Abstract
Adhesion of Plasmodium falciparum-infected erythrocytes to the endothelial ligand intercellular adhesion molecule 1 (ICAM-1) has been implicated in the pathogenesis of cerebral malaria. Recently, a high-frequency coding polymorphism in the N-terminal domain of ICAM-1 (ICAM-1Kilifi) that is associated with susceptibility to cerebral disease in Kenya has been described. Preliminary static adhesion assays suggested that two different selected P. falciparum lines, ITO4-A4 (A4) and ItG-ICAM (ItG), have different properties of binding to the natural variant proteins ICAM-1 and ICAM-1Kilifi. Using a flow adhesion assay system, we have confirmed differences between the two lines in binding of parasitized erythrocytes to the variant ICAM-1 proteins. Total adhesion of ItG-infected erythrocytes to ICAM-1 and ICAM-1Kilifi is greater than that of A4-infected erythrocytes, and erythrocytes infected by both parasite strains show reduced binding to ICAM-1Kilifi. However, under these physiologically relevant flow conditions, we have shown differences between A4 and ItG strains in dynamic rolling behavior on ICAM-1Kilifi. The percentage of erythrocytes infected with A4 that roll on both ICAM-1 and ICAM-1Kilifi is greater than that of those infected with ItG. Also, the rolling velocity of A4-infected erythrocytes on ICAM-1Kilifi is markedly increased compared to that on ICAM-1, in contrast to the rolling velocity of ItG-infected erythrocytes, which is similar on both proteins. These findings suggest that different parasite lines can vary in their avidity for the same host ligand, which may have important consequences for the pathophysiology of P. falciparum malaria.
Erythrocytes infected with mature forms of Plasmodium falciparum sequester in the vascular beds of a variety of organs by a process of cytoadherence to endothelial cells (for a review, see reference 6). It is thought that this phenomenon is responsible for many of the pathological features of severe clinical diseases, including cerebral malaria (28). Several endothelial cell adhesion molecules have been identified as ligands for parasitized erythrocytes (PRBC). These include thrombospondin (21), CD36 (1, 17, 20), intracellular adhesion molecule 1 (ICAM-1) (4), E-selectin, vascular cell adhesion molecule 1 (19), chondroitin sulfate A (23), P-selectin (29), PECAM-1 (platelet endothelial cell adhesion molecule 1) (26), and the integrin αvβ3 (24). The identity of the parasite-encoded receptor(s) remains to be fully elucidated, but P. falciparum erythrocyte membrane protein 1 (PfEMP-1) has been shown to mediate binding to several of the host ligands, including ICAM-1 (2, 12).
Some studies have attempted to link binding to a particular endothelial ligand with organ-specific disease, such as chondroitin sulfate A in placental sequestration (11). In vivo studies of animals and of humans support a role for ICAM-1 as a sequestration receptor, particularly in the brain, where it is implicated in the pathology of cerebral malaria (13, 27, 31). The most direct evidence comes from immunohistochemical staining, which has revealed up-regulation of ICAM-1 and colocalization with adherent PRBC in the cerebral vasculature of fatal cases of falciparum malaria (27). Also, a large case control study in Kenya indicated a predominance of ICAM-1 binding isolates in children with cerebral disease (16).
P. falciparum malaria places a selective pressure on the host genome such that high-frequency polymorphisms have become fixed in susceptible populations (30). Recently a lysine-to-methionine change at position 29 in the coding sequence of ICAM-1 in Kenya and The Gambia at a gene frequency of 30% has been described. This region of ICAM-1 contains the binding site for P. falciparum. Surprisingly, individuals homozygous for this mutation are more at risk of cerebral malaria (10), although this susceptibility was detected in Kenya but not in The Gambia (3). Understanding how this polymorphism might differentially affect malaria susceptibility in distinct populations clearly requires knowledge of the functional properties of the two different ICAM-1 alleles. Static binding assays with infected erythrocytes do reveal some differences in adhesion. This is evident for one parasite clone with a relatively low avidity for ICAM-1 (ITO4-A4 [A4]) but not for a second with a much higher avidity (ItG-ICAM [ItG]) (A. G. Craig, unpublished data). However, there is now a large body of data for both malaria and the wider field of leukocyte adhesion that shows that static binding assays can be misleading when applied to interactions that normally occur under physiological conditions of flow in the bloodstream. In the case of malaria this is epitomized by a knockout experiment in the KAHRP (knob-associated histidine-rich protein) gene, in which the mutant adhered normally in static assays but hardly at all under flow conditions (9). This is particularly important in the case of PRBC adhesion to ICAM-1 as A4-infected erythrocytes adhere statically to CD36 but roll on ICAM-1 under flow conditions (7).
