Abstract
Background
Patients with disorders of consciousness (DoC) are a challenging population prone to misdiagnosis with limited effective treatment options. Among neuromodulation techniques, transcutaneous auricular vagal nerve stimulation (taVNS) may act through a bottom-up manner to modulate thalamo-cortical connectivity and promote patients’ recovery. In this clinical trial, we aim to (1) assess the therapeutic clinical effects of taVNS in patients with DoC; (2) investigate the neural mechanisms underlying the effects of its action; (3) assess the feasibility and safety of the procedure in this challenging population; (4) define the phenotype of clinical responders; and (5) assess the long-term efficacy of taVNS in terms of functional outcomes.
Methods
We will conduct a prospective parallel randomized controlled double-blind clinical trial investigating the effects of taVNS as a treatment in DoC patients. Forty-four patients in the early period post-injury (7 to 90 days following the injury) will randomly receive 5 days of either active bilateral vagal stimulation (45 min duration with 30s alternative episodes of active/rest periods; 3mA; 200-300μs current width, 25Hz.) or sham stimulation. Behavioural (i.e., Coma Recovery Scale-Revised, CRS-R) and neurophysiological (i.e., high-density electroencephalography, hd-EEG) measures will be collected at baseline and at the end of the 5-day treatment. Analyses will seek for changes in the CRS-R and the EEG metrics (e.g., alpha band power spectrum, functional connectivity) at the group and individual (i.e., responders) levels.
Discussion
These results will allow us to investigate the vagal afferent network and will contribute towards a definition of the role of taVNS for the treatment of patients with DoC. We aim to identify the neural correlates of its action and pave the way to novel targeted therapeutic strategies.
Clinical trial registration
Clinicaltrials.gov n° NCT04065386.
Keywords: Coma, Disorders of consciousness, Minimally conscious state, Vegetative state, Unresponsive wakefulness syndrome, Vagal nerve stimulation, Electroencephalography, Randomized clinical trial, Treatment, Behavior
Introduction
Following the emergence from coma, patients with severe acquired brain injuries such as traumatic or anoxic brain injuries may not fully recover initial awareness and arousal levels for a certain period of time (Zeman, 2005) and thus enter the scope of “disorders of consciousness” (DoC). This medical state includes conditions such as the unresponsive wakefulness syndrome (UWS) (Laureys et al., 2010) and the minimally conscious state (MCS) (Giacino et al., 2002). Whilst UWS is defined by the presence of arousal (i.e., eye opening) but reflex behaviors only, MCS is defined by the presence of clearly discernible but fluctuant signs of awareness that can be observed at bedside (e.g., visual pursuit, command following). MCS can be subsequently distinguished in MCS minus (MCS-) and MCS plus (MCS+) depending on the preservation of language-related behaviors (Bruno et al., 2011). However, neither functional communication nor use of objects can be demonstrated at this stage (Giacino et al., 2002). Eventually, when the patient is functionally able to communicate or use objects again, one could say he/she has emerged from the MCS (eMCS), and from a more general point of view, from DoC (Giacino et al., 2002).
Treatment options for severely brain injured patients
An important challenge arising from this population of patients, both in chronic and acute care settings, is the limited therapeutic options. Several pharmacological interventions have been previously described (Thibaut et al., 2019), some of them providing promising results. Nevertheless, among them, amantadine is currently the only recommended medication, resulting in a faster consciousness recovery (Giacino et al., 2012), with potential side effects (e.g., seizure) (Estraneo et al., 2015). Additionally, non-pharmacological approaches (see review (Thibaut et al., 2019) have been studied including non-invasive brain stimulation therapies, such as transcranial direct current stimulation (Angelakis et al., 2018; Thibaut et al., 2019; Zhang & Song, 2018) and repetitive transcranial magnetic stimulation (Naro et al., 2015; Xia et al., 2017). However, these studies have reported small to moderate effect sizes (Thibaut et al., 2019). Thus, up to now, only a few studies have investigated potential therapies for DoC and only amantadine (Giacino et al., 2012) and transcranial direct current stimulation (Thibaut et al., 2014) provided class II evidence regarding treatment efficacy. Moreover, these strategies have never been implemented in the acute setting as most studies enrolled patients with prolonged DoC. Besides, some of these techniques (e.g., transcranial magnetic stimulation) require highly trained personnel and are difficult to integrate in routine care settings. It is therefore necessary to develop new therapeutic approaches that are realistic to include in the routine care of DoC patients in the early period following the injury. Indeed, as compared to the chronic stage, such early time window may be associated with interesting peaking neuroplastic modulations that could benefit the patients, which was suggested in animal models of recovery following cerebral damage (Jones & Schallert, 1992).
Vagal nerve stimulation: a possible therapeutic approach
Transcutaneous auricular vagal nerve stimulation (taVNS) represents a safe, non-invasive and easy to use therapeutic option. The device is usually small, requires minimal instruction to be used correctly and can be easily installed on the patient's ear. This technique has already been documented in numerous studies in healthy subjects and neurologic populations as an alternative to invasive VNS (Badran et al., 2018; Frangos et al., 2015; Garcia et al., 2017; Hamer & Bauer, 2019; Yakunina et al., 2018). Up to now, taVNS studies have reported promising results in patients with prolonged DoC but randomized controlled clinical trials are missing, and the underlying mechanisms of consciousness recovery remain unclear. To date, all taVNS studies in patients with DoC reported clinical improvement for a subset of the patients. In the first taVNS case report, Yu et al. (2017) demonstrated new signs of consciousness in a 73-year-old female with DoC due to cardiac arrest (50 days post-injury) following 30 minutes of twice a day 4-6 mA (20Hz, < 1ms pulse width) bilateral cymba conchae stimulation for four weeks. Following the treatment, fMRI data showed an increase in functional connectivity in the default mode network using the posterior cingulate cortex as a seed (Yu et al., 2017). However, since it is an uncontrolled case report, it cannot be excluded that such improvement could have been due to spontaneous recovery, especially given the acute to subacute context. In the second open-label study, 14 patients with prolonged (12.1 ± 6.4 months post-injury) DoC (n = 6 UWS, 8 MCS; n = 7 TBI, 3 hemorrhage, 4 anoxia) received 30 min of stimulation twice daily for four weeks at 1,5 mA (20Hz, 250μs pulse width) at the left tragus only. One of the MCS patients showed new signs of consciousness at the end of the four weeks of stimulation and four more patients showed new signs of consciousness at the four-week follow-up time point. No brain multimodal technique was used in this protocol. Side effects were described as mild and were considered common medical conditions without obvious relation to taVNS (Noé et al., 2019). In another uncontrolled study, five patients with DoC (56.8 ± 29.9 days post-injury) (n = 3 UWS, 2 MCS; n = 5 TBI) were stimulated over the left ear at 1 mA (25Hz, 250μs pulse width) for eight weeks. Three patients improved for more than three points as measured by the Coma Recovery Scale-Revised (CRS-R) during the experimental course (one UWS to eMCS, one UWS to MCS and one MCS to eMCS). Importantly, no serious side effects were reported, the most common undesired effect observed being skin irritation at the stimulation site (Hakon et al., 2020).
