Abstract
Anaplasma marginale is a tick-transmitted pathogen of cattle closely related to the human ehrlichiae, Ehrlichia chaffeensis and the agent of human granulocytic ehrlichiosis (HGE). These pathogens have in common a structurally conserved outer membrane protein (OMP) designated the major surface protein 2 (MSP-2) in A. marginale and HGE and OMP-1 in E. chaffeensis. Protective immunity against ehrlichial pathogens is believed to require induction of gamma interferon (IFN-γ) and opsonizing immunoglobulin (Ig) subclasses directed against OMP epitopes that, in concert, activate macrophages for phagocytosis and killing. Because interleukin-12 (IL-12) acts as an adjuvant for protein immunization to induce IFN-γ and protective immunity against intracellular pathogens, we hypothesized that as an adjuvant with MSP-2, IL-12 would augment type 1 recall responses to A. marginale. IL-12 was coadsorbed with MSP-2 to alum and shown to significantly enhance IFN-γ production by lymph node cells (LNC) and LNC-derived CD4+ T-cell lines from immunized calves following recall stimulation with A. marginale. LNC proliferation and IL-2 production were also enhanced in IL-12-treated calves. Elevated recall proliferative responses by peripheral blood mononuclear cells were still evident 9 months after immunization. Serum IgG levels were consistently increased in IL-12 immunized calves, predominantly due to higher IgG1 responses. The results support the use of IL-12 coadsorbed with OMP of ehrlichial pathogens in alum to amplify both antibody and type-1 cytokine responses important for protective immunity.
Anaplasma marginale is an important tick-transmitted rickettsial pathogen of cattle that invades and multiplies within erythrocytes, causing severe hemolytic anemia during acute infection. Recovery from acute disease leads to a state of persistent infection and allows subsequent transmission to immunologically naïve cattle. At least six outer membrane proteins (OMP) of A. marginale have been described (32, 34), and major surface protein 2 (MSP-2) is among the most immunodominant surface antigens. A. marginale is a member of the ehrlichial genogroup II, and MSP-2 shares significant structural and sequence homology with OMP homologues of other ehrlichiae. These include members of genogroup II, most notably the agent of human granulocytic ehrlichiosis (HGE), and the genogroup I pathogens Ehrlichia chaffeensis and Cowdria ruminantium (14, 18, 27, 30, 36, 51, 53).
Immunization with native MSP-2 derived from the Florida strain of A. marginale resulted in partial to complete protection in cattle challenged with homologous or heterologous strains (31). Immunization with outer membranes of A. marginale also provided protection to 70% of calves, and protection correlated with titers of antibody against MSP-2 (46). Furthermore, members of our group recently demonstrated complete protection against infection following homologous challenge in calves immunized with purified outer membranes of the Florida strain with saponin as an adjuvant (7). Complete protection was associated with development of immunoglobulin G2 (IgG2) responses directed predominantly against MSP-2 and with the production of gamma interferon (IFN-γ) by antigen-specific T cells. Several cloned CD4+ T-cell lines derived from the protected cattle were MSP-2 specific, but not strain specific, suggesting recognition of MSP-2 T-cell epitopes conserved among strains (9). This conservation is notable given the extensive structural variation encoded by the msp2 gene family, both within and between strains (15, 31, 34, 39). Thus, such conserved T-cell epitopes may be useful components of a subunit or nucleic acid vaccine designed to induce protective immunity against multiple strains of A. marginale.
CD4+ T cells are important for immunity against intraerythrocytic pathogens by activating macrophages through the production of IFN-γ (44) and by promoting enhanced IgG production (6). The importance of IFN-γ in protection against anaplasmosis has been recently reviewed (34). Briefly, in cattle IFN-γ enhances IgG2 production (6, 10), and activates macrophages to produce molecules, such as nitric oxide (NO), that are toxic for intraerythrocytic pathogens (references 20 and 44; L. K. M. Shoda, J. Florin-Christenson, M. Florin-Christenson, G. H. Palmer, and W. C. Brown, submitted for publication). In vitro incubation with A. marginale-immune serum neutralized the infectivity of A. marginale for cattle (33), and IgG2 may be involved in neutralization because of its superior ability to promote phagocytosis through opsonization (25). For these reasons, adjuvants that stimulate the production of IFN-γ during antigen priming and IgG antibodies are predicted to enhance protective immune responses.
