OBJECTIVES:
We aimed to describe the variation of hemostasis proteins in children with bacterial infections due to different pathogens (Neisseria meningitidis, Streptococcus pneumoniae, Staphylococcus aureus, and group A streptococcus [GAS]) and to study hemostasis proteins in relation to mortality.
DESIGN:
Preplanned analysis in prospective cohort study.
SETTING:
Hospitals in five European countries (Austria, The Netherlands, Spain, Switzerland, and the United Kingdom).
PATIENTS:
Admitted children (2012–2016) with community-acquired infections due to meningococci (n = 83), pneumococci (n = 64), S. aureus (n = 50), and GAS (n = 44) with available serum samples collected less than 48 hours after admission.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Fibronectin, plasminogen activator inhibitor type 1 (PAI-1), thrombomodulin, and a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) were measured in serum in 2019–2020. Additionally, von Willebrand factor, protein C, protein S, and factor IX were measured in citrate plasma available from a subset of patients. Outcome measures included in-hospital mortality and disease severity (need for ventilation/inotropes, Pediatric Index of Mortality score).
Of 241 children, 21 (8.7%) died and 177 (73.5%) were admitted to PICU. Mortality rate was similar for the pathogen groups. Levels of fibronectin and thrombomodulin differed for the different pathogens (p < 0.05). Fibronectin levels were lower in GAS infections than in S. pneumoniae and S. aureus infections but did not differ from meningococcal infections. Thrombomodulin levels in meningococcal infections were higher than in S. aureus and pneumococcal infections. Overall, the area under the curve for mortality was 0.81 (95% CI, 0.70–0.92) for thrombomodulin and 0.78 (95% CI, 0.69–0.88) for ADAMTS-13. The association of each hemostasis protein did not vary across pathogens for any of the outcome measures.
CONCLUSIONS:
Hemostatic disturbances in childhood bacterial infections are not limited to meningococcal sepsis but occur with a comparable severity across nonmeningococcal infections. High thrombomodulin and high ADAMTS-13 had good discriminative ability for mortality. Our results emphasize the importance of hemostatic disturbances in meningococcal and nonmeningococcal pediatric bacterial infections.
Keywords: bacterial infection, children, coagulation, hemostasis proteins, intensive care, mortality
RESEARCH IN CONTEXT
•Coagulation disorders and disseminated intravascular coagulation are a well-known feature of meningococcal infection and associated with morbidity and mortality.
•Little is known about hemostasis proteins in other common childhood bacterial infections.
•In a large European cohort, we describe the variation of hemostasis proteins in children with infections due to Neisseria meningitidis, Streptococcus pneumoniae, Staphylococcus aureus, and group A streptococcus and studied hemostasis proteins in relation to mortality and disease severity.
Sepsis is an important cause for mortality and morbidity in children and is estimated to contribute to 20% of childhood deaths (1–5). The inflammatory response to infection induces procoagulant and platelet activating pathways, whereas it reduces the functioning of anticoagulant pathways and fibrinolytic activity (6). These mechanisms result in coagulation abnormalities ranging from subtle derangements only detectable by highly sensitive assays to widespread deposition of fibrin throughout the microcirculation, manifesting as disseminated intravascular coagulation (DIC). Purpura is often present in DIC and is considered typical for meningococcal sepsis and is associated with septic shock, multiple organ dysfunction, and death. Nonmeningococcal infections, in which purpura do not commonly occur, can also cause mild to severe coagulopathies which influence disease severity (7, 8).
In addition to meningococcal infections, infections caused by Streptococcus pneumoniae, group A streptococcus (GAS), and Staphylococcus aureus comprise a large group of invasive community-acquired bacterial infections in children (1, 9, 10). Each pathogen might induce a coagulation host-response by different mechanisms, as bacteria are known to induce platelet activation or promote platelet adhesion, trigger activation of coagulation independently, or interact with the fibrinolytic system (11–13). For example, S. aureus secretes coagulases that directly activate thrombin (14), whereas meningococcal and streptococcal infections have been shown to be associated with activation of the contact pathway (15).
Although extensive research has been performed in describing hemostasis proteins in meningococcal sepsis or sepsis in general (16–29), the variation of hemostasis protein levels in infections across specific pathogen groups has not yet been described. Therefore, we aimed: 1) to study the variation of hemostasis protein levels in children with invasive bacterial infections due to either Neisseria meningitidis, S. pneumoniae, S. aureus, or GAS, and 2) to describe hemostasis protein levels and their relation with mortality and disease severity. Since severe DIC is typical for meningococcal infections, we hypothesize that disturbances in hemostasis protein levels are more pronounced in meningococcal infections than in nonmeningococcal infections and that mortality would be more common in meningococcal infections.
MATERIALS AND METHODS
Study Design and Population
This is a preplanned study embedded in the European Union Childhood Life-threatening Infectious Disease (EUCLIDS) study project, a European prospective multicenter cohort study that aimed to evaluate determinants of susceptibility and severity of severe pediatric bacterial infection. The EUCLIDS study protocol was approved by at least one ethical review board in every participating country: Austria—Ethikkomission Graz no. 24-116 ex 11/12; The Netherlands—CMO Regio Arnhem-Nijmegen no. 37986.091.11; Spain—Comité Ético de Investigación Clínica de Galicia no. 2011/298; Switzerland—Cantonal Ethics Committee, Inselspital, University of Bern no. KEK-029/11; and United Kingdom—NRES Committee London—Fulham no. 11/LO/1982. The local ethical review board was determined by the workplace of the local principal investigator. The EUCLIDS study design and methods have been described previously (1, 9, 30).
Briefly, patients less than 18 years admitted with community-acquired (suspected) sepsis or severe focal infection were prospectively included in 2012–2016. Informed consent was obtained from parents or care givers for inclusion in the study. This subanalysis focuses on children with sterile culture-proven infections caused by N. meningitidis, S. pneumoniae, S. aureus, or GAS included in Austria, The Netherlands, Spain, Switzerland, and the United Kingdom. Patients were selected if they had serum samples taken within 48 hours after study centre admission. In addition, we selected afebrile children as healthy controls (4:1). Collected clinical data included general characteristics, laboratory and microbiological results, disease severity score (Pediatric Index of Mortality [PIM]–2) (31), DIC score (32), treatments during admission, and mortality.