So far, the literature seems to provide evidence that taVNS is feasible and safe for patients with prolonged DoC (i.e., > 28 days post-onset) and that it might be beneficial to some of them through an activation of the thalamo-cortical loop. However, taVNS has never been tested in a controlled double-blind study in patients with DoC in the early period post-injury even given the core importance of this period on neuroplasticity, medical care decisions and survival rate. Therefore, the present study aims to demonstrate that it is also feasible to use taVNS for patients with DoC within 12 weeks post-injury in acute to subacute care units. In addition, we aim to investigate the clinical and neurophysiological effects of taVNS in this population.
The mesocircuit model for severe brain injuries and neural recovery
The fronto-parietal mesocircuit model relies on anatomopathological studies of severe brain injuries that have demonstrated the central role of the anterior forebrain in consciousness degradation following severe brain injuries (Schiff, 2010). This model suggests that all severe brain injuries share a common pathological basis involving widespread bilateral thalamic neural death (Adams et al., 2000), with major deafferentation of thalamic, striatal and (neo)cortical structures. More specifically, decreases in excitatory afferent drives from the central thalamus as well as insufficient top-down regulation from the frontal cortex to the striatum would lead to a sharp cutback of the medium spiny neurons activity that require high level of excitatory inputs to maintain their physiological firing rate (Grillner et al., 2005). The globus pallidus interna being under-inhibited by these neurons in turn over-inhibits the central thalamic neurons, thus promoting the circuit dysfunction and failing to maintain thalamic connections with the cortical fronto-parietal areas (Fridman et al., 2014). Overall, this model assumes that the disruption of the central thalamus activity and its connections to the cortical areas is critically involved in the network failure from which arises DoC and that these specific components’ integrity would conversely play a major role in the recovery process (Schiff, 2010; Thibaut et al., 2019). Such theory was further completed at the neurobiological level by the ABCD model, which provides deeper insights regarding the neural recovery processes (Schiff, 2016). The ABCD model allows to categorize the degree of cerebral deafferentation and predict the effect of such deafferentation on the neocortical neurons’ activity as measured by the EEG. Thus, the progression of EEG neural firing patterns that can be observed during the recovery process can be organized into four categories (i.e., corresponding to the four letters “A,B,C,D”), depending on the severity of the thalamo-cortical deafferentation, with each category corresponding to a clearly distinguishable level of functional thalamocortical network integrity. In practical terms, level ‘A’ would indicate highly hyperpolarized neocortical neurons suggesting complete or near-complete cortical deafferentation marked by low-frequency oscillations (i.e., < 1Hz, delta band), which can be associated with the patterns observed in some chronic UWS patients (Schiff et al., 2014). When neurons display lower polarity levels, this produces 5 to 9 Hz theta oscillations that are typical of B-type EEG resting state dynamics, indicating severely disconnected thalamocortical networks that can be observed in some MCS patients (Williams et al., 2013). In the C-type pattern, that usually appears in more restored or preserved networks, these 5 to 9 Hz theta oscillations get progressively associated with higher frequency rhythms, from 15 to 40 Hz (i.e., beta). Eventually, normal EEG activity can get back driven by functionally preserved interactions between the thalamus and the cortex, allowing the production of alpha oscillations (i.e., 8 to 12 Hz) with additional peak activity in higher frequency bands (Steriade et al., 2001). Overall, the mesocircuit hypothesis sets the frame for pathological brain network changes following traumatic brain injury, hypoxia or widespread ischemia while the ABCD model predicts relationships between the degree of functional or structural deafferentation to central thalamus, the shape of the EEG power spectra, and the associated behavioral level. Altogether, these models can be useful to study the neurophysiological correlates of recovery in the form of transitions from a category to another (e.g., switch from B-type to C-type EEG pattern) (Edlow et al., 2021) as it was shown in some treatment-responsive patients (Williams et al., 2013) and in spontaneous recovery from DoC (Claassen et al., 2016; Forgacs et al., 2017).
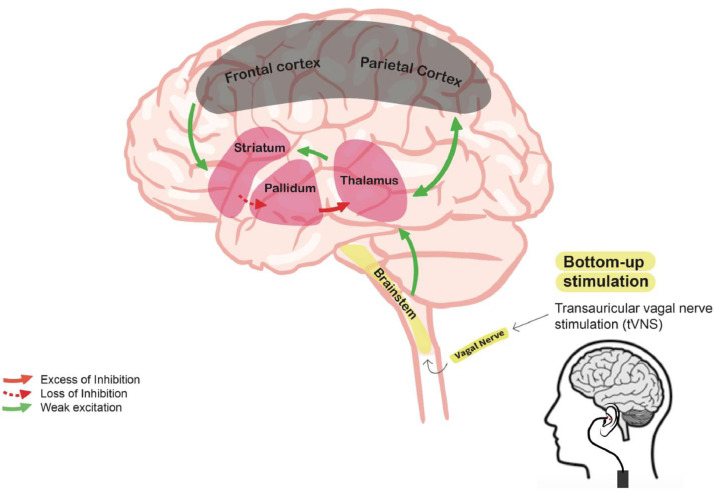
Among neuromodulation techniques, as opposed to other techniques such as transcranial electrical or magnetic simulations acting in a top-down manner (i.e., from the cortical to the subcortical structures), taVNS may be an interesting therapeutic candidate acting through a bottom-up manner to restore thalamocortical connectivity in post-coma patients as demonstrated in figure 1. At the theoretical level, to explain the mechanisms of action of taVNS in DoC patients, we developed the Vagal Cortical Pathways Model (Briand et al., 2020), which describes the potential influences of taVNS on the mesocircuit in severely injured brains. Mechanistically, taVNS is thought to stimulate the auricular branch of the vagus nerve which leads to the activation of the trigeminal nucleus and the nucleus of the solitary tract located in the lower brainstem areas. As a response, the activation of these nuclei would lead to the activation of the locus coeruleus and the dorsal raphe nucleus, both located in the upper brainstem structures and part of two major neurotransmitters systems. Following electrical inputs from the lower brainstem areas, the locus coeruleus is thought to increase its firing rates and release norepinephrine (Cao et al., 2017) that will modulate global brain activity, including the thalamus and that is linked to awareness of the environment (Aston-Jones & Cohen, 2005). Finally, the raphe nuclei will release serotonin, which also targets the brain, especially some structures involved in the awareness of the self (Hahn et al., 2012). Such assumptions are supported by both human and rodent studies that have demonstrated that VNS was likely to be associated with increased releases of endogenous norepinephrine and serotonin through the observation of a higher degree of activation of postsynaptic alpha(1)-adrenoceptors on 5-HT neurons (Dorr & Debonnel, 2006; Manta et al., 2009). Moreover, as compared to normal individuals, when rodents’ locus coeruleus was chemically damaged, the beneficial effects of VNS on seizure frequency appeared to be reduced (Krahl et al., 1998), thus supporting the involvement of the norepinephrine pathway in taVNS’ action over the brain. For more details regarding the hypotheses of action of taVNS neural mechanisms, see Briand et al. (2020).
Figure 1.
Postulated bottom-up effect influence of transcutaneous auricular vagal nerve stimulation on the mesocircuit in a severely damaged brain. The model suggests that the reduction of thalamocortical and thalamostriatal outflow following deafferentation and loss of neurons from the central thalamus withdraws important afferent drive to the medium spiny neurons of the striatum (green lines). Loss of active inhibition from the striatum (dashed red line) allows neurons of the globus pallidus interna (GPi) to tonically fire and provide active inhibition (red line) to their synaptic targets, including relay neurons of the already understimulated central thalamus, thus reducing thalamic activity and consequent thalamo-cortical connectivity. taVNS may hypothetically supply for the missing thalamic excitatory inputs by stimulating the lower and upper brainstem nuclei and thus promote the reinstatement of the thalamo-cortical connectivity. Adapted fromGiacino et al., 2014.
