Abstract
Little is known about the molecular pathogenesis of hepatitis and enterocolitis caused by enterohepatic Helicobacter species. Sonicates of the murine pathogen Helicobacter hepaticus were found to cause progressive cell distension, accumulation of filamentous actin, and G2/M cell cycle arrest in HeLa cell monolayers. The genes encoding this cytotoxic activity were cloned from H. hepaticus. Three open reading frames with closest homology to cdtA, cdtB, and cdtC from Campylobacter jejuni were identified. Sonicates of a laboratory strain of Escherichia coli carrying the cloned cdtABC gene cluster from H. hepaticus reproduced the cytotoxic activities seen with sonicates of H. hepaticus. Cytolethal distending toxin activity is a potential virulence determinant of H. hepaticus that may play a role in the pathogenesis of Helicobacter-associated hepatitis and enterocolitis.
Enterohepatic Helicobacter species (EHS) are emerging as important pathogens in the genus Helicobacter (15). In contrast to Helicobacter pylori and other gastric Helicobacter species, the EHS colonize the lower gastrointestinal tract, including the ileum, cecum, colon, and biliary tree. However, in a manner similar to the gastric Helicobacter species, the EHS cause persistent infections associated with chronic inflammation and epithelial cell hyperproliferation that can lead to neoplastic disease (15, 19, 36).
H. hepaticus is an EHS that causes chronic active hepatitis and typhlocolitis in immunocompetent mice (18, 37). In male mice of susceptible strains, H. hepaticus infection leads to chronic active hepatitis and liver cancer (16, 18, 20, 36). In addition, H. hepaticus infection is sufficient to induce inflammatory bowel disease in certain immunodeficient mice (9, 23, 34, 35).
Little is known about the molecular pathogenesis of the EHS. Urease, which has been demonstrated to be a virulence factor in the gastric Helicobacter species H. pylori and H. mustelae, is not present in all of the EHS. H. hepaticus strains do possess urease activity (16), but the role of urease in colonization or disease is not clear. Genes homologous to vacA, encoding the vacuolating cytotoxin (Vac) (13), and cag, the cytotoxin-associated genes that are part of a pathogenicity island in H. pylori (1, 10), have not been definitively identified in any EHS.
A cytolethal distending toxin (CDT) has been described in a number of mucosal pathogens, including Campylobacter jejuni (21) and other Campylobacter species (27), certain Escherichia coli strains (7, 22), Shigella dysenteriae (26), Haemophilus ducreyi (12), and Actinobacillus actinomycetemcomitans (31). CDT causes progressive cell enlargement and eventual death. The mechanism of CDT activity is reported to involve G2/M cell cycle arrest in target cells, possibly by preventing activation of cdc2 (11, 38). Additionally, the appearance of abnormal accumulations of polymerized actin has been reported in Chinese hamster ovary cells treated with the CDT from E. coli 9142-88 (5).
Campylobacter species are closely related to members of the genus Helicobacter. Members of both genera are microaerobic, motile, spiral- to curved-shaped, gram-negative bacteria that colonize the mucus of the gastrointestinal tract. C. jejuni and other Campylobacter species are an important cause of acute gastroenteritis (3). EHS have also been recognized to cause gastroenteritis, and similarities between these two groups of organisms have resulted in misidentification of some EHS as Campylobacter species in clinical and epidemiologic studies (6, 8, 28, 29). It has been suggested that CDT plays a role in the pathogenesis of C. jejuni-induced gastroenteritis (38). Given the similarities between campylobacters and helicobacters, particularly the EHS, we examined H. hepaticus for nucleotide sequence homology to the cdtABC gene cluster and for CDT activity.
MATERIALS AND METHODS
Bacteria and cell lines.
H. hepaticus ATCC 51449 was obtained from the American Type Culture Collection (ATCC), Manassas, Va., and was cultured on tryptic soy agar plates supplemented with 5% sheep blood. A microaerobic environment was maintained in vented GasPak jars which were evacuated to −20 mm Hg and then equilibrated with a gas mixture consisting of 80% N2, 10% H2, and 10% CO2. An incubation temperature of 37°C was used for growth. Long-term storage of bacteria was at −70°C in tryptic soy broth with 30% glycerol.
E. coli XL-1 Blue and SOLR were obtained from Stratagene (La Jolla, Calif.) and maintained on Luria-Bertani broth agar plates with the appropriate antibiotic selection.