In this study we have extended the functional analysis of ICAM-1Kilifi by investigating the interactions of the A4 and ItG lines on ICAM-1ref and ICAM-1Kilifi under flow conditions as well as by demonstrating differences in adhesion to ICAM-1 between the two variant parasite lines.
MATERIALS AND METHODS
Consumables.
Molecular biology-grade chemicals and sterile tissue culture media were obtained from Sigma Chemical Co. (Dorset, United Kingdom) unless otherwise stated. Falcon tissue culture plasticware was obtained from Becton Dickinson (Oxford, United Kingdom). Antibodies used were anti-ICAM-1 antibodies 15.2, a gift of Nancy Hogg (Imperial Cancer Research Fund, London, United Kingdom), and BBA4 (British Biotechnology, Cowley, United Kingdom) and an anti-CD31 antibody (JC70; Dako Ltd., High Wycombe, United Kingdom). The A4-specific anti-PfEMP-1 monoclonal antibody (MAb) BC6 was a gift from Dave Roberts (Molecular Parasitology Laboratory, Oxford, United Kingdom).
Malarial parasites.
P. falciparum lines A4, originally derived from the Brazilian line IT4/25/5 (22), and ItG (also derived from IT4/25/5 but with repeated selection on ICAM-1) were selected a further two times. A4 was selected on the MAb BC6 (which recognizes the A4-specific PfEMP-1 molecule), while ItG was selected on ICAM-1. Both selections were carried out as described previously (18). Subsequent fluorescence-activated cell sorter analysis of A4 with BC6 showed positive staining on the surface of 60% of infected erythrocytes. Selected parasite stabilates were frozen in batches of at least 40 to maintain phenotypic consistency in subsequent experiments.
Aliquots of parasites were thawed and cultured in RPMI 1640 medium supplemented with 2 mM l-glutamine, 37.5 mM HEPES, 10 mM glucose, 25 μg of gentamicin per ml, and 10% human serum at a pH of 7.2 under a gas mixture of 96% nitrogen, 3% carbon dioxide, and 1% oxygen (25) for 72 h before use. Parasites were then cultured for up to 5 days (with one sorbitol selection on the second day to maintain synchrony of the population).
Parasite cultures were washed twice in binding medium (RPMI 1640 medium supplemented with 25 mM HEPES and 10 mM glucose [no HCO3−] [final pH, 7.2]) prior to use. The cultures were then resuspended in the same medium to a final hematocrit of 1 or 3% parasitemia, as assessed by Coulter counting and Giemsa staining.
Preparation of protein-coated microslides.
Plastic microslides (Camlab, Cambridge, United Kingdom) were treated with 3-aminopropyltriethoxysilane (7). Recombinant protein chimeras of wild-type ICAM-1 (ICAM-1ref) (5) and ICAM-1Kilifi (10), consisting of the five extracellular domains of ICAM-1ref and ICAM-1Kilifi, respectively, linked to the Fc portion of human immunoglobulin G1, were purified from transiently transfected COS cells (5).
A range of concentrations of the proteins were prepared in phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA) and coated onto microslides for 2 h at 37°C in a humidified incubator. Slides were then washed twice and blocked for 1 h at 37°C with PBS plus 1% BSA.
Enzyme-linked immunosorbent assays (ELISAs) were performed with a range of protein concentrations to calculate the protein concentration necessary to give equal site densities of ICAM-1ref and ICAM-1Kilifi proteins on the microslides. Control microslides which had no protein coating but were blocked in PBS plus 1% BSA were included in the ELISAs and flow adhesion assays.
Flow-based adhesion assay.
The flow system was based on that described by Nash et al. (15). Briefly, the protein-coated or blocked microslide was placed on the stage of an inverted Leica microscope (Micro Instruments, Whitney, United Kingdom), and the ends were connected via silicone tubing to a three-way valve (Lee Products, Buckingham, United Kingdom). The valve allowed either cell-free binding medium or parasite solution to be drawn across the slide when connected to a Harvard constant infusion-withdrawal pump (Harvard Apparatus, Natick, Mass.). Flow rates were chosen to yield a wall shear stress level of either 0.05 or 0.1 Pa. The entire stage and the solutions were enclosed in an incubated Perspex case. This allowed the temperature of the contents to be maintained at 37°C.
After the microslide was washed for 1 min with cell-free binding medium, parasite suspension was drawn across the slide for a total of 6 min. Between 2 and 6 min, a video recording of binding PRBC was made, typically in nine separate fields of view. This allowed subsequent off-line analysis to determine the percentage of cells that were stationary and rolling and the velocity of the rolling cells. After 6 min, PRBC flow was exchanged for cell-free binding buffer and adherent PRBC (both rolling and static) were counted immediately in nine fields of view of a 0.25 mm2 graticule. Values were then normalized to the number of adherent cells/mm2/107 cells perfused.
