Abstract
C57BL/6, C3H, and BALB/c mice were vaccinated with plasmids encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2, previously described as strong inducers of immunity. Seroconversion for the relevant antigen was obtained in the majority of the animals. T. gondii lysate stimulated specific T-cell proliferation and secretion of gamma interferon (IFN-γ) in spleen cell cultures from vaccinated BALB/c and C3H mice but not in those from control mice. Although not proliferating, stimulated splenocytes from DNA-vaccinated C57BL/6 mice also produced IFN-γ. No interleukin-4 was detected in the supernatants of lysate-stimulated splenocytes from DNA-vaccinated mice in any of the mouse strains evaluated. As in infected animals, a high ratio of specific immunoglobulin G2a (IgG2a) to IgG1 antibodies was found in DNA-vaccinated C3H mice, suggesting that a Th1-type response had been induced. For BALB/c mice, the isotype ratio of the antibody response to DNA vaccination was less polarized. The protective potential of DNA vaccination was demonstrated in C3H mice. C3H mice vaccinated with plasmid encoding GRA1, GRA7, or ROP2 were partially protected against a lethal oral challenge with cysts of two different T. gondii strains: survival rates increased from 10% in controls to at least 70% after vaccination in one case and from 50% to at least 90% in the other. In vaccinated C3H mice challenged with a nonlethal T. gondii dose, the number of brain cysts was significantly lower than in controls. DNA vaccination did not protect BALB/c or C57BL/6 mice. Our results demonstrate for the first time in an animal model a partially protective effect of DNA vaccination against T. gondii.
Toxoplasma gondii most often causes subclinical infection; however, primary infection during pregnancy can induce fetal pathology and abortion in both humans and lower animals. In the chronic phase, reactivation of the infection can be life-threatening for immunocompromised individuals: 18 to 25% of U.S. AIDS patients suffer from Toxoplasma encephalitis (TE) (22). A vaccine against T. gondii would be extremely valuable for preventing both fetal infection and reactivation in immunocompromised individuals. It might also reduce economic losses due to abortion in farm animals.
It is well established that both humoral and cellular immune responses are elicited in Toxoplasma infection and that gamma interferon (IFN-γ) plays a predominant role in controlling both acute and chronic phases of T. gondii infections (reviewed in reference 10). Accumulating evidence indicates that vaccination with stage-specific antigens leads to stage-limited protection (reviewed in reference 1). Therefore, a vaccine inducing a Th1-type immune response against T. gondii antigens that are expressed during the different life stages of the parasite is likely to confer at least partial protection against T. gondii infections. Since the plasmid vectors used for DNA vaccination have been shown to contain immunostimulatory sequences favoring a Th1 response (43), we speculated that DNA vaccination of mice with suitable antigens might induce protective immunity against toxoplasmosis.
In this study, mice were immunized with plasmid DNA encoding three distinct Toxoplasma antigens: GRA1, GRA7, and ROP2. These antigens were chosen because they are expressed in the tachyzoite and bradyzoite life stages of the parasite (8, 15, 37) and because there is evidence that at least GRA1 and ROP2 can elicit potentially protective immune responses (14, 35). The 23-kDa calcium-binding protein GRA1 (antigen P24) is secreted by tachyzoites and bradyzoites (8). It induces humoral immune responses in mice and humans in the chronic phase of the infection (8). Moreover, GRA1 has shown to be protective in two animal models of infection (14, 40). Specific T-cell proliferation has been demonstrated in rats vaccinated with crude secreted antigens and with GRA1-expressing vaccinia virus. Adoptive transfer of T lymphocytes from these vaccinated rats conferred to nude rats partial protection against lethal challenge with the virulent RH strain of T. gondii (14). In addition, immunization of sheep with recombinant Mycobacterium bovis BCG producing and secreting GRA1 resulted in specific, partially protective cellular immune responses characterized by the production of IFN-γ (40). ROP2, a 54-kDa protein, was identified in a human T-cell clone that produced high levels of IFN-γ (35). T-cell-stimulatory peptides from ROP2 recognized by a high proportion of the infected human population have been identified (36). GRA7 is a recently discovered 29-kDa protein (15, 19). Like GRA1, it is secreted from the dense granules (15), and it reacts with sera from humans with acute and chronic infections (20).
In the present study, we used three strains of inbred mice with different major histocompatibility haplotypes and different levels of susceptibility to T. gondii-induced morbidity and mortality: C57BL/6 (H-2b), BALB/c (H-2d), and C3H (H-2k). C57BL/6 mice are highly susceptible, and oral infection with low numbers of encysted bradyzoites leads to a high mortality rate in the acute phase (29). Both H-2k and H-2d mice can survive oral infection (5). BALB/c mice can survive infection with larger numbers of parasites (3), and the cyst load in the brains of infected BALB/c mice is lower than in the intermediately resistant C3H mice (7, 41).
MATERIALS AND METHODS
Plasmid constructions.
All DNA constructs used for vaccination were based on the plasmid vector VR1020, obtained from Vical, Inc., San Diego, Calif. (27). The three genes encoding the antigens of interest (GRA1, GRA7, and ROP2) were PCR amplified (using Taq or Pfu DNA polymerase) from cloned DNA fragments, using a sense primer located at the start of the mature gene (after the putative signal sequence) and an antisense primer located at the end of the coding region, including the stop codon. Sense and antisense primers were designed to contain a BamHI (GRA7 and GRA1 genes) or BglII (ROP2 gene) restriction site to allow in-frame cloning of the gene fragment into the VR1020 vector. The amplified fragments were cloned into vector pGEMT (Promega, Madison, Wis.), and sequence analysis was performed on all three cloned genes to confirm that no PCR mutations were introduced. The genes were then recovered from the pGEMT vector by using either BamHI (GRA1 and GRA7) or BglII (ROP2) and cloned into the BamHI site of the expression vector VR1020 to generate an in-frame fusion with the vector-encoded signal sequence of human tissue plasminogen activator. Thus, the fusion proteins contained the 193, 210, and 535 carboxy-terminal amino acid residues of GRA1, GRA7, and ROP2 proteins, respectively. The orientation of the cloned genes was determined by restriction analysis, and one clone for each gene was selected for large-scale DNA preparation. All plasmids were propagated in Escherichia coli DH1 (4).
DNA for vaccination was purified by using EndoFree Plasmid Giga kits as instructed by the manufacturer (Qiagen, Hilden, Germany).
Vaccination.
