Abstract
Group A streptococcal (GAS) disease shows increasing incidence worldwide. We characterised children admitted with GAS infection to European hospitals and studied risk factors for severity and disability. This is a prospective, multicentre, cohort study (embedded in EUCLIDS and the Swiss Pediatric Sepsis Study) including 320 children, aged 1 month to 18 years, admitted with GAS infection to 41 hospitals in 6 European countries from 2012 to 2016. Demographic, clinical, microbiological and outcome data were collected. A total of 195 (61%) patients had sepsis. Two hundred thirty-six (74%) patients had GAS detected from a normally sterile site. The most common infection sites were the lower respiratory tract (LRTI) (22%), skin and soft tissue (SSTI) (23%) and bone and joint (19%). Compared to patients not admitted to PICU, patients admitted to PICU more commonly had LRTI (39 vs 8%), infection without a focus (22 vs 8%) and intracranial infection (9 vs 3%); less commonly had SSTI and bone and joint infections (p < 0.001); and were younger (median 40 (IQR 21–83) vs 56 (IQR 36–85) months, p = 0.01). Six PICU patients (2%) died. Sequelae at discharge from hospital were largely limited to patients admitted to PICU (29 vs 3%, p < 0.001; 12% overall) and included neurodisability, amputation, skin grafts, hearing loss and need for surgery. More patients were recruited in winter and spring (p < 0.001).
Conclusion: In an era of observed marked reduction in vaccine-preventable infections, GAS infection requiring hospital admission is still associated with significant severe disease in younger children, and short- and long-term morbidity. Further advances are required in the prevention and early recognition of GAS disease.
|
What is Known: • Despite temporal and geographical variability, there is an increase of incidence of infection with group A streptococci. However, data on the epidemiology of group A streptococcal infections in European children is limited. | |
|
What is New: • In a large, prospective cohort of children with community-acquired bacterial infection requiring hospitalisation in Europe, GAS was the most frequent pathogen, with 12% disability at discharge, and 2% mortality in patients with GAS infection. • In children with GAS sepsis, IVIG was used in only 4.6% of patients and clindamycin in 29% of patients. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04718-y.
Keywords: Streptococcus pyogenes, Child, Hospital, Outcome
Introduction
Group A streptococcal (GAS) infection is characterised by a wide variety of phenotypes, from upper respiratory tract and focal skin and soft tissue infections to necrotising fasciitis and streptococcal toxic shock syndrome (STSS), as well as peri-infectious phenomena such as rheumatic fever and post-streptococcal glomerulonephritis [1]. Extensive strain diversity—there are more than 200 emm-types—is presumed to contribute to the diversity of observed clinical syndromes [2–4].
In Western Europe, GAS was a leading cause of child death until the mid-twentieth century [5]. Although the incidence fell rapidly during the mid-twentieth century, it began rising again in the 1980s, and was estimated to be 3–4/100,000 in Northern Europe by the early 2000s [6]. Surges continue in Europe [7, 8], and further afield, for instance, South Korea [9, 10] and Utah, where a 2010 study found a rate of up to 14.1/100,000 cases in children [6, 11]. Severity of illness also seems to be increasing [12–14]. Mean case fatality rates in affluent countries remain relatively low at 8–16% [5]. However, the mortality of iGAS can rise rapidly up to 60–70% with any delay in antibiotics and interventions against toxins such as clindamycin and intravenous immunoglobulin [15–17].
Epidemiological studies capturing the full spectrum of GAS disease are challenging. Many cases never present to health care providers, or are treated in the community without microbiological diagnostics (tonsillitis, cellulitis). Studies have shown that microbiologically proven paediatric iGAS occurs most commonly with bacteraemia, soft tissue infections, STSS or necrotizing fasciitis [18], and that risk factors include other children in the household, preceding coryzal illness and varicella infection [19]. However, it has been difficult to study a wider population of children with severe bacterial infections where GAS is the likely cause but not isolated from a sterile site.
In this large study, we aimed to characterise children admitted with GAS infection to European hospitals and study risk factors for severity and disability. We gathered information about possible risk factors, clinical presentation, progress and outcomes for children presenting with suspected severe bacterial infection for whom the cause was proven or probable GAS.
Methods
Consortium and study sites
This study used data from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS) and the Swiss Paediatric Sepsis Study (SPSS) [20, 21]. EUCLIDS is a prospective, multicentre, cohort study aimed to identify genes and pathways determining susceptibility and severity of life-threatening bacterial infections. The network included 185 predominantly academic hospitals from 8 European countries. Details of EUCLIDS inclusion and exclusion criteria, as well as clinical definitions, have been published elsewhere [20]. In short, for this sub-cohort, children with suspected severe bacterial infection were recruited prospectively from 1 July 2012 to 31 December 2016, as early as possible in admission and before culture results became available. The SPSS is a prospective, national, observational, multicentre, cohort study investigating blood culture–proven sepsis in children under 17 years of age from all 10 major children’s hospitals in Switzerland from 1 January 2012 to 31 December 2015 [21]. In brief, children with blood culture–proven sepsis meeting the criteria for systemic inflammatory response syndrome (SIRS), as defined by the 2005 paediatric consensus definition [22] at the time of blood culture sampling, were included. Details of the study design and the study protocol have been published elsewhere [21].
Inclusion criteria
The combined EUCLIDS-SPSS database was used to identify all children for whom GAS was the most likely cause of their illness. Children were included if they met any of the following criteria:
Proven GAS: GAS grown from a normally sterile site or positive by pathogen-specific PCR (blood, CSF, joint fluid, pleural fluid, peritoneal fluid, tissue, urine, intra-operative pus or internal swab). GAS detection by PCR was performed according to local accredited hospital or specialised molecular microbiology laboratories.
- Probable GAS: Clinical symptoms consistent with GAS disease, NO other causative organism identified AND at least one of the following:
- GAS grown from a potential carriage site (throat, naso-pharynx, eye surface, ear, endo-tracheal tube, broncho-alveolar lavage, skin)
- Antistreptolysin O titre (ASOT) ≥ 300 IU/L [23]
- Local rapid streptococcal antigen test (RST) from pharyngeal sample positive.
