Abstract
Aiming to fill a gap in the literature, we aimed to identify the most promising EOs blocking in vitro cellular entry of SARS-CoV-2 delta variant without conferring human cytotoxicity and provide insights into the influence of their composition on these activities. Twelve EOs were characterized by gas chromatography coupled to mass spectrometry. The antiviral and cytotoxicity activities were determined using the cell-based pseudoviral entry with SARS-CoV-2 delta pseudovirus and the XTT assay in HeLa cells expressing human angiotensin-converting enzyme 2 (HeLa ACE-2), respectively. Syzygium aromaticum, Cymbopogon citratus, Citrus limon, Pelargonium graveolens, Origanum vulgare, “Illicium verum”, and Matricaria recutita showed EC50 lowered or close to 1 µg/mL but also the lowest CC50 (0.20–1.70 µg/mL), except “I. verum” (30.00 µg/mL). Among these, “I. verum”, C. limon, P. graveolens and S. aromaticum proved to be promising alternatives for SARS-CoV-2 delta variant inhibition (therapeutic index above 4), which possibly was related to the compounds (E)-anetole, limonene and beta-pinene, citronellol, and eugenol, respectively.
Subject terms: SARS-CoV-2, Mass spectrometry, Natural products
Introduction
Since the emergence of the COVID-19 pandemic, numerous measures to control this virus's spreading have been taken worldwide1. Although several countries are vaccinating their population, the pandemic is not over yet2, and the treatment of COVID-19 is still a real challenge3–5, mainly due to the new emerging variants.
The emergence of new variants contributes to the continued global circulation of SARS-CoV-2, and they are classified as variants of concern (VOCs) due to their easy transmission6. The VOCs have modifications in the spike protein and thus important implications for transmission rate, disease severity, and immune evasion, such as Omicron and, mainly, the Delta variant for its high infectivity7,8. Furthermore, the neutralizing antibodies produced from first-generation vaccination or natural infection with COVID-19 may not bind with the same affinity to VOCs9,10. Synthetic drugs such as chloroquine, hydroxychloroquine, and molnupiravir have been suggested as potential treatments against COVID-19. However, these drugs are doubtful in terms of effectiveness versus side effects and toxicity11, highlighting the need for continuous research to discover new natural alternatives for the treatment, control, and inactivation of the SARS-CoV-2 virus.
Natural compounds have been studied as potential antiviral agents against SARS-CoV-2 viruses5,12–15. Among the secondary metabolism products of plants, essential oils (EOs) have shown promising antiviral activity against several viruses16. For this reason, these oils have been considered a potential source of bioactive compounds for impairing SARS-CoV-2 replication or supporting the treatment of some symptoms of COVID-1917,18.
In the last two years, numerous review articles have hypothesized an effective action of EOs against SARS-CoV-2 based on their activity against other viruses such as human immunodeficiency virus (HIV), influenza A virus (H1N1), Zika virus, human herpes virus (HSV) and, avian influenza A virus (H5N1) and among others16,19–23. Similarly, in silico studies have addressed a high binding affinity of numerous subclasses of terpenes and sesquiterpenes in the main target proteins of the SARS-CoV-2 virus, such as main protease (Mpro/3CLpro), spike protein (Spro), angiotensin-converting enzyme-2 (ACE2), enzyme transmembrane protease serine-type 2 (TMPRSS2), RNA-dependent RNA polymerase, and others18,24–28. Beyond the favorable binding affinity of EOs on SARS-CoV-2 target proteins, in silico studies have demonstrated that their bioactive compounds have satisfactory pharmacokinetic and toxicity characteristics concerning absorption, distribution, metabolism, excretion, and toxicity18.
Nevertheless, despite this fact, there are only two in vitro studies with this approach to date. Senthil Kumar et al.29 observed significant ACE2 inhibitory effects in epithelial cells by Pelargonium graveolens and Citrus limon EOs, indicating a possible downregulating activity in the ACE2 receptor. These authors also reported successful results of both EOs for cytotoxicity in HT-29 cells. Ćavar Zeljković et al.12 evaluated the anti-SARS-CoV-2 activity of essential oils from Lamiaceae plant species and their monoterpenes and reported a notable activity for carvone, carvacrol, pulegone, menthofuran, and 1,8-cineole. According to these authors, while carvacrol and menthofuran presented good cytotoxicity, carvone, pulegone, and 1,8-cineole showed no activity in Vero 76 cells. In vitro studies are essential to validate in silico results, understand the real anti-SARS-CoV-2 potential of EOs, and boost in vivo studies for further commercial use.
We recently published a systematic and meta-analytic review from in silico studies, which aimed to identify the most EO promising molecules among more than 400 compounds and EO potential sources against SARS-CoV-2 based on their binding affinity by target proteins, pharmacokinetic, and toxicity properties18. Furthermore, EOs with potential antiviral activity against other viruses as described in the literature were also considered in this study. Therefore, this study aimed to assess the antiviral actitvity and cytotoxicity of twelve species of EOs against the SARS-CoV-2 delta variant. A chemometric approach was used to provide insights into the influence of EOs composition on their activity, which represents another area that needs more investigation.
Results and discussion
EC50, CC50, and TI values are shown in Table 1. The antiviral EC50 values varied from 0.09 to 57.1 µg/mL, revealing that the SARS-CoV-2 Delta variant was susceptible to all EOs tested. In a descending order of antiviral activity, the most active were S. aromaticum (0.09 µg/mL), C. citratus (0.1 µg/mL), C. limon (0.15 µg/mL), P. graveolens (0.2 µg/mL), and “I. verum” (0.5 µg/mL).
