Abstract
Background & Aims
A growing literature shows an improvement of chronic hepatitis C virus (HCV)-related depression after successful treatment with direct-acting antivirals. However, depression after HCV cure remains insufficiently documented in people living with HIV (PLWH) and HCV, a population with specific mental health challenges. This study aimed to (i) document the prevalence of moderate-to-severe depression (PHQ-9 score ≥10) across different age classes in HCV-cured PLWH; (ii) identify associated socio-behavioral correlates.
Methods
Descriptive analyses were performed on data collected during a cross-sectional survey (February 2018 - May 2019) nested in a prospective, multicenter cohort of individuals living with HIV and HCV (ANRS CO13 HEPAVIH). Socio-behavioral correlates of moderate-to-severe depression were identified using logistic regression.
Results
Among the 398 HCV-cured individuals in the study sample (median age [IQR]: 56 [53–59] years; 73.1% men), 23.9% presented with moderate-to-severe depression (PHQ-9 score ≥10). Depressive symptom prevalence rates were as follows: anhedonia: 52.3%; feeling ‘down’ or feelings of hopelessness: 48.3%; sleeping problems: 65.7%; lack of energy: 70.3%; eating disorders: 51.2%; lack of self-esteem: 34.3%; difficulty concentrating: 34.9%; sluggishness (in movement and voice) or restlessness: 24.6%; suicidal ideation: 17.1%. No significant difference was detected across age classes. Female sex, unhealthy alcohol use, sedentary lifestyle, and unhealthy eating behaviors were associated with increased odds of moderate-to-severe depression.
Conclusions
Depressive symptoms were common in this sample of HCV-cured PLWH. Unlike findings for the French general population, the prevalence of depression did not decrease with age class. Mental health remains a key issue for HIV-HCV-coinfected individuals, even after HCV cure, especially in women and in individuals with unhealthy behaviors.
Lay summary
Despite potential improvements in mental health after successful treatment with direct-acting antivirals, many people living with HIV (PLWH) and hepatitis C virus (HCV) - even in older age classes - still face depressive symptoms after HCV cure. In this population, women and people reporting unhealthy alcohol use, sedentary lifestyle, or unhealthy eating behaviors are more prone to report depressive symptoms after HCV cure. Mental health and lifestyle-related issues should be integrated in a global care model for PLWH living with or having a history of hepatitis C.
Keywords: Aging, Depression, Co-infection, HCV cure, HIV, Mental health
Abbreviations: HCV, hepatitis C virus; PHQ-9, Patient Heath Questionnaire-9; PLWH, people living with HIV
Graphical abstract
Highlights
-
•
Depressive symptoms are highly prevalent in people living with HIV (PLWH) after hepatitis C cure, especially in women.
-
•
Lifestyle-related factors, including alcohol use, level of physical activity and nutrition, correlate with depression.
-
•
A global care model is needed for PLWH living with or having a history of hepatitis C.
Introduction
Mental health disorders, including depression, are highly prevalent in people living with HIV (PLWH), with negative consequences on individuals’ perceived health and clinical outcomes.1 The prevalence of depression is even higher in people living with HIV and hepatitis C virus (HCV).2,3 This is because of several factors, including the impact of HCV on the central nervous system and, in many cases, vulnerabilities such as a history of injecting drug use and exposure to interferon-based therapy.
With life expectancy of PLWH approaching that of the general population, and HCV cure now possible for most individuals with HCV infection, addressing mental health needs has become a major challenge in the management of HIV-HCV coinfection. A growing literature shows significant improvements in mental health outcomes, including chronic HCV-related depression, after HCV cure thanks to successful treatment with direct-acting antivirals.4,5 However, depression after HCV cure remains insufficiently documented in individuals living with HIV and HCV, a population with specific mental health challenges. In addition, a recent survey showed a decline in the prevalence of depression with increasing age in the French general population.6 By contrast, in individuals living with HIV and HCV – who experience a faster aging process than the general population7,8 – there is a lack of data on the prevalence of depression across age classes. This study aimed to (i) document the prevalence of moderate-to-severe depression across different age classes in HCV-cured PLWH; (ii) identify associated socio-behavioral correlates.
Patients and methods
Data source
We used data from a multicenter cross-sectional survey conducted between February 2018 and May 2019 and nested in ANRS CO13 HEPAVIH, a French prospective cohort of people living with HIV and HCV.9 All patients enrolled in the ANRS CO13 HEPAVIH cohort study provided written informed consent. The cohort was designed and implemented in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Cochin University Hospital in Paris. Participation in the survey was proposed to all cohort patients. Survey participants answered a self-administered questionnaire to document their socio-behavioral characteristics, HCV status, and perceived health status. Information about cirrhosis status and time since HCV cure was also collected. The presence and severity of depressive symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9),10 a nine-item symptom checklist useable in routine HIV care11 which reflects criteria for major depression as laid out in DSM-IV (Fourth Edition of the Diagnostic and Statistical Manual of Mental Disorders). The PHQ-9 begins with a general question (“Over the past two weeks, how often have you been bothered by any of the following problems?”). Then, for each item, respondents are invited to choose the best possible answer among the following four modalities: “Never” (0); “Several days” (1); “More than half the days” (2); and “Nearly every day” (3) (Table 1). A score ranging from 0 to 27 can be calculated by summing the values associated with each answer. The higher the score, the more severe the level of depression, as follows: 0-4, none or minimal; 5-9, mild, 10-14, moderate; 15-19, moderately severe; and 20-27, severe. A PHQ-9 score equal to or greater than 10 therefore denoted moderate-to-severe depression.10 Socio-behavioral information collected using the self-administered questionnaire included sex, age, HCV transmission group, alcohol and tobacco use, physical activity, and eating behaviors. Unhealthy alcohol use was defined as an AUDIT-c (Alcohol Use Disorders Identification Test-Consumption)12 score above 4 for men, and above 3 for women. Not walking at least 10 consecutive minutes a day at least five days per week was considered a sedentary lifestyle. Eating behaviors were assessed using four questions addressing perceived unhealthy eating habits (eating too much, eating too fast, nibbling) and perceived diet balance. Reporting at least one unhealthy eating habit or unbalanced diet was considered reporting unhealthy eating behaviors. Plasma HIV viral load and CD4 T-cell count were described using data collected during the cohort follow-up visit closest to the date of the survey questionnaire administration.