HeLa cells (CCL-2) were obtained from the ATCC and cultured in Dulbecco's modified Eagle's medium supplemented with glutamine and 10% fetal calf serum.
PCR and DNA sequence determination.
Genomic DNA from plate-grown bacteria was isolated by using a Qiagen QIAamp kit for small-scale preparations or a Qiagen genomic G-100 kit for large-scale purification (Qiagen Inc., Santa Clarita, Calif.). Kits were used in accordance with the recommendations of the manufacturer.
The degenerate primers VAT2 and WMI1, originally used to identify the cdtB gene in C. jejuni (27), were synthesized (IDT, Coraville, Iowa) and used for PCR. PCR was performed by using Pharmacia Ready-To-Go PCR beads (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). Reactions were set up with 1 μl (approximately 100 ng) of template DNA, 20 pmol of each primer, and enough water for a total volume of 25 μl. This yielded a reaction containing 1.5 U of Taq polymerase, 10 mM Tris-HCl (pH 9.0 at room temperature), 50 mM KCl, 1.5 mM MgCl2, 200 μM each nucleotide, and stabilizers, including bovine serum albumin (BSA). The reaction mixtures were overlaid with 50 μl of mineral oil and subjected to amplification in a DNA thermal cycler (Perkin-Elmer model 480; PE Biosystems, Foster City, Calif.). The cycling conditions were as follows: initial denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 42°C for 1.5 min, and extension at 72°C for 1.5 min. A final extension at 72°C for 4 min was performed. Ten microliters of each reaction mixture was analyzed by electrophoresis in a 1.0% agarose gel and visualized after staining with ethidium bromide.
Bands of interest were excised from the gel and purified by using a gel band purification kit (Amersham Pharmacia Biotech) in accordance with the recommendations of the manufacturer. Purified fragments were ligated into the Promega pGEM T-easy vector (Promega, Madison, Wis.).
DNA sequencing on an ABI model 377 PRISM automated DNA sequencer was performed by the core laboratory at the Massachusetts General Hospital (Boston). DNA sequence analysis was performed on a Macintosh G3 computer using the MacVector 6.5 software package (Oxford Molecular, Campbell, Calif.).
Preparation of bacterial sonicates.
H. hepaticus cultures grown for 48 h on three 100-mm-diameter plates were harvested into 1 ml of phosphate-buffered saline (PBS). The bacteria were disrupted by six 30-s pulses on ice with a VirSonic 50 sonicator (Virtis, Gardiner, N.Y.). Debris was removed by centrifugation at 16,000 × g in an Eppendorf model 5415 centrifuge (Eppendorf Scientific, Westbury, N.Y.), followed by filtration through a 0.2-μm-pore-size filter. Aliquots of the preparations were stored at −70°C.
Cultures of E. coli harboring the cloned cdtABC gene cluster from H. hepaticus were grown for 18 h in 5 ml of Luria-Bertani broth supplemented with ampicillin at 100 μg/ml. Bacteria were harvested by centrifugation and then suspended in 1 ml of PBS. Preparation of sonicates was then performed as described above.
Tissue culture assay for CDT activity.
HeLa cells were seeded onto 13-mm-diameter circular glass coverslips in 24-well tissue culture plates at a density of 2 × 103 per well. Twenty microliters of bacterial sonicate was added to each well, and the plates were incubated in 5% CO2 at 37°C. At appropriate time points, coverslips were washed with PBS and then stained with Diff-Quik modified Wright stain (Baxter Healthcare, Miami, Fla.) and mounted for visualization by light microscopy.
Immunofluorescence microscopy.
Coverslips were washed with PBS and then fixed with a solution of 3.7% formaldehyde in PBS for 10 min at room temperature. After washing with PBS, cells were permeabilized with a solution of 0.1% Triton X-100 in PBS for 10 min at room temperature. The coverslips were washed again with PBS and then stored at 4°C in PBS with 0.5% BSA until they were stained.
Polymerized actin was stained with phalloidin labeled with Texas red (Molecular Probes, Eugene, Oreg.), and the nuclei were stained with Hoechst 33342 as described previously (39). Photographs were taken on a Nikon Labophot microscope (Nikon, USA, Melville, N.Y.) with T-Max 100 film (Kodak, Rochester, N.Y.).
Flow cytometry.
