Abstract
Aims
Recent technological advances enable diagnosing of obstructive coronary artery disease (CAD) from heart sound analysis with a high negative predictive value. However, the prognostic impact of this approach remains unknown. To investigate the prognostic value of heart sound analysis as two scores, the Acoustic-score and the CAD-score, in patients with suspected CAD which is treated according to standard of care.
Methods and results
Consecutive patients with angina symptoms referred for coronary computed tomography angiography (CTA) were enrolled. The Acoustic-score was developed from eight acoustic CAD-related features. This score was combined with risk factors to generate the CAD-score. A cut-off score >20 was pre-specified for both scores to indicate disease. If coronary CTA raised suspicion of obstructive CAD, patients were referred to invasive angiography and revascularized when indicated. Of 1675 enrolled patients, 1464 (87.4%) were included in this substudy. The combined primary endpoint was all-cause mortality and myocardial infarction (n = 26). Follow-up was 3.1 (2.7–3.4) years. Of patients with primary endpoints, the Acoustic-score was >20 in 25 (96%); the CAD-score was >20 in 22 (85%). In an unadjusted Cox analysis of the primary endpoints, the hazard ratio for scores >20 under current standard clinical care was 12.6 (1.7–93.2) for the Acoustic-score and 5.4 (1.9–15.7) for the CAD-score. The CAD-score contained prognostic information even after adjusting for lipid-lowering therapy initiation, stenosis at CTA, and early revascularization.
Conclusion
Heart sound analysis seems to carry prognostic information and may improve initial risk stratification of patients with suspected CAD.
Clinicaltrials.org ID
Keywords: Prognosis, Coronary stenosis, Cardiovascular diagnostic technique, Heart sound, Acoustic cardiography
Graphical Abstract
Introduction
Pre-test probability stratification of patients with suspected obstructive coronary artery disease (CAD) has been suboptimal for many years. Hence, most diagnostic tests to rule out CAD show normal conditions.1–3
Acoustic devices measuring the heart sound originating from turbulent blood flow in the coronary circulation and altered myocardial relaxation have therefore been developed. These devices have been shown to be effective in ruling out CAD, and to outperform other methods by being faster, risk-free and more cost-effective.4
The diagnostic performance of two acoustic algorithms was recently tested in two large studies of patients presenting with symptoms suggestive of obstructive CAD.5–7 The studies demonstrated that these algorithms were positively correlated with the extent of CAD in terms of coronary artery calcium score, maximal diameter stenosis, and number of vessels with disease. The studies demonstrated high sensitivities of 78–81%, moderate specificities of 35–53% but a high negative predictive value of 91–96%. It is therefore possible that these algorithms may serve as rule-out tests in patients with suspected stable CAD.
However, no study has investigated whether heart sound carries any prognostic information. The acoustic features included in these acoustic algorithms have been linked to coronary stenosis degree, coronary flow volume and velocity, vascular stiffness, left ventricular diastolic, and systolic dysfunction—all markers which are predictive of a poor prognosis.6,8–14
The aim of this study was to investigate the prognostic value of pre-specified heart sound analysis (Acoustic-score) and an acoustic pre-test probability score (CAD-score) in patients with symptoms suggestive of CAD referred for coronary computed tomography angiography (CTA) and subsequently treated according to standard of care.
Materials and methods
Study design and population
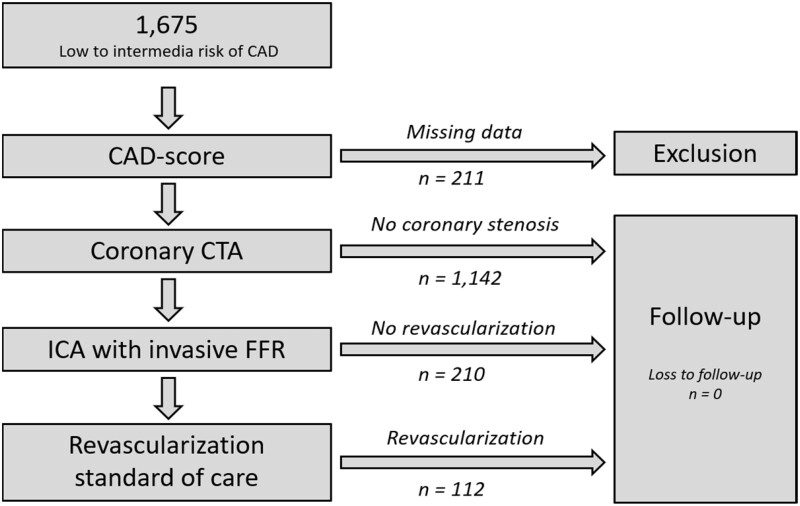
The present predefined substudy used patients from the Danish study of the Non-Invasive Testing in Coronary Artery Disease (Dan-NICAD) trial 1.15,16 Patients referred to coronary CTA due to symptoms suggestive of obstructive CAD were enrolled consecutively between September 2014 and March 2016. Patients were referred to coronary CTA after evaluation by a cardiologist in an outpatient cardiology unit. Decisions regarding referral to coronary CTA were based on patients’ history, symptoms, risk profile, and echocardiographic findings according to national and European Society of Cardiology guidelines. Exclusion criteria were: (i) age <40; (ii) previous known CAD; (iii) estimated glomerular filtration <40 mL/min; (iv) pregnancy; and (v) contra-indication for iodine-containing contrast medium, magnetic resonance imaging, or adenosine (severe asthma, advanced atrioventricular block, or critical aorta stenosis). All patients signed a written informed consent form. The Central Denmark Region Committees on Health Research Ethics, The Danish Medicines Agency, and The Danish Data Protection Agency approved the study, including the present 10-year follow-up substudy.
