Summary
Environmental pollution, inadequate eating habits and unhealthy lifestyles have led to a tremendous increase in ocular diseases worldwide. Given the costly treatments that are currently available for the most common and threatening eye diseases (such as cataract, dry eye disorder, or diabetic retinopathy), curing these diseases or preventing refractive errors by taking nutraceuticals and natural compounds that are present in our daily diet is a very valuable intervention. The eyes are the most important part of our visual system and require micronutrients such as vitamins, carotenoids, trace metals, and omega-3 fatty acids in order to function properly and to protect themselves against light-induced and age-mediated degenerative disorders. The Mediterranean Diet (MedDiet) has been in the limelight since the 1980s because of the several health benefits it provides, including eye health. MedDiet is characterized by the consumption of small amounts of red meat, while emphasizing the intake of fish, eggs, nuts, legumes, citrus fruits, green vegetables, olives and their derivatives, especially olive oil, and dairy products in a proportionate manner, in order to achieve the maximum health benefits. The antioxidant, anti-inflammatory, and neuroprotective properties of these foods – both when used as an ingredient in the dietary regime or as a source of nutritional supplements – have shown promising results in the management of chronic degenerative ocular diseases, both in animal models and in human subjects. In this chapter, we will focus on the importance of MedDiet and natural compounds for the visual system and its role in slowing down age-related ocular degeneration.
Keywords: Ocular diseases, Retinal diseases, Glaucoma, Med Diet, Antioxidants
Citation
How to cite this article: Medori MC, Naureen Z, Dhuli K, Placidi G, Falsini B, Bertelli M. Dietary supplements in retinal diseases, glaucoma, and other ocular conditions. J Prev Med Hyg 2022;63(suppl.3):E189-E199. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2760
Introduction
Eye health and vision have extensive and overwhelming effects on the overall quality of life, health, education, sustainable development, and even on the economy. Lacking the access to high quality treatment regimens and even to affordable eye care has rendered many people blind or visually impaired. In 2020 an estimated 596 million people in the world had distance vision impairment, out of which 90% of cases could be treated using high-cost treatment regimen [1, 2]. Out of these, 43 million suffered from complete or partial blindness. On the other hand, 510 million people could not improve near vision impairments because they could not afford reading glasses, showing the extremely poor socioeconomic conditions in which they live. These patients included all age groups, mostly affecting children and the elderly. In addition, eye diseases were more prevalent in women, ethnic minorities, and people living in rural areas. One of the major factors observed in these patients was that they were malnourished, due to the inadequate food supply because of overall poor socioeconomic conditions.
Diet, being a key lifestyle factor, can exert long-term effects even on ocular health and play a vital role in preventing visual dysfunction, which can lead to permanent visual impairment or blindness and it is estimated to affect 1 billion people worldwide by 2050 [1]. Consequently, the focus of the current chapter is how the Mediterranean dietary regime and the intake of supplements can prove to be beneficial for ocular health.
The visual system and vision impairment
Vision is the foremost among the human senses. It manifests through the coordinate function of the intricate visual pathways within the brain, interconnecting with eyes and their associated adnexal tissues. The cornea and lens of the eyes focus light onto the retinal photoreceptors, which then transduce light stimuli into neuronal impulses that lead to the development of a three-dimensional image by the brain. Clear vision requires structural and physiological integrity of all the components of the vision system (eyes, brain, and their pathways) and any abnormality results in visual dysfunction. Commonly, visual function is measured by distance visual acuity.
COMMON EYE CONDITIONS THAT LEAD TO VISUAL IMPAIRMENT
Many factors contribute to the development of visual dysfunction, such as genetic mutations, environment, lifestyle, age, and malnutrition [3-6]. The global trend in the population growth index indicates an increase in adult population with visual impairment and dysfunctions, caused by many ocular disorders: it could be because of refractive errors, dry eye disease (DED) [2, 7], diabetic retinopathy (DR) [8], ocular surface dysfunction (OSD), glaucoma, cataracts [2, 7], and age-related macular degeneration (AMD) [9]. Among children, on the other hand, the main causes of blindness and impaired vision are cerebral visual impairment, uncorrected refractive error, congenital ocular anomalies, cataract, retinopathy of prematurity, and corneal scarring. In addition, many conditions – such as allergic conjunctivitis, blepharitis, and dry eyes – can cause itching, pain and discharge in many people that do not suffer from visual dysfunction (Tab. I).
Tab. I.
Most prevalent eye conditions causing vision impairment.
| Ocular condition | Description | Epidemiology | Treatment | References |
|---|---|---|---|---|
| Cataract | Opacities in lens obstruct or scatter the incoming light | It is the most important cause of blindness worldwide (17.8 million in 2020) and the second leading cause of moderate or severe vision impairment (83.2 million). Cataract results from age-related changes, smoking, UV damage, dehydration crisis, diabetes, galactosemia, and steroid use | Surgery with intraocular lens (IOL) implantation | [2] |
| Refractive error | Blurred vision because light is not sharply focused on the retina, due to a mismatch between the eye axial length and the refractive power of the cornea and/or lens. Hypermetropia (long sight) and myopia (short sight) | It is the second main cause of blindness (3.7 million) and the main cause of vision impairment (157.5 million) | Refractive error can be corrected using spectacles, contact lenses, IOL implantation during cataract surgery or laser refractive surgery | [10, 11] |
| Glaucoma | Progressive damage of the optic nerve in one or both eyes, resulting in irreversible blindness | The third main cause of blindness (3.6 million) and the fourth cause of moderate or severe vision impairment (4.1 million). The risk of developing glaucoma increases with age and is increasing globally with population ageing | Medication, trabeculoplasty, iridotomy, or surgery | [2, 12] |
| AMD | Degeneration of the macula, resulting in loss of clear vision. It has both “dry” and “wet” forms | The fourth prominent cause of blindness globally (1.9 million) and the third in moderate to severe vision impairment (6.2 million). AMD is the primary cause of vision loss in high-income countries | Long term anti-VEGF intravitreal injections | [13-15] |
| DR | Damage to the small blood vessels in the retina leads to leakage of plasma fluid and blood, which may damage central vision (“diabetic macular oedema, DMO”) | The fifth cause of blindness (1.1 million) and vision impairment (3.8 million) | Retinal laser; intravitreal injection of anti-VEGF or steroid; retinal surgery | [8] |
THE EFFECT OF VISUAL IMPAIRMENT ON HEALTH AND WELL BEING
Vision impairment not only affects the normal day-to-day routine of an individual, but it also has social, psychological, and cognitive implications that affect the overall quality of life. Visually impaired people often experience reduced educational and employment prospects and are often forced to take up low-paid jobs [16]. Moreover, these people are often targeted with negative attitudes such as bullying, social exclusion, loneliness, violence, and sexual assault [17].
Good vision not only is closely associated with an improved quality of life, but it also exerts profound effects on the overall health of an individual. On the contrary, poor vision or visual impairment can predispose a person to several physiological and psychological complications, such as cardiovascular diseases, cancer, dementia, and depression [18]. Although the causal relationship of vision impairment with health conditions mentioned above is complex and difficult to assess precisely, it has been broadly categorized into three kinds:
direct effects of vision impairment on the individual’s health and wellbeing, such as injuries, and indirect effects caused by difficulties (or even impossibility) to access health care due to social isolation and limitations in mobility and physical activity;
other factors that are not specifically related to vision, like poor diet, poverty, smoking, sun exposure, ageing, etc;
effects of systemic health conditions on visual impairment such as dementia, diabetes, cancer, and ocular metastases.