Twenty-five-square-centimeter tissue culture flasks were seeded with 3 × 105 HeLa cells. One hundred microliters of bacterial sonicate was added to each flask, and then the flasks were incubated in 5% CO2 at 37°C. After 24, 48, and 72 h, cells were removed by trypsinization and transferred to a 1.5-ml microcentrifuge tube. Cells were pelleted by centrifugation at 735 × g for 3 min and resuspended in 3% polyethylene glycol 8000–2.5 μg of propidium iodide per ml–9 U of RNase per ml–0.1% Triton X-100–0.001% BSA in 4 mM sodium citrate. The cells were incubated for 20 min at 37°C and then mixed with an equal volume of 3% polyethylene glycol 8000–2.5 μg of propidium iodide per ml–0.1% Triton X-100–0.001% BSA in 0.4 M NaCl. Cells were incubated at 4°C for at least 1 h before performance of DNA content analysis on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, N.J.) using the Cell Quant software for data acquisition. Data analysis was performed by using the ModFit program on 104 cells for each experiment.
Genomic library construction and screening.
Two genomic H. hepaticus libraries were constructed by using the insertion vector λZAPII (Stratagene). Briefly, genomic DNA from H. hepaticus ATCC 51449 was partially digested with the restriction enzyme Tsp509I. DNA with a length of ≥5 kb was ligated into the EcoRI site of the vector. The libraries were screened by DNA hybridization using the PCR amplicon generated by amplification of H. hepaticus genomic DNA with the primers VAT2 and WMI1 (27). An [α-32P]dCTP-labeled probe was generated by using a random primer DNA labeling kit (Ready-To-Go DNA labeling beads; Amersham Pharmacia Biotech). The radioactive probe was used to screen 105 recombinant bacteriophage. Probe-positive plaques were identified and subcloned into the plasmid vector pBluescript SK(−) using the in vivo excision and recircularization features of the λZAPII vector. E. coli bacteria carrying these recombinant plasmids were further characterized by restriction mapping and by screening for CDT activity.
Nucleotide sequence accession number.
The nucleotide sequence of the H. hepaticus cdtABC gene cluster has been entered in the GenBank database under accession no. AF163667.
RESULTS
H. hepaticus possesses cdtABC nucleotide sequence homology.
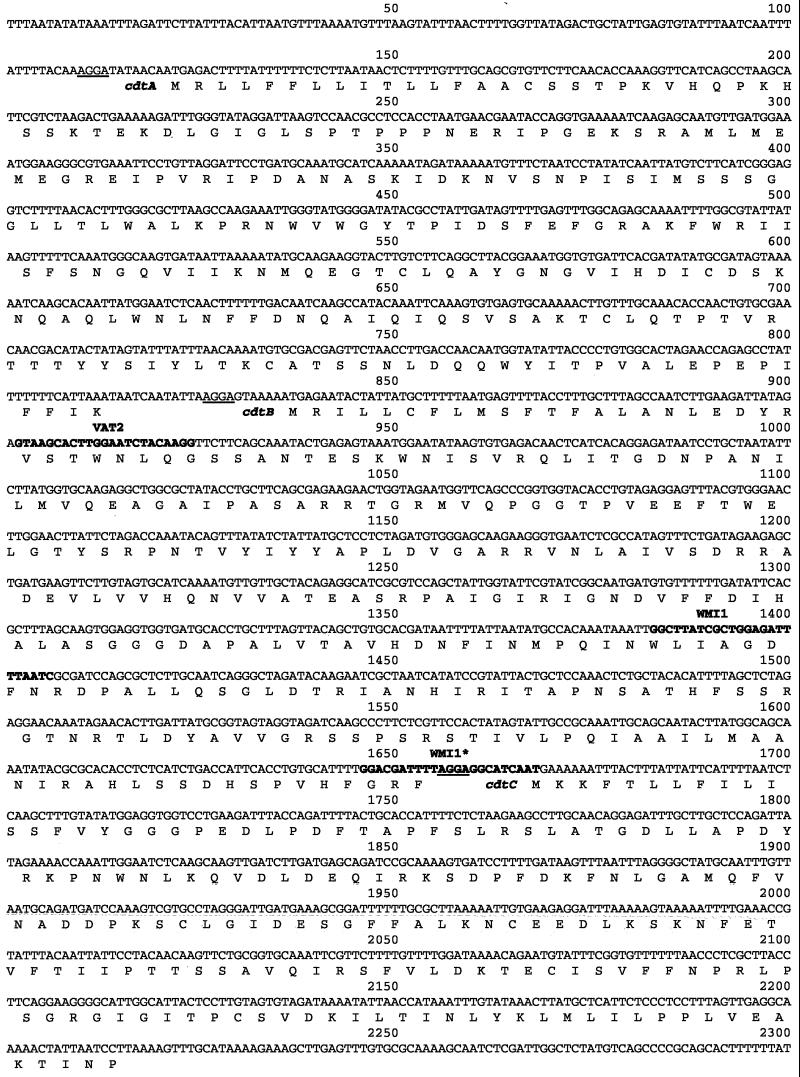
The degenerate primers VAT2 and WMI1 (27) amplify a 494-bp fragment of the cdtB gene from C. jejuni. Amplification of H. hepaticus genomic DNA with these primers produced a larger than expected amplicon of approximately 750 bp (data not shown). The complete nucleotide sequence of this amplicon was determined, and the deduced amino acid sequence exhibited significant homology to the published CdtB sequence of C. jejuni. The predicted H. hepaticus peptide fragment exhibited 57% identity and 72% similarity to the C. jejuni gene product. The larger than expected size of the amplicon was a consequence of the WMI1 primer annealing to a site 264 bp downstream of the anticipated target site (Fig. 1).
