Abstract
The association of respiratory mechanics, particularly respiratory system static compliance (CRS), with severity of hypoxaemia in patients with COVID-19-related acute respiratory distress syndrome (ARDS) has been widely debated, with some studies reporting distinct ARDS phenotypes based on CRS. Ascertaining whether such phenotypes exist is important, because they might indicate the need for ventilation strategies that differ from those used in patients with ARDS due to other causes. In a systematic review and meta-analysis of studies published between Dec 1, 2019, and March 14, 2022, we evaluated respiratory system mechanics, ventilator parameters, gas exchange parameters, and clinical outcomes in patients with COVID-19-related ARDS. Among 11 356 patients in 37 studies, mean reported CRS, measured close to the time of endotracheal intubation, was 35·8 mL/cm H2O (95% CI 33·9–37·8; I2=96·9%, τ2=32·6). Pooled mean CRS was normally distributed. Increasing ARDS severity (assessed by PaO2/FiO2 ratio as mild, moderate, or severe) was associated with decreasing CRS. We found no evidence for distinct CRS-based clinical phenotypes in patients with COVID-19-related ARDS, and we therefore conclude that no change in conventional lung-protective ventilation strategies is warranted. Future studies should explore the personalisation of mechanical ventilation strategies according to factors including respiratory system mechanics and haemodynamic status in patients with ARDS.
Introduction
Acute respiratory distress syndrome (ARDS) is a complex clinical syndrome with a variety of aetiologies, including infectious and non-infectious precipitating factors. ARDS is characterised by a combination of acute onset, hypoxaemia (ratio of partial pressure of oxygen in arterial blood [PaO2] to fractional inspired oxygen [FiO2] of ≤300 mm Hg), and bilateral pulmonary opacities on chest x-ray or CT that are not fully explained by cardiac failure or volume overload.1 ARDS severity is classified on the basis of the PaO2/FiO2 ratio as mild (>200 to 300 mm Hg), moderate (>100 to 200 mm Hg), or severe (≤100 mm Hg).1 Patients with ARDS usually have low respiratory system compliance, but this factor was excluded from the Berlin definition of ARDS because respiratory mechanics added little predictive value.1
The current protocolised approach to treating ARDS has attracted much scrutiny, especially during the COVID-19 pandemic, with calls being made for a more personalised approach.2 There was an initial suggestion, on the basis of small observational studies of patients with COVID-19-related ARDS, that the severity of hypoxaemia was out of proportion to impairment in respiratory system mechanics.3, 4 Despite meeting the criteria for moderate-to-severe ARDS, these patients were found to have relatively normal respiratory system compliance, and shunt fraction—ie, the percentage of cardiac output circulating through the lungs that is not completely oxygenated—that was out of proportion to non-aerated lung fraction. If this finding proved to be generalisable to the large population of patients with COVID-19-related ARDS, it could have implications for ventilation management, as suggested by others.3, 5, 6 However, these early observations have been challenged by several researchers and commentators.4, 7, 8, 9
It has also been proposed that severe hypoxaemia and high respiratory drive in patients with COVID-19-related and non-COVID-19-related ARDS with otherwise preserved respiratory system static compliance (CRS)—ie, respiratory system compliance when there is no airflow—could mediate ARDS progression via patient self-inflicted lung injury (P-SILI).2, 10 P-SILI refers to the lung injury that can develop from intense inspiratory effort. Although the existence of different phenotypes in patients with COVID-19-related ARDS based on CRS values seems plausible, measured CRS is intricately linked to extent of the aerated lung at the time of measurement, which depends on the timing of endotracheal intubation and invasive mechanical ventilation. Multiple small observational studies have not been able to identify such distinct phenotypes of CRS and reported a unimodal distribution of CRS in COVID-19-related ARDS.8, 11, 12 It is possible that patients with hypoxaemia who were intubated early and placed on mechanical ventilation during the first months of the pandemic, when less invasive respiratory supports were discouraged, could have exhibited high CRS. Subsequently, greater uptake of less invasive supports, less proactive endotracheal intubation, and the advent of disease-modifying therapies, such as corticosteroids, might all have affected the occurrence of the high-CRS phenotype.
A 2021 study that used dual-energy CT showed that, in critically ill patients with severe COVID-19-related ARDS, oxygenation impairment and the need for invasive mechanical ventilation were associated with a loss of lung aeration, greater shunt fraction, and the extent of ventilation–perfusion mismatch, which indicates a potential loss of hypoxic vasoconstriction.13 Patients with COVID-19-related ARDS could have varying degrees of CRS, recruitability, and hypoxaemia depending on the extent of ventilation–perfusion mismatch.
Key messages.
-
•
Acute respiratory distress syndrome (ARDS) is typically associated with reduced respiratory system compliance due to loss of surfactant, flooded alveoli, and compressive atelectasis
-
•
Reduced lung compliance in ARDS is usually a marker of decreased lung volume, and hence an indication that tidal volume should be reduced to prevent over-distension of healthy lung units; substantially reduced compliance in ARDS is associated with a high risk of lung injury from sheer strain, barotrauma, or volutrauma; lung-protective ventilation with high positive end-expiratory pressure and low tidal volume in patients with poor lung compliance has been shown to decrease lung injury
-
•
In the first months of the pandemic, when less invasive strategies for respiratory support were discouraged and disease-modifying therapies such as corticosteroids were not in use, some reports suggested that patients with COVID-19-related ARDS had higher respiratory system static compliance (CRS) measured soon after intubation than that reported for ARDS due to other causes, with potential implications for ventilation management
-
•
The suggestion that severity of hypoxaemia is out of proportion to impairment in respiratory system mechanics in patients with COVID-19-related ARDS was not borne out in our systematic review and meta-analysis, in which CRS measured soon after the initiation of invasive mechanical ventilation in patients with COVID-19-related ARDS was normally distributed and similar to CRS reported in clinical trials that recruited patients with conventional ARDS; we did not find evidence for distinct CRS-based clinical phenotypes in patients with COVID-19-related ARDS
-
•
Reported CRS in patients with COVID-19-related ARDS decreased as the severity of ARDS increased (assessed by PaO2/FiO2 ratio); positive end-expiratory pressure and tidal volume showed a positive association with CRS, whereas plateau pressure was negatively associated with CRS
-
•
Our study suggests that ventilatory strategies used in patients with non-COVID-19-related ARDS should be used in patients with COVID-19-related ARDS until there is evidence to the contrary; future research should focus on how best to individualise ventilatory strategy to the patient's specific respiratory mechanics and haemodynamic status
In view of uncertainty about respiratory system mechanics and phenotypes of CRS in patients with COVID-19-related ARDS—and their potential implications for ventilation management and outcomes—we aimed to characterise and evaluate basic respiratory system mechanics, ventilator parameters, gas exchange parameters, and clinical outcomes in a systematic review and meta-analysis of studies of patients with COVID-19-related ARDS. We used the earliest measures of respiratory variables taken (hours to days) after the initiation of invasive mechanical ventilation, because delayed findings would be influenced by disease progression and interventions or therapies administered to patients.9, 11 We discuss the implications of our findings for the management of individuals with COVID-19-related ARDS.
Methods
The study was conducted and the findings reported in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (appendix pp 2–3).14
Study selection
We carried out a literature search of MEDLINE through PubMed, Embase via Ovid, and the COVID-19 living systematic review on March 14, 2022. Living systematic reviews have been used during the Zika virus epidemic15 and during the COVID-19 pandemic.16 The key search terms “acute respiratory distress syndrome”; “intubate” or “mechanical ventilation”; and “coronavirus” were combined using the Boolean operators “OR” within search strings and using “AND” across search strings (appendix p 11).
