Abstract
Introduction
The variety, time patterns and long-term prognosis of persistent COVID-19 symptoms (long COVID-19) in patients who suffered from mild to severe acute COVID-19 are incompletely understood. Cohort studies will be combined to describe the prevalence of long COVID-19 symptoms, and to explore the pathophysiological mechanisms and impact on health-related quality of life. A prediction model for long COVID-19 will be developed and internally validated to guide care in future patients.
Methods and analysis
Data from seven COVID-19 cohorts will be aggregated in the longitudinal multiple cohort CORona Follow Up (CORFU) study. CORFU includes Dutch patients who suffered from COVID-19 at home, were hospitalised without or with intensive care unit treatment, needed inpatient or outpatient rehabilitation and controls who did not suffer from COVID-19. Individual cohort study designs were aligned and follow-up has been synchronised. Cohort participants will be followed up for a maximum of 24 months after acute infection. Next to the clinical characteristics measured in individual cohorts, the CORFU questionnaire on long COVID-19 outcomes and determinants will be administered digitally at 3, 6, 12, 18 and 24 months after the infection. The primary outcome is the prevalence of long COVID-19 symptoms up to 2 years after acute infection. Secondary outcomes are health-related quality of life (eg, EQ-5D), physical functioning, and the prevalence of thromboembolic complications, respiratory complications, cardiovascular diseases and endothelial dysfunction. A prediction model and a patient platform prototype will be developed.
Ethics and dissemination
Approval was obtained from the medical research ethics committee of Maastricht University Medical Center+ and Maastricht University (METC 2021-2990) and local committees of the participating cohorts. The project is supported by ZonMW and EuroQol Research Foundation. Results will be published in open access peer-reviewed scientific journals and presented at (inter)national conferences.
Trial registration number
Keywords: COVID-19, EPIDEMIOLOGY, Protocols & guidelines, Public health
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Survivors from seven existing COVID-19 cohorts will be asked to participate in CORona Follow Up (CORFU); clinical data will be aggregated and enriched with results from questionnaires on symptoms, health-related quality of life and societal impact at synchronised follow-up moments to estimate the prevalence and pathophysiological mechanisms of long COVID-19, the impact on health-related quality of life and their key determinants.
A control group of Dutch participants from the general population, who did not suffer from COVID-19, will be included for comparison with regard to the prevalence and health-related quality of life.
The heterogeneous cohort populations enable CORFU to investigate study aims in various subgroups (eg, home isolated vs hospitalised patients) and test pathophysiological hypotheses.
An over-representation of (former) COVID-19 patients admitted to the hospital (ward or intensive care unit) might exist, potentially resulting in over-representation of more severe cases of long COVID-19, all of which will be considered in the analysis and presentation.
Introduction
The WHO defines the post-COVID-19 condition, also known as long COVID, as a condition that occurs 3 months from the onset of infection, with symptoms that last for at least 2 months and are not explained by an alternative diagnosis.1 The prevalence of long COVID symptoms varies in literature, ranging from 40% to 68% six months after COVID diagnosis and up to 49% after 12 months.2–4 Frequently reported symptoms include fatigue, shortness of breath, headache, cognitive impairment (eg, concentration problems), muscle weakness and joint stiffness.5–8 Persistent long COVID symptoms are associated with poorer health-related quality of life.9 10 Furthermore, there is an increased risk of incident cardiovascular complaints and cardiovascular diseases for people who suffered from COVID beyond the first month of infection.11 12 Next to the physical and mental symptoms of long COVID, there is a psychological and emotional impact which might be induced by the social restrictions and financial impact (including income uncertainty) during the pandemic.13 14
Long COVID occurs both in patients with mild and with severe acute course. So far, the severity of the acute infection seems related to the risk of long COVID symptoms.15–17 Additional factors affecting the risk of long COVID are the presence of one or more pre-existent comorbidities and being a middle-aged female.16 18 19 Whether the type of SARS-CoV-2) strain relates to long COVID is unknown.
In patients suffering from critical COVID-19 in the intensive care unit (ICU), long COVID-19 symptoms may coexist with, or be indistinguishable from, the post-IC syndrome, defined as newly emerging physical, cognitive or mental limitations after suffering from severe disease during ICU stay.20
Due to the novelty of COVID-19, studies focus on short term physical functioning and mental well-being. However, less is known about persisting COVID-19 symptoms up to 2 years after acute infection, and the factors determining prognosis (if any). More knowledge will facilitate long COVID-19 (health)care and follow-up (eg, specific services such as (lung)rehabilitation and occupational support) for specific patient groups, guided by prognostic information. This knowledge may translate into national and international guidelines on the prevention, diagnosis and treatment of long COVID-19.
This paper describes the protocol of the CORona Follow Up (CORFU) study: a national longitudinal, multiple cohort study that aggregates data of existing cohorts and enriches these data with repeated digital follow-up questionnaires on long COVID symptoms and health-related quality of life up to 2 years after the first infection. Five aims, summarised in four work packages (WP), have been formulated:
-
WP1:
To describe the prevalence, severity, time patterns and duration of long COVID symptoms up to 2 years after acute infection and their relationship with health-related quality of life.