All included patients underwent a systematic interview to assess risk factors and symptoms and underwent coronary CTA. During their visit for coronary CTA, an examination with the acoustic microphone, CADScor®System (Acarix A/S, Denmark) was performed. Patients with suspicion of a coronary stenosis at coronary CTA all underwent invasive coronary angiography (ICA) with fractional flow reserve (ICA-FFR) measurement approximately 4 weeks after the coronary CTA. Patients without stenosis at coronary CTA did not undergo further testing. Follow-up data were obtained from electronic patient records and Danish national health registries (Figure 1).
Figure 1.
Flow chart of patients in the study. CAD, coronary artery disease; CTA, computed tomography angiography; FFR, fractional flow reserve; ICA, invasive coronary angiography.
The study design, imaging protocols, and analysis strategy have been published.16
CAD-score
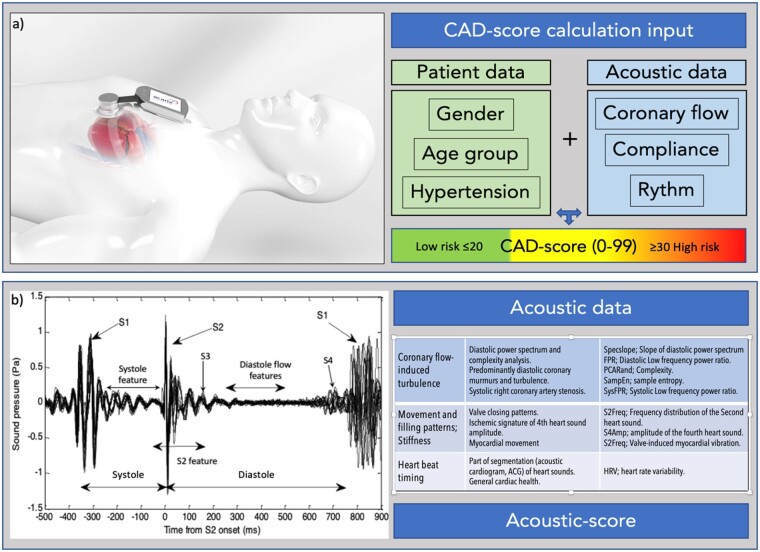
Heart sound recordings were obtained with the CADScor®System which is a small transportable electronic stethoscope device consisting of microphones and a micro-computer with a display. The microphone is mounted at the 4th intercostal space just to the left of the sternum with a dedicated adhesive patch during 5 min of rest. During the 3-min recording, the patient is asked to hold his/her breath for a period of 8 s, four times.
Recordings were conducted in an undisturbed room. The CADScor®System analyses the quality of the recording and in case of poor quality, the device requests a second recording.
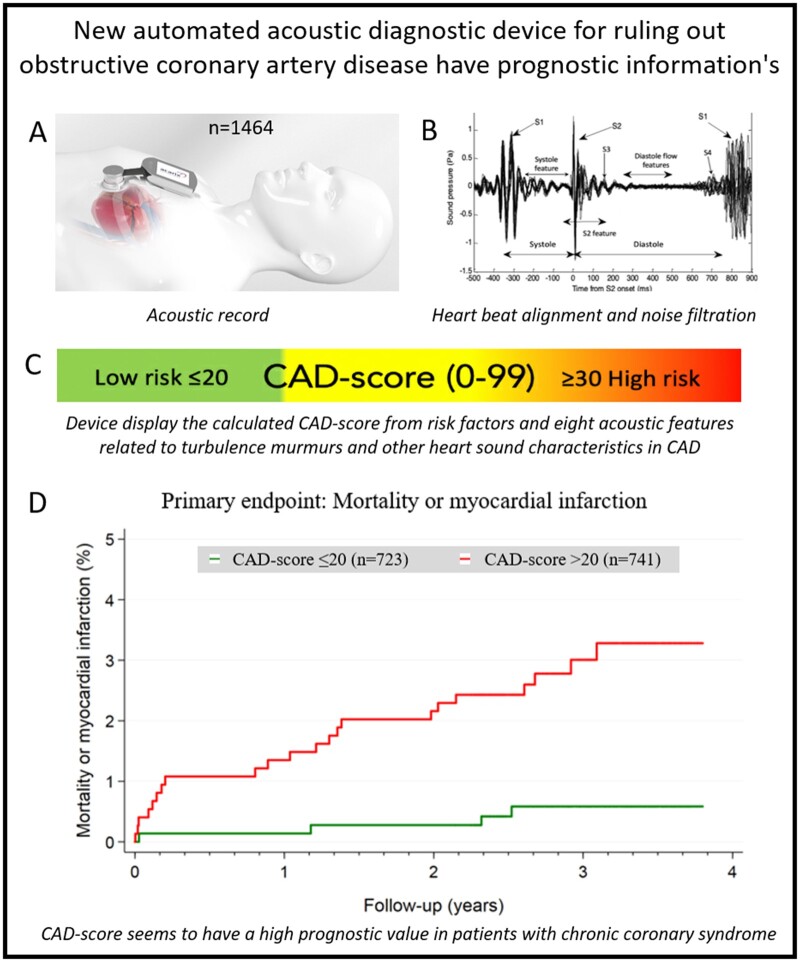
The CADScor®System algorithm Version 3 (V3) algorithm was utilized to calculate the Acoustic-score per se based on post-processing of audio recordings of the heart sound. In total, eight acoustic features related to turbulence murmurs and other heart sound characteristics in CAD were included in the Acoustic-score (Figure 2). The CAD-score V3 was generated by combining the Acoustic-score with the risk factors; gender, age, and hypertension (Figure 2).
Figure 2.
Depiction of the acoustic diagnostic method. (A) Coronary artery disease-score is calculated based on a 3-min recording session from the IC4-L region. Three patient risk factors are combined with acoustic derived data to generate a coronary artery disease-score. A score ≤20 is considered low risk and used to substantiate rule-out for coronary artery disease. (B) The acoustic data are derived from both the systolic and diastolic periods. The acoustic recording is segmented into discrete heart beats and aligned according to S2. Heart beats containing excess noise are filtered out, and the Acoustic-score is calculated. Several acoustic features having different, distinct coronary and myocardial origins are combined into the Acoustic-score.