We included several study types published during the COVID-19 pandemic period (from Dec 1, 2019, to March 14, 2022), including prospective cohort studies, case series with at least five patients, and studies of unspecified design. Studies were included if they involved adult patients (≥18 years) who received mechanical ventilation and if ventilator parameters were reported soon (hours to days) after the initiation of invasive mechanical ventilation. Studies were excluded if they did not present CRS data. Studies with a selected group of patients (eg, patients with moderate or severe ARDS or patients with obesity) or with patients who required special interventions (eg, prone positioning or inhaled nitric oxide) were excluded from the primary analysis. In the case of overlapping patient data (eg, overlapping enrolment period or same hospital) across two or more studies, we included the study with the largest sample size.
Data extraction and quality assessment
We extracted data for the included studies using a pre-specified datasheet (appendix p 12). Variables in the pre-specified datasheet included study characteristics, demographic data, clinical characteristics, interventions, and outcomes. For each selected observational study, the risk of bias was evaluated by two authors (MPR and CC) independently, using the five domains (selection, sample size, comparability, compliance reporting method, and quality of statistics; appendix p 13) of the modified Newcastle-Ottawa Scale (mNOS), which has a range of scores from 2 to 5.17 The mNOS was used because there were no control groups in the included studies. Certainty of evidence was assessed by four main factors (risk of bias, inconsistency, indirectness, and imprecision)18 using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) approach. The certainty of the evidence was rated from high (ie, we are very confident that the true effect lies close to that of the effect estimate) to very low (ie, we have very little confidence in the effect estimate: the true effect is likely to be substantially different; appendix pp 14–15).18, 19 The screening of studies, data collection, and risk-of-bias assessment were conducted independently and in duplicate by MPR and CC. Conflicts were resolved by consensus or by ASu.
Data synthesis
Given the substantial heterogeneity in patient demographics and treatment modalities for COVID-19, an inverse-variance random-effects meta-analysis was conducted. When pooling studies for meta-analysis of proportions, a distribution-free random-effects model was applied, using the standard method proposed by DerSimonian and Laird.20 Furthermore, the popular two-step arc-sine transformation described by Freeman and Tukey21 was applied to yield better final approximations of the normal distribution. We assessed the normal distribution of studies using a Shapiro-Wilk test. 95% CIs were computed using the Clopper-Pearson method.22
We assessed the possibility of publication bias via visual inspection of the funnel plot and Egger's test, and corrected for small-study effects using the random-effects trim-and-fill (R 0 estimator) procedure. We performed two sensitivity analyses. Firstly, we excluded studies with higher risks of bias (defined as an mNOS score of <3). Secondly, we repeated the primary meta-analysis using the fixed-effects model. Survival outcomes are presented as pooled proportions, and continuous outcomes are presented as pooled means, each with their respective 95% CI.
To explore potential sources of heterogeneity and to examine potential prognostically relevant covariates, we conducted subgroup analyses on the basis of ARDS severity (PaO2/FiO2 ratio of >200–300 mm Hg, >100 to 200 mm Hg, or ≤100 mm Hg), presence of hypercarbia (partial pressure of carbon dioxide in arterial blood [PaCO2] >45 mm Hg vs ≤45 mm Hg) and value of positive end-expiratory pressure (PEEP; >15 cm H2O vs ≤15 cm H2O). The random-effects χ2 test was used to assess differences between subgroups. We also conducted a summary-level meta-regression to investigate the effect of demographic factors, disease factors, and intervention factors on CRS. Demographic factors included mean BMI, average date of patient enrolment (defined as the midpoint between the first and last dates of patient enrolment in the study), disease factors (assessed by PaO2/FiO2 ratio and PaCO2), and intervention factors (PEEP, plateau pressure, driving pressure, and tidal volume [VT]). Subsequent regression results were reported as regression slopes (β) and 95% CIs.
We then pooled the following mortality rates: 28-day mortality, intensive care unit [ICU] mortality, in-hospital mortality, and cumulative mortality (defined as mortality at the latest point of recording). We also pooled the ICU length of stay, the hospital length of stay, and ventilator-free days. For continuous variables, means and SDs were derived from the aggregate data as per Luo and colleagues23 and Wan and colleagues.24 Because statistical heterogeneity can be overestimated by I 2 statistics in observational studies, we assessed interstudy variability as part of the assessment of the certainty of evidence outlined by the GRADE approach.25, 26 A post-hoc sensitivity analysis was done on the basis of individual study sample size (≤100 vs >100). All statistical analyses were done with R (version 4.0.2). A nominal p value of less than 0·05 was considered to be statistically significant in our study. The study protocol is registered with PROSPERO, CRD42020226124.
Results
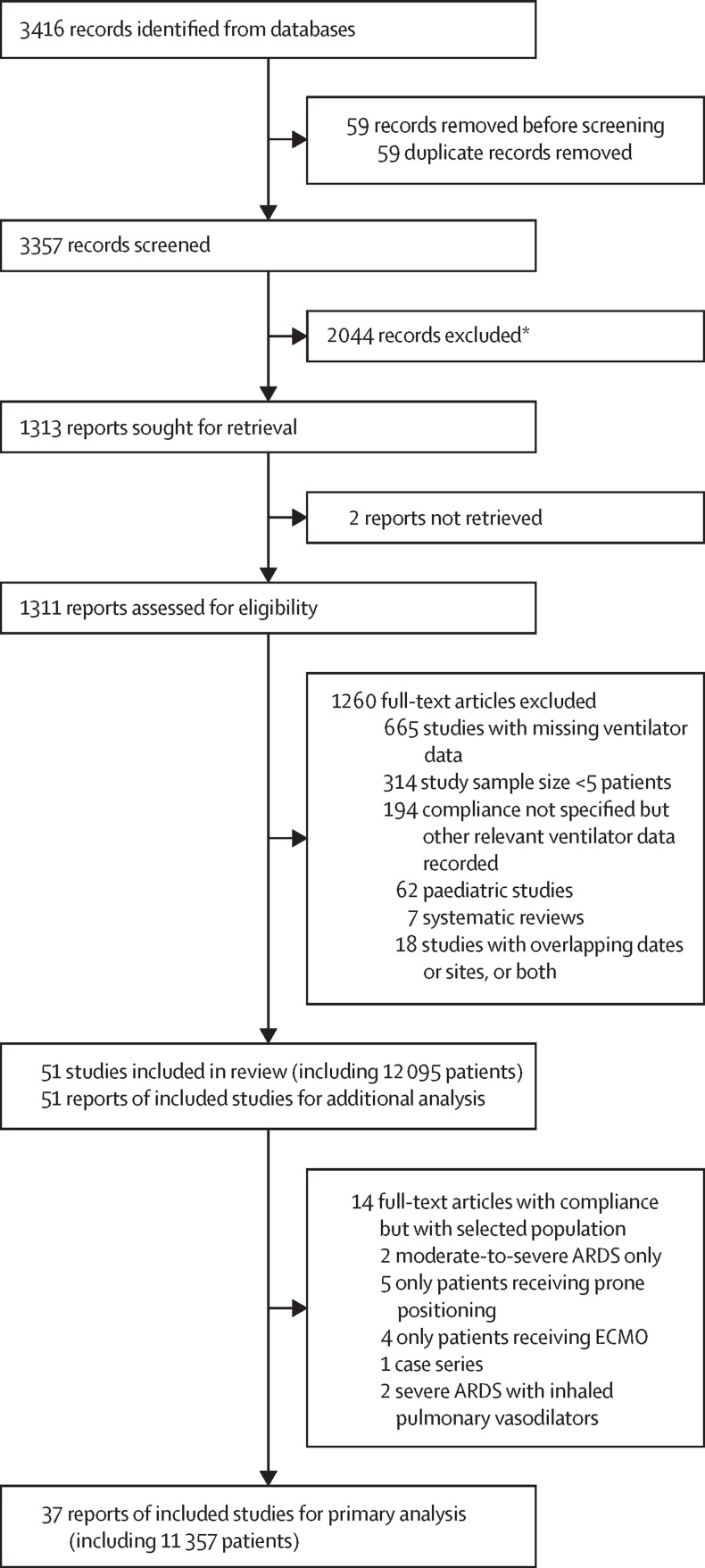
Of the 3416 references published until March 14, 2022, we assessed 1311 full-text articles for eligibility. We identified 12 095 patients receiving invasive ventilation for COVID-19-related ARDS from 51 observational studies that met our inclusion criteria. Of these studies, 37 unselected ARDS studies, involving a total of 11 356 patients (8861 male patients and 2495 female patients), were used for the primary analysis (figure 1 ). Among 51 studies of COVID-19-related ARDS, only nine had an mNOS score of less than 3; the others had an mNOS score that was 3 or more (23 studies had scores of 3, 11 studies had scores of 4, and eight studies had scores of 5; appendix p 13). The population was predominantly male (9268 [78·9%] of 11 742 patients; 45 studies). The mean BMI of patients was 25·9 kg/m2 (SD 2·45, range 25·3–34·7 kg/m2; appendix pp 18–20). GRADE assessments are illustrated in the appendix (pp 14–15). Ventilator parameters and respiratory variables are shown in the appendix (pp 21–23). Variations in CRS, PaCO2, VT, and PEEP are also shown in the appendix (p 9).