Table 1.
Characteristics of the study sample and socio-behavioral correlates of moderate-to-severe depression, defined as a PHQ-9 score ≥10.
| Variables (% of missing values) | % of individuals | Univariable logistic regression models, N = 398 |
Multivariable logistic regression model, n = 348 |
||
|---|---|---|---|---|---|
| OR [95% CI] | p value | aOR [95% CI] | p value | ||
| Female sex (0) | 26.9 | 2.41 [1.47–3.93] | <0.0001 | 2.90 [1.69–5.00] | <0.0001 |
| Age in years (0) | |||||
| <53 | 20.6 | 1 | 0.667 | ||
| 53-59 | 55.3 | 1.13 [0.62–2.06] | 0.683 | ||
| ≥60 | 24.1 | 0.87 [0.43–1.78] | 0.708 | ||
| HCV transmission group (0) | |||||
| Injecting drug use | 54.5 | 1 | 0.526 | ||
| Male-to-male sex | 9.3 | 0.67 [0.28–1.61] | 0.373 | ||
| Heterosexual behaviors | 10.8 | 1.11 [0.53–2.32] | 0.775 | ||
| Other | 25.4 | 0.71 [0.40–1.26] | 0.244 | ||
| Unhealthy alcohol usea (1.3) | 34.6 | 1.48 [0.92–2.38] | 0.109 | 1.72 [1.01–2.92] | 0.047 |
| Tobacco use (3.0) | 56.7 | 2.14 [1.30–3.53] | 0.003 | ||
| Sedentary lifestyleb (11.3) | 40.8 | 1.69 [1.03–2.78] | 0.039 | 1.75 [1.05 - 2.94] | 0.033 |
| Unhealthy eating behaviorsc (0.5) | 47.2 | 2.04 [1.27–3.27] | 0.003 | 2.08 [1.23–3.51] | 0.006 |
Cross-sectional survey nested within the ANRS CO13 HEPAVIH cohort study, February 2018 - May 2019, N = 398.
Global p values are presented in bold for variables with more than two categories.
(a)OR (adjusted) odds ratios; CI, confidence interval; HCV, hepatitis C virus; PHQ-9, Patient Heath Questionnaire-9.
AUDIT-c (Alcohol Use Disorders Identification Test-Consumption)12 score ≥4 (3) for men (women).
Not walking at least 10 consecutive minutes a day at least five days per week was considered a sedentary lifestyle.
Eating behaviors were assessed using four questions addressing perceived unhealthy eating habits (eating too much, eating too fast, nibbling) and perceived diet balance. Reporting at least one unhealthy eating habit or unbalanced diet was considered reporting unhealthy eating behaviors.
Statistical analyses
Our study sample comprised HCV-cured survey participants with available data on depression (i.e., who answered at least 5 of the 9 items in the PHQ-9). The main characteristics of patients were documented using descriptive statistics (number and percentage of individuals for categorical variables, median and interquartile range (IQR) for continuous variables). Age and sex were compared between HCV-cured patients included in the study population and those excluded. Three age classes were built using the first and third quartiles of the distribution of age in the study sample as cut-off values. The PHQ-9 score was calculated with missing values in the checklist of symptoms recoded as zero. Based on previous studies in the cohort,9,13 we hypothesized that individuals may only have answered items which they felt concerned them. We therefore interpreted the absence of a response to a given item as the absence of the associated symptom. The PHQ-9 score and patients’ answers to each item of the questionnaire were then compared between age classes. Comparisons were performed using either the Chi-square test or the exact Fisher test (if the sample size was lower than five for at least one variable category) for categorical variables, and the Kruskal-Wallis test for continuous variables. Univariable and multivariable logistic regression models were used to identify socio-behavioral correlates of moderate-to-severe depression (defined as a PHQ-9 score ≥10). We tested the variable ‘center’ as a random effect in logistic regression models in order to account for potential differences between centers participating in the study. In order to verify that different methods of handling missing PHQ-9 data provided the same results, we performed two sensitivity analyses. In the first, individuals with missing values in the checklist of symptoms were excluded. In the second, multiple imputation (MICE) was used. Lastly, we introduced clinical characteristics in logistic regression models in a final sensitivity analysis, using multiple imputation to account for missing data. Interactions between sex and the other variables were tested in the multivariable model. Finally, we performed sex-stratified analyses to describe the prevalence of moderate-to-severe depression for men and women in each age class. The significance threshold was set at 5%. Stata 16.1 for Windows (StataCorp, College Station, Texas, USA) software was used for the analyses.
Results
Characteristics of the study sample and prevalence of moderate-to-severe depression
Among the 435 cross-sectional survey participants, 425 were HCV-cured after successful treatment. Of the latter, those who did not answer any item in the PHQ-9 (n = 23) and those who answered fewer than 5 of the 9 items (n = 4) were excluded from the analyses. The remaining 398 individuals (91.5% of survey participants) constituted the study sample. No significant difference in terms of age class or sex was found between individuals in the study sample and excluded ones (data not shown). Most individuals in the study sample were men (73.1%), median [IQR] age was 56 [53–59] years (maximum: 75 years), and 54.5% had been HCV-infected through injecting drug use. More than one-third (34.6%) reported unhealthy alcohol use, 56.7% were tobacco smokers, 40.8% reported a sedentary lifestyle (to be noted, data were missing for 11.3% of individuals for the corresponding physical activity items), and 47.2% reported unhealthy eating behaviors (Table 1). Eight percent of individuals in the study sample had a detectable HIV viral load, 6.5% had a CD4 T-cell count lower than 200 cells/mm3, and 13.5% had cirrhosis. Median time since HCV cure [IQR] was 2 [2–4] years (data available for 223 individuals).