In line with the above, the postulated reinstatement of thalamo-cortical connectivity through taVNS afferent noradrenergic (i.e., locus coeruleus) and serotoninergic (i.e., dorsal raphe nucleus) drives to the thalamus and the whole brain would match the critical involvement of the central thalamus and its cortical projections in the recovery from DoC, and could induce both behavioural improvements at the clinical level and functional neural activity transition at the neurophysiological level.
Within these frameworks, this protocol proposition is a first step to investigate the therapeutic effects of taVNS in the early period following brain injury in a sham-controlled double-blind clinical trial, relying on both clinical assessments and neurophysiological measures.
Study objectives
This study aims to (1) evaluate the clinical effects of taVNS on consciousness recovery in patients with DoC (i.e., UWS and MCS patients) in a sham-controlled double-blind setting; (2) investigate the neural mechanisms underlying the action of taVNS on injured brains by investigating and comparing the neurophysiological correlates of taVNS with high-density resting-state EEG, using the ABCD model; (3) assess the feasibility of such treatment protocol through attrition rate as well as the safety of the procedure in patients with DoC and document proper stimulation of the vagus nerves through electrocardiogram (EKG) measurements; (4) define the phenotype of clinical responders by identifying biomarkers based on patient's demographical characteristics and functional impairments that may correlate with responsiveness to treatment; and (5) assess the long-term efficacy of taVNS in terms of functional outcomes.
Study hypotheses
Based on the Vagal Cortical Pathway and the mesocircuit models, our primary hypothesis is that active taVNS will significantly induce positive behavioural changes as compared to the sham stimulations. Furthermore, we expect that the improvement in the CRS-R (Giacino et al., 2004) total score and subsequent index score (Annen et al., 2019), which allows for correction of reflex behaviours, will lead to a change of diagnosis in a subset of patients. The secondary hypothesis is related to neural correlates. We hypothesize that taVNS will induce increased alpha and theta frequency power bands at the whole brain level as well as dynamic connectivity especially in the alpha band. Based on the ABCD model, we also expect that responders to treatment will present a shift in the EEG spectrum category, in line with a partially restored thalamocortical connectivity. In addition, we postulate that such modifications in brain activity patterns will precede observable behavioural changes at the bedside and will correlate with behavioural responsiveness. Furthermore, we postulate that our protocol will be feasible and safe to perform in the early period of DoC; and that the active taVNS procedure will modulate patients’ heart rate variability (HRV) to some extent as a result of the recruitment of parasympathetic innervations.
As exploratory hypotheses, we also expect that MCS patients will be more likely to respond to the taVNS treatment than patients in UWS. Moreover, we hypothesize that etiology may influence the response to taVNS stimulations, with traumatic etiologies having a higher ratio of responders as compared to hypoxic-ischemic etiologies. Eventually, we also expect patients who did receive the active treatment to obtain better outcome at three months following the end of the intervention.
Methods
Study design
We propose a prospective triple-blind parallel 2-arm randomized controlled trial opposing taVNS active stimulation to sham stimulation, with the experimenter, the patient and the person in charge of the data analyses being blind to the treatment allocation.
Study population and setting
Forty-four patients with DoC following severe brain damage will be included. All patients recruited will be DoC patients admitted to the intensive care unit (ICU) from the Centre Hospitalier Universitaire de Liège (CHU de Liège) in Liège, Belgium or from the Centre Neurologique William Lennox in Ottignies, Belgium. The main investigator will assess patients’ eligibility for the study at the time of admission, seek for the presence of any exclusion criterion and determine the current state of awareness using a minimum of two CRS-R assessments.
Inclusion and exclusion criteria are displayed in Table 1.
Table 1.
Study inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
UWS: unresponsive wakefulness syndrome; MCS: minimally conscious state; CRS-R: Coma Recovery Scale-Revised.
Power calculation
Sample size calculations were computed in G*Power (Faul et al., 2007) using a priori power analysis. Previous publications using taVNS in patients with DoC are limited, thus the effect size calculation was based on the best available data in the literature in line with the objectives and methods of this clinical trial (Hakon et al., 2020), leading to 0,96 effect size estimation. Using a two-tails t-test for two groups, we set the desired power at 0.8 with a Type-I error rate threshold of 0,05. Accounting for an allocation ratio of 1:1, this led to a minimum number of 19 participants per group. In line with previous studies conducted with this challenging population, we included a dropout rate of 15% in our calculation for a total of 22 participants per arm, that is, an ideal sample size of 44 patients to enroll. This decision was based on the fact that patients with DoC, especially in the ICU, represent a population at high risk for medical complications that could force discontinuation of the protocol and lead to a high attrition rate. Interim analyses of the study power will be conducted once 11 patients per arm will have completed the study to ensure safety, feasibility, and revaluation of the sample size.
Procedure
The study procedure will start as early as on the 7th day post-injury or until stable hemodynamics; and will have to be completed by the 90th day post-injury. Following open discussion about the study objectives, methods and potential risks, written informed consent will be obtained by the patients’ legal representative. He or she will be informed that withdraw from the study is possible at any time of the process without having to justify, including during the ongoing trial. If the participants were to recover consciousness and sufficient capacity for discernment during the study, they would be informed that the trial has been or is being performed, and consent will be obtained from them. Each party will keep a copy of the signed informed consent and will be able to refer to it for information.
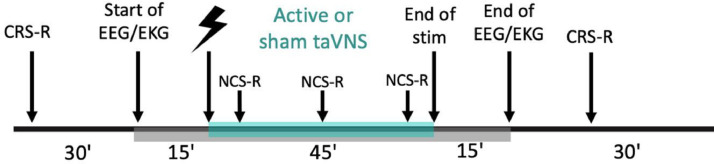
Of the 44 patients included, 22 will be allocated to the active group and 22 will be assigned to the sham group. A randomized order generator with a 1:1 allocation ratio will be used to determine the random allocation to each group. Only the investigator who generated the assignment sequence will be aware of the allocation and will then disclose the assigned intervention to the investigator in charge of the stimulation the day of the first session. Intervention allocation will be concealed from the patient, the family, the care providers from the medical team, and all investigators involved in the patient's assessment for the whole duration of the treatment phase. The evaluator will stay blind from the sequence as well as from the stimulation group during treatment and follow-up. Moreover, blinded analyses will be conducted as the data files’ names will be coded by the investigator who generated the assignment sequence. Figure 2 summarizes the protocol procedure.
Figure 2.
Study protocol's timeline of first and last day of treatment. taVNS: transcutaneous auricular vagal nerve stimulation; CRS-R: Coma Recovery Scale-Revised; EEG: electroencephalography; EKG: electrocardiogram; NCS-R: Nociception Coma Scale-Revised.
Screening and enrollment phase
During the screening phase, patients’ eligibility will be documented based on the medical record and insights from the medical team. The patient's state of awareness will be repeatedly assessed by a trained accredited examiner to confirm or disconfirm the DoC diagnosis by means of at least two CRS-R evaluations (see “behavioural assessments” for more details). Following approval from the referring physician, the trial will be explained to the family and consent will be obtained from the legal representative/substitute decision maker. Any patient from whom consent will be obtained will be considered enrolled in the study. All patients randomized will be included in the intention-to-treat (ITT) analyses.