Figure 1.
Study selection
ARDS=acute respiratory distress syndrome. ECMO=extracorporeal membrane oxygenation. *Studies were excluded because they were not about patients with COVID-19.
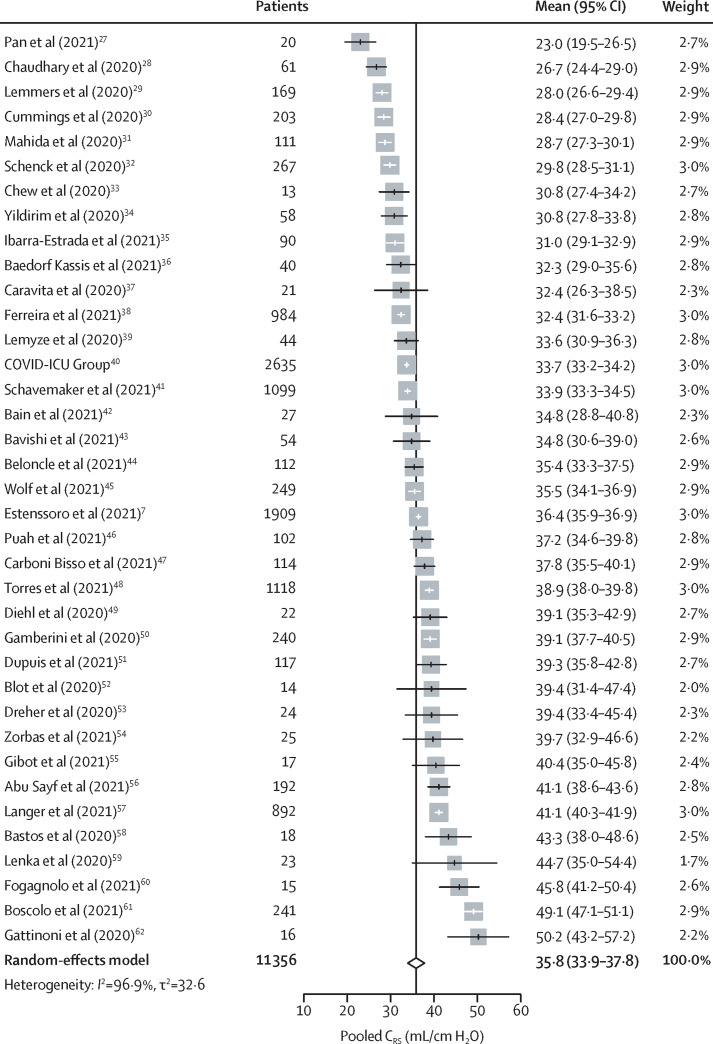
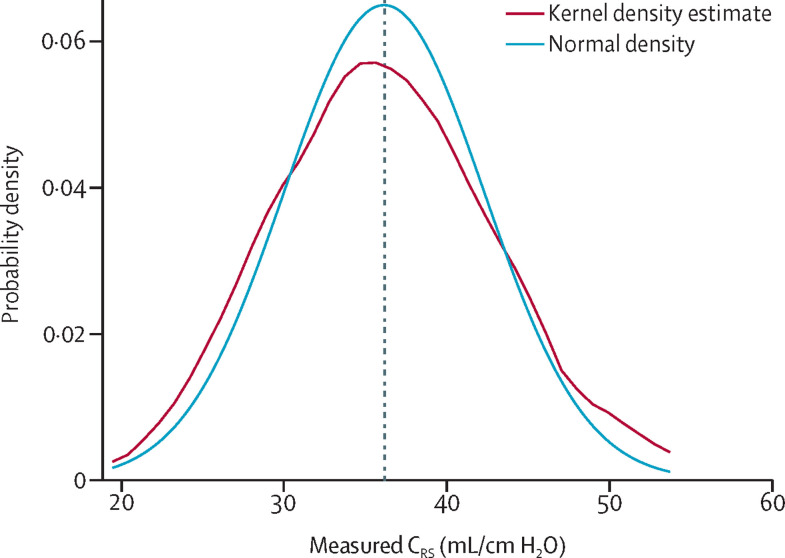
The pooled reported CRS in patients from studies of unselected COVID-19-related ARDS was 35·8 mL/cm H2O (95% CI 33·9–37·8; I 2=96·9%, τ2=32·6; high certainty; figure 2 ).27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 In these studies, CRS dispersion was not significantly different from a normal distribution (Shapiro-Wilk test p=0·92; figure 3 ). The pooled reported CRS in all patients from studies of selected and unselected COVID-19-related ARDS was 34·7 mL/cm H2O (32·8–36·6; 97·2%, 43·6; high certainty; appendix p 5). CRS of patients remained similar (33·9 mL/cm H2O, 32·0–35·9; 98·0%, 37·1) after excluding nine studies with mNOS scores of less than 3. Visual inspection did not reveal any significant asymmetry of the funnel plot (Egger's test p=0·95; appendix p 6). Furthermore, correction of small-study effects using the R 0 estimator procedure did not change the pooled estimate of CRS (34·7 mL/cm H2O, 32·8–36·6).
Figure 2.
Forest plot showing pooled CRS from studies of unselected patients with COVID-19-related ARDS
Data from 37 unselected ARDS studies, with a total of 11 356 patients, are shown. ARDS=acute respiratory distress syndrome. CRS=respiratory system static compliance.
Figure 3.
Kernel density plot showing the distribution of CRS means
CRS means in 37 unselected ARDS studies were normally distributed (Shapiro-Wilk test p=0·92). Pooled mean CRS was 35·8 mL/cm H2O (vertical dashed line). ARDS=acute respiratory distress syndrome. CRS=respiratory system static compliance.
