Abstract
The development of an effective preerythrocytic vaccine against Plasmodium falciparum malaria is likely to require inclusion of components from several preerythrocytic antigens. The association of HLA-B53 with resistance to severe malaria in West Africa provided evidence that HLA class I-restricted CD8+ T-cell responses play a role in protective immunity in African children, supporting data from rodent models of malaria. Previously, a single epitope from liver-stage-specific antigen 1 (LSA-1) has been shown to be recognized by HLA-B53-specific cytotoxic T lymphocytes (CTL), but HLA-B53 epitopes were not found in four other antigens. In this study we measured CTL responses to peptides from the recently sequenced antigen liver-stage antigen 3 (LSA-3) and identified in it a new epitope restricted by HLA-B53. Several CTL epitopes restricted by other class I types were also identified within LSA-3 in studies in The Gambia and Tanzania. CTL were also identified to an additional P. falciparum antigen, exported protein 1 (Exp-1), the homologue of which is a protective antigen in a rodent model of malaria. These findings emphasize the diversity of P. falciparum antigens recognized by CD8+ T cells in humans and support the inclusion of components from several antigens in new CTL-inducing vaccines against malaria.
Preerythrocytic immunity to Plasmodium falciparum infection is mediated in part by T lymphocytes acting against the liver-stage parasite. These T cells must recognize parasite-derived peptides on infected host cells in the context of major histocompatibility complex antigens. T-cell-mediated immunity appears to target several parasite antigens expressed during the sporozoite and liver stages of the infection (13). Complete protection against sporozoite challenge observed in irradiated Plasmodium berghei sporozoite-immunized mice and P. falciparum sporozoite-immunized humans results from immune responses to antigens expressed by the parasite at the preerythrocytic stages of its life cycle (20). Although antibody and CD4+ and CD8+ T cells all have been implicated in preerythrocytic immunity, protection mainly or entirely dependent on CD8+ T cells (25, 27) is found in several rodent host-parasite combinations. Recently, it has been found that it is possible to immunize perforin- and Fas-deficient mice with irradiated Plasmodium berghei sporozoites, indicating that CD8+ T-cell-mediated protection against this parasite is probably not related to the lytic functions of these cells (23). However, in humans, lysis assays correlate well with other measures of CD8+ T-cell function, such as gamma interferon (IFN-γ) secretion (15).
We (1, 12, 17, 21) and others (4, 6, 7, 26) have previously identified in malaria-endemic areas several epitopes of cytotoxic T-lymphocytes (CTL), restricted by a variety of HLA class I molecules, in each of five preerythrocytic P. falciparum antigens: circumsporozoite protein, thrombosponding related adhesion protein, liver-stage antigen 1 (LSA-1), Pfs16, and sporozoite threonine and asparagine-rich protein. These CTL recognize antigens presented by recombinant vaccinia viruses (2) and are present in the peripheral blood at measured precursor frequencies of 17 to 98 per million peripheral blood mononuclear cells (21). In this study, we extend this work to ask whether, through natural malaria exposure, CTL are induced by two additional preerythrocytic antigens of P. falciparum that have recently been advocated as promising vaccine candidates, liver-stage antigen 3 (LSA-3) (18; P. Druihle, unpublished data) and exported protein 1 (Exp-1) (5, 14).
LSA-3 is a 1,786-amino-acid protein in the K1 strain of P. falciparum. This antigen was selected for study based on its abundance on both sporozoites and liver-stage parasites and its consistent recognition by sera from humans protected from sporozoite challenge following irradiated sporozoite immunization, whereas sera from similarly immunized but nonprotected individuals did not recognize the antigen (P. Druihle, unpublished data). LSA-3 is expressed on sporozoites and in liver-stage but not blood-stage parasites and contains a central repeat region which defines one of the B-cell epitopes recognized by antibodies from individuals in malaria-endemic areas. Proliferative T helper cell responses to peptides from LSA-3 in 84% of naturally exposed West Africans have been observed (P. Druihle, unpublished data), and these responses were found to correlate with IFN-γ secretion. Although polymorphic in length in the central repeat region, the large 5′ and 3′ regions of LSA-3 are markedly conserved between the K1 strain and the T9/96 strain of P. falciparum, in contrast to many malarial antigens and some of the other preerythrocytic antigens.