Table 1.
In vitro antiviral and cytotoxic activities of twelve essential oils expressed in half-maximal effective concentration (EC50), half-maximal cytotoxic concentration (CC50), and therapeutic index (TI).
| Essential oils | Concentrations in µg/mL (95% confidence interval ) | TI (CC50/EC50) | |
|---|---|---|---|
| EC50 | CC50 | ||
| Zingiber officinale | 33.7 (20.9–55.3) | 38.6 (22.3–70.2) | 1.12 |
| Eucalipto globulus | 12.9 (6.4–25.3) | 23.9 (15.2–37.7) | 1.85 |
| Thymus vulgaris | 3.1 (1.3–7.4) | 2.0 (1.5–2.8) | 0.67 |
| “Illicium verum” | 0.5 (0.3–0.8) | 29.5 (17.7–51.7) | 60.00 |
| Rosmarinus officinalis | 57.1 (35.2–93.3) | 81.1 (43.2–169.0) | 1.42 |
| Melaleuca alternifolia | 29.2 (18.6–46.7) | 41.0 (27.9–60.9) | 1.41 |
| Syzygium aromaticum | 0.09 (0.07–0.13) | 0.42 (0.30–0.58) | 4.44 |
| Cymbopogon citratus | 0.11 (0.07–0.19) | 0.20 (0.12–0.22) | 2.00 |
| Origanum vulgare | 0.36 (0.23–0.57) | 0.41 (0.31–0.56) | 1.00 |
| Citrus limon | 0.15 (0.09–0.22) | 1.3 (0.7–2.3) | 8.67 |
| Pelargonium graveolens | 0.20 (0.14–0.29) | 1.7 (1.1–2.6) | 8.50 |
| Matricaria recutita | 1.1 (0.6–1.9) | 1.2 (0.8–1.7) | 1.09 |
The antiviral activity of drugs against SARS-CoV-2 is widely documented in the literature. For example, in Vero E6 cells, hydroxychloroquine and chloroquine showed an EC50 of 1.5 µg/mL and 2.1 µg/mL, respectively30. In the study of Pasquereau et al.31, chloroquine showed an EC50 of 5 µg/mL, while lopinavir exhibited an EC50 of 8.81 µg/mL in MRC5 cells. Regarding artemisinin and arbidol, the EC50 values were 64.45 µg/mL and 10.70 µg/mL in Vero E6 cells, respectively32,33. Therefore, our results indicate that most of the EOs evaluated in this study had similar or better EC50 values than the main antiviral drugs already used for the COVID-19 treatment, especially those EOs showing EC50 values close or below 1 µg/mL (S. aromaticum, C. citratus, C. limon, P. graveolens, O. vulgare, “I. verum”, and M. recutita). It is worth noting that among the diverse cell lines used in pseudoviral assays, HeLa-ACE2 cells proved to be more sensitive in detecting replicating viruses and provided more reproducible titers34,35, justifying the choice of the cell line used in our studies.
EOs are well-known for their broad biological spectrum and antiviral potential18. Nevertheless, studies evaluating in vitro antiviral activity of EOs against SARS-CoV-2 are very sparse in the literature. The antiviral mechanisms of EOs are still poorly understood, but it is already known that their activity depends mainly on EO compounds and their interactions and some inherent factors concerning the virus, such as the viral load kinetic and viral protein structure. Based on that, the antiviral mechanisms of EOs can be classified into three main ones: target sites during the viral lifecycle (intracellular, intercellular or multiple), morphological alteration (binding to or masking viral structures or destroying them), and protein inhibition through a hydrogen bond, hydrophobic or ionic interactions among others16,18. However, it is not yet entirely known for sub-family coronavirinae.