To describe the received rehabilitation and paramedical support in relation to the persisting symptoms and health-related quality of life.
WP2:
To investigate the pathophysiological mechanisms that may cause long COVID symptoms and the role of vulnerability/resilience factors.
WP3:
To develop and validate a prediction model for the persistence of symptoms, stratified by severity of COVID.
WP4 (in collaboration with EuroQol Research Foundation):
Develop a patient platform prototype where patients can digitally consult their reported outcomes, compare them with previous outcomes, relate to reference information and find reported information that fits their situation.
Methods and analysis
Study design
The CORFU study is a longitudinal multiple cohort study that aggregates data of seven existing Dutch COVID-19 cohorts, prospectively complemented with routinely collected outcome data on long COVID-19, with a maximum follow-up of 24 months after initial infection. Data will be collected between 1 October 2021 and 31 December 2022.
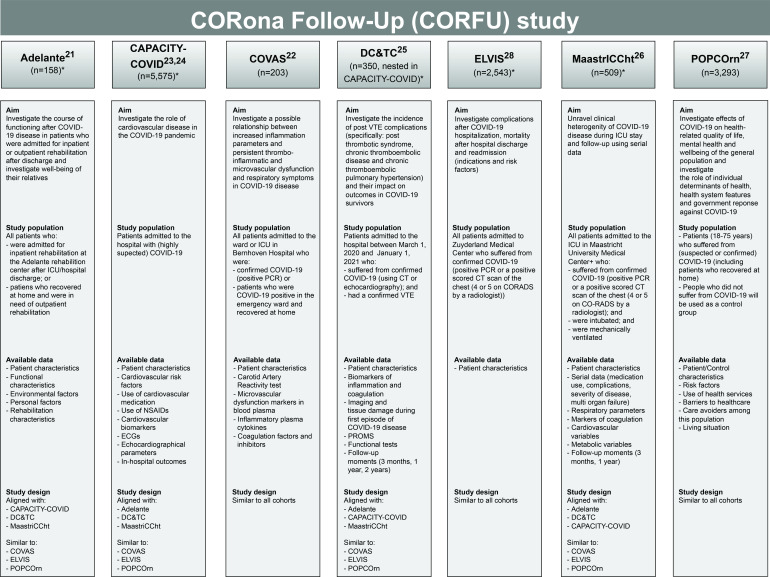
All cohorts were initiated and designed to conduct COVID-19 research. Six cohorts will collect data according to their individual clinical focus (figure 1). In addition, participants from the community-based POPulation health impact of the COVID-19 pandemic (POPCOrn) cohort will serve as a control group as this cohort partly consists of controls who did not suffer from COVID-19. However, at present, many of the POPCOrn participants could have suffered from (mild) COVID-19. Therefore, all POPCOrn participants will be asked repeatedly to report whether or not they suffered from (confirmed of suspected) COVID-19 and only the participants who did not suffer from COVID-19 will serve as a control. In the POPCOrn cohort, similar outcome data will be collected as in the other participating cohorts. As CORFU is open to new collaborations, it is likely that additional cohorts will join CORFU in the future. Participation of new cohorts will be reported when presenting the CORFU study findings.
Figure 1.
Overview of the participating COVID-19 cohorts in the CORFU study. The source population included in the individual cohorts is an estimate as of May 2022. The total number of CORFU study participants might not add up to the total source population (n=12 631) due to non-survivors, participants included in multiple cohorts and participants who might not want to participate in the CORFU study. *At the time of manuscript preparation, prospective inclusion is ongoing in five cohorts: Adelante, CAPACITY-COVID, DC&TC, ELVIS and MaastrICCht cohort. CAPACITY-COVID, Cardiac complications in patients with COVID-19 cohorts; CORADS, COVID-19 Reporting and Data System Score; COVAS, Bernhoven Early detection of Vascular damage after COVID-19 cohort; DC&TC cohort, Dutch COVID and Thrombosis Consortium cohort; ELVIS, ZuydErLand COVID-19 regiStry; ICU, Iintensive care unit; MaastrICCht, Maastricht Intensive Care COVID cohort; NSAID, non-steroidal anti-inflammatory drug; POPCOrn, POPulation health impact of the COVID-19 pandemic; PROMS, Patient-Reported Outcome Measures; VTE, venous thromboembolic.
The cohort-specific follow-up measurements will be complemented by a repeatedly administered CORFU questionnaire covering the full array of long COVID symptoms, health-related quality of life effects and their key determinants. Furthermore, (clinical) data that have already been collected in the participating cohorts during the acute COVID stage will be used to investigate the CORFU study aims.