Samples and Laboratory Assay for Hemostasis Proteins
Venous blood was drawn for collection of serum and citrate plasma. All samples were stored at –80°C until analysis which was performed in 2019 and 2020. We selected eight proteins for measurement: fibronectin, thrombomodulin, a disintegrin and metalloprotease with thrombospondin type-1 motif, member 13 (ADAMTS-13), plasminogen activator inhibitor type 1 (PAI-1) (measured in serum), and factor IX, protein C, protein S, von Willebrand factor (vWF) (measured in a subset of patients with available citrated plasma samples [n = 146]). This selection was based on previously reported associations with disease severity in sepsis (Fig. 1; and Appendix 1, http://links.lww.com/PCC/C167) (16–28). All the proteins were measured with Luminex technology. Assays for fibronectin, PAI-1, and ADAMTS-13 were obtained from R&D Systems (Abingdon, United Kingdom). Factor IX, protein C, protein S, and vWF were measured with the human coagulation 4-plex ProcartaPlex panel 3 (ThermoFisher Scientific, Vienna, Austria). Thrombomodulin levels were measured with a laboratory developed Luminex assay based on capture antibody, detection antibody, and recombinant human thrombomodulin (Thrombomodulin DuoSet ELISA assay; R&D Systems), with a lower limit of detection 10 pg/mL. Laboratory technicians conducting the assays were blinded for the clinical information.
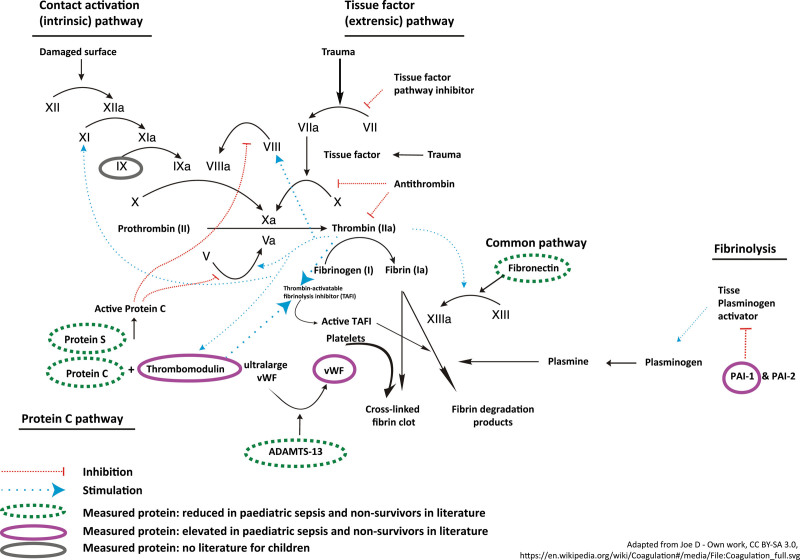
Figure 1.
Schematic simplified presentation of the coagulation pathways. PAI-1 = plasminogen activator inhibitor type 1, TAFI = thrombin activatable fibrinolysis inhibitor, vWF = von Willebrand factor.
Clinical Definitions
The primary outcome measure was in-hospital mortality. Secondary outcome measures included the following markers of disease severity: need for mechanical ventilatory support or inotrope/vasoactive drug requirement (33) and PIM2 score (31). PIM2 scores were only calculated for patients admitted to PICU. DIC scores were calculated for patients with at least one parameter (platelet count, d-dimer, prothrombin time, or fibrinogen) measured in routine care ± 12 hours of the drawn research sample. DIC was defined as a score greater than or equal to 5 according to the International Society of Thrombosis and Hemostasis Scoring System for DIC (32).
Data Analysis
First, we compared clinical characteristics and hemostasis protein levels between the patients with invasive bacterial infections and controls. Second, we studied the variation of hemostasis protein levels between pathogens by comparing protein levels between groups of patients with different causative pathogens. To study whether the association of each protein level depended on pathogen type, we used logistic regression to test the interaction of the pathogen group with the log-transformed value for each protein for the primary outcome measure (mortality) and secondary outcome (need for invasive ventilation/inotropes). We tested the significance of the interaction using the likelihood ratio (LR) test. This analysis was performed for each protein separately as our aim was to study the variation of each protein between pathogen groups. Log-transformed values were used as these resulted in a better fit of the data. Third, in the total cohort of invasive bacterial infections, we assessed hemostasis protein levels in relation to mortality and disease severity (need for invasive ventilation/inotropes, PIM2 score). Differences were assessed using Mann-Whitney U test and Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. For continuous outcomes, we assessed correlations with Spearman rank test. Based on the Spearman rho, significant correlations were classified as very weak (0–0.19), weak (0.2–0.39), moderate (0.4–0.59), strong (0.6–0.79), and very strong (0.8–1) (34). Last, we performed a multivariable logistic regression analysis including only the proteins (log-transformed) that differed for survivors and nonsurvivors. For the proteins that remained significant in the multivariable analysis, we assessed their discriminative ability for mortality by area under the receiver operating curves (AUROC) and identified cut-off values using the Youden’s index as an exploratory analysis. We assessed the predictive performance of these cut-off values for mortality by sensitivity, specificity, and positive/negative LRs. Positive LRs greater than 5 and negative LRs less than 0.2 were considered clinically relevant (35).
Significance level was defined at p value of less than 0.05. To account for multiple testing of hemostasis proteins, we applied the false discovery rate method (36, 37). All analyses were performed in R Version 3.6 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). For this study, a convenience sample was used. A post hoc power calculation showed that the post hoc power was 0.78 for fibronectin, 0.56 for PAI-1, 1 for thrombomodulin, and 0.82 for ADAMTS-13.