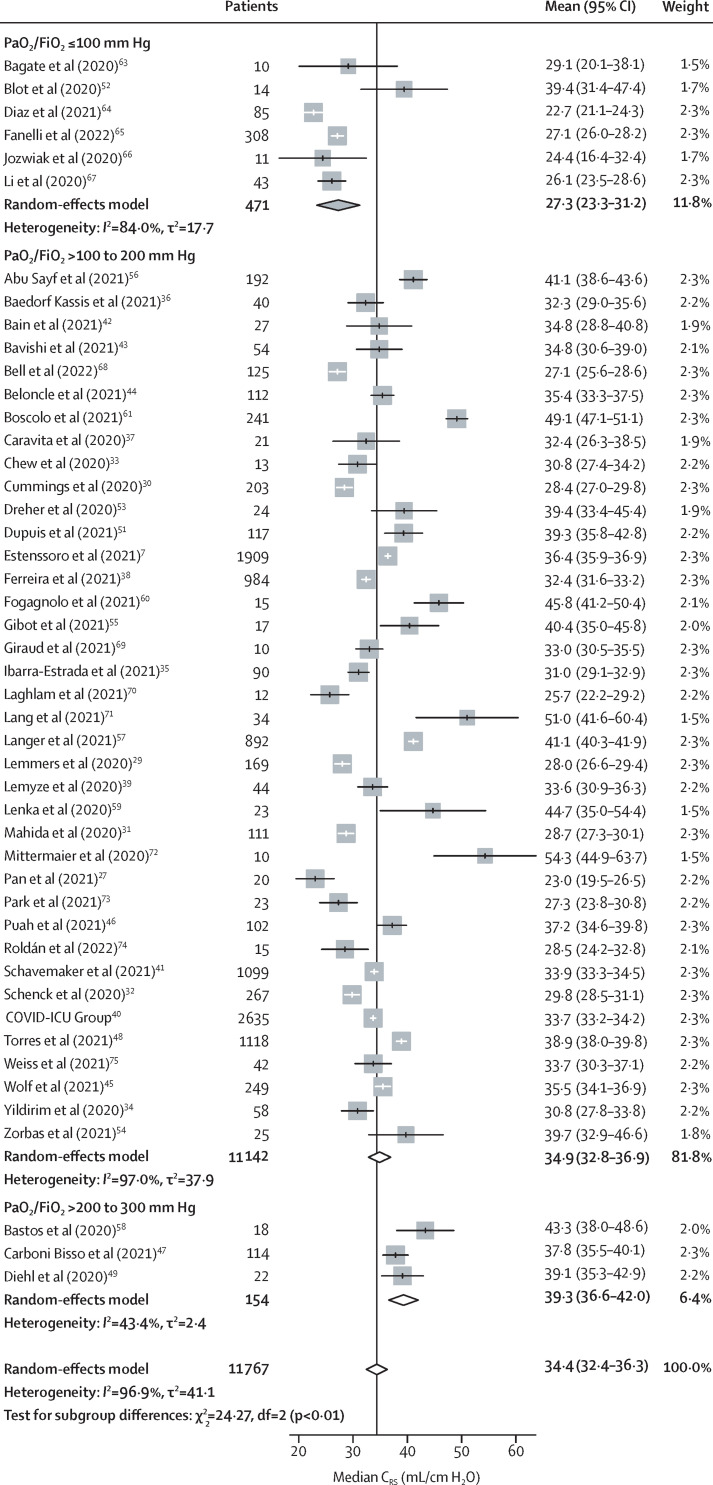
The overall pooled mean PaO2/FiO2 ratio of patients in unselected studies was 149·1 mm Hg (95% CI 135·4–162·9; 34 studies). Increasing severity of COVID-19-related ARDS, assessed by PaO2/FiO2, was associated with reduced CRS (pinteraction<0·0001): CRS progressively decreased from 39·3 mL/cm H2O (95% CI 36·6–42·0) in patients with mild ARDS, to 34·9 mL/cm H2O (32·8–36·9) in patients with moderate ARDS, and 27·3 mL/cm H2O (23·3–31·2) in patients with severe ARDS (table 1 , figure 4 ).63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 There was no significant association between CRS and PaCO2 (31·5 [95% CI 27·7–35·4] in patients with PaCO2 >45 mm Hg vs 36·5 [33·3–39·6] in those with PaCO2 ≤45 mm Hg; pinteraction=0·052; table 1) or between CRS and PEEP (35·2 [95% CI 27·6–42·8] in patients with PEEP >15 cm H2O vs 34·2 [32·3–36·2] in those with PEEP ≤15 cm H2O; pinteraction=0·80; table 1). Further details of the association between CRS and PEEP are provided in the appendix (pp 8–9).
Table 1.
Interaction of CRS with the severity of ARDS, the presence of hypercarbia, and the value of PEEP
| Number of studies | Number of patients | Pooled estimate of CRS, mL/cm H2O (95% CI) | Heterogeneity and between-studies variance | |
|---|---|---|---|---|
| PaO2/FiO2 ratio (pinteraction<0·0001) | ||||
| >200 to 300 mm Hg | 3 | 154 | 39·3 (36·6–42·0) | I2=43·4%, τ2=2·4 |
| >100 to 200 mm Hg | 38 | 11 142 | 34·9 (32·8–36·9) | I2=97·0%, τ2=37·9 |
| ≤100 mm Hg | 6 | 471 | 27·3 (23·3–31·2) | I2=84·3%, τ2=17·7 |
| PaCO2 (pinteraction=0·052) | ||||
| >45 mm Hg | 16 | 2814 | 31·5 (27·7–35·4) | I2=98·2%, τ2=56·6 |
| ≤45 mm Hg | 13 | 7738 | 36·5 (33·3–39·6) | I2=97·8%, τ2=31·7 |
| PEEP (pinteraction=0·80) | ||||
| >15 cm H2O | 7 | 410 | 35·2 (27·6–42·8) | I2=92·3%, τ2=95·5 |
| ≤15 cm H2O | 41 | 11 685 | 34·2 (32·3–36·2) | I2=97·6%, τ2=37·9 |
Severity of ARDS was assessed using the PaO2/FiO2 ratio (mild, >200 to 300 mm Hg; moderate, >100 to 200 mm Hg; or severe, ≤100 mm Hg). The presence of hypercarbia was assessed using PaCO2 (>45 mm Hg vs ≤45 mm Hg). PEEP values were high or low (>15 cm H2O vs ≤15 cm H2O) compared with patients with mild ARDS (PaO2/FiO2 ratio >200 to 300 mm Hg). ARDS=acute respiratory distress syndrome. CRS=respiratory system static compliance. FiO2=fractional inspired oxygen. PaCO2=partial pressure of carbon dioxide in arterial blood. PaO2=partial pressure of oxygen in arterial blood. PEEP=positive end-expiratory pressure.
Figure 4.
Forest plot comparing mean CRS of each study with the severity of ARDS
Data from 47 of the 51 studies of patients with COVID-19-related ARDS are shown, with severity of ARDS assessed by the mean PaO2/FiO2 ratio of each study. ARDS=acute respiratory distress syndrome. CRS=respiratory system static compliance. FiO2=fractional inspired oxygen. PaO2=partial pressure of oxygen in arterial blood.
