −0.948 | −2.962 to 1.065 | 0.356 |
| HDL‐cholesterol, mg/dL | −0.279 | −0.183 | −0.096 | −0.885 to 0.692 | 0.811 |
| Additional* and post hoc outcomes | |||||
| Body weight, kg | 0.382 | 0.378 | 0.004 | −0.310 to 0.318 | 0.978 |
| Body mass index, kg/m2 | 0.142 | 0.134 | 0.009 | −0.103 to 0.120 | 0.880 |
| Insulin, μIU/mL* | 0.120 | 0.615 | −0.495 | −2.089 to 1.098 | 0.542 |
| Total cholesterol, mg/dL* | −4.908 | −1.968 | −2.940 | −5.535 to −0.346 | 0.026† |
| LDL‐cholesterol, mg/dL* | −4.947 | −2.482 | −2.465 | −4.796 to −0.134 | 0.038† |
| Very LDL‐cholesterol, mg/dL | 0.592 | 0.814 | −0.222 | −1.525 to 1.081 | 0.738 |
| Total cholesterol: HDL‐cholesterol | −0.083 | −0.028 | −0.055 | −0.119 to 0.009 | 0.095 |
| Health‐related quality of life (0–100)* | 0.164 | −0.320 | −0.481 | −1.615 to 0.653 | 0.405 |
BP indicates blood pressure; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
Pre‐specified.
Figure 2. Visceral adipose tissue by subgroup.
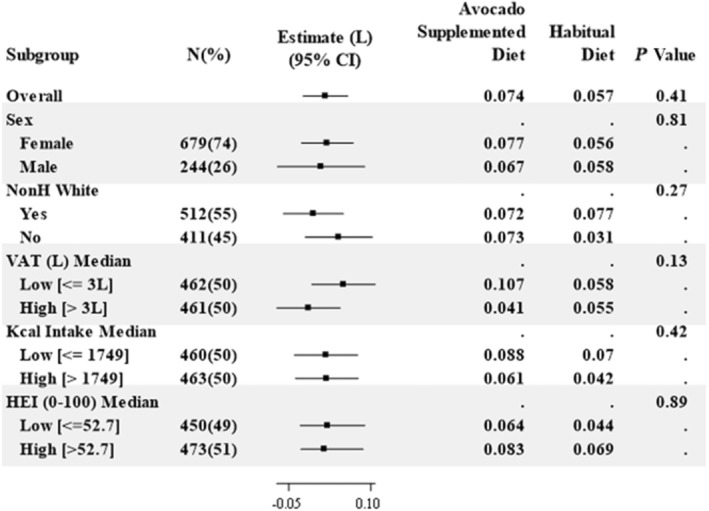
For each subgroup, the estimated difference of the 6‐month effects on VAT between the Avocado Supplemented Diet group and the Habitual Diet group is shown as a solid square with a horizontal line showing its 95% CI. At the right, the estimated 6‐month effect on VAT from the mixed effects model is shown for each group along with a P value for the interaction terms, except for Overall, which is the P value for the difference. HEI indicates Healthy Eating Index (range from 0 to 100); Kcal, kilocalories; NonH White, Non‐Hispanic White; and VAT, visceral adipose tissue volume (in liters).
Providing 1 avocado per day for 6 months resulted in no significant differences in changes between the 2 groups for the secondary outcomes of hepatic fat fraction, hsCRP, or components of the metabolic syndrome (waist circumference, systolic and diastolic blood pressure, or triglyceride, glucose, and HDL‐C concentrations) (Table 3). Of the additional outcomes, only total cholesterol and LDL‐C were nominally significant, modestly lower in the Avocado Supplemented compared than Habitual Diet Group (2.9 [P=0.026] and 2.5 mg/dL [P=0.038], respectively). The other additional and post hoc outcomes, body mass index, insulin, and very LDL‐C concentrations, and total cholesterol:HDL‐C ratio were similar in the Avocado Supplemented and Habitual Diet Groups. There was a significant increase in fiber and the HEI in the Avocado Supplemented Diet Group compared with the Habitual Diet Group and no significant effect on body weight, which remained stable during the intervention period in both groups (Tables 3 and 4, Table S2).