RESULTS
Study Population
Of the 4,739 patients included in the EUCLIDS study, 2,062 patients had a sterile site proven infection of which 1,443 were infections caused by any of the bacteria of interest for the current study. Of those, we included 241 patients with available serum sample collected less than 48 hours after hospital admission (Appendix 2, http://links.lww.com/PCC/C167). This comprised 83 N. meningitidis infections (34.4%), 64 S. pneumoniae infections (26.6%), 50 S. aureus infections (20.7%), and 44 GAS infections (18.3%). In addition, we collected 64 healthy controls (female [n = 30, 47%]; median age 5.4 yr [interquartile range (IQR), 3.1–12.3 yr]). Compared with healthy controls, patients with invasive bacterial infections were younger (median age 3.3 yr [IQR, 1.3–9.2 yr]; p < 0.05) but had similar sex distribution (110 female [47%]) (Appendix 3, http://links.lww.com/PCC/C167). Of 241 patients with invasive bacterial infections, 21 (8.7%) died and 177 (73.5%) were admitted to the PICU (Table 1).
TABLE 1.
Descriptive Characteristics for All Patients and Stratified for Neisseria meningitidis, Streptococcus pneumoniae, Staphylococcus aureus, and Group A Streptococcus
| Patient Characteristics | Invasive Bacterial Infections, N = 241 n (%) | Missing n (%) | Neisseria meningitidis, N = 83 n (%) | Streptococcus pneumoniae, N = 64 n (%) | Staphylococcus aureus, N = 50 n (%) | Group A streptococcus, N = 44 n (%) | p |
|---|---|---|---|---|---|---|---|
| Female | 112 (46.9) | 33 (39.8) | 25 (39.1) | 25 (50) | 27 (61.4) | 0.07 | |
| Age in yr, median (IQR) | 3.3 (1.3–9.2) | 1.8 (0.7–5.3) | 2.5 (1.3–5.3) | 9.9 (4.3–13.0) | 3.7 (1.8–7.6) | < 0.01 | |
| Immunizations up to date | 179 (74.3) | 52 (21.6) | 63 (75.9) | 50 (78.1) | 32 (64) | 34 (77.3) | 0.56 |
| Any chronic underlying condition | 102 (42.3) | 27 (32.5) | 30 (46.9) | 27 (54) | 18 (40.9) | 0.08 | |
| Duration of symptoms at presentation, d, median (IQR) | 2.7 (1.5-5.6) | 36 (14.9) | 1.5 (1.1-2.6) | 3.7 (2.1–6.8) | 3.6 (2.4–4.9) | 4.6 (2.3–7.7) | < 0.01 |
| Severity of illness | |||||||
| Sepsis | 159 (66.0) | 65 (78.3) | 36 (56.2) | 25 (50.0) | 33 (75) | < 0.01 | |
| Admitted to PICU | 177 (73.4) | 73 (88) | 40 (62.5) | 24 (48) | 40 (90.9) | < 0.01 | |
| Need for invasive ventilation or inotropes | 123 (51.0) | 54 (65.1) | 20 (31.2) | 17 (34) | 32 (72.7) | < 0.01 | |
| Need for invasive ventilation | 106 (44.0) | 37 (15.4) | 45 (54.2) | 18 (28.1) | 13 (26) | 30 (68.2) | < 0.01 |
| Days on invasive ventilation, median (IQR) | 4 (3–7) | 5 (3–6) | 3 (2–9) | 4 (3–12) | 4 (2–8) | 0.7 | |
| Need for inotropes | 105 (43.6) | 38 (15.8) | 51 (61.4) | 12 (18.8) | 15 (30) | 27 (61.4) | < 0.01 |
| Days on inotropes, median (IQR) | 3 (2–5) | 3 (2-4) | 2 (4-5) | 3 (3-6) | 4 (2–5) | 0.51 | |
| Pediatric Index of Mortality 2 score (%), median (IQR) | 3.5 (0.8–11.5) | 3.5 (0.8–12.6) | 2.7 (0.8–8.9) | 2.6 (0.8–4.1) | 6.2 (1.1–12.3) | 0.32 | |
| PICU-free days at day 28, median (IQR) | 25 (21-28) | 2/177 | 24 (21–26) | 26 (21–28) | 28 (20–28) | 23 (17-26) | < 0.01 |
| Death | 21 (8.7) | 7 (8.4) | 7 (10.9) | 4 (8) | 3 (6.8) | 0.89 | |
| Routine chemistry and hematological testsa | |||||||
| Platelets (10^9/L), median (IQR) | 174 (103–270) | 54 (22.4) | 140 (86–196) | 249 (172–385) | 196 (137–254) | 174 (66–293) | < 0.01 |
| d-dimer (ng/mL), median (IQR) | 5733 (2650–11,711) | 219 (90.9) | 7,970 (2,384–22,819) | 3,189 (1,595–4,783) | 5,090 (3,380–8,930) | 4,497 (4,195–31,929) | 0.5 |
| Prothrombin time (s), median (IQR) | 19 (14–27) | 128 (53.1) | 22 (18–31) | 15 (13–24) | 16 (13–26) | 15 (12–20) | < 0.01 |
| Fibrinogen (g/L), median (IQR) | 3.9 (2.5–5.5) | 146 (60.6) | 3.6 (2.6–4.7) | 4.9 (3.5–6.6) | 4.9 (3.2–6.1) | 2.9 (1.8–4.7) | 0.09 |
| DIC score ≥ 5 | 18 (7.5) | 53 (22.0) | 10 (12.0) | 2 (3.1) | 3 (6) | 3 (6.8) | 0.38 |
| DIC score, median (IQR) | 2 (0–2) | 53 (22.0) | 2 (2–4) | 2 (0–2) | 1.5 (0–2) | 2 (0–2) | < 0.01 |
DIC = disseminated intravascular coagulation, IQR = interquartile range.
± 12 hr from blood sample.