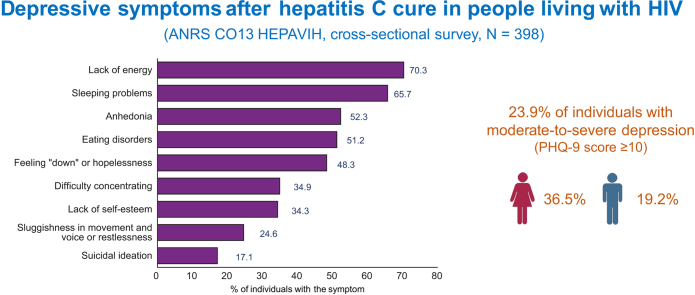
Ninety-five individuals (23.9%, 95% CI 19.8–28.4) presented moderate-to-severe depression (36.5% in women, 19.2% in men). Mild, moderate, moderately severe and severe depression concerned 31.2%, 14.8%, 6.5%, 2.5% of the study sample, respectively.
Socio-behavioral correlates of moderate-to-severe depression
Results of logistic regression models showed no significant relationship between age and the presence of moderate-to-severe depression (Table 1). In the multivariable analysis, being a woman (adjusted odds ratio [95% CI] 2.90 [1.69–5.00], p <0.0001), unhealthy alcohol use (1.72 [1.01–2.92]; p = 0.047), sedentary lifestyle (1.75 [1.05–2.94], p = 0.033), and unhealthy eating behaviors (2.08 [1.23–3.51], p = 0.006) were identified as independent correlates of moderate-to-severe depression (Table 1). Inclusion of the variable ‘center’ as a random effect in the model confirmed the robustness of this multivariable analysis (no change in the list of variables retained in the model, no substantial modification of estimated adjusted odds ratios and associated confidence intervals). Interactions between sex and the other variables were tested in the multivariable model, but were not significant (data not shown).
Depressive symptoms across age classes
No significant difference was detected between age classes either for the prevalence of moderate-to-severe depression or for the level of depression (Table 2). Just over half (52.3%) of the study sample had anhedonia, 48.3% felt ‘down’ or had feelings of hopelessness, 65.7% had sleeping problems, 70.3% a lack of energy, 51.2% eating disorders, 34.3% a lack of self-esteem, 34.9% difficulty concentrating, 24.6% sluggishness (in movement and voice) or restlessness, and 17.1% suicidal ideation (Fig. 1). No significant difference was detected between age classes in terms of the frequency of these symptoms, or the percentage of patients receiving treatment for depression (Table 2). In the sex-stratified analyses, no significant difference was detected between age classes for the prevalence of moderate-to-severe depression (40.7%, 34.6%, and 35.7% of women with moderate-to-severe depression in the three age classes, p = 0.862; and 14.6%, 22.6%, and 14.7% of men with moderate-to-severe depression in the three age classes, p = 0.233).
Table 2.
Depressive symptoms across age classes in HCV-cured people living with HIV.
| Whole study sample (N = 398) |
Age classes (in years) |
p valuea | |||
|---|---|---|---|---|---|
| Under 53 (n = 82) |
Between 53 and 59 (n = 220) |
Over 59 (n = 96) |
|||
| Variables (% of missing values) | No. of individuals (%) or median [IQR] | ||||
|
PHQ-910depression score | |||||
| Continuous score (0) | 5 [2–9] | 5 [2–9] | 6 [2–10] | 4 [1–9] | 0.612 |
| Severity of depressionb (0) | 0.802 | ||||
| None or minimal (0–4) | 179 (45.0) | 37 (45.1) | 91 (41.4) | 51 (53.1) | |
| Mild (5–9) | 124 (31.2) | 26 (31.7) | 73 (33.2) | 25 (26.0) | |
| Moderate (10–14) | 59 (14.8) | 12 (14.6) | 36 (16.4) | 11 (11.5) | |
| Moderately severe (15–19) | 26 (6.5) | 5 (6.1) | 15 (6.8) | 6 (6.3) | |
| Severe (20–27) | 10 (2.5) | 2 (2.4) | 5 (2.3) | 3 (3.1) | |
|
Moderate-to-severe depression (score ≥10) (0) |
95 (23.9) |
19 (23.2) |
56 (25.5) |
20 (20.8) |
0.666 |
|
Answers to the nine items in PHQ-9 | |||||
| 1. Little interest or pleasure in doing things (1.9) | 0.598 | ||||
| Never | 186 (47.7) | 34 (43.0) | 104 (47.7) | 48 (51.6) | |
| Several days | 117 (30.0) | 26 (32.9) | 67 (30.7) | 24 (25.8) | |
| More than half the days | 52 (13.3) | 14 (17.7) | 28 (12.8) | 10 (10.8) | |
| Nearly every day | 35 (9.0) | 5 (6.3) | 19 (8.7) | 11 (11.8) | |
| 2. Feeling ‘down’, depressed, or hopeless (2.2) | 0.278 | ||||
| Never | 201 (51.7) | 40 (50.0) | 113 (52.1) | 48 (52.2) | |
| Several days | 126 (32.4) | 31 (38.8) | 67 (30.9) | 28 (30.4) | |
| More than half the days | 41 (10.5) | 6 (7.5) | 28 (12.9) | 7 (7.6) | |
| Nearly every day | 21 (5.4) | 3 (3.8) | 9 (4.2) | 9 (9.8) | |
| 3. Trouble falling asleep or staying asleep, or sleeping too much (1.0) | 0.467 | ||||
| Never | 135 (34.3) | 28 (34.2) | 67 (30.5) | 40 (43.5) | |
| Several days | 131 (33.5) | 29 (35.4) | 77 (35.0) | 25 (27.2) | |
| More than half the days | 61 (15.5) | 11 (13.4) | 38 (17.3) | 12 (13.0) | |
| Nearly every day | 67 (17.0) | 14 (17.1) | 38 (17.3) | 15 (16.3) | |
| 4. Feeling tired or having little energy (1.7) | 0.639 | ||||
| Never | 116 (29.7) | 23 (28.1) | 62 (28.8) | 31 (33.0) | |
| Several days | 169 (43.2) | 36 (43.9) | 98 (45.6) | 35 (37.2) | |
| More than half the days | 63 (16.1) | 16 (19.5) | 29 (13.5) | 18 (19.2) | |
| Nearly every day | 43 (11.0) | 7 (8.5) | 26 (12.1) | 10 (10.6) | |
| 5. Poor appetite or overeating (2.2) | 0.493 | ||||
| Never | 190 (48.8) | 33 (40.2) | 107 (49.5) | 50 (55.0) | |
| Several days | 100 (25.7) | 27 (32.9) | 55 (25.5) | 18 (19.8) | |
| More than half the days | 47 (12.1) | 11 (13.4) | 24 (11.1) | 12 (13.2) | |
| Nearly every day | 52 (13.4) | 11 (13.4) | 30 (13.9) | 11 (12.1) | |
| 6. Feeling bad about yourself, or that you are a failure or have let yourself or your family down (1.4) | 0.984 | ||||
| Never | 258 (65.6) | 56 (68.3) | 138 (63.3) | 64 (68.8) | |
| Several days | 76 (19.3) | 15 (18.3) | 45 (20.6) | 16 (17.2) | |