Hence, vision impairment negatively affects the patients’ mobility and mental health, even exacerbating the risk of dementia, it increases the likelihood of injuries like falls and road traffic crashes, thus increasing also the patients’ need for social care, ultimately leading to higher mortality rates [2, 19].
Diet and eye health
Several population-based studies have revealed the importance of diet, specifically of micronutrients, in the management of age-related ocular diseases. These studies have led researchers to focus their studies on a wide variety of nutrients in the management of eye problems, such as retinal conditions (light mediative oxidative damage), refractive errors, age-related macular degeneration, cataract, glaucoma, and dry eye [1].
Nutrient deficiencies led to manifestations of ocular diseases in the adults and the effects of malnutrition on the development of newborns’ visual system have suggested that nutrition plays an important role not only in the proper development of the visual system, but also in enhancing or suppressing the various ocular manifestations leading to visual impairment and blindness. For instance, studies have demonstrated that taking vitamin A in recommended doses has beneficial effects on retinitis pigmentosa, while vitamin E is detrimental to the same condition [20]. In addition, lutein and docosahexaenoic acid (DHA) supplementation, along with vitamin A, was found to delay retinal degeneration and visual decline. Similarly, several studies have reported a link between vitamin A deficiency and night blindness and xerophthalmia [21]. On the other hand, vitamin B deficiency due to poor intake or absorption results in nutritional amblyopia, which causes blurred vision and reduced visual acuity [22]. Furthermore, carotenoids have been useful in improving the vision throughout life, as well as in reducing retinal and lens degeneration – leading to AMD and cataract in the elderly population [23].
MEDDIET AND EYE HEALTH
A healthy lifestyle, indicated by healthy eating habits and plenty of physical activity, offers a valuable intervention to fight against the tremendously increasing risk of eye disorders, such as myopia progression, dry eye disorders, diabetic retinopathy, cataracts, glaucoma, or AMD. Providing certain micronutrients such as minerals, Omega 3 fatty acids, vitamins, and carotenoids in recommended daily doses ensures an overall good health and exerts positive effects on eye health and on the visual system. However, including these micronutrients in the diet as supplements might raise concerns in the underdeveloped countries, where most of the population lives below poverty line and are thus unable to afford nutritional supplements. In this regard, diet regimes that are affordable by a wide range of socioeconomic groups are a method of choice to introduce beneficial nutrients in the body.
The MedDiet in this regard has received considerable attention in the past few decades, mainly because people dwelling in the Mediterranean region tend to have lower incidence of cardiovascular diseases and other chronic disorders [24]. Furthermore, adherence to the MedDiet is linked with increased antioxidant activity, reduced incidence of several diseases, reduced inflammation, and eventually reduced mortality [25]. A number of studies has shown that adherence to the MedDiet is also associated with reduced frailty [26]. In addition, people following this diet regime experience fewer visual disorders as well as a higher lifespan. This can be because this diet regime favors an enhanced consumption of whole meal bread, cereals, olive oil, fruits, legumes, and vegetables, and a low consumption of saturated fat, avoiding or reducing red meat consumption and taking with chicken and fish in moderate proportions, and an adequate intake of cheese and yogurt. Besides that, regular physical activity also helps enhance the beneficial effects of the MedDiet.
A recent retrospective clinical trial involving 7756 participants, with a mean age of 71 years, showed in 56.5% of cases the beneficial effects of following the MedDiet on AMD. The results indicated that closely following the MedDiet reduced the risk of progression to late AMD and to large drusen. This association was greater for geographic atrophy (GA) than neovascular AMD. In addition to that, fish intake contributed to a great extent in association [27].
In another prospective study, the researchers investigated the association between following the MedDiet and the incidence of advanced AMD in 4446 European people, aged 55 and older: it was observed that the likelihood of developing AMD in subjects that followed the diet was a 41% lower as compared to those who did not [28].
These findings establish the important role of a nutrient-rich MedDiet – containing whole grains, olive oil, fruits, vegetables, legumes, and fish – in AMD prevention. Although these studies could not prove a direct cause and effect relationship between MedDiet and AMD, it still highlighted that the reduced risk of developing AMD was due to the overall diet and not to the individual components (Tab. II).
Tab. II.
Dietary patterns as interventions to age-related eye diseases.
| Diet pattern | Ocular diseases | ||||
|---|---|---|---|---|---|
| Cataract | Refractive error | Glaucoma | AMD | Diabetic retinopathy | |
| MedDiet | A small study on 500 African male subjects indicated a lower risk of developing cataract upon adherence to the MedDiet [25] Lack of evidence due to scarcity of clinical trials | Not assessed | Reduced risk of developing glaucoma [29] | Decreased risk of late AMD | Prevents diabetes and therefore diabetic retinopathy and lowers the chances of retinal degeneration during DR |
| Western diet | Not assessed | Not assessed | Not assessed | Increased risk of AMD | Not assessed |
| Prudent diet | Not assessed | Not assessed. | Not assessed | Lower incidence of AMD in some studies. However, results are variable [30] | Not assessed |
MEDITERRANEAN DIET– GUT MICROBIOTA – EYE AXIS
Gut dysbiosis has been recently observed to increase the predisposition to various ocular diseases, such as dry eye, uveitis, macular degeneration, and glaucoma [31]. This indicates the existence of a gut-eye axis (or gut-retina axis), mediated by the gut microbiota [32]. It is a well-known fact that alterations in the gut microbiota result in many intestinal and extra-intestinal diseases, including non-alcoholic fatty liver disease (NAFLD) [33], metabolic and inflammatory diseases, obesity, and cancer [34]. An increased intestinal permeability (termed as “leaky gut”) allows leakage of bacterial endotoxin lipopolysaccharides (LPS) and pathogen-associated molecular pattern molecules (PAMPs), which activate pattern recognition receptors (PRRs), resulting in low-grade inflammation in several tissues. This crosstalk can extend to dendritic cells, perivascular macrophages, and retinal pigment epithelium (RPE) cells, and might end up in ocular inflammation. In addition, the gut microbial metabolites might regulate retina-specific immune cells [35]. A recent study has shown that obesity-associated gut microbiota is the major role player in pathological angiogenesis of choroidal neovascularization (CNV) in retinal tissue [35], reinstating the existence of a gut-retina axis. Gut microbiota undergoes significant changes in respect to age and dietary patterns and greatly influences the overall health. MedDiet has been shown to alter the gut microbiota in many ways, such as increasing the microbial diversity, enhancing the Bifidiobacteria, and Lactobacillus sp population while reducing the proteobacteria [36]. Studies have shown that people following MedDiet had a better gut microbiota composition as compared to those following a Western diet regime [37]. As this diet regime avoids high caloric fatty foods and promotes fresh leafy vegetables and fruits, it results in higher proportion of short-chain fatty acids (SCFAs) and fiber-degrading bacteria in the feces [38]. In addition, some studies have reported an association between gut microbiome and ocular surface microbiome with ocular surface diseases. These studies have been recently reviewed by [39], and they suggest a convincing link between gut microbiota-eye health and gut microbiota-ocular surface microbiota-eye health. Whatever the case, the MedDiet and dietary intake of probiotics have been known to modulate a healthy gut (Fig. 1.).
Fig. 1.