Across the different pathogens, distribution of sex was similar, but patients with S. aureus (median 9.9 yr [IQR, 4.3–13.0 yr] were older than patients with infections due to GAS (median 3.7 yr [IQR, 1.8–7.6 yr], meningococcus (median 1.8 yr [IQR, 0.7–5.3 yr]), and pneumococcus (median 2.5 yr [IQR, 1.3–5.3 yr]). Patients with meningococcal infection had a shorter duration of symptoms at presentation to the hospital (1.5 d [IQR, 1.1–2.6 d]; p < 0.01) compared with patients with infections due to S. pneumoniae (3.7 d [IQR, 2.1–6.8 d]), S. aureus (3.6 d [IQR, 2.4–4.9 d]), and GAS (4.6 d [IQR, 2.3–7.7 d]). In addition, proportion of PICU admissions differed across the pathogen group (p < 0.01, N. meningitidis 88% [73/83]; S. pneumoniae 62.5% [40/64]; S. aureus 48% [24/50]; and GAS 90.9% [40/44]). For patients admitted to PICU, PIM2 scores were similar across the pathogen groups.
Overall mortality was comparable (p = 0.89) between the different infections (GAS 7% [n = 3], meningococcal 8% [n = 7], pneumococcal 11% [n = 7], and S. aureus 8% [n = 4]). Numeric DIC scores differed between the different infections with higher abnormal DIC scores in the meningococcal patients (meningococcal 2 [IQR, 2–4], pneumococcal 2 [IQR, 0–2],S. aureus 1.5 [IQR, 0–2]), GAS 2 [IQR, 0–2]). Frequency of DIC (score > 5), however, was similar across pathogens (p = 0.38).
Variation of Hemostasis Protein Levels Across Pathogens
In patients, levels of PAI-1, thrombomodulin, ADAMTS-13, and vWF were higher than in controls, whereas levels of fibronectin and protein C were lower in patients than in controls. Levels of factor IX and protein S did not differ for patients and controls (Appendix 4, http://links.lww.com/PCC/C167). Between the four pathogen groups, levels of fibronectin (p = 0.03) and thrombomodulin (p < 0.001) differed across the pathogens (Table 2; and Appendix 5, http://links.lww.com/PCC/C167). Levels of the other proteins did not vary for the different pathogens. Thrombomodulin levels in N. meningitidis infections were higher than in S. aureus and S. pneumoniae infections. Fibronectin levels were lower in GAS infections compared with S. aureus and S. pneumoniae infections.
TABLE 2.
Hemostasis Protein Levels in Invasive Bacterial Infections: Stratified by Pathogen Group
| Hemostasis Protein | Group A streptococcus, n = 44 Median (IQR) | Neisseria meningitides, n = 83 Median (IQR) | Streptococcus pneumoniae, n = 64 Median (IQR) | Staphylococcus aureus, n = 50 Median (IQR) | p a |
|---|---|---|---|---|---|
| Serum | |||||
| Fibronectin (µg/mL) | 22 (6–61) | 43 (14–91) | 67 (20–123) | 51 (21–150) | 0.03 |
| Plasminogen activator inhibitor type 1 (ng/mL) | 87 (58–159) | 83 (24–208) | 130 (56–198) | 87 (12–186) | 0.52 |
| Thrombomodulin (pg/mL) | 409 (10–1,559) | 847 (10 – 2,861) | 10 (10–918) | 10 (10–703) | < 0.001 |
| ADAMTS-13 (ng/mL) | 9 (2–60) | 33 (7–87) | 19 (5–45) | 12 (2–45) | 0.13 |
| Citrate plasma | n = 20 | n = 43 | n = 34 | n = 25 | |
| Factor XI (%) | 53 (25–289) | 50 (35–95) | 110 (53–300) | 55 (35–300) | 0.11 |
| Protein C (%) | 40 (23–81) | 40 (25–50) | 55 (40–79) | 35 (25–65) | 0.11 |
| Protein S (%) | 65 (46–136) | 95 (65–148) | 155 (85–315) | 95 (75–240) | 0.14 |
| von Willebrand factor (%) | 300 (103–300) | 300 (183–300) | 300 (103–300) | 300 (300–300) | 0.38 |
ADAMTS-13 = a disintegrin and metalloprotease with thrombospondin type-1 motif, member 13, IQR = interquartile range.
p values adjusted for multiple testing.
Hemostasis Protein Levels and Relation With Mortality and Disease Severity
In all patients, thrombomodulin and ADAMTS-13 were higher in nonsurvivors compared with survivors, whereas levels of fibronectin were lower in nonsurvivors than in survivors. PAI-1, factor IX, protein C, protein S, and vWF were not related to survival status (Table 3; and Appendix 6, http://links.lww.com/PCC/C167). The association of each protein for mortality or need for invasive ventilation/inotropes did not vary between the different pathogens (interaction p > 0.05).
TABLE 3.
Hemostasis Protein Levels for Survivors and Nonsurvivors
| Hemostasis Protein | Survivors, n = 220 Median (IQR) | Nonsurvivors, n = 21 Median (IQR) | p a |
|---|---|---|---|
| Serum | |||
| Fibronectin (ug/mL) | 46 (15–109) | 17 (7–22) | 0.03 |
| Plasminogen activator inhibitor type 1 (ng/mL) | 93 (31–180) | 197 (5–552) | 0.06 |
| Thrombomodulin (pg/mL) | 10 (10–1,105) | 3,022 (1,320–8,990) | 0.00 |
| ADAMTS-13 (ng/mL) | 16 (2–53) | 86 (38–201) | 0.00 |
| Citrate plasma | n = 114 | n = 8 | |
| Factor IX (%) | 60 (35–300) | 35 (24–49) | 0.10 |
| Protein C (%) | 40 (26–65) | 30 (15–35) | 0.08 |
| Protein S (%) | 110 (65–315) | 68 (44–88) | 0.12 |
| von Willebrand factor (%) | 300 (125–300) | 300 (265–300) | 0.52 |
ADAMTS-13 = a disintegrin and metalloprotease with thrombospondin type-1 motif, member 13, IQR = interquartile range.
aAdjusted for multiple testing.