To minimize variation during examination of the effect of protein concentration, for each condition, the same slide was used for both cultures. Adherent PRBC from each line were removed by discharging cell-free binding medium from a syringe at a high level of shear stress across the microslide. Visual observation confirmed the removal of cells. Parallel experiments in which A4- or ItG-infected cells were perfused across the washed slide were carried out to ensure that the adherence of one strain and its subsequent removal had no effect on adherence of the other (data not shown).
Antibodies.
Antibodies were diluted in PBS supplemented with 1% BSA to final concentrations of 5 to 100 μg/ml. These were then incubated on protein-coated, blocked microslides at 37°C for 1 h prior to adhesion assays. After each assay was completed, an indirect ELISA was performed to confirm that antibody was still bound to the slide.
Off-line measurement of rolling velocity and statistical analyses.
Sequential still frames of the video recording were digitized by using the built-in video input of a Macintosh G3/233 computer. The acquisition of images and measurement of the movement of selected individual cells were automated with a macro written for the public domain Image program (developed at the U.S. National Institutes of Health and available on the Internet [http://rsb.info.nih.gov/nih-image/]). The linear distance between the locations of individual cells in two sequential frames was measured. The velocity was then calculated from the interval between the frames. The macro is available by e-mail from the authors. Data was analyzed with the Statview 4.1 program by using multiple analysis of variance to compare groups, with Bonferroni's correction for multiple comparisons.
RESULTS
Binding to ICAM-1ref under flow conditions.
In order to establish appropriate parameter values for the assays, we first examined the adhesion of both A4- and ItG-infected erythrocytes to ICAM-1ref under a range of shear stress levels and at a range of protein coating concentrations.
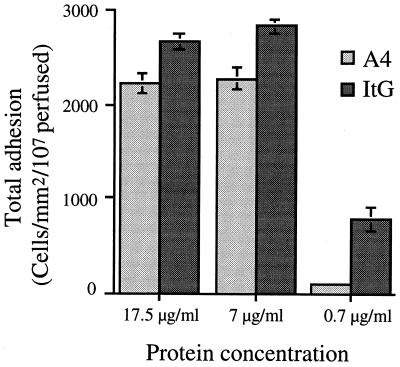
Figure 1 shows that at an inflow shear stress level of 0.05 Pa the total amount of adhesion of ItG-infected erythrocytes (rolling and static) was significantly higher than that of A4-infected erythrocytes at all concentrations of ICAM-1 (P < 0.0002). Binding of each clone was identical at 17.5 and 7 μg of ICAM-1 per ml (for ItG, P = 0.1993; for A4, P = 0.7495). At the lowest concentration of ICAM-1 tested (0.7 μg/ml), adhesion was almost completely abolished for A4-infected erythrocytes but was still detectable for ItG-infected erythrocytes. Increasing the inflow level to 0.1 Pa resulted in a five- to sevenfold decrease in binding of both lines, but no difference in relative levels of adhesion between the two lines was apparent (data not shown). Under these conditions, no binding of either clone was detectable at the lowest receptor concentration.
FIG. 1.
Differential total binding (rolling and static) of A4- and ItG-infected PBRC to ICAM-1ref at an inflow shear stress of 0.05 Pa. Experiment and analysis were carried out as described in Materials and Methods, and data represent the means ± standard errors (error bars) of the results of 3 to 10 experiments.
This established that at an inflow shear stress level of 0.05 Pa, binding to ICAM-1ref of both A4- and ItG-infected erythrocytes was saturated at a receptor concentration of 17.5 μg/ml. These conditions were therefore used in subsequent antibody blocking studies.
Inhibition of adhesion by MAbs under flow conditions.
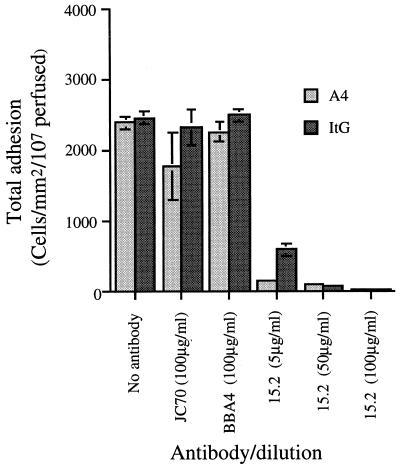
Two anti-ICAM-1 MAbs, both of which map to domain 1, were used in these experiments. MAb 15.2 blocks adhesion of both the leukocyte integrin LFA-1 (lymphocyte function-associated antigen 1) and erythrocytes infected with the A4 line of P. falciparum in static assays (5). BBA4 is an antibody which maps to the K29 region, since it binds to ICAM-1ref but not to ICAM-1Kilifi (10) and has been shown to cause partial inhibition of the binding of A4-infected erythrocytes to ICAM-1ref (A. G. Craig, unpublished data).