Treatment phase
All patients included will receive daily sessions of 45 minutes 3 mA active taVNS (i.e., over the cymba conchae) or sham (i.e., over the ear lobe) stimulation for five consecutive days. During the whole protocol, an experimenter will stay at the patients’ bedside to ensure proper stimulation parameters through adequate impedance and electrode placement. The first and last day of the treatment phase, behavioural assessments (CRS-R) will be performed before and after the stimulation to seek for behavioural signs of consciousness. Neurophysiological measures (i.e., hd-EEG) as well as bioelectrical signals (i.e., EKG) will be recorded continuously from 15 minutes just before the start of the stimulation to 15 minutes just after the end of the stimulation. Arousal will be monitored and patients will be kept awake using the arousal protocol from the CRS-R (Giacino et al., 2004) during both the entire stimulation periods and the EEG recordings. To assess taVNS tolerance, patient's comfort will be monitored using an appropriate pain scale (Nociception Coma Scale-Revised, NCS-R,(Chatelle et al., 2012)) that will be performed three times during the stimulation time frame as well as at baseline. Moreover, the retrospective treatment feasibility will be assessed and calculated as the percentage of completed sessions over planned sessions.
Follow-up
At 90 days following the end of the treatment, patient's functional outcome will be collected through follow-up assessments. These evaluations will be carried out by means of structured phone interviews with the patient's relatives/care givers using the Disability Rating Scale (DRS) and the Glasgow Outcome Scale-Extended (GOS-E). Moreover, the number of days spent in the current care unit until discharge will also be collected at that time point.
Materials
taVNS and sham stimulations
Stimulations will be delivered by a transcutaneous auricular vagal nerve stimulation device (Cerbomed GmbH, Germany, NEMOS/tVNS®), using Titanium/Iridium electrodes. Typically, in taVNS studies, the stimulation current intensity is set according to the patient's perceptual threshold (e.g., 200% of the perceptual threshold (Badran et al., 2018)) or pain threshold (i.e., below pain threshold). However, such methods cannot be used in the present protocol due to DoC patients’ characteristics. Indeed, in these challenging conditions, perception and pain processing are usually altered and patients are non-communicative (Chatelle et al., 2014), thus leaving the clinicians and researchers unable to collect such thresholds. Therefore, all patients allocated to the active taVNS group will receive bilateral cymba conchae stimulations using a two-phase signal (45 min duration with 30s alternative episodes of active/rest periods; 3mA; 200-300μs current width, 25Hz). The sham equivalent will consist of stimulations sharing the exact same technical parameters but located on the ear lobes, as the use of this location as an active control was supported by recent montages studies (Kreisberg et al., 2021). The choice of stimulating bilaterally, both over the right and the left ears can be explained by the following: invasive VNS studies usually implant the stimulator on the left side only due to an asymmetrical innervation of the heart from the vagus nerves. Indeed, it has been documented that stimulating the right cervical vagus nerve can induce direct electrical inputs to the sino-atrial node, which can produce undesired cardiac events such as arrythmias that are less observed when stimulating the left side (Yuan & Silberstein, 2016). However, these concerns might not be relevant for non-invasive stimulation of the auricular branch of the vagus nerves as it is thought to involve signals sent bilaterally to the heart surface following brain integration first instead of directly stimulating the pacemaking node (Chen et al., 2015). Thus, in this protocol, we will provide bilateral stimulation to boost our chances of success as previous studies did before us without reporting any side effects (Yap et al., 2020). To ensure adequate contact between the electrodes and the ear skin for the entire duration of the stimulations, the device is equipped with an alarm feature that produces a sound when the anode and the cathode's impedances with the skin are not optimal (i.e., > 18 kOhm).
Behavioural assessments
The recommended scale to determine patients’ level of consciousness and achieve a final diagnosis at the bedside is currently the CRS-R (Giacino et al., 2018; Kondziella et al., 2020). It is subdivided in six subscales investigating the auditory, visual, motor and oromotor functions as well as the patient's communication skills and arousal for a total of 23 points. This scale is used to diagnose the level of consciousness (coma, UWS, MCS minus or plus, eMCS) according to the presence of specific items (Giacino et al., 2004; Kondziella et al., 2020). To consider a patient as a clinical responder, the patient should display new sign(s) of consciousness following taVNS that was not present at baseline nor during screening phase.
Pain assessment is a challenging task in this population due to the absence of communication. The NCS-R is a validated scale that was designed to detect and assess pain in patients with DoC through three axes: motor, verbal and facial responses to noxious stimulations. The total score ranges from 0 to 9, the first indicating the absence of pain and the latter expressing maximum pain (Chatelle et al., 2012). In this protocol, the NCS-R will be performed at baseline, as well as at the beginning, halfway and at the end of each stimulation session to ensure patient's comfort. To assess the tolerance to taVNS, a score of 4 has been set as our cut-off value to distinguish painful versus non-painful behavior. The NCS-R scores during stimulation will be reported, and if superior to 4 and different from baseline, the current intensity of taVNS will be reduced by 0,5 mA, and the NCS-R will be performed again. All scores will be documented as well as the final current intensity used to perform the stimulation. All patients still displaying significant signs of pain at a low threshold of 0.5 mA will be excluded from the study.
To assess patient's functional outcome following cessation of treatment, structured phone interviews will be conducted at three months post-intervention. The Glasgow Outcome Scale-Extended (GOS-E) is used to classify global outcomes in traumatic brain injury survivors within 8 categories: dead, vegetative state, lower severe disability, upper severe disability, lower moderate disability, upper moderate disability, lower good recovery and upper good recovery (Levin et al., 2001). The Disability Rating Scale (DRS) is another validated scale that provides insights regarding patient's impairment and progress overtime following severe brain injury. The scale investigates behaviors within 8 domains, namely eye opening, communication, motor response, feeding, toileting, grooming, level of functioning and employability. The total outcome score ranges from 0 to 29 and is therefore classified in a category according to the level of disability, the higher the score, the bigger the disability: None (0), Mild (1), Partial (2-3), Moderate (4-6), Moderately severe (7-11), Severe (12-16), Extremely severe (17-21), Vegetative state (22-24) and Extreme vegetative state (25-29) (Rappaport et al., 1982). Phone-adapted versions of these scales will be used to conduct phone interviews with relatives or care providers.
Neurophysiological assessments
To ensure the robustness of our connectivity analyses, 128 channels high-density resting state EEG will be recorded using BrainVision device and software (ActiCHamp Plus, Brain Products GmbH, Gilching, Germany). During the 75 minutes of recording (i.e., 15 minutes prior stimulation, 45 minutes of stimulation and 15 minutes following the end of stimulation), patients will be kept awake (e.g., eyes open) and still by the experimenter. EEG signals are sensitive to movements artifacts, therefore, recording times could be adapted according to the data's quality to obtain sufficient usable materials. EEG signals will be measured in microvolts, sampled at 500 Hz and referenced to the vertex (Cz) using 128 passive sponge-based channels nets. The resting-state data will be used to obtain spectral power and spectral connectivity using the graph theory, which have proven to correlate with behavioral recovery of patients with DoC (Chennu et al., 2017). From a neurobiological perspective, it will also allow to classify each patient's EEG dominant rhythm in one of the ABCD classifications according to the ABCD model previously mentioned (Edlow et al., 2021) and thus allow to characterize the outcomes at the network level. This will also provide insights in potential changes in thalamo-cortical deafferentation following taVNS, allowing to test the assumptions of the Vagal Cortical Pathways Model at the cortical level.