Exp-1, a P. falciparum antigen, was originally described by Hope and colleagues (14) as a 23-kDa secreted blood-stage malaria antigen, Ag5.1. This antigen was found to accumulate at the membrane of the parasitophorous vacuole and other compartments associated with it in infected erythrocytes (14). This antigen possesses a B-cell epitope (amino acids 120 to 137) with sequence homology to the tandem tetrapeptide repeat of P. falciparum CSP, and a monoclonal antibody, McAb5.1, was found to recognize this region in both CSP and Exp-1 (14). Sanchez et al. (24) showed that hepatocytes of mice immunized with recombinant Exp-1 expressed the antigen late in the liver stage of the infection, raising the possibility that peptides from Exp-1 could be processed and expressed on the hepatocyte surface in the context of HLA class I molecules in humans and thus become targets for CTL recognition. Several peptides from this antigen have recently been found to be frequently recognized by CTL from irradiated sporozoite-immunized volunteers, but responses in a Kenyan population were much weaker (6). Doolan et al. (8) found that immunization with a DNA vaccine encoding the P. yoelii homologue of Exp-1, PyHEP17 (5), induced significant protection against sporozoite challenge in several strains of mice and suggested that Exp-1 might be a protective antigen in P. falciparum.
MATERIALS AND METHODS
Peptides.
By using published peptide binding motifs for HLA molecules (22), short peptides (8 or 9 amino acids long) with binding motifs for molecules HLA-A2, HLA-B8, HLA-B53, and HLA-B58 were identified from the amino acid sequences of LSA-3 and Exp-1. No peptides with a clear HLA-B35 binding motif were identified in either antigen. Peptides were synthesized by F-moc chemistry with the Zinsser Analytic automatic synthesizer. Freeze-dried peptides were dissolved in 10 to 20 μl of dimethyl sulfoxide, and phosphate-buffered saline was added to bring the dimethyl sulfoxide concentration to 0.01%. The peptide concentration was determined by a micro-bicinchoninic acid protein assay (Pierce, Rockford, Ill.). All peptides were confirmed to be >70% pure by high-performance liquid chromatography analysis.
Peptide binding studies.
Peptide binding to class I HLA molecules was determined by using the HLA assembly assay described by Elvin et al. (9). Briefly, radiolabeled T2 cells transfected with the relevant HLA molecule were lysed in the presence of peptide. The HLA-peptide complex formed was immunoprecipitated with conformation-dependent antibody W6/32 and electrophoresed on an isoelectric focusing gel. HLA assembly, measured by the amount of radioactivity, was quantitated by autoradiography. Chinese hamster ovary cells transfected with HLA-B8 (CHO-B8) were used for HLA-B8 assembly assays as described by McAdam et al. (19).
Donors.
Adult volunteers from the village of Brefet, The Gambia, and a smaller number of adult blood donors from the capital of The Gambia, Banjul, were studied from 1994 to 1996. Also, a small number of healthy Tanzanian adults were evaluated as part of a study described previously (17). All of the donors were residents of these malaria-endemic areas, but none was parasitemic at the time of blood sampling. This study was approved by the Gambian government-MRC joint ethical committee.
CTL restimulation.
Peptides were initially tested combined in groups, or pools. The number of peptides in a pool used to restimulate peripheral blood lymphocytes (PBL) varied with the number of peptides synthesized for a particular HLA molecule. The rationale for testing many peptides in a pool was that any responses identified would be those to a single or few relatively immunodominant peptides. Thus, for example, 49 HLA-A2 peptides were divided into four pools, three pools with 12 peptides each and one pool with 13 peptides. For HLA-B53 where only three peptides were synthesized, all three were used in a single pool. There were three pools of peptides each for HLA-B8 and HLA-B58. HLA-B8 pools had seven peptides in each pool. For HLA-B58, two of the three pools had 10 peptides each and the third had 11 peptides. In the pools each peptide was used at a concentration of 20 μM, and PBL from malaria-immune donors were incubated with one or many peptides, each at 20 μM, in a small volume (100 μl) of tissue culture medium R10 (RPMI 1640 [Sigma] supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 4 mM l-glutamine [GIBCO], and 10% fetal calf serum) for 1 h at 37°C in the presence of 5% CO2 and humidity. Cells were grown at a concentration of 1 × 106 to 1.5 × 106/ml in R10 in 24-well tissue culture plates (Falcon). Plates were incubated in a humidified incubator at 37°C and 5% CO2. Human recombinant interleukin 2 (10 U/ml; Cetus) was added to the cultures after 72 h, and cytotoxic assays were performed on day 8. Restimulated cells not used for the week 1 assay were maintained on a weekly dose of 10 U of IL-2 per ml until tested for cytotoxicity.
