Abstract
This work is a literature review, presenting the current state of the use of cannabinoids on neurodegenerative diseases. The emphasis is on Parkinson's (PD) and Alzheimer's (AD) diseases, the two most prevalent neurological diseases. The review goes from Cannabis sativa and its hundreds of bioactive compounds to Δ<sup>9</sup>-tetrahydrocannabinol (THC) and mainly cannabidiol (CBD) and their interactions with the endocannabinoid receptors (CB1 and CB2). CBD molecular targets were also focused on to explain its neuroprotective action mechanism on neurodegenerative diseases. Although THC is the main psychoactive component of C. sativa, and it may induce transient psychosis-like symptoms, growing evidence suggests that CBD may have protective effects against the psychotomimetic effects of THC and therapeutic properties. Furthermore, a great number of recent works on the neuroprotective and anti-inflammatory CBD effects and its molecular targets are also reviewed. We analyzed CBD actions in preclinical and in clinical trials, conducted with PD and AD patients. Although the data on preclinical assays are more convincing, the same is not true with the clinical data. Despite the consensus among researchers on the potential of CBD as a neuroprotective agent, larger and well-designed randomized clinical trials will be necessary to gather conclusive results concerning the use of CBD as a therapeutic strategy for the treatment of diseases such as PD and AD.
Keywords: Cannabis sativa, Cannabidiol, Neurodegeneration, Neuroprotection
Introduction
Cannabis sativa L. (fam. Cannabaceae) is a medicinal plant cultivated throughout millennia, for agricultural, industrial, and medicinal purposes, among others. Over the years, it became a controversial plant, due to its psychoactive effects. Since then, C. sativa has moved back and forth from the category of herbal medicine to an illicit drug and again to a medicinal product [1].
According to the literature, there are more than 550 chemical compounds in C. sativa and more than 100 phytocannabinoids among which are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). These phytocannabinoids work by binding to cannabinoid receptors (CB1 and CB2), as well as to other receptor systems [2].
THC is the main psychotropic constituent of C. sativa and a CB1 and CB2 receptor partial agonist. Its effects appear to be influenced by the expression and by the signaling efficiency of cannabinoid receptors, as well as by endogenous cannabinoids (endocannabinoids) release. CBD displays unexpectedly high potency as an antagonist of CB1/CB2 receptor agonists [3].
CB1 receptors are present at very high levels in the brain and mediate many of the psychoactive effects of cannabinoids, while CB2 receptors have a more restricted distribution, being present mainly in the peripheral system, i.e., immune cells, and in a few neurons. Both CB1 and CB2 couple primarily to inhibitory G proteins and, thus, partial agonism, functional selectivity, and inverse agonism play important roles in determining the cellular response to specific cannabinoid receptor ligands [4].
Due to their expression and localization in the CNS, the CB1 receptor, endocannabinoids, and the enzymes involved in the synthesis and degradation of these endocannabinoids are implicated in multiple pathophysiological events, ranging from memory deficits to neurodegenerative diseases [5]. Furthermore, the phytocannabinoids and terpenoids present in C. sativa may act in concert to elicit therapeutic effects. While THC directly activates CB1 and CB2 receptors, CBD is known to modulate the activity of many cellular effectors, including CB1 and CB2 receptors, 5-HT1A receptors, GPR55, μ- and δ-opioid receptors, transient receptor potential vanilloid 1 (TRPV1) cation channels, PPARγ, and the fatty acid amide hydrolase enzyme known to break down the endocannabinoid, anandamide [6].
Evidence has raised the possibility that CBD can act as a negative allosteric modulator of CB1. Results from computational methods offer a possible explanation of how CBD can directly modulate the effects of THC on CB1 receptors [7]. Besides, several studies have described CBD as a multitarget molecule, acting as an adaptogen and as a modulator, in different ways, depending on the type and location of disequilibrium, both in the brain and in the body, mainly interacting with specific CB1 and CB2 receptor proteins [8, 9].
CBD is being pursued as a therapeutic treatment for multiple conditions, usually by oral delivery. Despite animal studies suggesting a low oral bioavailability, the literature on humans is not sufficient. According to Millar and coworkers, 2018 [10], of the 792 articles retrieved, only 24 included pharmacokinetic parameters in humans. The half-life of CBD was reported between 1.4 and 10.9 h after oromucosal spray, 2–5 days after chronic oral administration, 24 h after i.v., and 31 h after smoking. The authors conclude that understanding properties, such as bioavailability and half-life, is critical to future therapeutic success.
CBD has received great scientific interest, due to its medical applications. This compound showed efficacy as an anti-seizure, antipsychotic, neuroprotective, antidepressant, and anxiolytic. The neuroprotective activity appears linked to its excellent anti-inflammatory and antioxidant properties [11]. The objectives of the present literature review are focused on the neuroprotective potential of C. sativa, with an emphasis on CBD as a therapeutic strategy for the management of neurodegenerative diseases such as Parkinson's (PD) and Alzheimer's diseases (AD).
CBD and Neurodegenerative Diseases
Neurodegenerative diseases represent one of the main causes of death in industrialized countries and are characterized by a loss of neurons, in particular regions of the central nervous system [12]. The increase in life expectancy and the prevalence of neurodegenerative diseases are rapidly growing worldwide. Evidence indicates that the pathophysiology of neurodegenerative diseases may overlap at molecular levels and pathways, leading to cell death. Oxidative stress (OS) and inflammation, which are tightly linked and interdependent, are regarded as playing a key role in neurodegeneration pathogenesis [13].
In neurodegenerative diseases, altered proteins undergo an unfolding process followed by the formation of β-structures and a pathological tendency to self-aggregate, which is a characteristic of tau protein (TAU) in AD and α-synuclein in PD. It is believed that this nerve cell loss underlies the subsequent decline in cognitive and motor function. Thus, neuroinflammation is common among neurodegenerative diseases and has been implicated as a critical mechanism responsible for progressive neurodegeneration [14].
Increased reactive oxygen species (ROS) and OS have been implicated in the pathogenesis of neurodegenerative conditions, including AD and PD. The endogenous antioxidant response pathway protects cells from OS, by increasing the expression of cytoprotective enzymes, and is regulated by Nrf2 (nuclear factor erythroid 2-related factor 2). Nrf2 regulates cellular resistance to oxidants and detoxifying and antioxidant defense gene expressions [15]. Nrf2 has also been shown to exert anti-inflammatory effects and modulates both mitochondrial function and biogenesis. Mitochondrial dysfunction and neuroinflammation are key players in AD and PD [16].
These neurodegenerative diseases are characterized by the accumulation of misfolded proteins, contributing to mitochondrial fragmentation, OS, and neuroinflammation. In this context, Nrf2 has a pivotal role in redox homeostasis and anti-inflammatory functions in neurodegenerative diseases. Nrf2 activation has been shown to mitigate several pathologic mechanisms associated with neurological diseases and thus could be a novel therapeutic approach to target neurodegenerative pathogenesis such as AD and PD [17, 18].