Participants
The study population consists of Dutch (former) COVID-19 survivors and non-COVID-19 controls, who have been included in one of the cohorts. Former COVID-19 cases are either confirmed by a positive PCR test for SARS-CoV-2 and/or a positive CT scan of the chest based on the COVID-19 Reporting and Data System (CO-RADS) Score (score 4–5 by a radiologist), or likely based on self-reported questionnaires (ie, unspecified positive COVID-19 test or the presence of COVID-19-related symptoms). The study population is categorised into five subgroups:
Patients who suffered from confirmed COVID-19 admitted to the hospital ward.
Patients who suffered from confirmed COVID-19 admitted to the ICU.
Patients who suffered from either confirmed or likely (ie, self-reported) COVID-19 at home.
Patients who suffered from confirmed COVID-19 and needed inpatient or outpatient rehabilitation after infection at home or in the hospital (ward and/or ICU).
Controls who (likely, ie, self-reported) did not suffer from COVID-19.
Adult participants (≥18 years) with confirmed or suspected COVID-19 and non-COVID-19 controls who sufficiently master the Dutch language will be eligible for inclusion in the CORFU study. No additional exclusion criteria will be used. In addition to provided consent or no declared objection for initial cohort participation, all participants will be asked to give written and/or digital informed consent prior to the first CORFU follow-up questionnaire (if not already covered by the specific cohort inclusion scheme).
Data sources: COVID-19 cohorts
Data will be derived from the following seven COVID-19 cohorts:
Adelante cohort.21
Bernhoven early detection of vascular damage after COVID-19 (COVAS) cohort.22
Cardiac complications in patients with COVID-19 (CAPACITY-COVID) cohort.23 24
Dutch COVID-19 and Thrombosis Consortium cohort.25
MaastrICCht cohort.26
POPulation health impact of the COVID-19 pandemic (POPCOrn) cohort.27
ZuydErLand COVID-19 regiStry (ELVIS) cohort.28
Figure 1 shows the aims, study population and outcomes of each cohort. Electronic case report forms will be used to facilitate data aggregation in order to answer the CORFU study aims. Moreover, the study designs of the majority of the individual cohorts have been aligned in the conceptualisation phase. This includes synchronisation of the follow-up moments during which CORFU data will be collected prospectively, as well as a synchronisation of the additional (clinical) data that will be collected in the individual cohorts, including their level of measurement.
In May 2022, the total source population of the participating cohorts included 12 631 participants. However, the total number of CORFU participants will be lower, as it depends on the survival and the CORFU response rates in the individual cohorts, as well as the number of participants which are included in multiple cohorts. In addition, five out of seven cohorts are prospectively including new patients. The CORFU study population will be described in more detail when reporting the study findings.
Data collection: CORFU questionnaire
Besides the clinical data collection in the cohorts, the CORFU questionnaire will be periodically administered to study participants up to 2 years after suffering from COVID-19. The CORFU questionnaire is based on an internationally developed basic questionnaire on persistent symptoms after COVID-19.27 It is digitally adaptive and includes questions on the following outcomes and determinants:
Outcomes
Long COVID symptoms, with a five-level severity scale.
Health-related quality of life (EuroQol 5 Dimenions 5 Levels (EQ-5D-5L), EuroQol Visual Analogue Scale (EQ-VAS)).
Anxiety and depression (Hospital Anxiety and Depression Scale (HADS), Generalized Anxiety Disorder 2-item (GAD-2), Patient Health Questionnaire-2 (PHQ-2)).
Social participation and connectedness.
Experienced stigmatisation and resilience.
Consequences for employment status and personal income.
Determinants
COVID-19-related factors (eg, date of diagnosis and/or clinical admission, severity, wave as surrogate for SARS-CoV-2 strain).
Sociodemographic and diversity factors (eg, age, sex, gender, socioeconomic).
Presence of chronic disease or pre-existing vulnerability.
Impact on healthcare access and experienced quality, healthcare avoidance and self-care.
Vaccination status at the moment of acute infection.
The CORFU questionnaire will be digitally administered at 3, 6, 12, 18 and 24 months after COVID-19 via a web-based survey or, if requested, on paper. On an individual level, the follow-up moments on which the CORFU questionnaire will be administered depends on the date of first infection (diagnosis and/or admission). In retrospect, not all follow-up moments will apply to all participants. As the CORFU study duration is 15 months, participants will receive a maximum of three CORFU questionnaires. Completing the questionnaire takes, on average, 20–25 min, and participants will receive regular reminders to optimise the response rate.
As study participants were included at different time point in the COVID-19 pandemic, depending on their date of first infection, different contextual factors might apply such as lockdowns, the availability of testing material and testing policy, and the vaccination strategy at that time. These factors are presented in detail in online supplemental table S1.
bmjopen-2022-065142supp001.pdf (254.9KB, pdf)
Outcome variables
The primary outcome is the prevalence of long COVID symptoms up to 2 years after infection. Symptoms include, but are not limited to, fatigue, muscle weakness, respiratory complaints, cardiovascular complaints, cognitive impairment, anxiety and depression. Secondary outcomes are health-related quality of life, physical functioning, and the prevalence of thromboembolic complications, respiratory complications, cardiovascular diseases and endothelial dysfunction.