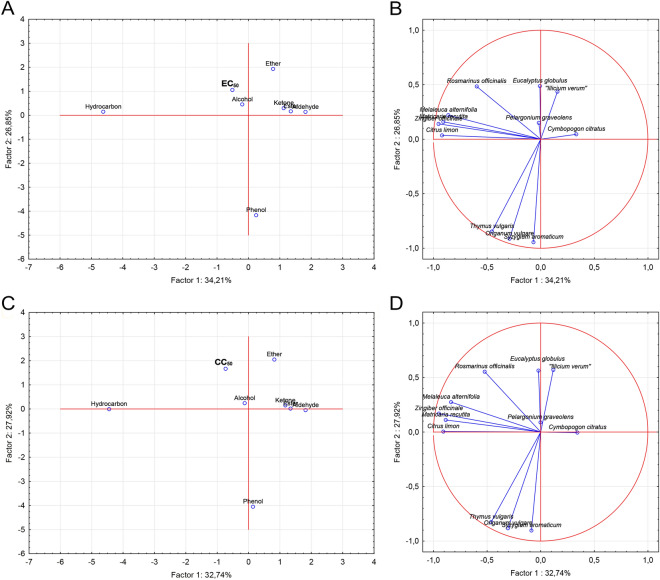
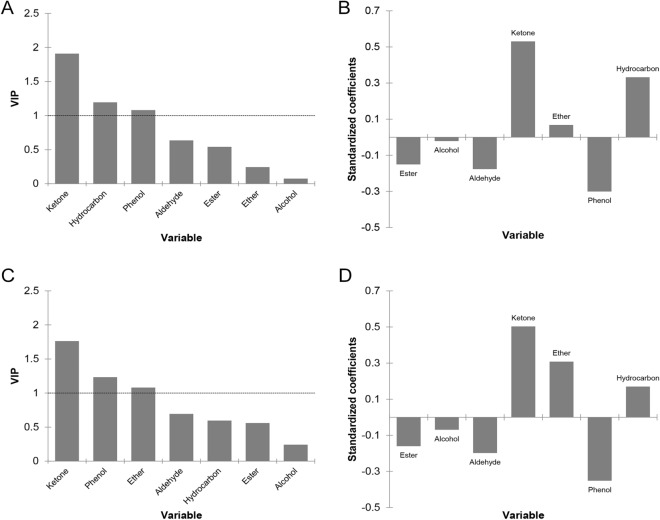
Through PCA analysis, the influence of the majority functional groups within each EO on EC50 and CC50 values can be better understood. Concerning EC50 values, PCA 1 and PCA 2 explained 34.21% and 26.85% of the total variance, respectively, and separated the functional groups into three categories: hydrocarbons and alcohol (category 1), phenols (category 2), ethers, aldehydes, esters and ketones (category 3; Fig. 1A). Among these groups, ketones (VIP: 1.910), hydrocarbons (VIP: 1.195) and phenols (VIP: 1.079) had VIP values above 1, indicating a more significant influence on the EC50 values (Fig. 2A). However, both ketones and hydrocarbons contributed to an increase in EC50 values and, consequently, to a decreased anti-SARS-CoV-2 activity, whereas phenols contributed to a decreased in EC50 values and, to an increased antiviral activity (Fig. 2B). Likewise, EC50 values were not affected by ether (VIP: 0.243; Fig. 2A), but it revealed a trend decrease in antiviral activity (Fig. 2B). In contrast, although aldehydes, esters, and alcohols have shown no influence on the EC50 values (VIP from 0.076 to 0.635; Fig. 2A), they exhibited a potential to decrease EC50 values or increase the anti-SARS-CoV-2 activity (Fig. 2B).
Figure 1.
Principal component analysis (PCA) of the functional groups (ketone, alcohol, phenol, aldehyde, hydrocarbon, ether, and ester) within the essential oils regarding the EC50 (A, B) and CC50 (C, D) values with 95% confidence interval.
Figure 2.
VIP values and standardized coefficients of the functional groups (ketone, alcohol, phenol, aldehyde, hydrocarbon, ether, and ester) within essential oils from partial least-squares regression (PLSR) analysis regarding EC50 (A, B) and CC50 (C, D) values with 95% confidence interval.
There are no reports about the influence of EO functional groups on their antiviral activity. In the present study, most of the EOs had a predominance of hydrocarbons (Supplementary Table 1, Fig. 1A,B), which contributed to a low activity against SARS-CoV-2. A hypothesis is the absence of reactive groups in their structure, such as oxygen or hydroxyls. However, it is worth noting that C. limon EO presented 96% of hydrocarbons and showed EC50 values of 0.15 µg/mL. It may be attributed to a high concentration of specific hydrocarbons, such as limonene (68.5%) and beta-pinene (10.4%). A previous study reported that limonene compound (73%) was the main responsible for the inhibitory activity of C. limon EO against the ACE2 enzyme29. Moreover, it is well-known that the EOs may have synergistic or additive interactions due to presenting a broad composition of bioactive compounds. C. limon EO was also composed of aldehydes, such as geranial (0.7%) and neral (0.5%), a functional group related to increased antiviral activity against SARS-CoV-2. It also indicates the need for further studies about this EO's composition to understand its activity against ACE2 better. The same was observed for M. recutita EO with 56.7% of hydrocarbons, of which 50.6% was (E)-beta-farnesene. There are no in vitro reports about the anti-SARS-CoV-2 activity of geranial, neral, and (E)-beta-farnesene. However, the last one exhibited a high binding affinity for Spro, ACE2, and Mpro/3CLpro24, emphasizing the need for further in vitro studies to better understand the individual activity of the compounds. Within alcohol, citronellol (41.5%) was mainly observed in P. graveolens (EC50: 0.2 µg/mL) (Supplementary Table 1, Fig. 1A, B). A promising antiviral activity was observed for citronellol through binding affinity to the receptor-binding domain (RBD)36. Despite that, our findings from PLSR suggest a weak potential anti-SARS-CoV-2 activity by alcohols (Fig. 2A,B). Additionally, this functional group was found as secondary compounds in other EOs with discrepant EC50 values as M. alternifolia (40.7%; EC50: 29 µg/mL) and M. recutita (24.1%; EC50: 1.1 µg/mL) (Table 1, Supplementary Table 1), reinforcing its low influence on antiviral activity.
The S. aromaticum, T. vulgaris and O. vulgare EOs presented mainly eugenol, carvacrol and/or thymol (Supplementary Table 1, Fig. 1A,B), respectively, which are phenols widely known for their antiviral potential16. There are no in vitro studies related to S. aromaticum or eugenol, however, this compound was already shown to have binding affinity to to RBD, Mpro/3CLpro, Spro, and ACE237–39. Regarding anti-SARS-CoV-2 in vitro action, O. vulgare EO presented a lower EC50 value (0.4 µg/mL) than T. vulgaris EO (3 µg/mL). This may be due to the higher amount of phenols in O. vulgare EO (70.3%) than T. vulgaris (56.7%), which were compounds that tended to increase antiviral activity (Fig. 2B). Moreover, T. vulgaris EO showed 25.5% of carvacrol, a lower concentration than O. vulgare EO (70.3%). According to Ćavar Zeljković et al.12, carvacrol alone exhibited activity against SARS-CoV-2, while it was not observed for thymol.