Adhesion of both parasites to ICAM-1ref was completely blocked by MAb 15.2, showing that adherence measured under flow conditions was of the correct specificity (Fig. 2). The same antibody also blocked adhesion of A4- and ItG-infected erythrocytes to ICAM-1Kilifi but was completely effective at only 5 μg/ml, whereas an antibody concentration of 50 μg/ml was required to inhibit adhesion to ICAM-1ref (data not shown).
FIG. 2.
Effect of antibody blockade on the total adhesion (rolling and static) of A4- and ItG-infected PBRC to ICAM-1ref at an ICAM-1 coating concentration of 17.5 μg/ml and an inflow shear stress of 0.05 Pa. Experimental details are described in Materials and Methods, and data represent the means ± standard errors (error bars) of the results of two experiments.
Unlike the partial inhibition of A4 binding seen in static adhesion assays, MAb BBA4 had no effect on total adhesion of erythrocytes infected by either parasite to ICAM-1ref, nor did it affect the percentage of the population that was rolling or the rolling velocity of either clone (data not shown).
Comparative binding of A4- and ItG-infected PRBC to ICAM-1Kilifi and ICAM-1ref under flow conditions. (i) Total adhesion.
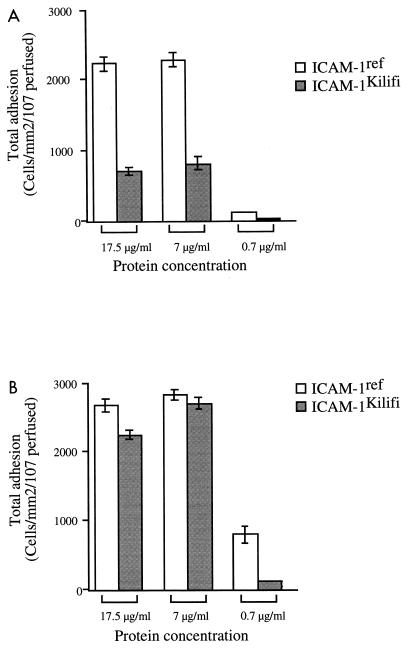
Next we compared binding of the two parasite clones to both ICAM-1 variants under the same conditions, to determine whether the change in structure of the ICAM-1Kilifi protein had any effect on binding of the parasite clones under flow conditions. At 0.05 Pa, the total adhesion of A4-infected erythrocytes to ICAM-1Kilifi was reduced significantly to approximately 30% of that seen with the reference protein (P < 0.0001), whereas binding of ItG-infected erythrocytes to ICAM-1Kilifi was between 85 and 95% of binding to ICAM-1ref at similar receptor concentrations (Fig. 3).
FIG. 3.
Total adhesion (rolling and static) of A4-infected (A) and ItG-infected (B) PBRC on ICAM-1ref and ICAM-1Kilifi at an inflow shear stress of 0.05 Pa. Experiment and analysis were carried out as described in Materials and Methods, and data represent the means ± standard errors (error bars) of the results of 3 to 10 experiments.
In common with binding of A4- and ItG-infected erythrocytes to ICAM-1ref, total adhesion to ICAM-1Kilifi was also saturating at the two higher receptor concentrations and fell off rapidly at 0.7 μg/ml, with ItG being, once again, less sensitive to this change. At the higher shear stress level of 0.1 Pa, the same trends were observed but at greatly reduced levels of adhesion. No adhesion of either parasite at the lowest concentration of ICAM-1Kilifi was detected (results not shown).
(ii) Rolling adhesion.
In these experiments rolling was usually observed as a flipping motion of the PRBC across the ICAM-1-coated surface. However, for both lines of parasite, when the receptor concentration and/or the flow rate reduced the amount of adhesion, the rolling behavior became mixed. Some PRBC rolled very quickly for short periods of time before becoming detached from the substrate. Other PRBC tethered transiently to ICAM-1 such that an infected cell would adhere to the receptor briefly and then detach without rolling.