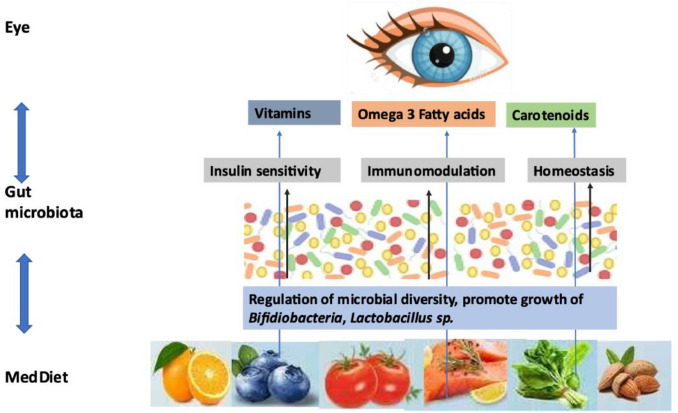
MedDiet- Gut Microbiota-Eye Axis.
As the MedDiet modulates gut microbiota, which in turn influences the onset of ocular diseases, it seems to exist a MedDiet-gut microbiota-eye axis. Further studies in this area would be beneficial for the development of diet-mediated management of various ocular diseases.
Natural molecules
Food contains many essential vitamins, micronutrients, and biologically active compounds that can play a pivotal role in maintaining eye health. Some selected natural molecules and their effect on ocular diseases is presented in Table III.
Tab. III.
The effects of selected nutritional supplements on various ocular manifestations and their food sources.
| Natural compounds | Food sources | Benefits for the eyes | References |
|---|---|---|---|
| Vitamin A | Almonds, carrots, tuna | Beneficial in retinitis pigmentosa | [31] |
| Melatonin | Fish, milk, eggs, seeds, pistachio | Neuroprotectant, IOP regulation | [34] |
| Taurine | Sea food, turkey, seaweed | Prevents retinal degeneration and light-induced damages | [52] |
| Omega-3 fatty acids | Fishes (mackerel, sardine, salmon) | Prevent inflammatory damage, reduce IOP, beneficial in dry eye disease and AMD | [66] |
| B-Vitamins | Milk, cheese, eggs, fishes, leafy vegetables, chicken | Antioxidant, neuroprotective, decrease cataract incidence | [60] |
| Palmitoylethanolamide (PEA) | Egg yolk, peanuts | Anti-inflammatory, retino-protectant in glaucoma and diabetic retinopathy | [78] |
| Saffron | Flower of Crocus sativus | Anti-inflammatory, anti-oxidant, protection against light damage to photoreceptors | [90] |
MELATONIN
Melatonin is a hormone secreted by the pineal gland and regulated by the hypothalamic suprachiasmatic nucleus (SCN) [40]. It plays an important role in circadian rhythms, as shown by its enhanced secretion at night and vice versa [41]. In addition to that melatonin has antioxidant, immunomodulation, and neuroprotective properties [42, 43]. Melatonin binds to two G-protein coupled receptors, MT1 and MT2, which are widely distributed in different tissues and it works in both a receptor-dependent manner and receptor-independent one [44]. Melatonin has low affinity towards another receptor, MT3, acting as an enzyme with different kinetic and ligand association and different pharmacological characteristics as compared to MT1 and MT2 [45]. In the past two decades, much attention has been paid to the relationship between melatonin and eye in connection with SCN, circadian rhythm, and ocular diseases [46]. Many researchers have reported melatonin production in various ocular tissues following the circadian rhythms, such as lachrymal gland, crystalline lens, retina, ciliary body, and iris [47]. Furthermore, studies have shown the presence of melatonin receptors in choroid, sclera, cornea, and retina, indicating an important role of melatonin in visual system [48]. Several studies have elucidated a link between melatonin and various ocular conditions, with a focus on melatonin as part of the treatment regime for glaucoma and for inflammatory and age-related diseases [49, 50]. Melatonin has been proven to be a strong neuroprotective agent when applied exogenously to control intra ocular pressure and preventing intraocular hypertension related retinal injury and glaucoma in animal models and clinical trials [49, 51, 52]. Furthermore, melatonin is a strong antioxidant, anti-inflammatory, and immunomodulation agent showing a therapeutic potential in AMD, diabetic retinopathy, and uveitis [53, 54]. In addition, melatonin protects the retina from oxidative stress by regulating the secretion of vascular endothelial growth factor (VEGF) in the retina [55]. Although it has been proven beneficial in the management of several ocular diseases, the causality between melatonin and ocular diseases is yet to be unidentified, testifying the dire need of more prospective clinical studies.
TAURINE
Taurine is the most abundant amino acid in the retina of mammals, with taurine concentration reaching 50 mmol/g tissue higher than in any other ocular structure or in the brain [56]. The retinal pigment epithelium (RPE) and Müller cells provide taurine to the retina, which then collects and transfer it to photoreceptor cells [57]. Photoreceptors require an adequate amount of taurine from extracellular environment and its transport is dependent upon osmoregulation by high and low-affinity Na+ and Cl−-dependent taurine transporters present in the retina [58]. Although the function of taurine in the retina is still uncertain, a lack of taurine in nutrition has resulted in reduced plasma taurine concentration and atypical electroretinograms in children and adolescents [56].
Taurine depletion has been observed to increase light-induced photoreceptor degeneration in rats, indicating the role of taurine in retinal survival and photoreceptor protection [59]. Accordingly, the administration of taurine supplement might prove beneficial in preventing and counteracting retinal degeneration and light-induced pathologies.
Being a strong antioxidant, taurine exhibits cytoprotective effects by reacting and counteracting the neutrophil oxidant, hypochlorous acid. This reaction lead to the production of taurine chloramine, which inhibits the inflammatory pathway [60]. Moreover, taurine reduces the mitochondrial production of superoxide, prevents mitochondrial ROS induced oxidative stress, and protects the antioxidant enzymes [56]. The protective role of taurine in photoreceptor degeneration, retinal ganglion cell loss, and other retinal morphological injuries in in vitro and in vivo disease models shows that it plays an essential role in retinal function preservation, so it can be administered as a supplement or as part of a taurine-rich diet to prevent retinal degeneration in various ocular diseases [61]. Taurine has been observed to hamper the progression of retinal diseases, highlighting its promising candidature in preventing or treating retinal diseases.
SPEARMINT
Even when not consumed in large quantities, spearmint has plenty of vitamins and minerals that are beneficial for the body. The spearmint extract – rich in fiber, vitamin A, iron, manganese, and folate – along with forskolin, homotaurine, and B vitamins, has been recently observed as beneficial in counteracting the retinal dysfunction in retinal ganglion cell (RGC) death caused by optic nerve crush [60]. As the spearmint extract is rich in polyphenols, it contains flavonoids that exhibit neuroprotective effects on RGC loss by reducing inflammation and oxidative stress [62, 63]. Further research is required in order to determine the beneficial effects of spearmint extract in ocular diseases; however, as it is a regular dietary ingredient of MedDiet, it holds significant potential in managing various ocular and other pathological conditions.
B VITAMINS
The B-vitamins, comprising eight water-soluble vitamins, perform an essential role in cell function, acting as coenzyme in various metabolic reactions. They are specifically involved in energy production, DNA/RNA synthesis/repair, synthesis of numerous neurochemicals, genomic and non-genomic methylation, and cellular signaling, thus maintaining vital functions in the brain. B vitamins (like B6, B12, and folic acid) are beneficial in eye health, as these prevent AMD by reducing homocysteine levels in blood.