Concerning aldehydes, esters, ketones and ethers categories, the first two showed a tendency to increase the anti-SARS-CoV-2 activity, while the last two demonstrated an opposite trend (Figs. 1A, 2B). The aldehydes geranial and neral were identified as major compounds in C. citratus EO (EC50 of 0.1 µg/mL) at 45.5% and 33.7%, respectively (Supplementary Table 1, Fig. 1A,B). There are no in vitro reports concerning the activity of this EO against SARS-CoV-2. Nevertheless, geranial and neral are reported to have high in silico affinity for the main protease and for ACE2 (Thuy et al.40), corroborating our in vitro results. Furthermore, it is reported that these compounds have a similar mechanism to drugs already known (e.g., hydroxychloroquine and chloroquine) interfering with glycosylation of the SARS-CoV-2 viral spike protein to its receptor (ACE2)30,41. It reinforces our findings regarding the tendency of aldehydes, mainly geranial and neral, to increase the antiviral activity of EOs against SARS-CoV-2.
The ester category was represented by P. graveolens, C. citratus, and R. officinalis EOs, which showed this functional group as secondary compounds in their composition (20.7%, 1.7%, and 1.1%, respectively; Supplementary Table 1). Also, P. graveolens EO had the widest variety of ester compounds, followed by C. citratus and R. officinalis EOs. Although they have not been the majority compounds of these EOs, it is suggested that the amount and diversity of esters may have influenced the EC50 values. This is because, among three EOs within the ester category, R. officinalis showed the lowest concentration and the poorest variety of these compounds, and the lowest antiviral activity (EC50: 57 µg/mL). Asif et al.23 report a high binding affinity of ester geranyl formate against Spro. Additionally, esters have been reported to potentially reverse symptoms related to COVID-1942. Despite this, there is very little data in the literature regarding the anti-SARS-CoV-2 action of esters within the EOs, including in vitro studies. The R. officinalis EO also presented the highest concentration of ketones (17.3%), emphasizing that it contributed to a lowered anti-SARS-CoV-2 activity (Table 1, Supplementary Table 1, Fig. 2A,B).
The ether category was represented by E. globulus (EC50: 13 µg/mL) and labeled as I. verum (EC50: 0.5 µg/mL), which presented mostly1,8-cineole (86.2%) and (E)-anethole (90.7%), respectively (Supplementary Table 1, Fig. 1A,B). Although in silico studies have revealed some affinity of the 1,8-cineole for Spro37, RBD28,43, Mpro/3CLpro44, and TMPRSS243, its in vitro activity against SARS-CoV-2 in Vero 76 cells was very low (EC50 = 835.85 µg/mL)12. Furthermore, E. globulus had a reasonable amount of hydrocarbons (13.9%), which may explain the contrasting EC50 values compared to “I. verum”, two EOs containing mostly ether grouped within the same PCA category. This fact associated with our findings on ether tending to decrease the anti-SARS-CoV-2 activity (Fig. 2B) may justify the high EC50 values for E. globulus. Otherwise, in vitro studies evaluating antiviral activity from compound (E)-anethole has not yet been reported. Therefore, (E)-anethole seems to be a specific ether compound with potential activity against SARS-CoV-2 when predominantly present in an EO (about or above 90%). It is worth highlighting that 9% of triacetin (a not natural compound) was found in product labeled as “I. verum”. However, it did not demonstrate in vitro anti- SARS-CoV-2 activity (EC50: 2400 µg/mL), indicating that (E)-anethole was indeed responsible for the antiviral activity.
Aside from antiviral activity, the cell viability study is an important parameter to ensure that effective EOs against viral agents do not exert toxicity on the human host cells16,45. In the PCA analysis concerning CC50 values (Fig. 1C,D), the first and the second components explained 32.74% and 27.92% of the total variance, respectively, and separated the functional group treatments into three categories, likewise for EC50 values: hydrocarbons and alcohols (category 1), phenols (category 2), and aldehydes, ethers, esters and ketones (category 3; Fig. 1C). Among these groups, ketones (VIP: 1.763), phenols (VIP: 1.232) and ether (VIP: 1.080) had VIP values above 1, indicating a more significant influence on the CC50 values (Fig. 2C). In Fig. 2D, it was observed that ketones and ether contributed to an increase in CC50 values and, consequently, to a decreased cytotoxicity, however, the presence of phenols contributed to increase the cytotoxicity. In addition, despite hydrocarbons having a VIP value of 0.596, this functional group also showed a trend of reduced cytotoxicity (Fig. 2C,D). Likewise, CC50 values were not affected by alcohols (VIP: 0.243), esters (VIP: 0.560), and aldehydes (VIP: 0.694; Fig. 2C), but they revealed a trend increasing in cytotoxicity (Fig. 2D).
Based on Fig. 1C,D, it is possible to observe that hydrocarbons also strongly influenced the grouping of the EOs considering CC50 values. The influence of the composition of EOs is complex, where each compound acts individually and/or interacts46. Furthermore, the compound cytotoxicity can be based on mechanisms such as cell death by apoptosis and/or necrosis, cell cycle arrest, loss of key organelles function, and damage of cell membranes, causing reduced production of ATP, pH gradient changes, and loss of mitochondrial potential, among others46–48. In this context, the cytotoxic activity of EOs may be related to properties of mono and sesquiterpenes, such as lipophilicity and molecular weight, justifying PCA grouping.