Cases were excluded if they had been enrolled retrospectively to avoid selection bias, or were from the non-European EUCLIDS sites. In addition, patients in whom other potential causative pathogens were detected from sterile or non-sterile site cultures were excluded.
Sepsis, severe sepsis and septic shock were defined according to Goldstein criteria, and focal infection was used for patients with an organ system identifiable febrile illness not matching sepsis according to Goldstein criteria [22].
Clinical data collection
Data on demographics, clinical presentation, underlying disease, exposure to varicella-zoster virus (VZV), smoking, recent surgery, illness severity, management, microbiological results and outcome were collected prospectively. Exposure to VZV or smoking was not available for the Swiss patients. Underlying diseases at admission to hospital were classified using the Paediatric Complex Chronic Conditions classification system [24]. Illness severity in PICU patients was measured by the Paediatric Risk of Mortality score (EUCLIDS patients only) [25] and the Paediatric Index of Mortality-2 (PIM2) [26]. Lactate values were obtained on PICU admission only, concomitant with PIM2 data collection. Outcome included mortality, disability, PICU-free days and length of hospital stay. Disability was defined as a Pediatric Overall Performance Category (POPC) score greater than 1 [27], need for skin graft, amputation, hearing loss, neurodisability or need for surgery. The POPC score was determined either by direct observation or by chart review and ranges from 1 to 6, varying from (1) good overall performance to (6) brain death [27] (Supplementary Table 1 for description of categories). PICU-free days (days alive and free from the need for intensive care) were censored at day 28. In patients who died, PICU-free days were considered zero.
Patients were grouped as no focus (primary bloodstream infection and sepsis without a known source) versus patients with a clinical focus of infection. All data were collected in web-based case report forms. Monthly telephone conferences, biannual meetings, clinical protocols including case definitions, data audits and monitoring ensured uniform procedures amongst study sites.
Statistical analysis
Categorical variables were presented as counts (percentages). The chi-square or Fisher’s exact test was used to compare frequency distributions between two categorical variables. Continuous variables were presented as median (interquartile range (IQR)) for non-parametric data. ANOVA, Kruskal–Wallis, Student’s t, or Mann–Whitney U tests were used to test differences between groups, as appropriate. Statistical analysis was performed with IBM® SPSS version 24 (Armonk, USA). A p value of less than 0.05 was considered statistically significant.
Results
During the study period, 346/4025 (9%) of the children prospectively enrolled at any of the participating hospitals had proven/probable GAS disease. Other commonly identified pathogens were Neisseria meningitidis, Staphylococcus aureus and Streptococcus pneumoniae in about 8% of patients each [20]. Twenty-two GAS patients were excluded because they had been recruited retrospectively as part of the genetics study, and 4 because they were from non-European EUCLIDS sites (Fig. 1), leaving 320 patients for analyses.
Fig. 1.
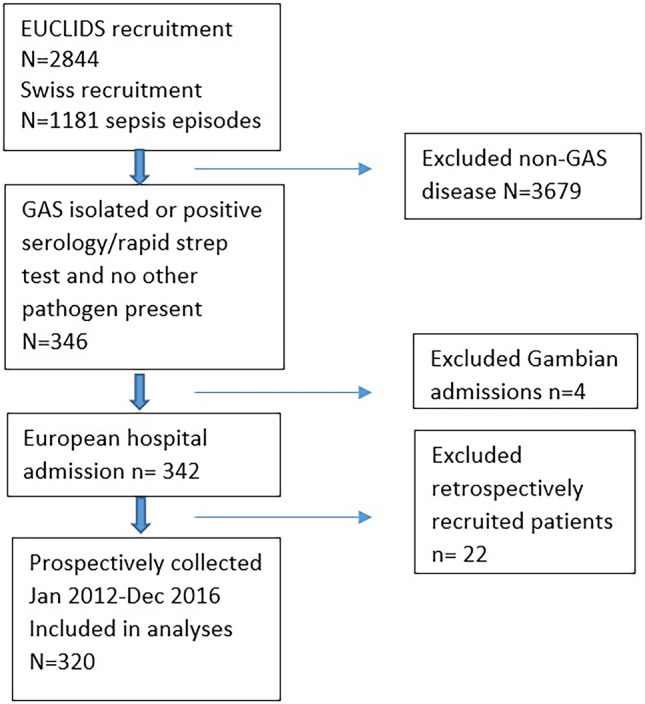
Study design. CONSORT flow chart including and excluding patients
Demographics and clinical spectrum
A total of 161 (50%) were male, with median age 47 (IQR 27–84) months. 47 (15%) presented without a clinical focus of infection. In children with a focus of infection, bone/joint, soft tissue or respiratory tract infection was the predominant presentation. One or more underlying conditions were present in 117 (37%) patients of which the most common were recent VZV (n = 21, 6.6%), congenital or genetic conditions (n = 18, 5.6%) and eczema (n = 11, 3.4%). Further details are presented in Table 1.
Table 1.