| More than half the days | 36 (9.2) | 7 (8.5) | 21 (9.6) | 8 (8.6) | |
| Nearly every day | 23 (5.9) | 4 (4.9) | 14 (6.4) | 5 (5.4) | |
| 7. Difficulty concentrating on things, such as reading the newspaper or watching television (1.2) | 0.479 | ||||
| Never | 256 (65.1) | 56 (69.1) | 134 (61.5) | 66 (70.2) | |
| Several days | 85 (21.6) | 18 (22.2) | 51 (23.4) | 16 (17.0) | |
| More than half the days | 27 (6.9) | 2 (2.5) | 18 (8.3) | 7 (7.5) | |
| Nearly every day | 25 (6.4) | 5 (6.2) | 15 (6.9) | 5 (5.3) | |
| 8. Moving or speaking so slowly that people could have noticed. Or the opposite: being so fidgety or restless you have been moving around a lot more than usual (2.9) | 0.813 | ||||
| Never | 291 (75.4) | 61 (78.2) | 161 (75.6) | 69 (72.6) | |
| Several days | 48 (12.4) | 8 (10.3) | 29 (13.6) | 11 (11.6) | |
| More than half the days | 32 (8.3) | 7 (9.0) | 16 (7.5) | 9 (9.5) | |
| Nearly every day | 15 (3.9) | 2 (2.6) | 7 (3.3) | 6 (6.3) | |
| 9. Thoughts that you would be better off dead, or of hurting yourself in some way (1.4) | 0.719 | ||||
| Never | 325 (82.9) | 65 (81.3) | 183 (84.3) | 77 (81.1) | |
| Several days | 42 (10.7) | 11 (13.8) | 18 (8.3) | 13 (13.7) | |
| More than half the days | 17 (4.3) | 3 (3.8) | 11 (5.1) | 3 (3.2) | |
| Nearly every day |
8 (2.0) |
1 (1.3) |
5 (2.3) |
2 (2.1) |
|
|
Treatment for depression | |||||
| Receiving treatment for depression (5.8) | 60 (16.0) | 11 (14.3) | 34 (16.0) | 15 (17.4) | 0.860 |
Cross-sectional survey nested within the ANRS CO13 HEPAVIH cohort study, February 2018 - May 2019, N = 398.
HCV, hepatitis C virus; IQR, interquartile range; PHQ-9, Patient Heath Questionnaire-9.
Comparison between age classes using the Chi-square or Fisher’s exact test (for categorical variables) and the Kruskal-Wallis test (for continuous variables).
PHQ-9 score range for the different degrees of depression severity: none or minimal (0-4), mild (5-9), moderate (10-14), moderately severe (15-19), severe (20-27).
Fig. 1.
Percentage of HCV-cured people living with HIV affected by the nine depressive symptoms assessed by the PHQ-9.
PHQ-9, Patient Heath Questionnaire-9 (cross-sectional survey nested within the ANRS CO13 HEPAVIH cohort study, February 2018–May 2019, N = 398).
Sensitivity analyses
In the sensitivity analysis, which excluded the 44 individuals with missing values in the PHQ-9 checklist of symptoms, the prevalence of moderate-to-severe depression was estimated at 23.5% (95% CI 19.3–28.2). In the multiple imputation sensitivity analysis, this prevalence was estimated at 23.9% (95% CI 19.9–28.4). The results of the main analysis concerning comparisons between age classes and identification of socio-behavioral correlates of moderate-to-severe depression were confirmed in these two sensitivity analyses (data not shown).
When included in the models, plasma HIV viral load detectability, CD4 T-cell count, time since HCV cure and cirrhosis status were not significantly associated with moderate-to-severe depression (data not shown).
Discussion
In this sample of HCV-cured PLWH with a median age of 56 years, the prevalence of depression was high, with more than one-quarter of the sample having moderate-to-severe depression. This is more than three times the prevalence of depression in the French general population (estimated at 7.03% according to a recent European survey using the PHQ14). Interestingly, unlike findings in a recent general population-based survey led in France,6 neither the prevalence nor severity of depression varied significantly with age. In that survey, prevalence decreased linearly after 45 years of age. Our results reflect clinical mental health data collected in a previous study on the same cohort.15 However, comparisons are difficult due to differences in the scales used to assess depression and in the HCV treatments available at the time of each study. Our results are consistent with those of a recent review study3 which showed that people living with HIV and HCV were significantly more likely to report depressive symptoms than HIV- or HCV-monoinfected people. We found no significant difference between age classes in terms of the presence of each depressive symptom studied. These findings confirm that mental health remains a key issue for individuals living with HIV and HCV, even after HCV cure. Promising results from the literature demonstrate that depressive symptoms decrease in many individuals with chronic HCV infection successfully treated with direct-acting antiviral therapy.4,5 However, real-life data are still lacking to document depression after HCV cure in previously HIV-HCV-coinfected individuals.
In the present study, the odds of having moderate-to-severe depression were nearly three times higher in women compared with men. This is in line with what is observed in the French general population.6 Differences in the prevalence of depression between men and women are well-reported, with both psychosocial and biological mechanisms potentially at play.[16], [17], [18] Findings from sex-stratified analyses suggested a potential decrease in the prevalence of moderate-to-severe depression with age among women (as observed in the French general population). However, sex-stratified comparisons of prevalence rates between age classes showed no statistically significant difference.