Table 4.
Descriptive Statistics of Secondary and Additional Measures
| Variable | Avocado Supplemented Diet Group (n=505) | Habitual Diet Group (n=503) | ||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |||
| 3 mo | 6 mo | 3 mo | 6 mo | |||
| Anthropometric | ||||||
| Body weight, kg | 93.2±19.0 | 93.8±19.3 | 93.4±19.4 | 93.1±18.9 | 93.6±19.1 | 93.4±19.1 |
| Body mass index, kg/m2 | 32.9±5.3 | 33.1±5.5 | 33.0±5.5 | 33.2±5.6 | 33.3±5.7 | 33.2±5.6 |
| Waist circumference, cm* | ||||||
| Women | 106.0±11.9 | 106.1±12.2 | 106.3±12.6 | 106.6±12.2 | 106.6±12.6 | 106.3±3.0 |
| Men | 117.6±11.5 | 117.6±11.8 | 116.8±12.0 | 117.6±12.2 | 117.6±12.6 | 117.2±12.7 |
| Cardiometabolic risk factors | ||||||
| Systolic BP, mm Hg* | 123±16 | 121±15 | 122±16 | 123±16 | 122±15 | 123±16 |
| Diastolic BP, mm Hg* | 77±10 | 75±10 | 76±15 | 76±11 | 76±10 | 76±10 |
| High‐sensitivity C‐reactive protein, mg/L* | 3.7 (1.5–7.8) | 3.7 (1.5–7.6) | 3.6 (1.6–7.6) | 3.7 (1.6–7.3) | 3.7 (1.4–7.7) | 3.8 (1.6–7.9) |
| Total cholesterol, mg/dL† | 185±40 | 181±40 | 180±38 | 190±39 | 189±40 | 189±38 |
| LDL‐cholesterol, mg/dL† | 110±34 | 106±33 | 104±33 | 114±34 | 112±34 | 111±33 |
| HDL‐cholesterol, mg/dL* | ||||||
| Women | 55±13 | 55±13 | 54±13 | 56±13 | 56±12 | 56±13 |
| Men | 44±9 | 45±10 | 44±9 | 45±10 | 46±11 | 46±11 |
| Very LDL‐cholesterol, mg/dL | 21 (16–29) | 20 (15–29) | 21 (15–30) | 21 (15–29) | 21 (15–30) | 21 (16–29) |
| Triglyceride, mg/dL* | 106 (79–145) | 102 (73–144) | 103 (75–148) | 104 (73–145) | 105 (76–149) | 104 (80–146) |
| Total cholesterol:HDL‐cholesterol | 3.8±1.0 | 3.7±1.1 | 3.7±1.0 | 3.7±1.0 | 3.7±1.0 | 3.7±1.0 |
| Glucose, mg/dL* | 100 (93–109) | 100 (93–108) | 101 (94–111) | 101 (93–110) | 100 (93–109) | 101 (94–110) |
| Insulin, μIU/mL† | 14 (9–20) | 14 (9–20) | 13 (9–21) | 13 (8–21) | 13 (9–22) | 13 (8–22) |
Data are presented as mean±SD, or median (interquartile range). BP indicates blood pressure; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
Secondary outcome.
Additional prespecified outcome.
Adverse Events
There were 21 adverse events reported during the trial, 16 occurred in the Avocado Supplemented Diet Group and 5 occurred in Habitual Diet Group (Table 5). Twelve events were unrelated to trial participation. Nine events were identified as possibly or definitely related to the intervention, all in the Avocado Supplemented Diet Group, and included experiencing skin rash or breathing difficulties (n=3) and gastrointestinal upset (gas, bloating, diarrhea) (n=6). Two were classified as serious, 1 for noncardiac chest pain and 1 for E coli infection. Both resolved and did not recur. No deaths occurred during the trial.