In this paper, we present data from the Acoustic-score version 3 without risk factor modification and the CAD-score version 3. Both an Acoustic-score and a CAD-score value >20 were pre-specified as abnormal.6
Coronary computed tomography angiography
All patients in the Dan-NICAD trial underwent coronary CTA scans on a 320-slice volume CT scanner (Aquillion One, Toshiba Medical Systems, Japan) according to clinical guidelines.
CT imaging analysis included an Agatston calcium score and evaluation of CAD including luminal diameter stenosis estimation in each segment of the coronary tree using an 18-segment model. Coronary lesions were evaluated blinded to patient history. Stenosis severity was classified in all segments with a reference vessel diameter >2 mm. Severe stenosis was defined as 50–100% diameter reduction (≈70% to 100% area reduction). Segments with suspected severe stenosis and non-evaluable segments with a reference vessel diameter >2 mm were defined as having obstructive CAD by coronary CTA and referred to ICA.
Invasive coronary angiography
Patients with suspected obstructive CAD at the coronary CTA were referred to ICA. ICA-FFR was measured in lesions with a visually estimated 30–90% diameter stenosis located in vessels with a reference diameter >2.0 mm using a clinical ICA-FFR system (Aeris, St. Jude Medical, Minnetonka, MN, USA). Haemodynamically obstructive CAD was identified in a blinded core lab as: (i) ICA-FFR value <0.80, (ii) luminal diameter stenosis reduction >90%, or (iii) luminal diameter stenosis reduction ≥50% if ICA-FFR was indicated but not performed for technical, anatomical, or other reasons.
Revascularizations were performed according to standard clinical practice after the ICA. Decisions were made blinded for CAD-score and other diagnostic test results.
Follow-up
Clinical endpoint data were extracted from patient records and Danish national health databases. Using the Civil Personal Registration number assigned to each Danish citizen at birth, we obtained information regarding mortality, diagnosed diseases, in-hospital procedures, and medical treatment. The CAD-score remained blinded to clinicians during follow-up. End of follow-up was set to 30 June 2018.
Information on mortality, myocardial infarction, and revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) was obtained from the Western Denmark Heart Registry, the Danish National Patient Registry, and/or Danish Civil Personal Register. Data on Medical treatment and compliance were extracted from reimbursed medical prescriptions at Danish pharmacies through the Danish National Health Service Prescription Database. Changes in lipid lowering therapy after primary cardiac evaluation were defined as continued, discontinued, initiated, or no therapy according to both pre- and post-test-issued prescriptions within a window of 180 days before and 120 days after the examination.
Statistical analysis
The primary study endpoint was defined as a composite endpoint of all-cause mortality and myocardial infarction blinded for the first 120 days after coronary CTA. Secondary endpoints were early and late revascularization with a cut-off of 120 days with death as competing risk, and any coronary events blinded for the first 120 days after coronary CTA (all-cause mortality, myocardial infarction, and late revascularization). Patients who were revascularized early in relation to the baseline cardiac diagnostic evaluation were not excluded from the follow-up.
The primary aim was to compare scores with a cut-off value of 20, however for the CAD-score, we also considered the three groups: CAD-scores ≤20 (reference), CAD-scores 21–29, and CAD-scores ≥30, as well as an increment of the CAD-score of 10 units.
Time-to-event analysis was performed with univariate and multivariate Cox regression of the cause-specific hazard ratios (HRs). Cumulative incidence functions for each endpoint were generated to illustrate the risk over time. For Cox multiple regression analysis, we pre-specified models which included an updated Diamond–Forrester score, coronary disease severity at coronary CTA with stratification for change in medication, and revascularization as part of the Dan-NICAD trial’s cardiac evaluation. However, post hoc we chose to develop several models with the endpoint of any cardiac event due to the low number of events. Cox multiple regression analysis was pre-specified to be blinded for the period of the first 120 days from baseline to enable adjustment of change in medication and revascularization as part of the baseline cardiac evaluation. In the blinded period, two patients died. They were the only ones excluded in the Cox multiple regression analysis.
For all statistical analyses, 95% confidence intervals (CIs) were reported when appropriate. The statistical analyses were performed using STATA-15.
Results
Of 1675 patients enrolled in the Dan-NICAD trial, 1464 (87.4%) were included in this study. In total, 211 (12.6%) were excluded due to missing CAD-score V3 (characteristics specified in Supplementary material online, Table S1). Baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristic of included patient demographics (N = 1464)
| Total | CAD-score ≤20 | CAD-score >20 | |
|---|---|---|---|
| Number of patients | 1464 | 723 (49.3%) | 741 (50.7%) |
| Characteristic | |||
| Race, Caucasian | 1454 (99.3%) | 717 (99.2%) | 737 (99.5%) |
| Sex, male | 716 (48.9%) | 257 (35.6%) | 459 (62.9%) |
| Age (years) | 57.1 ± 8.7 | 53.3 ± 8.0 | 60.8 ± 7.8 |
| Body mass index (kg/m²) | 26.6 ± 4.1 | 26.3 ± 4.1 | 27.0 ± 4.1 |
| Abdominal circumference (cm) | 93.0 ± 12.6 | 90.6 ± 12.3 | 95.3 ± 12.5 |
| Blood pressure | |||
| Systolic | 138 ± 19 | 129 ± 14 | 148 ± 18 |
| Diastolic | 83 ± 11 | 79 ± 10 | 86 ± 11 |
| Heart ratea | 65 ± 11 | 65 ± 11 | 66 ± 11 |
| Smoking | |||
| Never | 697 (47.6%) | 343 (47.4%) | 354 (47.8%) |
| Former | 536 (36.6%) | 245 (33.9%) | 291 (39.3%) |
| Active | 231 (15.8%) | 135 (18.7%) | 96 (13.0%) |
| Diabetes | 79 (5.4%) | 24 (3.3%) | 55 (7.4%) |
| Symptoms | |||
| Typical chest pain | 407 (27.8%) | 203 (28.1%) | 204 (27.5%) |
| Atypical chest pain | 493 (33.7%) | 259 (35.8%) | 234 (31.6%) |
| Non-specific | 564 (38.5%) | 261 (36.1%) | 303 (40.9%) |
| Updated Diamond Forrester score | 39% (20–54%) | 28% (16–45%) | 42% (29–59%) |
| Low risk (<15%) | 210 (14.3%) | 168 (23.2%) | 42 (5.7%) |
| Moderate risk (≥15% to 85%) | 1220 (83.3%) | 555 (76.8%) | 665 (89.7%) |
| High risk (≥85%) | 34 (2.3%) | 0 (0.0%) | 34 (4.6%) |
| Medication | |||
| Hypertension | 491 (33.8%) | 86 (12.1%) | 405 (45.9%) |
| Mean number of antihypertensives | 1.9 ± 1.0 | 1.9 ± 0.9 | 1.8 ± 1.0 |
| Hypercholesterolaemia | 398 (27.2%) | 145 (20.0%) | 253 (34.1%) |
| Biochemistryb | |||
| Cholesterol (total, mmol/L) | 5.4 ± 1.1 | 5.4 ± 1.1 | 5.3 ± 1.1 |
| HbA1c (mmol/mol) | 37.7 ± 7.2 | 37.1 ± 7.0 | 38.3 ± 7.2 |
| Creatinine (mmol/L) | 75.4 ± 14.6 | 72.4 ± 12.8 | 78.3 ± 15.6 |
Values are n (%) or mean ±SD or median (IQR).