Target cells.
Target cells were Epstein-Barr virus-transformed autologous or HLA-matched B-cell lines. Target cells (1 × 106 to 2 × 106) were radioactively labeled with 100 μCi of 51Cr for 1 h at 37°C, washed once in R10, and pulsed with 10 to 20 μM of peptide for an additional 1 h. Cells were washed once, diluted in R10 to 105/ml, and plated at 50 μl/well in 96-well round-bottom tissue culture plates. 51Cr-labeled target cells not pulsed with peptide were also set up as nonpeptide-specific controls.
51Cr release assay.
Cytotoxicity assays were performed as described previously (12) on day 8 and again at 2 and 3 weeks if sufficient effector cells were available. All assays were performed at a standardized effector-to-target-cell ratio of 50:1. Briefly, 5 × 103 target cells were incubated with CTL in duplicate, with only R10 medium in quadruplicate, and with 5% Triton X-100 in quadruplicate. All wells had a total volume of 150 μl in round-bottom 96-well tissue culture plates. Plates were incubated at 37°C, and radioactivity in the supernatant from each well was measured after 4 h. Supernatant (20 μl) was transferred onto spot-on filter mats (Wallac, Oy Turku, Finland) and dried, and radioactivity was measured in a betaplate scintillation counter (Wallac LKB 1205). The percentage of 51Cr release was calculated as described previously (12, 21). A positive result was defined as at least 10% more lysis of peptide-pulsed target cells than peptide-unpulsed target cells, provided that lysis with peptide was at least double that without peptide, an operational definition of positivity based on extensive experience with malaria CTL assays for this population. A result of 10% lysis was used as the cut off for statistical significance at the 5% level based on analysis of the magnitude of counts in duplicate wells, in accordance with established convention. Spontaneous 51Cr release in the absence of CTL was always less than 25% of maximum release by 5% Triton X.
Sequence analysis.
A 580-bp fragment of LSA-3 was PCR amplified with the primers AAAGAAGAGGTTAAAGAAGAACCAAAG (5′) and TGAAGAAGCTATTGTTACACATGATGA (3′). The PCR mixture contained 10 mM Tris (pH 8.3), 3.5 mM MgCl2, 75 mM KCl, 0.2 mM (each) deoxynucleoside triphosphates, 0.5 μM (each) primer, and 1 U of Taq gold. Cycling conditions were 1 cycle of 84°C for 18 min followed by 34 cycles of 94°C for 10 s, 56°C for 30 s, and 72°C for 60 s. The PCR products were cloned into pGEM-T (Promega) and sequenced with M13 forward and reverse primers on an ABI 373 sequencer.
RESULTS
Peptides.
Sequences of 8 or 9 amino acids were selected to match the peptide binding motifs for the class I molecules HLA-A2, HLA-B8, HLA-B53, and HLA-B58, and 104 peptides from LSA-3 and 26 peptides from Exp-1 were synthesized. Because of this large number and the limited volume of blood available from each donor, peptides were initially tested after being pooled according to HLA type.
HLA-A2-restricted CTL responses.
Screening for responses to pools of LSA-3 peptides identified two Gambian individuals (Table 1) with positive CTL responses from 12 donors studied. CTL studies were also carried out in Ifakara, Tanzania, an area of high malaria transmission in Tanzania, using pools of LSA-3 peptide to which CTL responses had been demonstrated in The Gambia. Of two individuals with HLA-A2 tested, one had strong CTL responses to two sets of peptides (Table 1).
TABLE 1.