CBD has been shown to influence interactions of transcription factors Nrf2-NFκB by inhibiting the NF-κB pathway, increasing the expression of Nrf2 activators, and stimulating the transcription activity of Nrf2. Moreover, the antioxidant and anti-inflammatory activities of CBD are manifested through Nrf2 activation and an inhibitor of NF-κB, respectively [19]. In addition, CBD induces the expression of several Nrf2 target genes [20, 21], becoming an important molecular target for both AD and PD.
A contribution of neuroprotective and anti-inflammatory therapeutic strategies for these diseases is important since actual conventional treatments do not stop the neurodegenerative progression. The neuroprotective potential of CBD, resulting from its anti-inflammatory and antioxidant properties, is under intense preclinical research for use in numerous neurodegenerative diseases [22]. Thus, CBD, which lacks any unwanted psychotropic effect, may represent a very promising agent [23, 24].
Furthermore, neurodegeneration leading to PD and AD has become a major health burden, not only in low- and middle-income countries but in the developed world as well. Among all neurodegenerative diseases, 1.8% accounts for PD, while AD for 12%, with the current rates reporting disease incidences higher in low- and middle-income countries, is imperative to the demand for novel research on these two diseases [25].
Recently, the neuroprotective capability of CBD against OS, as well as its toxicity profile on in vitro culture, is systematically pursued. Although CBD showed both neurotoxic and neuroprotective effects on hippocampal neurons, the use of low-concentrated (i.e., 5 μM) CBD did not cause toxic effects and significantly rescued the neurons from OS, confirming its neuroprotection capability [26].
CBD may represent a prototype for anti-inflammatory drug development for human pathologies, where both inflammation and OS play an important role, as in neurodegenerative diseases. In this regard, AD and PD, characterized by extensive oxidative damage to different biological substrates, lead to cell death by different pathways. These diseases present a complex etiology, with a variety of factors contributing to the progression of their neurodegenerative processes and then the treatment strategies should target multiple substrates to stop and/or slow down the neurodegeneration. In this context, CBD, interacting with the ECB system, has also a cannabinoid receptor-independent mechanism and might be a good candidate for antioxidant drug development, for both PD and AD [27].
The endocannabinoid system (ECS) is currently being studied as a PD and AD drug target, where the overexpression of ECS receptors exerts neuroprotection against PD and reduces neuroinflammation in AD. THC and CBD have shown neuroprotection in PD and AD animal models, although sometimes trigger toxic effects on patients when administered directly. Therefore, it is important to know the molecular cascade following cannabinoid treatment focusing especially on gene expression to identify drug targets for preventing and repairing neurodegeneration [25].
Furthermore, a recent systematic review and meta-analysis [28] showed that cannabinoid-based medicines (CBMs) are being used worldwide, although their safety and tolerability in older adults are still not well known. These data from randomized controlled trials (RCTs) suggest that although THC-containing CBMs are associated with side effects, CBMs, in general, are safe and acceptable in older adults, pointing out the potential of CBM and CBD for neurodegenerative diseases treatment. Besides, according to the WHO Report [29], CBD is generally well tolerated with a good safety profile, which emphasizes the need to consider CBM and mainly CBD promising agents for neurodegenerative diseases treatments.
CBD and Parkinson's Disease
PD is a major neurodegenerative disease characterized by progressive degeneration of the nervous system, the primary locus of the disease being the loss of dopamine in the substantia nigra pars compacta (SNpc). Dopamine-based therapies typically help initial motor symptoms. Nonmotor symptoms require nondopaminergic approaches (e.g., selective serotonin reuptake inhibitors for psychiatric symptoms and cholinesterase inhibitors for cognition) [30]. Levodopa remains the most potent drug for controlling PD symptoms, yet it is associated with significant complications such as the “wearing off” effect, levodopa-induced dyskinesias, and other motor complications [31].
Epidemiological, clinical trials, and experimental evidence implicate inflammatory processes, in the degeneration of dopaminergic neurons in the nigrostriatal pathway. Cellular mechanisms activated or enhanced by inflammatory processes may contribute to mitochondrial dysfunction, OS, or apoptosis of dopaminergic (DA) neurons. Epigenetic factors have the potential to trigger neuroinflammation, including environmental exposures and age-associated chronic inflammatory conditions [14, 32].
Wingless/integrated (Wnt) proteins are secreted lipid-modified glycoproteins belonging to a group of signal transduction pathways. The Wnt signaling mainly consists of three pathways, and the canonical Wnt pathway leads to the regulation of gene transcription. Signaling by the Wnt canonical pathway, via the transcription co-activator beta-catenin (β-catenin), controls embryonic development and adult homeostasis [33]. The hallmark of the canonical Wnt/β-catenin signaling pathway is the activation of β-catenin-mediated transcriptional activity [34]. The Wnt pathway and the components of Wnt/β-catenin signaling are widely expressed in the midbrain and control of DA neurons.
Evidence suggests that mitochondrial dysfunction plays a key role in the pathogenesis of PD, and Axin-2, a negative regulator of Wnt/β-catenin signaling, affects mitochondrial biogenesis. In addition, the unilateral 6-hydroxydopamine (6-OHDA) injection into the medial forebrain bundle (a model of PD) was shown to potentially dysregulate Wnt/β-catenin signaling in the SNpc, suggesting that the manipulation of Wnt signaling may enhance the endogenous regenerative capacity of DA neurons [35]. CBD was shown to downregulate glycogen synthase kinase 3 beta (GSK-3β), the main inhibitor of the Wnt/β-catenin pathway. The activation of the Wnt/β-catenin could be associated with the control of OS and inflammation [36], and its dysregulation could lead to diseases, including neurodegenerative diseases [37]. In the presence of neuroinflammation, the Janus kinase/signal transducer and activator of the transcription signaling pathway and other transcription factors are upregulated and induce microglial activation, contributing to PD [38].
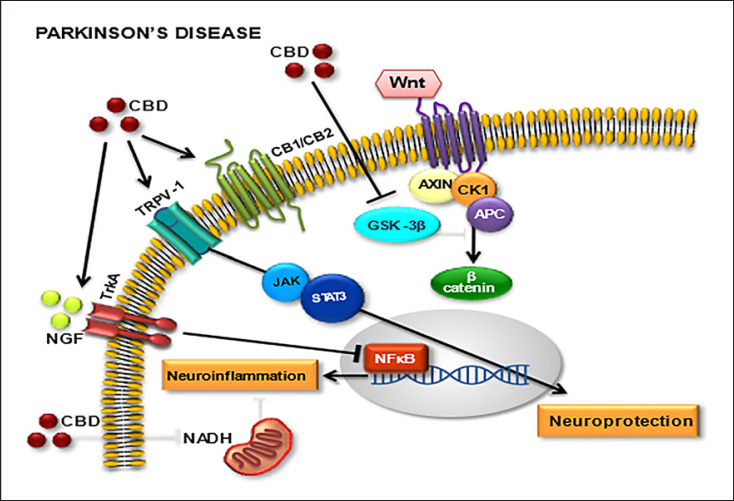
CBD acts as an agonist of TRPV1, PPARγ, and 5-HT1A receptors and as an antagonist of GPR55 receptor. It also antagonizes the action of CB1 and CB2 receptor agonists, acting as an inverse agonist and negative allosteric modulator [39]. Furthermore, CBD exerts its neuroprotective effects through three G protein-coupled receptors (adenosine receptor subtype 2 A, serotonin receptor subtype 1 A, and G protein-coupled receptor 55), one ligand-gated ion channel (transient receptor potential vanilloid channel-1), and one nuclear factor (peroxisome proliferator-activated receptor γ). Moreover, the therapeutical properties of CBD are also due to GABAergic modulation [11]. Figure 1 shows the main molecular targets involved with the CBD neuroprotective action in PD.