Table 5.
Safety: Adverse Events, by Arm
| Event | Avocado Supplemented Diet* | Habitual Diet |
|---|---|---|
| Breathing difficulties, skin rash | 3 | 0 |
| Gastrointestinal | 6 | 0 |
| Other | 7 | 5 |
| Total | 16 | 5 |
Two adverse events that occurred in the Avocado Supplemented Diet arm were deemed “possibly” linked to the intervention. One was for noncardiac chest pain and the other for E coli infection. Both resolved without further medical intervention and did not recur during the trial.
DISCUSSION
In a large multicenter study of 1008 free‐living participants with elevated waist circumference, addition of 1 avocado a day for 6 months to habitual diets had no effect on VAT volume, the primary study end point, despite a positive effect on diet quality. Subgroup analyses showed a consistently null effect across sex, race or ethnicity, baseline VAT, baseline energy intake, and baseline HEI and health‐related quality of life. Changes in most secondary and additional outcome measures (cardiometabolic risk factors), except for modest decreases in total cholesterol and LDL‐C concentrations, were not statistically significant.
The hypothesis that avocado intake would reduce VAT was based on prior clinical trials reporting that diets high in MUFA lowered the upper body fat (android and abdominal) to lower body fat (gynoid) ratio, both with and without weight loss. 25 , 26 , 27 , 28 There were differences between the interventions among these studies. Those reporting this finding involved dietary interventions that achieved levels of MUFA >22% of total caloric intake, used a different source of MUFA, and/or replaced the MUFA with saturated fat. 26 , 27 , 28 Notably, relative to prior studies, the present study had a larger sample size, which increased the statistical power to detect true effects if they occurred, had a longer intervention period, and employed a more realistic dietary modification, a single avocado a day with no additional dietary advice, mimicking real world conditions.
A recent 12‐week intervention study of individuals with a body mass index ≥25 kg/m2, who consumed 1 avocado a day compared with an isocaloric diet with no avocados, also did not observe significant differences in change in abdominal adiposity as measured by DEXA. 14 In that study, exploratory analyses indicated there was a significant decrease in abdominal adiposity and in VAT to subcutaneous abdominal adipose tissue ratio in women but not men. A direct comparison between the 2 studies cannot be made because the data for our study are expressed as the total intake whereas the data provided in the earlier publication are only for the single meal that contained an avocado. In addition, DEXA was used to assess adiposity in the prior study, whereas multislice 3‐dimensional MRI was used to measure VAT in the current study. Although cheaper and faster, DEXA measures are limited to providing a single 2‐dimensional projection image. Although comparison studies have shown DEXA to perform similarly to single‐slice MRI in cross‐sectional studies, 29 it does not perform well in longitudinal studies and is likely to underestimate small longitudinal changes. 30 , 31 Despite providing no guidance with regard to offsetting the energy content of the avocados, there was no significant effect of the avocados on body weight, suggesting participant nonintentional compensation. The compensation resulted in a shift in macronutrient content of the diet during the 6‐month intervention period. The participants in the Avocado Supplemented Group increased their fat and lowered their carbohydrate and protein intake.