FIG. 1.
Nucleotide sequence of the cdtABC gene cluster from H. hepaticus. The three presumed ribosomal binding sites (underlined) for each open reading frame are shown along with the deduced amino acid sequences. Also indicated (bold) are the sites at which the degenerate PCR primers VAT2 and WMI1 bind. Forward primer VAT2 and the predicted site of reverse primer WMI1 are expected to yield a 505-bp amplicon. The actual binding site of the reverse primer WMI1* along with forward primer VAT2 produced an observed product of 768 bp.
By using this PCR product, lambda clones containing homology to cdtB were isolated and corresponding plasmid clones were generated. DNA sequence analysis of these clones revealed that H. hepaticus has three closely linked open reading frames corresponding to the entire cdtABC gene cluster (Fig. 1). The deduced amino acid sequences had the closest homology to the proteins encoded by the cdtABC gene cluster from C. jejuni (Fig. 2). The amino acid homologies were as follows: CdtA, 30% identity and 44% similarity; CdtB, 56% identity and 72% similarity; CdtC, 40% identity and 55% similarity.
FIG. 2.
Comparison of the predicted amino acid sequences of CdtA, CdtB, and CdtC from H. hepaticus ATCC 51449 (Hh) and C. jejuni 81-176 (Cj). Colons indicate identical amino acids, and conserved amino acids are indicated by periods. Dashes indicate gaps introduced by the MacVector ClustalW alignment program.
Sonicates of H. hepaticus cause morphologic changes in HeLa cell monolayers.
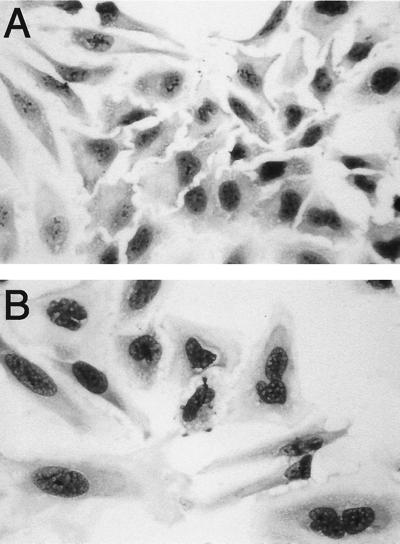
Because the presence of cdtABC nucleotide sequence homology was demonstrated in DNA from H. hepaticus, we sought to determine whether sonicates of this organism exhibited CDT activity. HeLa cells treated with sonicates of H. hepaticus showed marked cellular distension (Fig. 3B). The distended cells also exhibited nuclear enlargement, and approximately 15% of the affected cells were found to be multinucleated. Occasionally, nuclear irregularities and fragmentation were also seen in HeLa cells treated with sonicates of H. hepaticus.
FIG. 3.
Cytopathic effect of H. hepaticus CDT on HeLa cells. Compared to untreated cells (A), cells treated with sonicates from H. hepaticus (B) exhibited marked cytoplasmic distension along with nuclear enlargement, multinucleation, and nuclear fragmentation. Magnification, ×250.