Mean heart rate at the time of CAD-score measurement was: 54 ± 7 b.p.m. and at the time of CTA: 56 ± 7 b.p.m.
Data available in: cholesterol 94%, glucose fasting 8%, HbA1c 78%, and creatinine 99% of the cohort.
The median Acoustic-score was 24 (14–29). The Acoustic-score correlated vaguely with age, Spearman’s rho 0.11 (P < 0.001), and was higher in males than in females, 27 (21–32) vs. 21 (16–25) (P < 0.001). A total of 488 (33%) patients had an Acoustic-score ≤20, indicating low risk.
The median CAD-score was 20 (10–31). The CAD-score was positively correlated with age, Spearman’s rho 0.50 (P < 0.001), and was higher in males than in females: 28 (15–31) vs. 15 (7–24) (P < 0.001), respectively. A total of 723 (49%) patients had a CAD-score ≤20, indicating low risk. The CAD-score was positively correlated with the Acoustic-score, Spearman’s rho 0.54 (P < 0.001). Furthermore, 2 × 2 tables for the scores are available in Supplementary material online, Table S2).
Diagnostic imaging characteristics of the baseline cardiac evaluation including echo, coronary CTA, ICA-FFR measurements, and revascularization related to the baseline cardiac evaluation, which were part of the Dan-NICAD trial, are summarized in Table 2. The median inter-test interval between coronary CTA and ICA was 30 days (10th and 90th percentiles: 14 and 50 days).
Table 2.
Imaging study characteristics from the baseline cardiac evaluation which were protocolized in the Dan-NICAD trial
| Total | CAD-score ≤20 | CAD-score >20 | |
|---|---|---|---|
| Number of patients | 1464 | 723 (49.3%) | 741 (50.7%) |
| Echo (n = 1438) | |||
| Left ventricular ejection fraction | 59.9% ± 3.4 | 60.0% ± 2.6 | 59.5% ± 4.0 |
| Cardiac valve disease, any | 69 (4.7%) | 23 (3.2%) | 46 (6.2%) |
| Coronary artery calcium score (n = 1459) | |||
| Median | 0 (0–81) | 0 (0–13) | 25 (0–192) |
| Coronary artery calcium score (CACS) groups | |||
| None (CACS = 0) | 747 (51.2%) | 472 (65.7%) | 275 (37.2%) |
| Low/moderate (CACS: 1–399) | 564 (38.7%) | 219 (30.5%) | 345 (46.6%) |
| High (CACS ≥400) | 148 (10.1%) | 28 (3.9%) | 120 (16.2%) |
| Cardiac computed tomography angiography (n = 1457) | |||
| Coronary artery disease severity | |||
| Non (stenosis 0% and CACS = 0) | 696 (47.8%) | 449 (62.5%) | 247 (33.5%) |
| Mild (stenosis 0–30%) | 306 (21.0%) | 126 (17.5%) | 180 (24.4%) |
| Moderate (stenosis 30–50%) | 111 (7.6%) | 51 (7.1%) | 60 (8.1%) |
| Severe (stenosis 50–100%) | 344 (23.6%) | 93 (12.9%) | 251 (34.0%) |
| Radiation dose per coronary CTA (mSv) | 2.7 ± 1.7 | 2.4 ± 1.5 | 2.9 ± 1.8 |
| Invasive coronary angiography with fractional flow reserve (n = 321)a | |||
| Coronary artery disease severity | |||
| Obstructive stenosis | 140 (9.7%) | 27 (3.8%) | 113 (15.6%) |
| Number of coronary vessels with severe stenosis | |||
| One-vessel disease | 85 (5.9%) | 16 (2.3%) | 69 (9.5%) |
| Two-vessel disease | 37 (2.6%) | 8 (1.1%) | 29 (4.0%) |
| Three-vessel disease or left main disease | 18 (1.3%) | 3 (0.4%) | 15 (2.1%) |
Values are n (%) or mean ± SD or median (IQR).
CACS, Coronary artery calcium score.
ICA were only indicated in patient with severe stenosis at coronary CTA.
Treatments initiated at baseline
Lipid-lowering therapy was initiated based on baseline cardiac evaluation in 103 (14.3%) patients with a CAD-score ≤20 and in 184 (24.8%) with a CAD-score >20 (Supplementary material online, Figure S1). However, at baseline, plasma cholesterol levels were also higher in patients initiating lipid lowering therapy than in others, 5.8 ± 1.1 vs. 5.3 ± 1.0 mmol/L, P < 0.001.