Screening of CTL responses to peptide pools consisting of peptides synthesized according to peptide binding motifs of common African HLA class I molecules
| Donor | LSA-3 Peptide pool | % specific lysisd |
|---|---|---|
| Z24a | HLA-A2 pool A | 27 |
| Z26a | HLA-A2 pool A | 25 |
| F28c | HLA-A2 pool A | 26 |
| F28c | HLA-A2 pool D | 39 |
| Z42a | HLA-B8 pool A | 11 (×2)b |
| Z42a | HLA-B8 pool B | 17 |
| Z175a | HLA-B8 pool B | 22 |
| Z14a | HLA-B8 pool B | 10 |
| F28c | HLA-B8 pool A | 10 |
| F28c | HLA-B8 pool B | 24 |
| F28c | HLA-B8 pool C | 26 |
Donors from The Gambia.
Z42 responded on two separate occasions to LSA3 B8 pool A.
Donors from Tanzania.
Percent specific lysis was calculated by using duplicate test wells with differences in chromium release of not more than 25%.
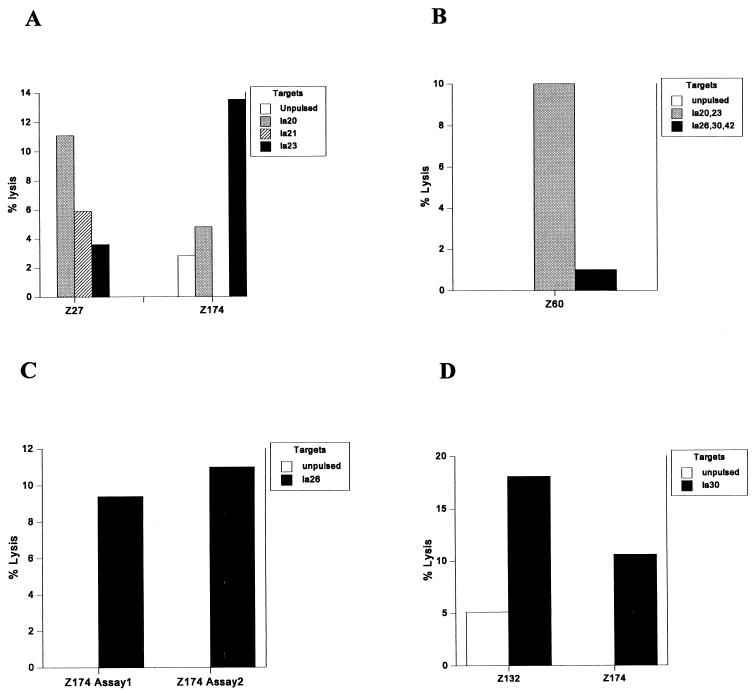
Fourteen Gambian HLA-A2 donors were studied 10 months after the initial CTL testing in order to identify the particular peptide epitope(s) within the pools. Restimulated PBL from donor Z27 of HLA subtype HLA-A*0202 showed 11% specific lysis on target cells pulsed with la20 (Fig. 1A). PBL from another donor, Z174, with the same HLA subtype lysed target cells pulsed with la23 (11%) (Fig. 1A) but not target cells pulsed with either la20 or la21. Restimulated PBL from another HLA-A2 donor, Z60, lysed target cells pulsed with the pool consisting of peptides la20, la21, and la23 (10%) (Fig. 1B), but it was not possible to test this response on single peptides due to the limited number of cells available. In separate assays, PBL from donor Z174 restimulated with other peptides also lysed target cells pulsed with la26 (11%) and la30 (10%) (Fig. 1C and D, respectively). Cells from another donor, Z132, subtyped as HLA-A*0205 also lysed target cells pulsed with la30 (13% specific lysis) (Fig. 1D). Interestingly, all of the peptides found to be HLA-A2 epitopes bound to HLA-A*0201 in peptide binding assays (Table 2), even though the subtype specificity of the response was often through other HLA-A2 subtypes.
FIG. 1.
CTL responses to HLA-A2 binding peptides from LSA-3 by HLA-A2 donors from The Gambia. Cells were tested at an effector-to-target-cell ratio of 50:1, and the percent lysis was calculated from duplicate wells (each) of peptide-pulsed and peptide-unpulsed target cells.
TABLE 2.