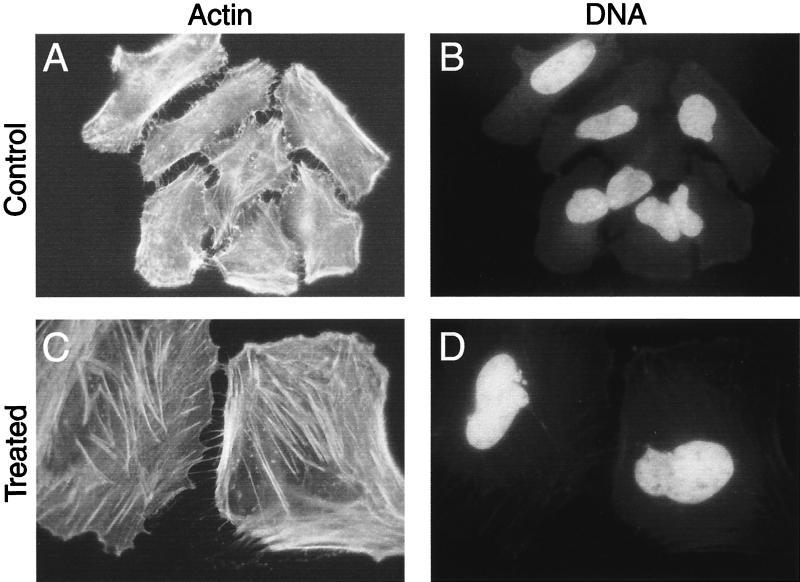
The cytoplasmic distension induced by sonicates of H. hepaticus was readily apparent, but the extent of cellular margins was underestimated by using light microscopy following the application of this modified Wright stain. To further examine the cellular structure of treated cells and to detect associated cytoskeletal changes, monolayers were stained with fluorescently labeled phalloidin to visualize filamentous actin (F-actin). Phalloidin staining revealed the full extent of the enlargement of cells in monolayers treated with sonicates of H. hepaticus (Fig. 4). There also appeared to be an increase in the amount of F-actin present in cells in affected monolayers. Nuclear staining with the fluorescent DNA stain Hoechst 33342 confirmed the nuclear enlargement, multinucleation, and nuclear fragmentation visualized by light microscopy.
FIG. 4.
Cytoplasmic and nuclear distension of HeLa cells treated with sonicates of H. hepaticus. Photomicrographs of untreated cells (A and B) and cells treated with sonicates of H. hepaticus (C and D) for 72 h are shown. Fixed and permeabilized cells were double labeled for epifluorescence microscopy with Texas red-labeled phalloidin (A and C) and Hoechst 33342 (B and D). Treated cells exhibited increased cell size and prominent stress fiber-like structures (C) as well, as increased nuclear size (D). Magnification, ×500.
Sonicates of H. hepaticus cause G2/M cell cycle arrest in cultured cells.
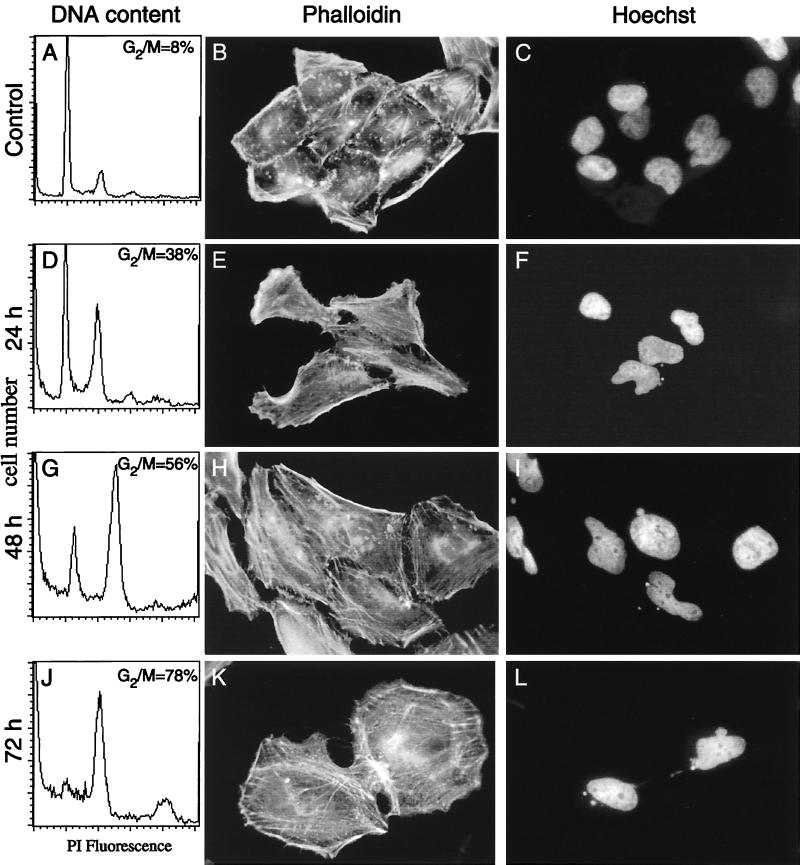
To examine whether the morphologic changes observed in cells treated with sonicates of H. hepaticus were also associated with cell cycle arrest, the DNA content of treated cells was determined by flow cytometry. In untreated cell monolayers, the fraction of cells with a DNA content of 4N was consistently 8 to 10% (Fig. 5A). In monolayers treated with sonicates of H. hepaticus, an increase in the fraction of cells with a DNA content of 4N was seen 24 h after sonicate addition (Fig. 5D). The fraction of cells with a DNA content of 4N increased at 48 h and reached a maximum by 72 after sonicate addition (Fig. 5G and J). In addition, by 72 h, there was a significant fraction of cells with a DNA content of 8N among cells treated with H. hepaticus sonicates. Examination of the size of these cells, as judged by fluorescent width, indicates that these are probably multinucleated cells, as opposed to cellular aggregates. This is consistent with the observation that multinucleated cells were present in treated monolayers (Fig. 3).
