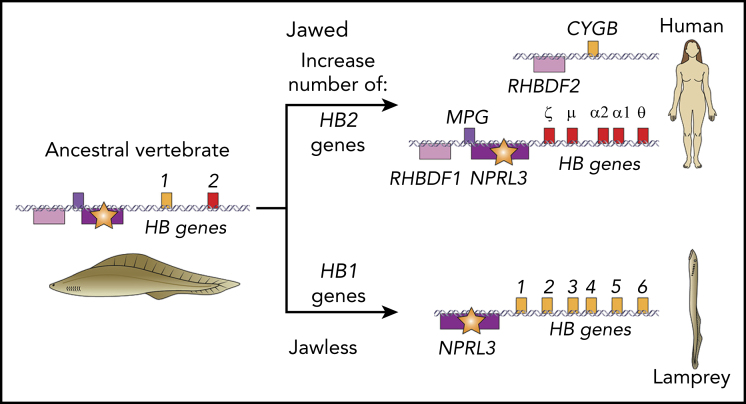
A globin gene regulatory element discovered in lampreys suggests an ancient origin in ancestral vertebrates. The Miyata et al study of lampreys showed that genes encoding globin polypeptides of the oxygen transporter hemoglobin (HB genes, light orange and red boxes) are adjacent to a ubiquitously expressed NPRL3 gene (violet box) in both major branches of vertebrates, jawed and jawless, despite the separate, convergent evolution of HB genes in each branch, with HB genes in jawless vertebrates more related to CYGB (light orange boxes). Furthermore, an intron of lamprey NPRL3 contains a major regulatory element for globin genes (star), as is the situation in humans. These maps in extant species suggest that the linkage of NPRL3, containing a strong regulatory element, to HB genes occurred in an ancestral vertebrate, represented as Haikouichthys ercaicunensis.10 By hypothesizing multiple ancestral HB genes in the linkage group, one related to CYGB and another related to canonical vertebrate HB (red box), the model can explain convergent evolution of different oxygen-transporting globins as selective expansions of one or the other gene while maintaining strong regulation from the NPRL3 intronic enhancer. Additional genes characteristic of this locus are also shown; boxes above the illustrative DNA helices are transcribed left to right, and those below the DNA are transcribed right to left. The CYBG gene and HB genes are on different chromosomes in humans.
In this issue of Blood, Miyata et al report on their studies in lampreys, a vertebrate far distant from mammals, and infer that a core component of globin gene regulation became active long ago, likely in the Cambrian Period (about 430 to 540 million years ago).1
Comparisons of hemoglobin gene loci across a broad range of mammalian species have revealed much about both their evolutionary histories and their regulation (eg, locations of many cis-regulatory elements were predicted by strong noncoding sequence conservation). However, equivalent studies in vertebrate species more distant from humans and mice are more challenging, mainly because regulatory element sequences evolve rapidly compared with the slower rate of evolution of protein-coding sequences. The exons of homologous protein-coding genes can align well in comparisons of genomic sequences from humans to invertebrates or beyond, but human regulatory regions rarely align with sequences more distant than marsupials.2 Thus, comparative genomic analyses relevant to the regulation of human genes can be applied across mammalian species (perhaps out to 160 million years ago), but the challenges for examining longer histories are formidable.
One question requiring a long evolutionary view is the origin of regulatory elements that direct the high-level production of the globin polypeptides of hemoglobins (Hgb's, encoded by HB genes) in vertebrate erythroid cells. Efforts to manipulate the sequences of such regulatory elements, or proteins acting at them, are at the heart of multiple efforts to redirect globin expression for therapeutic purposes, such as reactivation of fetal Hgb in adults with sickle cell disease or other hemoglobinopathies. This keen interest in globin gene regulatory elements has driven decades of research that has revealed that the introns of the gene NPRL3 are needed for high-level expression of the genes encoding the α-globin subunits of Hgb in humans and mice3 (see figure). An intron of the homologous NPRL3 gene in zebrafish also enhances expression of the linked HB genes.4 Notably, the intron in fish NPRL3 shares a common function (enhancement) with equivalent introns in mammals, despite a lack of sequence similarity.5
Miyata et al extended our view of globin regulatory elements much further back in history. They examined the lamprey, a member of the vertebrate clade most distant from mammals. About 500 million years ago, ancestral vertebrates separated into 2 major groups, the jawed vertebrates (leading to the evolution of mammals, birds, and fish) and the jawless vertebrates (leading to the evolution of hagfish and lampreys). The Hgb's in both jawed and jawless vertebrates transport oxygen within erythrocytes, but surprisingly, the globin genes in the jawless species are more similar to the CYGB gene encoding cytoglobin,6 a protein widely distributed across cell types in most vertebrates that usually does not have a role in oxygen transport.7 Thus, it seems that the oxygen-transporting activity of HB genes arose by convergent evolution in the 2 branches of vertebrates, from a CYGB-like ancestral gene in jawless vertebrates and from an ancestral gene related to canonical HB genes (eg, HBA and HBB genes in mammals) in jawed vertebrates.