Female inbred mice (C57BL/6, BALB/c, and C3H) were purchased from Harlan (Horst, The Netherlands). In addition, some C57BL/6 were purchased from IFFA-Credo (L'Arbresle, France). Vaccination was started when the animals were 6 weeks old. Mice were anesthetized by intramuscular injection of ketamine (100 mg/kg)-xylazine (3 mg/kg) (Rhône-Mérieux, Lyon, France, and Bayer, Leverkusen, Germany) and injected three times (at 3-week intervals) in both quadriceps with 100 μg of DNA, using a 0.3-ml syringe (Becton Dickinson, Paramus, N.J.). As a negative control, the empty vector VR1020 was injected. Mice were bled 3 weeks after the last DNA dose and in some experiments (Fig. 2) also 3 weeks following the first and second doses.
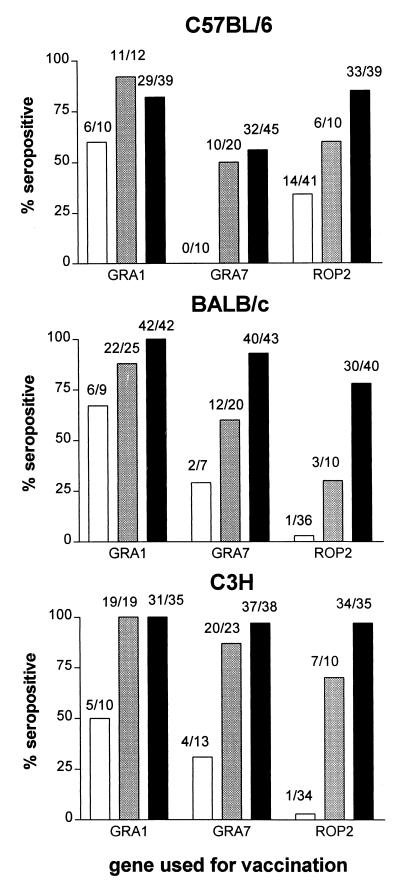
FIG. 2.
Seroconversion rates of C57BL/6, BALB/c, and C3H mice (serum samples from individual animals) 3 weeks after 1 (white bars), 2 (grey bars), and 3 (black bars) intramuscular injections of 100 μg of plasmid DNA encoding GRA1, GRA7, or ROP2, with a 3-week interval between two DNA administrations. Serum samples were considered positive when their absorbance values in ELISA exceeded at least by a factor of 2 the absorbance from a pool of preimmune sera at a dilution 1/200 or further. Numbers above the bars show the absolute numbers of positive/tested animals.
T. gondii strains and doses for peroral challenge of DNA-vaccinated mice.
Two T. gondii strains, 76K and IPB-G, were used for challenge. Strain 76K was obtained from a guinea pig (24) and propagated in Swiss mice by the peroral administration of brain cysts of infected animals every 2 months (9). Strain IPB-G, isolated from the placenta of a patient with congenital toxoplasmosis, is a zymodeme II type strain (45) that has been passaged in Swiss mice by intraperitoneal inoculation of brain cysts once a year. It induces mortality in 20% of these animals. C3H mice were challenged perorally with 50 cysts of strain IPB-G or 76K. BALB/c mice received perorally either 50 or 200 cysts, and C57BL/6 mice received 10 cysts, of strain IPB-G. DNA-vaccinated animals were challenged at 6 or 9 weeks after the third administration of DNA. Brain cyst numbers in surviving animals were assessed at 6 or 8 weeks after challenge.
T. gondii infection.
Female inbred BALB/c and C3H mice were infected perorally with 25 cysts, and C57BL/6 mice were infected intraperitoneally with 10 cysts, of strain 76K. The mice were used at least 2 months following infection.
Preparation of TLA.
Tachyzoites of the virulent T. gondii strain RH were obtained from the peritoneal fluid of infected Swiss mice. The material was passed twice through a 26-gauge needle. The parasites were washed, resuspended in phosphate-buffered saline (PBS), and sonicated (1-min burst, 1-min cooling, 150 W) in an Ultrasonic disintegrator (MSE, Leicester, United Kingdom). The protein concentration of the Toxoplasma lysate (TLA) was determined by using the Bio-Rad DC protein assay and albumin (fraction V; Boehringer Mannheim, GmbH, Mannheim, Germany) to generate a standard curve.
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of TLA was carried out as described by Laemmli (23), using the Bio-Rad (Hercules, Calif.) minigel system (12% polyacrylamide gel). The BenchMark prestained protein ladder (Life Technologies, Grand Island, N.Y.) was used for molecular weight standards. Electrophoretic transfer onto nitrocellulose membranes (Hybond-C; Amersham Pharmacia Biotech, Rainham, United Kingdom) was done with a mini Trans-Blot electrophoretic cell system (Bio-Rad) as instructed by the manufacturer. The membrane was blocked by incubation with 5% dried skimmed milk in TBS-T (10 mM Tris-HCl [Bio-Rad], 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. Antisera were diluted 1:100 in TBS-T and incubated overnight at 4°C. The monoclonal antibody against GRA1 (BATO 35) (34) was used at a dilution of 1:400. Peroxidase-labeled anti-mouse antibody (diluted 1:1,000; Amersham) was used as the secondary antibody (1-h incubation at room temperature). α-1-Naphthol (0.03 g; Bio-Rad) in ice-cold methanol (10 ml; Merck, Darmstadt, Germany) was added to a mixture of 50 ml of TBS and 300 μl of 3% H2O2 solution (Merck); 500 μl of this mixture was added to each membrane strip and further incubated for 10 to 15 min. The reaction was stopped by washing in distilled water.
Enzyme-linked immunosorbent assay (ELISA) for GRA1, GRA7, and ROP2.
To measure total antigen-specific antibodies, Nunc immunoplates (Life Technologies) were coated either overnight at 4°C with crolac-GRA1 (5 μg/ml) (44) and tumor necrosis factor-GRA7 (19) (6 μg/ml) or for 1.5 h at 37°C with reduced crolac-ROP2 (44) in 50 mM carbonate buffer (pH 9.6). Crolac is a 48-amino-acid fusion protein derived from the phage lambda protein Cro and the Escherichia coli protein LacI. Plates were washed in PBS–0.1% Tween, and blocked for 1 h at 37°C in PBS containing 10% fetal calf serum (FCS) (Life Technologies) (GRA1 and GRA7) or in PBS with 0.5% casein (ROP2). After washing, serum samples were diluted either in PBS with 10% FCS (GRA1 and GRA7) or in PBS containing 0.5% casein supplemented with Triton X-705 (2.86 g/liter; Sigma Chemical Co., St. Louis, Mo.) (ROP2) and incubated again for 1 h at 37°C. Plates were then washed and supplemented with a peroxidase-conjugated anti-kappa light chain of mouse immunoglobulin (Ig; 1/1,000) (Experimental Immunology Unit, Université Catholique de Louvain, Louvain Belgium) for 1 h. After washing, o-phenylenediamine dihydrochloride tablets (Sigma Fast; Sigma) in H2O2 were used for development. The reaction was stopped by addition of 2 N H2SO4. Absorbance was read at 450/692 nm in a Titertek Multiskan MCC/340 (Labsystems, Espoo, Finland). Samples were considered positive if at the same dilution of at least 1/200 the optical density (OD) exceeded the OD of the preimmune serum by a factor 2.