The EOs showed CC50 values ranging from 0.2 to 81 µg/mL (Table 1). Overall, functional groups that increased or tended to increase EC50 values also increased or tended to increase CC50 values (Fig. 2C,D). In other words, functional groups that contributed to a decreased activity against SARS-CoV-2 were those that contributed to a decreased cytotoxicity for human cells. It may be clearly noted in our findings. Z. officinale, M. alternifolia, and R. officinalis presented the lowest cytotoxicity but also the lowest anti-SARS-CoV-2 activity (Table 1). Likewise, EOs exhibiting the highest antiviral activity were the most cytotoxic (S. aromaticum, C. citratus, C. limon, P. graveolens, O. vulgare, and M. recutita), except the “I. verum”. This EO demonstrated effectiveness against SARS-CoV-2 and low cytotoxicity (Table 1), suggesting the great potential of the (E)-anethole alone (without the influence of other EO compounds) to block cellular entry of SARS-CoV-2 with no human cell damage.
Among the EOs with higher cytotoxicity, those that showed the highest ones were C. citratus, O. vulgare, and S. aromaticum. Like antiviral activity, the EOs cytotoxicity is attributed to their major chemical constituents16. C. citratus EO was mainly composed of geranial and neral compounds (aldehydes), O. vulgare EO of carvacrol (phenol), and S. aromaticum EO of eugenol (phenol). All of these compounds have already shown high cytotoxicity in HeLa cells in previous studies49,50, corroborating our findings. However, it is worth mentioning that more studies need to be carried out to understand the cytotoxic activity of EOs, including evaluating secondary compounds present in their composition and their influence on anti-SARS-CoV-2 activity.
Regarding the therapeutic index (TI), this parameter depends on the EC50 and CC50 values aiming exactly to attain EOs with higher antiviral activity and lower cytotoxicity. Stránská et al.51 reported that a TI greater than 4 is satisfactory for natural products. In this way, the EOs that presented acceptable values of TI in descending order were “I. verum” (TI: 60), C. limon (TI: 8.67), P. graveolens (TI: 8.50), and S. aromaticum (TI: 4.44). The low cytotoxicity in HT-29 cells of C. limon and P. graveolens EOs has already been reported in the literature by Senthil Kumar et al.29, which also suggested that both EOs may impair the viral cellular entry during SARS-CoV-2 infection through an anti-ACE2 activity in vitro assay. Nevertheless, these authors did not determine TI values. Regarding the product labeled as “I. verum”, composed of ((E)-anetole compound) and triacetin, and S. aromaticum EOs, no study has yet reported neither anti-SARS-CoV-2 activity or TI values. The contaminant triacetin present in “I. verum” composition showed EC50 of 2400 µg/mL, CC50 of 1600 µg/mL, and TI of 0.66. Therefore, the antiviral and cytotoxicity activities of this EO were due to its main compound ((E)-anethole), which was responsible for the highest value of TI in our study, revealing its potential against SARS-CoV-2. The high TI found for C. limon EO may be attributed to potential specific hydrocarbons (limonene and beta-pinene), and their interaction with other compounds from another functional group, such as geranial and neral (aldehydes), wherein all of them conferred better anti-SARS-CoV-2 activity to EOs. P. graveolens and S. aromaticum presented a predominance of citronellol and eugenol, respectively, that contributed to strongly reducing EC50 values and obtaining good TI values.
The activity of functional groups, specific compounds, and their interactions directly influenced the anti-SARS-CoV-2 and HeLa cells' cytotoxicity actions and, consequently, the TI values. The functional groups able to improve activity against SARS-CoV-2 also increased the cytotoxicity of the EOs. Hydrocarbons, ketones, and ethers decreased or tended to decrease the anti-SARS-CoV-2 activity and cytotoxicity, while the opposite was observed for aldehydes, phenols, alcohols, and esters. Some specific compounds, such as (E)-anetole, eugenol, limonene, beta-pinene, and citronellol, can be highlighted due to their majority presence in EOs with greater activity against the SARS-CoV-2 delta variant. From interactions, product labeled as “I. verum”, C. limon, P. graveolens, and S. aromaticum were the EOs most effective against SARS-CoV-2 while minimizing cytotoxicity (TI above 4). In this context, they could be helpful in different ways, such as developing new drugs, including the use of nanoencapsulation, or using their volatile characteristics through spray formulation for the nasal and oral cavities to suppress COVID-19 infection52. However, more in vitro studies are needed to understand the compound interactions in these four potential EOs leading to great TI against SARS-CoV-2 and its variants.
Methods
Plant material
The essential oils of Zingiber officinale, Eucalyptus globulus, Thymus vulgaris, Rosmarinus officinalis, Melaleuca alternifolia, Syzygium aromaticum, Cymbopogon citratus, Origanum vulgare, Citrus limon, Pelargonium graveolens, Matricaria recutita and product labeled as Illicium verum (see Supplementary Table 1) were acquired from Quinari (Quinari—Óleos e essências, Paraná, Brazil). According to the manufacturer, the parts used for extracting the EOs were the leaves (C. citratus, E. globulus, R. officinalis, M. alternifolia, O. vulgare, and T. vulgaris), seeds (I. verum), rhizome (Z. officinale), shells (C. limon), flowers (M. recutita), seeds and leaves (S. aromaticum), and flowers and leaves (P. graveolens). Triacetin (99%) was purchased from the Sigma Aldrich ® (St. Louis, MO).