If the oxygen-transporting function of Hgb's arose twice in vertebrates, did the elements responsible for high-level expression in erythroid cells also arise twice? Miyata et al tackled this difficult question with a mix of genomics, biochemistry, and genetics. After assembling a DNA sequence of the globin gene locus from the river lamprey (Lampetra fluviatilis), the authors discovered 6 HB genes with an NPRL3 gene just upstream (see figure). The arrangement and orientations of the genes were similar to those in humans, raising the intriguing possibility that the introns of lamprey NRPL3 could harbor a critical regulatory element. The authors searched the locus for a biochemical signature common to most regulatory elements, accessibility of chromatin, and they found an accessible chromatin site in intron 7 of NRPL3 in lamprey erythrocytes. They then tested the function genetically, using transient transgenesis in lampreys to show that intron 7 of lamprey NPRL3 could drive erythroid-specific expression of a reporter gene. Importantly, lamprey intron 7 was not active in transgenic assays in more conventional model species such as zebrafish, showing the need for conducting functional analyses in an appropriate context, in this case transgenesis in a closely related species, the sea lamprey. Thus, an intronic enhancer in NPRL3 is regulating the expression of linked HB genes in erythroid cells of both major clades of vertebrates, despite the fact that the genes in those HB clusters are derived from different ancestral genes.
These results led to a model (see figure) to explain the conundrum of convergent evolution of protein-coding genes while sharing an ancestral regulatory element. More than 500 million years ago, during the enormous diversification of animals in the seas of the Cambrian Period, an NPRL3 gene with a strong regulatory element became linked to at least 2 different globin genes in the ancestor to vertebrates. Hints from gene arrangements in tunicates suggest that NPRL3 was not linked to globin genes before this time. That strong regulatory element remained active, leading to high-level expression of the linked HB genes in both clades of vertebrates. However, in adapting to the need for oxygen transport, the ancestral globin gene corresponding to canonical hemoglobin genes expanded and diversified in jawed vertebrates, whereas the ancestral globin gene corresponding to CYGB had a similar fate in jawless vertebrates.
The new results show that gene regulatory elements can be deeply preserved over evolutionary time, long past the time frame illuminated by comparative genomics, but only careful biochemical and genetic analyses will reveal them. However, it is important to keep in mind that many regulatory elements are not strongly conserved; perhaps a majority of regulatory elements arose recently within specific lineages or have been repurposed for new functions.8, 9 The authors deduce a model that helps resolve a previously difficult evolutionary scenario. Still, many questions remain, including how the globin gene clusters arose within each clade and how various globins have been adapted to different functions across clades. It is likely that comparative biochemical and functional analyses of globin genes will remain fruitful for many future studies.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Miyata M, Gillemans N, Hockman D, et al. An evolutionary ancient mechanism for regulation of hemoglobin expression in vertebrate red cells. Blood. 2020;136(3):269–278. doi: 10.1182/blood.2020004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller W, Rosenbloom K, Hardison RC, et al. 28-way vertebrate alignment and conservation track in the UCSC Genome Browser. Genome Res. 2007;17(12):1797–1808. doi: 10.1101/gr.6761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay D, Hughes JR, Babbs C, et al. Genetic dissection of the α-globin super-enhancer in vivo. Nat Genet. 2016;48(8):895–903. doi: 10.1038/ng.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganis JJ, Hsia N, Trompouki E, et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev Biol. 2012;366(2):185–194. doi: 10.1016/j.ydbio.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philipsen S, Hardison RC. Evolution of hemoglobin loci and their regulatory elements. Blood Cells Mol Dis. 2018;70:2–12. doi: 10.1016/j.bcmd.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann FG, Opazo JC, Storz JF. Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc Natl Acad Sci U S A. 2010;107(32):14274–14279. doi: 10.1073/pnas.1006756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol. 2002;19(4):416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 8.Vierstra J, Rynes E, Sandstrom R, et al. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science. 2014;346(6212):1007–1012. doi: 10.1126/science.1246426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denas O, Sandstrom R, Cheng Y, et al. Genome-wide comparative analysis reveals human-mouse regulatory landscape and evolution. BMC Genomics. 2015;16(1):87. doi: 10.1186/s12864-015-1245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu D-G, Luo H-L, Morris SC, et al. Lower Cambrian vertebrates from south China. Nature. 1999;402(6757):42–46. [Google Scholar]