Deficiency of B vitamins results in reduced vision or formation of blind spots because the optic nerve is compromised. Several observational studies have reported the protective role of a high blood level of B vitamins, such as riboflavin, thiamin, and niacin, against the development of lens opacities [64]. Riboflavin and niacin also have protective effects against oxidative stress by acting as coenzymes of oxidative enzymes. Administering these two B vitamins in undernourished Chinese population resulted in decreasing the incidence of cataract [65]. An epidemiological observation study, involving 5442 female health professionals with preexisting cardiovascular diseases, has shown that vitamin B12 deficiency in women is linked with an increased risk of AMD, which are twice as much as those having normal B12 levels [64]. The randomized, double-masked, placebo-controlled trial data indicated that subjects with daily supplementation of 50mg B6, 1mg B12, and 2.5mg folate supplements for two years had 35% to 40% chances to be less prone to AMD. However, in another study, supplementation of vitamin B6, B12, and folate exceeding the recommended dietary doses resulted in an increased risk of causing cataract in U.S. physicians in a timespan of over 7 years [66]. Consistently with these findings, a high dietary folate intake in participants using multivitamins is associated with an increased risk of posterior subcapsular cataract (PSC) in Age-Related Eye Disease Study (AREDS) patients [67]. Another study reported a two-fold increase in cataracts in the PSC region with high folate intake [68].
OMEGA-3 FATTY ACIDS
Evidence suggests that the essential omega-3 fatty acids, obtained from a healthy diet or supplementation, provide benefits to the visual system. Omega-3 fatty acids can be obtained from a variety of food sources: for instance, the short-chain omega-3 called alpha-linoleic acid (ALA) is abundant in flaxseed and chia seeds, while marine-based foods are rich in long-chain omega-3 fatty acids, like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). The absorption of long-chain omega-3 fatty acids in the cellular membranes is based on their competition with the long-chain omega-6 fatty acid called arachidonic acid (AA) [69], which is a pro-inflammatory agent. As a result, increasing omega-3 fatty acid intake regulates a systemic inflammation via production of anti-inflammatory metabolites. As the optimal dietary ratio of omega-6 to omega-3 fatty acid should be 4:1, a diet rich in omega-6 fatty acids – such as western fried foods – results in an imbalance of the two, thus leading to an appropriate absorption of omega-3 fatty acids in the cellular membranes [70]. This results in deficiency of omega-3 fatty acids in approximately 80% of the adult population of developed countries [71]. Balanced diets, having plentiful long-chain omega-3 fatty acids, have been demonstrated to be beneficial in several chronic ocular conditions, including dry eye disease and AMD [72]. Moreover, long-chain omega-3 fatty acids are beneficial in reducing ocular surface inflammation in dry eye disease, improving tear-lipid profile [73]. Studies have shown that women with a low dietary intake of omega-3 fatty acids tend to have higher chances to develop dry eye disease [74]. However, clinical trials conducted to assess the potential benefits of omega-3 fatty acids in dry eye disease resulted in contradictory findings. DHA, specifically, is implicated in structural and functional maintenance of retina [75].
In addition, diets rich in polyunsaturated fatty acids enhance the retinal cellular response in AMD animal models to oxidative, ischemic, and inflammatory damage [76]. Although epidemiological studies have shown the potentials of dietary long-chain omega-3 intake in lowering the risk of early-stage AMD [77] and of progression to late-stage visual impairment [78], the omega-3 supplements have not shown the same efficacy as whole foods [79]. On the contrary, a double-masked placebo-controlled randomized clinical trial conducted in Australia concluded that oral omega-3 supplementation for 3 months significantly reduced IOP in normotensive adults [80]. However, the synergistic interactions of different nutrients in the whole food is essential for harvesting their full benefits and this should be given due consideration during clinical recommendations.
PALMITOYLETHANOLAMIDE (PEA)
Palmitoylethanolamide (PEA) is a pleiotropic naturally occurring endogenous N-acetylethanolamine cell-protective lipid found in several foods and in many living organisms. Numerous studies have reported its anti-inflammatory and neuroprotective characteristics; however, its beneficial effects are dose-dependent and mediated via receptors such as PPAR-α, PPAR-γ, PPAR-δ, GPR, and TRPV1 [81]. In the past 50 years, many clinical trials for various ocular diseases (such as glaucoma, diabetic retinopathy, uveitis, and pathological conditions involving inflammation) have tested PEA effectiveness. This molecule is available both as via dietary sources rich in PEA and as via supplement. Several PEA supplementary products (like Normast, PeaVera, and Visimast) are administered to glaucoma and neuroinflammation patients in Italy for nutritional support. PEA holds promise in treating many retinopathies and has been evaluated in many double-blind placebo-controlled studies to be safe, effective, and tolerable up to 1.8 g/day. Moreover, as PEA downregulates proinflammatory genes, it has been beneficial as an anti-inflammatory and retino-protectant compound in glaucoma and diabetic retinopathy [82].
SAFFRON
AMD is a retinal neurodegenerative disease characterized in its early stage by large soft drusen and hypo-hyperpigmentation of the retinal pigment epithelium (RPE). Late AMD, the potentially blinding stage of disease, includes geographic atrophy of the RPE (“dry” age-related macular degeneration), or subretinal neovascular membranes (“wet” age-related macular degeneration) [83].
AMD is generally considered a multifactorial disease, whose development and progression are the results of a complex interaction between genetic and environmental risk factors. Both oxidative stress [84] and chronic inflammation [85] seem to play a significant role in the pathogenesis of AMD together with many risk factors [86-89]. The final outcome of all neurodegenerative retinal diseases is the death of photoreceptors, consequently visual functions progressively deteriorate up to complete blindness. Recently, new strategies able to mitigate photoreceptor death using natural products have been explored. Among the others, Maccarone et al. (2008) provided data showing that l’Aquila saffron is protective against light damage to photoreceptors, in a rat model [90]. A proof-of-principle clinical trial in AMD patients confirmed the potentiality of saffron treatment in neurodegenerative diseases and its consistency in time [91,92] and in patients carrying genetic mutations [93].
Saffron is a well-known spice, used widely in different cuisines. It has also long been used in traditional medical practice [93,94]. Egyptian healers used it to treat gastrointestinal ailments; in Roman times it was used to promote wound healing and relieve upper respiratory complaints. In more recent times it has been used as an anti-inflammatory, anticonvulsive and anti-tumor agent; it has been investigated in the treatment of cognitive defects. Because of this long history of medical use associated to a variety of different clinical applications it is essential to make an accurate analysis of relevant data specifically in the field of retinal diseases. Saffron is the commercial name of the dried red stigmas of Crocus sativus flowers. It is produced in many areas all over the world, but the bulbs may present different genetical characteristics and the cultivar and the drying strategies are quite different; this makes each single production to be unique and this could explain the variety of effects and some discrepancies in the literature. Chemically, saffron is known to contain more than 150 volatile and aroma-yielding compounds and many non-volatile biologically active components, including carotenoids (zeaxanthin and crocetin) and various alpha- and beta-carotenes. Its golden yellow-orange colour comes from alpha-crocin, a water soluble biogentobiose (sugar) ester of crocetin. Its flavor arises from the glycoside picrocrocin, a molecule containing safranal and a carbohydrate. Its most potent anti-oxidant ingredients appear to be crocin, and crocetin, a carotenoid dicarboxylic acid which forms the core of crocin. Several actions of crocin on mammalian tissues have been reported including anti-apoptotic activity and increased oxygen diffusivity [90, 95]. Kanakis et al. (2007) showed that metabolites of saffron bind directly to DNA and induce its partial conformation to beta-DNA, thereby protecting the cell from damage [96]. Saffron has been shown to have anti-inflammatory actions, including for example the inhibition of tissue necrosis factor [97]. Based on these observation it comes clear that the saffron extract does not act as a simple antioxidant. The peculiar characteristics of saffron components support the hypothesis of an involvement of very different ways of action going from antioxidant activity to direct control of gene expression as it is also suggested by microarray experiments [98].