Initially, the WHO definition will be used to define long COVID.1 Potentially identified long COVID phenotypes as part of WP1 and other international developments within the field will also be further considered throughout the study.
Data management and data safety
The data will be stored and accessible according to Findability, Accessibility, Interoperability and Reusability (FAIR) data standards.29 For this, we will apply a machine-readable metadata scheme. Two trusted third parties will administer the digital questionnaires in the individual cohorts: Durrer Center for Cardiovascular Research, Amsterdam, the Netherlands, and Triqs, Zwolle, the Netherlands. Durrer Center facilitates autonomous and secure data management and is founded by the Netherlands Heart Institute. Triqs is an innovative research agency facilitating data collection through digital questionnaires.
The data flow is as follows. First, Durrer Center will receive participants’ contact details from the participating cohorts, check these for any flaws (eg, missing contact details and duplications) and encrypt the contact details except email prior to sharing these with Triqs. Next, Triqs will invite the participants for consent and, subsequently, for participation in the CORFU questionnaire. On consent, participants will digitally receive the questionnaires. After that, Triqs will store the resulting data records (still encrypted) and send these to Durrer Center. Durrer Center will decrypt and subsequently verify each data record and create a pseudoanonymised dataset which will be made available to the CORFU research group and the participating cohorts. As part of the data process, obligatory General Data Protection Regulation contracts will be created between the participating hospitals (care units), Durrer Center, Triqs and the CORFU study unit. In addition, data access agreements will be arranged between the CORFU study unit and the participating cohorts; poststudy secondary analysis of the survey data has been agreed on in collaboration with the EuroQol Research Foundation. Both Durrer Center and Triqs work processes and facilities meet the Dutch privacy legislation standards (International Organization for Standardization and NEderlandse Norm (NEN) norms).
WPs and data analysis
This paragraph describes a generic outline of aims and methods of the WPs. Future manuscripts on CORFU WP findings will describe the aims and used methods in more detail. Table 1 displays the WPs and the corresponding aims and involved cohorts. Within all WPs, baseline data of participants will be described in detail, stratified by subgroup if necessary. Missing data will be imputed if the percentage of incomplete records exceeds 5% using multiple imputation with fully conditional specification. The number of imputations will be set to the percentage of incomplete records, and values will be drawn using predictive mean matching.30 The primary analyses of the WPs 1–3 aims will be performed with data from CORFU participants who (likely) suffered from COVID-19. Sensitivity analyses will be performed for the subgroup of participants who suffered from confirmed COVID-19 (ie, positive PCR test for SARS-CoV-2 and/or a CO-RADS Score of 4–5).
Table 1.
Overview of the work packages, including study aims and cohorts
| WP | Aim | Cohorts involved* |
| WP1 | 1. Describe the prevalence, severity, time patterns and duration of long COVID symptoms up to 2 years after acute infection and their relationship with health-related quality of life. 2. Describe the received rehabilitation and paramedical support in relation to the persisting symptoms and health-related quality of life. |
Data from all cohorts will be used. |
| WP2 | 3. Describe the pathophysiological mechanisms that may cause long COVID symptoms and the role of vulnerability/resilience factors. | Every cohort will deliver results for specific pathophysiological hypotheses. |
| WP3 | 4. Develop and validate a prediction model for the persistence of symptoms, stratified by severity of COVID-19. | Data from all cohorts will be used. |
| WP4 | 5. Develop a patient platform prototype where patients can digitally consult their reported outcomes, compare them with previous outcomes, relate to reference information and find reported information that fits their situation. | The platform will be developed and tested in a limited no of cohorts (to be determined). |
*CORFU cohorts include Adelante, CAPACITY-COVID, COVAS, DC&TC, ELVIS, MaastrICCht and POPCOrn.
CAPACITY-COVID, Cardiac complications in patients with COVID-19 cohorts; DC&TC cohort, Dutch COVID and Thrombosis Consortium cohort; COVAS, Bernhoven Early detection of Vascular damage after COVID-19 cohort; ELVIS, ZuydErLand COVID-19 regiStry; MaastrICCht, Maastricht Intensive Care COVID cohort; POPCOrn, POPulation health impact of the COVID-19 pandemic; WP, work package.