IL-12 is a cytokine that when used as an adjuvant with a protein antigen can augment protective immunity against intracellular pathogens by stimulating IFN-γ production (1, 26). Numerous studies in mice have verified that interleukin-12 (IL-12), produced by dendritic cells and other antigen-presenting cells (APC) during T-cell priming, promotes a biased or enhanced Th1 cytokine response (17, 24, 40, 41, 43, 45, 47). When adsorbed together with a soluble protein in aluminum hydroxide (alum), IL-12 stimulated a polarized type 1 cytokine response but enhanced both type 1 (IgG2 and IgG3) and type 2 (IgG1) antibody responses in mice (19). Adsorption of IL-12 to alum appeared critical for maintaining serum IFN-γ levels, likely by prolonging the in vivo half-life of IL-12. Recently, it was demonstrated that the recall T-cell response to a protein antigen administered with IL-12 in phosphate buffered saline (PBS) during the primary antigen inoculation featured the type 1 cytokine and antibody responses observed immediately after priming. Interestingly, the memory response to antigen was additionally characterized by the development of type 2 cytokine and antibody responses not observed after priming (3). In vivo experiments performed with IL-12 as an adjuvant for cattle have not been reported. However, IL-12 stimulated enhanced IFN-γ production by mitogen- or antigen-stimulated bovine peripheral blood mononuclear cells (PBMC) and antigen-stimulated effector CD4+ T-cell clones (4). Furthermore, when added during in vitro activation and differentiation of memory T cells cultured with antigen, IL-12 induced production of IFN-γ that was significantly enhanced compared to that of cells cultured without exogenous IL-12 (49). In the present study, we hypothesized that IL-12 administered as an adjuvant to cattle with MSP-2 by coadsorption to alum would prime for enhanced type 1 recall responses characterized by increased production of IFN-γ in response to A. marginale stimulation by memory or effector CD4+ T cells. We observed that IL-12 stimulated enhanced IFN-γ and IL-2 secretion and transcript expression by antigen-primed lymph node cells (LNC) as well as enhanced serum IgG1 titers. Transcript levels of IL-4 and IL-10 were also elevated in LNC from IL-12-primed calves. Contrary to what was predicted, IL-12 did not uniformly stimulate IgG2 production, and the upregulation of IgG2 by one calf given IL-12 was not associated with high levels of IFN-γ production. These data suggest that factors in addition to IFN-γ may be important for promoting isotype switching to IgG2.
MATERIALS AND METHODS
Preparation of A. marginale antigens.
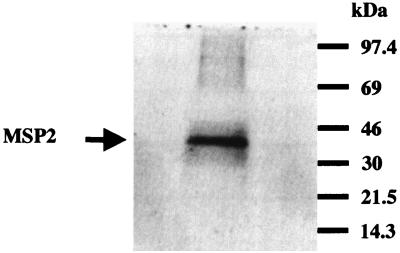
A splenectomized Holstein calf was infected with A. marginale (Florida strain) to propagate organisms for preparation of outer membranes and purified MSP-2. Native MSP-2 was gel purified to ensure that all of the potentially expressed MSP-2 copies in the Florida strain were represented in the immunogen. A. marginale organisms were isolated from thawed, infected bovine erythrocytes by sonication and differential ultracentrifugation as previously described (33), suspended in electrophoresis sample buffer, and separated on multiple preparative sodium dodecyl sulfate (SDS)–10% polyacrylamide gels. One gel was transferred and blotted with MSP-2-specific monoclonal antibody (MAb) as described previously (31) to orient the MSP-2 on the preparative gels. The MSP-2 band was excised from multiple gels, and the protein was electroeluted from the gel fragments as described previously (29). Eluted protein was concentrated and dialyzed against PBS (pH 7.2) and repurified a second time on preparative gels. Figure 1 shows the result of a SDS–12% polyacrylamide gel electrophoresis (SDS–12% PAGE) analysis of 3 μg of gel-purified MSP-2 used for inoculation. MSP-2 was verified by immunoblotting to be reactive with the antibodies specific for MSP-2 but unreactive with antibodies to other A. marginale MSP (data not shown).
FIG. 1.
SDS-PAGE analysis of gel-purified native MSP-2 from the Florida strain of A. marginale. MSP-2 (3 μg) was visualized on a Coomassie blue-stained 12% acrylamide gel (arrow). Molecular masses are indicated on the right of the gel.