IgG1 and IgG2a antibody determinations were performed as described for total antibodies. After incubation of serum samples, an anti-mouse IgG1 or anti-mouse IgG2a antibody labeled with biotin (Pharmingen, San Diego, Calif.) was added at 1/1,000 dilution for 1 hour, followed by washing, addition of streptavidin-peroxidase (1/2,000; Pharmingen) for 20 min, and development as described above. Samples were considered positive if the OD exceeded 0.3. Sera from BALB/c mice vaccinated with DNA encoding antigen 85A from M. tuberculosis via a gene gun were kindly provided by A. Tanghe (A. Tanghe, O. Denis, B. Lambrecht, V. Motte, T. Van Den Berg, and K. Huygen, submitted for publication).
In vitro spleen cell proliferation.
Two months after the last DNA injection, seropositive animals were sacrificed. Single-cell suspensions of splenocytes from DNA-vaccinated mice were obtained by gentle squeezing of whole spleens in erythrocyte lysis buffer (155 mM ammonium chloride, 10 mM potassium hydrogen carbonate, 0.1 mM EDTA [pH 7.4]) (Merck). Residual debris was removed by passage through a nylon gaze. The recovered cell suspension was washed once in RPMI 1640 (Life Technologies), and the cells were resuspended and plated in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine 1640 (Life Technologies), 0.05 mM 2-mercaptoethanol (Sigma), and penicillin-streptomycin (100 U/ml; Life Technologies). The viability of the cells used in the experiments was always higher than 80% as determined by trypan blue exclusion (BDH Chemicals, Dorset, United Kingdom). Splenocytes were stimulated with total T. gondii lysate used at a concentration of 25 μg/ml. As controls, the cells were also stimulated either with pokeweed mitogen (Life Technologies) prepared as instructed by the manufacturer and further diluted 1/5 in RPMI 1640 or with concanavalin A (Sigma) used at a final concentration of 2.5 μg/ml. Cells were cultured for 4 days in flat-bottomed microwell plates at 5 × 105 cells/ml, and [3H]thymidine (Amersham Pharmacia Biotech) was added at 1 μCi/well during the last 18 h. The cells were harvested onto glass fiber mats (Wallac, Turku, Finland) by using an automatic cell harvester (Skatron, Lier, Norway), and radioactivity was measured in a liquid scintillation counter (Betaplate; Wallac).
ELISA for IFN-γ and IL-4.
Supernatants from 72-h spleen cell cultures from mice 2 months following the third DNA dose were harvested and stored at −20°C until IFN-γ content was measured by ELISA. Briefly, Nunc immunoplates were coated overnight (4°C) with the capturing rat anti-mouse-IFN-γ monoclonal antibody (18181D; Pharmingen) diluted 1:1,000 in 50 mM sodium bicarbonate buffer, pH 9.6. The wells were washed thoroughly with 0.05% Tween 20 in PBS. Empty binding sites were blocked by 1 h of incubation at 37°C with 10% FCS in PBS. The supernatants from the cell cultures were tested in triplicate (100 μl per well) by incubation for 1 h at 37°C. After five washes, biotinylated rat anti-mouse IFN-γ monoclonal antibody (18112D; Pharmingen) was added (1:1,000 dilution; 100 μl per well) for 1 h at 37°C. Streptavidin-peroxidase conjugate (Jackson ImmunoResearch, West Grove, Pa.) was added (1:2,000) to the washed wells and allowed to react for 20 min at room temperature. Bound complexes were detected by reaction with the Sigma Fast substrate. The reaction was stopped by addition of 2 N H2SO4. Absorbance was read at 450/692 nm in a Titertek Multiskan. IFN-γ content was calculated as picograms per milliliter, using recombinant murine IFN-γ (Life Technologies) as a standard. The detection limit was 94 pg/ml. Interleukin-4 (IL-4) determination was carried out with the mouse IL-4 Quantikine M from R&D Systems (Minneapolis, Minn.) as instructed by the manufacturer. The detection limit was 4 pg/ml.
Enumeration of T. gondii cysts in the mouse brain.
Mouse brains were homogenized with a mortar and pestle in 2 ml of PBS. Then 100 μl (four aliquots of 25 μl each) of this suspension was counted in a phase-contrast microscope at a magnification of ×40.
QC-PCR.
One milliliter of brain suspension was used for DNA extraction. A detailed description of the method will be provided elsewhere (16a). In brief, a T. gondii-specific repetitive DNA fragment of 529 bp was amplified for 40 cycles, in competition with a 410-bp fragment that is recognized by the same primers. This 410-bp competitor DNA was prepared by PCR cloning the 529-bp fragment of T. gondii into a pUC19 plasmid and deleting an internal fragment of 119 bp. DNA was extracted from the brain homogenates used for cyst counting by using a QIAamp tissue kit (Qiagen) as instructed by the manufacturer. In each quantitative competitive PCR (QC-PCR) sample, an amount of DNA equivalent to 1/1,000 of a complete mouse brain was included, along with a known copy number (3 × 106) of the plasmid with its 410-bp competitor fragment.
The PCR products were separated on a polyacrylamide gel. After staining with ethidium bromide, images of the gel were digitized and analyzed with the public domain program NIH Image (developed at the National Institutes of Health; available on the Internet at http://rsb.info.nih.gov/nih-image/). The ratio between the integrated fluorescence levels of the 529-bp band containing genomic T. gondii DNA and the 410-bp band containing competitor plasmid DNA was calculated and is indicated in the text as the relative amount of T. gondii DNA.
Statistical analysis.
For statistical evaluation of data from proliferation assays, IFN-γ and IL-4 production, brain cyst counting, and QC-PCR, the results for vaccinated mice were compared to those for controls by a two-sided Student t test. Survival curves for vaccinated mice were compared to those for controls by the Mantel-Haenszel test. Statistical analyses and graphics were carried out with the Prism 2.01 software (GraphPad, San Diego, Calif.).
RESULTS
Humoral immune response induced by DNA vaccination.