Nominally significant greater decreases in total cholesterol and LDL‐C concentrations were observed in the Avocado Supplemented compared with Habitual Diet Group. The between‐group differences in total cholesterol and LDL‐C align with the observed dietary fiber differences between groups. A single avocado has ≈3.3 g of soluble fiber. 32 Other factors may have also contributed to the differences in total cholesterol and LDL‐C concentrations, such as changes in the gut microbiota 33 and the phytosterol content of the avocadoes. 34 The findings of the current study are in contrast with those of a systematic review that included data up to February 2015, 35 which concluded avocados decreased total cholesterol and LDL‐C and resulted in no significant change in HDL‐C, and one that included data up to September 2017, 36 which concluded avocados did not decrease total and LDL cholesterol but increased HDL‐cholesterol concentrations. Differences in inclusion and exclusion criteria, in addition to the period searched, may explain the differences. An observational study published in 2022 focusing on 2 large US cohorts concluded that dietary patterns containing avocados were associated with lower cardiovascular disease risk and coronary heart disease when replacing foods such as margarine, butter, egg, yogurt, cheese, and processed meats. 37
The estimated between‐group differences in mean energy intake were significantly higher in the Avocado Supplemented than Habitual Diet Group (117 kilocalories); however, there was no significant difference in change in mean body weight between groups. Self‐reports indicated >90% compliance with the intervention. Energy intake was estimated using a subjective measure, 38 the mean of 4 24‐hour dietary recalls collected during the 6‐month intervention period, whereas body weight was assessed objectively, using a calibrated scale. One large avocado provided in this study contained 280 kilocalories suggesting that avocados at least partially replaced a component of habitual intake. Although the specific food groups replaced by the avocados were not determined, the macronutrient profile data suggest avocados replaced foods relatively high in carbohydrate. Our results are consistent with a systematic review published this year that concluded avocado consumption did not promote weight gain. 39
The HAT study was a large multicenter, investigator‐blinded, randomized clinical trial. The retention rate was high (92%), despite the extraordinary challenges associated with the COVID‐19 pandemic (Table S5). Changes in the dietary intake of the Avocado Supplemented Diet Group were consistent with what would be predicted based on the macronutrient composition of the fruit. The primary outcome, VAT, was measured prospectively with a high level of precision using a multislice 3‐dimensional MRI technique. This trial was not without limitations. Medication usage was not collected. Given the intervention period and frequency of physician visits it is unlikely there were major changes in medication usage in the vast majority of participants. As with all food‐based diet trials, participants could not be blinded to the dietary intervention. The 6‐month intervention period may have been insufficient to detect a change in VAT. However, a longer intervention period might have compromised compliance. Although an uninterrupted supply of avocados was provided until arrangements for final study measures could be made, the lives and diets of those last enrolled in the trial may have been affected by the COVID‐19 pandemic. Were there a change, the concurrent control design likely balanced a potential temporal bias between the 2 groups. The commitment of subjects and research teams at all sites made it possible to successfully complete the study and maintain a high level of dietary compliance.
CONCLUSIONS
In conclusion, in this randomized controlled multicenter parallel trial, the addition of 1 avocado per day to the habitual diet for 6 months in individuals with visceral adiposity did not reduce VAT and had minimal effect on risk factors associated with cardiometabolic disorders.
Sources of Funding
This work was supported by the Avocado Nutrition Center.
Disclosures
None.
Supporting information
Data S1
Tables S1–S5
Figures S1–S2
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025657