Characteristics of children admitted with GAS infection
| All patients (n = 320) | No PICU admission (n = 172) | PICU admission (n = 148) | p | |
|---|---|---|---|---|
| Sex (male, n, %) | 161 (50%) | 86 (50%) | 75 (51%) | 0.9 |
| Age (months) (IQR) | 47 [27–84] | 57 [36–85] | 40 [21–83] | 0.01 |
| Time interval onset symptoms to hospital admission (n = 251, days) | 3.0 [1.8–6.0] | 3.5 [1.8–6] | 3.0 [1.8–5.7] | 0.67 |
| Immunizations up-to-date (n = 222) | 211 (95%) | 103/110 (94%) | 108/112 (96%) | 0.06 |
| Number of underlying conditions | ||||
| None | 203 (63%) | 107 (62%) | 96 (65%) | 0.11 |
| 1 | 77 (24%) | 48 (28%) | 29 (20%) | |
| ≥ 2 | 40 (13%) | 17 (10%) | 23 (16%) | |
| Underlying conditions | ||||
| Congenital or genetic defect | 18 (5.6%) | 4 (2.3%) | 14 (9.5%) | 0.006 |
| Prematurity | 10 (3.1%) | 5 (2.9%) | 5 (3.4%) | 0.11 |
| Immunodeficiency | 6 (1.9%) | 1 (0.6%) | 5 (3.4%) | 0.19 |
| Cardiac condition | 5 (1.6%) | 2 (1.2%) | 3 (2.0%) | 0.47 |
| Epilepsy | 5 (1.6%) | 0 | 5 (3.4%) | 0.01 |
| Respiratory | 9 (2.8%) | 6 (3.5%) | 3 (2.0%) | 0.12 |
| Haematological | 1 (0.3%) | 0 | 1 (0.7%) | 0.28 |
| Oncological | 1 (0.3%) | 1 (0.6%) | 0 | 0.35 |
| Inflammatory | 2 (0.6%) | 1 (0.6%) | 1 (0.7%) | 0.36 |
| Liver disease | 1 (0.3%) | 0 | 1 (0.7%) | 0.28 |
| Renal disease | 0 | 0 | 0 | NA |
| Metabolic disease | 2 (0.6%) | 1 (0.6%) | 1 (0.7%) | 0.36 |
| Recent surgery | 3 (0.9%) | 1 (0.6%) | 2 (1.4%) | 0.48 |
| Eczema/dermatitis | 11 (3.4%) | 6 (3.5%) | 5 (3.4%) | 0.96 |
| Recent chickenpox | 21 (6.6%) | 10 (5.8%) | 11 (7.4%) | 0.09 |
| Primary infection site | < 0.001 | |||
| None | 47 (15%) | 14 (8%) | 33 (22%) | |
| Lower respiratory tract | 71 (22%) | 14 (8%) | 57 (39%)* | |
| Skin/Soft tissue | 73 (23%) | 50 (29%) | 23 (16%) | |
| Bone/joint | 60 (19%) | 52 (30%) | 8 (5%) | |
| Upper respiratory tract | 46 (14%) | 34 (20%) | 12 (8%) | |
| Intracranial | 18 (6%) | 5 (3%) | 13 (9%) | |
| Peritoneal | 3 (1%) | 1 (1%) | 2 (1%) | |
| Renal | 2 (1%) | 2 (1%) | 0 | |
| Microbiology | 0.11 | |||
| Sterile site positive culture or PCRa | 236 (74%) | 120 (70%) | 116 (78%) | |
| Blood | 145 (61%) | 80 (67%) | 65 (56%) | |
| CSF | 5 (2%) | 2 (2%) | 3 (3%) | |
| Joint fluid | 18 (8%) | 17 (14%) | 1 (1%) | |
| Pleural fluid | 37 (16%) | 3 (3%) | 34 (30%) | |
| Peritoneal fluid | 1 (0.4%) | 0 | 1 (1%) | |
| Tissue | 4 (2%) | 1 (1%) | 3 (3%) | |
| Urine | 1 (0.4%) | 1 (1%) | 0 | |
| Abscess/pus | 26 (11%) | 15 (13%) | 11 (10%) | |
| Intra-operative swab | 13 (6%) | 6 (5%) | 7 (6%) | |
| GAS clinical syndrome, AND | 84 (26%) | 52 (30%) | 32 (22%) | |
| Potential carriage site positive | 68 (21%) | 40 (23%) | 28 (19%) | |
| Elevated ASOT | 7 (2%) | 4 (2%) | 3 (2%) | |
| Pharyngeal RST positive | 9 (3%) | 8 (5%) | 1 (1%) | |
| Inflammatory markers | ||||
| Max CRP (n = 256, mg/L) | 185 (80–286) | 119 (62–228) | 256 (149–328) | < 0.001 |
| Hospital length of stay (n = 318, days) | 11 [6-18] | 8 [4-13] | 17 [11-26] | < 0.001 |
| PICU-free days at day 28 (n = 318) | 28 (23–28) | 28 | 23 [18-25] | < 0.001 |
ASOT antistreptolysin O titre, CRP C-reactive protein, CSF cerebrospinal fluid, GAS group A streptococcal, PCR polymerase chain reaction, PICU paediatric intensive care unit, RST rapid streptococcal antigen test
*34/57 ((69%) patients in PICU and 4/14 (29%) patients not requiring PICU for lower respiratory tract infection had pleural empyema
aBreakdown exceeds 100% as GAS could have been identified from multiple sources per patient
Characteristics of PICU cases and risk factors for severe disease
A total of 148 (46%) children were admitted to PICU. One hundred five (71%) patients in PICU required invasive ventilation, with a median (IQR) of 5 (3–8) days (n = 92). Eighty-eight (59%) PICU patients required inotropes, with a median (IQR) of 3 (2–4.3) days (n = 74). None of the patients required extracorporeal membrane oxygenation. The median (IQR) PRISM score was 14 (8–20, n = 72) and PIM2 6.1% (1.4–11.5, n = 88) predicted death rate. Median lactate on PICU admission was 1.5 mmol/L (IQR 0.9–2.4, n = 90). The number of PICU-free days at day 28 in the PICU group was 23 days (IQR 18–25) (Table 1).
The proportion of patients admitted to PICU differed between countries (p = 0.026; Supplementary Fig. 1). Patients admitted to PICU, compared to those not admitted to PICU, were younger (40 [21–83] vs 57 [36–85] months, respectively, p = 0.01), had more frequent epilepsy (p = 0.01) and congenital and genetic defects (p = 0.005) as underlying conditions, and more often had LRTI, intracranial infection and infection without a focus, whereas SSTI and bone and joint infections were more common in patients not requiring PICU (p < 0.001) (Table 1). Also, the maximum CRP in PICU patients was significantly higher and hospital admission was associated with a two-fold increase in duration. Time from onset of symptoms to admission to hospital did not differ between PICU and non-PICU patients. Eczema (n = 11) and recent VZV infection (n = 21) were not associated with PICU admission. Also, there was no difference in exposure to smoking between PICU and non-PICU patients (20 (12%) and 29 (20%), respectively, p = 0.13).