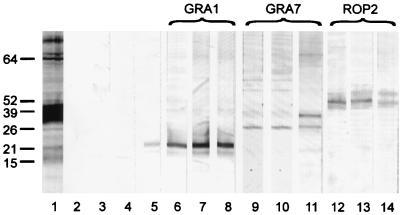
Vaccination with plasmid DNA encoding GRA1, GRA7, and ROP2 induced a strong antibody response. In Western blot analysis of TLA after SDS-PAGE, pools of sera from the three mouse strains used reacted with a single protein band at the expected molecular weight of the corresponding antigen (Fig. 1). A second, higher-molecular-weight band was also detected in C3H mice vaccinated with GRA7 DNA.
FIG. 1.
Reactivity of sera from DNA-vaccinated mice with TLA. Western blots of total parasite lysate were developed with a pool of 10 antisera from GRA1 DNA-vaccinated mice (lane 6, C57BL/6; lane 7, BALB/c; lane 8, C3H), GRA7 DNA-vaccinated mice (lane 9, C57BL/6; lane 10, BALB/c; lane 11, C3H), and ROP2 DNA-vaccinated mice (lane 12, C57BL/6; lane 13, BALB/c; lane 14, C3H). Reactivity from mice receiving empty vector DNA is shown in lanes 2, 3, and 4 (C57BL/6, BALB/c, and C3H, respectively). As controls, an anti-GRA1 monoclonal antibody (lane 5) and a pool from T. gondii chronically infected Swiss mice (lane 1) were included. The positions and molecular masses (in kilodaltons) of protein standards are also shown.
As shown in Fig. 2, significant antibody responses were observed in a high percentage of animals in all vaccine-host combinations, although there were differences depending on the antigen and the mouse strain. C3H mice were found to be good responders for all three antigens: seroconversion was found in more than 90% of the animals. Almost all BALB/c mouse had antibodies against GRA1 and GRA7, and about 80% had antibodies against ROP2. C57BL/6 mice responded slightly better to ROP2 and to GRA1 than to the GRA7 antigen. In general, the number of positive mice increased with the number of DNA doses. This was particularly the case for ROP2, which induced very weak responses after a single dose.
The magnitude of the titers found in vaccinated animals also differed depending on the antigen and mouse strain. The highest titers were found with GRA7 (13,000 ± 9,000 [mean ± standard error of the mean {SEM}] in C57BL/6, 530,000 ± 120,000 in C3H, and 160,000 ± 77,000 in BALB/c). The titers against GRA1 were intermediate (3,600 ± 1,400 in C57BL/6, 79,000 ± 28,000 in BALB/c, and 8,400 ± 2,900 in C3H), and the lowest titers were against ROP2 (1,100 ± 250 in C57BL/6, 1,890 ± 430 in BALB/c, and 4,800 ± 1,600 in C3H). The titers for C57BL/6 were always lower than those for the other two strains. The IgG1 and IgG2a humoral isotype responses against GRA1, GRA7, and ROP2 were analyzed individually in sera from five DNA-vaccinated BALB/c and five DNA-vaccinated C3H mice. For comparison, sera from gene gun-vaccinated BALB/c mice receiving the gene encoding the Mycobacterium tuberculosis 85A antigen were included because gene gun immunization has been shown to induce pronounced Th2-type humoral responses (28, 32, 39). As shown in Table 1, gene gun-vaccinated BALB/c mice had titers of IgG1 that were severalfold higher than the IgG2a titers. In contrast, in C3H mice vaccinated against T. gondii antigens, the titers of IgG2a were substantially higher than those for IgG1, both in the pool of sera from infected mice and in at least four of five vaccinated mice, for all antigens tested. This predominance of IgG2a over IgG1 antibodies suggests that in the DNA-vaccinated C3H animals as in chronic infections, a pronounced Th1 response is achieved. In vaccinated BALB/c mice, less polarized IgG2a/IgG1 ratios were found, and the IgG1 titers for all three antigens were higher than titers observed during infection (Table 1).
TABLE 1.
Titers of IgG1 and IgG2a antibodies in chronically T. gondii-infected mice and DNA-vaccinated mice against GRA1, GRA7, and ROP2 from T. gondii and Ag85A from M. tuberculosis
| Group | No. | Titera
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
T. gondii
|
M. tuberculosis Ag85A
|
||||||||||||
| GRA1
|
GRA7
|
ROP2
|
|||||||||||
| IgG2a | IgG1 | IgG2a/IgG1 | IgG2a | IgG1 | IgG2a/ IgG1 | IgG2a | IgG1 | IgG2a/IgG1 | IgG2a | IgG1 | IgG2a/IgG1 | ||
| C3Hb | |||||||||||||
| Infected | 12,800 | 1,600 | 8 | 409,600 | 6,400 | 64 | 800 | 0 | |||||
| Vaccinated | 1 | 409,600 | 6,400 | 64 | 25,600 | 6,400 | 4 | 3,200 | 100 | 32 | |||
| 2 | 409,600 | 12,800 | 32 | 51,200 | 800 | 64 | 1600 | 100 | 16 | ||||
| 3 | 102,400 | 25,600 | 4 | 6,400 | 0 | 800 | 200 | 4 | |||||
| 4 | 102,400 | 102,400 | 1 | 3,200 | 12,800 | 1/4 | 400 | 0 | |||||
| 5 | 409,600 | 25,600 | 16 | 102,400 | 800 | 128 | 400 | 0 | |||||
| BALB/c | |||||||||||||
| Infected | 1,600 | 3,200 | 1/2 | 204,800 | 1,600 | 128 | 800 | 50 | 16 | ||||
| Vaccinatedc | 1 | 204,800 | >819,200 | <1/4 | 102,400 | 204,800 | 1/2 | 1,600 | 400 | 4 | |||
| 2 | 204,800 | 25,600 | 8 | 25,600 | 25,600 | 1 | 800 | 3,200 | 1/4 | ||||
| 3 | 204,800 | 102,400 | 2 | 6,400 | 3,200 | 2 | 3,200 | 3,200 | 1 | ||||
| 4 | 51,200 | 51,200 | 1 | 12,800 | 12,800 | 1 | 25,600 | 800 | 32 | ||||
| 5 | 25,600 | 25,600 | 1 | 6,400 | 1,600 | 4 | 25,600 | 800 | 32 | ||||
| Vaccinatedd | |||||||||||||
| 1 | <50 | 800 | <1/16 | ||||||||||
| 2 | 400 | >51,200 | <1/128 | ||||||||||
| 3 | 800 | 6,400 | 1/8 | ||||||||||
| 4 | 800 | 25,600 | 1/32 | ||||||||||
| 5 | 400 | 25,600 | 1/64 | ||||||||||
Samples were considered positive at a given dilution if the OD exceeded 0.3.