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Kim D, Hou W, Wang F, Arcan C. Factors affecting obesity and waist circumference among US adults. Prev Chronic Dis. 2019;16:E02. doi: 10.5888/pcd16.180220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 3. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all‐cause, cardiovascular, and cancer mortality: sixteen years of follow‐up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 4. Song X, Jousilahti P, Stehouwer CD, Söderberg S, Onat A, Laatikainen T, Yudkin JS, Dankner R, Morris R, Tuomilehto J, et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr. 2013;67:1298–1302. doi: 10.1038/ejcn.2013.203 [DOI] [PubMed] [Google Scholar]
- 5. Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan‐European case‐cohort analysis. Eur Heart J. 2018;39:397–406. doi: 10.1093/eurheartj/ehx448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177–189. doi: 10.1038/s41574-019-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lassale C, Fezeu L, Andreeva VA, Hercberg S, Kengne AP, Czernichow S, Kesse‐Guyot E. Association between dietary scores and 13‐year weight change and obesity risk in a French prospective cohort. Int J Obes. 2012;36:1455–1462. doi: 10.1038/ijo.2011.264 [DOI] [PubMed] [Google Scholar]
- 8. Paradis AM, Godin G, Pérusse L, Vohl MC. Associations between dietary patterns and obesity phenotypes. Int J Obes. 2009;33:1419–1426. doi: 10.1038/ijo.2009.179 [DOI] [PubMed] [Google Scholar]
- 9. Schwingshackl L, Hoffmann G, Kalle‐Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta‐analysis of prospective cohort studies. PLoS One. 2015;10:e0140846. doi: 10.1371/journal.pone.0140846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fulgoni V III, O'Neil CE, Nicklas TA. Avocado consumption by adults is associated with better nutrient intake, diet quality, and some measures of adiposity: National Health and Nutrition Examination Survey, 2001–2012. Intern Med Rev. 2017;3:2–23. doi: 10.18103/imr.v3i4.422 [DOI] [Google Scholar]
- 11. Fulgoni VL III, Dreher M, Davenport AJ. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr J. 2013;12:1. doi: 10.1186/1475-2891-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wien M, Haddad E, Oda K, Sabaté J. A randomized 3×3 crossover study to evaluate the effect of Hass avocado intake on post‐ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr J. 2013;12:155. doi: 10.1186/1475-2891-12-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henning SM, Yang J, Woo SL, Lee RP, Huang J, Rasmusen A, Carpenter CL, Thames G, Gilbuena I, Tseng CH, et al. Hass avocado inclusion in a weight‐loss diet supported weight loss and altered gut microbiota: a 12‐week randomized, parallel‐controlled trial. Curr Dev Nutr. 2019;3:nzz068. doi: 10.1093/cdn/nzz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan NA, Edwards CG, Thompson SV, Hannon BA, Burke SK, Walk ADM, Mackenzie RWA, Reeser GE, Fiese BH, Burd NA, et al. Avocado consumption, abdominal adiposity, and oral glucose tolerance among persons with overweight and obesity. J Nutr. 2021;151:2513–2521. doi: 10.1093/jn/nxab187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reboussin DM, Kris‐Etherton PM, Lichtenstein AH, Li Z, Sabate J, Matthan NR, Petersen K, Rajaram S, Vitolins M, Ford N. The design and rationale of a multi‐center randomized clinical trial comparing one avocado per day to usual diet: the Habitual Diet and Avocado Trial (HAT). Contemp Clin Trials. 2021;110:106565. doi: 10.1016/j.cct.2021.106565 [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute Division of Cancer Control and Population Sciences . ASA24 resources related to the Healthy Eating Index (HEI). Available at: https://epi.grants.cancer.gov/hei/sas‐code.html. Accessed December 17, 2021.
- 17. Stewart A, Ware J. Measuring Functioning and Well‐Being: The Medical Outcomes Study Approach. Duke University Press; 1992. doi: 10.7249/CB361 [DOI] [Google Scholar]
- 18. So R, Sasai H, Matsuo T, Tsujimoto T, Eto M, Saotome K, Tanaka K. Multiple‐slice magnetic resonance imaging can detect visceral adipose tissue reduction more accurately than single‐slice imaging. Eur J Clin Nutr. 2012;66:1351–1355. doi: 10.1038/ejcn.2012.147 [DOI] [PubMed] [Google Scholar]