WP1 will investigate the first and second study aims. First, data of the seven cohorts will be aggregated and used to estimate the prevalence and severity of long COVID symptoms, expressed as a percentage with a 95% CI and as a distribution of severity scores at every follow-up moment. Next, the association between symptom severity and health-related quality of life will be quantified using linear regression analysis and repeated measurements analysis, adjusted for potential confounders. Finally, rehabilitation and paramedical support will be described using descriptive statistics. Analyses will be stratified by participant subgroups (ie, suffered from COVID at home, admitted to hospital ward, admitted to ICU). Furthermore, long COVID ‘phenotypes’ (ie, subtypes: patients with similar expressions of symptoms) will be estimated. Important phenotypes may depend on combinations of previously reported domains of symptoms (eg, respiratory, cardiovascular), but phenotypes may also depend on previously unidentified combinations. Clusters of patients will be estimated using unsupervised machine learning techniques with K-means and hierarchical clustering, which are data supportive and thereby not confirmatory (of prior hypotheses) in nature.31
WP2 will investigate the third study aim. Each cohort will formulate specific long COVID research questions related to various pathophysiological mechanisms and data availability. Table 2 shows examples of research questions that will be studied. To explore these pathophysiological mechanisms, data of different cohorts will be aggregated when possible. Next, we will develop directed acyclic graphs (DAGs), presenting (presumed) causal relationships based on current knowledge and new hypotheses while considering long COVID phenotypes identified in WP1. Subsequently, multivariable regression modelling will be used to test the various causal models (expressed in DAGs). Confounding, effect modification and mediation will be considered by testing as model parameters. Associations will be presented as regression coefficients or ORs, including 95% CIs. Analyses will be performed separately for the individual cohort data and for the joint cohort data in which the same outcome measures were used.
Table 2.
Overview of work package 2 (WP2) hypotheses on pathophysiological mechanisms that might cause long COVID symptoms
| Pathophysiological mechanism | Main research questions include, but are not limited to: | Cohort (minimally) involved |
| Thromboembolic complications | 1. What is the impact of venous thromboembolic complications on long-term functional outcomes in COVID-19 survivors? | DC&TC |
| Cardiovascular diseases | 2. What is the impact of myocardial damage during hospital ward or ICU stay due to COVID-19 on angina pectoris and dyspnoea over time? | CAPACITY-COVID |
| Endothelial dysfunction | 3. What is the relationship between elevated inflammation parameters and persistent thrombo-inflammation, coagulation, microvascular and macrovascular dysfunction, and respiratory symptoms after COVID-19 disease? | COVAS |
| Multi organ failure | 4. What is the impact of multiorgan failure during ICU stay on long-term functional outcomes and (health-related) quality of life in COVID-19 survivors? | MaastrICCht |
| Pre-existing coronary atherosclerosis | 5. What is the relationship between pre-existing clinical and subclinical coronary atherosclerosis, angina pectoris and respiratory symptoms after COVID-19 infection? | ELVIS |
| NA | 6. What is the level of functioning during the course of disease in patients following a rehabilitation programme after COVID-19-related ICU admission? | Adelante |
CAPACITY-COVID, Cardiac complications in patients with COVID-19 cohorts; DC&TC cohort, Dutch COVID and Thrombosis Consortium cohort; COVAS, Bernhoven Early detection of Vascular damage after COVID-19 cohort; ELVIS, ZuydErLand COVID-19 regiStry; ICU, intensive care unit; MaastrICCht, Maastricht Intensive Care COVID cohort; NA, not applicable; POPCOrn, POPulation health impact of the COVID-19 pandemic.
WP3 will investigate the fourth study aim. In order to develop a prediction model, we will aim to identify the set of predictors, measured at time of COVID-19 diagnosis and during the course of the disease, that will maximise the ability to discriminate patients who experience long COVID-19 symptoms from patients who do not experience these symptoms. Potential predictors will be selected from the living review by Wynants et al and recent literature.32 Using backward stepwise elimination on the Akaike information criterion in logistic regression analysis, the initial model structure and parameters (including follow-up period) will be estimated. In addition, the model will be internally validated using bootstrapping techniques.
WP4 will address the fifth study aim: developing and testing a patient platform prototype. As the patient platform will be connected to the digital questionnaire platform, individual CORFU questionnaire responses can be presented individually to the corresponding patient. It offers the possibility for patients to consult their own situation, compare this with the past and with the situation of similar patients, profit from suggestions of other patients in similar situations, and gain insight into their future health. The specific content (eg, which symptom domains and other domains of interest) to be presented in the patient platform will be based on focus groups with healthcare professionals, (former) patients and patient representatives. Patient platforms aim to increase empowerment and reassurance (outcomes tested) and might provide guidance in healthcare-seeking and self-care. By providing feedback on the given answers, the platform increase patients’ knowledge and self-consciousness about the potential existence of long COVID-19 symptoms and change over time, thereby putting them in own data-driven control on their health situation.
Sample size calculation
The sample size for this study is established pragmatically. A heterogeneous sample of COVID-19 patients is included by choosing different cohorts with considerable heterogeneity in the severity of the disease, national coverage and without any exclusion criteria. This resulted in a collection of seven small to very large cohorts. Our sample size calculations of WP1, WP2 and WP3 are based on the smallest subgroup of patients that will be analysed in this study.