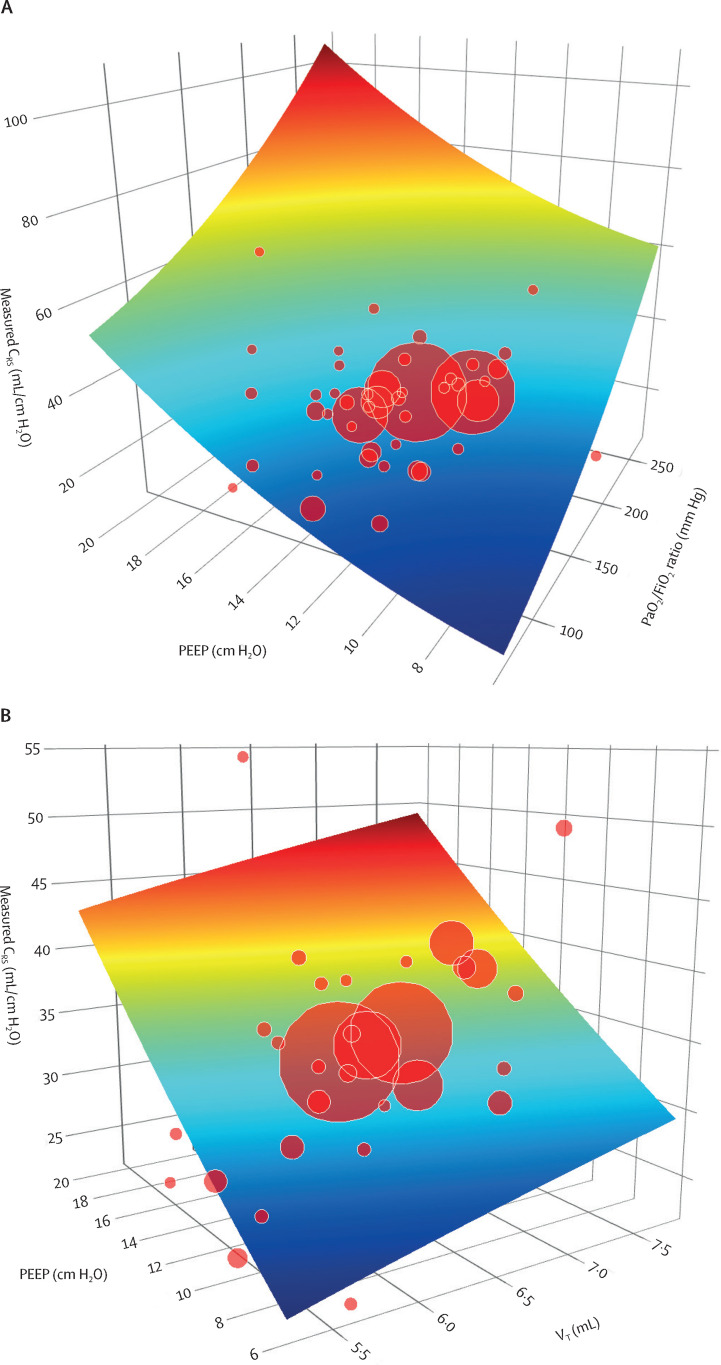
The complex interactions of CRS with PaO2/FiO2 ratio and PEEP are shown in figure 5A ; the interactions of CRS with VT and PEEP are shown in figure 5B. In patients with moderate-to-severe ARDS (PaO2/FiO2 ratio ≤200 mm Hg), increased PEEP was associated with higher compliance than in patients with mild ARDS (PaO2/FiO2 ratio >200 to 300 mm Hg).
Figure 5.
Three-dimensional plots showing the complex interactions between CRS and related variables
(A) Interactions between CRS, PaO2/FiO2 ratio, and PEEP. As oxygenation worsened (ie, PaO2/FiO2 ratio decreased), CRS also decreased. As PEEP increased, CRS also increased. (B) Interactions between CRS, VT, and PEEP. CRS increased with an increase in VT or PEEP. CRS=respiratory system static compliance. FiO2=fractional inspired oxygen. PaO2=partial pressure of oxygen in arterial blood. PEEP=positive end-expiratory pressure. VT=tidal volume.
In univariable meta-regression analyses, there was a statistically significant positive correlation between CRS (the dependent variable) and PaO2/FiO2 ratio (β=0·06, 95% CI 0·02 to 0·11), between CRS and PEEP (0·88, 0·07 to 1·68), and between CRS and VT (6·28, 3·37 to 9·19). CRS had a statistically significant negative correlation with driving pressure (–2·49, –3·12 to –1·86), with plateau pressure (–0·77, –1·53 to –0·02), and with PaCO2 (–0·39, –0·71 to –0·07). There was no significant correlation between CRS and BMI (–0·02, –1·09 to 1·05) or between CRS and average date of patient enrolment (–0·005, –0·04 to 0·03). The results of the meta-regression analyses are presented in table 2 and in the appendix (p 9).
Table 2.
Univariable meta-regression analysis with CRS as the dependent variable
| Number of studies | Regression coefficient, β (95% CI) | p value | |
|---|---|---|---|
| PaO2/FiO2ratio (mm Hg) | 47 | 0·06 (0·02 to 0·11) | 0·0070 |
| PaCO2(mm Hg) | 29 | −0·39 (−0·71 to −0·07) | 0·018 |
| PEEP (cm H2O) | 48 | 0·88 (0·07 to 1·68) | 0·033 |
| VT (mL) | 31 | 6·28 (3·37 to 9·19) | <0·0001 |
| Driving pressure (cm H2O) | 36 | −2·49 (−3·12 to −1·86) | <0·0001 |
| Plateau pressure (cm H2O) | 36 | −0·77 (−1·53 to −0·02) | 0·045 |
| BMI (kg/m2) | 30 | −0·02 (−1·09 to 1·05) | 0·97 |
| Average date of patient enrolment (days from Jan 1, 2020)* | 47 | −0·005 (−0·04 to 0·03) | 0·76 |
Average date of patient enrolment was calculated by taking the midpoint of the first and final dates of patient enrolment. ARDS=acute respiratory distress syndrome. CRS=respiratory system static compliance. FiO2=fractional inspired oxygen. PaCO2=partial pressure of carbon dioxide in arterial blood. PaO2=partial pressure of oxygen in arterial blood. PEEP=positive end-expiratory pressure. VT=tidal volume.
The pooled mean reported ICU mortality was 35·7% (95% CI 29·4–42·2; I 2=97%, τ2=41·1; moderate certainty; 27 studies), whereas the pooled in-hospital mortality was 39·1% (32·6–45·8; moderate certainty; 14 studies). The overall pooled 28-day mortality was 43·2% (32·6–54·1; moderate certainty; 13 studies). Pooled cumulative mortality was 40·3% (35·1–45·6; moderate certainty; 43 studies). CRS had no significant correlation with cumulative morality (β=–0·005, 95% CI –0·01 to 0·002). The pooled mean ICU length of stay was 19 days (95% CI 15–22; high certainty; 25 studies), whereas the pooled mean hospital length of stay was 28 days (23–34; high certainty; 13 studies). The pooled mean ventilator-free days (up to day 28) was 5·2 days (2·9–7·6; high certainty; seven studies). The appendix shows the results for secondary outcomes (p 24).
Post-hoc sensitivity analysis conducted on the basis of the individual study sample size (≤100 patients in 31 studies vs >100 patients in 20 studies) found no difference in CRS (34·6 mL/cm H2O [95% CI 31·8–37·3; I 2=91·9%, τ2=55·5] for sample ≤100 vs 35·0 mL/cm H2O [32·4–37·5; 98·5%, 32·5] for sample >100; appendix p 29). Another post-hoc sensitivity analysis using the fixed-effects model yielded a value that was similar to that of the random-effects model (CRS 34·1 mL/cm H2O, 33·9–34·3; 97·0%, 43·6; appendix p 10).