CTL epitopes identified in LSA-3 and Exp-1
| Peptide | HLA restriction | Sequence | Position | Binding assay | No. of responders/no. tested |
|---|---|---|---|---|---|
| la20 | HLA-A2 | DLLEEGNTL | 111–119 | + | 2/14 |
| la23 | HLA-A2 | KLEELHENV | 893–901 | + | 1/14 |
| la26 | HLA-A2 | VLDKVEETV | 981–989 | + | 1/14 |
| la30 | HLA-A2 | GLLNKLENI | 1060–1068 | + | 2/14 |
| la72 | HLA-B8 | MEKLKELEK | 1260–1268 | + | 1/6 |
| la90 | HLA-B53 | EPKDEIVEV | 1524–1532 | + | 1/6 |
| ex23 | HLA-B58 | ATSVLAGL | 77–84 | NDa | 2/6 |
ND, not done.
HLA-B8-restricted CTL responses.
In screening assays, with 21 peptides synthesized to conform to the HLA-B8 binding motif grouped in pools, three of five HLA-B8 individuals from Brefet, The Gambia, responded to one or more pools of peptides (Table 1). One of the two HLA-B8 donors tested in Ifakara responded, and this donor's cells recognized target cells pulsed with all three HLA-B8 LSA-3 peptide pools (pools A, B, and C) (Table 1). Thus, as found for HLA-A2 in both The Gambia and Ifakara and for HLA-B8 in The Gambia, individuals who showed positive CTL responses often responded to more than one peptide pool. In this Tanzanian individual, specific lysis was 10, 24, and 28% for pools A, B, and C, respectively.
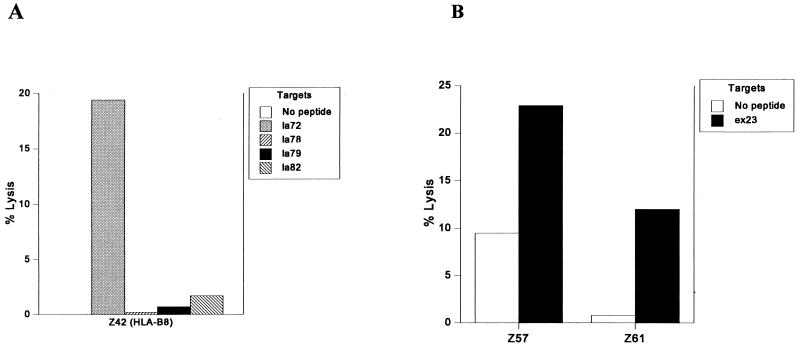
Of six Gambian HLA-B8 donors tested the following year for CTL responses to determine peptide epitopes within the peptide pools, one donor (Z42) made a response (19%) to a single peptide la72 (Fig. 2A). Z42 had previously responded twice (11% specific lysis on each occasion) to LSA-3 HLA-B8 pool A, a pool of seven peptides including only one HLA-B8 binding peptide, la72, and once (17% specific lysis) to LSA-3 HLA-B8 pool B, also consisting of seven peptides including the other HLA-B8 binding peptides la78, la79, and la82. We were unable to test HLA-B8 peptide pool C for binding.
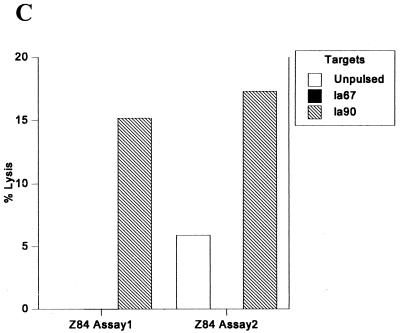
FIG. 2.
CTL responses to LSA-3 peptides by HLA-B8 (A) and HLA-B53 (C) donors from The Gambia. (B) Responses to the liver-stage and blood-stage antigen Exp-1 by two HLA-B58 Gambian adults. Effector-to-target-cell ratio was 50:1, and the percent specific lysis was calculated from duplicate wells (each) of peptide-pulsed and peptide-unpulsed target cells.
HLA-B58-restricted CTL responses.
Five of 18 peptides with the HLA-B58 binding motif synthesized from LSA-3 had the ability to induce assembly of HLA-B58 (data not shown). However, no HLA-B58-specific CTL responses to LSA-3 were identified in the study. Four peptides with the binding motif for HLA-B58 were synthesized according to the sequence of Exp-1 and tested in six Gambian adults with this HLA type. Two donors (Z57 and Z61) responded to peptide ex23 (13 and 11% lysis, respectively) (Fig. 2B). One of these donors (Z61) had previously been found to respond to an HLA-B58-restricted epitope in the antigen LSA-1 (1). ex23 overlaps two recently described peptide epitopes, Exp-180 and Exp-183, by five and two amino acids, respectively (6). CTL to these peptides were detected in an irradiated-sporozoite-immunized volunteer but not in naturally exposed individuals.