Fig. 1.
Main molecular targets for cannabidiol (CBD) in Parkinson's disease (PD). Cannabinoid receptors (CB1/CB2), transient receptor potential cation channel subfamily V member 1 (TRPV1), Wnt/GSK-3β (Wnt/glycogen synthase kinase 3 beta pathway), nerve growth factor (NGF), neurotrophic tyrosine kinase receptor member (TrkA), Janus kinase (JAK), signal transducer and activator of transcription proteins (SATAxin3), nicotinamide adenine dinucleotide (NADH), adenomatous polyposis coli (APC), casein kinase 1 (CK1), beta-catenin (β-catenin), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).
Although the depletion of DA neurons is the most important neurotransmitter alteration in PD, other neurochemical changes occur and contribute to its symptomatology. The underlying molecular pathogenesis involves multiple pathways and mechanisms, such as α-synuclein proteostasis, mitochondrial function, OS, calcium homeostasis, axonal transport, and mainly neuroinflammation [37, 40]. Other alterations include the increased levels of proinflammatory cytokines in the CSF and nigrostriatal regions of PD brains, leading to reactive microglia in the SNpc. This may chronically produce ROS, resulting in OS and mitochondrial dysfunction [41].
In the past decade, CBD has been shown to have compensatory effects both on the ECS and as a neuromodulator and neuroprotector, in models such as 6-OHDA, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, and reserpine, as well as other PD models. Although the CBD-induced neuroprotection observed in animal models of PD has been attributed to the activation of the CB1 receptor, a recent work proposed that CBD is able of activating other receptors, such as CB2 and the TRPV1 receptor, both of which are expressed in DA neurons of the nigrostriatal pathway [42].
Several in vitro experiments have demonstrated promising neuroprotective effects of CBD in PD models. In PC12 and SH-SY5Y cells treated with MPP+, CBD increased cell viability, differentiation, and the expression of axonal and synaptic proteins [43, 44], and these neuroprotective effects depend on the activation of tropomyosin receptor kinase A receptors. CBD also protected SH-SY5Y cells (an in vitro model of PD) against LPS- and b-amyloid-induced decreases in cell viability. The mechanism is independent of nerve growth factor but involves the receptors of nerve growth factor, tropomyosin receptor kinase A, and an increased expression of axonal and synaptic proteins suggesting that CBD has a neurorestorative potential that might contribute to its neuroprotective action [see ref. 43 for a review]. More recently [45], CBD was shown to decrease the loss of tyrosine hydroxylase expression and cytotoxicity in the MPP+-induced SH-SY5Y cells. These authors showed that CBD protects cells from mitochondrial dysfunction, by upregulating SIRT1 and inhibiting NF-κB and NOTCH pathways.
Recently, CBD was shown to exhibit a preferential action on astrocytes, activating the astrocytic transient receptor potential cation channel vaniloid 1 (TRPV1) and enhancing the endogenous neuroprotective response of ciliary neurotrophic factor (CNF) [46]. These results overall support the potential therapeutic utility of CBD in PD, as both a neuroprotective and symptomatic agent.
CBD (1–10 μM) was shown to inhibit the release of proinflammatory cytokines (TNF-α and IL-1β). CBD also inhibits glutamate, a mediator of inflammation on microglial cells stimulated by LPS in a receptor-independent effect manner [47]. According to these authors, CBD exerts its anti-inflammatory actions by an antioxidant effect, which is amplified through the inhibition of glucose-dependent NADPH synthesis. These results confirm that CBD may have a therapeutic benefit in the presence of neuroinflammatory processes.
Furthermore, a neurotoxin model of PD, using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, demonstrated that the administration of CBD (5 mg/kg, i.p.), for 5 weeks, did not reduce motor deficits or DA neuronal loss in the nigrostriatal pathway. On the other hand, daily administration of CBD (3 mg/kg, i.p.), for 14 days, decreased both DA depletion and tyrosine hydroxylase expression, in the striatum of rats that received 6-OHDA [48].
Importantly, the interactions between cannabinoids and DA neurons in the basal ganglia involve not only the modulation of other neurotransmitters (γ-aminobutyric acid, glutamate, opioids, and peptides) but also the activation of CB1 and CB2 receptors. Despite new CBMs have been proposed for motor and nonmotor symptoms of PD, so far, results from clinical studies are controversial and inconclusive and additional clinical studies involving larger samples of patients, appropriate molecular targets, and specific clinical outcome measures are needed to clarify the effectiveness of CBM therapies on PD symptoms [49].
As far as clinical trials are concerned, evidence points to a possible effect of CBD in improving quality of life measures, in PD patients with no psychiatric comorbidities [50]. However, the authors conclude that studies with larger samples and specific objectives are required, before definitive conclusions can be drawn. Crippa and coworkers, 2019 [51], found four RCTs involving the administration of agonists/antagonists of the CB1 receptor, showing that these compounds were well tolerated. Three trials involving CBD and PD: an open-label study, a case series, and an RCT showed that CBD was well tolerated and presented significant therapeutic effects in nonmotor symptoms. However, in these clinical trials, sample sizes were small and CBD treatment was short (up to 6 weeks). The authors concluded that large-scale RCTs are needed to try to replicate these results and to assess the long-term safety of CBD.
Furthermore, an open-label study with fifteen participants showed that CBD, in the form of Epidiolex, was efficacious in PD, but the relatively high dose used was associated with liver enzyme elevations [52]. The authors conclude that RCT is needed for investigating various forms of Cannabis in PD. Another review [53] present clinical trial data on the benefits and potential side effects of CBD-based drug products, specifically Epidiolex showing the state of the art and the primary and secondary outcomes. Besides the study designs are inconsistent, the drug and dosage used also varies significantly, what could explain differences in side effects. While CBD has been proven to be well tolerated in a wide range of patients suffering from various ailments, there is certainly need for better-designed clinical trials. Furthermore, although observational studies establish subjective symptom alleviation and interest in medical Cannabis or its derivatives among PD patients, there is insufficient evidence to support its integration into clinical practice for motor symptom treatment [54].
A systematic search of the literature, conducted in June 2021 [55], presented five RCT and eighteen nonrandomized studies investigating cannabis treatments in PD patients. According to the authors, although no compelling evidence was found to recommend the use of cannabis in PD patients, a potential benefit was identified with respect to alleviation of PD-related tremor, anxiety, pain, improvement of sleep quality and quality of life.