Forty-two children (13%) had a severe infection (defined as a clinical syndrome suspected for severe invasive bacterial disease such as septicaemia, toxic shock syndrome, pneumonia, empyema, meningitis, osteomyelitis and septic arthritis) in the previous medical history, but this was not related to increased risk of PICU admission. In fact, those with a severe infection in the past were less often admitted to PICU (7.4 vs 18%, p = 0.02). This could not be explained by a difference in onset of symptoms until admission (p = 1.0).
Microbiology
The diagnosis of GAS infection was based on a positive culture and/or PCR from a normally sterile site in 236 (74%) patients, of which 145 (61%) in blood and 37 (16%) in pleural fluid were the most common sites (Table 1). For the remaining patients, GAS clinical syndrome was determined by the local team based on positive culture and/or PCR from a potential carriage site (n = 68, 21%), elevated ASOT (n = 7, 2%) and a positive pharyngeal RST (n = 9, 3%). Streptococcal titres were measured with a median of 16 days (IQR 10–28) after onset of symptoms and 8 days (IQR 5–12) after admission to the hospital. There was no difference in the means of GAS identification between non-PICU and PICU patients (p = 0.11).
Seasonality
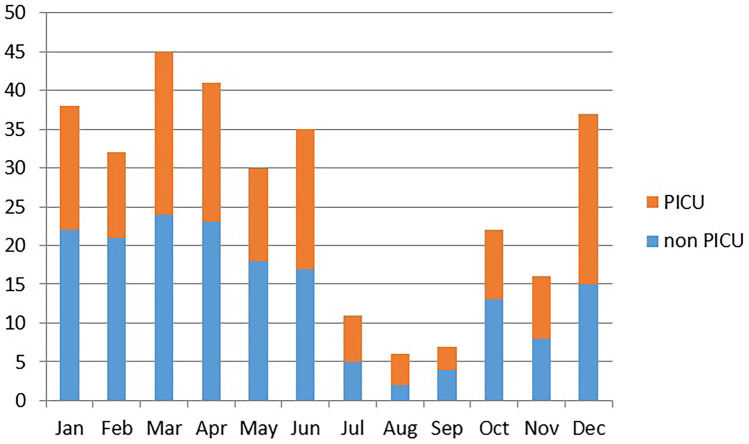
A seasonal pattern was noted with more GAS-infected patients recruited in the winter and spring months (n = 223 (70%), December–May) compared to the rest of the year (n = 97 (30%), June–November, p < 0.001; Fig. 2).
Fig. 2.
Numbers of patients with GAS disease per month (2012–2016 combined). Accumulated numbers of patients with GAS disease over the 5-year study period per month of presentation. Total n = 320
Sepsis versus focal infection
A total of 195 (61%) patients had sepsis, of which 47 (24%) without a focus, and in 125 (39%) patients disease was limited to a focal infection. Patients with sepsis tended to be younger (median 44 months) compared to patients with focal infection (median 57 months, p = 0.07). Sex distribution was not significantly different for patients with sepsis or focal infection. Patients with sepsis relatively more often had LRTI (25.1 vs 17.6%) as focus of infection compared to the other foci (p < 0.001). Sepsis was associated with a higher CRP than focal infection (median 228 (IQR 114–303) vs 111 (IQR 53–211); p < 0.001). The proportion of patients with sepsis was higher in those requiring PICU (n = 116, 78%) than in those not requiring PICU admission (n = 79, 46%, p < 0.001) (Table 2). All but one (73/74 (99%)) patient with septic shock or toxic shock were admitted to PICU. Patients with sepsis more often had GAS identified from a normally sterile site than those with focal infection (n = 168, 86% vs n = 68, 54%; p < 0.001).
Table 2.
Sepsis in iGAS infection
| Sepsis severity | No PICU, N = 172 | PICU, N = 148 | p |
|---|---|---|---|
| None | 93 (54%) | 32 (22%) | p < 0.001 |
| Sepsis | 74 (43%) | 34 (23%) | |
| Severe sepsis | 4 (2%) | 9 (6%) | |
| Septic shock | 1 (1%) | 58 (40%) | |
| Toxic shock syndrome | 15 (10%) |
iGAS invasive group A streptococcal, PICU paediatric intensive care unit
With regard to adjunctive treatment of patients with sepsis, intravenous immunoglobulin (IVIG) was administered in 9/195 (4.6%) patients and administration did not differ between countries (7/86 (8.1%) patients with sepsis from the UK and 2/39 (5.1%) patients with sepsis from the Netherlands; p = 0.32). However, clindamycin was prescribed in 57/195 (29%) sepsis patients and prescription rate was different between countries (39/86 (45%) from the UK, 8/39 (21%) from the Netherlands, 4/7 (57%) from Spain, 4/56 (7%) from Switzerland, 1/3 (33%) from Austria and 1/4 (25%) from Germany; p < 0.001).
Outcome
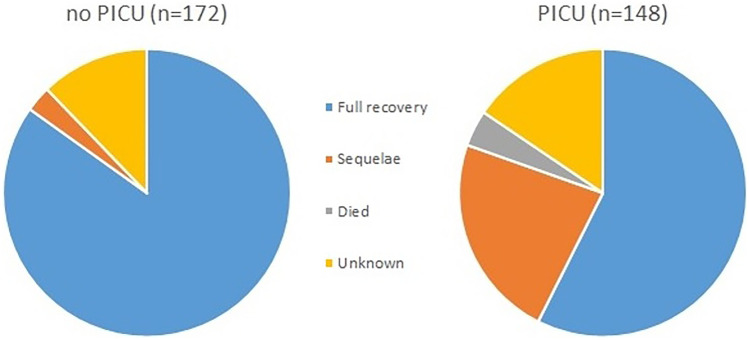
Six children died, reflecting a crude mortality of 2% (Table 3). Overall, 231 (72%) children survived without disability and 39 (12%) with disability, including 23/168 (14%) children who did not have an underlying condition at hospital admission, i.e. previously healthy children. For the remaining 44 (14%) patients, information on disability was not classified. The majority of patients where disability was not classified were transferred back to their local hospital for ongoing care, from which point no reliable judgement could be made regarding full recovery. The proportion of survivors without disability was lower for those admitted to PICU (57%, p < 0.001). Age was not associated with outcome (disability vs no disability). Skin graft and need for surgery were seen in patients with sepsis and focal infection, but other complications, such as death, amputation and neurodisability, were observed at discharge in patients with sepsis only, and limited to those admitted to PICU (Fig. 3).