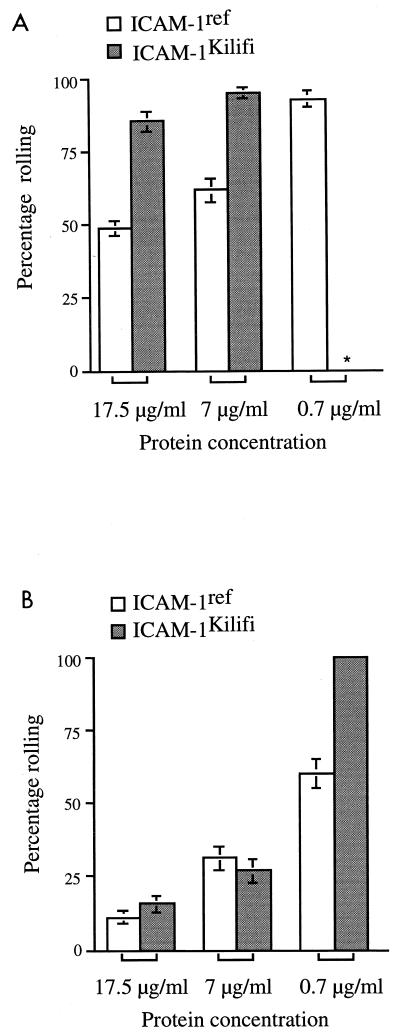
The percentage of the A4 and ItG populations that rolled on ICAM-1ref and ICAM-1Kilifi increased steadily with decreasing protein concentration at 0.05 Pa (Fig. 4). This was particularly noticeable at the lowest receptor concentration at which total adhesion was significantly reduced (Fig. 1). Additionally, the percentage of A4-infected erythrocytes rolling on both ICAM-1ref and ICAM-1Kilifi was consistently higher than that of ItG-infected erythrocytes (P < 0.0001), but this difference became less apparent when the receptor concentration was decreased, producing an increase in the rolling fraction of ItG-infected erythrocytes.
FIG. 4.
The percentages of A4-infected (A) and ItG-infected (B) populations that roll on ICAM-1ref and ICAM-1Kilifi at different concentrations and at an inflow shear stress of 0.05 Pa. ∗, no measurable adhesion (rolling or static). The experiment and analysis were carried out as described in Materials and Methods, and data represent the means ± standard errors (error bars) of the results of 3 to 10 experiments.
The percentage of A4-infected erythrocytes that rolled on ICAM-1Kilifi was significantly greater than that which rolled on ICAM-1ref (P < 0.0001) (Fig. 4A), and this percentage was relatively unaffected by variations in protein concentration (at which binding was detectable). In contrast, ItG-infected erythrocytes showed similar low levels of rolling at the two highest protein concentrations (P = 0.1189 at 17 μg/ml, P = 0.4393 at 7 μg/ml). However, at a 0.7-μg/ml coating concentration, the fraction of ItG-infected erythrocytes which rolled on ICAM-1Kilifi approached 100%, a value significantly higher than that seen for ICAM-1ref (P < 0.0001), implying that the Kilifi allele does affect this interaction (Fig. 4B).
Effect of flow rate on rolling velocity.
Because erythrocytes infected by the two parasite clones showed differences in degree of rolling on the variant proteins, we then measured the rolling velocity. At the two highest concentrations of ICAM-1ref, A4-infected erythrocytes rolled two to three times faster than ItG-infected erythrocytes (P = 0.0109 at 17 μg/ml, P = 0.0001 at 7 μg/ml) (Table 1). This trend and the absolute values for rolling velocity of the two lines was unaffected by increasing the flow rate. Velocity, like the percentage of rolling and total adhesion, only changed significantly when the receptor concentration was very low, when there was a marked increase for both A4- and ItG-infected cells. At this point, under low flow rates, erythrocytes infected by A4 were more significantly affected than those infected by ItG, such that there was a 10- to 15-fold difference in absolute rolling velocity between the two lines compared to a difference of two- to threefold at higher receptor concentrations.
TABLE 1.
The effect of various ICAM-1ref and ICAM-1Kilifi concentrations and inflow shear stress levels on the velocity of rolling PRBC
| Flow rate (Pa) | Receptor concentration (μg/ml) | Velocity (μm/s)a of PBRC infected by:
|
|||
|---|---|---|---|---|---|
| A4
|
ItG
|
||||
| ICAM-1ref | ICAM-1Kilifi | ICAM-1ref | ICAM-1Kilifi | ||
| 0.05 | 17.5 | 14.9 ± 1.5 | 130.2 ± 10.2 | 7 ± 1.6 | 2.1 ± 0.3 |
| 7 | 15.1 ± 0.6 | 125.8 ± 12.6 | 4 ± 0.3 | 3.7 ± 0.6 | |
| 0.7 | 160 ± 25.6 | 24 ± 2.8 | 251.2 ± 108.2 | ||
| 0.1 | 17.5 | 16.4 ± 2.5 | 116.5 ± 23.8 | 5 ± 1.8 | 2.7 ± 0.9 |
| 7 | 13.1 ± 1 | 75.5 ± 12.3 | 4.2 ± 0.8 | 3.7 ± 0.9 | |
| 0.7 | |||||
Experimental details are given in Materials and Methods, and values are the means ± standard errors of the results of 3 to 10 experiments.