A recent literature review identified 569 papers on PD and cannabinoid treatments [56]. Of these, there were only seven papers featuring RCT on the effects of different cannabinoids on PD. The results of these trials did not support the efficacy of cannabinoids in the treatment of motor signs of PD, and the authors concluded that there is currently insufficient data for supporting the administration of cannabinoids to PD patients. In conclusion, there is a consensus in the literature that larger, RCT on cannabis use in PD should be conducted, to show convincing data concerning the benefits of CBD in PD patients.
CBD and Alzheimer's Disease
AD is a chronic neurodegenerative disease affecting the central nervous system and leading to decline of cognitive functions. One of the causes is the decrease of the neurotransmitter acetylcholine levels in the brain, in part due to a higher activity of acetylcholinesterase (AChE), the enzyme responsible for acetylcholine degradation [57].
AD can be considered as a multifactorial pathology that depends on a combination of both genetic and environmental factors. There are several possible causes associated with AD onset, besides alterations in the cholinergic system, such as deposition of beta-amyloid (Aβ) aggregates, precipitation of intracellular neurofibrillary tangles (NFTs) (due to hyperphosphorylation of protein Tau), OS, neuroinflammation by microglial activation, high concentrations of heavy metals, metabolic diseases (such as those provoked by dysregulation of cholesterol homeostasis), type 2 diabetes, and obesity [58]. Therefore, many pharmacological strategies have been designed, aiming at slowing down AD symptoms, but such strategies have been mostly ineffective.
The characteristic neuropathological aspects of AD are senile plaques, NFTs, and amyloid angiopathy. These brain lesions associated with AD are characterized by the presence of a broad spectrum of inflammatory mediators produced by cells residing in the brain, including neurons [41]. Senile plaques in AD patients were shown to express CB1 and CB2 cannabinoid receptors together with markers of microglial activation. However, CB1-positive neurons, present in high numbers in control cases, are greatly reduced in areas of microglial activation. Furthermore, G protein coupling and CB1 receptor protein expression were found to be markedly decreased in AD brains [59]. These results indicate that cannabinoid receptors are important in the pathology of AD and that cannabinoids succeed in preventing the neurodegenerative process occurring in the disease.
AD is associated with OS due, in part, to the membrane action of Aβ peptide aggregates. Thus, the effect of CBD was also studied on Aβ peptide-induced toxicity on PC12 cells [60]. These earlier results indicate that CBD exerts a combination of neuroprotective, anti-oxidative, and anti-apoptotic effects against Aβ peptide toxicity and that inhibition of caspase 3 appearance from its inactive precursor, pro-caspase 3, by CBD, is involved in this neuroprotection.
Some years ago, CBD was studied on PC12 cells and the results showed that Aβ-induced TAU hyperphosphorylation was inhibited by CBD [61]. This inhibition was seen to be associated with a downregulation of p-GSK-3β, an inhibitor of the Wnt pathway. CBD may also increase Wnt/β-catenin by stimulation of PPARγ, inhibition of Aβ, and ubiquitination of the amyloid precursor protein. CBD attenuated OS and diminished mitochondrial dysfunction and ROS generation. CBD suppressed, through activation of PPARγ, proinflammatory signaling and, thus, the above authors concluded that CBD may be a potential candidate for AD therapy.
In the case of AD treatment, CBD can rescue the production of NFTs and inhibit neuronal apoptosis, acting indirectly as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. A recent work [37] showed that CBD promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the GSK-3β hyperphosphorylation caused by Aβ and may be a new therapeutic candidate for AD.
Besides, recent findings in AD rodent models have demonstrated promising effects of cannabinoids in reducing amyloid plaque deposition and stimulating hippocampal neurogenesis [62]. Beneficial effects on several dementia-related symptoms have also been reported in clinical trials after cannabinoid treatments. Accordingly, future studies should address the correct therapeutic dosage and timing of treatment, related to the use of cannabinoids in AD therapy.
AD is characterized by the accumulation of amyloid-β and tau hyperphosphorylation, neuroinflammation, and OS. CBD has demonstrated neuroprotective, anti-inflammatory, and antioxidant properties in vitro; thus, it is being investigated as a potential multifunctional treatment option for AD. The current status quo of in vivo effects of CBD, in pharmacological and transgenic animal models for AD, demonstrates its ability to reduce reactive gliosis and the neuroinflammatory response, as well as to promote neurogenesis [63]. Importantly, CBD also reverses and prevents the development of cognitive deficits in AD rodent models [64, 65].
Interestingly, combination therapies of CBD and THC show that CBD can antagonize the psychoactive effects associated with THC and possibly mediate greater therapeutic benefits than phytocannabinoids alone [63]. According to these authors, this study provides “proof of principle” that CBD and possibly CBD-THC combinations are potential candidates for novel AD therapies. However, further investigations should address the long-term potential of CBD and evaluate mechanisms involved in the therapeutic effects described.
CBD has been shown to reverse cognitive deficits of AD transgenic mice and to exert neuroprotective, anti-oxidative, and anti-inflammatory properties in vitro and in vivo [65]. This study was the first to demonstrate the ability of CBD to prevent the development of a social recognition deficit in AD transgenic mice and provides the first evidence that CBD may have the potential as a preventive treatment for AD.
Furthermore, cannabinoids present neuroprotective properties, reduce neuroinflammation, and enhance neurogenesis, and evidence suggests that the utilization of marijuana products containing both THC and CBD or CBD alone is effective and safe for use in older people with agitation associated with dementia [66]. A recently conducted review [67] summarized positive findings for the therapeutic benefits of cannabinoids in the agitation of AD and dementia, but there was no definitive conclusion because of varying cannabinoid products.
Cannabinoids seem to be well tolerated, with few short-term side effects, differing from first-line medications utilized for dementia behaviors, which can have unwanted side effects. Cannabinoid-based medicines (CBM) have shown an ability to inhibit some symptoms associated with dementia with few adverse effects. Although there are several studies and recent reviews focused on these issues, further research regarding the safety, efficacy, and variability of phytocannabinoids is needed [24, 65, 66, 67, 68, 69].
The use of nanoparticle drugs can give better effects on the target tissue, since AD due to its high prevalence requires more appropriate treatments. A previous study investigated the effect of CBD, coated by nano-chitosan, on learning and memory, hippocampal CB1 and CB2 receptors, and amyloid plaques in an AD rat model [70]. According to these authors, it seems that CBD coated by nano-chitosan has good potential for reducing Aβ plaques, increasing brain CB1 and CB2 levels, and improving learning and memory in AD rats.
A prospective observational study [71] tested the acceptability, practical aspects, and clinical outcomes of a THC/CBD-based oral medication in severely demented patients, in a specialized nursing home in Geneva. The authors concluded that an oral cannabis extract with THC/CBD, at higher dosages, was well tolerated and greatly improved behavior problems and rigidity, in severely demented patients.
Computational modeling of the THC-AChE interaction revealed that THC binds in the peripheral anionic site of AChE [72], the critical region involved in amyloid genesis. Compared to currently approved drugs, prescribed for the treatment of AD, THC is a considerably superior inhibitor of Aβ aggregation, and this study provides a previously unrecognized molecular mechanism through which cannabinoid molecules may directly impact the progression of AD.