Our study also identified several behavioral correlates of depressive symptoms, including unhealthy alcohol use, sedentary lifestyle, and unhealthy eating behaviors. While we may hypothesize the existence of a bidirectional relationship between such behavioral factors and depression, our findings underline the need for a global care model for the follow-up of PLWH treated and cured of hepatitis C. Since HCV cure is associated with significant improvements in quality of life, the period post-HCV cure may be an opportunity to manage addictive behaviors and adopt healthier lifestyles.9,[19], [20], [21]
This study is limited by its cross-sectional nature. Future studies should characterize the clinical profile of PLWH with moderate-to-severe depression after HCV cure, and investigate whether the observed sex effect on the prevalence of depression may be partially attributable to HIV- or HCV-related characteristics. Despite this limitation, our results are timely and provide a warning about the high prevalence of depression after HCV cure in HIV-HCV-coinfected individuals. The use of the PHQ-9 – a depression scale not specifically developed for aging people6 – may constitute another study limitation. However, a previous study found it was a valid instrument in aging and geriatric populations.22 Finally, the low number of women in the study sample prevented analysis of sex-specific correlates of moderate-to-severe depression. Larger studies are needed to identify potential differences between men and women in the patterns of correlates of depression after HCV cure.
To conclude, our results highlight the heavy burden of depression in aging PLWH with a history of hepatitis C, even after HCV cure, especially in women. Though bidirectional relationships exist between behavioral factors and depression, specific lifestyle-related behaviors such as unhealthy alcohol use, lack of physical activity, and nutrition should be addressed during post-cure follow-up, and integrated together with mental health in a global care model.
Financial support
This work was supported by the ANRS Emerging Infectious Diseases, with the participation of SIDACTION; Abbott France; Glaxo-Smith-Kline; Roche; Schering-Plough, BMS, Merck-Serono).
Conflict of interest
The authors have no conflict of interest to declare concerning this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Conceptualization: FM, PC, TB. Data curation: FM, CR, CP. Formal analysis: CR. Funding acquisition: FM, MBS, LW, DS, PC, PS. Investigation: SB, CG, CD, ER, DS, PS. Methodology: FM, CR, VdB, CP. Project administration: MBS, LW, DS, PC, PS. Resources: SB, CG, CD, ER, LW, DS, PS. Software: CR, VdB. Supervision: FM, CP, PC. Validation: FM, CR, CP, TB. Visualization: FM, CR, TB. Writing–original draft: FM, CR, TB. Writing–review and editing: FM, SB, CR, CP, CG, VdB, CD, MBS, ER, LW, DS, PC, PS, TB.
Data availability statement
Data are available upon request to the scientific committee of the ANRS CO13 HEPATHER cohort, which includes the authors of the manuscript (contact: pmcarrieri@aol.com).
Acknowledgements
The ANRS CO13 HEPAVIH cohort and the nested cross-sectional survey presented here were sponsored and funded by ANRS Emerging Infectious Diseases. Our thanks to all the members of the ANRS-CO13-HEPAVIH study group, the physicians and nurses involved in the cohort follow-up, and the patients who participated in the present survey. We also thank Jude Sweeney (Milan, Italy) for the English revision and copyediting of the manuscript.
The ANRS CO13 HEPAVIH Study Group:
Scientific Committee: D.Salmon (co-Principal investigator), L.Wittkop (co-Principal Investigator & Methodologist), P.Sogni (co-Principal Investigator), L. Esterle (project manager), P.Trimoulet, J.Izopet, L.Serfaty, V.Paradis, B.Spire, P.Carrieri, M.A.Valantin, G.Pialoux, J.Chas, O.Zaegel-Faucher, K.Barange, A.Naqvi, E.Rosenthal, A.Bicart-See, O.Bouchaud, A.Gervais, C.Lascoux-Combe, C.Goujard, K.Lacombe, C.Duvivier, D.Neau, P.Morlat, F.Bani-Sadr, L.Meyer, F.Boufassa, B.Autran, A.M.Roque, C.Solas, H.Fontaine, D.Costagliola, L.Piroth, A.Simon, D.Zucman, F.Boué, P.Miailhes, E.Billaud, H.Aumaître, D.Rey, G.Peytavin, V.Petrov-Sanchez, A. Levier.
Clinical Centers (ward: participating physicians): APHP, Hôpitaux Universitaires Paris Centre, Paris (Médecine Interne et Maladies Infectieuses: D. Salmon, R.Usubillaga; Hépato-gastro-entérologie: P.Sogni; Anatomo-pathologie: B.Terris; Virologie: P.Tremeaux); APHP Pitié-Salpétrière, Paris (Maladies Infectieuses et Tropicales: C.Katlama, M.A.Valantin, H.Stitou; Médecine Interne: A.Simon, P.Cacoub, S.Nafissa; Hépato-gastro-entérologie: Y.Benhamou; Anatomo-pathologie: F.Charlotte; Virologie: S.Fourati); AP-HM Sainte-Marguerite, Marseille (Service d'Immuno-Hématologie Clinique: I.Poizot-Martin, O.Zaegel, H.Laroche; Virologie: C.Tamalet); APHP Tenon, Paris (Maladies Infectieuses et Tropicales: G.Pialoux, J.Chas; Anatomo-pathologie: P.Callard, F.Bendjaballah; Virologie: C.Amiel, C.Le Pendeven); CHU Purpan, Toulouse (Maladies