The Kilifi mutation caused a 10-fold increase in the rolling velocity of A4-infected erythrocytes (in line with a decreased overall adhesion and increased proportion of rolling parasites) which was independent of flow rate and was maintained until adhesion was lost at the lowest concentration of receptor. In contrast, the rolling velocity of ItG-infected erythrocytes was slightly reduced (compared with no overall changes in total adhesion or rolling population) but, as for A4-infected erythrocytes, these values were maintained until the receptor was maximally diluted, when there was a dramatic increase in rolling velocity which was unaffected by increases in shear stress levels.
DISCUSSION
The clinical effects of malaria infection are mediated by a number of different mechanisms, including cytoadherence and induction of a host cytokine response (6). Adhesion of infected erythrocytes to endothelial cells is of prime importance in causing sequestration and mediating the pathological effects of cerebral malaria in the brain. Many endothelial ligands are known to mediate binding of PBRC, and a number of studies support a role for ICAM-1 binding in the pathology of cerebral malaria (13, 27, 31). Recently, an allelic form of this protein (ICAM-1Kilifi) (10) which has been associated with increased susceptibility to cerebral malaria in Kenya, but not The Gambia, has been described (3). We hypothesized that functional differences between the variant proteins might affect the binding characteristics of P. falciparum. Hence, we investigated the adhesion of specific strains of P. falciparum to both forms of ICAM-1 under flow conditions.
The strains of P. falciparum were selected to represent two adhesive phenotypes. Firstly, A4 represented a common adhesive phenotype, binding to ICAM-1 but also to other receptors, such as CD36. Secondly, we used the line ItG, which has been selected for increased binding to ICAM-1 (18). Since these experiments were carried out with laboratory-selected isolates, any results must be interpreted with caution, since in vivo there is marked heterogeneity in the binding phenotype of clinical PRBC isolates (8, 29). This may arise due either to the high frequency of antigenic switching (22) or to multiple infection within an individual. We attempted to simplify our system by the use of selected isolates and pure ICAM-1, but as was previously mentioned, there was still significant variation within the parasite populations.
ICAM-1ref bound both A4- and ItG-infected erythrocytes, but A4 binding was consistently lower than that of ItG. The adhesion of the latter was also more resistant to changes in receptor concentration and changing flow rates (Fig. 1). Several interpretations for these results are possible. Firstly, even a selected parasite population shows some degree of heterogeneity; hence, the higher levels of adhesion of ItG-infected erythrocytes may represent a greater percentage of ICAM-1 binding parasites in the perfused population. Secondly, ItG may bind to ICAM-1 with either a higher affinity, due to the characteristics of the expressed PfEMP-1 molecule, or a higher avidity, resulting from greater levels of expression of PfEMP-1 on the cell surface. This would imply a difference in the var gene expressed by the two strains, and it has been shown by PCR analysis that the PfEMP-1 molecule expressed by A4 is distinct from that expressed by ItG (S. Kyes, personal communication).
In addition to demonstrating differences in total binding, flow assays also reveal differences in the characteristics of adhesive interactions. Hence, a greater percentage of adherent A4-infected PBRC were observed to be rolling on ICAM-1ref compared to ItG-infected PBRC, but the difference became less marked when receptor concentration was reduced and the proportion of rolling ItG-infected PRBC increased (Fig. 4). The rolling velocities of the two strains on ICAM-1ref were also very different and not appreciably affected by protein concentration or increasing flow rate until very low coating concentrations were reached (Table 1). This suggests that the kinetics of binding may be different for the two lines. The ability to adhere from flow is associated with a high on rate, while rolling requires relatively rapid bond dissociation. Since the only difference between the interactions studied here is the identity of the PfEMP-1 molecule expressed by the two strains, this would suggest that the rolling phenotype and velocity of rolling are factors that are determined by the parasite ligand. Further studies will be required to elucidate the kinetic differences that underlie the binding properties of these two parasite strains.