To prepare antigen for in vitro assays, A. marginale Florida organisms were resuspended in PBS containing the protease inhibitors antipain and E-64 (Boehringer Mannheim, Indianapolis, Ind.) at a concentration of 25 μg/ml and phenylmethylsulfonyl fluoride (Sigma Chemical Co., St. Louis, Mo.) at a concentration of 300 μg/ml and were homogenized by two passages through a French pressure cell (SLM Instruments, Inc., Urbana, Ill.) at a pressure level of 1,500 lb/in2. Either homogenate or membranes prepared from the homogenate by sucrose density gradient centrifugation (7, 46) were used. Protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, Calif.).
Cattle and immunization with MSP-2.
Nine age-matched Holstein steers 4 to 5 months old and weighing approximately 150 to 200 kg at the start of the experiment were verified to be serologically negative for A. marginale by competitive inhibition enzyme-linked immunosorbent assay (ELISA) (21) and were assigned randomly to three groups. All calves received six 2-ml subcutaneous inoculations in the right and left sides of the neck as detailed in Table 1. Calves were administered alum and IL-12 (group I); alum and MSP-2 (group II); or alum, MSP-2, and IL-12 (group III). Human recombinant IL-12 was kindly provided by Genetics Institute, Inc., Cambridge, Mass. The dosage of IL-12 (approximately 50 ng/kg of body weight) used was 10-fold less than that used in human trials where toxicity was observed (22). In initial inoculations, 20 μg of MSP-2 was given, since this amount of antigen induced vigorous responses in other immunization studies (reference 37; W. C. Brown, T. C. McGuire, and G. H. Palmer, unpublished observations). After two injections, the amount of MSP-2 was increased, since weak antibody and proliferation responses were generated. Gel-purified MSP-2 and/or IL-12 was adsorbed in 20 mg of alum (Rehydragel, low viscosity sterile gel; Reheis, Inc., Berkeley Heights, N.J.) in 2 ml of sterile PBS according to the manufacturer's instructions. Serum samples and PBMC were collected before and at 2 weeks following each inoculation and assayed for A. marginale-specific antibody and proliferation, respectively. Once specific lymphocyte proliferation was observed, the animals were given a final antigen inoculation and the antigen-draining (right) and contralateral (left) prescapular lymph nodes (LN) were surgically biopsied to remove approximately one half of the LN. The timing of the sixth inoculation was staggered so that one calf per group was used at each time point (Table 1). LNC suspensions were prepared (38) and either were used immediately for proliferation assays, surface phenotype analysis, and cytokine determination or were cryopreserved in liquid nitrogen in a solution of 10% dimethylsulfoxide in fetal bovine serum.
TABLE 1.
Immunization protocol
| Week(s) | Amt of material in inoculationa
|
|||
|---|---|---|---|---|
| Leftc | Right
|
|||
| MSP-2 (μg) | IL-12 (μg) | Alum (mg) | ||
| 0 | 20 | 20 | 10 | 20 |
| 4 | 20 | 20 | 10 | 20 |
| 8 | 20 | 40 | 10 | 20 |
| 11 | 20 | 40 | 0 | 20 |
| 14.5 | 20 | 100 | 0 | 20 |
| 26, 31, 35b | 20 | 100 | 0 | 20 |
Calves were inoculated subcutaneously in the left side of the neck with a 2-ml inoculation containing alum alone and in the right side of the neck with a 2-ml inoculation containing alum adsorbed with the indicated amount of IL-12 and/or MSP-2 per injection. For the right inoculation, calves in group I (calves 56, 58, and 62) were administered alum and IL-12, calves in group II (calves 57, 63, and 65) were administered alum and MSP-2, and calves in group III (calves 59, 60, and 61) were administered alum, MSP-2, and IL-12.
The time of the final inoculation was staggered so that one calf from each group was inoculated at 26, 31, or 35 weeks. The lymph nodes were biopsied 3 days following the last antigen inoculation. Calves were inoculated as follows: on week 26, calves 62, 65, and 61; on week 31, calves 56, 63, and 60; and on week 35, calves 58, 57, and 59.
Values indicate amount of alum in inoculation (in milligrams).
Analysis of A. marginale-specific IgG1 and IgG2 responses.