First, for estimating the prevalence of long COVID symptoms (WP1), for the least favourable percentage of 50% (the variance of a percentage is highest at 50%), the maximum width of the 95% CI will be approximately plus and minus 5%. For all other percentages, the CI will be even smaller. Second, for investigating the pathophysiological mechanisms that may cause long COVID symptoms (WP2), there will be sufficient power to detect associations with symptom severity, expressed in standardised effect size (Cohen’s d) of 0.3, with a power of 80% and a type-I error of 5%. Third, for the development of a prediction model for long COVID complaints (WP3), we anticipate a large number of cases, taking into account that 57% of patients suffered from at least one long COVID complaint up to 6 months after infection.15 Depending on the response rate and resulting sample size, we will determine the maximum number of candidate predictors we can use for multivariable modelling with the method of Riley et al, allowing a maximum shrinkage of predictor coefficients of 0.9.33
Statistical tests or estimations are not part of WP4. Therefore, sample size or statistical power calculations are not applicable for WP4.
Patient and public involvement
Patient organisations (family and patient centred intensive care, IC Connect and the ‘Hartenraad’) and patients of the Maastricht University Medical Centre+ (MUMC+) intensive care panel were involved in the design of the CORFU study. Patients were involved in the development and testing of the international basic questionnaire on persistent symptoms after COVID-19, which serves as the basis for the CORFU questionnaire. In addition, patients provided feedback on the phrasing of questions, the fill-out time of the questionnaire and the willingness to fill out the questionnaire periodically. Participants will be able to provide feedback on the (missing) content of the CORFU questionnaire through an open-ended question. Comments will be discussed and implemented prospectively when deemed relevant, making the CORFU questionnaire a continuously developing measurement instrument.
Patients will have an advisory role in developing the patient platform prototype (WP4), which allows patients to digitally consult their answers in real time and compare them with reference populations. In addition, advice will be asked on the (type of) provided feedback questions, the formatting and visualisation of answers, and the relevant reference groups to be considered. Eventually, CORFU findings will be presented in a lay summary, and a flyer on long COVID will be developed in close collaboration with patients. The dissemination strategy of CORFU findings and the long COVID flyer will be based on patient and public preferences, in which also the involved patient organisations will have an important role.
Discussion
The CORFU study has the opportunity to investigate the prevalence, pathophysiological mechanisms, and prediction of long COVID, and its relationship with health-related quality of life. CORFU will aggregate data from seven existing COVID cohorts and will enrich the data with prospective follow-up of long COVID outcomes and determinants up to a maximum of 2 years after acute infection. A prediction model and patient platform prototype will be developed to guide future patient care.
Estimation of the prevalence of long COVID symptoms up to 2 years after infection will be based on the multidimensional CORFU questionnaire, including physical and psychological complaints after COVID. The extensive set of physical complaints gives the opportunity to study symptoms in great detail. The additional focus on psychological impact addresses the call to take COVID psychopathology into account when designing new studies. It reflects the current knowledge that mental well-being is worse in patients who suffered from COVID compared with healthy respondents, and that there is a high rate of mental health complaints up to 1 year after acute infection.27 34
CORFU findings may be used to inform national and international guidelines on diagnostics, treatment and follow-up of long COVID and contribute to developing a (new) more accurate long COVID definition, likely differentiating long COVID phenotypes. Available guidelines and definitions on long COVID are currently, as expected, based on short-term follow-up studies, whereas CORFU will report long COVID symptoms up to 2 years after infection.1 35 Besides, the current WHO long COVID definition remains broad and unspecific, thereby lacking accurate differentiation of its heterogeneous appearance into clinical phenotypes. Defining such phenotypes with potentially adding clinical parameters (biomarkers, imaging, etc) might enhance clinical workability, and thereby diagnostics, and the development of tailored therapies based on underlying pathophysiology.
An important strength of the CORFU study is that data will be aggregated from seven cohorts of (former) COVID-19 patients. Due to mortality and non-response, the effective number might be slightly lower. Nevertheless, the large CORFU sample size allows for robust analysis. Furthermore, the difference in designs of the cohorts allows us to answer study aims for various subgroups (eg, COVID-19 at home vs hospitalised (ward and/or ICU)) and to test multiple, detailed, pathophysiological hypotheses, depending on the characteristics of the patients included in the participating cohorts, also related to COVID-19 variants by using wave at the time of infection as a surrogate marker. Furthermore, the ability to aggregate data of multiple cohorts is an efficient way of (close) national collaboration which contributes to more robust and reliable findings compared with multiple, parallel, single cohort studies with smaller sample sizes.
Another strength is that CORFU will use data from a large control group of respondents who did not suffer from COVID-19. This allows the comparison of the prevalence of long COVID-19 symptoms with the prevalence of these symptoms in the general Dutch, non-COVID-19 population, potentially highlighting the secondary impacts of the pandemic. This is a crucial comparison currently lacking in the majority (79%) of long COVID-19 research but required to identify and quantify attributable symptoms objectively.36 For instance, the (social) restrictions may significantly impact the quality of life and (mental) well-being of the general population.13 27 37 38 These factors need to be considered when analysing and interpreting the CORFU findings. Lastly, as part of WP4, patients will receive personalised feedback on their own questionnaire outcomes and those of other patients in similar situations.