In the multivariable analysis, log-thrombomodulin (adjusted odds ratio [aOR], 1.43 [95% CI, 1.11–1.95]) and log-ADAMTS-13 (aOR, 1.59 [95% CI, 1.09–2.43]) were associated with mortality, whereas log-fibronectin was not (aOR, 0.89 [95% CI, 0.75–1.10]). The discriminative ability (AUROC) between survivors and nonsurvivors was 0.81 (95% CI, 0.70–0.92) for thrombomodulin and 0.78 (95% CI, 0.69–0.88) for ADAMTS-13. For thrombomodulin and ADAMTS-13, the optimal cut-offs were 2,733 pg/mL and 20 ng/mL, respectively. The cut-off for ADAMTS-13 yielded the best rule-out value for mortality (sensitivity 0.95 [95% CI, 0.76–1.00]; negative LR 0.09 [95% CI, 0.01–0.61]), whereas thrombomodulin has good rule-in value (specificity 0.89 [95% CI, 0.84–0.92]; positive LR 5.8 [95% CI, 3.6–9.4]) (Table 4).
TABLE 4.
Predictive Performance of Fibronectin, Thrombomodulin, and ADAMTS-13 for Mortality (8.7%), n = 241
| Hemostasis Protein and Cut-Off Value | Sensitivity (95% CI) | Specificity (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) | Probability of Death With Abnormal Value, % | Probability of Death With Normal Value, % |
|---|---|---|---|---|---|---|
| Thrombomodulin ≥ 2,733 pg/mL | 0.67 (0.43–0.85) | 0.89 (0.84–0.92) | 5.8 (3.6–9.4) | 0.38 (0.21–0.69) | 35.6 | 3.5 |
| ADAMTS-13, ≥ 20 ng/mL | 0.95 (0.76–1.00) | 0.53 (0.47–0.60) | 2.0 (1.7–2.4) | 0.09 (0.01–0.61) | 16.3 | 0.8 |
ADAMTS-13 = a disintegrin and metalloprotease with thrombospondin type-1 motif, member 13, LR = likelihood ratio.
Compared with patients without invasive ventilation/inotropes, patients in need for invasive ventilation/inotropes had higher levels of thrombomodulin and lower levels for fibronectin, factor IX, protein C, and protein S. Levels of fibronectin, thrombomodulin, factor IX, protein C, and protein S were correlated with PIM2 score although correlations were very weak to weak (Appendix 7, http://links.lww.com/PCC/C167).
AT THE BEDSIDE
•Hemostatic disturbances were not limited to meningococcal infections but occurred with a comparable frequency and severity in nonmeningococcal infections.
•Higher levels of thrombomodulin and ADAMTS-13 were associated with mortality in children admitted to the hospital with invasive infections.
•Our study emphasizes the importance of hemostatic disturbances in meningococcal and nonmeningococcal pediatric bacterial infections.
DISCUSSION
In this large European cohort, we studied the variation of hemostasis protein levels across invasive pediatric community-acquired infections caused by N. eningitides, S. pneumoniae, S. aureus, and GAS and assessed the association of hemostasis protein levels with mortality and disease severity. Contrary to our hypothesis that meningococcal infection would result in greater mortality, we found that mortality was similar across all pathogen groups. Hemostatic derangements were not limited to meningococcal infections but occurred with a comparable frequency and severity in nonmeningococcal infections. Higher levels of thrombomodulin and ADAMTS-13 were associated with mortality in children admitted to the hospital with invasive infections.
Hemostatic disturbances and purpura fulminans are typical for meningococcal infections. Although the highest DIC scores were more frequent in patients with meningococcal disease, we found similar frequency and severity of hemostatic disturbances across the different pathogens. Apart from thrombomodulin, the levels of hemostasis proteins did not differ specifically for meningococcal infections. Although less visible on physical examination than purpura fulminans, hemostatic derangements may result in microthrombi which could negatively influence microcirculatory perfusion and reduce oxygen delivery thereby influencing disease severity (38). In our study, data on purpura or petechiae and skin necrosis were unfortunately not available.
Previous studies found higher PAI-1 levels and lower protein C levels in nonsurvivors (18–20, 26, 39). This is in contrary to our study, where an association of PAI-1 or protein C with nonsurvivors was not observed. Our study focused on children with different pathogens that needed hospital admission, whereas previous studies have focused on meningococcal infections only or children with septic shock with more severe disease reflected by higher mortality rate (up to 29%) (26, 39). Another possible explanation for an absent association with mortality is that we measured PAI-1 in serum, whereas previous studies measured PAI-1 in citrate plasma. Additional measurements of PAI-1in citrate plasma samples (from the same moment as the serum samples) revealed poor correlation (n = 30, Spearman r = 0.47, data not shown) between PAI-1 level in serum and citrate plasma samples.
Why infections with the same pathogen cause mild disease in one child but can lead to severe sepsis in another child is not completely understood. Sepsis has a multifactorial etiology based on pathogen-dependent factors and host-dependent factors, which includes genetic predisposition and the host-specific immune and hematological response (28). The release of tissue factor following inflammation related endothelial damage activates the hemostatic response. This hematological response subsequently limits pathogen movement and is thus important and beneficial for containment of the infection (40, 41).
Each Pathogen Might Influence the Coagulation System Using Different Mechanisms
S. aureus, for instance, is able to produce coagulase factors which eventually can convert fibrinogen into fibrin, thereby manipulating the coagulation system (14). GAS, on the other hand, expresses streptokinase which activates plasminogen, an important part of the fibrinolytic pathway (42). For N. meningitidis, the lipopolysaccharide on the outer membrane is essential in activating the immune system and by up-regulating tissue factor, stimulating the coagulation system (43). In our study, we expected to find more severe hemostatic disturbances in meningococcal infections, but only higher levels of thrombomodulin were observed for meningococcal infections. Similar to our study, Tan et al (44) found that patients with different types of bacterial infections had alterations in hemostatic mechanisms measured by clot waveform analysis. Differences between Gram-positive infections and Gram-negative infections were also not observed in their study, although they included a different variety of pathogens and did not include N. meningitidis. Although pathogen factors play an important role, we speculate that the host response and disbalance of the immune and hemostatic responses are responsible for severe disease outcomes. Future studies will need to further unravel this complex interplay between pathogens and the host response.