Table 3.
Outcome of patients with GAS disease
| All patients (n = 320) | No PICU admission (n = 172) | PICU admission (n = 148) | p | |
|---|---|---|---|---|
| Died | 6 (2%) | 0 | 6 (4%) | < 0.001 |
| Survived with disability | 39 (12%) | 5 (3%) | 34 (23%) | |
| Mild overall disability | 20 (6%) | 2 (1%) | 18 (12%) | |
| Moderate overall disability | 5 (2%) | 0 | 5 (3%) | |
| Severe overall disability | 3 (1%) | 0 | 3 (2%) | |
| Amputation | 4 (1%) | 0 | 4 (3%) | |
| Skin graft | 9 (3%) | 0 | 9 (6%) | |
| Amputation and skin graft | 2 (1%) | 0 | 2 (1%) | |
| Need for surgery | 10 (3%) | 4 (2%) | 6 (4%) | |
| Neurodisability | 2 (1%) | 0 | 2 (1%) | |
| Survived without disability | 231 (72%) | 146 (85%) | 85 (57%) | |
| Unknown | 44 (14%) | 21 (12%) | 23 (16%) |
GAS group A streptococcal, PICU paediatric intensive care unit
Fig. 3.
Outcome of GAS disease in PICU and ward patients. Relative outcomes of patients with GAS per admission category (PICU or non-PICU)
Discussion
In this European cohort, GAS is a significant cause of probable or confirmed severe bacterial infection, with a significant burden of mortality and persistent morbidity. Risk factors for PICU admission were lower age, LRTI, intracranial infection and infection without a focus. The need for PICU admission did not seem to be related to delayed presentation. The proportion of patients admitted to PICU differed between countries. A survey amongst participating centres showed that criteria for PICU admission and availability of resources differed between centres. In some centres, non-invasive ventilation (e.g. CPAP) was only supported in PICU, whilst in others it could be offered on a paediatric ward or high dependency unit (unpublished). In addition, except for Switzerland, the participating centres were not representing the entire population, and selection bias may contribute to differences.
Data from this study originates from two separate cohorts: EUCLIDS and SPSS. In EUCLIDS, recruitment of patients took place on admission and largely before the causative pathogen was known. Only patients with suspected severe bacterial infection admitted to the hospital were recruited, which means those with milder infections not requiring admission were not included. Also, due to the nature of the study, it is not clear exactly what proportion of overall eligible children was recruited for the study. In SPSS, only children with blood culture–proven sepsis were recruited, meaning that children with GAS disease and negative blood culture were omitted. Therefore, data from our study could be an underestimation of the true impact of GAS disease in Europe and should be interpreted with caution.
The overall mortality (2%) was comparable to other studies on bacteraemic children including all patients in hospital [21, 28], but was lower than most previously reported mortality rates in patients admitted to PICU [5]. When assessing severity, considering mortality alone risks underestimating the true impact of iGAS. Whilst for most patients full recovery at hospital discharge was noted, significant sequelae were identified in 12% overall, increasing to 23% for those admitted to PICU. As patients were not followed after hospital discharge, no information is available on potential resolution of some of the sequelae and longer term morbidity and functional outcome related to iGAS disease. In addition, no data on baseline POPC scores were available, which means pre-existing comorbidity could not be taken into account assessing the difference in functioning pre- and post-infection.
Overall, 74% of patients had GAS identified from a sterile site. For 26% of patients, a positive potential carriage site, a rapid antigen test or raised ASOT was the only method of microbiological GAS confirmation. We acknowledge these diagnostic methods as a limitation of our study. However, whilst analysis of proven GAS infections might be the gold standard, it is recognised that GAS cannot be cultured in all patients. By including probable cases, we better reflected the actual demographics of GAS disease. We reduced the risk of including non-GAS cases by excluding patients in whom other potential causative pathogens were detected from sterile or non-sterile site cultures. Only patients in whom diagnostics exclusively identified GAS were included.
As not all GAS isolates were kept, we were unable to obtain their M types to assess potential association with phenotype and severity. The variability in these proteins, associated with diversity in disease phenotypes, makes development of a generic GAS vaccine challenging [2, 29, 30]. Future research focussing on bacterial phenotypes related to LRTI, intracranial infection and sepsis might help prioritise vaccine development, in order to prevent most severe disease.
Interestingly, only a few patients with recent VZV infection were identified. Whilst it is well-known that VZV increases vulnerability to iGAS infection [16], our study confirms that GAS infection predominantly occurs in individuals with no obvious risk factors. It has to be acknowledged that for patients recruited in Switzerland, unfortunately, VZV and other exposures were not recorded.
Despite the fact that this study was not purposely designed to study epidemiology, a seasonal variation was noted. Patients were recruited to the study early during the admission when the cause of infection was not yet known, limiting recruitment bias. Most patients with GAS infection were recruited in winter and spring, with a clear reduction in the summer months, whilst recruitment took place year-round. Seasonal increase has also been noted in Australian children, where iGAS coincides with the influenza season [31]. An increased incidence over the winter months has also been reported in Hong Kong, South Korea, the USA, Iceland and other European countries [6, 10, 32–36].
Conclusion
Our study showed that LRTI, intracranial infection and infection without a focus more commonly resulted in severe GAS disease requiring admission to PICU. PICU admission for GAS infection was associated with worse outcomes with regard to mortality and disability. With increasing incidence of iGAS disease worldwide, and increased morbidity and mortality in those requiring PICU, future research should focus on prevention of iGAS infection. Vaccination development should target iGAS serotypes associated with severe disease requiring PICU admission.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
A list of authors and their affiliations appears in the Supplementary Information.