Pool from three animals.
Individual animals vaccinated by intramuscular injection.
Individual animals vaccinated using plasmid-coated gold particles and a gene gun.
Cellular immune response induced by DNA vaccination.
To evaluate cellular anti-Toxoplasma immune responses in the DNA-vaccinated mice, seropositive animals were selected and sacrificed 2 months after the last DNA injection. Spleen cell suspensions from individual mice were stimulated in vitro with T. gondii RH TLA. Substantial specific lymphoproliferation was observed in spleen cell cultures from vaccinated BALB/c mice after 72 h of culture (Fig. 3). Specific but less vigorous cellular responses were also observed in spleen cell cultures from vaccinated C3H mice. In contrast, splenocytes from vaccinated seropositive C57BL/6 mice did not proliferate when stimulated with TLA (data not shown).
FIG. 3.
In vitro proliferation of splenocytes from DNA-vaccinated BALB/c and C3H mice after stimulation with TLA. Splenocytes from individual mice were harvested 2 months after the third DNA dose. Following 72 h of stimulation with TLA, [3H]thymidine was added for 18 h. The incorporated radioactivity was then measured. Control splenocytes were from mice receiving the plasmid DNA without insert. For comparative purposes, splenocytes from BALB/c and C3H mice chronically infected with strain 76K (25 cysts per os) were included in the experiment. ∗, P < 0.05; ∗∗, P < 0.01.
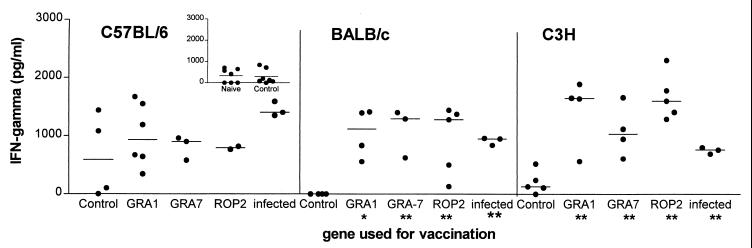
Compared to controls, spleen cells from coding-DNA-vaccinated BALB/c and C3H mice produced significant levels of IFN-γ when stimulated with TLA (Fig. 4). In TLA-stimulated splenocytes from C57BL/6 mice, IFN-γ production was induced in two of four control vaccinated mice as well as in the coding-DNA-vaccinated animals. Additional control as well as naive C57BL/6 mice were tested to confirm TLA-induced IFN-γ production by splenocytes from nonimmunized and control-vaccinated animals (Fig. 4, inset). Eventually, no statistically significant differences could be found between coding-DNA-vaccinated, control DNA-vaccinated, and naive animals.
FIG. 4.
IFN-γ production by splenocytes from DNA-vaccinated C57BL/6, BALB/c, and C3H mice after stimulation with TLA. Splenocytes from individual mice were cultured as for Fig. 3. Following 72 h of stimulation with TLA, supernatants from the cultures were harvested and analyzed for the presence of IFN-γ by ELISA. Unstimulated splenocytes did not secrete detectable IFN-γ. Control splenocytes were from mice receiving the plasmid DNA without insert. Chronically infected BALB/c and C3H mice are as in Fig. 3. Chronically infected C57BL/6 mice were infected with 10 cysts of strain 76K by intraperitoneal injection. ∗, P < 0.05; ∗∗, P < 0.01. The inset shows an additional experiment comparing IFN-γ secretion after TLA stimulation of splenocytes from control DNA-vaccinated and naive C57BL/6 mice.
We investigated whether splenocytes from the seropositive DNA-vaccinated mice secreted the Th2-associated cytokine IL-4 when stimulated with TLA. IL-4 was undetectable in splenocyte supernatants from both vaccinated and control mice of the three mouse strains analyzed. This was in contrast to the cytokine pattern induced during T. gondii chronic infection: TLA-stimulated spleen cells from all strains produced IL-4 upon infection, but splenocytes from BALB/c mice secreted significantly more IL-4 (130 ± 8.7 pg/ml, n = 5) compared to infected C57BL/6 (10 ± 1 pg/ml, n = 3) and C3H (12 ± 5 pg/ml, n = 4) mice.
Protective efficacy of DNA vaccination in mice.
Preliminary experiments were performed with C3H, BALB/c, and C57BL/6 mice to determine the lethal dose of T. gondii cysts by intragastric gavage. In C57BL/6 mice, a dose as low as 10 cysts of either strain IPB-G or strain 76K killed the majority of the animals. For C3H mice, 50 cysts of strain IPB-G were required to induce at least 50% mortality. Selection of the size of the inoculum was critical for BALB/c mice. This mouse strain showed marked changes in susceptibility with relatively low changes in the inoculum dose from a certain threshold onward: doses of 50 or 100 cysts resulted in 20% mortality, whereas 200 cysts killed all the infected mice.
C3H mice vaccinated with GRA1, GRA7, or ROP2 showed significant resistance to challenge with 50 cysts of strain IPB-G 6 weeks after the last injection of DNA. This challenge dose killed 9 of 10 mice that had been injected with control plasmid, whereas 9 of 10 mice vaccinated with DNA encoding GRA7 or ROP2 survived (P < 0.001), as did 7 of 10 animals vaccinated with DNA encoding GRA1 (P < 0.003) (Fig. 5A).
FIG. 5.
Survival curves of C3H (A and B), C57BL/6 (C), and BALB/c (D and E) mice vaccinated with plasmid encoding GRA1, GRA7, or ROP2 or with control plasmid without insert (three 100-μg doses of DNA with 3-week interval) and challenged perorally between 6 and 9 weeks after the third DNA dose. T. gondii cysts (strain IPB-G) were administered to C3H (50 cysts in panel A), C57BL/6 (10 cysts in panel C), and BALB/c mice (200 cysts in panel D and 50 cysts in panel E) mice. T. gondii 76K was used for challenging C3H mice (50 cysts in panel B). The number of mice for each condition is indicated in parentheses.
To extend this observation to a different T. gondii strain, 10 C3H mice of each vaccination group were submitted to an oral challenge with strain 76K (50 cysts) 9 weeks after the third DNA dose (Fig. 5B). Again, significantly higher survival rates were obtained in T. gondii DNA-vaccinated mice. All GRA7 and all ROP2 DNA-vaccinated mice survived (P < 0.02), as did 9 of 10 GRA1-vaccinated animals (P < 0.05), compared to only 50% of the empty vector-vaccinated mice.