In total, 140 patients were diagnosed with haemodynamically obstructive CAD at ICA based on the baseline cardiac evaluation; and 112 patients were subsequently early revascularized according to standard clinical practice, 62 with PCI and 50 with CABG (secondary endpoint, Tables 3 and 4). CAD-scores were significantly higher in the 112 revascularized patients than in the 1352 patients without revascularization related to the primary cardiac evaluation, 31 (21–40) vs. 19 (10–30) (P < 0.001), respectively. A detailed description of clinical information and imaging characteristics of the 26 patients who were revascularized and had a CAD-score ≤20 is presented in Supplementary material online, Table S3.
Table 3.
Tables of events during follow-up according to the Acoustic-score
| Total | Acoustic-score ≤20 | Acoustic-score >20 | |
|---|---|---|---|
| Number of patients | 1464 | 448 (33.3%) | 667 (66.7%) |
| Primary endpoint | |||
| Mortality and myocardial infarction | 26 (1.8%) | 1 (0.2%) | 25 (2.6%) |
| Secondary endpoints | |||
| Mortality, all-cause | 16 (1.1%) | 1 (0.2%) | 15 (1.5%) |
| Myocardial infarction | 10 (0.7%) | 0 (0.0%) | 10 (1.0%) |
| Procedure related/spontaneous | 2/8 | 0/0 | 2/8 |
| Early/late (cut-off 120 days) | 7/3 | 0/0 | 7/3 |
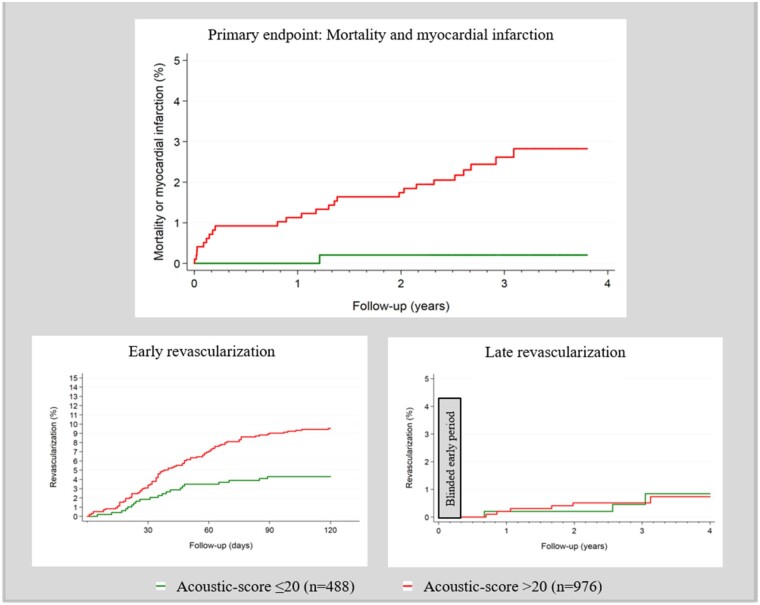
| Early revascularization (≤120 days) | 115 (7.9%) | 21 (4.3%) | 94 (9.6%) |
| Part of the primary Dan-NICAD evaluation | 112 | 21 | 91 |
| Acute | 3 | 0 | 3 |
| Late revascularization (>120 days) | 9 (0.6%) | 3 (0.6%) | 6 (0.6%) |
Values are n (%).
Prognostic value of heart sound analysis
The combined primary endpoint occurred in 26 patients; 16 patients died and 10 patients had a myocardial infarction within a median follow-up time of 3.1 (2.7–3.4) years. In total, nine patients had late revascularization (>120 days after inclusion) (Supplementary material online, Table S4).
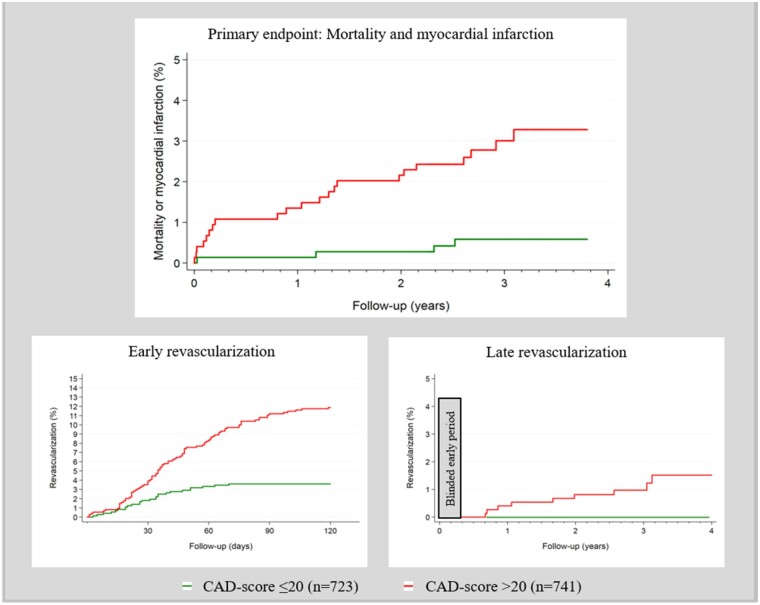
The isolated Acoustic-score without inclusion of risk factors was >20 in 25 out of 26 (96%) patients with the primary endpoint, mortality and myocardial infarction (Table 3). The unadjusted HR was 12.6 (1.7–93.2), P < 0.05. The HR of the Acoustic-score did not increase for Acoustics-scores ≥30 compared with scores in the 21–29 range. Acoustic-score >20 had an HR for early revascularization within 120 days of 2.3 (1.4–3.6) but did not predict late revascularization (Figure 3, Supplementary material online, Figure S2).
Figure 3.
Primary and secondary endpoints according to Acoustic-score with a pre-specified cut-off >20.