Conclusion
Eyes are an integral part of our lives, well-being, development, and happiness. It has been proven now that a healthy diet helps maintain a healthy vision system, avoiding many ocular problems. Scientific evidence suggests that micronutrients, vitamins, and natural compounds with antioxidant and anti-inflammatory properties play a pivotal role in alleviating and protecting age-related ocular degeneration. Therefore, with aging we require an adequate supply of good fats, controlled carbohydrates, fibres, and proteins, along with micronutrients and vitamins to maintain our health. In addition, consuming a good amount of probiotics helps regulating the gut microbiota, which in turn mediates the ocular surface microbiota and prevents many ocular diseases. The Mediterranean diet, which is a blend of all these ingredients, holds significant potential as a dietary regime for better eye care and therapy and should be given due consideration.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; MCM, ZN, KD, GP, BF: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Figures and tables
References
- [1].Valero-Vello M, Peris-Martínez C, García-Medina JJ, Sanz-González SM, Ramírez AI, Fernández-Albarral JA, Galarreta-Mira D, Zanón-Moreno V, Casaroli-Marano RP, Pinazo-Duran MD. Searching for the antioxidant, anti-inflammatory, and neuroprotective potential of natural food and nutritional supplements for ocular health in the mediterranean population. Foods 2021;10:1231. https://doi.org/ 10.3390/foods10061231 10.3390/foods10061231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, Ah Tong BAM, Arunga S, Bachani D, Bascaran C, Bastawrous A, Blanchet K, Braithwaite T, Buchan JC, Cairns J, Cama A, Chagunda M, Chuluunkhuu C, Cooper A, Crofts-Lawrence J, Dean WH, Denniston AK, Ehrlich JR, Emerson PM, Evans JR, Frick KD, Friedman DS, Furtado JM, Gichangi MM, Gichuhi S, Gilbert SS, Gurung R, Habtamu E, Holland P, Jonas JB, Keane PA, Keay L, Khanna RC, Khaw PT, Kuper H, Kyari F, Lansingh VC, Mactaggart I, Mafwiri MM, Mathenge W, McCormick I, Morjaria P, Mowatt L, Muirhead D, Murthy GVS, Mwangi N, Patel DB, Peto T, Qureshi BM, Salomão SR, Sarah V, Shilio BR, Solomon AW, Swenor BK, Taylor HR, Wang N, Webson A, West SK, Wong TY, Wormald R, Yasmin S, Yusufu M, Silva JC, Resnikoff S, Ravilla T, Gilbert CE, Foster A, Faal HB. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health 2021:9:e489-e551. https://doi.org/10.1016/S2214-109X(20)30488-5 10.1016/S2214-109X(20)30488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Falsini B, Placidi G, De Siena E, Savastano MC, Minnella AM, Maceroni M, Midena G, Ziccardi L, Parisi V, Bertelli M, Maltese PE, Chiurazzi P, Rizzo S. USH2A-Related Retinitis Pigmentosa: staging of disease severity and morpho-functional studies. Diagnostics (Basel) 2021;11:213. https://doi.org/10.3390/diagnostics11020213 10.3390/diagnostics11020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marino V, Dal Cortivo G, Maltese PE, Placidi G, De Siena E, Falsini B, Bertelli M, Dell’Orco D. Impaired Ca2+ sensitivity of a novel GCAP1 variant causes cone dystrophy and leads to abnormal synaptic transmission between photoreceptors and bipolar cells. Int J Mol Sci 2021;22:4030. https://doi.org/10.3390/ijms22084030 10.3390/ijms22084030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Falsini B, Placidi G, De Siena E, Chiurazzi P, Minnella AM, Savastano MC, Ziccardi L, Parisi V, Iarossi G, Percio M, Piteková B, Marceddu G, Maltese PE, Bertelli M. Genetic characteristics of 234 Italian patients with macular and cone/cone-rod dystrophy. Sci Rep 2022;12:3774. https://doi.org/10.1038/s41598-022-07618-1 10.1038/s41598-022-07618-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colombo L, Maltese PE, Castori M, El Shamieh S, Zeitz C, Audo I, Zulian A, Marinelli C, Benedetti S, Costantini A, Bressan S, Percio M, Ferri P, Abeshi A, Bertelli M, Rossetti L. Molecular epidemiology in 591 italian probands with nonsyndromic retinitis pigmentosa and usher syndrome. Invest Ophthalmol Vis Sci 2021;62:13. https://doi.org/10.1167/iovs.62.2.13 10.1167/iovs.62.2.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang J, Tuo J, Wang Z, Zhu A, Machalińska A, Long Q. Pathogenesis of Common Ocular Diseases. J Ophthalmol 2015:734527. https://doi.org/10.1155/2015/734527 10.1155/2015/734527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lanzetta P, Sarao V, Scanlon PH, Barratt J, Porta M, Bandello F, Loewenstein A, Vision Academy. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol 2020;57:785-98. https://doi.org/10.1007/s00592-020-01506-8 10.1007/s00592-020-01506-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pondorfer SG, Terheyden JH, Heinemann M, Wintergerst MWM, Holz FG, Finger RP. association of vision-related quality of life with visual function in age-related macular degeneration. Sci Rep 2019;9:15326. https://doi.org/10.1038/s41598-019-51769-7 10.1038/s41598-019-51769-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Glewwe Paul, West KL, Lee J. The Impact of Providing Vision screening and free eyeglasses on academic outcomes: evidence from a randomized trial in title i elementary schools in Florida. J Policy Anal Manage 2018;37:265-300. https://doi.org/10.1002/pam.22043 10.1002/pam.22043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hannum E, Zhang Y. poverty and proximate barriers to learning: vision deficiencies, vision correction and educational outcomes in rural northwest China. World Dev 2012;40:1921-31. https://doi.org/10.1016/j.worlddev.2012.04.029 10.1016/j.worlddev.2012.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinazo-Durán MD, Muñoz-Negrete FJ, Sanz-González SM, Benítez-Del-Castillo J, Giménez-Gómez R, Valero-Velló M, Zanón-Moreno V, García-Medina JJ. The role of neuroinflammation in the pathogenesis of glaucoma neurodegeneration. Prog Brain Res 2020;256:99-124. https://doi.org/10.1016/bs.pbr.2020.07.004 10.1016/bs.pbr.2020.07.004 [DOI] [PubMed] [Google Scholar]
- [13].Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol 2019;4:e000398. https://doi.org/10.1136/bmjophth-2019-000398 10.1136/bmjophth-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gahn G, Khanani A. New therapies of neovascular AMD beyond Anti-VEGF injections. Vision 2018;2:15. https://doi.org/10.3390/vision2010015 10.3390/vision2010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yerramothu P. New therapies of neovascular AMD–beyond anti-VEGFs. Vision 2018;2:31. https://doi.org/10.3390/vision2030031 10.3390/vision2030031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].French S. visual impairment and work: experiences of visually impaired people. 1st Edition. Routledge; 2017. https://doi.org/10.4324/9781315569536 10.4324/9781315569536 [DOI] [Google Scholar]