In AD, the Wnt/β-catenin pathway is downregulated; PPARγ is increased. Downregulation of Wnt/β-catenin, through activation of GSK-3β by Aβ, and inactivation of PI3K/Akt signaling involves OS in AD. GSK-3β phosphorylation activates tau hyperphosphorylation, which induces NFTs and neuroinflammation [61]. Wnt proteins participate in the remodeling of pre- and post-synaptic regions, thus modulating synaptic function, and are constantly released in the brain to maintain basal neural activity [73]. The Wnt family of secreted glycolipoproteins via the transcription co-activator β-catenin controls embryonic development and adult homeostasis [34]. In the brain, Wnt/β-catenin signaling is not only crucial for neuronal survival and neurogenesis, but it plays important roles in regulating synaptic plasticity and blood-brain barrier integrity and function [74].
Moreover, activation of Wnt/β-catenin signaling inhibits amyloid-β production and TAU hyperphosphorylation in the brain. Wnt/β-catenin signaling is greatly suppressed in AD brain, via multiple pathogenic mechanisms, and, as such, restoring Wnt/β-catenin signaling represents a unique opportunity for the rational design of novel AD therapies [75]. Evidence suggests that synaptic signaling is compromised in the aging brain and in AD, contributing to the synaptic decline. Thus, Wnt signaling is a prominent synaptic pathway and is required for synaptic plasticity in the adult brain [76, 77]. Emerging studies suggest that enhancing Wnt signaling could boost synaptic function during aging, ameliorate synaptic pathology in AD, and by targeting Wnt signaling components, may provide novel therapeutic avenues for synapse protection or restoration in the brain [78].
Specifically, the Wnt/β-catenin axis is pivotal to the development and homeostasis of the central nervous system, and its dysregulation has been associated with various neurological diseases, including neurodegenerative diseases [79]. Therefore, this signaling pathway has been proposed as a potential therapeutic target against neurodegeneration. The increasing interest in the role of the Wnt/β-catenin pathway, on the onset of neurodegenerative diseases, demonstrates how targeting this signaling for therapeutic purposes could be a great opportunity for neuroprotection and neuro repair. Furthermore, by restoring this signaling, one may strongly increase the chance to develop disease-modifying treatments for these brain pathologies [77].
Impaired Wnt signaling pathways are associated with enhanced levels of amyloid-β, reduced β-catenin levels, and increased expression of the GSK-3β enzyme, suggesting their direct association with the pathogenesis of AD. Importantly, various natural and synthetic molecules, including CBD, have been shown to modulate Wnt signaling in the adult brain, leading to a process of neurogenesis and alleviating behavioral dysfunction [78].
The peroxisome proliferator-activated receptor (PPAR) family of nuclear receptors, consisting of three subtypes (PPARα, PPARγ, and PPARβ/δ), plays a major regulatory role in energy homeostasis and metabolic function and, also, important functions in neurodegenerative diseases, among others [80]. PPARγ has been reported to be involved in the etiology of pathological features of AD. In addition, PPARδ with a potent anti-inflammatory activation property and PPARδ agonism were shown to reduce the brain Aβ levels, in a transgenic mouse model of AD [81].
Moreover, due to its interaction at PPARγ, CBD was observed to stimulate hippocampal neurogenesis. PPARγ coordinates lipid, glucose, and energy metabolism and is found, in elevated levels, in the brains of AD patients [82]. Furthermore, a physiological function of PPARγ is its ability to modulate inflammatory responses. In animal models of AD, the PPARγ agonist treatment results in the reduction of amyloid plaque burden, reduced inflammation, and reversal of disease-related behavioral impairment. In addition, a phase II clinical trial showed that the use of the PPARγ agonist rosiglitazone was associated with improved cognition and memory, in patients with mild to moderate AD, and may represent an attractive therapeutic target for the treatment of the disease [82].
Besides, PPARγ agonists have been shown to reduce inflammatory responses, in several animal models of neurological diseases, and decrease amyloid burden in transgenic mice. Thus, GW742 (a PPARδ agonist) was shown to reduce amyloid plaque burden and astrocyte activation in glia cells [83]. These results suggest that PPARδ agonists can also reduce amyloid burden, likely to be mediated by effects on amyloid clearance. PPARγ has been reported to be involved in the etiology of pathological features of AD. CBD, devoid of psychomimetic effects, has attracted much attention because of its promising neuroprotective properties in rat AD models, even though the mechanism responsible for such actions remains unknown. Results showed that the blockade of PPARγ was able to significantly blunt CBD effects on reactive gliosis and subsequently on neuronal damage. Moreover, due to its interaction with PPARγ, CBD was observed to stimulate hippocampal neurogenesis [84].
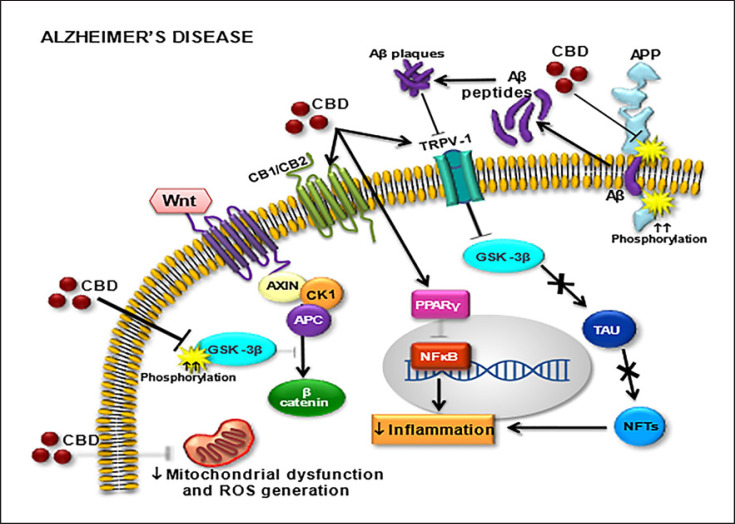
Activation of PPARγ mediates neuroprotective and anti-inflammatory actions of cannabinoids, among others, in conjunction with activation of the more traditional target sites of action, such as CB1 and CB2 receptors and the TRPV1 ion channel. PPARs also mediate some of the effects of inhibitors of endocannabinoid degradation or transport [85, 86]. Figure 2 shows the main CBD molecular targets which possibly justify its neuroprotective action by decreasing mitochondrial dysfunction and ROS generation.
Fig. 2.
Molecular targets for cannabidiol (CBD) in Alzheimer's disease (AD). Cannabinoid receptors (CB1/CB2), transient receptor potential cation channel subfamily V member 1 (TRPV1), Wnt/GSK-3β (Wnt/glycogen synthase kinase 3 beta pathway), amyloid precursor protein (APP), beta-amyloid (Aβ) peptide, peroxisome proliferator-activated receptors (PPARγ), beta-catenin (β-catenin), neurofibrillary tangles (NFTs), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), tau protein (TAU), reactive oxygen species (ROS).