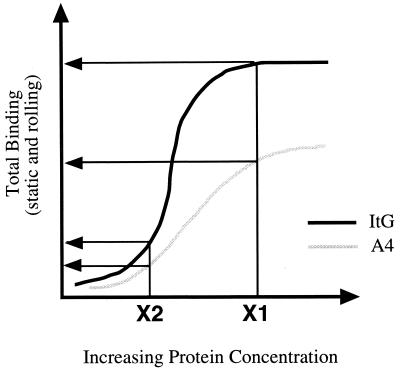
Our results show that rolling is not a prerequisite for static PRBC adhesion to ICAM-1. It has been previously suggested that ICAM-1 may act in concert with static receptors by anchoring the PRBC prior to firm adhesion in much the same way as selectins do during leukocyte capture from flow (7). Within the two populations, gradations in adhesion could be seen such that some cells adhered statically while others rolled, at a range of velocities. As such, A4 and ItG binding could be represented as two separate binding curves, such that ItG is displaced from A4 due to a higher average level of binding within the population (Fig. 5). For example, at a given concentration of receptor a level of binding would be attained which is higher for ItG. However, when protein concentration is reduced, stressing the binding, ItG behaves more like A4.
FIG. 5.
Hypothetical representation of the binding curves of erythrocytes infected by A4 and ItG strains on ICAM-1ref. The curves show what may happen to the total adhesion (rolling and static) when the receptor concentration and/or the flow rate is varied.
The K29M Kilifi mutation greatly reduced adhesion of A4-infected PRBC to ICAM-1, in contrast to ItG-infected PRBC, which showed no significant change in total binding (Fig. 3). In addition, while the percentage of A4-infected cells rolling and the rolling velocity both increased on ICAM-1Kilifi compared to ICAM-1ref, no change was observed for ItG-infected cells (Fig. 4). The decrease in total adhesion of A4-infected cells on ICAM-1Kilifi is in agreement with results obtained by a static binding assay (A. G. Craig, unpublished data), but only the flow-based assay was able to show differences in the rolling phenotype and thus a functional difference in binding to the two protein variants.
The effect of ICAM-1Kilifi on the binding of A4- and ItG-infected PRBC suggests differences in the binding interaction with ICAM-1 for these two parasite strains. In one model, the Kilifi mutation could either affect a residue important for A4 binding or alter the conformation of ICAM-1 in such a way as to destabilize the A4 interaction while not affecting the binding site for ItG. However, ELISA data previously obtained with a panel of ICAM-1 antibodies have revealed no gross structural alterations, suggesting that any such change would be subtle (10). Fine antibody mapping and site-directed mutagenesis would be needed to establish the validity of this hypothesis. However, the fact that antibody 15.2 can totally abrogate binding of both A4- and ItG-infected erythrocytes on both receptor types suggests that at least part of the binding site may be common for the two parasite strains. Alternatively, the differences seen between A4- and ItG-infected erythrocytes in adhesion to ICAM-1 could be due solely to differences in affinity or avidity of the different PfEMP1 molecules on the surface of erythrocytes infected with these two antigenically variant laboratory lines.
Although simplified, our observations may have implications for disease pathogenesis. In Kenya, the Kilifi variant is associated with increased susceptibility to cerebral malaria (10). In an individual homozygous for the Kilifi variant, high-affinity ICAM-1 binding lines (such as ItG) could have a competitive advantage over A4-like strains due to selection of high-affinity binding parasites within cerebral vessels. During severe malaria these vessels express high levels of ICAM-1 in the absence of significant levels of CD36 (27), in contrast with other vascular beds in the body. Selection of high-affinity ICAM-1 binding strains by ICAM-1Kilifi would promote sequestration while increasing clearance of low-affinity-binding parasites. A greater density of parasites may then prevail in the cerebral vasculature, resulting in the symptoms of cerebral malaria.
In summary, we have shown that different parasite strains binding to the same receptor, ICAM-1, show different binding phenotypes. Binding of erythrocytes infected by A4 parasites to the variant form ICAM-1Kilifi is reduced, whereas binding of erythrocytes infected by ItG is unaffected. These differences become most apparent under physiological flow conditions and may arise due to variations in binding affinity or avidity, differences in binding sites on the ICAM-1 molecule, or heterogeneity within the parasite population. Our results raise the possibility that host sequestration receptors may be able to select populations of highly adhesive parasites in vivo. Future work must therefore focus on examining flow adhesion of PRBC to ICAM-1 expressed in a cellular context, where the effects of lateral mobility within the membrane and synergy with other sequestration receptors (14) can be examined more closely.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust of Great Britain (grant no. 044055/Z/95/Z/140, 044508, and 045135/Z/95/Z) and the Royal Society of Great Britain (grant no. 574006.G503/19335).
We thank Andrew Gearing (British Biotechnology, Oxford, United Kingdom) and Nancy Hogg (ICRF Lincoln's Inn Field, London, United Kingdom) for the gifts of MAb BBA4 and MAb 15.2, respectively, and Chris Ockenhouse (WRAIR, Washington, D.C.) for providing parasite line ItG. We also thank Phil Stone, Robert Pinches, and Sarah Lee for expert technical assistance.