Infectieuses et Tropicales: B. Marchou; Médecine interne: L.Alric; Hépato-gastro-entérologie: K.Barange, S.Metivier; Anatomo-pathologie: J.Selves; Virologie: F.Larroquette); CHU Archet, Nice (Médecine Interne: E.Rosenthal; Infectiologie: A.Naqvi, V.Rio; Anatomo-pathologie: J.Haudebourg, M.C.Saint-Paul; Virologie: A. De Monte, V.Giordanengo, C.Partouche); APHP Avicenne, Bobigny (Médecine Interne–Unité VIH: O.Bouchaud; Anatomo-pathologie: A.Martin, M.Ziol; Virologie: Y.Baazia, V.Iwaka-Bande, A.Gerber); Hôpital Joseph Ducuing, Toulouse (Médecine Interne: M.Uzan, A.Bicart-See, D.Garipuy, M.J.Ferro-Collados; Anatomo-pathologie: J.Selves; Virologie: F.Nicot); APHP Bichat–Claude-Bernard, Paris (Maladies Infectieuses: A.Gervais, Y.Yazdanpanah; Anatomo-pathologie: H.Adle-Biassette; Virologie: G.Alexandre, Pharmacologie: G.Peytavin); APHP Saint-Louis, Paris (Maladies infectieuses: C.Lascoux-Combe, J.M.Molina; Anatomo-pathologie: P.Bertheau; Virologie: M.L.Chaix, C. Delaugerre, S. Maylin); APHP Saint-Antoine (Maladies Infectieuses et Tropicales: K. Lacombe, J. Bottero; J. Krause, P.M. Girard, Anatomo-pathologie: D. Wendum, P. Cervera, J. Adam; Virologie: C. Viala); APHP, Hôpitaux Paris Sud, Bicêtre, Paris (Maladies Infectieuses et Tropicales: D. Vittecocq; Médecine Interne: C. Goujard, Y. Quertainmont, E. Teicher; Virologie: C. Pallier); APHP Necker, Paris (Maladies Infectieuses et Tropicales: O. Lortholary, C. Duvivier, C. Rouzaud, J. Lourenco, F. Touam, C. Louisin: Virologie: V. Avettand-Fenoel, E. Gardiennet, A. Mélard); CHU Bordeaux Hôpital Pellegrin, Bordeaux (Maladies Infectieuses et Tropicales: D. Neau, A. Ochoa, E. Blanchard, S. Castet-Lafarie, C. Cazanave, D. Malvy, M. Dupon, H. Dutronc, F. Dauchy, L. Lacaze-Buzy, A. Desclaux; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas); CHU Bordeaux Hôpital Saint-André, Bordeaux (Médecine Interne et Maladies Infectieuses: P. Morlat, D. Lacoste, F. Bonnet, N. Bernard, M. Hessamfar, J, F. Paccalin, C. Martell, M. C. Pertusa, M. Vandenhende, P. Mercié, D. Malvy, T. Pistone, M.C. Receveur, M. Méchain, P. Duffau, C Rivoisy, I. Faure, S. Caldato; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas, P. Bellecave, C. Tumiotto); CHU Bordeaux Hôpital du Haut-Levêque, Bordeaux (Médecine Interne: J.L. Pellegrin, J.F. Viallard, E. Lazzaro, C. Greib; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimoulet, S. Reigadas); Hôpital FOCH, Suresnes (Médecine Interne: D. Zucman, C. Majerholc; Virologie: M. Brollo, E. Farfour); APHP Antoine Béclère, Clamart (Médecine Interne: F. Boué, J. Polo Devoto, I. Kansau, V. Chambrin, C. Pignon, L. Berroukeche, R. Fior, V. Martinez, S. Abgrall, M. Favier; Virologie: C. Deback); CHU Henri Mondor, Créteil (Immunologie Clinique: Y. Lévy, S. Dominguez, J.D. Lelièvre, A.S. Lascaux, G. Melica); CHU Nantes Hôpital Hôtel Dieu, Nantes (Maladies Infectieuses et Tropicales: E. Billaud, F. Raffi, C. Allavena, V. Reliquet, D. Boutoille, C. Biron; M. Lefebvre, N. Hall, S. Bouchez; Virologie: A. Rodallec, L. Le Guen, C. Hemon); Hôpital de la Croix Rousse, Lyon (Maladies Infectieuses et Tropicales: P. Miailhes, D. Peyramond, C. Chidiac, F. Ader, F. Biron, A. Boibieux, L. Cotte, T. Ferry, T. Perpoint, J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, M. Amiri, F Valour; Hépato-gastro-entérologie: J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, C. Augustin-Normand; Virologie: C. Scholtes, T.T. Le-Thi); CHU Dijon, Dijon (Département d'infectiologie: L. Piroth, P. Chavanet M. Duong Van Huyen, M. Buisson, A. Waldner-Combernoux, S. Mahy, A. Salmon Rousseau, C. Martins); CH Perpignan, Perpignan (Maladies infectieuses et tropicales: H. Aumaître, Virologie: S. Galim); CHU Robert Debré, Reims (Médecine interne, maladies infectieuses et immunologie clinique: F. Bani-Sadr, D. Lambert, Y Nguyen, J.L. Berger, M. Hentzien, Virologie: V. Brodard); CHRU Strasbourg (Le Trait d’Union: D Rey, M Partisani, ML Batard, C Cheneau, M Priester, C Bernard-Henry, E de Mautort, P Fischer, Virologie: P Gantner et S Fafi-Kremer).
Data collection: F.Roustant, P. Platterier, I. Kmiec, L. Traore, S. Lepuil, S. Parlier, V. Sicart-Payssan, E. Bedel, S. Anriamiandrisoa, C. Pomes, F. Touam, C. Louisin, M. Mole, C. Bolliot, P Catalan, M. Mebarki, A. Adda-Lievin, P. Thilbaut, Y. Ousidhoum, F.Z. Makhoukhi, O. Braik, R. Bayoud, C. Gatey, M.P. Pietri, V. Le Baut, R. Ben Rayana, D. Bornarel, C. Chesnel, D. Beniken, M. Pauchard, S. Akel, S. Caldato, C. Lions, A. Ivanova, A-S. Ritleg, C. Debreux, L. Chalal, J.Zelie, H. Hue, A. Soria, M. Cavellec, S. Breau, A. Joulie, P. Fisher, S. Gohier, D. Croisier-Bertin, S. Ogoudjobi, C. Brochier, V. Thoirain-Galvan, M. Le Cam.
Management, statistical analyses: P. Carrieri, M. Chalouni, V. Conte, L. Dequae-Merchadou, M. Desvallées, L. Esterle, C. Gilbert, S. Gillet, Q. Guillochon, C. Khan, R. Knight, F. Marcellin, L. Michel, M. Mora, C. Protopopescu, P. Roux, B. Spire, T. Barré, C. Ramier, A. Sow, C. Lions, V. Di Beo, M. Bureau, L Wittkop.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100614.