SDS-PAGE and immunoblotting were performed with MAb specific for bovine IgG, IgG1, and IgG2 to determine the appearance of MSP-2-specific antibody and the subclass of the specific IgG response. The procedures were performed as described recently (7), with the following modifications. A. marginale (Florida strain) homogenate (100 μg of protein) was applied in a single lane to a 7.5 to 17.5% polyacrylamide gradient gel and electrophoresed, and the proteins were transferred to nitrocellulose membranes. The membranes were air-dried and immersed for 2 h in blocking solution consisting of PBS (pH 7.4) supplemented with 0.02% sodium azide, 0.1% Tween 20, and 10% normal horse serum (Vector Laboratories, Inc., Burlingame, Calif.) with gentle agitation. Following extensive washing in PBS-Tween (PBS [pH 7.4]–0.02% sodium azide–0.1% Tween 20), the membranes were placed in a Miniblotter 25 apparatus (Immunetics, Cambridge, Mass.) and 250 μl of bovine sera serially diluted (1:100 to 1:100,000) in blocking solution per well was added according to the manufacturer's protocol. The membranes were incubated for 2 h at room temperature on a rocking platform. Following successive washes in PBS-Tween with 0.1% NP-40 and PBS-Tween alone, the membranes were incubated overnight at 4°C and for an additional 1.5 h at room temperature on a rocker platform with murine anti-bovine IgG, IgG1, or IgG2 MAb (Serotec Ltd., Oxford, United Kingdom) diluted 1:100 in PBS-Tween with 2% horse serum. The membranes were washed extensively, and the reaction mixtures were then labeled for 1 h at room temperature with peroxidase-conjugated affinity-purified donkey anti-mouse IgG (heavy plus light chains; Jackson Immunoresearch Laboratories, West Grove, Pa.) diluted 1:5,000 in TNT buffer containing 1% horse serum. The membranes were washed repeatedly with TNT buffer, and the chemiluminescence was developed with a Renaissance Western blot chemiluminescence reagent (NEN Life Science Products, Boston, Mass.) according to the manufacturer's instructions.
A. marginale-specific T-cell lines.
Short-term T-cell lines were established from PBMC of A. marginale MSP-2-immunized calves from 3 to 5 weeks following the fifth immunization or from cryopreserved LNC. Briefly, 4 × 106 PBMC or LNC per well were cultured in 24-well plates (Costar, Cambridge, Mass.) in 1.5 ml of complete RPMI 1640 medium (5) with 3 to 5 μg of A. marginale membrane antigen per ml. After 7 days, cells were subcultured to a density of 5 × 105 cells per well and cultured with antigen and 2 × 106 irradiated (3,000 rads) autologous PBMC as a source of APC. T-cell lines were maintained for up to 5 weeks by weekly stimulation with antigen and APC, and cells were assayed for antigen-dependent proliferation 7 days following the last stimulation.
Lymphocyte proliferation assays.
Proliferation assays were carried out in replicate wells of round-bottomed 96-well plates (Costar) for 5 to 6 days when PBMC were used or for 4 days when LNC or short-term T-cell lines were used, essentially as described previously (7, 9). PBMC or LNC (2 × 105) were cultured in triplicate wells with antigen in a total volume of 100 μl of complete medium. T-cell lines were assayed 7 days after the last stimulation with antigen and APC. T-cell lines (3 × 104 cells) were cultured in duplicate wells in a total volume of 100 μl of complete medium containing 2 × 105 APC and antigen. Antigens consisted of 0.2 to 25.0 μg of A. marginale Florida homogenate or membranes per ml, and as a control, membranes prepared from uninfected bovine red blood cells (URBC) (6) were used. Protein concentrations in all antigen preparations were determined by the Bradford assay. To determine proliferation, cells were radiolabeled for the last 18 h of culture with 0.25 μCi of [3H]thymidine (Dupont New England Nuclear, Boston, Mass.), radiolabeled nucleic acids were harvested onto glass filters, and radionucleotide incorporation was measured with a Betaplate 1205 liquid scintillation counter (Wallac, Gaithersburg, Md.). Results presented are the mean number of counts per minute of replicate cultures ± the standard error of the mean (SEM) or the stimulation index (SI), which represents the mean number of counts per minute of replicate cultures of cells plus antigen/the mean number of counts per minute of replicate cultures of cells plus medium or URBC. An SI of ≥2.0 was considered statistically significant.
Cell-surface phenotypic analysis.