Recent studies [87], using an AD mouse model, suggested that CBD can reverse cognitive deficits along with Aβ-induced neuroinflammatory, oxidative responses, and neuronal death. Furthermore, CBD can reduce the accumulation of Aβ and hyperphosphorylation of tau, possibly delaying AD progression. Besides, the non-cannabinoid receptor, PPARγ, has been suggested to be involved in multiple actions of CBD. Considering their anti-inflammatory and neuroprotective properties, PPAR-β/δ agonists are promising treatments for AD and other neurodegenerative diseases. Therefore, future studies should test PPAR-β/δ ligands, in combination with ligands of other PPARs, in these neurological diseases and in inflammation [88].
CBD can potentially enhance neuroprotection, by reducing inflammation, regulating cerebral blood flow, increasing neurogenesis, and protecting the brain against ROS. Interestingly, Shelef et al. [89], recruited 11 AD patients to an open-label, 4 weeks, in a prospective trial type of study. The objective was to measure the efficacy and safety of medical cannabis oil containing THC, in relieving behavioral and psychological symptoms of dementia. The authors concluded that adding cannabis oil containing THC to AD patients' pharmacotherapy is safe and a promising treatment option. However, double-blind RCTs are still required for validating the use of CBD as a medication for dementia [90].
Conclusions
The pathways to the development of AD and PD share similarities, though the specific components or proteins involved may differ. A unifying feature of these neurodegenerative diseases is the abnormal accumulation and processing of mutant or damaged intra- and extracellular proteins leading to brain neuronal vulnerability and dysfunction. A detailed review of ubiquitin-proteasome, mRNA splicing, mitochondrial dysfunction, and OS pathway interrelation on neurodegeneration can improve the understanding of the action mechanism of these diseases. The identified pathways common to AD and PD nominate promising new targets for further studies as well as biomarkers that may require simultaneous targeting of multiple components [91].
While the efficacy of cannabinoids was not proven in a robust RCT, observational studies showed promising results, especially for patients whose symptoms were refractory. In addition, the safety profile is favorable as most of the ADEs reported were mild. Future trials may want to consider dose escalation and formulations with improved bioavailability [92]. A large, well-conducted study is needed for a better understanding of whether cannabinoids are a useful treatment for people living with dementia [93]. Furthermore, many pharmacological details are yet to be determined, such as dosing, treatment duration, and concentrations of active compounds such as CBD [94].
The most consistent results, including some recent ones [11, 27], concern the neuroprotective and antioxidant action of CBD, which could represent a real therapeutic resource to limit the extent and severity of neuronal damage, typical of neurodegenerative diseases such as PD and AD. Despite the wealth of preclinical studies showing the efficacy of both Δ9-THC and CBD, still relatively few well-designed clinical trials with these compounds have been carried out. Phytocannabinoids led humanity to discover one of the most intriguing and pleiotropic endogenous signaling systems, the ECS, and are targets to develop new therapeutic strategies for the treatment of neurodegenerative diseases such as PD and AD [95].
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
The authors are grateful to the financial support from the Foundation for Support to Scientific and Technological Development of the State do Ceará (FUNCAP) and to the Brazilian National Research Council (CNPq).
Author Contributions
Milena de Barros Viana: helped with the project coordination, Pedro Everson Alexandre de Aquino and Daniel Araki Ribeiro: helped with the manuscript formatting, Débora Estadella: made the figures, and Glauce Socorro de Barros Viana: was responsible for the coordination and wrote the manuscript.
Funding Statement
The authors are grateful to the financial support from the Foundation for Support to Scientific and Technological Development of the State do Ceará (FUNCAP) and to the Brazilian National Research Council (CNPq).
References
- 1.Hussain T, Jeena G, Pitakbut T, Vasilev N, Kayser O. Cannabis sativa research trends, challenges, and new-age perspectives. iScience. 2021;24((12)):103391. doi: 10.1016/j.isci.2021.103391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock EM, Parker LA. Constituents of cannabis sativa. Adv Exp Med Biol. 2021;1264:1–13. doi: 10.1007/978-3-030-57369-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Pertwee RG. The diverse CB1and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153((2)):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20((S1)):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 5.Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci. 2016;10:294. doi: 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y, Tajima M, Sugiyama E, Sato VH, Sato H. Development of a novel nano-emulsion formulation to improve intestinal absorption of cannabidiol. Med Cannabis Cannabinoids. 2019;2((1)):35–42. doi: 10.1159/000497361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung H, Fierro A, Pessoa-Mahana CD. Cannabidiol binding and negative allosteric modulation at the cannabinoid type 1 receptor in the presence of delta-9-tetrahydrocannabinol: an in-silico study. PLoS One. 2019;14((7)):e0220025. doi: 10.1371/journal.pone.0220025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellati F, Borgonetti V, Brighenti V, Biagi M, Benvenuti S, Corsi L. Cannabis sativa L. and nonpsychoactive cannabinoids: their chemistry and role against oxidative stress, inflammation, and cancer. Biomed Res Int. 2018;2018:1691428. doi: 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19((3)):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millar SA, Stone NL, Yates AS, O'Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365. doi: 10.3389/fphar.2018.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules. 2019;24((8)):1459. doi: 10.3390/molecules24081459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111((1)):3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kujawska M, Domanskyi A, Kreiner G. Editorial: common pathways linking neurodegenerative diseases- the role of inflammation. Front Cell Neurosci. 2021;15:15754051. doi: 10.3389/fncel.2021.754051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N. Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. 2019;10:1008. doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53((1)):401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandes MS, Gray NE. Nrf2 as a therapeutic target in neurodegenerative diseases. ASN Neuro. 2020;12:1759091419899782. doi: 10.1177/1759091419899782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson DA, Johnson JÁ. Nrf2 − a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Buttari B, Profumo E, Tucci P, Saso L. A perspective on Nrf2 signaling pathway for neuroinflammation: a potential therapeutic target in Alzheimer's and Parkinson's diseases. Front Cell Neurosci. 2021;15:787258. doi: 10.3389/fncel.2021.787258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastrząb A, Jarocka-Karpowicz I, Markowska A, Wroński A, Gęgotek A, Skrzydlewska E. Antioxidant and anti-inflammatory effect of cannabidiol contributes to the decreased lipid peroxidation of keratinocytes of rat skin exposed to UV radiation oxidative medicine and cellular longevity. Oxid Med Cell Longev. 2021:1–13. [Google Scholar]