- [17].Brunes AB, Hansen M, Heir T. Loneliness among adults with visual impairment: prevalence, associated factors, and relationship to life satisfaction. Health Qual Life Outcomes 2019;17:24. https://doi.org/10.1186/s12955-019-1096-y 10.1186/s12955-019-1096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bernabei V, Morini V, Moretti F, Marchiori A, Ferrari B, Dalmonte E, De Ronchi D, Atti AR. Vision and hearing impairments are associated with depressive–anxiety syndrome in Italian elderly. Aging Ment Health 2011;15:467-74. https://doi.org/10.1080/13607863.2011.562483 10.1080/13607863.2011.562483 [DOI] [PubMed] [Google Scholar]
- [19].Kuźma E, Littlejohns TJ, Khawaja AP, Llewellyn DJ, Ukoumunne OC, Thiem U. Visual impairment, eye diseases, and dementia risk: a systematic review and meta-analysis. J Alzheimers Dis 2021;83:1073-87. https://doi.org/10.3233/JAD-210250 10.3233/JAD-210250 [DOI] [PubMed] [Google Scholar]
- [20].Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 1993;111:761-72. https://doi.org/10.1001/archopht.1993.01090060049022 10.1001/archopht.1993.01090060049022 [DOI] [PubMed] [Google Scholar]
- [21].West K. Vitamin A deficiency disorders in children and women. Food Nutr Bull 2003;24:S78-S90. https://doi.org/10.1177/15648265030244s204 10.1177/15648265030244s204 [DOI] [PubMed] [Google Scholar]
- [22].Semba RD. Nutritional amblyopia and B complex vitamin deficiencies. In: Handbook of Nutrition and Ophthalmology 2007:281-354. https://doi.org/10.1007/978-1-59259-979-0_7 10.1007/978-1-59259-979-0_7 [DOI] [Google Scholar]
- [23].Vishwanathan R, Johnson EJ. Lutein and zeaxanthin and eye disease. In: Tanumihardjo S, eds. Carotenoids and Human Health. Nutrition and Health. Totowa, NJ: Humana Press; 2013. https://doi.org/10.1007/978-1-62703-203-2_13 10.1007/978-1-62703-203-2_13 [DOI] [Google Scholar]
- [24].Georgousopoulou EN, Mellor DD, Naumovski N, Polychronopoulos E, Tyrovolas S, Piscopo S, Valacchi G, Anastasiou F, Zeimbekis A, Bountziouka V, Gotsis E, Metallinos G, Tyrovola D, Foscolou A, Tur JA, Matalas AL, Lionis C, Sidossis L, Panagiotakos D; MEDIS study group. Mediterranean lifestyle and cardiovascular disease prevention. Cardiovasc Diagn Ther 2017;7:S39-S47. https://doi.org/10.21037/cdt.2017.03.11 10.21037/cdt.2017.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;337:a1344-a1344. https://doi.org/10.1136/bmj.a1344 10.1136/bmj.a1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feldman E. Mediterranean diet and frailty risk. Integrative Medicine Alert 2018;21. Available at: https://www.reliasmedia.com/articles/142449-mediterranean-diet-and-frailty-risk. Accessed on: 02/08/2022
- [27].Keenan TD, Agrón E, Mares J, Clemons TE, van Asten F, Swaroop A, Chew EYage-related eye disease studies (AREDS) 1 and 2 Research Groups. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020;127:1515-28. https://doi.org/10.1016/j.ophtha.2020.04.030 10.1016/j.ophtha.2020.04.030 [DOI] [PubMed] [Google Scholar]
- [28].Merle B, Colijn JM, Cougnard-Grégoire A, de Koning-Backus A, Delyfer MN, Kiefte-de Jong JC, Meester-Smoor M, Féart C, Verzijden T, Samieri C, Franco OH, Korobelnik JF, Klaver C, Delcourt CEYE-RISK Consortium. Mediterranean Diet and incidence of advanced age-related macular degeneration: The EYE-RISK Consortium. Ophthalmology 2019;126:381-90. https://doi.org/10.1016/j.ophtha.2018.08.006 10.1016/j.ophtha.2018.08.006 [DOI] [PubMed] [Google Scholar]
- [29].Moïse MM, Benjamin LM, Doris TM, Dalida KN, Augustin NO. Role of Mediterranean diet, tropical vegetables rich in antioxidants, and sunlight exposure in blindness, cataract and glaucoma among African type 2 diabetics. Int J Ophthalmol 2012;5:231-7. https://doi.org/10.3980/j.issn.2222-3959.2012.02.23 10.3980/j.issn.2222-3959.2012.02.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dighe S, Zhao J, Steffen L, Mares JA, Meuer SM, Klein B, Klein R, Millen AE. Diet patterns and the incidence of age-related macular degeneration in the Atherosclerosis Risk in Communities (ARIC) study. Br J Ophthalmol 2020;104:1070-6. https://doi.org/10.1136/bjophthalmol-2019-314813 10.1136/bjophthalmol-2019-314813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Napolitano P, Filippelli M, Davinelli S, Bartollino S, dell’Omo R, Costagliola C. Influence of gut microbiota on eye diseases: an overview. Ann Med 2021;53:750-61. https://doi.org/10.1080/07853890.2021.1925150 10.1080/07853890.2021.1925150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rinninella E, Mele M, Merendino N, Cintoni M, Anselmi G, Caporossi A, Gasbarrini A, Minnella A. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut–retina axis. Nutrients 2018;10:1677. https://doi.org/10.3390/nu10111677 10.3390/nu10111677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khan A, Ding Z, Ishaq M, Bacha AS, Khan I, Hanif A, Li W, Guo X. understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci 2021;17:818-33. https://doi.org/10.7150/ijbs.56214 10.7150/ijbs.56214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55-71. https://doi.org/10.1038/s41579-020-0433-9 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- [35].Meng X, Li Y, Li S, Zhou Y, Gan RY, Xu DP, Li HB. Dietary sources and bioactivities of melatonin. Nutrients 2017;9:367. https://doi.org/10.3390/nu9040367 10.3390/nu9040367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 2018;9:890. https://doi.org/doi:10.3389/fmicb.2018.00890 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, Kyriacou A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr 2017;117:1645-55. https://doi.org/10.1017/S0007114517001593 10.1017/S0007114517001593 [DOI] [PubMed] [Google Scholar]
- [38].De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812-21. https://doi.org/10.1136/gutjnl-2015-309957 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- [39].Cavuoto KM, Banerjee S, Galor A. Relationship between the microbiome and ocular health. Ocul Surf 2019;17:384-92. https://doi.org/10.1016/j.jtos.2019.05.006 10.1016/j.jtos.2019.05.006 [DOI] [PubMed] [Google Scholar]
- [40].Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res 1996;73:135-9. https://doi.org/10.1016/0166-4328(96)00085-x 10.1016/0166-4328(96)00085-x [DOI] [PubMed] [Google Scholar]
- [41].Mul Fedele ML, Galiana MD, Golombek DA, Muñoz EM, Plano SA. Alterations in metabolism and diurnal rhythms following bilateral surgical removal of the superior cervical ganglia in rats. Front Endocrinol (Lausanne) 2018;8:370. https://doi.org/10.3389/fendo.2017.00370 10.3389/fendo.2017.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mayo JC, Sainz RM. Melatonin from an antioxidant to a classic hormone or a tissue factor: experimental and clinical aspects 2019. Int J Mol Sci 2020;21:3645. https://doi.org/10.3390/ijms21103645 10.3390/ijms21103645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Khoei HH, Asghari MH. Immuno-regulatory role of melatonin in cancer. J Cell Physiol 2019;235:745-57. https://doi.org/10.1002/jcp.29036 10.1002/jcp.29036 [DOI] [PubMed] [Google Scholar]
- [44].Legros C, Dupré C, Brasseur C, Bonnaud A, Bruno O, Valour D, Shabajee P, Giganti A, Nosjean O, Kenakin TP, Boutin JA. Characterization of the various functional pathways elicited by synthetic agonists or antagonists at the melatonin MT1 and MT2 receptors. Pharmacol Res Perspect 2019;8:e00539. https://doi.org/10.1002/prp2.539 10.1002/prp2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol 2001;61:1369-79. https://doi.org/10.1016/s0006-2952[01]00615-3 10.1016/s0006-2952[01]00615-3 [DOI] [PubMed] [Google Scholar]