The CAD-score was >20 in 22 of 26 (85%) patients with the combined primary endpoint—in 12 of 16 (75%) of patients who died, and in all 10 patients who had myocardial infarction. In addition, CAD-score was >20 in all patients who underwent late revascularization (>120 days after inclusion) (Table 4).
Table 4.
Tables of events during follow-up according to the CAD-score
| Total | CAD-score ≤20 | CAD-score >20 | |
|---|---|---|---|
| Number of patients | 1464 | 723 (49.3%) | 741 (50.7%) |
| Primary endpoint | |||
| Mortality and myocardial infarction | 26 (1.8%) | 4 (0.6%) | 22 (3.0%) |
| Secondary endpoints | |||
| Mortality, all-cause | 16 (1.1%) | 4 (0.6%) | 12 (1.6%) |
| Myocardial infarction | 10 (0.7%) | 0 (0.0%) | 10 (1.4%) |
| Procedure related/spontaneous | 2/8 | 0/0 | 2/8 |
| Early/late (cut-off 120 days) | 7/3 | 0/0 | 7/3 |
| Early revascularization (≤120 days) | 115 (7.9%) | 26 (3.6%) | 89 (12.0%) |
| Part of the primary Dan-NICAD evaluation | 112 | 26 | 86 |
| Acute | 3 | 0 | 3 |
| Late revascularization (>120 days) | 9 (0.6%) | 0 (0.0%) | 9 (1.2%) |
Values are n (%).
In an unadjusted Cox regression analysis of the combined primary endpoint, CAD-scores >20 had an HR of 5.4 (1.9–15.7), P < 0.01. The HR increased significantly with higher CAD-score; with CAD-scores ≤20 as reference, CAD-scores 21–29 and CAD-scores ≥30 had a HR of 3.0 (0.9–10.8), P = 0.09, and 7.7 (2.6–22.9), P < 0.001, respectively (Figure 4 and Supplementary material online, Figure S2).
Figure 4.
Primary and secondary endpoints according to coronary artery disease-score with a pre-specified cut-off >20.
It was not possible to calculate the HR for late revascularization as there were no events in patients with CAD-scores ≤20. However, the log-rank test for late revascularization was significant, P < 0.001 (Figure 4).
In Cox regression analysis of any cardiac events after 120 days (secondary endpoint: death, myocardial infarction, or revascularization >120 days), a CAD-score increment of 10 units had an HR of 1.8 (1.3–2.5) P < 0.01, which was not influenced by adjustment for sex and age. In contrast to the updated Diamond–Forrester score, the CAD-score remained significant after including the updated Diamond–Forrester score in the model. Finally, the CAD-score remained significantly associated with events after adjusting for (i) coronary stenosis at coronary CTA and (ii) lipid lowering therapy initiation and revascularization (Table 5).
Table 5.
Cox regression analysis of any cardiac events after 120 days (dead, myocardial infarction or late revascularization >120 days)
| Univariate | Multivariate | |
|---|---|---|
| Model A | ||
| CAD-score per 10 unit | 1.8 (1.3–2.5) P < 0.01 | 1.8 (1.1–2.8) P < 0.05 |
| Sex, male | 2.1 (0.9–5.0) P = 0.08 | 1.1 (0.4–3.0) P = 0.84 |
| Age per 10 years | 1.5 (0.9–2.3) P = 0.11 | 1.0 (0.6–1.7) P = 0.95 |
| Model B | ||
| CAD-score per 10 unit | 1.8 (1.3–2.5) P < 0.01 | 1.7 (1.2–2.4) P < 0.01 |
| Diamond–Forrester score 10 unit | 1.2 (1.0–1.4) P < 0.05 | 1.1 (0.9–1.3) P = 0.52 |
| Model C | ||
| CAD-score per 10 unit | 1.8 (1.3–2.5) P < 0.01 | 1.6 (1.1–2.4) P < 0.05 |
| Diamond–Forrester score 10 unit | 1.2 (1.0–1.4) P < 0.05 | 1.0 (0.8–1.2) P = 0.88 |
| Severe stenosis at coronary CTA | 6.1 (2.6–14.3) P < 0.001 | 4.2 (1.7–10.4) P < 0.01 |
| Model D | ||
| CAD-score per 10 unit | 1.8 (1.3–2.5) P < 0.01 | 1.5 (1.1–2.1) P < 0.05 |
| Lipid lowering therapy initiated | 4.1 (1.9–9.2) P < 0.01 | 2.0 (0.8–4.9) P = 0.13 |
| Revascularization at baseline | 8.9 (3.9–20.0) P < 0.001 | 4.6 (1.8–11.6) P < 0.01 |
CAD-score and updated Diamond–Forrester scores are analysed as a continuous variable per 10 units.
Values are hazard ratios (CI 95%).
In a stratified analysis of patients not revascularized as part of the baseline cardiac evaluation (n = 1352), a CAD-score >20 had an HR of 3.9 (1.1–13.9), P < 0.05, and a CAD-score increase of 10 units had an HR of 1.5 (1.0–2.3), P = 0.05, for events after 120 days (dead, myocardial infarction or revascularization >120 days). Similarly, in patients (n = 112) who were revascularized, all patients who died or had myocardial infarction or revascularization >120 days had a CAD-score >20, and a CAD-score increment of 10 units had an HR of 1.5 (0.8–2.6), P = 0.17, for events after 120 days.
In a ‘worst case scenario’ simulation, an event was assigned to all patients with early revascularization as part of the primary baseline cardiac evaluation and with a false negative CAD-score ≤20 (n = 26). In this scenario, CAD-scores >20 had similarly 5-year events rates, for any mortality, myocardial infarction, late revascularization not part of the primary baseline evaluation, and early revascularization as part of the primary cardiac evaluation with CAD-score ≤20 (Supplementary material online, Figure S3).