We focused our study on children with invasive bacterial infections as these are known to be associated with hemostatic disturbances. We acknowledge that severe viral infections are also able to cause DIC, such as in Ebola and dengue hemorrhagic fever. Similar to bacterial infections, the involvement of the coagulation system in viral infections most likely limits the spread of the infection (45).
The inflammatory response as result of infection can lead to coagulation abnormalities, ranging from subtle to more severe derangements. As the overall prevalence of DIC was low in our population, our results confirm that disturbances in hemostatic proteins may not necessarily lead to DIC. Disturbances in hemostatic proteins do influence disease severity as our results show that increased thrombomodulin and ADAMTS-13 levels were associated with mortality. The proteins thrombomodulin and ADAMTS-13 are discussed in more detail below.
Our study confirms previous findings (18, 21, 46) and shows higher thrombomodulin levels in nonsurvivors compared with survivors and good discriminative ability of thrombomodulin levels for nonsurvivors and survivors. Additionally, serum thrombomodulin is correlated to PIM2 scores and is higher in patients needing invasive ventilation or inotropes, thereby underscoring a clear association of high thrombomodulin with severity of disease.
This study is the first to compare thrombomodulin levels across different pathogen groups. A study in adults with sepsis reported higher thrombomodulin levels in patients with DIC compared with those without, but the disease-causing pathogens were not reported (46). In our cohort, thrombomodulin levels in meningococcal infections were higher compared with S. pneumonia and S. aureus infections, but the presence of DIC did not differ between pathogen groups. Possibly, higher thrombomodulin levels in meningococcal infections could reflect more endothelial injury, without leading to more DIC. Importantly, our study shows the potential of serum thrombomodulin as prognostic marker for disease severity and mortality.
In our study, serum ADAMTS-13 was higher in bacterial infections compared with healthy children and higher in nonsurvivors than in survivors. This is in contrast to previous studies which found lower plasma ADAMTS-13 in pediatric meningococcal sepsis than in healthy adults and also lower plasma ADAMTS-13 in nonsurvivors compared with survivors (23, 24). Comparability of these studies is not straightforward due to differences in measurement (assay, serum/plasma), controls (children vs adults), and patients (septic shock vs hospital admissions).
How could the elevations in thrombomodulin and ADAMTS-13 contribute to hemostatic disturbances and dysfunction? Thrombomodulin is an endothelial cell surface glycoprotein activating the protein C pathway and sheds from the surface after endothelial injury (47–49). Elevated thrombomodulin could reflect more endothelial injury. The endothelial injury could subsequently promote inflammatory and microthrombotic pathways including platelet activation and release of unusually large vWF multimers (ULVWF) (50). The ULVWF are anchored to the endothelium, will recruit even more platelets, and trigger microthrombosis. Furthermore, elevated thrombomodulin inhibits fibrinolysis by activation of thrombin activatable fibrinolysis inhibitor (51). ADAMTS-13 is known to cleave vWF multimers. The general hypothesis is that depletion of ADAMTS-13 or ADAMTS-13 deficiency provokes microvascular thrombosis as vWF multimers continue to attract more platelets. Our study, however, found elevated levels of ADAMTS-13 in nonsurvivors. We speculate that elevated ADAMTS-13 could lead to increased degradation of vWF. The increased degradation of vWF could reduce platelet adhesion and subsequent clot forming. Future studies will need to address the role of ADAMTS-13 in severe pediatric infections.
Strengths of our study include the large prospective European cohort of pediatric bacterial infections caused by different pathogens. Second, we used detailed clinical data to study hemostasis proteins in relation to mortality and disease severity. Third, hemostasis proteins were chosen according to reported associations with disease severity in literature.
Our study has limitations. First, we selected patients with samples drawn within 48 hours after study centre admission. As our population consists of almost 75% of PICU admissions, our results may not be generalizable to all bacterial infections admitted to the general ward. The higher proportion of PICU admissions, however, may reflect the population at risk for development of hemostatic abnormalities. These data provide us insight in the disease course for infections due to different pathogens with variations in the proportion of PICU admissions: patients with S. aureus and S. pneumoniae infections were less likely to be admitted to the PICU. Although the proportion of PICU admission varied across the pathogen groups, disease severity measured by PIM2 score was similar for the different pathogens in the PICU group. In addition, patients with meningococcal infections had a shorter duration of symptoms at presentation which emphasizes the rapidly progressive disease course for this group (52). Second, the different timings of the measurement and influence of treatment effects before or during admission were not taken into account. However, as greater than or equal to 70% of the samples included were obtained within the first 24 hours of hospital admission, we consider the influence of sample timing and treatment influences to be limited. Furthermore, plasma required for measurement of factor IX, protein C, protein S, and vWF was only available from a subset of the included patients with similar distribution of pathogens. However, this subset consisted of sicker patients with higher rate of PICU admissions and more requirement of ventilation/inotropes (Appendix 8, http://links.lww.com/PCC/C167). In addition, our measurements of vWF were not able to distinguish multimer sizes of vWF. Last, assessment of the relation between hemostasis protein levels and presence of DIC or hemorrhage was not possible as these variables are very closely correlated.
CONCLUSIONS
In this large European cohort of severe invasive pediatric bacterial infections, hemostasis disturbances were not limited to meningococcal infections but occurred with a comparable frequency and severity in nonmeningococcal sepsis. Mortality was similar across all pathogens. In all infections, high thrombomodulin levels and high ADAMTS-13 levels were associated with mortality. Thrombomodulin levels discriminated well for mortality. Our results emphasize the importance of hemostatic disturbances in severe pediatric bacterial infections.
ACKNOWLEDGMENTS
We acknowledge Daan Nieboer, MSc, Erasmus University for his help on the statistical analysis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Drs. Driessen and Emonts are contributed equally.