Abbreviations
- ANOVA
Analysis of variance
- ASOT
Antistreptolysin O titre
- CPAP
Continuous positive airway pressure
- CRP
C-reactive protein
- CSF
Cerebrospinal fluid
- EUCLIDS
European Childhood Life-threatening Infectious Disease Study
- GAS
Group A Streptococcus
- ICD-10
International Classification of Diseases 2010
- iGAS
Invasive group A Streptococcus
- IQR
Interquartile range
- IVIG
Intravenous immunoglobulin
- LRTI
Lower respiratory tract infection
- PCR
Polymerase chain reaction
- PICU
Paediatric intensive care unit
- PIM2
Paediatric Index of Mortality
- POPC
Pediatric Overall Performance Category
- PRISM
Paediatric Risk of Mortality score
- RST
Rapid streptococcal antigen test
- SIRS
Systemic inflammatory response syndrome
- SPSS
Swiss Pediatric Sepsis Study
- STSS
Streptococcal toxic shock syndrome
- VZV
Varicella-zoster virus
Authors’ contributions
FMT, JH, EDC, ME, RdG, WZ and ML designed the study and obtained funding. JH, NPB, PA, LJS, GJD, STA, ME, LJS and EDC assisted with recruitment of patients, data collection and sample collection. ME and NPB did the statistical analyses. LA and RG provided database and informatics support. LA, ME, NPB, LJS and GJD wrote the first draft of the manuscript. FMT, JAH, RdG, WZ, EDC, STA, FMT, PA, RG, JH and ML contributed to writing of the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the European Seventh Framework Programme for Research and Technological Development (FP7) under EUCLIDS Grant Agreement no. 279185. The Swiss Pediatric Sepsis Study was funded by grants from the Swiss National Science Foundation (342730_153158/1), the Swiss Society of Intensive Care, the Bangerter Foundation, the Vinetum and Borer Foundation and the Foundation for the Health of Children and Adolescents.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by at least one ethical review board in every country (Coordinating Center Research Ethics Committee reference: 11/LO/1982). Written informed consent was obtained from parents or legal guardians. In the Swiss study, consent was obtained for collection of research blood, but waiver of consent for collection of anonymized epidemiological data was approved.
Competing interests
The authors declare no competing interests.
Disclaimer
The funders were not involved in the design of the study, collection, analysis, interpretation of data or in writing the manuscript.
Footnotes
The original online version of this article was revised due to missing collaborators.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Navin P. Boeddha and Lucy Atkins contributed equally.
Change history
1/23/2023
A Correction to this paper has been published: 10.1007/s00431-022-04787-z
Contributor Information
Marieke Emonts, Email: marieke.emonts@ncl.ac.uk.
EUCLIDS consortium:
Michael Levin, Lachlan Coin, Stuart Gormley, Shea Hamilton, Jethro Herberg, Bernardo Hourmat, Clive Hoggart, Myrsini Kaforou, Vanessa Sancho-Shimizu, Victoria Wright, Amina Abdulla, Paul Agapow, Maeve Bartlett, Evangelos Bellos, Hariklia Eleftherohorinou, Rachel Galassini, David Inwald, Meg Mashbat, Stefanie Menikou, Sobia Mustafa, Simon Nadel, Rahmeen Rahman, Clare Thakker, S Bokhandi, Sue Power, Heather Barham, N Pathan, Jenna Ridout, Deborah White, Sarah Thurston, S Faust, S Patel, Jenni McCorkell, P Davies, Lindsey Crate, Helen Navarra, Stephanie Carter, R Ramaiah, Rekha Patel, Catherine Tuffrey, Andrew Gribbin, Sharon McCready, Mark Peters, Katie Hardy, Fran Standing, Lauran O’Neill, Eugenia Abelake, Akash Deep, Eniola Nsirim, A Pollard, Louise Willis, Zoe Young, C Royad, Sonia White, PM Fortune, Phil Hudnott, Federico Martinón-Torres, Antonio Salas, Fernando Álvez González, Ruth Barral-Arca, Miriam Cebey-López, María José Curras-Tuala, Natalia García, Luisa García Vicente, Alberto Gómez-Carballa, Jose Gómez Rial, Andrea Grela Beiroa, Antonio Justicia Grande, Pilar Leboráns Iglesias, Alba Elena Martínez Santos, Federico Martinón-Torres, Nazareth Martinón-Torres, José María Martinón Sánchez, Beatriz Morillo Gutiérrez, Belén Mosquera Pérez, Pablo Obando Pacheco, Jacobo Pardo-Seco, Sara Pischedda, Irene Rivero Calle, Carmen Rodríguez-Tenreiro, Lorenzo Redondo-Collazo, Antonio Salas Ellacuriaga, Sonia Serén Fernández, María del Sol Porto Silva, Ana Vega, Lucía Vilanova Trillo, Susana Beatriz Reyes, María Cruz León León, Álvaro Navarro Mingorance, Xavier Gabaldó Barrios, Eider Oñate Vergara, Andrés Concha Torre, Ana Vivanco, Reyes Fernández, Francisco Giménez Sánchez, Miguel Sánchez Forte, Pablo Rojo, J. Ruiz Contreras, Alba Palacios, Cristina Epalza Ibarrondo, Elizabeth Fernandez Cooke, Marisa Navarro, Cristina Álvarez Álvarez, María José Lozano, Eduardo Carreras, Sonia Brió Sanagustín, Olaf Neth, Ma del Carmen Martínez Padilla, Luis Manuel Prieto Tato, Sara Guillén, Laura Fernández Silveira, David Moreno, R. de Groot , A. M. Tutu van Furth, M. van der Flier, N. P. Boeddha, G. J. A. Driessen, M. Emonts, J. A. Hazelzet, T. W. Kuijpers, D. Pajkrt, E. A. M. Sanders, D. van de Beek, A. van der Ende, H. L. A. Philipsen, A. O. A. Adeel, M. A. Breukels, D. M. C. Brinkman, C. C. M. M. de Korte, E. de Vries, W. J. de Waal, R. Dekkers, A. Dings-Lammertink, R. A. Doedens, A. E. Donker, M. Dousma, T. E. Faber, G. P. J. M. Gerrits, J.A.M. Gerver, J. Heidema, J. Homan-van der