Discussion
In this study, we present prognostic data showing that an Acoustic-score derived from heart sound has prognostic impact in patients with symptoms suggestive of CAD referred to cardiac CT. This Acoustic-score combined with risk factors, the CAD-score, demonstrated an HR >5 for the primary endpoint, a composite of myocardial infarction and all-cause mortality, at a pre-specified binary cut-point of CAD-score of 20. The prognostic value rose with higher CAD-score, HR >7.5 for CAD-score ≥30 compared with ≤20. In addition, the CAD-core remained a prognostic predictor after adjusting for the updated Diamond–Forrester score and coronary stenosis at coronary CTA.
Acoustic detection of CAD
Acoustic detection of turbulent blood flow in coronary stenosis was first described in 1967 as a high-frequency diastolic murmur.17 Since then, heart sound analysis has allowed differentiation between no, early, and advanced stages of CAD, as illustrated by diastolic frequency spectrum plots.5,18 Technological improvements in heart sound recording, segmentation, and analysis have enabled the development of an acoustic device for ruling out obstructive CAD.
Two large studies have tested the diagnostic accuracy of these acoustic rule-out devices in stable CAD. The CADence device was evaluated in the TURBULENCE study, a 21-sites multicentre study in the USA.7 The CADence device uses multiple recording positions and is handheld during the recording. Patients (n = 785) referred for nuclear stress testing due to chest pain symptoms and two or more CAD risk factors were included. Nuclear stress testing was followed by either coronary CTA or ICA as a reference standard with ≥70% diameter stenosis indicating obstructive CAD. Disease prevalence was 15%, and the CADence device had a sensitivity of 78%, a specificity of 35%, and a positive and negative predictive value of 17% and 91%, respectively.
In the Dan-NICAD trial, the CADScor®System was tested in 1,675 patients with predominately intermediate risk of CAD who had been referred for coronary CTA.6 Patients were referred from five Danish hospitals, and coronary CTA was performed at two high-volume centres. Patients with a coronary stenosis at coronary CTA underwent ICA with FFR, and haemodynamically obstructive CAD was defined as ICA-FFR <0.80. In Denmark, coronary CTA is the first-line test for ruling out CAD in de novo patients, which results in a low disease prevalence of 10% in the Dan-NICAD trial compared with 15% in TURBULENCE. The CADScor®System had sensitivity of 81%, a specificity of 53%, and a positive and negative predictive value of 16% and 96%, respectively; hence, similar sensitivities and positive predictive values but higher specificities and negative predictive values than the TURBULENCE study.
Prognostic value of acoustic analysis of heart sound
The present study is the first study to evaluate the prognostic value of an acoustic analysis of heart sounds. An isolated Acoustic-score of eight heart sound features related to CAD predicted 25 out of 26 myocardial infarctions or deaths. Combining the Acoustic-score with risk factors to obtain the CAD-score increased the proportion of patients with a score ≤20, and thus a low probability of CAD, from 33% to nearly 50%. Nonetheless, the CAD-score remains highly predictive for both deaths, myocardial infarctions, and late revascularization.
The prognostic value of the CAD-score remained significant after adjustment for coronary stenosis, which is a very strong prognostic predictor in stable CAD. This is due to specific acoustic features related to, e.g. high-risk plaque characteristic. However, further research is needed regarding the origin of these acoustic features and their correlation with specific pathologies.
The Dan-NICAD trial was designed to evaluate the diagnostic accuracy of the CAD-score with a reference of haemodynamic coronary stenosis at ICA.6 Decisions regarding medical treatment and revascularization were based on standard clinical care; thus, no decision was taken based on the CAD-score. In patients with a CAD-score ≤20, lipid lowering therapy was initiated in 103 (14.3%) and 26 (3.6%) were revascularized. These patients had a therapy that might have altered their prognosis and affected the outcome of the present study. We performed multiple regression and stratified analyses to adjust for this confounder, and the conclusion on the prognosis was unaffected.
Finally, we performed a ‘worst case scenario’ presuming the situation that all early revascularized patients with CAD-scores ≤20 had an event of death, myocardial infarction, or late revascularization—as mimic the most severe consequence if we had not offered early revascularization to these patients due to rule-out based on CAD-score. In this scenario, a CAD-score >20 was no longer a predictor of prognosis. Contradict, 723 patients with a CAD-score ≤20 would not have need coronary CTA for ruling out obstructive CAD. Hence, a reduction in contrast agent and iodinated radiation of >1700 mSv (723 patients with an averaged radiation dose of 2.4 mSv per coronary CTA) could be achieved in this study despite we used newer generations of CT scanners.
Limitations
This cohort comprised almost exclusively Caucasians with low to intermedia pre-test probability of CAD and none had previously documented CAD. Patients were referred to coronary CTA as the first-line diagnostic test according to Danish standard of care. This approach might have introduced referral bias base on pre-test probability and due to the limitations of coronary CTA, e.g. irregular heart rate and severe obesity.
The development of CAD-score V3 required splitting the cohort into a consecutive training (n = 593) and a blinded validation (n = 1082) cohort, which might have over-fitted the results in the total cohort analysis. However, diagnostic accuracy in the validation vs. training cohort indicated no over-fitting.6
The CAD-score does not distinguish between diastolic murmurs origination. Hence, diastolic valve disease may decrease CAD-score specificity as it increases the false positive rate but sensitivity remains high.19
Finally, this study is limited by the low event rates reflecting an overall favourable prognosis of patients with stable CAD. Hence, the results of this study are promising but should be interpreted with caution and final conclusions regarding the safety of acoustic rule-out strategies should await results of randomized controlled strategy trials.
Conclusion
The Acoustic-score derived from heart sound seems to give prognostic information about mortality, myocardial infarction, and late revascularization in patients with symptoms suggestive of CAD which is treated according to standard of care. Diagnostic algorithms of this Acoustic-score combined with risk factors developed for ruling out CAD, the CAD-score, risk-stratified patients even further. These findings are useful as new automated acoustic diagnostic devices have emerged for ruling out obstructive CAD.
Supplementary material
Supplementary material is available at European Heart Journal is available at online.