All authors have read and approved the article for submission. Drs. Hagedoorn, Boeddha, Kohlfuerst, Hazelzet, Zenz, Dik, Driessen, and Emonts contributed to conceptualization. All authors contributed to data curation. Drs. Hagedoorn, Boeddha, Vermont, Zenz, Dik, Driessen, and Emonts contributed to data analysis. Drs. van Rijswijk, Dik, and Driessen contributed to sample analysis. Drs. Dik, Driessen, and Emonts contributed to supervision. Dr. Hagedoorn contributed to writing the original draft. Drs. Boeddha, Kohlfuerst, Anderson, Carrol, Agapow, van der Flier, Hazelzet, Herberg, Kuijpers, Levin, Martinon-Torres, van Rijswijk, Schlapbach, Vermont, Zenz, Dik, Driessen, and Emonts contributed to writing—review and editing.
Supported, in part, from the European Union’s Seventh Framework program under number 279185 (European Union Project European Union Childhood Life-threatening Infectious Disease study).
Drs. Hagedoorn’s and Zenz’s institutions received funding from the European Union (EU). Dr. Boeddha received funding from GlaxoSmithKline. Drs. Kohlfuerst’s, Hazelzet’s, and Emonts’ institutions received funding from EU Project European Union Childhood Life-threatening Infectious Disease study (EUCLIDS). Dr. Kohlfuerst received support for article research from EU Project EUCLIDS. Drs. Carrol, van der Flier, and Herberg received support for article research from EU Horizon 2020. Dr. Hazelzet’s institution received funding from Erasmus Medical Centre. Dr. Zenz received support for article research from the EU. Dr. Driessen’s institution received funding from Merck and the Elizabeth von Freyburg Foundation. Dr. Emonts’ institution received funding from the EU, FP7, EU H2020 Personalized Risk assessment in Febrile illness to Optimize Real-life Management across the European Union (PERFORM), and EU H2020 Diagnosis and Management of Febrile Illness using RNA Personalised Molecular Signature Diagnosis (DIAMONDS). The remaining authors have disclosed that they do not have any potential conflicts of interest.
The European Union Project European Union Childhood Life-threatening Infectious Disease study protocol was approved by at least one ethical review board in every participating country: Austria—Ethikkomission Graz no. 24-116 ex 11/12; the Netherlands—Commissie Medisch-wetenschappelijk onderzoek (CMO) Regio Arnhem-Nijmegen no. 37986.091.11; Spain—Comité Ético de Investigación Clínica de Galicia no. 2011/298; Swiss—Cantonal Ethics Committee, Inselspital, University of Bern no. KEK-029/11; United Kingdom—National Research Ethics Service (NRES) Committee London—Fulham no. 11/LO/1982. Written informed consent was obtained from parents or legal guardians.
A data set containing individual participant data will be made available in a public data repository containing a specific DOI upon publication. The data will be anonymized and will not contain any identifiable data.
REFERENCES
- 1.Boeddha NP, Schlapbach LJ, Driessen GJ, et al. ; EUCLIDS Consortium: Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: A prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Crit Care 2018; 22:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreras-Antolín L, Oligbu G, Okike IO, et al. : Infection is associated with one in five childhood deaths in England and Wales: Analysis of national death registrations data, 2013-15. Arch Dis Child 2020; 105:857–863 [DOI] [PubMed] [Google Scholar]
- 5.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons J, Pittet JF: The coagulopathy of acute sepsis. Curr Opin Anaesthesiol 2015; 28:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gorp EC, Suharti C, ten Cate H, et al. : Review: Infectious diseases and coagulation disorders. J Infect Dis 1999; 180:176–186 [DOI] [PubMed] [Google Scholar]
- 8.Nadel S, Goldstein B, Williams MD, et al. ; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group: Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet 2007; 369:836–843 [DOI] [PubMed] [Google Scholar]
- 9.Martinón-Torres F, Salas A, Rivero-Calle I, et al. ; EUCLIDS Consortium: Life-threatening infections in children in Europe (the EUCLIDS Project): A prospective cohort study. Lancet Child Adolesc Health 2018; 2:404–414 [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach LJ, Straney L, Alexander J, et al. ; ANZICS Paediatric Study Group: Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: A multicentre retrospective cohort study. Lancet Infect Dis 2015; 15:46–54 [DOI] [PubMed] [Google Scholar]
- 11.Cox D, Kerrigan SW, Watson SP: Platelets and the innate immune system: Mechanisms of bacterial-induced platelet activation. J Thromb Haemost 2011; 9:1097–1107 [DOI] [PubMed] [Google Scholar]
- 12.Shannon O, Herwald H, Oehmcke S: Modulation of the coagulation system during severe streptococcal disease. Curr Top Microbiol Immunol 2013; 368:189–205 [DOI] [PubMed] [Google Scholar]
- 13.Lähteenmäki K, Kuusela P, Korhonen TK: Bacterial plasminogen activators and receptors. FEMS Microbiol Rev 2001; 25:531–552 [DOI] [PubMed] [Google Scholar]
- 14.Liesenborghs L, Verhamme P, Vanassche T: Staphylococcus aureus, master manipulator of the human hemostatic system. J Thromb Haemost 2018; 16:441–454 [DOI] [PubMed] [Google Scholar]
- 15.Oehmcke S, Herwald H: Contact system activation in severe infectious diseases. J Mol Med (Berl) 2010; 88:121–126 [DOI] [PubMed] [Google Scholar]
- 16.Blanco A, Guisasola JA, Solís P, et al. : Fibronectin in meningococcal sepsis. Correlation with antithrombin III and protein C. Acta Paediatr Scand 1990; 79:73–76 [DOI] [PubMed] [Google Scholar]
- 17.Riordan FA, Bestwick K, Thomson AP, et al. : Plasma fibronectin levels in meningococcal disease. Eur J Pediatr 1997; 156:451–453 [DOI] [PubMed] [Google Scholar]
- 18.Faust SN, Levin M, Harrison OB, et al. : Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med 2001; 345:408–416 [DOI] [PubMed] [Google Scholar]