Other bioelectrical signal
Although unlikely, the continuous monitoring of EKG will allow real-time detection of arrhythmias (such as bradycardia), which could potentially be induced by bilateral taVNS, similarly to what has been observed in VNS implantable devices (Ardesch et al., 2007). EKG adhesive foam electrodes (Suretrace RTL adult ECG electrodes, ConMed, United-States) will be placed below the left clavicle and at the fourth intercostal space at the left sternal border. A third electrode will be placed at the right ankle for ground. In addition to our recordings, patients’ EKG will also be monitored for all stimulation sessions by the hospital unit in which they will be hospitalized. Any cardiac event (e.g., bradycardia, arrhythmia) will be noted and reported to the medical team by the experimenter present during the stimulation sessions. EKG will also be used as a bioelectrical marker to document a proper stimulation of the vagus nerves through an observable modulation of autonomic function towards parasympathetic predominance. This will be achieved by computing parameters of the heart rate variability (HRV) recorded during the stimulations, as taVNS was shown to modulate the HRV in some studies (Machetanz et al., 2021).
Data management
In addition to the above-mentioned measures, collection of 1) socio-demographic characteristics: age, sex, occupation, level of education, ethnicity, languages spoken, handedness, pre-morbid medical history (including hearing, neurocognitive, previous history of concussion/head trauma and psychiatric disorders); and 2) acute critical illness characteristics: mechanism of injury, Glasgow Coma Scale score at the emergency room and at one week, results from general neurologic examination, CT scan and/or MRI findings (time window: max. 30 days from enrollment), clinical EEG findings, magnitude of intracranial pressure (if monitored, and presence and duration of increased intracranial pressure), duration of sedation, duration of loss of consciousness, brain injury severity, and ICU stay duration will be done systematically since these independent variables could act as covariates for patients’ recovery.
Data management will comply with the General Data Protection Regulation (EU 2016/679) and patients and/or their legal representative will be made aware of their rights regarding these data. All clinical data collected will be stored in their anonymous version onto Research Space – RSpace© - an online secured server providing database security and protection against malicious use. RSpace uses Scalable Storage in Cloud provided by Amazon Web Services. Only the investigators in charge of the study will get access to the secured server and its content.
Analyses
Intention-to-treat analyses will be conducted for all study outcomes. Analyses will focus on the detection of changes induced by the taVNS intervention both at the individual level (comparing data before and after treatment) and at the group level (comparing the sham arm to the treatment arm). Behavioral CRS-R total scores and subsequent index scores will be defined as our primary outcome. Group treatment effects will be evaluated with calculation of the difference between each group post-treatment and pre-treatment score means. The change in parameters (i.e., CRS-R scores, index scores) between pre and post sessions within individual patients will also be compared. Moreover, clinical responders to taVNS will be identified as patients who will display new sign(s) of consciousness following treatment that was not present at baseline nor during the screening phase. In that context, further sub-group analyses will also be conducted based on the patients’ diagnosis an etiology to better characterize the responders’ profile.
EEG metrics and behavioural outcome measures will be our secondary outcome. EEG data preprocessing in MATLAB/EEGLAB will involve data to be filtered between 0.5 and 45 Hz and segmented into 10-seconds epochs. Downsampling to 250 Hz and Notch filtering for power line interference will also be automatically applied at that stage. Then, channel and epoch variance thresholds will be set to identify abnormally noisy channels and epochs. A maximum of 15% of the channels will be rejected and interpolated. If data quality is not sufficient, the session will be excluded. Artefacts and noisy epochs will then be rejected based on visual inspection. Independent Components Analysis (ICA) based on the Infomax ICA algorithm (Bell & Sejnowski, 1995) will then be run to identify and further manually remove components that were generated by non-neural noise sources. For the estimation of spectral power, EEG data will be preprocessed and analyzed with EEGLAB (https://sccn.ucsd.edu/eeglab/) and the FieldTrip toolbox (http://www.fieldtrip toolbox.org/) using spectral and cross-spectral decompositions from cleaned data for delta (0-4 Hz), theta (4-8 Hz), alpha (8-13 Hz), beta (13-25 Hz) and gamma (25-40 Hz) bans. Relative percentage contributions to the total power over each band frequency will then be calculated. We will also use the debiased weighted Phase Lag Index (dwPLI) to estimate spectral connectivity between pairs of channels (Peraza et al., 2012). The dwPLI is a robust estimator of scalp-level connectivity that is more invariant to volume conduction in comparison to other estimators. Median spectral connectivity and graph-theoretic topology metrics such as clustering coefficient, path length, modularity and participation coefficient within the bands of interest, will also be estimated. Results will be corrected using Holm correction for multiple comparisons and considered statistically significant at p < 0.05.
Finally, EKG data analyses will be performed in MATLAB. Artifacts rejections will first occur and parameters and metrics of HRV (RR mean, SDNN, RMSSD, NN50, pNN50, SD1, SD2 rrHRV) (see Supplementary Materials for abbreviation description) will then be computed using compatible toolboxes (HRV toolbox) as it was done in previous taVNS studies investigating HRV (Machetanz et al., 2021).
Discussion
Despite the clinical and scientific challenges, considering the early period post-injury in DoC research becomes an increasingly crucial matter since this is the most critical period for neuroplasticity and medical care decisions having an undeniable impact on patient's survival (Turgeon et al., 2011). This protocol describes the first double-blind parallel randomized-controlled trial that aims to determine the safety and feasibility of using taVNS in patients with DoC in the early period following injury (7-90 days post-injury) and assess its efficacy for consciousness recovery.
This study will rely on the mechanisms described by the Vagal Cortical Pathways Model and will shed new light on this bottom-up therapeutic approach for the treatment of acute to sub-acute DoC. This protocol will involve behavioral and neurophysiological measures to test our hypothesis and bring evidence on taVNS’ clinical effects and neural mechanisms of recovery. At the behavioural level, it aims to confirm previous preliminary results from small-sample open-label studies using taVNS in DoC and showing clinical improvement following the intervention by means of significantly greater CRS-R scores post-taVNS compared to sham. We also expect to observe better outcome assessed at follow-up in the active compared to the sham groups.