FIG. 5.
Time dependence of DNA content and cytopathic effect of HeLa cells treated with bacterial sonicates with CDT activity. Compared to control cells (A), cells treated with sonicates from H. hepaticus (D, G, and J) showed a progressive increase in the fraction of cells with a DNA content of 4 N, with a maximal effect being reached 72 h after sonicate addition (J). Compared to control cells (B and C), cells treated with sonicates from an E. coli strain harboring the cloned H. hepaticus cdt locus showed a progressive increase in size with accumulations of polymerized actin (E, H, and K) and progressive nuclear abnormalities (F, I, and L). These changes in morphology mirrored the progressive cell cycle block observed by flow cytometry. Magnification, ×400. PI, propidium iodide.
Cytopathic activity of E. coli carrying the cdtABC gene cluster from H. hepaticus.
Sonicates of the 15 E. coli clones harboring the recombinant plasmids generated from the genomic library screen were examined for a cytopathic effect on HeLa cell monolayers. Sonicates of 3 of the 15 clones produced a cytopathic effect on cultured HeLa cell monolayers which was indistinguishable from that produced by sonicates of H. hepaticus. Representative clones were selected for further characterization. HeLa cell monolayers treated with sonicates of E. coli carrying the H. hepaticus cdt locus were examined for cytoskeletal and nuclear rearrangements over time by fluorescence microscopy. At 24 h after sonicate addition, nuclear fragmentation was observed (Fig. 5F) but the majority of cells still had a normal size and actin ultrastructure. By 48 h, more nuclear abnormalities were observed (Fig. 5I) and cell distension became apparent, along with an increase in the amount of F-actin (Fig. 5H). At 72 h, the cells in monolayers treated with sonicates of an E. coli CDT clone (Fig. 5K and L) were indistinguishable from those treated with H. hepaticus sonicates (Fig. 3).
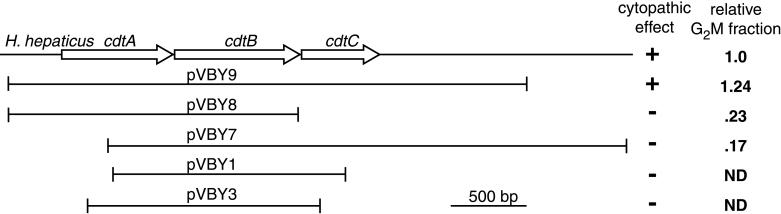
Cell cycle analysis demonstrated that the cytopathic effect produced by the E. coli clones was also accompanied by G2/M cell cycle arrest (Fig. 6). Mapping of the inserts from a number of clones demonstrated that induction of a cytopathic effect and cell cycle arrest required the presence of the entire cdtABC gene cluster (Fig. 6).