Veen, M. A. M. Jacobs, N. J. G. Jansen, P. Kawczynski, K. Klucovska, M. C. J. Kneyber, Y. Koopman-Keemink, V. J. Langenhorst, J. Leusink, B. F. Loza, I. T. Merth, C. J. Miedema, C. Neeleman, J. G. Noordzij, C. C. Obihara, A. L. T. van Overbeek - van Gils, G. H. Poortman, S. T. Potgieter, J. Potjewijd, P. P. R. Rosias, T. Sprong, G. W. ten Tussher, B. J. Thio, G. A. Tramper-Stranders, M. van Deuren, H. van der Meer, A. J. M. van Kuppevelt, A. M. van Wermeskerken, W. A. Verwijs, T. F. W. Wolfs, Luregn J Schlapbach, Philipp Agyeman, Christoph Aebi, Eric Giannoni, Martin Stocker, Klara M Posfay-Barbe, Ulrich Heininger, Sara Bernhard-Stirnemann, Anita Niederer-Loher, Christian Kahlert, Paul Hasters, Christa Relly, Walter Baer, Christoph Berger, Enitan D Carrol, Stéphane Paulus, Hannah Frederick, Rebecca Jennings, Joanne Johnston, Rhian Kenwright, Colin G Fink, Elli Pinnock, Marieke Emonts, Rachel Agbeko, Suzanne Anderson, Fatou Secka, Kalifa Bojang, Isatou Sarr, Ngane Kebbeh, Gibbi Sey, Momodou Saidykhan, Fatoumatta Cole, Gilleh Thomas, Martin Antonio, Werner Zenz, Daniela S. Klobassa, Alexander Binder, Nina A. Schweintzger, Manfred Sagmeister, Hinrich Baumgart, Markus Baumgartner, Uta Behrends, Ariane Biebl, Robert Birnbacher, Jan-Gerd Blanke, Carsten Boelke, Kai Breuling, Jürgen Brunner, Maria Buller, Peter Dahlem, Beate Dietrich, Ernst Eber, Johannes Elias, Josef Emhofer, Rosa Etschmaier, Sebastian Farr, Ylenia Girtler, Irina Grigorow, Konrad Heimann, Ulrike Ihm, Zdenek Jaros, Hermann Kalhoff, Wilhelm Kaulfersch, Christoph Kemen, Nina Klocker, Bernhard Köster, Benno Kohlmaier, Eleni Komini, Lydia Kramer, Antje Neubert, Daniel Ortner, Lydia Pescollderungg, Klaus Pfurtscheller, Karl Reiter, Goran Ristic, Siegfried Rödl, Andrea Sellner, Astrid Sonnleitner, Matthias Sperl, Wolfgang Stelzl, Holger Till, Andreas Trobisch, Anne Vierzig, Ulrich Vogel, Christina Weingarten, Stefanie Welke, Andreas Wimmer, Uwe Wintergerst, Daniel Wüller, Andrew Zaunschirm, Ieva Ziuraite, Veslava Žukovskaja, Claudia Mikula, Gebhard Feierl, Alexander Binder, Werner Zenz, Wolfgang Walcher, Gotho Geishofer, Daniela Klobassa, Müller Martin, Klaus Pfurtscheller, Karl Reiter, Siegfried Rödl, Gerfried Zobel, Bettina Zöhrer, Bärbel Töpke, Peter Fucik, Markwart Gabriel, Johann M. Penzien, Gedeon Diab, Robert Miething, K.H. Deeg, Jürg Hammer, Ulrich Heininger, Verena Varnholt, Andreas Schmidt, Lutz Bindl, Ursula Sillaber, Christian Huemer, Primrose Meier, G. Simic-Schleicher, Markus Markart, Eberhard Pfau, Hans Broede, Bernd Ausserer, Hermann Kalhoff, Volker Arpe, Susanne Schweitzer-Krantz, Johannes-Martin Kasper, Kathrin Loranth, Hans J. Bittrich, Burkhard Simma, Jens Klinge, Michael Fedlmaier, Nicola Weigand, Egbert Herting, Regina Grube, Christoph Fusch, Alois Gruber, Ulf Schimmel, Suzanne Knaufer-Schiefer, Wolfgang Lässig, Axel Hennenberger, Axel von der Wense, Roland Tillmann, Jürgen Schwarick, Friedrich C. Sitzmann, Werner Streif, Herbert Müller, Peter Kurnik, Peter Groneck, Ute Weiss, Helene Gröblacher-Roth, Jürgen Bensch, Reinhard Moser, Rudolf Schwarz, Kurt Lenz, Thomas Hofmann, Wolfgang Göpel, Dietrich Schulz, Thomas Berger, Erwin Hauser, Kai Martin Förster, Jochen Peters, Thomas Nicolai, Björn Kumlien, Regina Beckmann, Christiane Seitz, D. Hüseman, Roland Schürmann, Van Hop Ta, Eckart Weikmann, W. Evert, Jürgen Hautz, Jürgen Seidenberg, Lucia Wocko, Petra Luigs, Hans-Ludwig Reiter, J. Quietzach, Michael König, Johanna Herrmann, Horst Mitter, Ekkehard Seidler, Bernhard Maak, Wolfgang Sperl, Karl Zwiauer, Manfred Meissl, Reinhard Koch, Manfred Cremer, H. A. Breuer, W. Görke, Robert Nossal, Walter Pernice, Ralf Brangenberg, Hans R. Salzer, Hartmut Koch, Gerhard Schaller, Franz Paky, Friedrich Straßer, Franz Eitelberger, D. Sontheimer, Andreas Lischka, Martina Kronberger, Alfred Dilch, Christian Scheibenpflug, Robert Bruckner, Klaus Mahler, Klaus Runge, Wolfgang Kunze, and Peter Schermann
References
- 1.Dietrich ML, Steele RW. Group A Streptococcus. Pediatr Rev. 2018;39(8):379–391. doi: 10.1542/pir.2017-0207. [DOI] [PubMed] [Google Scholar]
- 2.Shulman ST, Tanz RR, Dale JB, Steer AC, Smeesters PR. Added value of the emm-cluster typing system to analyze group A Streptococcus epidemiology in high-income settings. Clin Infect Dis. 2014;59(11):1651–1652. doi: 10.1093/cid/ciu649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MR, McIntyre L, Mutreja A, Lacey JA, Lees JA, Towers RJ, et al. Atlas of group A streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat Genet. 2019;51(6):1035–1043. doi: 10.1038/s41588-019-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson-Smith M, De Oliveira DM, Guglielmini J, McMillan DJ, Vu T, Holien JK, et al. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis. 2014;210(8):1325–1338. doi: 10.1093/infdis/jiu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steer AC, Lamagni T, Curtis N, Carapetis JR. Invasive group a streptococcal disease: epidemiology, pathogenesis and management. Drugs. 2012;72(9):1213–1227. doi: 10.2165/11634180-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46(7):2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plainvert C, Loubinoux J, Bidet P, Doloy A, Touak G, Dmytruk N, et al. Epidemiology of Streptococcus pyogenes invasive diseases in France (2007–2011) Arch Pediatr. 2014;21(Suppl 2):S62–S68. doi: 10.1016/S0929-693X(14)72262-6. [DOI] [PubMed] [Google Scholar]