- 19.Leclerc F, Hazelzet J, Jude B, et al. : Protein C and S deficiency in severe infectious purpura of children: A collaborative study of 40 cases. Intensive Care Med 1992; 18:202–205 [DOI] [PubMed] [Google Scholar]
- 20.Samransamruajkit R, Hiranrat T, Prapphal N, et al. : Levels of protein C activity and clinical factors in early phase of pediatric septic shock may be associated with the risk of death. Shock 2007; 28:518–523 [DOI] [PubMed] [Google Scholar]
- 21.Lin JJ, Hsiao HJ, Chan OW, et al. : Increased serum thrombomodulin level is associated with disease severity and mortality in pediatric sepsis. PLoS One 2017; 12:e0182324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krafte-Jacobs B, Brilli R: Increased circulating thrombomodulin in children with septic shock. Crit Care Med 1998; 26:933–938 [DOI] [PubMed] [Google Scholar]
- 23.Lin JJ, Chan OW, Hsiao HJ, et al. : Decreased ADAMTS 13 activity is associated with disease severity and outcome in pediatric severe sepsis. Medicine (Baltimore) 2016; 95:e3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongers TN, Emonts M, de Maat MP, et al. : Reduced ADAMTS13 in children with severe meningococcal sepsis is associated with severity and outcome. Thromb Haemost 2010; 103:1181–1187 [DOI] [PubMed] [Google Scholar]
- 25.Hermans PW, Hazelzet JA: Plasminogen activator inhibitor type 1 gene polymorphism and sepsis. Clin Infect Dis 2005; 41(Suppl 7):S453–S458 [DOI] [PubMed] [Google Scholar]
- 26.Kornelisse RF, Hazelzet JA, Savelkoul HF, et al. : The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis 1996; 173:1148–1156 [DOI] [PubMed] [Google Scholar]
- 27.Green J, Doughty L, Kaplan SS, et al. : The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost 2002; 87:218–223 [PubMed] [Google Scholar]
- 28.Boeddha NP, Emonts M, Cnossen MH, et al. : Gene variations in the protein C and fibrinolytic pathway: Relevance for severity and outcome in pediatric sepsis. Semin Thromb Hemost 2017; 43:36–47 [DOI] [PubMed] [Google Scholar]
- 29.Boeddha NP, Driessen GJ, Cnossen MH, et al. : Circadian variation of plasminogen-activator-inhibitor-1 levels in children with meningococcal sepsis. PLoS One 2016; 11:e0167004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agyeman PKA, Schlapbach LJ, Giannoni E, et al. ; Swiss Pediatric Sepsis Study: Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: A population-based cohort study. Lancet Child Adolesc Health 2017; 1:124–133 [DOI] [PubMed] [Google Scholar]
- 31.Slater A, Shann F, Pearson G; Paediatric Index of Mortality (PIM) Study Group: PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29:278–285 [DOI] [PubMed] [Google Scholar]
- 32.Levi M, Toh CH, Thachil J, et al. : Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 2009; 145:24–33 [DOI] [PubMed] [Google Scholar]
- 33.Pollack MM, Ruttimann UE, Getson PR: Pediatric risk of mortality (PRISM) score. Crit Care Med 1988; 16:1110–1116 [DOI] [PubMed] [Google Scholar]
- 32.Wechsler S: Statistics at square one. In: Statistics in Medicine. Ninth Edition, revised by Campbell MJ, Swinscow TDV, London, UK, BMJ Publ Group, 1996, p. 140. Price: £11. ISBN 0-7279-0916-9. 1997; 16:2629–2630 [Google Scholar]
- 33.Van den Bruel A, Haj-Hassan T, Thompson M, et al. ; European Research Network on Recognising Serious Infection Investigators: Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: A systematic review. Lancet 2010; 375:834–845 [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B: Stat Methodol 1995; 57:289–300 [Google Scholar]
- 35.Glickman ME, Rao SR, Schultz MR: False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014; 67:850–857 [DOI] [PubMed] [Google Scholar]
- 36.Erdem Ö, Kuiper JW, Tibboel D: Hemodynamic coherence in critically ill pediatric patients. Best Pract Res Clin Anaesthesiol 2016; 30:499–510 [DOI] [PubMed] [Google Scholar]
- 39.Leclerc F, Cremer R, Leteurtre S, et al. : Fibronectin and meningococcal disease. Eur J Pediatr 1999; 158:81–82 [DOI] [PubMed] [Google Scholar]
- 40.Antoniak S: The coagulation system in host defense. Res Pract Thromb Haemost 2018; 2:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaertner F, Massberg S: Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol 2016; 28:561–569 [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Wang X, Degen JL, et al. : Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood 2009; 113:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandtzaeg P, Bjerre A, Øvstebø R, et al. : Neisseria meningitidis lipopolysaccharides in human pathology. J Endotoxin Res 2001; 7:401–420 [PubMed] [Google Scholar]
- 44.Tan CW, Wong WH, Cheen MHH, et al. : Assessment of aPTT-based clot waveform analysis for the detection of haemostatic changes in different types of infections. Sci Rep 2020; 10: 14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoniak S, Mackman N: Multiple roles of the coagulation protease cascade during virus infection. Blood 2014; 123:2605–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin SM, Wang YM, Lin HC, et al. : Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med 2008; 36:683–689 [DOI] [PubMed] [Google Scholar]
- 47.Ito T, Thachil J, Asakura H, et al. : Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit Care 2019; 23:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boehme MW, Galle P, Stremmel W: Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology 2002; 107:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conway EM: Thrombomodulin and its role in inflammation. Semin Immunopathol 2012; 34:107–125 [DOI] [PubMed] [Google Scholar]
- 50.Chang JC: Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J 2019; 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouma BN, Mosnier LO: Thrombin activatable fibrinolysis inhibitor (TAFI)–how does thrombin regulate fibrinolysis? Ann Med 2006; 38:378–388 [DOI] [PubMed] [Google Scholar]
- 52.Thompson MJ, Ninis N, Perera R, et al. : Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006; 367:397–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.