Discussion
In this systematic review and meta-analysis, we evaluated respiratory mechanics, gas exchange, and outcomes in a very large group of mechanically ventilated patients with COVID-19-related ARDS. Our main findings, based on data from 11 356 patients in 37 unselected studies, were as follows: pooled mean CRS, measured close to the time of endotracheal intubation, was normally distributed; CRS decreased progressively with increasing severity of ARDS (assessed by decreasing PaO2/FiO2 ratio); higher PEEP and higher VT were associated with greater CRS; and higher plateau pressure and driving pressure were associated with lower CRS.
Respiratory system mechanics in COVID-19-related ARDS
We found that pooled mean CRS, measured close to the time of the initiation of invasive mechanical ventilation, was 35·8 mL/cm H2O (95% CI 33·9–37·8) across 37 unselected studies and the reported mean CRS across the 51 studies ranged from 22·7 to 54·3 mL/cm H2O. In comparison, the reported mean CRS in landmark ARDS clinical trials published between 2004 and 2017 that included patients with all severities of ARDS, or moderate-to-severe ARDS, ranged from 23·2 to 41·0 mL/cm H2O.76, 77, 78, 79, 80, 81, 82, 83, 84 These ARDS trials are useful comparators because they applied lung-protective ventilation strategies (ie, low VT and moderate-to-high PEEP) similar to those applied in COVID-19 studies, given that such a strategy is now standard practice. In this setting, studies of patients with COVID-19-related ARDS that reported higher CRS than is typically seen in patients with ARDS due to other causes might have included patients who were treated with an early intubation strategy at the beginning of the COVID-19 pandemic. Non-invasive ventilation and high-flow oxygen ventilation were not preferentially used during the first months of the pandemic due to concerns of viral aerosolisation and resultant safety concerns for health-care workers. As such, patients were intubated at the early stages of the disease process, when CRS was still preserved.
In addition, we found that the mean PaO2/FiO2 ratio of patients from unselected COVID-19-related ARDS studies, obtained at the time of CRS measurements, was 149·1 mm Hg (95% CI 135·4–162·9; range 95·3–271·8). In comparison, the range of mean PaO2/FiO2 ratios in non-COVID-19-related ARDS clinical trials was 100–200 mm Hg.76, 77, 78, 79, 80, 81, 82, 83, 84 The range of mean VT values in COVID-19-related ARDS studies was 5·6–6·4 mL/kg, compared with a mean VT range of 5·8–8·6 mL/kg in ARDS clinical trials (appendix pp 31–33). The mean PEEP range in COVID-19 ARDS studies was 6·0–20·1 cm H2O, compared with a range of 7·5–16·1 cm H2O in ARDS clinical trials.76, 77, 78, 79, 80, 81, 82, 83, 84 These data indicate that the suggested existence of distinct CRS-based phenotypes in patients with COVID-19-related ARDS could simply have been based on observations from small datasets during an early phase of the pandemic. Reduction in lung compliance in ARDS is attributed to a decrease in aerated lung volume due to fluid-filled alveoli from inflammatory oedema, decreased surfactant function, and atelectasis. We believe that patients with COVID-19 also have similar pathophysiological changes. However, in addition to the timing of intubation, ARDS severity, and the ventilation strategy applied, factors such as viral mutations, vaccination uptake, and increased use of disease-modifying drugs as the pandemic progressed could all contribute to the measures obtained and could not be controlled for in our analysis.
The association of higher PEEP with greater CRS might be related to lung recruitment. There is an ongoing debate about optimal PEEP and lung tissue available for recruitment in COVID-19-related ARDS, and about the routine application of low VT in all patients with ARDS, regardless of respiratory mechanics. Many have argued that the routine application of higher PEEP and low VT in all patients with COVID-19-related ARDS, regardless of their respiratory mechanics, could be detrimental.85, 86 Greater CRS was seen in patients who received higher PEEP, higher VT, or a combination of both. Application of higher PEEP in patients with relatively high CRS might have been driven partly by the severity of hypoxaemia, which suggests a possible dissociation between gas exchange and mechanics. These observations, in a small group of patients, might have led to the CRS-based phenotype hypothesis. It is possible that hypoxaemia and CRS could at times be decoupled, albeit transiently, in patients with ARDS, and this observation might not be specific to COVID-19-related ARDS. Similarly, the higher VT found in patients with greater CRS might indicate that clinicians applied both VT and PEEP—based on an assessment of respiratory mechanics—to maintain safe driving and plateau pressures, instead of undertaking a routine low-VT, high-PEEP approach. Establishing whether either of these approaches was independently detrimental is beyond the scope of our analysis given the scarcity of individual patient data. Higher driving pressures seen in patients with lower CRS, although expected, are challenging to decipher further in the absence of individual patient data and measurements over time. Clinicians might have further optimised PEEP and VT to reduce driving pressure in the patients most severely affected by COVID-19-related ARDS.
Personalised ventilation management in ARDS
The COVID-19 pandemic has re-kindled the concept that mechanical ventilation should be personalised for every patient according to several factors, including a patient's CRS, PEEP, overall respiratory system mechanics, acid-base balance, and haemodynamic status. Studies during the initial stages of the COVID-19 pandemic suggested the existence of different ARDS phenotypes with either normal CRS 5, 62, 87 or very low CRS.88 Subsequent studies addressed the existence of these COVID-19-related ARDS phenotypes, but the studies were limited by relatively small sample sizes.8, 9, 12 Early in the pandemic, many guidelines across the globe recommended early intubation to minimise the risk of SARS-CoV-2 infection among health-care workers.89, 90, 91, 92 As such, many patients might have been intubated and mechanically ventilated early; alternatively, they might have faced unnecessary delays owing to resource constraints. Either of these scenarios could have influenced measurements of CRS and might have led to potential artifactual clinical phenotypes.
Mechanical ventilation supports should be tailored to patient physiology rather than to a pathological condition. Making changes to conventional ventilatory strategies or adopting newer strategies on the basis of scarce evidence in emerging infectious respiratory pathologies can be deleterious to patients. Future studies should explore personalised strategies, which might be informed by multivariable in silico modelling of the dynamic variables related to respiratory mechanics and gas exchange.93 These strategies could be augmented by cardiopulmonary computational models and artificial intelligence, which might provide safer ventilatory approaches in patients with ARDS of any aetiology. Ideally, these approaches would be tested in clinical trials prior to widespread use.
Study strengths and limitations
Our systematic review and meta-analysis had many strengths. To our knowledge, this is the first systematic review evaluating respiratory characteristics and mechanics of patients with COVID-19-related ARDS. Rigorous search criteria were used, and appropriate statistical analyses were done to evaluate the outcomes. Of note, 42 of 51 COVID-19-related ARDS studies were of acceptable quality, with an mNOS score that was greater than 3. In addition, our meta-analysis summarised data from more than 2 years across the COVID-19 pandemic. Through this analysis, we can derive a broader, longitudinal view of how respiratory mechanics change in patients who receive mechanical ventilation for COVID-19, which other studies have not been able to investigate. Furthermore, appropriate sensitivity analyses were conducted, and the GRADE approach was used to rate the certainty of the evidence, allowing us to communicate our findings in an efficient and standardised manner.