Funding
The research reported here is partly financed by the Danish Heart Foundation (15-R99-A5837-22920), the Hede Nielsen Foundation, and by an institutional research grant from Acarix A/S, Denmark.
Conflict of interest: S.W., S.E.S., and M.B. acknowledges support from Acarix in form of an institutional research grant. N.R.H. received institutional research grant and speakers fee from Abbott, Boston Scientific, and Medis medical imaging. M.B. disclose advisory board participation for NOVO Nordisk, Astra-Zeneca, Bayer, Sanofi, and Acarix. B.S.L. is an industrial PhD fellow at Acarix and Aalborg University. S.E.S. is a part-time consultant in Acarix. B.S.L. and S.E.S. are minor shareholders of Acarix. The remaining authors have nothing to disclose.
Data availability
Data can be shared on request to the corresponding author with permission from Acarix A/S.
Supplementary Material
References
- 1. Patel MR, Peterson ED, Dai D, Brennan JM,, Redberg RF, Anderson HV, Brindis RG, Douglas PS.. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 3. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas JL, Winther S, Wilson RF, Bottcher M.. A novel approach to diagnosing coronary artery disease: acoustic detection of coronary turbulence. Int J Cardiovasc Imaging 2017;33:129–136. [DOI] [PubMed] [Google Scholar]
- 5. Winther S, Schmidt SE, Holm NR, Toft E, Struijk JJ, Botker HE, Bottcher M.. Diagnosing coronary artery disease by sound analysis from coronary stenosis induced turbulent blood flow: diagnostic performance in patients with stable angina pectoris. Int J Cardiovasc Imaging 2016;32(2):235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winther S, Nissen L, Schmidt SE, Westra JS, Rasmussen LD, Knudsen LL, Madsen LH, Kirk Johansen J, Larsen BS, Struijk JJ, Frost L, Holm NR, Christiansen EH, Botker HE, Bottcher M.. Diagnostic performance of an acoustic-based system for coronary artery disease risk stratification. Heart 2018;104:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas JL, Ridner M, Cole JH, Chambers JW, Bokhari S, Yannopoulos D, Kern M, Wilson RF, Budoff MJ.. The clinical evaluation of the CADence device in the acoustic detection of coronary artery disease. Int J Cardiovasc Imaging 2018;34:1841–1848. [DOI] [PubMed] [Google Scholar]
- 8. Thomas JL, Winther S, Wilson RF, Bottcher M.. A novel approach to diagnosing coronary artery disease: acoustic detection of coronary turbulence. Int J Cardiovasc Imaging 2017;33:129–136. [DOI] [PubMed] [Google Scholar]
- 9. Azimpour F, Caldwell E, Tawfik P, Duval S, Wilson RF.. Audible coronary artery stenosis. Am J Med 2016;129:515–521.e3. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt S, Holst-Hansen C, Hansen J, Toft E, Struijk J.. Acoustic features for the identification of coronary artery disease. IEEE Trans Biomed Eng 2015;62:2611–2619. [DOI] [PubMed] [Google Scholar]
- 11. Becker M, Roehl AB, Siekmann U, Koch A, de la Fuente M, Roissant R, Radermacher K, Marx N, Hein M.. Simplified detection of myocardial ischemia by seismocardiography. Differentiation between causes of altered myocardial function. Herz 2014;39:586–592. [DOI] [PubMed] [Google Scholar]
- 12. Silvestre A, Sandhu G, Desser KB, Benchimol A.. Slow filling period/rapid filling period ratio in the apexcardiogram: relation to the diagnosis of coronary artery disease. Am J Cardiol 1978;42:377–382. [DOI] [PubMed] [Google Scholar]
- 13. Akay M, Semmlow JL, Welkowitz W, Bauer MD, Kostis JB.. Noninvasive detection of coronary stenoses before and after angioplasty using eigenvector methods. IEEE Trans Biomed Eng 1990;37:1095–1104. [DOI] [PubMed] [Google Scholar]
- 14. Wang JZ, Tie B, Welkowitz W, Semmlow JL, Kostis JB.. Modeling sound generation in stenosed coronary arteries. IEEE Trans Biomed Eng 1990;37:1087–1094. [DOI] [PubMed] [Google Scholar]
- 15. Nissen L, Winther S, Westra J, Ejlersen JA, Isaksen C, Rossi A, Holm NR, Urbonaviciene G, Gormsen LC, Madsen LH, Christiansen EH, Maeng M, Knudsen LL, Frost L, Brix L, Botker HE, Petersen SE, Bottcher M.. Diagnosing coronary artery disease after a positive coronary computed tomography angiography: the Dan-NICAD open label, parallel, head to head, randomized controlled diagnostic accuracy trial of cardiovascular magnetic resonance and myocardial perfusion scintigraphy. Eur Heart J Cardiovasc Imaging 2018;19:369–377. [DOI] [PubMed] [Google Scholar]
- 16. Nissen L, Winther S, Isaksen C, Ejlersen JA, Brix L, Urbonaviciene G, Frost L, Madsen LH, Knudsen LL, Schmidt SE, Holm NR, Maeng M, Nyegaard M, Botker HE, Bottcher M.. Danish study of Non-Invasive testing in Coronary Artery Disease (Dan-NICAD): study protocol for a randomised controlled trial. Trials 2016;17:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dock W, Zoneraich S.. A diastolic murmur arising in a stenosed coronary artery. Am J Med 1967;42:617–619. [DOI] [PubMed] [Google Scholar]
- 18. Semmlow J, Rahalkar K.. Acoustic detection of coronary artery disease. Annu Rev Biomed Eng 2007;9:449–469. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt SE, Winther S, Larsen BS, Groenhoej MH, Nissen L, Westra J, Frost L, Holm NR, Mickley H, Steffensen FH, Lambrechtsen J, Norskov MS, Struijk JJ, Diederichsen ACP, Boettcher M.. Coronary artery disease risk reclassification by a new acoustic-based score. Int J Cardiovasc Imaging 2019;35:2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be shared on request to the corresponding author with permission from Acarix A/S.