HLA-B53-restricted CTL responses.
Three peptides were identified and synthesized with the peptide binding motif for HLA-B53. Six donors with this HLA type from Brefet, The Gambia, were studied, and one donor, Z84, made a repeatable lytic response (15 and 11%) to the peptide la90 (Fig. 2C). This peptide was subsequently shown to bind to HLA-B53 in a binding (assembly) assay (Table 2).
Sequence analysis of the HLA-B53 LSA-3 epitope.
To assess the extent of polymorphism in the HLA-B53-restricted epitope la90, this epitope was sequenced in DNA samples from 18 Gambian children with malaria. Only a single base change at the third position, CCA→CCC, of the codon specifying the proline residue at position 2 of the peptide epitope was found in 12 of the 18 individuals. This change does not alter the amino acid sequence.
To address the question of possible cross-reactive CTL responses, we ran all epitopes identified in this study through the BLAST search in GenBank and did not find any non-malaria-associated organism with similar sequences.
DISCUSSION
The finding of CTL responses to both LSA-3 and Exp-1 in Africans naturally exposed to malaria supports suggestions that these antigens may play a role in protective immunity to P. falciparum. Previous evidence for a protective role of LSA-3 has come from studies in the chimpanzee model of P. falciparum infection and from the frequency of recognition of this antigen by naturally exposed Africans in antibody and proliferative T-cell assays (3; P. Druihle, unpublished data). The homologue of LSA-3 in rodent malaria parasites has not been identified, but the P. yoelii homologue of PfExp-1, known as PyHEP17, has been shown to be a protective antigen in some strains of mice (8).
The present report brings to seven the number of P. falciparum preerythrocytic antigens that have been identified as targets of CTL responses. Indeed, CTL responses to all preerythrocytic antigens that have been investigated in detail have been detected. This finding diminishes the prospect of including all identified CTL target antigens in subunit vaccines and suggests that the actual number of target antigens that are naturally recognized might be quite large.
Reviewing the studies performed to date in The Gambia suggests that all these target antigens may not be recognized equally. Numerous epitopes have been identified in P. falciparum thrombosponding related adhesion protein (1) and now several in LSA-3, and these antigens appear to be recognized rather frequently. The circumsporozoite protein appears to have the most polymorphic CTL epitopes, but polymorphism in this antigen has been investigated more thoroughly than in the others. The lower frequency of responses to Exp-1 and Pfs16 may simply reflect the smaller sizes of these antigens. LSA-3 is the largest preerythrocytic antigen investigated to date, which may in part explain its relatively frequent recognition by CTL.
In keeping with previous reports, the proportion of individuals studied who showed positive CTL responses was low both in The Gambia and in Tanzania. This in part reflects the low precursor frequency of malaria CTL in malaria-endemic populations in general (1, 4, 6, 7, 12, 17, 21, 26) compared to the higher levels observed in irradiated-sporozoite-immunized volunteers (6, 28, 29). The relative insensitivity of chromium release assays also reduces the observed positivity rate, and preliminary data on the use of IFN-γ ELISPOT assays for detection of human CD8+ T cells suggest that this may be a more useful screening approach (16; M. Plebanski et al., unpublished data).
We noted a tendency for individuals who did have detectable CTL to respond to more than one CTL epitope, as seen in some previous studies (17). Whether this reflects individual variability in CTL precursor frequencies or other factors is unclear. The detection of CTL responses restricted by multiple subtypes of HLA-A2 emphasizes the importance of precise subtyping of class I alleles prior to matching of effectors and target cell lines.
A search of GenBank database sequences revealed no homologies with the new epitopes described here. However, we cannot rule out the possibility that cross-reactive sequences from other infectious pathogens may exist. Analysis of malaria-naïve individuals might give some insight into possible cross-reactive epitopes, but the low malaria CTL response rate observed even in malaria-endemic settings would require a very large sample size to show a difference in response rate in such naïve controls.
Due to the limited number of cells available, we were unable to test for possible HLA class II restriction. To overcome this limitation all epitopes were shown to bind the relevant class I allele, and for the majority of our experiments we matched effector and target cells only at a single class I locus. In the few cases where we used autologous target cells, however, there is a remote possibility that some of the responses observed may be HLA class II restricted. However, for this to occur would require an unprecedented nonamer malaria epitope which binds to both HLA class I and class II molecules and can induce cytotoxicity.