On the other hand, regarding the clinical and neurophysiological effects of taVNS on patients with DoC, as we will assess both effects after one day of treatment and after five days of treatment, we expect that the neurophysiological effects will be observed before any clinical effects are manifest. This is based on results from top-down stimulation techniques (e.g., repetitive transcranial magnetic stimulation, transcranial direct current stimulation) that have suggested early brain responses to treatments in the absence of any observable clinical changes (Bai et al., 2018; Liu et al., 2016)
Regarding the neurophysiological outcome more specifically, based on the available literature, an increase in the theta frequency band power is expected, in line with evidence from implanted VNS device which reported behavioral improvements following treatment. Such increase could firstly be explained by the relationship between the theta frequency band power and the neuronal activity in the thalamus and posterior cingulate cortex, which are considered hubs of consciousness (Gusnard & Raichle, 2001; Schiff, 2009) and involved in the default mode network (Leech & Sharp, 2014). Nonetheless, in the framework of the ABCD model for neural recovery, it could also be explained by the return of higher average membrane potentials permitting spontaneous oscillations by the pyramidal cells (i.e., physiological intrinsic frequency of 5 to 9 Hz), which are proposed to account for the 7 Hz rhythm pattern that dominates the resting activity of the B-type EEG spectrum as observed in some MCS patients. Moreover, taVNS may facilitate the reinstatement of thalamo-cortical afferences that characterizes recovery from DoC and thus allow the return of higher oscillatory patterns (i.e., beta and alpha) in some patients as predicted in the C and D-types EEG patterns. Referring to the vagal cortical pathway, this could be explained by the reinstatement of bursting to tonic firing modes of the thalamic cells that could be the result of taVNS afferent noradrenergic drives from the upper brainstem nucleus (i.e., locus coeruleus) since its firing rates were found to be significantly higher following short-term vagus nerve treatments (Dorr & Debonnel, 2006). In addition, improvement in clinical signs of consciousness has been previously documented in DoC patients and correlated to an increase in the alpha frequency power spectrum, that could be linked to enhanced thalamo-cortical interactions (Straudi et al., 2019). Other than frequency, the functional connectivity has been shown to match behavioral consciousness in patients with DoC (Chennu et al., 2017). If taVNS has positive effect on the level of consciousness, this should be reflected by an increase in the alpha band connectivity of the EEG signal following active taVNS as compared to sham. Moreover, such increase should be of a greater magnitude in clinical responders compared to non-responders.
Another challenge remains to develop patient-fitted therapeutic strategies based on responder's profile, which will also be addressed with the present protocol. In that matter, we expect to observe differences in response to treatment based on patients’ etiology and diagnosis. Indeed, since MCS patients display higher levels of plastic changes and reorganization in the awareness networks than UWS patients (Bagnato, 2022; Estraneo & Trojano, 2017; Voss et al., 2006), we expect them to benefit more from brain stimulation interventions as it was demonstrated in previous therapeutic works (Thibaut et al., 2014). Regarding to the pathological mechanisms leading to such disorders, anoxic brain injuries usually lead to diffuse brain damage that generally affects sub-cortical regions such as the basal ganglia and deeper white matter structures (Caine & Watson, 2000) while traumatic etiologies may display more focal lesions. In that context, since taVNS is thought to reinstate the thalamo-cortical flow in a bottom-up manner by reaching subcortical structures first, it may be more effective in traumatic lesions that have structurally spared the thalamus and brainstem nuclei. Eventually, on a more exploratory basis, EKG metrics could allow us to attest the proper activation of the vagus nerves and subsequent cardiac parasympathetic related activity.
Our results will bring objective evidence to further assess bottom-up non-invasive brain stimulation techniques and their relevance in modulating the thalamo-cortical connectivity to promote patients’ recovery. This protocol stands as an important milestone in the development of new therapeutic approaches in the early care of DoC.
Author contributions
MMV, MMB and AT were involved in conception and methodology design of the study, ethical and trial registration procedures, and manuscript writing. MMV and AT lead the implementation and the coordination of the trial. OG, JA, DL and RET participated in trial methodology design and provided technical expertise regarding the clinical trial materials and settings. DL and DM helped implementing the trial in the hospital settings. MMB, AT, SL and OG participated in study conception and helped defining its theoretical framework. All authors contributed to manuscript revision.
Ethics statement
This study was approved by the University and University Hospital of Liège Ethics Committee under the reference number 2019/91 (B707201939853).
Declaration of Competing Interest
Authors have no conflict of interest to declare.
Acknowledgment
We would like to express our gratitude to the University and University Hospital of Liège, the patients and their families and the ICU staff. The Belgian National Funds for Scientific Research (FRS-FNRS) in the framework of the research project No PDR/BEJ T.0134.21, the European Union's Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 945539 (Human Brain Project SGA3), the European Space Agency (ESA) and the Belgian Federal Science Policy Office (BELSPO) in the framework of the PRODEX Programme, the BIAL Foundation, the Mind Science Foundation, the fund Generet of the King Baudouin Foundation, the Mind Care International foundation and AstraZeneca Foundation. We also thank the University of Laval (Québec, Canada), the Canadian Institutes of Health Research and the Fonds de Recherche du Québec en Santé for their financial support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijchp.2022.100360.
Appendix. Supplementary materials
References
- Adams J.H., Graham D.I., Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain : A Journal of Neurology. 2000;123(7):1327–1338. doi: 10.1093/BRAIN/123.7.1327. Pt 7. [DOI] [PubMed] [Google Scholar]
- Angelakis E., Konstantinidi M., Sakas D. Cognitive rehabilitation in disorders of consciousness with transcranial direct current stimulation. Dialogues in Clinical Neuroscience & Mental Health. 2018;1:1. doi: 10.26386/obrela.v1is3.54. [DOI] [Google Scholar]
- Annen, J., Filippini, M.M., Bonin, E., Cassol, H., Aubinet, C., Carrière, M., Gosseries, O., Thibaut, A., Barra, A., Wolff, A., Sanz, L.R.D., Martial, C., Laureys, S., & Chatelle, C. (2019). Diagnostic accuracy of the CRS-R index in patients with disorders of consciousness. 10.1080/02699052.2019.1644376, 33(11), 1409–1412. 10.1080/02699052.2019.1644376 [DOI] [PubMed]
- Ardesch J.J., Buschman H.P.J., van der Burgh P.H., Wagener-Schimmel L.J.J.C., van der Aa H.E., Hageman G. Cardiac responses of vagus nerve stimulation: Intraoperative bradycardia and subsequent chronic stimulation. Clinical Neurology and Neurosurgery. 2007;109:849–852. doi: 10.1016/j.clineuro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-noepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28(1):403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Badran B.W., Dowdle L.T., Mithoefer O.J., LaBate N.T., Coatsworth J., Brown J.C., DeVries W.H., Austelle C.W., McTeague L.M., George M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimulation. 2018;11(3):492–500. doi: 10.1016/J.BRS.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato S. The role of plasticity in the recovery of consciousness. Handbook of Clinical Neurology. 2022;184:375–395. doi: 10.1016/B978-0-12-819410-2.00020-5. [DOI] [PubMed] [Google Scholar]