- 19. d'Assignies G, Ruel M, Khiat A, Lepanto L, Chagnon M, Kauffmann C, Tang A, Gaboury L, Boulanger Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur Radiol. 2009;19:2033–2040. doi: 10.1007/s00330-009-1351-4 [DOI] [PubMed] [Google Scholar]
- 20. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. doi: 10.1093/clinchem/20.4.470 [DOI] [PubMed] [Google Scholar]
- 21. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. doi: 10.1093/clinchem/19.5.476 [DOI] [PubMed] [Google Scholar]
- 22. Stein M. Clinical methods of enzymatic analysis. Vol 117. Academic Press; 1965:435–452. [Google Scholar]
- 23. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 24. Babson A. The cirrus IMMULITE automated immunoassay system. J Clin Immunoassay. 1991;14:83–88. [Google Scholar]
- 25. Bowen KJ, Kris‐Etherton PM, Shearer GC, West SG, Reddivari L, Jones PJH. Oleic acid‐derived oleoylethanolamide: a nutritional science perspective. Prog Lipid Res. 2017;67:1–15. doi: 10.1016/j.plipres.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 26. Paniagua JA, Gallego de la Sacristana A, Romero I, Vidal‐Puig A, Latre JM, Sanchez E, Perez‐Martinez P, Lopez‐Miranda J, Perez‐Jimenez F. Monounsaturated fat‐rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate‐rich diet in insulin‐resistant subjects. Diabetes Care. 2007;30:1717–1723. doi: 10.2337/dc06-2220 [DOI] [PubMed] [Google Scholar]
- 27. Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr. 2005;82:320–326. doi: 10.1093/ajcn/82.2.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4‐week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717–727. doi: 10.1079/BJN2003948 [DOI] [PubMed] [Google Scholar]
- 29. Neeland IJ, Grundy SM, Li X, Adams‐Huet B, Vega GL. Comparison of visceral fat mass measurement by dual‐X‐ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016;6:e221. doi: 10.1038/nutd.2016.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dias KA, Ramos JS, Wallen MP, Davies PSW, Cain PA, Leong GM, Ingul CB, Coombes JS, Keating SE. Accuracy of longitudinal assessment of visceral adipose tissue by dual‐energy X‐ray absorptiometry in children with obesity. J Obes. 2019;2019:2193723–2193712. doi: 10.1155/2019/2193723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor JL, Holland DJ, Coombes JS, Keating SE. Accuracy of dual‐energy x‐ray absorptiometry for assessing longitudinal change in visceral adipose tissue in patients with coronary artery disease. Int J Obes. 2021;45:1740–1750. doi: 10.1038/s41366-021-00840-3 [DOI] [PubMed] [Google Scholar]
- 32. Marlett JA, Cheung TF. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J Am Diet Assoc. 1997;97:1139–1148, 1151; quiz 1149‐1150. doi: 10.1016/S0002-8223(97)00275-7 [DOI] [PubMed] [Google Scholar]
- 33. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol‐lowering effects of dietary fiber: a meta‐analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30 [DOI] [PubMed] [Google Scholar]
- 34. Salazar‐López NJ, Domínguez‐Avila JA, Yahia EM, Belmonte‐Herrera BH, Wall‐Medrano A, Montalvo‐González E, González‐Aguilar GA. Avocado fruit and by‐products as potential sources of bioactive compounds. Food Res Int. 2020;138:109774. doi: 10.1016/j.foodres.2020.109774 [DOI] [PubMed] [Google Scholar]
- 35. Peou S, Milliard‐Hasting B, Shah SA. Impact of avocado‐enriched diets on plasma lipoproteins: a meta‐analysis. J Clin Lipidol. 2016;10:161–171. doi: 10.1016/j.jacl.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 36. Mahmassani HA, Avendano EE, Raman G, Johnson EJ. Avocado consumption and risk factors for heart disease: a systematic review and meta‐analysis. Am J Clin Nutr. 2018;107:523–536. doi: 10.1093/ajcn/nqx078 [DOI] [PubMed] [Google Scholar]
- 37. Pacheco LS, Li Y, Rimm EB, Manson JE, Sun Q, Rexrode K, Hu FB, Guasch‐Ferré M. Avocado consumption and risk of cardiovascular disease in US adults. J Am Heart Assoc. 2022;11:e024014. doi: 10.1161/JAHA.121.024014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TI, Speakman JR, Jeansonne M, Allison DB. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–1113. doi: 10.1038/ijo.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conceição AR, Fraiz GM, Rocha DMUP, Bressan J. Can avocado intake improve weight loss in adults with excess weight? A systematic review and meta‐analysis of randomized controlled trials. Nutr Res. 2022;102:45–58. doi: 10.1016/j.nutres.2022.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S2