Characterization of EOs by GC–MS and GC-FID
The composition of EOs was determined using gas chromatography coupled to mass spectrometry (GC–MS). The analyses were performed in an Agilent 7890A gas chromatography coupled to a 5975C mass detector and equipped with a 5% diphenyl–95% dimethylpolysiloxane capillary column (DB-5 MS, 30 m × 0.25 mm × 0.25 µm). The oven temperature was programmed to rise from 60 to 240 °C at 3 °C/min. The carrier gas was helium at 1.0 mL/min. Each pure oil was dissolved in hexane (0.1%), and 1.0 µL was injected through an Agilent 7693A autosampler in split mode (1:50) with the injector at 250 °C. The transfer line was kept at 260 °C, the ion source at 230 °C, and the analyzer at 150 °C. The mass detector was operated in electron ionization mode (70 eV), with 3.15 scans/second, and data was collected in the 40–350 m/z range. The EO compounds were identified by comparing their mass spectra with those found in the Wiley Registry of Mass Spectral Data53 and Adam's library54, and their linear retention indices were calculated according to55. For quantitation, the samples were injected in an Agilent 7890A gas chromatograph equipped with a flame ionization detector (FID) operating at 280 °C, using the same column and analytical conditions as described for GC–MS, except for the carrier gas, which, in this case, was hydrogen (1.5 mL/min). An internal standard (methyl octanoate) was added to all samples. The raw peak areas were normalized using the internal standard area56 and corrected using predicted response factors57. All calculations were performed using a series of pre-programmed Excel electronic sheets58. The FID analyses were carried out in triplicate.
Pseudovirus (PsV) production and cell line
The HeLa cells expressing human angiotensin-converting enzyme 2 (HeLa ACE-2) were provided by Dr. Dennis Burton (The Scripps Research Institute, La Jolla, CA). SARS-CoV-2 pseudovirus was produced from plasmids containing the SARS-CoV-2 spike genes [pSARS-CoV2-Strunc delta, pCRV1NHG GagPol, and pNanoLuc2AEGFP] following procedures described by Schmidt et al.59 and modified by Alsaidi et al.60.
Antiviral and cytotoxicity assays
The antiviral and cytotoxicity activities were determined using the cell-based pseudoviral entry and the XTT assay, respectively60. All samples were first diluted in complete medium (Dulbecco’s Modified Eagles Medium, 10% FBS, 1% Penicillin + Streptomycin, Thermofisher Scientific, Waltham, MA) containing 20% Dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO). From this stock, nine different dilutions were prepared in the complete medium. For Z. officinale, E. globulus, T. vulgaris, I. verum, R. officinalis, and M. alternifolia, the dilutions ranged from 10,000 to 1.52 µg/mL and for S. aromaticum, C. citratus, O. vulgare, C. limon, P. graveolens, and M. recutita from 500 to 0.08 µg/mL. For cytotoxicity assay, these different dilutions were added in triplicates to clear bottom 96-well microplates containing HeLa ACE-2 cells, and it was incubated at 37 °C, 5% CO2, and 98% humidity for 72 h. XTT (ThermoFisher Scientific, Waltham, MA) was added to all wells after 72-h incubation, and the absorbance was measured at 450 nm using a Spectramax iD3 (Molecular Devices, San Jose, CA). For the antiviral assay, different dilutions of each sample were pre-incubated in triplicates with SARS-CoV-2 Delta PsV at 37 °C, 5% CO2, and 98% humidity for 30 min at 37 °C, 5% CO2, and 98% humidity. The mixture was then transferred to 96-well white opaque microplates containing HeLa ACE-2 cells and the plates were incubated under the same conditions previously mentioned. The TurboLuc™ Luciferase One-Step Glow Assay Kit (ThermoFisher Scientific) was used to determine the percentage entry of PsV in each sample dilution versus the virus control. The half-maximal effective concentration (EC50), half-maximal cytotoxic concentration (CC50), and therapeutic index (TI = CC50/EC50) were determined for each sample.
Data analysis and chemometrics approach
For EC50 and CC50, all samples' dilutions were tested in triplicate in each independent experiment (n = 2). For dose–response–inhibition analysis, the GraphPad Prism software was used (version 9.0.2, GraphPad Sofware, San Diego, California, USA). The principal component analysis (PCA) was used to identify associations between EOs and their composition with EC50 and CC50 values on Statistica 10.0 software (StatSoft, Oklahoma, USA). The partial least-squares regression (PLSR) was applied to determine which functional groups were determinants to increase or decrease the EC50 and CC50 values, considering the partial least square standardized coefficients and the variable importance in projection (VIP) > 1. For PLSR analysis, it was used the XLSTAT software, version 2021.1 (Addinsoft, New York, New York, USA). All statistical analyses were carried out at a 5% significance level.
Supplementary Information
Acknowledgements
The authors are thankful for the financial support provided by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) Brazil—Grant No. [E-26/200.891/2021], [E-26/010.000148/2020], and [E-26/201.790/2020]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Grant No. [313119/2020-1]; and the Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) Brazil Grant No. [88887.518752/2020-00].