Contributor Information
Patrizia Carrieri, Email: pmcarrieri@aol.com.
the ANRS CO13 HEPAVIH Study Group:
D. Salmon, R. Usubillaga, P. Sogni, B. Terris, P. Tremeaux, C. Katlama, M.A. Valantin, H. Stitou, A. Simon, P. Cacoub, S. Nafissa, Y. Benhamou, F. Charlotte, Virologie: S. Fourati, I. Poizot-Martin, O. Zaegel, H. Laroche, C. Tamalet, G. Pialoux, J. Chas, P. Callard, F. Bendjaballah, C. Amiel, C. Le Pendeven, B. Marchou, L. Alric, K. Barange, S. Metivier, J. Selves, F. Larroquette, E. Rosenthal, Infectiologie, A. Naqvi, V. Rio, J. Haudebourg, M.C. Saint-Paul, A. De Monte, V. Giordanengo, C. Partouche, O. Bouchaud, A. Martin, M. Ziol, Y. Baazia, V. Iwaka-Bande, A. Gerber, M. Uzan, A. Bicart-See, D. Garipuy, M.J. Ferro-Collados, J. Selves, Virologie, F. Nicot, A. Gervais, Y. Yazdanpanah, H. Adle-Biassette, G. Alexandre, G. Peytavin, C. Lascoux-Combe, J.M. Molina, P. Bertheau, M.L. Chaix, C. Delaugerre, S. Maylin, K. Lacombe, J. Bottero, J. Krause, P.M. Girard, D. Wendum, P. Cervera, J. Adam, C. Viala, D. Vittecocq, C. Goujard, Y. Quertainmont, E. Teicher, C. Pallier, O. Lortholary, C. Duvivier, C. Rouzaud, J. Lourenco, F. Touam, C. Louisin, V. Avettand-Fenoel, E. Gardiennet, A. Mélard, D. Neau, A. Ochoa, E. Blanchard, S. Castet-Lafarie, C. Cazanave, D. Malvy, M. Dupon, H. Dutronc, F. Dauchy, L. Lacaze-Buzy, A. Desclaux, P. Bioulac-Sage, P. Trimoulet, S. Reigadas, P. Morlat, D. Lacoste, F. Bonnet, N. Bernard, M. Hessamfar, J, F. Paccalin, C. Martell, M.C. Pertusa, M. Vandenhende, P. Mercié, D. Malvy, T. Pistone, M.C. Receveur, M. Méchain, P. Duffau, C. Rivoisy, I. Faure, S. Caldato, P. Bioulac-Sage, P. Trimoulet, S. Reigadas, P. Bellecave, C. Tumiotto, J.L. Pellegrin, J.F. Viallard, E. Lazzaro, C. Greib, P. Bioulac-Sage, P. Trimoulet, S. Reigadas, D. Zucman, C. Majerholc, M. Brollo, E. Farfour, F. Boué, J. Polo Devoto, I. Kansau, V. Chambrin, C. Pignon, L. Berroukeche, R. Fior, V. Martinez, S. Abgrall, M. Favier, C. Deback, Y. Lévy, S. Dominguez, J.D. Lelièvre, A.S. Lascaux, G. Melica, E. Billaud, F. Raffi, C. Allavena, V. Reliquet, D. Boutoille, C. Biron, M. Lefebvre, N. Hall, S. Bouchez, A. Rodallec, L. Le Guen, C. Hemon, P. Miailhes, D. Peyramond, C. Chidiac, F. Ader, F. Biron, A. Boibieux, L. Cotte, T. Ferry, T. Perpoint, J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, M. Amiri, F. Valour, J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, C. Augustin-Normand, C. Scholtes, T.T. Le-Thi, L. Piroth, P. Chavanet, M. Duong Van Huyen, M. Buisson, A. Waldner-Combernoux, S. Mahy, A. Salmon Rousseau, C. Martins, H. Aumaître, S. Galim, F. Bani-Sadr, D. Lambert, Y. Nguyen, J.L. Berger, M. Hentzien, V. Brodard, D. Rey, M. Partisani, M.L. Batard, C. Cheneau, M. Priester, C. Bernard-Henry, E. de Mautort, P. Fischer, P. Gantner, and S. Fafi-Kremer
Scientific Committee:
F. Roustant, P. Platterier, I. Kmiec, L. Traore, S. Lepuil, S. Parlier, V. Sicart-Payssan, E. Bedel, S. Anriamiandrisoa, C. Pomes, F. Touam, C. Louisin, M. Mole, C. Bolliot, P. Catalan, M. Mebarki, A. Adda-Lievin, P. Thilbaut, Y. Ousidhoum, F.Z. Makhoukhi, O. Braik, R. Bayoud, C. Gatey, M.P. Pietri, V. Le Baut, R. Ben Rayana, D. Bornarel, C. Chesnel, D. Beniken, M. Pauchard, S. Akel, S. Caldato, C. Lions, A. Ivanova, A.-S. Ritleg, C. Debreux, L. Chalal, J. Zelie, H. Hue, A. Soria, M. Cavellec, S. Breau, A. Joulie, P. Fisher, S. Gohier, D. Croisier-Bertin, S. Ogoudjobi, C. Brochier, V. Thoirain-Galvan, M. Le Cam, D. Salmon, L. Wittkop, P. Sogni, L. Esterle, P. Trimoulet, J. Izopet, L. Serfaty, V. Paradis, B. Spire, P. Carrieri, M.A. Valantin, G. Pialoux, J. Chas, O. Zaegel-Faucher, K. Barange, A. Naqvi, E. Rosenthal, A. Bicart-See, O. Bouchaud, A. Gervais, C. Lascoux-Combe, C. Goujard, K. Lacombe, C. Duvivier, D. Neau, P. Morlat, F. Bani-Sadr, L. Meyer, F. Boufassa, B. Autran, A.M. Roque, C. Solas, H. Fontaine, D. Costagliola, L. Piroth, A. Simon, D. Zucman, F. Boué, P. Miailhes, E. Billaud, H. Aumaître, D. Rey, G. Peytavin, V. Petrov-Sanchez, and A. Levier
Clinical Centers (ward: participating physicians):
P. Carrieri, M. Chalouni, V. Conte, L. Dequae-Merchadou, M. Desvallées, L. Esterle, C. Gilbert, S. Gillet, Q. Guillochon, C. Khan, R. Knight, F. Marcellin, L. Michel, M. Mora, C. Protopopescu, P. Roux, B. Spire, T. Barré, C. Ramier, A. Sow, C. Lions, V. Di Beo, M. Bureau, and L. Wittkop
Supplementary data
The following are the supplementary data to this article:
References
- 1.Remien R.H., Stirratt M.J., Nguyen N., Robbins R.N., Pala A.N., Mellins C.A. Mental health and HIV/AIDS: the need for an integrated response. AIDS Lond Engl. 15 juill 2019;33(9):1411–1420. doi: 10.1097/QAD.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adinolfi L.E., Nevola R., Rinaldi L., Romano C., Giordano M. Chronic hepatitis C virus infection and depression. Clin Liver Dis. 2017;21(3):517–534. doi: 10.1016/j.cld.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Fialho R., Pereira M., Rusted J., Whale R. Depression in HIV and HCV co-infected patients: a systematic review and meta-analysis. Psychol Health Med. 2017;22(9):1089–1104. doi: 10.1080/13548506.2017.1280177. [DOI] [PubMed] [Google Scholar]