- 20.Kozela E, Juknat A, Gao F, Kaushansky N, Coppola G, Vogel Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13((1)):136. doi: 10.1186/s12974-016-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casares L, Garcia V, Garrido-Rodriguez M, Millán E, Collado JÁ, Garcia-Martin A, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321. doi: 10.1016/j.redox.2019.101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative diseases: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75((2)):323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative diseases? CNS Neurosci Ther. 2009;15((1)):65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maroon J, Bost J. Review of the neurological benefits of phytocannabinoids. Surg Neurol Int. 2018;9((1)):91. doi: 10.4103/sni.sni_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooray R, Gupta V, Suphioglu C. Current aspects of the endocannabinoid system and targeted THC and CBD phytocannabinoids as potential therapeutics for Parkinson's and Alzheimer's diseases: a review. Mol Neurobiol. 2020;57((11)):4878–4890. doi: 10.1007/s12035-020-02054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Choi H, Kang EK, Ji GY, Kim Y, Choi IS. In vitro studies on therapeutic effects of cannabidiol in neural cells: neurons, glia, and neural stem cells. Molecules. 2021;26((19)):6077. doi: 10.3390/molecules26196077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassano T, Villani R, Pace L, Carbone A, Bukke VN, Orkisz S, et al. From Cannabis sativa to cannabidiol: promising therapeutic candidate for the treatment of neurodegenerative diseases. Front Pharmacol. 2020;11:124. doi: 10.3389/fphar.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velayudhan L, McGoohan K, Bhattacharyya S. Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: a systematic review and meta-analysis. Plos Med. 2021;18((3)):e1003524. doi: 10.1371/journal.pmed.1003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO: CANNABIDIOL (CBD) Critical review report expert committee on drug dependence fortieth meeting Geneva. 2018. pp. p. 4–7. [Google Scholar]
- 30.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson's disease: a Review. JAMA. 2020;323((6)):548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 31.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008;4:743–757. doi: 10.2147/ndt.s2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald BT, Tamai K, He X. Wnt/β-Catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17((1)):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K, Wang X, Zhang H, Wang Z, Nan G, Li Y, et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest. 2016;96((2)):116–136. doi: 10.1038/labinvest.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Mishra A, Mohanbhai SJ, Tiwari V, Chaturvedi RK, Khurana S, et al. Axin-2 knockdown promote mitochondrial biogenesis and dopaminergic neurogenesis by regulating Wnt/β-catenin signaling in rat model of Parkinson's disease. Free Radic Biol Med. 2018;129:73–87. doi: 10.1016/j.freeradbiomed.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 36.Vallée A, Lecarpentier Y, Vallée JN. Cannabidiol and the canonical WNT/β-catenin pathway in glaucoma. Int J Mol Sci. 2021;22((7)):3798. doi: 10.3390/ijms22073798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Liu Y, Tian D, Tian L, Ju X, Qi L, et al. Overview of cannabidiol (CBD) and its analogues: structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer's disease. Eur J Med Chem. 2020;192:112163. doi: 10.1016/j.ejmech.2020.112163. [DOI] [PubMed] [Google Scholar]
- 38.Lashgari NA, Roudsari NM, Momtaz S, Sathyapalan T, Abdolghaffari AH, Sahebkar A. The involvement of JAK/STAT signaling pathway in the treatment of Parkinson's disease. J Neuroimmunol. 2021;361:577758. doi: 10.1016/j.jneuroim.2021.577758. [DOI] [PubMed] [Google Scholar]
- 39.Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement diseases? Front Pharmacol. 2018;11:9–482. doi: 10.3389/fphar.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieder CR. Cannabidiol in Parkinson's disease. Braz J Psychiatry. 2020;42((2)):126–127. doi: 10.1590/1516-4446-2019-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milano W, Capasso A. Neuroprotection by cannabinoids in neurodegenerative diseases. Alzheimers Dement Cogn Neurol. 2018;2:1–7. [Google Scholar]
- 42.Patricio F, Morales-Andrade AA, Patricio-Martínez A, Limón ID. Cannabidiol as a therapeutic target: evidence of its neuroprotective and neuromodulatory function in Parkinson's disease. Front Pharmacol. 2020;11:595635. doi: 10.3389/fphar.2020.595635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos NAG, Martins NM, Sisti FM, Fernandes LS, Ferreira RS, Queiroz RHC, et al. The neuroprotection of cannabidiol against MPP+ -induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson's disease. Toxicol In Vitro. 2015;30((1)):231–240. doi: 10.1016/j.tiv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Janefjord E, Mååg JLV, Harvey BS, Smid SD. Cannabinoid effects on beta amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell Mol Neurobiol. 2014;34((1)):31–42. doi: 10.1007/s10571-013-9984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang S, Li J, Yao Z, Liu J. Cannabidiol induces autophagy to protects neural cells from mitochondrial dysfunction by upregulating SIRT1 to inhibits NF-κB and NOTCH pathways. Front Cell Neurosci. 2021;15:654340. doi: 10.3389/fncel.2021.654340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giuliano C, Francavilla M, Ongari G, Petese A, Ghezzi C, Rossini N, et al. Neuroprotective and symptomatic effects of cannabidiol in an animal model of Parkinson's disease. Int J Mol Sci. 2021;22((16)):8920. doi: 10.3390/ijms22168920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.dos-Santos-Pereira M, Guimarães FS, Del-Bel E, Raisman-Vozari R, Michel PP. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia. 2020;68((3)):561–573. doi: 10.1002/glia.23738. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira-Junior NC, Campos AC, Guimarães FS, Del-Bel E, Zimmermann PMR, Brum Junior L, et al. Biological bases for a possible effect of cannabidiol in Parkinson's disease. Braz J Psychiatry. 2020;42((2)):218–224. doi: 10.1590/1516-4446-2019-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stampanoni Bassi M, Sancesario A, Morace R, Centonze D, Iezzi E. Cannabinoids in Parkinson's disease. Cannabis Cannabinoid Res. 2017;2((1)):21–29. doi: 10.1089/can.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chagas MHN, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28((11)):1088–1098. doi: 10.1177/0269881114550355. [DOI] [PubMed] [Google Scholar]
- 51.Crippa JAS, Hallak JEC, Zuardi AW, Guimarães FS, Tumas V, Dos Santos RG. Is cannabidiol the ideal drug to treat non-motor Parkinson's disease symptoms? Eur Arch Psychiatry Clin Neurosci. 2019;269((1)):121–133. doi: 10.1007/s00406-019-00982-6. [DOI] [PubMed] [Google Scholar]
- 52.Leehey MA, Liu Y, Hart F, Epstein C, Cook M, Sillau S, et al. Safety and tolerability of cannabidiol in Parkinson disease: an open label, dose-escalation study. Cannabis Cannabinoid Res. 2020;5((4)):326–336. doi: 10.1089/can.2019.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pauli CS, Conroy M, Vanden Heuvel BD, Park SH. Cannabidiol drugs clinical trial outcomes and adverse effects. Front Pharmacol. 2020;11:63. doi: 10.3389/fphar.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanabalasingam SJ, Ranjith B, Jackson R, Wijeratne DT. Cannabis and its derivatives for the use of motor symptoms in Parkinson's disease: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2021;14:17562864211018527. doi: 10.1177/17562864211018561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbi B, Corbett J, Hughes I, Owusu MA, Thorning S, Broadley SA, et al. Effects of cannabis in Parkinson's disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12((2)):495–508. doi: 10.3233/JPD-212923. [DOI] [PubMed] [Google Scholar]
- 56.Figura M, Koziorowski D, Sławek J. Cannabis in Parkinson's disease - the patient's perspective versus clinical trials: a systematic literature review. Neurol Neurochir Pol. 2022;56((1)):21–27. doi: 10.5603/PJNNS.a2022.0004. [DOI] [PubMed] [Google Scholar]