REFERENCES
- 1.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Investig. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch D I, Gormely J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein-1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule-1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy R, Kwiatkowski D, Hill A V. Absence of an association between intercellular adhesion molecule-1, complement receptor-1 and interleukin-1 receptor antagonist gene polymorphisms and severe malaria in a West African population. Trans R Soc Trop Med Hyg. 1998;92:312–316. doi: 10.1016/s0035-9203(98)91026-4. [DOI] [PubMed] [Google Scholar]
- 4.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 5.Berendt A R, McDowall A, Craig A G, Bates P A, Sternberg M J E, Marsh K, Newbold C I, Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1 binding site. Cell. 1992;68:71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- 6.Berendt A R, Ferguson D J, Gardner J, Turner G, Rowe A, McCormick C, Roberts D, Craig A, Pinches R, Elford B C, et al. Molecular mechanisms of sequestration in malaria. Parasitology. 1994;108:S19–S28. doi: 10.1017/s0031182000075685. [DOI] [PubMed] [Google Scholar]
- 7.Cooke B M, Berendt A R, Craig A G, MacGregor J, Newbold C I, Nash G B. Rolling and stationary cytoadhesion of red blood cells parasitized by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br J Haematol. 1994;87:162–170. doi: 10.1111/j.1365-2141.1994.tb04887.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooke B M, Morris-Jones S, Greenwood B M, Nash G B. Mechanisms of cytoadhesion of flowing, parasitized red blood cells from Gambian children with falciparum malaria. Am J Trop Med Hyg. 1995;53:29–35. [PubMed] [Google Scholar]
- 9.Crabb B S, Cooke B M, Reeder J C, Waller R F, Caruana S R, Davern K M, Wickham M E, Brown G V, Coppel R L, Cowman A F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Reyes D, Craig A G, Kyes S A, Peshu N, Snow R W, Berendt A R, Marsh K, Newbold C I. A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum Mol Genet. 1997;6:1357–1360. doi: 10.1093/hmg/6.8.1357. [DOI] [PubMed] [Google Scholar]
- 11.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 12.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul D K, Liu X D, Nagel R L, Shear H L. Microvascular hemodynamics and in vivo evidence for the role of intercellular adhesion molecule-1 in the sequestration of infected red blood cells in a mouse model of lethal malaria. Am J Trop Med Hyg. 1998;58:240–247. doi: 10.4269/ajtmh.1998.58.240. [DOI] [PubMed] [Google Scholar]
- 14.McCormick C J, Craig A, Roberts D, Newbold C I, Berendt A R. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Investig. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash G B, Cooke B M, Marsh K, Berendt A, Newbold C I, Stuart J. Rheological analysis of the adhesive interactions of red blood cells parasitized by Plasmodium falciparum. Blood. 1992;79:798–807. [PubMed] [Google Scholar]
- 16.Newbold C I, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 17.Ockenhouse C F, Tandon N N, Magowan C, Jamieson G A, Chulay J D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989;243:1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- 18.Ockenhouse C F, Ho M, Tandon N N, Van Seventer G A, Shaw S, White N J, Jamieson G A, Chulay J D, Webster H K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 19.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 21.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 22.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G B, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siano J P, Grady K K, Millet P, Wick T M. Short report. Plasmodium falciparum: cytoadherence to αvβ3 on human microvascular endothelial cells. Am J Trop Med Hyg. 1998;59:77–79. doi: 10.4269/ajtmh.1998.59.77. [DOI] [PubMed] [Google Scholar]
- 25.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 26.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 27.Turner G D H, Morrison H, Jones M, Davis T M, Looareesuwan S, Buley I D, Gatter K C, Newbold C I, Pukritayakamee S, Nagachinta B, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 28.Turner G D H. Cerebral malaria. Brain Pathol. 1997;7:569–582. doi: 10.1111/j.1750-3639.1997.tb01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udomsangpetch R, Reinhardt P H, Schollaardt T, Elliott J F, Kubes P, Ho M. Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J Immunol. 1997;158:4358–4364. [PubMed] [Google Scholar]
- 30.Weatherall D J. Common genetic disorders of the red cell and the ‘malaria hypothesis.’. Ann Trop Med Parasitol. 1987;81:539–548. doi: 10.1080/00034983.1987.11812155. [DOI] [PubMed] [Google Scholar]
- 31.Willimann K, Matile H, Weiss N A, Imhof B A. In vivo sequestration of Plasmodium falciparum-infected human erythrocytes: a severe combined immunodeficiency mouse model for cerebral malaria. J Exp Med. 1995;182:643–653. doi: 10.1084/jem.182.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]