- 4.Kaur H., Dhiman R.K., Kulkarni A.V., Premkumar M., Singh V., Duseja A.K., et al. Improvement of chronic HCV infection-related depression, anxiety, and neurocognitive performance in persons achieving SVR-12: a real-world cohort study. J Viral Hepat. 2022;29(5):395–406. doi: 10.1111/jvh.13668. [DOI] [PubMed] [Google Scholar]
- 5.Pericot-Valverde I., Heo M., Niu J., Norton B.L., Akiyama M.J., Agyemang L., et al. Declines in depressive symptoms among people who inject drugs treated with direct-acting antivirals while on opioid agonist therapy. Open Forum Infect Dis. oct 2020;7(10):ofaa380. doi: 10.1093/ofid/ofaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Léon C., Chan Chee C., du Roscoät E., le groupe Baromètre, santé 2017. La dépression en France chez les 18-75 ans: résultats du Baromètre santé 2017. Bull Epidémiol Hebd. 2018;32–33:637–644. http://invs.santepubliquefrance.fr/beh/2018/32-33/2018_32-33_1.html [in French] [Google Scholar]
- 7.Pfefferbaum A., Zahr N.M., Sassoon S.A., Kwon D., Pohl K.M., Sullivan E.V. Accelerated and premature aging characterizing regional cortical volume loss in human immunodeficiency virus infection: contributions from alcohol, substance use, and hepatitis C coinfection. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(10):844–859. doi: 10.1016/j.bpsc.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Francesco D., Wit F.W., Bürkle A., Oehlke S., Kootstra N.A., Winston A., et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS Lond Engl. 01 2019;33(2):259–268. doi: 10.1097/QAD.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcellin F., Di Beo V., Esterle L., Abgrall S., Pialoux G., Barré T., et al. Post-HCV cure self-reported changes in physical activity, eating behaviours, and fatigue in people living with HIV (ANRS CO13 HEPAVIH) J Viral Hepat. nov 2021;28(11):1665–1667. doi: 10.1111/jvh.13605. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. sept 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards M., Quinlivan E.B., Bess K., Gaynes B.N., Heine A., Zinski A., et al. Implementation of PHQ-9 depression screening for HIV-infected patients in a real-world setting. J Assoc Nurses AIDS Care JANAC. juin 2014;25(3):243–252. doi: 10.1016/j.jana.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 13.Barré T., Mercié P., Lions C., Miailhes P., Zucman D., Aumaître H., et al. HCV cure: an appropriate moment to reduce cannabis use in people living with HIV? (ANRS CO13 HEPAVIH data) AIDS Res Ther. 15 mars 2022;19(1):15. doi: 10.1186/s12981-022-00440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias-de la Torre J., Vilagut G., Ronaldson A., Serrano-Blanco A., Martín V., Peters M., et al. Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. Lancet Public Health. oct 2021;6(10) doi: 10.1016/S2468-2667(21)00047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel L., Lions C., Winnock M., Lang J.P., Loko M.A., Rosenthal E., et al. Psychiatric and substance use disorders in HIV/hepatitis C virus (HCV)-coinfected patients: does HCV clearance matter? [Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS) HEPAVIH CO13 cohort] HIV Med. nov 2016;17(10):758–765. doi: 10.1111/hiv.12382. [DOI] [PubMed] [Google Scholar]
- 16.Shi P., Yang A., Zhao Q., Chen Z., Ren X., Dai Q. A hypothesis of gender differences in self-reporting symptom of depression: implications to solve under-diagnosis and under-treatment of depression in males. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.589687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labaka A., Goñi-Balentziaga O., Lebeña A., Pérez-Tejada J. Biological sex differences in depression: a systematic review. Biol Res Nurs. juill 2018;20(4):383–392. doi: 10.1177/1099800418776082. [DOI] [PubMed] [Google Scholar]
- 18.Salk R.H., Hyde J.S., Abramson L.Y. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. août 2017;143(8):783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight R., Roux P., Vilotitch A., Marcellin F., Rosenthal E., Esterle L., et al. Significant reductions in alcohol use after hepatitis C treatment: results from the ANRS CO13-HEPAVIH cohort. Addict Abingdon Engl. sept 2017;112(9):1669–1679. doi: 10.1111/add.13851. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.P., Edwards A., Reeve B.B., Golin C.E., Evon D.M. Symptoms and functioning improve after chronic hepatitis C cure as assessed by the memorial symptom assessment scale and PROMIS measures. J Viral Hepat. 29 Juin 2021 doi: 10.1111/jvh.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evon D.M., Sarkar S., Amador J., Lok A.S., Sterling R.K., Stewart P.W., et al. Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: the PROP UP study. J Hepatol. sept 2019;71(3):486–497. doi: 10.1016/j.jhep.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Wang S., Wang L., Yi X., Jia X., Jia C. Comparison of the geriatric depression scale-15 and the patient health questionnaire-9 for screening depression in older adults. Geriatr Gerontol Int. févr 2020;20(2):138–143. doi: 10.1111/ggi.13840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the scientific committee of the ANRS CO13 HEPATHER cohort, which includes the authors of the manuscript (contact: pmcarrieri@aol.com).