- Bai Y., Xia X., Wang Y., Guo Y., Yang Y., He J., Li X. Fronto-parietal coherence response to tDCS modulation in patients with disorders of consciousness. The International Journal of Neuroscience. 2018;128(7):587–594. doi: 10.1080/00207454.2017.1403440. [DOI] [PubMed] [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7(6):1129–1159. doi: 10.1162/NECO.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Briand M.M., Gosseries O., Staumont B., Laureys S., Thibaut A. Transcutaneous auricular vagal nerve stimulation and disorders of consciousness: A hypothesis for mechanisms of action. Frontiers in Neurology. 2020;11 doi: 10.3389/FNEUR.2020.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.A., Vanhaudenhuyse A., Thibaut A., Moonen G., Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. Journal of Neurology. 2011;258(7):1373–1384. doi: 10.1007/S00415-011-6114-X. [DOI] [PubMed] [Google Scholar]
- Caine D., Watson J.D.G. Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review. Journal of the International Neuropsychological Society. 2000;6(1):86–99. doi: 10.1017/S1355617700611116. [DOI] [PubMed] [Google Scholar]
- Cao J., Lu K.H., Powley T.L., Liu Z. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS ONE. 2017;12(12):1–17. doi: 10.1371/journal.pone.0189518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelle C., Majerus S., Whyte J., Laureys S., Schnakers C. A sensitive scale to assess nociceptive pain in patients with disorders of consciousness. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83(12):1233–1237. doi: 10.1136/JNNP-2012-302987. [DOI] [PubMed] [Google Scholar]
- Chatelle, C., Thibaut, A., Whyte, J., de Val, M.D., Laureys, S., & Schnakers, C. (2014). Pain issues in disorders of consciousness. 10.3109/02699052.2014.920518, 28(9), 1202–1208. 10.3109/02699052.2014.920518 [DOI] [PubMed]
- Chen M., Yu L., Ouyang F., Liu Q., Wang Z., Wang S., Zhou L., Jiang H., Zhou S. The right side or left side of noninvasive transcutaneous vagus nerve stimulation: Based on conventional wisdom or scientific evidence? International Journal of Cardiology. 2015;187(1):44–45. doi: 10.1016/J.IJCARD.2015.03.351. [DOI] [PubMed] [Google Scholar]
- Chennu S., Annen J., Wannez S., Thibaut A., Chatelle C., Cassol H., Martens G., Schnakers C., Gosseries O., Menon D., Laureys S. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain : A Journal of Neurology. 2017;140(8):2120–2132. doi: 10.1093/BRAIN/AWX163. [DOI] [PubMed] [Google Scholar]
- Claassen J., Velazquez A., Meyers E., Witsch J., Falo M.C., Park S., Agarwal S., Michael Schmidt J., Schiff N.D., Sitt J.D., Naccache L., Sander Connolly E., Frey H.P. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Annals of Neurology. 2016;80(4):541–553. doi: 10.1002/ANA.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr A.E., Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. The Journal of Pharmacology and Experimental Therapeutics. 2006;318(2):890–898. doi: 10.1124/JPET.106.104166. [DOI] [PubMed] [Google Scholar]
- Edlow B.L., Claassen J., Schiff N.D., Greer D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nature Reviews Neurology. 2021;17(3):135–156. doi: 10.1038/S41582-020-00428-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estraneo A., Pascarella A., Moretta P., Loreto V., Trojano L. Clinical and electroencephalographic on–off effect of amantadine in chronic non-traumatic minimally conscious state. Journal of Neurology. 2015;262:1584–1586. doi: 10.1007/s00415-015-7771-y. [DOI] [PubMed] [Google Scholar]
- Estraneo A., Trojano L. Prognosis in disorders of consciousness. Coma and Disorders of Consciousness: Second Edition. 2017:17–36. doi: 10.1007/978-3-319-55964-3_2/TABLES/3. [DOI] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. 2007 39:2. [DOI] [PubMed] [Google Scholar]
- Forgacs P.B., Frey H.P., Velazquez A., Thompson S., Brodie D., Moitra V., Rabani L., Park S., Agarwal S., Falo M.C., Schiff N.D., Claassen J. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Annals of Clinical and Translational Neurology. 2017;4(2):119–129. doi: 10.1002/ACN3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos E., Ellrich J., Komisaruk B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: FMRI evidence in humans. Brain Stimulation. 2015;8(3):624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E.A., Beattie B.J., Broft A., Laureys S., Schif N.D. Proceedings of the national academy of sciences of the United States of America. Vol. 111. 2014. Role of anterior forebrain mesocircuit dysfunction in the severely injured brain; pp. 6473–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R.G., Lin R.L., Lee J., Kim J., Barbieri R., Sclocco R., Wasan A.D., Edwards R.R., Rosen B.R., Hadjikhani N., Napadow V. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain. 2017;158(8):1461–1472. doi: 10.1097/j.pain.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R.D., Zasler N.D. The minimally conscious state: Definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/WNL.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Fins J.J., Laureys S., Schiff N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nature Reviews Neurology. 2014;10(2):99–114. doi: 10.1038/nrneurol.2013.279. 2014 10:2. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK coma recovery scale-revised: Measurement characteristics and diagnostic utility. Archives of Physical Medicine and Rehabilitation. 2004;85(12):2020–2029. doi: 10.1016/J.APMR.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Katz D.I., Schiff N.D., Whyte J., Ashman E.J., Ashwal S., Barbano R., Hammond F.M., Laureys S., Ling G.S.F., Nakase-Richardson R., Seel R.T., Yablon S., Getchius T.S.D., Gronseth G.S., Armstrong M.J. Practice guideline update recommendations summary: Disorders of consciousness: Report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology; the American congress of rehabilitation medicine; and the national institute on disability, independent living, and rehabilitation research. Archives of Physical Medicine and Rehabilitation. 2018;99(9):1699–1709. doi: 10.1016/j.apmr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Whyte J., Bagiella E., Kalmar K., Childs N., Khademi A., Eifert B., Long D., Katz D.I., Cho S., Yablon S.A., Luther M., Hammond F.M., Nordenbo A., Novak P., Mercer W., Maurer-Karattup P., Sherer M. Placebo-controlled trial of amantadine for severe traumatic brain injury. New England Journal of Medicine. 2012;366(9):819–826. doi: 10.1056/NEJMOA1102609/SUPPL_FILE/NEJMOA1102609_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hellgren J., Ménard A., Saitoh K., Wikström M.A. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends in Neurosciences. 2005;28(7):364–370. doi: 10.1016/J.TINS.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hahn A., Wadsak W., Windischberger C., Baldinger P., Hof̈lich A.S., Losak J., Nics L., Philippe C., Kranz G.S., Kraus C., Mitterhauser M., Karanikas G., Kasper S., Lanzenberger R. Proceedings of the national academy of sciences of the United States of America. Vol. 109. 2012. Differential modulation of the default mode network via serotonin-1A receptors; pp. 2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakon J., Moghiseh M., Poulsen I., Øland C.M.L., Hansen C.P., Sabers A. Transcutaneous vagus nerve stimulation in patients with severe traumatic brain injury: A feasibility trial. Neuromodulation : Journal of the International Neuromodulation Society. 2020;23(6):859–864. doi: 10.1111/NER.13148. [DOI] [PubMed] [Google Scholar]
- Hamer H.M., Bauer S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS) Epilepsy Research. 2019;153:83–84. doi: 10.1016/j.eplepsyres.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Research. 1992;581(1):156–160. doi: 10.1016/0006-8993(92)90356-E. [DOI] [PubMed] [Google Scholar]