DNA vaccination did not protect C57BL/6 mice from challenge with 10 cysts of strain IPB-G. This dose induced more than 60% mortality in all conditions tested (Fig. 5C). No protection was observed in BALB/c DNA-vaccinated mice either. Inoculation with 200 cysts of strain IPB-G led to mortality rates of 100% in the control group and at least 80% in the vaccinated group (Fig. 5D). A decrease of the dose by a factor 4 (50 cysts) led to at least 70% survival in both control and vaccinated animals (Fig. 5E).
To evaluate the effect of vaccination on T. gondii brain cyst development in the chronic phase of infection, C3H mice were infected with a nonlethal dose (25 cysts of strain IPB-G) 3 weeks following the last administration of DNA. The mice survived and were sacrificed 2 months after the infection, and cysts in the brain were counted by microscopy and analyzed by PCR. The average number of cysts ± SEM was 2,650 ± 412 per brain of mice injected with control DNA (n = 6). The brains of T. gondii DNA-vaccinated mice contained a significantly lower cyst burden (P < 0.05 in all cases). In the brains of GRA1-vaccinated C3H mice, an average of 1,225 cysts ± 80 (n = 4) was found, whereas the brains of GRA7 and ROP2 DNA-vaccinated mice contained 983 ± 153 (n = 7) and 869 ± 162 (n = 7) cysts, respectively. QC-PCR confirmed this lower infectious load in vaccinated animals: the relative amount of T. gondii DNA detected in the brains of vaccinated C3H mice was significantly lower (0.169 ± 0.026 [mean ± SEM] with GRA1, 0.189 ± 0.026 with GRA7, and 0.204 ± 0.034 with ROP2) than in the brains of mice injected with control plasmid (0.723 ± 0.179). In BALB/c mice, we detected no effect of vaccination on the development of T. gondii. The cyst burden was measured in the brains of vaccinated and control BALB/c mice surviving challenge with 50 cysts of strain IPB-G strain (Fig. 5E). Six weeks after infection, the number of brain cysts in these mice was very low and not different in the T. gondii DNA-vaccinated animals (for GRA1, 266 ± 137 [mean ± SEM] [n = 7]; for GRA7, 152 ± 45 [n = 10); for ROP2, 134 ± 54 [n = 7]) than in the control group (103 ± 49 cysts/brain [n = 8]).
DISCUSSION
DNA vaccination has been shown to be a powerful method for the induction of specific humoral and cellular immune responses in a number of vertebrate host species (11, 17). The number of preclinical models in which genetic immunization has been applied has increased steadily over the last 5 years (reviewed in references 18 and 43). With respect to parasitic infections, progress has been made to develop vaccines against malaria, cryptosporidiosis, leishmaniasis, and schistosomiasis (12, 13, 16, 21, 30, 31, 38, 46–48). We know of only one previous report on DNA vaccination with a T. gondii surface antigen, SAG1. In that study, a humoral response was found, but data on cellular responses or protection were not presented (2). In this study, we show that DNA immunization with potentially protective T. gondii antigens (GRA1, GRA7, and ROP2) induces both humoral and cellular immune responses in mice of three different genetic backgrounds. In addition, we show that in one mouse strain, DNA vaccination not only reduces the mortality associated with the acute phase of infection but also limits the parasite load during the chronic phase of the disease.
Very high specific antibody titers could be achieved, especially after three injections of DNA. BALB/c and C3H mice exhibited the highest specific antibody titers, which in some GRA7 DNA-vaccinated C3H mice exceeded 1:106. In ROP2-vaccinated mice, the number of seroconverting animals and the titers were lower than with the other two antigens. However, this may be due to an underestimation, as the recombinant ROP2 protein used for detection in ELISA contained only the 330 C-terminal amino acids, compared to 535 codons in the construct used for vaccination. In any case, our results confirm that seroconversion can readily be obtained by DNA vaccination. We also evaluated the isotype nature of the IgG response achieved during vaccination. C3H mice exhibited a high ratio of IgG2a to IgG1 antibody titers, characteristic of Th1-type responses and comparable to those in chronically infected animals. This was not the case for vaccinated BALB/c animals, in which the IgG2a/IgG1 ratio was less polarized: the IgG1 levels in the sera from these animals were consistently higher than those in the infected control pool.
Immunized BALB/c and C3H mice vaccinated with any of the three DNA constructs also showed specific cellular immune responses characterized by significantly increased splenocyte proliferation and secretion of IFN-γ in response to TLA. In contrast, cells from vaccinated C57BL/6 mice failed to proliferate when stimulated with TLA. Moreover, IFN-γ production was induced by the lysate in splenocyte cultures from both vaccinated and control mice. We cannot exclude that the lack of proliferation is due to the presence of inhibitory components in the parasite lysate, and IFN-γ production by antigen-specific T cells may have been obscured by the high level of nonspecific production induced by total lysate. Purified antigens will be needed to resolve this problem.
We also investigated whether IL-4, which plays a major role in controlling the development of cell-mediated immunity (25, 33, 42), was produced by TLA-stimulated splenocytes of vaccinated mice and whether host strain-dependent differences were observed at this level. Intramuscular DNA vaccination failed to induce IL-4 production in any of the mouse strains evaluated. In contrast, IL-4 was produced by spleen cells from mice chronically infected with T. gondii. It is noteworthy that IL-4 production by spleen cells from infected BALB/c mice was much higher than that by C57BL/6 and C3H spleen cells, again indicating that the former strain is more prone to a Th2-type response than the latter two.
The major purpose of the present work was to see whether DNA vaccination could positively influence the outcome of T. gondii infections in vaccinated mice. We evaluated the protective nature of the immune responses induced by vaccination by orally infecting seropositive vaccinated mice with T. gondii. In the C3H strain, protection induced by vaccination was demonstrated in three independent experiments. Immunization with DNA encoding all three antigens partially protected C3H mice against an otherwise lethal dose of T. gondii IPB-G that killed 90% of the control vaccinated mice. After a sublethal challenge with the same T. gondii strain, parasite burden, measured as numbers of cysts and amount of T. gondii DNA in the brains of the surviving mice, was significantly lower for all mice receiving the Toxoplasma genes compared to controls. DNA vaccination also reduced mortality upon lethal challenge with the less virulent strain 76K. These results indicate that vaccination not only limited the death associated with the acute phase of infection but also conferred partial protection during the chronic phase of the disease. This is of particular relevance considering that C3H mice are susceptible to TE and have markedly more Toxoplasma cysts in the brain than the resistant BALB/c (41). It will be of interest to evaluate the effect of DNA vaccination on the development of TE in the chronic phase.