Potential limitations of the CORFU study also merit consideration. First, combining data from seven cohorts is challenging. As each cohort has specific study aims regarding the pathophysiological processes causing long COVID symptoms, not all cohorts collect the same (clinical) information from their participants. To ensure that data from all cohorts can be integrated and that between-cohort comparisons are possible, a minimal set of variables was (post-hoc) harmonised among the participating cohorts regarding background characteristics (eg, sociodemographics, employment status, social, economic status, cultural background), comorbidities and potential confounders. Furthermore, to optimise the data integration process, data received from the cohorts will be transferred into a machine-readable metadata scheme prior to merging the various datasets. Second, there will be an over-representation of (former) COVID patients admitted to the hospital ward and/or ICU compared with those who suffered from COVID at home. This affects estimations of long COVID prevalence, which can be evaluated by post hoc stratification using community-based cases. The epidemiological basis used for the statistical models to develop prediction models is independent of inpatient/outpatient distribution and depends solely on the associations and interactions found within specific patient groups, as COVID severity will be added as a covariate. Moreover, analyses will be stratified by disease severity for subgroup-specific conclusions. Third, especially in the first COVID wave (1 March 2020–30 June 2020) for non-hospitalised patients, not all suspected COVID cases were tested due to capacity and test-material constraints in the Netherlands. For CORFU, this means that there is a lack of confirmed infections for participants of the community-based POPCORN cohort who self-reported to have (likely) suffered from COVID based on an unspecified positive test or the presence of COVID-19-related symptoms. In order to reduce the impact of this limitation, the primary analyses will be repeated in the sensitivity analyses on the subgroup of COVID cases with a confirmed infection. The same holds for controls from the POPCORN cohort who (likely) did not suffer from COVID: there is a possibility that these controls did suffer from COVID, but that they, for example, did not test due to the absence of symptoms or test-material constraints, or that their tests were false negative (online supplemental table S1). This might result in some misclassification of cases that had no or only very mild symptoms, never got tested, and will likely report never having suffered from COVID. This will be described when reporting CORFU study results, and, when deemed relevant, additional (stratified) analyses will be conducted.
Ethics and dissemination
This study will be conducted according to the latest update of the Declaration of Helsinki and is registered at ClinicalTrials.gov (NCT05240742). Ethics approval was obtained from the medical research ethics committee (MREC) of Maastricht University Medical Center+ and Maastricht University (committee reference number METC2021-2990) and the local MRECs of the participating cohorts (online supplemental table S2). Participants will be asked for written or digital informed consent, by the cohort in which they are participating, prior to administering the first CORFU questionnaire and will be informed that participation is voluntary. Data will be made available (Open Science/FAIR) subject to ethical approval and standard access and anonymisation procedures.
Supplementary Material
Footnotes
Contributors: BCTvB, BH, BLJHK, CG-D, EB, FAK, FWA, GJB, HTC, ICCvdH, JH, JWLC, KV, MW and SMJvK conceived and designed the study. CG-D, DK, EBNJJ, MSJNW, SCMH and SMJvK drafted the manuscript. BCTvB, BH, BLJHK, EB, FAK, FWA, GJB, HTC, ICCvdH, JH, JWLC, KV, LHW, MW, MDdK, ML, RW and SvS critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding: This work is supported by The Netherlands Organization for Health Research and Development (ZonMW), grant number 10430302110005, and an unrestricted grant has been received from the EuroQol Research Foundation. Individual COVID-19 cohorts contributing to the CORFU study may have been funded independently from this grant.