We have proposed previously that the association of HLA-B53 with resistance to severe malaria in The Gambia (11), together with the identification of HLA-B53-restricted CTL responses to an epitope in LSA-1, gave priority to the selection of this antigen for inclusion in CTL-inducing subunit vaccines against malaria (12). Over the last few years it has become apparent that several malaria preerythrocytic antigens are recognized by CTL and that several epitopes may be recognized by the CD8+ T cells from a single individual. In this study we identify an additional HLA-B53 epitope, which, in this case, is present in the antigen LSA-3. Thus, the case for inclusion of LSA-1 in subunit vaccines might now be applied equally to LSA-3. However, although the HLA-B53-restricted epitope ls6 in LSA-1 is strongly conserved (12, 30), further information is required on the extent of conservation of the HLA-B53-restricted epitope la90 in LSA-3. Despite the small (n = 18) number of parasite samples sequenced in this study, the available data suggest that, at least in parasites from The Gambia, la90 shows limited variation.
The increasing number of CTL target antigens in P. falciparum will preclude the incorporation of all of these in most currently available vectors. An alternative approach is to design epitope-based rather than antigen-based vaccines that include epitopes restricted by a range of common HLA types. This approach allows conserved epitopes to be chosen and is now being pursued for P. falciparum with the incorporation of CTL and other epitopes from a variety of preerythrocytic antigens (10). The epitopes identified here will allow components from two additional potentially protective antigens to be included in these new polyepitope candidate vaccines.
ACKNOWLEDGMENTS
We thank all the blood donors for their participation, M. Sanyang and P. Kibatala for assistance, and B. Greenwood and M. Tanner for encouragement and support.
This study was funded by the Wellcome Trust, the MRC, and EC-INCO-DC. A.V.S.H. is a Wellcome Trust Principal Research Fellow.
REFERENCES
- 1.Aidoo M, Lalvani A, Allsopp C E, Plebanski M, Meisner S J, Krausa P, Browning M, Morris Jones S, Gotch F, Fidock D, Takiguchi M, Robson K J, Greenwood B M, Druilhe P, Whittle H C, Hill A V S. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet. 1995;345:1003–1007. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 2.Aidoo M, Lalvani A, Whittle H C, Hill A V, Robson K J. Recombinant vaccinia viruses for the characterization of Plasmodium falciparum-specific cytotoxic T lymphocytes: recognition of processed antigen despite limited re-stimulation efficacy. Int Immunol. 1997;9:731–737. doi: 10.1093/intimm/9.5.731. [DOI] [PubMed] [Google Scholar]
- 3.BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. 1997;27:1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- 4.Blum-Tirouvanziam U, Servis C, Habluetzel A, Valmori D, Men Y, Esposito F, Del Nero L, Holmes N, Fasel N, Corradin G. Localization of HLA-A2.1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J Immunol. 1995;154:3922–3931. [PubMed] [Google Scholar]
- 5.Doolan D L, Hedstrom R C, Rogers W O, Charoenvit Y, Rogers M, de la Vega P, Hoffman S L. Identification and characterization of the protective hepatocyte erythrocyte protein 17 kDa gene of Plasmodium yoelii, homolog of Plasmodium falciparum exported protein 1. J Biol Chem. 1996;271:17861–17868. doi: 10.1074/jbc.271.30.17861. [DOI] [PubMed] [Google Scholar]
- 6.Doolan D L, Hoffman S L, Southwood S, Wentworth P A, Sidney J, Chesnut R W, Keogh E, Appella E, Nutman T B, Lal A A, Gordon D M, Oloo A, Sette A. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity. 1997;7:97–112. doi: 10.1016/s1074-7613(00)80513-0. [DOI] [PubMed] [Google Scholar]
- 7.Doolan D L, Houghten R A, Good M F. Location of human cytotoxic T cell epitopes within a polymorphic domain of the Plasmodium falciparum circumsporozoite protein. Int Immunol. 1991;3:511–516. doi: 10.1093/intimm/3.6.511. [DOI] [PubMed] [Google Scholar]