- 8.Scaber J, Saeed S, Ihekweazu C, Efstratiou A, McCarthy N, O’Moore E (2011) Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill 16(5) [PubMed]
- 9.Filleron A, Jeziorski E, Michon AL, Rodiere M, Marchandin H. Current insights in invasive group A streptococcal infections in pediatrics. Eur J Pediatr. 2012;171(11):1589–1598. doi: 10.1007/s00431-012-1694-8. [DOI] [PubMed] [Google Scholar]
- 10.Park DW, Kim SH, Park JW, Kim MJ, Cho SJ, Park HJ, et al. Incidence and characteristics of scarlet fever, South Korea, 2008–2015. Emerg Infect Dis. 2017;23(4):658–661. doi: 10.3201/eid2304.160773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockmann C, Ampofo K, Hersh AL, Blaschke AJ, Kendall BA, Korgenski K, et al. Evolving epidemiologic characteristics of invasive group a streptococcal disease in Utah, 2002–2010. Clin Infect Dis. 2012;55(4):479–487. doi: 10.1093/cid/cis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lithgow A, Duke T, Steer A, Smeesters PR. Severe group A streptococcal infections in a paediatric intensive care unit. J Paediatr Child Health. 2014;50(9):687–692. doi: 10.1111/jpc.12601. [DOI] [PubMed] [Google Scholar]
- 13.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA. 2014;111(17):E1768–E1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Shahib A, Underwood A, Afshar B, Turner CE, Lamagni T, Sriskandan S, et al. Emergence of a novel lineage containing a prophage in emm/M3 group A Streptococcus associated with upsurge in invasive disease in the UK. Microb Genom. 2016;2(6):e000059. doi: 10.1099/mgen.0.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358–365. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 16.Laupland KB, Davies HD, Low DE, Schwartz B, Green K, McGeer A (2000) Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Ontario Group A Streptococcal Study Group. Pediatrics 105(5):E60 [DOI] [PubMed]
- 17.Whitehead BD, Smith HV, Nourse C. Invasive group A streptococcal disease in children in Queensland. Epidemiol Infect. 2011;139(4):623–628. doi: 10.1017/S0950268810001378. [DOI] [PubMed] [Google Scholar]
- 18.Zachariadou L, Stathi A, Tassios PT, Pangalis A, Legakis NJ, Papaparaskevas J, et al. Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol Infect. 2014;142(3):512–519. doi: 10.1017/S0950268813001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Factor SH, Levine OS, Harrison LH, Farley MM, McGeer A, Skoff T, et al. Risk factors for pediatric invasive group A streptococcal disease. Emerg Infect Dis. 2005;11(7):1062–1066. doi: 10.3201/eid1107.040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinon-Torres F, Salas A, Rivero-Calle I, Cebey-Lopez M, Pardo-Seco J, Herberg JA, et al. Life-threatening infections in children in Europe (the EUCLIDS Project): a prospective cohort study. Lancet Child Adolesc Health. 2018;2(6):404–414. doi: 10.1016/S2352-4642(18)30113-5. [DOI] [PubMed] [Google Scholar]
- 21.Agyeman PKA, Schlapbach LJ, Giannoni E, Stocker M, Posfay-Barbe KM, Heininger U, et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc Health. 2017;1(2):124–133. doi: 10.1016/S2352-4642(17)30010-X. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6(1):2–8. 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed]
- 23.Pagana KD, Pagana TJ, Pagana TN (2019) Mosby’s diagnostic & laboratory test reference. 14 ed. Elsevier
- 24.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G (2003) PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29(2):278–85. 10.1007/s00134-002-1601-2 [DOI] [PubMed]
- 27.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 28.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 29.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 30.Safar A, Lennon D, Stewart J, Trenholme A, Drinkovic D, Peat B, et al. Invasive group A streptococcal infection and vaccine implications, Auckland. New Zealand Emerg Infect Dis. 2011;17(6):983–989. doi: 10.3201/eid/1706.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver J, Thielemans E, McMinn A, Baker C, Britton PN, Clark JE, et al. Invasive group A Streptococcus disease in Australian children: 2016 to 2018 - a descriptive cohort study. BMC Public Health. 2019;19(1):1750. doi: 10.1186/s12889-019-8085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CF, Cowling BJ, Lau EHY. Epidemiology of reemerging scarlet fever, Hong Kong, 2005–2015. Emerg Infect Dis. 2017;23(10):1707–1710. doi: 10.3201/eid2310.161456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeesters PR, Laho D, Beall B, Steer AC, Van Beneden CA. Seasonal, geographic, and temporal trends of emm clusters associated with invasive group A streptococcal infections in US multistate surveillance. Clin Infect Dis. 2017;64(5):694–695. doi: 10.1093/cid/ciw807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, Lynfield R, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis. 2016;63(4):478–486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45(7):853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 36.Olafsdottir LB, Erlendsdottir H, Melo-Cristino J, Weinberger DM, Ramirez M, Kristinsson KG, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill. 2014;19(17):5–14. doi: 10.2807/1560-7917.ES2014.19.17.20784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.