Competing interests: BCTvB, BH, BLJHK, JH, DK, EB, EBNJJ, GJB, ICCvdH, JH, JWLC, LHW, MSJNW, MW, RW, SCMH, SMJK, SvS and BH declare no competing interests. FAK received research support from Bayer, BMS, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, Boston Scientific, The Netherlands Organization for Health Research and Development (ZonMW), The Dutch Thrombosis Association, and The Dutch Heart Foundation. FWA is supported by the National Institute of Health Research University College London Hospitals Biomedical Research Centre. For the CAPACITY-COVID cohort participating in CORFU, FWA and ML received support from Dutch Heart Foundation (2020B006 CAPACITY) an The Netherlands Organization for Health Research and Development (ZonMW) (grant number 10430102110006 DEFENCE). HTC received support from Bayer, received consulting fees from Pfizer, Leo, Alveron, Viatris, Astra Zeneca, and Galapagos, and has stock (options) in Coagulation Profile. KV has royalities or licences for Philips, Medtronic, Abbott, and Biosense Webster, received consulting fees from Philips, Biosense Webster, Boston Scientific, and Medtronic, and has a role in the European Heart Rhythm Association congress organization and digital committee. MDdK received a presentation fee from Glaxo Smith Kline. ML is supported by the Alexandre Suerman Stipend of the University Medical Center Utrecht.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus World Health Organization; 2021. [Google Scholar]
- 2.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021;27:1607–13. 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747–58. 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021;27:1507–13. 10.1016/j.cmi.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv 2021. doi: 10.1101/2021.01.27.21250617. [Epub ahead of print: 30 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun 2022;101:93–135. 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttery S, Philip KEJ, Williams P, et al. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Resp Res 2021;8:e001075. 10.1136/bmjresp-2021-001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han JH, Womack KN, Tenforde MW, et al. Associations between persistent symptoms after mild COVID‐19 and long‐term health status, quality of life, and psychological distress. Influenza Other Respi Viruses 2022;16:680–9. 10.1111/irv.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Kelly B, Vidal L, Avramovic G, et al. Assessing the impact of COVID-19 at 1-year using the SF-12 questionnaire: data from the anticipate longitudinal cohort study. Int J Infect Dis 2022;118:236–43. 10.1016/j.ijid.2022.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583–90. 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Network Open 2021;4:e2128568–e68. 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrosa AL, Bitencourt L, Fróes ACF, et al. Emotional, behavioral, and psychological impact of the COVID-19 pandemic. Front Psychol 2020;11:566212–12. 10.3389/fpsyg.2020.566212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 2020;78:185–93. 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynberg E, van Willigen HDG, Dijkstra M, et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis 2021. 10.1093/cid/ciab759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanoski N, Chen X, Becker U, et al. Severity of COVID-19 and adverse long-term outcomes: a retrospective cohort study based on a US electronic health record database. BMJ Open 2021;11:e056284. 10.1136/bmjopen-2021-056284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect 2021. 10.1016/j.cmi.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC who clinical characterisation protocol. Lancet Reg Health Eur 2021;8:100186. 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med 2012;40:502–9. 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 21.Wiertz CMH, Vints WAJ, Maas GJCM, et al. COVID-19: patient characteristics in the first phase of Postintensive care rehabilitation. Arch Rehabil Res Clin Transl 2021;3:100108. 10.1016/j.arrct.2021.100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willems LH, Nagy M, ten Cate H, et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res 2022;209:106–14. 10.1016/j.thromres.2021.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linschoten M, Asselbergs FW. CAPACITY-COVID: a European registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur Heart J 2020;41:1795–6. 10.1093/eurheartj/ehaa280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linschoten M, Uijl A, Schut A, et al. Clinical presentation, disease course, and outcome of COVID-19 in hospitalized patients with and without pre-existing cardiac disease: a cohort study across 18 countries. Eur Heart J 2022;43:1104–20. 10.1093/eurheartj/ehab656 [DOI] [PubMed] [Google Scholar]
- 25.Kruip MJHA, Cannegieter SC, ten Cate H, et al. Caging the dragon: research approach to COVID‐19–related thrombosis. Res Pract Thromb Haemost 2021;5:278–90. 10.1002/rth2.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tas J, van Gassel RJJ, Heines SJH, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht intensive care COVID cohort (MaastrICCht). BMJ Open 2020;10:e040175. 10.1136/bmjopen-2020-040175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long D, Haagsma JA, Janssen MF, et al. Health-related quality of life and mental well-being of healthy and diseased persons in 8 countries: does stringency of government response against early COVID-19 matter? SSM Popul Health 2021;15:100913. 10.1016/j.ssmph.2021.100913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leijte WT, Wagemaker NM, van Kraaij TD, et al. Sterfte en heropname Na ziekenhuisopname Met covid-19. Ned Tijdschr Geneeskd 2020. [PubMed] [Google Scholar]
- 29.Wilkinson MD, Dumontier M, Aalbersberg IJsbrandJ, et al. The fair guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castela Forte J, Perner A, van der Horst ICC. The use of clustering algorithms in critical care research to unravel patient heterogeneity. Intensive Care Med 2019;45:1025–8. 10.1007/s00134-019-05631-z [DOI] [PubMed] [Google Scholar]
- 32.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ 2020;6:m1328. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley RD, Snell KIE, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med 2019;38:1276–96. 10.1002/sim.7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazza MG, Palladini M, De Lorenzo R, et al. One-year mental health outcomes in a cohort of COVID-19 survivors. J Psychiatr Res 2022;145:118–24. 10.1016/j.jpsychires.2021.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, Royal College of General Practitioners . COVID-19 rapid guideline: managing the long-term effects of COVID-19; 2021.
- 36.Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 2021;27:1129–30. 10.1038/s41591-021-01402-w [DOI] [PubMed] [Google Scholar]
- 37.May T, Aughterson H, Fancourt D, et al. ‘Stressed, uncomfortable, vulnerable, neglected’: a qualitative study of the psychological and social impact of the COVID-19 pandemic on UK frontline keyworkers. BMJ Open 2021;11:e050945. 10.1136/bmjopen-2021-050945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epifanio MS, Andrei F, Mancini G, et al. The impact of COVID-19 pandemic and Lockdown measures on quality of life among Italian general population. JCM 2021;10:289. 10.3390/jcm10020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065142supp001.pdf (254.9KB, pdf)