- [46].Andrews CD, Foster RG, Alexander I, Vasudevan S, Downes SM, Heneghan C, Plüddemann A. Sleep-wake disturbance related to ocular disease: a systematic review of phase-shifting pharmaceutical therapies. Transl Vis Sci Technol 2019;8:49. https://doi.org/10.1167/tvst.8.3.49 10.1167/tvst.8.3.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Carracedo G, Carpena C, Concepción P, Díaz V, García-García M, Jemni N, Lledó VE, Martín M, Pastrana C, Pelissier R, Veselinova A, Wang X, Pintor J. Presence of melatonin in human tears. J Optom 2017;10:3-4. https://doi.org/10.1016/j.optom.2016.03.002 10.1016/j.optom.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rada JA, Wiechmann AF. Melatonin receptors in chick ocular tissues: implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci 2006;47:25-33. https://doi.org/10.1167/iovs.05-0195 10.1167/iovs.05-0195 [DOI] [PubMed] [Google Scholar]
- [49].Alkozi HA, Navarro G, Franco R, Pintor J. Melatonin and the control of intraocular pressure. Prog Retin Eye Res 2020;75:100798. https://doi.org/10.1016/j.preteyeres.2019.100798 10.1016/j.preteyeres.2019.100798 [DOI] [PubMed] [Google Scholar]
- [50].Aranda ML, Fleitas MFG, Dieguez H, Iaquinandi A, Sande PH, Dorfman D, Rosenstein RE. Melatonin as a Therapeutic Resource for Inflammatory Visual Diseases. Curr Neuropharmacol 2017;15:951-62. https://doi.org/10.2174/1570159X15666170113122120 10.2174/1570159X15666170113122120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Martínez-Águila A, Martín-Gil A, Carpena-Torres C, Pastrana C, Carracedo G. Influence of circadian rhythm in the eye: significance of melatonin in glaucoma. Biomolecules 2021;11:340. https://doi.org/10.3390/biom11030340 10.3390/biom11030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gubin D, Neroev V, Malishevskaya T, Cornelissen G, Astakhov SY, Kolomeichuk S, Yuzhakova N, Kabitskaya Y, Weinert D. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J Pineal Res 2021;70:e12730. https://doi.org/10.1111/jpi.12730 10.1111/jpi.12730 [DOI] [PubMed] [Google Scholar]
- [53].Diéguez HH, González Fleitas MF, Aranda ML, Calanni JS, Keller Sarmiento MI, Chianelli MS, Alaimo A, Sande PH, Romeo HE, Rosenstein RE, Dorfman D. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J Pineal Res 2020;68:e12643. https://doi.org/10.1111/jpi.12643 10.1111/jpi.12643 [DOI] [PubMed] [Google Scholar]
- [54].Ferreira de Melo IM, Martins Ferreira CG, Lima da Silva Souza EH, Almeida LL, Bezerra de Sá F, Cavalcanti Lapa Neto CJ, Paz de Castro MV, Teixeira VW, Coelho Teixeira ÁA. Melatonin regulates the expression of inflammatory cytokines, VEGF and apoptosis in diabetic retinopathy in rats. Chem Biol Interact 2020;327:109183. https://doi.org/10.1016/j.cbi.2020.109183 10.1016/j.cbi.2020.109183 [DOI] [PubMed] [Google Scholar]
- [55].Klettner A, Kampers M, Töbelmann D, Roider J, Dittmar M. The Influence of Melatonin and Light on VEGF Secretion in Primary RPE Cells. Biomolecules 2021;11:114. https://doi.org/10.3390/biom11010114 10.3390/biom11010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Castelli V, Paladini A, d’Angelo M, Allegretti M, Mantelli F, Brandolini L, Cocchiaro P, Cimini A, Varrassi G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci Ther 2021;27:403-12. https://doi.org/10.1111/cns.13610 10.1111/cns.13610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].El-Sherbeny A, Naggar H, Miyauchi S, Ola MS, Maddox DM, Martin PM, Ganapathy V, Smith SB. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and müller cells. Invest Ophthalmol Vis Sci 2004;45:694-701. https://doi.org/10.1167/iovs.03-0503 10.1167/iovs.03-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tomi M, Tajima A, Tachikawa M, Hosoya K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim Biophys Acta 2008;1778:2138-42. https://doi.org/10.1016/j.bbamem.2008.04.012 10.1016/j.bbamem.2008.04.012 [DOI] [PubMed] [Google Scholar]
- [59].García-Ayuso D, Di Pierdomenico J, Hadj-Said W, Marie M, Agudo-Barriuso M, Vidal-Sanz M, Picaud Serge, Villegas-Pérez MP. Taurine depletion causes ipRGC loss and increases light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci 2018;59:1396. https://doi.org/10.1167/iovs.17-23258 10.1167/iovs.17-23258 [DOI] [PubMed] [Google Scholar]
- [60].Cammalleri M, Dal Monte M, Amato R, Bagnoli P, Rusciano D. A dietary combination of forskolin with homotaurine, spearmint and b vitamins protects injured retinal ganglion cells in a rodent model of hypertensive glaucoma. Nutrients 2020;12:1189. https://doi.org/10.3390/nu12041189 10.3390/nu12041189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Froger N, Moutsimilli L, Cadetti L, Jammoul F, Wang QP, Fan Y, Gaucher D, Rosolen SG, Neveux N, Cynober L, Sahel JA, Picaud S. Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog Retin Eye Res 2014;41:44-63. https://doi.org/10.1016/j.preteyeres.2014.03.001 10.1016/j.preteyeres.2014.03.001 [DOI] [PubMed] [Google Scholar]
- [62].Bastaki SM, Adeghate E, Amir N, Ojha S, Oz M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am J Transl Res 2018;10:4210-22. [PMC free article] [PubMed] [Google Scholar]
- [63].Kiani AK, Falsini B, Ziccardi L, Gusson E, Mangialavori D, Allegrini F, Colao E, Bertelli M. Flavonoid supplements increase neurotrophin activity to modulate inflammation in retinal genetic diseases. Acta Biomed 2020;91:e2020014. https://doi.org/10.23750/abm.v91i13-S.10683 10.23750/abm.v91i13-S.10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the women’s antioxidant and folic acid cardiovascular study. Arch Intern Med 2009;169:335-41. https://doi.org/10.1001/archinternmed.2008.574 10.1001/archinternmed.2008.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang XB, Qiao C, Wei L, Han YD, Cui NH, Huang ZL, Li ZH, Zheng F, Yan M. Associations of polymorphisms in mthfr gene with the risk of age-related cataract in chinese han population: a genotype-phenotype analysis. PLoS One 2015;10:e0145581. https://doi.org/10.1371/journal.pone.0145581 10.1371/journal.pone.0145581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Christen WG, Glynn RJ, Manson JE, MacFadyen J, Bubes V, Schvartz M, Buring JE, Sesso HD, Gaziano JM. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology 2014;121:525-34. https://doi.org/10.1016/j.ophtha.2013.09.038 10.1016/j.ophtha.2013.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Glaser TS, Doss LE, Shih G, Nigam D, Sperduto RD, Ferris FL, 3rd, Agrón E, Clemons TE, Chew EY; Age-Related Eye Disease Study Research Group. The association of dietary lutein plus zeaxanthin and B vitamins with cataracts in the age-related eye disease study: AREDS Report No. 37. Ophthalmology 2015;122:1471-9. https://doi.org/10.1016/j.ophtha.2015.04.007 10.1016/j.ophtha.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tan AG, Mitchell P, Rochtchina E, Flood VM, Cumming RG, Wang JJ. Serum homocysteine, vitamin B12, and folate, and the prevalence and incidence of posterior subcapsular cataract. Invest Ophthalmol Vis Sci 2014;56:216-20. https://doi.org/10.1167/iovs.14-15531 10.1167/iovs.14-15531 [DOI] [PubMed] [Google Scholar]