However, some limitations of our study need to be acknowledged. First, there was substantial heterogeneity, not only in patient management with regard to mechanical ventilation, but also in the way and time of reporting of the analysed variables in the included studies. Variable effects of the pandemic on different countries, and variable resource availability during the COVID-19 era, might understandably have further influenced the timing of commencement of invasive mechanical ventilation and, therefore, might have affected measured CRS. Nonetheless, the subgroup and meta-regression analyses were able to identify several covariates (eg, PaO2/FiO2 ratio, PEEP, PaCO2, VT, plateau pressure, and driving pressure) that might have influenced CRS, explaining the possible sources of heterogeneity in our analysis. Second, there was wide variation in sample size among studies, ranging from 11 to 2635 patients. This heterogeneity, especially in small studies, could lead to selection bias and could increase the risk of random error.94, 95 However, our sensitivity analysis showed that the study sample size and fixed-effects model did not affect the derived mean CRS. Third, deriving the mean (SD) CRS from studies that reported CRS as median (IQR) using validated methods, despite being a well described practice, might have influenced the distribution pattern of CRS. Fourth, we did not have individual patient data, which would have provided us with a richer analysis, allowing us to investigate patient-level variability and possible factors related to mean CRS. In addition, the absence of individual patient data meant that we could not precisely delineate the association of an individual patient's mechanics with their clinical and functional outcomes. We performed subgroup and meta-regression analyses to look for potential sources of heterogeneity and prognostically relevant study-level covariates. Finally, we did not have large datasets with approximate physiology to comment on phenotypes. Despite these limitations, our analysis was a comprehensive analysis of large sample size and provided an estimate of CRS with a relatively narrow confidence interval.
Conclusions and future directions
In this large systematic review and meta-analysis, the pooled CRS in patients with COVID-19-related ARDS, measured close to the time of the initiation of invasive mechanical ventilation, was normally distributed. We did not observe any distinct CRS-based clinical phenotypes in patients with COVID-19-related ARDS. Furthermore, the association of greater CRS with higher PEEP or VT, or both, indicates that clinicians might have applied either of these ventilator settings on the basis of respiratory mechanics, instead of using a routine low-VT, high-PEEP approach. Similarly, the association of higher plateau pressure with lower CRS also supports the utility of plateau pressure as a guide to PEEP and VT optimisation in patients with COVID-19-related ARDS. Future studies that use patient-level data should explore the complex inter-relationship and trajectory of respiratory system mechanics, gas exchange, and control of breathing to assess the effect of these factors on clinical outcomes in patients with COVID-19-related ARDS, with a view to developing a personalised and safe approach to ventilation management.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Suei Nee Wong from the Medical Resource Team at the National University of Singapore Libraries for her input on the search criteria. We acknowledge research support from Metro North Hospital and Health Service and the Canadian Institutes of Health Research. No funding was received for this study.
Contributors
MPR, ASu, and KS designed the study. MPR, CC, and ASu contributed to the search strategy, screening of articles, data collection, and risk-of-bias assessment. RRL, CA, MPR, CC, ASu, KR, ASS, and KS contributed to the data analysis and interpretation. RRL, CA, MPR, and CC created the tables and figures. MPR drafted the manuscript. ASu, CC, RRL, CA, KR, ASS, and KS contributed to the critical revision of the manuscript for intellectually important content. All authors provided critical conceptual input, interpreted results of the data analysis, and read and approved the final draft of the manuscript. MPR and CC accessed and verified the data. All authors were responsible for the decision to submit the manuscript for publication.
Supplementary Material
References
- 1.The ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Marini JJ. Isn't it time to abandon ARDS? The COVID-19 lesson. Crit Care. 2021;25:326. doi: 10.1186/s13054-021-03748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiumello D, Busana M, Coppola S, et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46:2187–2196. doi: 10.1007/s00134-020-06281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021;47:83–85. doi: 10.1007/s00134-020-06320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 7.Estenssoro E, Loudet CI, Ríos FG, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9:989–998. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos LDJ, Sjoding M, Sinha P, et al. Longitudinal respiratory subphenotypes in patients with COVID-19-related acute respiratory distress syndrome: results from three observational cohorts. Lancet Respir Med. 2021;9:1377–1386. doi: 10.1016/S2213-2600(21)00365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 11.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenbunder B, Ehrmann S, Piagnerelli M, et al. Static compliance of the respiratory system in COVID-19 related ARDS: an international multicenter study. Crit Care. 2021;25:52. doi: 10.1186/s13054-020-03433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball L, Robba C, Herrmann J, et al. Lung distribution of gas and blood volume in critically ill COVID-19 patients: a quantitative dual-energy computed tomography study. Crit Care. 2021;25:214. doi: 10.1186/s13054-021-03610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 15.Counotte MJ, Egli-Gany D, Riesen M, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barre syndrome: from systematic review to living systematic review. F1000Res. 2018;7:196. doi: 10.12688/f1000research.13704.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim ZJ, Subramaniam A, Reddy MP, et al. Case fatality rates for COVID-19 patients requiring invasive mechanical ventilation: a meta-analysis. Am J Respir Crit Care Med. 2020;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314:2373–2383. doi: 10.1001/jama.2015.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook. 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.9rdbelsnu4iy
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Miller JJ. The inverse of the Freeman-Tukey double arcsine transformation. Am Stat. 1978;32:138. [Google Scholar]
- 22.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 23.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 27.Pan C, Lu C, She X, et al. Evaluation of positive end-expiratory pressure strategies in patients with coronavirus disease 2019-induced acute respiratory distress syndrome. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.637747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhary S, Lo KB, Matta A, Azmaiparashvili Z, Benzaquen S, Patarroyo-Aponte G. Ventilator mechanics and outcomes in critically ill patients with COVID-19 infection. Chest. 2020;158:A627. [Google Scholar]
- 29.Lemmers DHL, Abu Hilal M, Bnà C, et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00385-2020. 00385-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahida RY, Chotalia M, Alderman J, et al. Characterisation and outcomes of ARDS secondary to pneumonia in patients with and without SARS-CoV-2: a single-centre experience. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17:1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chew SY, Lee YS, Ghimiray D, Tan CK, Chua GS. Characteristics and outcomes of COVID-19 patients with respiratory failure admitted to a “pandemic ready” intensive care unit—lessons from Singapore. Ann Acad Med Singap. 2020;49:434–448. [PubMed] [Google Scholar]
- 34.Yildirim M, Kesikli B, Talan L, et al. Determination of serum zinc and copper levels and their relation with mortality in critically ill patients. Intensive Care Med Exp. 2020;9 [Google Scholar]
- 35.Ibarra-Estrada M, García-Salas Y, Mireles-Cabodevila E, et al. Use of airway pressure release ventilation in patients with acute respiratory failure due to coronavirus disease 2019: results of a single-center randomized controlled trial. Crit Care Med. 2021;50:586–594. doi: 10.1097/CCM.0000000000005312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baedorf Kassis E, Schaefer MS, Maley JH, et al. Transpulmonary pressure measurements and lung mechanics in patients with early ARDS and SARS-CoV-2. J Crit Care. 2021;63:106–112. doi: 10.1016/j.jcrc.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caravita S, Baratto C, Di Marco F, et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail. 2020;22:2228–2237. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira JC, Ho YL, Besen BAMP, et al. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11:92. doi: 10.1186/s13613-021-00882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemyze M, Courageux N, Maladobry T, et al. Implications of obesity for the management of severe coronavirus disease 2019 pneumonia. Crit Care Med. 2020;48:e761–e767. doi: 10.1097/CCM.0000000000004455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schavemaker R, Schultz MJ, Lagrand WK, van Slobbe-Bijlsma ER, Serpa Neto A, Paulus F. Associations of body mass index with ventilation management and clinical outcomes in invasively ventilated patients with ARDS related to COVID-19-insights from the PRoVENT-COVID study. J Clin Med. 2021;10 doi: 10.3390/jcm10061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain W, Yang H, Shah FA, et al. COVID-19 versus non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc. 2021;18:1202–1210. doi: 10.1513/AnnalsATS.202008-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bavishi AA, Mylvaganam RJ, Agarwal R, Avery RJ, Cuttica MJ. Timing of intubation in coronavirus disease 2019: a study of ventilator mechanics, imaging, findings, and outcomes. Crit Care Explor. 2021;3:e0415. doi: 10.1097/CCE.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beloncle F, Studer A, Seegers V, et al. Longitudinal changes in compliance, oxygenation and ventilatory ratio in COVID-19 versus non-COVID-19 pulmonary acute respiratory distress syndrome. Crit Care. 2021;25:248. doi: 10.1186/s13054-021-03665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf M, Alladina J, Navarrete-Welton A, et al. Obesity and critical illness in COVID-19: respiratory pathophysiology. Obesity (Silver Spring) 2021;29:870–878. doi: 10.1002/oby.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puah SH, Cove ME, Phua J, et al. Association between lung compliance phenotypes and mortality in COVID-19 patients with acute respiratory distress syndrome. Ann Acad Med Singap. 2021;50:686–694. [PubMed] [Google Scholar]