- 57.Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer's disease. Neuropharmacology. 2020;1:190–208. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 58.Vecchio I, Sorrentino L, Paoletti A, Marra R, Arbitrio M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer's disease. J Cent Nerv Syst Dis. 2021;13:11795735211029113. doi: 10.1177/11795735211029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramírez BG, Blázquez C, Gómez-Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25((8)):1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89((1)):134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 61.Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer's disease. Acta Biochim Biophys Sin. 2017;49((10)):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 62.Abate G, Uberti D, Tambaro S. Potential and limits of cannabinoids in Alzheimer's disease therapy. Biology. 2021;10((6)):542. doi: 10.3390/biology10060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watt G, Karl T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer's disease. Front Pharmacol. 2017;8:20. doi: 10.3389/fphar.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng D, Spiro AS, Jenner AM, Garner B, Karl T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer's disease transgenic mice. J Alzheimers Dis. 2014;42((4)):1383–1396. doi: 10.3233/JAD-140921. [DOI] [PubMed] [Google Scholar]
- 65.Coles M, Watt G, Kreilaus F, Karl T. Medium-dose chronic cannabidiol treatment reverses object recognition memory deficits of APPswe/PS1ΔE9 transgenic female mice. Front Pharmacol. 2020;11:587604. doi: 10.3389/fphar.2020.587604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Premoli M, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Pina SD, et al. Cannabidiol: recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019;224:120–127. doi: 10.1016/j.lfs.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 67.Mueller A, Fixen DR. Use of cannabis for agitation in patients with dementia. Sr Care Pharm. 2020;35((7)):312–317. doi: 10.4140/TCP.n.2020.312. [DOI] [PubMed] [Google Scholar]
- 68.Timler A, Bulsara C, Bulsara M, Vickery A, Smith J, Codde J. Use of cannabinoid-based medicine among older residential care recipients diagnosed with dementia: study protocol for a double-blind randomised crossover trial. Trials. 2020;21((1)):188. doi: 10.1186/s13063-020-4085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahji A, Meyyappan AC, Hawken ER. Cannabinoids for the neuropsychiatric symptoms of dementia: a systematic review and meta-Analysis. Can J Psychiatry. 2020;65((6)):365–376. doi: 10.1177/0706743719892717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amini M, Abdolmaleki Z, The effect of cannabidiol coated by nano-chitosan on learning and memory Hippocampal CB1 and CB2 levels, and amyloid plaques in an Alzheimer's disease rat model. Neuropsychobiology. 2022;81((3)):171–183. doi: 10.1159/000519534. [DOI] [PubMed] [Google Scholar]
- 71.Broers B, Patà Z, Mina A, Wampfler J, de Saussure C, Pautex S. Prescription of a THC/CBD-based medication to patients with dementia: a pilot study in Geneva. Med Cannabis Cannabinoids. 2019;2((1)):56–59. doi: 10.1159/000498924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eubanks LM, Rogers CJ, Beuscher AE, Koob GF, Olson AJ, Dickerson TJ, et al. A molecular link between the active component of marijuana and Alzheimer's disease pathology. Mol Pharm. 2006;3((6)):773–777. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oliva CA, Vargas JY, Inestrosa NC. Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front Cell Neurosci. 2013;7:224. doi: 10.3389/fncel.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang N, Parr CJC, Birch AM, Goldfinger MH, Sastre M. The amyloid precursor protein binds to β-catenin and modulates its cellular distribution. Neurosci Lett. 2018;685:190–195. doi: 10.1016/j.neulet.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 75.Jia L, Piña-Crespo J, Li Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer's disease. Mol Brain. 2019;12((1)):104. doi: 10.1186/s13041-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serafino A, Giovannini D, Rossi S, Cozzolino M. Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: recent approaches and current challenges. Expert Opin Drug Discov. 2020;15((7)):803–822. doi: 10.1080/17460441.2020.1746266. [DOI] [PubMed] [Google Scholar]
- 77.Nagu P, Sharma V, Behl T, Pathan AKA, Mehta V. Molecular insights to the Wnt signaling during Alzheimer's disorder: a potential target for therapeutic interventions. J Mol Neurosci. 2022;72((4)):679–690. doi: 10.1007/s12031-021-01940-5. [DOI] [PubMed] [Google Scholar]
- 78.Palomer E, Buechler J, Salinas PC. Wnt signaling deregulation in the aging and Alzheimer's brain. Front Cell Neurosci. 2019;13:227. doi: 10.3389/fncel.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sig Transduct Target Ther. 2022;7:3–23. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tyagi S, Kaushal C, Gupta P, Saini A, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2((4)):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malm T, Mariani M, Donovan LJ, Neilson L, Landreth GE. Activation of the nuclear receptor PPARδ is neuroprotective in a transgenic mouse model of Alzheimer's disease through inhibition of inflammation. J Neuroinflammation. 2015;12((1)):7–14. doi: 10.1186/s12974-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer's disease: therapeutic implications. CNS Drugs. 2008;22:1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 83.Kalinin S, Richardson JC, Feinstein DL. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2009;6((5)):431–437. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- 84.Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6((12)):e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173((12)):1899–910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lago-Fernandez A, Zarzo-Arias S, Jagerovic N, Morales P. Relevance of peroxisome proliferator activated receptors in multitarget paradigm associated with the endocannabinoid system. Int J Mol Sci. 2021;22((3)):1001. doi: 10.3390/ijms22031001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong Y, Lim CS. Understanding the modulatory effects of cannabidiol on Alzheimer's disease. Brain Sci. 2021;11((9)):1211. doi: 10.3390/brainsci11091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strosznajder AK, Wójtowicz S, Jeżyna MJ, Sun GY, Strosznajder JB. Recent insights on the role of PPAR-β/δ in neuroinflammation and neurodegeneration, and its potential target for therapy. Neuromolecular Med. 2021;23((1)):86–98. doi: 10.1007/s12017-020-08629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J Alzheimers Dis. 2016;51((1)):15–19. doi: 10.3233/JAD-150915. [DOI] [PubMed] [Google Scholar]
- 90.Singh J, Neary JP. Neuroprotection following concussion: the potential role for cannabidiol. Can J Neurol Sci. 2020;47((3)):289–300. doi: 10.1017/cjn.2020.23. [DOI] [PubMed] [Google Scholar]
- 91.Tan SH, Karri V, Tay NWR, Chang KH, Ah HY, Ng PQ, et al. Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in Alzheimer's disease, Parkinson's disease. Biomed Pharmacother. 2019;111:765–777. doi: 10.1016/j.biopha.2018.12.101. [DOI] [PubMed] [Google Scholar]
- 92.Hillen JB, Soulsby N, Alderman C, Caughey GE. Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: a systematic review. Ther Adv Drug Saf. 2019 May 15;10:204209861984699. doi: 10.1177/2042098619846993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bosnjak KD, Markovic D, Brkovic T, Jeric KM, Rubic Z, Vuica VA, et al. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2021;9:12–18. doi: 10.1002/14651858.CD012820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stella F, Valiengo LCL, Paula VJR, Lima CAM, Forlenza OV. Medical cannabinoids for treatment of neuropsychiatric symptoms in dementia: a systematic review. Trends Psychiatry Psychother. 2021;43((4)):243–255. doi: 10.47626/2237-6089-2021-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ligresti A, De-Petrocellis L, Di -Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96:1593–659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]