FIG. 6.
Cytolethal distending toxin activity exhibited by sonicates of E. coli clones carrying plasmids containing various sections of the H. hepaticus cdtABC gene cluster. The ability of sonicates of each clone to cause a typical CDT cytopathic effect and cell cycle arrest in cultured HeLa cell monolayers is indicated. Cell cycle arrest is indicated by the percentage of cells in G2/M relative to the percentage seen in monolayers treated with H. hepaticus sonicates. Only sonicates of a clone (pVBY9) that carries a plasmid with the entire cdtABC gene cluster were able to cause a cytopathic effect or cell cycle arrest. ND, not done.
DISCUSSION
In this study, we identified and characterized CDT activity in the EHS H. hepaticus. The term CDT was coined to describe an activity in culture supernatants of certain E. coli strains, as well as C. jejuni, that causes progressive distension and eventually death of cultured mammalian cells (21, 22). We demonstrate here that sonicates of H. hepaticus induce G2/M cell cycle arrest in cultured HeLa cells and cause progressive cellular enlargement of HeLa cells, accompanied by the appearance of abnormal accumulations of F-actin. Coupled with the presence of DNA sequences homologous to the cdtABC gene cluster from C. jejuni and the cytopathic activity of E. coli strains carrying the cloned H. hepaticus genes, these results indicate that H. hepaticus possesses a toxin that is a novel member of the CDT family.
The predicted CDT gene products from H. hepaticus had the closest homology to the CDT from C. jejuni. As reported previously (27, 31), the greatest homology is seen in the CdtB amino acid sequence. This may indicate a conserved function for the CdtB subunit; however, our results are in agreement with others indicating that all three gene products are necessary for cytotoxic activity in laboratory strains of E. coli.
The exact role of CDT in pathogenesis has not been clearly determined. It has been proposed that CDT plays a role in the pathogenesis of diarrheal illness. CDT activity in E. coli was originally described in clinical isolates associated with gastroenteritis (22). A study of children with acute diarrhea showed a trend toward an increased rate of isolation of CDT-producing E. coli among children with diarrhea compared to controls, but this did not reach statistical significance (2). It should be noted that several different virotypes of E. coli are associated with diarrheal illness, each with distinct virulence determinants (24). The presence of CDT in a particular E. coli strain may represent only one of several virulence factors required for gastrointestinal pathogenesis. Conversely, CDT has been demonstrated in all C. jejuni isolates, as well as in other members of the genus Campylobacter (14, 27). Demonstration of a role for CDT in gastrointestinal pathogenesis mediated by C. jejuni has not been reported. The well-characterized colonization models for C. jejuni may not be optimal for establishing the contribution of CDT to disease outcome, since they do not reproduce the clinical syndrome of gastroenteritis associated with C. jejuni infection. Partially purified preparations of the CDT from S. dysenteriae expressed in a laboratory strain of E. coli have been shown to induce watery diarrhea in suckling mice (25). However, the role of CDT in intact S. dysenteriae or in diarrheagenic E. coli has not been demonstrated. An isogenic H. ducreyi cdtC mutant has been shown to maintain virulence in the temperature-dependent rabbit model of experimental chancroid (30).
CDT is a candidate virulence factor in the EHS H. hepaticus that may play a role in the pathogenesis of gastrointestinal disease caused by these organisms. In mice, infection with H. hepaticus is associated with a proliferative typhlitis and proliferative hepatitis. In vitro, CDT appears to induce cell cycle arrest, which suggests that the true targets of CDT activity are not enterocytes or hepatocytes. It is possible that CDT causes arrest of a cell type that inhibits epithelial cell proliferation. Alternatively, CDT in H. hepaticus may have a role in modulation of the immune response that allows persistence of the organism. However, it should be noted that CDT activity and nucleotide sequence homology do not appear to be present in all EHS that cause gastroenteritis. We have identified CDT activity and a homologue of the cdtB locus in the EHS H. pullorum but have failed to demonstrate either CDT activity or DNA homology to cdtB in the EHS H. cinaedi or H. fennelliae (data not shown). It also remains to be determined if CDT is a candidate virulence determinant for any of the gastric Helicobacter species, but CDT homology is not present in the genomic sequence of H. pylori (4, 33).