Author contributions
L.T.N: Conceptualization, Formal analysis, Data Curation, Writing- original draft. M.L.G.M.: Conceptualization, Formal analysis, Data Curation, Writing-review, and editing. J.F.R.: Formal analysis, Data Curation, Writing-review, and editing. N.T.: Formal analysis, Data Curation, Writing-review, and editing. J.S.: Funding acquisition, Writing-review, and editing. C.A.C.J.: Funding acquisition, project administration, supervision, and writing–review, and editing.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luiz Torres Neto, Email: luiztorresneto@ufrj.br.
Maria Lúcia Guerra Monteiro, Email: marialuciaguerra@yahoo.com.br.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25342-8.
References
- 1.Torres Neto L, Monteiro MLG, Viana FM, Conte-Junior CA. COVID-19 contamination through food: A study with Brazilian consumers of different socioeconomic and demographic characteristics. J. Sens. Stud. 2022 doi: 10.1111/joss.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav, R. & Moon, S. Opinion: Is the pandemic ending soon? WHO Philippineshttps://www.who.int/philippines/news/detail/11-03-2022-opinion-is-the-pandemic-ending-soon (2022).
- 3.Santos S, et al. Cannabidiol and terpene formulation reducing SARS-CoV-2 infectivity tackling a therapeutic strategy. Front. Immunol. 2022;13:841459. doi: 10.3389/fimmu.2022.841459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma J, et al. An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chem. 2021;346:128933. doi: 10.1016/j.foodchem.2020.128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Emergency Situational Updates. Weekly epidemiological update on COVID-19–30 November 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---30-november-2021 (2021).
- 7.Simon-Loriere E, Schwartz O. Towards SARS-CoV-2 serotypes? Nat. Rev. Microbiol. 2022;20:187–188. doi: 10.1038/s41579-022-00708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puhach O, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022 doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg. Microbes Infect. 2022;11:277–283. doi: 10.1080/22221751.2021.2023329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L-L, et al. Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant-associated receptor binding domain (RBD) mutations on the susceptibility to serum antibodies elicited by coronavirus disease 2019 (COVID-19) infection or vaccination. Clin. Infect. Dis. 2022;74:1623–1630. doi: 10.1093/cid/ciab656. [DOI] [PubMed] [Google Scholar]
- 11.Das S, et al. The controversial therapeutic journey of chloroquine and hydroxychloroquine in the battle against SARS-CoV-2: A comprehensive review. Med. Drug Discov. 2021;10:100085. doi: 10.1016/j.medidd.2021.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ćavar Zeljković S, Schadich E, Džubák P, Hajdúch M, Tarkowski P. Antiviral activity of selected lamiaceae essential oils and their monoterpenes against SARS-Cov-2. Front. Pharmacol. 2022;13:1589. doi: 10.3389/fphar.2022.893634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singla RK, et al. Natural products for the prevention and control of the COVID-19 pandemic: Sustainable bioresources. Front. Pharmacol. 2021;12:3215. doi: 10.3389/fphar.2021.758159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejera E, et al. Computational modeling predicts potential effects of the herbal infusion “horchata” against COVID-19. Food Chem. 2022;366:130589. doi: 10.1016/j.foodchem.2021.130589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahun M, et al. Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 2022;373:131594. doi: 10.1016/j.foodchem.2021.131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Yao L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules. 2020;25:2627. doi: 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wani AR, Yadav K, Khursheed A, Rather MA. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021;152:104620. doi: 10.1016/j.micpath.2020.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres Neto L, Monteiro MLG, Galvan D, Conte-Junior CA. An evaluation of the potential of essential oils against SARS-CoV-2 from in silico studies through the systematic review using a chemometric approach. Pharmaceuticals. 2021;14:1138. doi: 10.3390/ph14111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mezarina JPIM, et al. Antiviral effect of mouthwashes against SARS-COV-2: A systematic review. Saudi Dent. J. 2022;34:167–193. doi: 10.1016/j.sdentj.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojah EO. Exploring essential oils as prospective therapy against the ravaging Coronavirus (SARS-CoV-2) Iberoam. J. Med. 2020;4:322–330. doi: 10.53986/ibjm.2020.0056. [DOI] [Google Scholar]
- 21.Tshibangu DST, et al. Possible effect of aromatic plants and essential oils against COVID-19: Review of their antiviral activity. J. Complement. Altern. Med. Res. 2020 doi: 10.9734/jocamr/2020/v11i130175. [DOI] [Google Scholar]
- 22.Valussi M, Antonelli M, Donelli D, Firenzuoli F. Appropriate use of essential oils and their components in the management of upper respiratory tract symptoms in patients with COVID-19. J. Herb. Med. 2021;28:100451. doi: 10.1016/j.hermed.2021.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asif M, Saleem M, Saadullah M, Yaseen HS, Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28:1153–1161. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da Silva JKR, Figueiredo PLB, Byler KG, Setzer WN. Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: An in-silico investigation. Int. J. Mol. Sci. 2020;21:3426. doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salim, B. & Noureddine, M. Identification of compounds from Nigella Sativa as new potential inhibitors of 2019 Novel Coronasvirus (Covid-19): Molecular docking study. ChemRxiv. 10.26434/chemrxiv.12055716.v1 (2020).
- 26.Thuy BTP, et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasanth, D. S. N. B. K. et al. In-silico strategies of some selected phytoconstituents from Melissa officinalis as SARS CoV-2 main protease and spike protein (COVID-19) inhibitors. Mol. Simul.47, 457–470 (2021).