In BALB/c mice, it was difficult to assess the protective effect of DNA vaccination. BALB/c mice, considered naturally resistant, readily survive T. gondii infections, which lead to the formation of very low numbers of brain cysts. BALB/c mice are also resistant to TE. The development of TE and brain cyst burden during T. gondii infection has been comprehensively characterized and linked to the major histocompatibility complex class I coding complex (5, 6, 41). However, BALB/c mice can succumb to T. gondii infections when relatively high doses of parasite are inoculated. It is striking that that mortality in these mice sharply increases from a certain threshold dose (3). Therefore, it is difficult to define a suitable condition for the assessment of protective immunity. In this study, gavage with 200 T. gondii cysts induced high mortality in both control and vaccinated groups, whereas a dose of 50 cysts caused very limited mortality. Animals that survived all had a low number of cysts in the brain. In these particular circumstances, improved protection due to vaccination could not be demonstrated in the BALB/c mice: there was no further decrease in the already low cyst load in animals surviving a low-dose challenge, nor was there improved survival in the acute phase after a high-dose challenge. In view of the difference in natural resistance between C3H and BALB/c mice, it is difficult to link the lack of protection in BALB/c to differences in the type of immune response. Nevertheless, the higher IgG1 titers after vaccination and the higher IL-4 production after infection suggest a tendency toward a Th2-type response in BALB/c mice, whereas a more pronounced Th1-type response as observed in C3H mice might confer better protection. However, it should be stressed that the role of IL-4 and Th2 cellular responses during toxoplasmosis is still unclear. Some studies suggest that IL-4 is necessary to avoid the induction of pathology, while others indicate that its absence might be beneficial for resolution of the acute phase (10).
No protection was observed in vaccinated C57BL/6 mice. On the contrary, a somewhat higher mortality was observed in two of the three vaccinated groups. Again, this may be related to the particular course of the disease in this strain. Recent studies on inflammatory mediator production indicate that T-cell-derived cytokines may promote pathological changes during T. gondii infection of C57BL/6 mice: the major pathological finding in C57BL/6 mice succumbing to oral T. gondii infection is the inflammation of the ileum due to IFN-γ produced by CD4+ lymphocytes, predominantly of the α/β type (26). Thus, the high mortality in infected C57BL/6 mice could be due to an uncontrolled pathological Th1-type response. DNA vaccination may stimulate IFN-γ production even further, thereby exacerbating the immunopathology rather than conferring protection. In C3H mice, which succumb due to the parasite multiplication rather than to immunopathology (26), the strong induction of IFN-γ by DNA immunization should be beneficial instead.
The three antigens, including the novel GRA7, were immunogenic in all three mouse strains, and in C3H mice they produced similar protective effects. The fact that T. gondii stimulates strong immunity in natural infections suggests that the parasite possesses a battery of highly immunogenic antigens, among them the antigens selected for our research. This fact combined with the strong adjuvant activity of bacterial plasmid DNA may explain the powerful anti-toxoplasma immunity that we obtained. Nevertheless, more vaccination and challenge experiments with these and other antigens, either separately or in combination, are needed to elucidate the relative vaccination potential of each of the genes. In addition, it will be necessary to differentiate the effects that the vaccine-induced immune responses have on the parasite survival from those associated with immunopathology.
ACKNOWLEDGMENTS
Martine Vercammen and Tatiana Scorza contributed equally to this work.
This work was supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (grant GO40598).
We are very much indebted to R. Zaugg (Vical, Inc.) for giving us the opportunity to work with the VR1020 plasmid. We thank A. Laeremans, F. Van Ackeleyen, and F. Crabbé for valuable contributions in maintaining T. gondii strains. We are grateful to Isabelle Bourgain, who provided T. gondii 76K. We very much appreciate the gift of sera from mice vaccinated with M. tuberculosis Ag85A provided by A. Tanghe.
REFERENCES
- 1.Alexander J, Jebbari H, Bluethmann H, Satoskar A, Roberts C W. Immunological control of Toxoplasma gondii and appropriate vaccine design. Curr Top Microbiol Immunol. 1996;219:188–190. doi: 10.1007/978-3-642-51014-4_17. [DOI] [PubMed] [Google Scholar]
- 2.Angus C W, Klivington D, Wyman J, Kovacs J A. Nucleic acid vaccination against Toxoplasma gondii in mice. J Eukaryot Microbiol. 1996;43:117S. doi: 10.1111/j.1550-7408.1996.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 3.Araujo F G, Williams D M, Grumet F C, Remington J. Strain-dependent differences in murine susceptibility to Toxoplasma. Infect Immun. 1976;13:1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann B J. Derivations and genotypes of some mutant derivations of Escherichia coli K-12. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1190–1219. [Google Scholar]
- 5.Blackwell J M, Roberts C W, Alexander J. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 1993;15:317–324. doi: 10.1111/j.1365-3024.1993.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown C R, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 7.Brown C R, Hunter C A, Estes R G, Beckmann E, Forman J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against toxoplasmosis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 8.Cesbron-Delauw M F, Guy B, Torpier G, Pierce R J, Lenzen G, Cesbron J Y, Charif H, Lepage P, Darcy F, Lecocq J P, Capron A. Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proc Natl Acad Sci USA. 1989;86:7537–7541. doi: 10.1073/pnas.86.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chardès T, Bourguin I, Mevelec M-N, Dubremetz J-F, Bout D. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect Immun. 1990;58:1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkers E Y, Gazzinelli R T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly J J, Ulmer J B, Liu M A. Immunization with DNA. J Immunol Methods. 1994;176:145–152. doi: 10.1016/0022-1759(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 12.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunisation: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupre L, Poulain-Godefroy O, Ban E, Ivanoff N, Mekranfar M, Schacht A M, Capron A, Riveau G. Intradermal immunization of rats with plasmid DNA encoding Schistosoma mansoni 28 kDa glutathione S-transferase. Parasite Immunol. 1997;19:505–513. doi: 10.1046/j.1365-3024.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 14.Duquesne V, Auriault C, Gras-Masse H, Boutillon C, Darcy F, Cesbron-Delauw M-F, Tartar A, Capron A. Identification of T cell epitopes within a 23-kD antigen (p24) of Toxoplasma gondii. Clin Exp Immunol. 1991;84:527–534. [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer H-G, Stachelhaus S, Sahm M, Meyer H E, Reichmann G. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol Biochem Parasitol. 1998;91:251–262. doi: 10.1016/s0166-6851(97)00227-2. [DOI] [PubMed] [Google Scholar]