- Kondziella D., Bender A., Diserens K., van Erp W., Estraneo A., Formisano R., Laureys S., Naccache L., Ozturk S., Rohaut B., Sitt J.D., Stender J., Tiainen M., Rossetti A.O., Gosseries O., Chatelle C. European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. European Journal of Neurology. 2020;27(5):741–756. doi: 10.1111/ENE.14151. [DOI] [PubMed] [Google Scholar]
- Krahl S.E., Clark K.B., Smith D.C., Browning R.A. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709–714. doi: 10.1111/J.1528-1157.1998.TB01155.X. [DOI] [PubMed] [Google Scholar]
- Kreisberg E., Esmaeilpour Z., Adair D., Khadka N., Datta A., Badran B.W., Bremner J.D., Bikson M. High-resolution computational modeling of the current flow in the outer ear during transcutaneous auricular Vagus Nerve Stimulation (taVNS) Brain Stimulation. 2021;14(6):1419–1430. doi: 10.1016/j.brs.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S., Celesia G.G., Cohadon F., Lavrijsen J., León-Carrión J., Sannita W.G., Sazbon L., Schmutzhard E., von Wild K.R., Zeman A., Dolce G. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Medicine. 2010;8 doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S., Boake C., Song J., McCauley S., Contant C., Diaz-Marchan P., Brundage S., Goodman H., Kotrla K.J. Validity and sensitivity to change of the extended glasgow outcome scale in mild to moderate traumatic brain injury. Journal of Neurotrauma. 2001;18(6):575–584. doi: 10.1089/089771501750291819. [DOI] [PubMed] [Google Scholar]
- Liu P., Gao J., Pan S., Meng F., Pan G., Li J., Luo B. Effects of high-frequency repetitive transcranial magnetic stimulation on cerebral hemodynamics in patients with disorders of consciousness: A sham-controlled study. European Neurology. 2016;76(1–2):1–7. doi: 10.1159/000447325. [DOI] [PubMed] [Google Scholar]
- Machetanz K., Berelidze L., Guggenberger R., Gharabaghi A. Transcutaneous auricular vagus nerve stimulation and heart rate variability: Analysis of parameters and targets. Autonomic Neuroscience. 2021;236 doi: 10.1016/J.AUTNEU.2021.102894. [DOI] [PubMed] [Google Scholar]
- Manta S., Dong J., Debonnel G., Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. Journal of Psychiatry & Neuroscience : JPN. 2009;34(4):272–280. https://pubmed.ncbi.nlm.nih.gov/19568478/ [PMC free article] [PubMed] [Google Scholar]
- Naro A., Russo M., Leo A., Bramanti P., Quartarone A., Calabrò R.S. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: Preliminary results. Neurorehabilitation and Neural Repair. 2015;29(7):603–613. doi: 10.1177/1545968314562114. [DOI] [PubMed] [Google Scholar]
- Noé E., Ferri J., Colomer C., Moliner B., O'Valle M., Ugart P., Rodriguez C. Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimulation. 2019 doi: 10.1016/j.brs.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Peraza L.R., Asghar A.U.R., Green G., Halliday D.M. Volume conduction effects in brain network inference from electroencephalographic recordings using phase lag index. Journal of Neuroscience Methods. 2012;207(2):189–199. doi: 10.1016/J.JNEUMETH.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Rappaport M., Hall K.M., Hopkins K., Belleza T., Cope D.N. Disability rating scale for severe head trauma: Coma to community. Archives of Physical Medicine and Rehabilitation. 1982;63(3):118–123. https://pubmed.ncbi.nlm.nih.gov/7073452/ [PubMed] [Google Scholar]
- Schiff N.D. Recovery of consciousness after brain injury : A mesocircuit hypothesis. Cell Press. 2009;33(1):1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends in Neurosciences. 2010;33(1):1–9. doi: 10.1016/J.TINS.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D. Mesocircuit mechanisms underlying recovery of consciousness following severe brain injuries: Model and predictions. Brain Function and Responsiveness in Disorders of Consciousness. 2016:195–204. doi: 10.1007/978-3-319-21425-2_15/FIGURES/2. [DOI] [Google Scholar]
- Schiff N.D., Nauvel T., Victor J.D. Large-scale brain dynamics in disorders of consciousness. Current Opinion in Neurobiology. 2014;25:7–14. doi: 10.1016/J.CONB.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Timofeev I., Grenier F. Natural waking and sleep states: A view from inside neocortical neurons. Journal of Neurophysiology. 2001;85(5):1969–1985. doi: 10.1152/JN.2001.85.5.1969/ASSET/IMAGES/LARGE/9K0511653012.JPEG. [DOI] [PubMed] [Google Scholar]
- Straudi S., Bonsangue V., Mele S., Craighero L., Montis A., Fregni F., Lavezzi S., Basaglia N. Bilateral M1 anodal transcranial direct current stimulation in post traumatic chronic minimally conscious state: A pilot EEG-tDCS study. Brain Injury. 2019;33(4):490–495. doi: 10.1080/02699052.2019.1565894. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Bruno M.A., Ledoux D., Demertzi A., Laureys S. tDCS in patients with disorders of consciousness. Neurology. 2014;82(13):1112–1118. doi: 10.1212/WNL.0000000000000260. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Piarulli A., Martens G., Chatelle C., Laureys S. Effect of multichannel transcranial direct current stimulation to reduce hypertonia in individuals with prolonged disorders of consciousness: A randomized controlled pilot study. Annals of Physical and Rehabilitation Medicine. 2019;62(6):418–425. doi: 10.1016/j.rehab.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Schiff N., Giacino J., Laureys S., Gosseries O. Vol. 18. Lancet Publishing Group; 2019. Therapeutic interventions in patients with prolonged disorders of consciousness; pp. 600–614. (The lancet neurology). [DOI] [PubMed] [Google Scholar]
- Turgeon A.F., Lauzier F., Simard J.-F., Scales D.C., Burns K.E.A., Moore L., Zygun D.A., Bernard F., Meade M.O., Dung T.C., Ratnapalan M., Todd S., Harlock J., Fergusson D.A. Withdrawing life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. Cmaj. 2011;183(14):1581–1588. doi: 10.1503/cmaj.110974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss H.U., Ulǔg A.M., Dyke J.P., Watts R., Kobylarz E.J., McCandliss B.D., Heier L.A., Beattie B.J., Hamacher K.A., Vallabhajosula S., Goldsmith S.J., Ballon D., Giacino J.T., Schiff N.D. Possible axonal regrowth in late recovery from the minimally conscious state. The Journal of Clinical Investigation. 2006;116(7):2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.T., Conte M.M., Goldfine A.M., Noirhomme Q., Gosseries O., Thonnard M., Beattie B., Hersh J., Katz D.I., Victor J.D., Laureys S., Schiff N.D. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. ELife. 2013;2013(2) doi: 10.7554/ELIFE.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Bai Y., Zhou Y., Yang Y., Xu R., Gao X., Li X., He J. Effects of 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Frontiers in Neurology. 2017;8:1–8. doi: 10.3389/fneur.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Id E.N. BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PLoS ONE. 2018;13(11):1–18. doi: 10.1371/journal.pone.0207281. Editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap J.Y.Y., Keatch C., Lambert E., Woods W., Stoddart P.R., Kameneva T. Critical review of transcutaneous vagus nerve stimulation: Challenges for translation to clinical practice. Frontiers in Neuroscience. 2020;14 doi: 10.3389/FNINS.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Yang Y., Wang L., Fang J., Chen Y., He J., Rong P. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI : The first case report. Brain Stimulation. 2017;10(2):328–330. doi: 10.1016/j.brs.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Yuan H., Silberstein S.D. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache. 2016;56(2):259–266. doi: 10.1111/HEAD.12650. [DOI] [PubMed] [Google Scholar]
- Zeman A. Vol. 150. Elsevier; 2005. The boundaries of consciousness: Neurobiology and neuropathology. (Progress in brain research). [DOI] [Google Scholar]
- Zhang Y., Song W. Transcranial direct current stimulation in disorders of consciousness: A review. International Journal of Neuroscience. 2018;128(3):255–261. doi: 10.1080/00207454.2017.1381094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.