- 28.Kulkarni SA, et al. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020;1221:128823. doi: 10.1016/j.molstruc.2020.128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senthil Kumar KJ, et al. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants. 2020;9:770. doi: 10.3390/plants9060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gendrot M, et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med. Infect. Dis. 2020;37:101873. doi: 10.1016/j.tmaid.2020.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquereau S, et al. Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro. Viruses. 2021;13:354. doi: 10.3390/v13020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touret F, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao R, et al. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 2020;6:2524–2531. doi: 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- 34.Rogers TF, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science (80-) 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stelzer-Braid S, et al. Virus isolation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for diagnostic and research purposes. Pathology. 2020;52:760–763. doi: 10.1016/j.pathol.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadalam PK, et al. Antiviral essential oil components against SARS-CoV-2 in pre-procedural mouth rinses for dental settings during COVID-19: A computational study. Front. Chem. 2021;9:1–11. doi: 10.3389/fchem.2021.642026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santra S, et al. Structure-based assortment of herbal analogues against spike protein to restrict COVID-19 entry through hACE2 receptor: An in-silico approach. Acta Biol. Szeged. 2021;64:159–171. doi: 10.14232/abs.2020.2.159-171. [DOI] [Google Scholar]
- 38.Altayeb, H. et al. Potential activity of a selected natural compounds on SARS-CoV-2 RNA-dependent-RNA polymerase, and binding affinity of the receptor-binding domain (RBD). 10.21203/rs.3.rs-32971/v1. (2020)
- 39.Rout J, Swain BC, Tripathy U. In silico investigation of spice molecules as potent inhibitor of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1819879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thuy BT, Vo Du Nhan VD, Quang NM, Duoc NT, Tat PV. Evaluation of SARS-CoV-2 inhibition of some compounds inCYMBOPOGON CITRATUS oil combining docking and molecular dynamics simulations. Vietnam J. Chem. 2021;59:790–799. [Google Scholar]
- 41.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama S, Kondo K, Ueha R, Kashiwadani H, Heinbockel T. Possible use of phytochemicals for recovery from COVID-19-induced anosmia and ageusia. Int. J. Mol. Sci. 2021;22:8912. doi: 10.3390/ijms22168912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Jesus, M., Gaza, J. T., Junio, H. & Nellas, R. Molecular docking studies of aromatherapy oils against SARS-COV-2 a preprint (2020).
- 44.Panikar S, et al. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health. 2021;14:601–610. doi: 10.1016/j.jiph.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseliou M, Pirintsos SA, Lionis C, Castanas E, Sourvinos G. Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. F., Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 2019;17–18:100288. doi: 10.1016/j.hermed.2019.100288. [DOI] [Google Scholar]
- 46.Russo R, Corasaniti MT, Bagetta G, Morrone LA. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid.-Based Complement. Altern. Med. 2015;2015:1–9. doi: 10.1155/2015/397821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei A, Shibamoto T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010;58:7218–7225. doi: 10.1021/jf101077s. [DOI] [PubMed] [Google Scholar]
- 48.Tuttolomondo T, et al. Biomolecular characterization of wild sicilian oregano: Phytochemical screening of essential oils and extracts, and evaluation of their antioxidant activities. Chem. Biodivers. 2013;10:411–433. doi: 10.1002/cbdv.201200219. [DOI] [PubMed] [Google Scholar]
- 49.Mesa-Arango AC, et al. Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: Composition, cytotoxicity and antifungal activity. Mem. Inst. Oswaldo Cruz. 2009;104:878–884. doi: 10.1590/S0074-02762009000600010. [DOI] [PubMed] [Google Scholar]
- 50.Ranjitkar S, et al. Cytotoxic effects on cancerous and non-cancerous cells of trans-cinnamaldehyde, carvacrol, and eugenol. Sci. Rep. 2021;11:16281. doi: 10.1038/s41598-021-95394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stránská R, et al. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: Prevalence and characterization. J. Clin. Virol. 2005;32:7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Pelvan E, et al. Development of propolis and essential oils containing oral/throat spray formulation against SARS-CoV-2 infection. J. Funct. Foods. 2022;97:105225. doi: 10.1016/j.jff.2022.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLafferty FW, Stauffer DB. Registry of Mass Spectral Data. Wiley; 1994. [DOI] [PubMed] [Google Scholar]
- 54.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publ. Corp; 2007. [Google Scholar]
- 55.van Den Dool H, Dec Kratz P. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 56.Bicchi C, et al. Quantitative analysis of essential oils: A complex task. Flavour Fragr. J. 2008;23:382–391. doi: 10.1002/ffj.1905. [DOI] [Google Scholar]
- 57.Cachet T, et al. IOFI recommended practice for the use of predicted relative-response factors for the rapid quantification of volatile flavouring compounds by GC-FID. Flavour Fragr. J. 2016;31:191–194. doi: 10.1002/ffj.3311. [DOI] [Google Scholar]
- 58.Bizzo H, Barboza E, Santos M, Gama P. Um conjunto de planilhas eletrônicas para identificação e quantificação de constituintes de óleos essenciais. Quim. Nova. 2020 doi: 10.21577/0100-4042.20170458. [DOI] [Google Scholar]
- 59.Schmidt F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217:e20201181. doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alsaidi S, et al. Griffithsin and Carrageenan combination results in antiviral synergy against SARS-CoV-1 and 2 in a pseudoviral model. Mar. Drugs. 2021;19:418. doi: 10.3390/md19080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.