- 8.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ T cell-, interferon γ-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elvin J, Cerundolo V, Elliott T, Townsend A A. A quantitative assay of peptide-dependent class I assembly. Eur J Immunol. 1991;21:2025–2031. doi: 10.1002/eji.1830210909. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert S C, Plebanski M, Harris S J, Allsopp C E, Thomas R, Layton G T, Hill A V S. A protein particle vaccine containing multiple malaria epitopes. Nat Biotechnol. 1997;15:1280–1284. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- 11.Hill A V, Allsopp C E, Kwiatkowski D, Anstey N M, Twumasi P, Rowe P A, Bennett S, Brewster D, McMichael A J, Greenwood B M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 12.Hill A V, Elvin J, Willis A C, Aidoo M, Allsopp C E, Gotch F M, Gao X M, Takiguchi M, Greenwood B M, Townsend A R, McMichael A J, Whittle H C. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman S L, Franke E D, Hollingdale M R, Druilhe P. Attacking the infected hepatocyte. In: Hoffman S L, editor. Malaria vaccine development. Washington, D.C.: American Society for Microbiology; 1997. pp. 35–76. [Google Scholar]
- 14.Hope I A, Hall R, Simmons D L, Hyde J E, Scaife J G. Evidence for immunological cross-reaction between sporozoites and blood stages of a human malaria parasite. Nature. 1984;308:191–194. doi: 10.1038/308191a0. [DOI] [PubMed] [Google Scholar]
- 15.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V S. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalvani A, Hurt N, Aidoo M, Kibatala P, Tanner M, Hill A V S. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur J Immunol. 1996;26:773–779. doi: 10.1002/eji.1830260408. [DOI] [PubMed] [Google Scholar]
- 18.Marchand C, Druilhe P. How to select P. falciparum antigens in an expression library without defined probe. Bull W H O. 1990;68:158–164. [PMC free article] [PubMed] [Google Scholar]
- 19.McAdam S, Klenerman P, Tussey L, Rowland Jones S, Lalloo D, Phillips R, Edwards A, Giangrande P, Brown A L, Gotch F, McMichael A J. Immunogenic HIV variant peptides that bind to HLA-B8 can fail to stimulate cytotoxic T lymphocyte responses. J Immunol. 1995;155:2729–2736. [PubMed] [Google Scholar]
- 20.Nardin E H, Nussenzweig R S. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 21.Plebanski M, Aidoo M, Whittle H C, Hill A V S. Precursor frequency analysis of cytotoxic T lymphocytes to pre-erythrocytic antigens of Plasmodium falciparum in West Africa. J Immunol. 1997;158:2849–2855. [PubMed] [Google Scholar]
- 22.Rammensee H G, Friede T, Stevanovich S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 23.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 1997;19:145–148. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez G I, Rogers W O, Mellouk S, Hoffman S L. Plasmodium falciparum: exported protein-1, a blood stage antigen, is expressed in liver stage parasites. Exp Parasitol. 1994;79:59–62. doi: 10.1006/expr.1994.1060. [DOI] [PubMed] [Google Scholar]
- 25.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 26.Sedegah M, Sim B K, Mason C, Nutman T, Malik A, Roberts C, Johnson A, Ochola J, Koech D, Were B, Hoffman S L. Naturally acquired CD8+ cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. J Immunol. 1992;149:966–971. [PubMed] [Google Scholar]
- 27.White K L, Snyder H L, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156:3374–3381. [PubMed] [Google Scholar]
- 28.Wizel B, Houghten R, Church P, Tine J A, Lanar D, Gordon D M, Ballou W R, Sette A, Hoffman S L. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J Immunol. 1995;155:766–775. [PubMed] [Google Scholar]
- 29.Wizel B, Houghten R A, Parker K C, Coligan J E, Church P, Gordon D M, Ballou W R, Hoffman S L. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J Exp Med. 1995;182:1435–1445. doi: 10.1084/jem.182.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Shi Y P, Udhayakumar V, Alpers M P, Povoa M M, Hawley W A, Collins W E, Lal A A. Sequence variations in the non-repetitive regions of the liver stage-specific antigen-1 (LSA-1) of Plasmodium falciparum from field isolates. Mol Biochem Parasitol. 1995;71:291–294. doi: 10.1016/0166-6851(95)00069-d. [DOI] [PubMed] [Google Scholar]