- 47.Carboni Bisso I, Huespe I, Lockhart C, et al. Clinical characteristics of critically ill patients with COVID-19. Medicina (B Aires) 2021;81:527–535. [PubMed] [Google Scholar]
- 48.Torres A, Motos A, Riera J, et al. The evolution of the ventilatory ratio is a prognostic factor in mechanically ventilated COVID-19 ARDS patients. Crit Care. 2021;25:331. doi: 10.1186/s13054-021-03727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diehl JL, Peron N, Chocron R, et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: a hypothesis-generating study. Ann Intensive Care. 2020;10:95. doi: 10.1186/s13613-020-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamberini L, Tonetti T, Spadaro S, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupuis C, Bouadma L, de Montmollin E, et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease-2019 pneumonia. Crit Care Explor. 2021;3:e0329. doi: 10.1097/CCE.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blot M, Jacquier M, Aho Glele LS, et al. CXCL10 could drive longer duration of mechanical ventilation during COVID-19 ARDS. Crit Care. 2020;24:632. doi: 10.1186/s13054-020-03328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dreher M, Kersten A, Bickenbach J, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zorbas JS, Ho KM, Litton E, Wibrow B, Fysh E, Anstey MH. Airway pressure release ventilation in mechanically ventilated patients with COVID-19: a multicenter observational study. Acute Crit Care. 2021;36:143–150. doi: 10.4266/acc.2021.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibot S, Conrad M, Courte G, Cravoisy A. Positive end-expiratory pressure setting in COVID-19-related acute respiratory distress syndrome: comparison between electrical impedance tomography, PEEP/FiO2 tables, and transpulmonary pressure. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.720920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abu Sayf A, Ouellette D, Fadel R. Mechanical ventilation and COVID-19: a case-control analysis of clinical characteristics, lung mechanics, and mortality. Chest. 2021;3:e0377. doi: 10.1097/CCE.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langer T, Brioni M, Guzzardella A, et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021;25:128. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bastos GAN, Azambuja AZ, Polanczyk CA, et al. Clinical characteristics and predictors of mechanical ventilation in patients with COVID-19 hospitalized in southern Brazil. Rev Bras Ter Intensiva. 2020;32:487–492. doi: 10.5935/0103-507X.20200082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenka J, Chhabria MS, Sharma N, et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 in a tertiary community hospital in upstate New York. J Community Hosp Intern Med Perspect. 2020;10:491–500. doi: 10.1080/20009666.2020.1811070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fogagnolo A, Grasso S, Dres M, et al. Focus on renal blood flow in mechanically ventilated patients with SARS-CoV-2: a prospective pilot study. J Clin Monit Comput. 2021;36:1–7. doi: 10.1007/s10877-020-00633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boscolo A, Sella N, Lorenzoni G, et al. Static compliance and driving pressure are associated with ICU mortality in intubated COVID-19 ARDS. Crit Care. 2021;25:263. doi: 10.1186/s13054-021-03667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gattinoni L, Quintel M, Marini JJ. “Less is more” in mechanical ventilation. Intensive Care Med. 2020;46:780–782. doi: 10.1007/s00134-020-05981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagate F, Tuffet S, Masi P, et al. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann Intensive Care. 2020;10:151. doi: 10.1186/s13613-020-00769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diaz RA, Graf J, Zambrano JM, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204:34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fanelli V, Giani M, Grasselli G, et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: a multicenter retrospective cohort study. Crit Care. 2022;26:34. doi: 10.1186/s13054-022-03906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jozwiak M, Chiche JD, Charpentier J, et al. Use of venovenous extracorporeal membrane oxygenation in critically-ill patients with COVID-19. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.614569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Fink JB, Augustynovich AE, Mirza S, Kallet RH, Dhand R. Effects of inhaled epoprostenol and prone positioning in intubated coronavirus disease 2019 patients with refractory hypoxemia. Crit Care Explor. 2020;2:e0307. doi: 10.1097/CCE.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell J, William Pike C, Kreisel C, Sonti R, Cobb N. Predicting impact of prone position on oxygenation in mechanically ventilated patients with COVID-19. J Intensive Care Med. 2022;37:883–889. doi: 10.1177/08850666221081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giraud R, Legouis D, Assouline B, et al. Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol Rep. 2021;9 doi: 10.14814/phy2.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laghlam D, Rahoual G, Malvy J, Estagnasié P, Brusset A, Squara P. Use of almitrine and inhaled nitric oxide in ARDS due to COVID-19. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.655763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang CN, Zotzmann V, Schmid B, et al. Intensive care resources and 60-day survival of critically-ill COVID-19 patients. Cureus. 2021;13 doi: 10.7759/cureus.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mittermaier M, Pickerodt P, Kurth F, et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J, Lee HY, Lee J, Lee SM. Effect of prone positioning on oxygenation and static respiratory system compliance in COVID-19 ARDS vs. non-COVID ARDS. Respir Res. 2021;22:220. doi: 10.1186/s12931-021-01819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roldán R, Rodriguez S, Barriga F, et al. Sequential lateral positioning as a new lung recruitment maneuver: an exploratory study in early mechanically ventilated Covid-19 ARDS patients. Ann Intensive Care. 2022;12:13. doi: 10.1186/s13613-022-00988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss TT, Cerda F, Scott JB, et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth. 2021;126:48–55. doi: 10.1016/j.bja.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 77.Cavalcanti AB, Suzumura É A, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low peep on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failurea randomized controlled trial. JAMA. 2004;292:2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 79.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 80.Hodgson CL, Cooper DJ, Arabi Y, et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP). A phase II, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200:1363–1372. doi: 10.1164/rccm.201901-0109OC. [DOI] [PubMed] [Google Scholar]
- 81.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 82.Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 83.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 84.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 85.Pelosi P, Ball L, Barbas CSV, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25:250. doi: 10.1186/s13054-021-03686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan C, Chen L, Lu C, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuo M-Z, Huang Y-G, Ma W-H, et al. Expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J. 2020;35:105–109. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown CA, 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1:80–84. doi: 10.1002/emp2.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brewster DJ, Chrimes N, Do TB, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212:472–481. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das A, Saffaran S, Chikhani M, et al. In silico modeling of Coronavirus disease 2019 acute respiratory distress syndrome: pathophysiologic insights and potential management implications. Crit Care Explor. 2020;2:e0202. doi: 10.1097/CCE.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126:619–625. doi: 10.1097/PRS.0b013e3181de24bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tripepi G, Jager KJ, Dekker FW, Zoccali C. selection bias and information bias in clinical research. Nephron Clin Pract. 2010;115:c94–c99. doi: 10.1159/000312871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.