Another cytotoxic activity called granulating cytotoxin has been described previously in H. hepaticus (32). This activity is distinct from the Vac characterized in H. pylori. This cytotoxic activity can be demonstrated on the CCL9.1 mouse liver cell line and is characterized by the appearance of cytoplasmic granules in intoxicated cells. The role of this toxin in pathogenesis is also unknown. Other cell lines, including HeLa cells, do not display cytopathic effects when treated with granulating cytotoxin. The lack of effect on HeLa cells, which were used to demonstrate CDT activity, indicates that the previously described granulating cytotoxin is distinct from the CDT activity described here.
We are currently characterizing CDT genes and activities in other Helicobacter species. We have also developed methods for targeted gene disruption in EHS (unpublished results). By using these techniques and our well-characterized small-animal models of EHS infection and disease (9, 17, 37), we expect to define the role of CDT in the pathogenesis of Helicobacter-associated experimental inflammatory bowel disease and hepatic disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI01398 to V.B.Y. and DK52413 to D.B.S. from the National Institutes of Health.
We thank James G. Fox and Stephen B. Calderwood for critical review of the manuscript and Glenn Paradis for help with flow cytometry.
REFERENCES
- 1.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Albert M J, Faruque S M, Faruque A S, Bettelheim K A, Neogi P K, Bhuiyan N A, Kaper J B. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J Clin Microbiol. 1996;34:717–719. doi: 10.1128/jcm.34.3.717-719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allos B M, Blaser M J. Campylobacter jejuni and the expanding spectrum of related infections. Clin Infect Dis. 1995;20:1092–1099. doi: 10.1093/clinids/20.5.1092. [DOI] [PubMed] [Google Scholar]
- 4.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 5.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atabay H I, Corry J E, On S L. Identification of unusual Campylobacter-like isolates from poultry products as Helicobacter pullorum. J Appl Microbiol. 1998;84:1017–1024. doi: 10.1046/j.1365-2672.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 7.Bouzari S, Varghese A. Cytolethal distending toxin (CLDT) production by enteropathogenic Escherichia coli (EPEC) FEMS Microbiol Lett. 1990;59:193–198. doi: 10.1016/0378-1097(90)90055-u. [DOI] [PubMed] [Google Scholar]
- 8.Burnens A P, Stanley J, Morgenstern R, Nicolet J. Gastroenteritis associated with Helicobacter pullorum. Lancet. 1994;344:1569–1570. doi: 10.1016/s0140-6736(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 9.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 14.Eyigor A, Dawson K A, Langlois B E, Pickett C L. Cytolethal distending toxin genes in Campylobacter jejuni and Campylobacter coli isolates: detection and analysis by PCR. J Clin Microbiol. 1999;37:1646–1650. doi: 10.1128/jcm.37.5.1646-1650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J G. The expanding genus of Helicobacter: pathogenic and zoonotic potential. Semin Gastrointest Dis. 1997;8:124–141. [PubMed] [Google Scholar]
- 16.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J G, Yan L L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hailey J R, Haseman J K, Bucher J R, Radovsky A E, Malarkey D E, Miller R T, Nyska A, Maronpot R R. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol Pathol. 1998;26:602–611. doi: 10.1177/019262339802600503. [DOI] [PubMed] [Google Scholar]
- 21.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 23.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 27.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley J, Linton D, Burnens A P, Dewhirst F E, On S L, Porter A, Owen R J, Costas M. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology. 1994;140:3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 29.Steinbrueckner B, Haerter G, Pelz K, Weiner S, Rump J A, Deissler W, Bereswill S, Kist M. Isolation of Helicobacter pullorum from patients with enteritis. Scand J Infect Dis. 1997;29:315–318. doi: 10.3109/00365549709019053. [DOI] [PubMed] [Google Scholar]
- 30.Stevens M K, Latimer J L, Lumbley S R, Ward C K, Cope L D, Lagergard T, Hansen E J. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect Immun. 1999;67:3900–3908. doi: 10.1128/iai.67.8.3900-3908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor N S, Fox J G, Yan L. In-vitro hepatotoxic factor in Helicobacter hepaticus, H. pylori and other Helicobacter species. J Med Microbiol. 1995;42:48–52. doi: 10.1099/00222615-42-1-48. [DOI] [PubMed] [Google Scholar]
- 33.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 34.von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161:5673–5680. [PubMed] [Google Scholar]
- 35.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 36.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 37.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young V B, Falkow S, Schoolnik G K. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;116:197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]