- 16.Gardner M J, Doolan D L, Hedstrom R C, Wang R, Sedegah M, Gramzinski R A, Aguiar J C, Wang H, Margalith M, Hobart P, Hoffman S L. DNA vaccines against malaria: immunogenicity and protection in a rodent model. J Pharm Sci. 1996;85:1294–300. doi: 10.1021/js960147h. [DOI] [PubMed] [Google Scholar]
- 16a.Homan, W., M. Vercammen, J. De Braekeleer, and H. Verschueren. Identification of a 200- to 300-fold repetitive 529-bp DNA fragment in Toxoplasma gondii and its possible use for diagnostic and quantitative PCR. Int. J. Parasitol., in press. [DOI] [PubMed]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Randall Deck R, De Witt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K. DNA vaccines: application to tuberculosis. Int J Tuberc Lung Dis. 1998;2:1–8. [PubMed] [Google Scholar]
- 19.Jacobs D, Dubremetz J-F, Loyens A, Bosman F, Saman E. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol Biochem Parasitol. 1998;91:237–249. doi: 10.1016/s0166-6851(97)00204-1. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin Diagn Lab Immunol. 1999;6:24–29. doi: 10.1128/cdli.6.1.24-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins M, Kerr D, Fayer R, Wall R. Serum and colostrum antibody responses induced by jet-injection of sheep with DNA encoding a Cryptosporidium parvum antigen. Vaccine. 1995;13:1658–1664. doi: 10.1016/0264-410x(95)00121-g. [DOI] [PubMed] [Google Scholar]
- 22.Kasper L H, Buzoni-Gatel D. Some opportunistic parasitic infections in AIDS: candidiasis, pneumocystosis, cryptosporidiosis, toxoplasmosis. Parasitol Today. 1998;14:150–156. doi: 10.1016/s0169-4758(97)01212-x. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Laugier M, Quillici M. Intérêt expérimental d'une souche de toxoplasmose peu pathogène pour la souris. Ann Parasitol Hum Comp. 1970;45:389–403. [PubMed] [Google Scholar]
- 25.Le Gros G, Ben-Sasson S W, Seder R, Finkelman F D, Paul W E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro; IL-2 and IL-4 are required for in vitro generation of IL-4 producing cell. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T-cell dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke C J, Carmer K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 28.McCluskie M J, Brazolot M C L, Gramzinski R A, Robinson H L, Santoro J C, Fuller J T, Widera G, Haynes J R, Purcell R H, Davis H L. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol Med. 1999;5:287–300. [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitainy G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989;142:3247–3255. [PubMed] [Google Scholar]
- 30.Mor G, Klinman D M, Shapiro S, Hagiwara E, Sedegah M, Norman J A, Hoffman S L, Steinberg A D. Complexity of the cytokine and antibody response elicited by immunising mice with Plasmodium yoelii circumsporozite protein plasmid DNA. J Immunol. 1995;155:2039–2046. [PubMed] [Google Scholar]
- 31.Nara T, Tanabe K, Mahakunkijcharoen Y, Osada Y, Matsumoto N, Kita K, Kojima S. The B cell epitope of paramyosin recognised by a partially protective monoclonal IgE antibody to Schistosoma japonicum. Vaccine. 1997;15:79–84. doi: 10.1016/s0264-410x(96)00100-4. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira S C, Rosinha G M, de-Brito C F, Fonseca C T, Afonso R R, Costa M C, Goes A M, Rech E L, Azevedo V. Immunological properties of gene vaccines delivered by different routes. Braz J Med Biol Res. 1999;32:207–214. doi: 10.1590/s0100-879x1999000200009. [DOI] [PubMed] [Google Scholar]
- 33.Quelle F W, Shimoda K, Thierfelder W, Fisher C, Kimm A, Ruben S M, Cleveland J L, Pierce J H, Keegan A D, Nelms K. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in response to IL-4 and IL-3 but not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saavedra R, De Meuter F, Hérion P. Monoclonal antibodies identify new Toxoplasma gondii soluble antigens. Hybridoma. 1990;9:453–463. doi: 10.1089/hyb.1990.9.453. [DOI] [PubMed] [Google Scholar]
- 35.Saavedra R, De Meuter F, Decourt J-L, Hérion P. Human T cell clone identifies a potentially partially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991;147:1975–1982. [PubMed] [Google Scholar]
- 36.Saavedra R, Becerill M A, Dubeaux C, Lippens R, De Vos M-J, Hérion P, Bollen A. Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun. 1996;64:3858–3862. doi: 10.1128/iai.64.9.3858-3862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadak A Z, Taghy B, Fortier B, Dubremetz J-F. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1988;29:203–211. doi: 10.1016/0166-6851(88)90075-8. [DOI] [PubMed] [Google Scholar]
- 38.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smooker P M, Steeper K R, Drew D R, Strugnell R A, Spithill T W. Humoral responses in mice following vaccination with DNA encoding glutathione S-transferase of Fasciola hepatica: effects of mode of vaccination and the cellular compartment of antigen expression. Parasite Immunol. 1999;21:357–364. doi: 10.1046/j.1365-3024.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- 40.Supply P, Sutton P, Coughlan S N, Bilo K, Saman E, Trees A J, Cesbron-Delauw M L, Locht C. Immunogenicity of recombinant BCG producing the GRA1 antigen from Toxoplasma gondii. Vaccine. 1999;17:705–714. doi: 10.1016/s0264-410x(98)00255-2. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Joh K, Orellana M A, Conley F K, Remington J S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74:732–739. [PMC free article] [PubMed] [Google Scholar]
- 42.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 43.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 44.Van Gelder F, Bosman F, De Meuter F, Van Heuverswyn H, Hérion P. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J Clin Microbiol. 1993;31:9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vercammen M, Dardé M-L, El Bouhdidi A, Ben Messaoud A, De Meuter F, Dubremetz J-F, Carlier Y. Proceedings of the ICOPA IX, 9th International Congress of Parasitology. Bologna, Italy: Monduzzi Editore; 1998. Fc receptor activity of Toxoplasma gondii tachyzoites: immunoglobulin binding by T. gondii strains with different isoenzyme patterns, and by SAG1 and SAG2 deficient mutants; pp. 1027–1032. [Google Scholar]
- 46.Xu D, Liew F Y. Genetic vaccination against leishmaniasis. Vaccine. 1994;12:1534–1536. doi: 10.1016/0264-410x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 47.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W, Waine G J, McManus D P. Antibodies to Schistosoma japonicum (Asian bloodfluke) paramyosin induced by nucleic acid vaccination. Biochem Biophys Res Commun. 1995;212:1029–1039. doi: 10.1006/bbrc.1995.2073. [DOI] [PubMed] [Google Scholar]