- [69].Molina-Leyva I, Molina-Leyva A, Bueno-Cavanillas A. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmol 2017;95:e677-e685. https://doi.org/10.1111/aos.13428 10.1111/aos.13428 [DOI] [PubMed] [Google Scholar]
- [70].Zhang AC, Singh S, Craig JP, Downie LE. Omega-3 Fatty Acids and Eye Health: Opinions and Self-Reported Practice Behaviors of Optometrists in Australia and New Zealand. Nutrients 2020;12:1179. https://doi.org/10.3390/nu12041179 10.3390/nu12041179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Meyer BJ. Australians are not meeting the recommended intakes for omega-3 long chain polyunsaturated fatty acids: results of an analysis from the 2011-2012 national nutrition and physical activity survey. Nutrients 2016;8:111. https://doi.org/10.3390/nu8030111 10.3390/nu8030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol 2011;22:279-82. https://doi.org/10.1097/ICU.0b013e3283477d23 10.1097/ICU.0b013e3283477d23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. ω-3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci 2016;57:2472-8. https://doi.org/10.1167/iovs.16-19131 10.1167/iovs.16-19131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr 2005;82:887-93. https://doi.org/10.1093/ajcn/82.4.887 10.1093/ajcn/82.4.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, Dammeier S, Meester-Smoor M, Pool FM, de Jong EK, Delcourt C, Rodrigez-Bocanegra E, Biarnés M, Luthert PJ, Ueffing M, Klaver CCW, Nogoceke E, den Hollander AI, Lengyel I. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res 2018;67:56-86. https://doi.org/10.1016/j.preteyeres.2018.04.006 10.1016/j.preteyeres.2018.04.006 [DOI] [PubMed] [Google Scholar]
- [76].Simón MV, Agnolazza DL, German OL, Garelli A, Politi LE, Agbaga MP, Anderson RE, Rotstein NP. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J Neurochem 2016;136:931-46. https://doi.org/10.1111/jnc.13487 10.1111/jnc.13487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 2009;127:656-65. https://doi.org/10.1001/archophthalmol.2009.76 10.1001/archophthalmol.2009.76 [DOI] [PubMed] [Google Scholar]
- [78].Wu J, Cho E, Giovannucci EL, Rosner BA, Sastry SM, Willett WC, Schaumberg DA. Dietary intakes of eicosapentaenoic acid and docosahexaenoic acid and risk of age-related macular degeneration. Ophthalmology 2017;124:634-43. https://doi.org/10.1016/j.ophtha.2016.12.033 10.1016/j.ophtha.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lawrenson JG, Evans JR. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2015;2015:CD010015. https://doi.org/10.1002/14651858.CD010015.pub3 10.1002/14651858.CD010015.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Downie LE, Vingrys AJ. Oral omega-3 supplementation lowers intraocular pressure in normotensive adults. Transl Vis Sci Technol 2018;7:1. https://doi.org/10.1167/tvst.7.3.1 10.1167/tvst.7.3.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Keppel Hesselink JM, Costagliola C, Fakhry J, Kopsky DJ. Palmitoylethanolamide, a natural retinoprotectant: its putative relevance for the treatment of glaucoma and diabetic retinopathy. J Ophthalmol 2015;2015:430596. https://doi.org/10.1155/2015/430596 10.1155/2015/430596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ye S, Chen Q, Jiang N, Liang X, Li J, Zong R, Huang C, Qiu Y, Ma JX, Liu Z. PPARα-Dependent effects of palmitoylethanolamide against retinal neovascularization and fibrosis. Invest Ophthalmol Vis Sci 2020;61:15. https://doi.org/10.1167/iovs.61.4.15 10.1167/iovs.61.4.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R.The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol 1995;39:367-74. https://doi.org/10.1016/s0039-6257(05)80092-x 10.1016/s0039-6257(05)80092-x [DOI] [PubMed] [Google Scholar]
- [84].Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115-34. https://doi.org/10.1016/s0039-6257(00)00140-5 10.1016/s0039-6257(00)00140-5 [DOI] [PubMed] [Google Scholar]
- [85].Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 2011;95:14-25. https://doi.org/10.1016/j.pneurobio.2011.05.011 10.1016/j.pneurobio.2011.05.011 [DOI] [PubMed] [Google Scholar]
- [86].Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci 2010;51:1275-81. https://doi.org/10.1167/iovs.09-4478 10.1167/iovs.09-4478 [DOI] [PubMed] [Google Scholar]
- [87].Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med 2012;33:399-417. https://doi.org/10.1016/j.mam.2012.03.009 10.1016/j.mam.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Marangoni D, Falsini B, Piccardi M, Ambrosio L, Minnella AM, Savastano MC, Bisti S, Maccarone R, Fadda A, Mello E, Concolino P, Capoluongo E. Functional effect of Saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J Transl Med 2013;11:228. https://doi.org/10.1186/1479-5876-11-228 10.1186/1479-5876-11-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet 200;10:19-43. https://doi.org/10.1146/annurev.genom.9.081307.164350 10.1146/annurev.genom.9.081307.164350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthal Visual Sci 2008;49:1254-61. https://doi.org/10.1167/iovs.07-0438 10.1167/iovs.07-0438 [DOI] [PubMed] [Google Scholar]
- [91].Falsini B, Piccardi M, Minnella A, Savastano C, Capoluongo E, Fadda A, Balestrazzi E, Maccarone R, Bisti S. Influence of saffron supplementation on retinal flicker sensitivity in early age related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:6118-24. https://doi.org/10.1167/iovs.09-4995 10.1167/iovs.09-4995 [DOI] [PubMed] [Google Scholar]
- [92].Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, Capoluongo E, Maccarone R, Bisti S, Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med 2012;429124. https://doi.org/10.1155/2012/429124 10.1155/2012/429124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr 2004;44:155-72. https://doi.org/10.1080/10408690490441433 10.1080/10408690490441433 [DOI] [PubMed] [Google Scholar]
- [94].Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochem Biophys Acta 2007;1770:578-84. https://doi.org/10.1016/j.bbagen.2006.11.012 10.1016/j.bbagen.2006.11.012 [DOI] [PubMed] [Google Scholar]
- [95].Di Marco F, Romeo S, Nandasena C, Purushothuman S, Adams C, Bisti S, Stone J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am J Neurodegener Dis 2013;2:208-20. [PMC free article] [PubMed] [Google Scholar]
- [96].Kanakis CD, Tarantilis PA, Tajimir-Riahi HA, Polissiou MG. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: stability and antioxidative properties. J Agric Food Chem 2007;55:970-7. https://doi.org/10.1021/jf062638l 10.1021/jf062638l [DOI] [PubMed] [Google Scholar]
- [97].Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I, Hong JW, Lee EH. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 2010;648:110-6. https://doi.org/10.1016/j.ejphar.2010.09.003 10.1016/j.ejphar.2010.09.003 [DOI] [PubMed] [Google Scholar]
- [98].Natoli R, Zhu Y, Valter K, Bisti S, Eells J, Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol Vis 2010;16:1801-22. [PMC free article] [PubMed] [Google Scholar]