Differentiation markers on LNC and T-cell lines were analyzed by indirect immunofluorescence and flow cytometry as previously described (8). The MAbs used were specific for bovine CD2 (MAb MUC2A), CD3 (MAb MM1A), CD4 (MAb CACT 138A), CD8 (MAbs CACT 80C and BAT 82B), and the δ chain of the γ/δ T-cell receptor (TcR) (MAb CACT 61A). These MAbs were kindly provided by William C. Davis, Washington State University, Pullman, Wash.
Detection of IFN-γ and IL-2 in supernatants of LNC and T-cell lines.
Fresh LNC were cultured for 96 h in complete medium with 5 μg of A. marginale membrane antigen per ml, and supernatants were collected at 72 (first set of calves) or 96 h. T-cell lines were established from cryopreserved, antigen-draining LNC of calves in groups II and III by weekly stimulation with membrane antigen and APC. After 3 weeks, the cells were washed and cultured for 48 h at a density of 2 × 106 cells per ml with 2 × 106 APC per ml and 3 μg of membranes prepared from the Florida strain of A. marginale per ml. Supernatants were harvested by centrifugation and stored frozen at −20°C. The bovine IFN-γ assay was performed with a commercial ELISA kit (BOVIGAM; CSL Limited, Parkville, Victoria, Australia) according to the manufacturer's protocol. The IFN-γ activity in culture supernatants diluted 1:5 to 1:2,000 was determined by comparison with a standard curve obtained with a supernatant from a Mycobacterium bovis purified protein derivative-specific Th cell clone that contained 440 U of IFN-γ per ml (previously determined by the neutralization of vesicular stomatitis virus [8]). In our assay, 0.6 U corresponds to 1 ng of IFN-γ (2). The results are presented in units or nanograms of IFN-γ per milliliter. The IL-2 bioassay was performed as described previously (48) in triplicate with supernatants diluted 1:2 with an IL-2-dependent bovine CD8+ T-cell clone (G4.3D1) for responder cells and recombinant human IL-2 (Boehringer Mannheim) as a standard. The results are presented in picograms of IL-2 produced per milliliter of cell supernatant.
Analysis of neutralizing antibody against human IL-12.
Heat-inactivated sera obtained from calves before and following the third inoculation of MSP-2 and IL-12 were serially diluted from 1:200 to 1:64,000 and tested for neutralization of human IL-12. An IL-12 bioassay based on induction of IFN-γ by IL-12- and phytohemagglutinin (PHA)-stimulated PBMC was used (42). Diluted sera were incubated with 0.02 ng of IL-12 at 37°C for 1 h and then added along with 1 μg of PHA (Sigma Chemical Co.) to 2 × 106 PBMC cultured in 1 ml of complete medium. Supernatants were harvested 48 h later and tested for IFN-γ by ELISA. At dilutions of 1:200 or greater, preimmunization sera had no inhibitory activity. As a positive control, serial dilutions of goat anti-human IL-12 IgG (R & D Systems, Minneapolis, Minn.) were also mixed with 0.02 ng of IL-12 and tested for induction of IFN-γ, and the dose that neutralized 50% of the biological activity (ND50) was 0.5 μg.
QC-RT-PCR analysis of cytokine transcripts in the LNC from immunized calves.
As ELISA-based assays to quantify bovine IL-4 and IL-10 are not commercially available, quantitative, competitive reverse transcription-PCR (QC-RT-PCR) analysis of cytokine mRNA was performed. RNA was prepared from antigen-draining and contralateral prescapular LNC from all calves prior to or following culture for 6 or 12 h of 2.7 × 106 cells per ml with 5 μg of A. marginale membrane antigen per ml. Total cellular RNA was extracted using the Trizol reagent (GIBCO-BRL, Gaithersburg, Md.), and RNA samples were stored at −80°C. QC-RT-PCR was performed as described previously (48–50), with minor modifications. Briefly, competitor molecules (mimics) for bovine IL-2, IL-4, IL-10, and IFN-γ were kindly provided by Dante Zarlenga (Department of Immunobiology and Disease Resistance, U.S. Department of Agriculture, Agriculture Research Service, Beltsville, Md.), and the bovine β-actin mimic was generated in our laboratory as described by Zarlenga et al. (52). The primer sequences used to amplify the cytokine cDNAs were exactly as described previously (48–50), with the exception of the IL-2 reverse primer, for which the correct sequence is 5′ TCA AGT CAT TGT TGA GTA GAT GCT T 3′. The resultant IL-2, IL-4, IL-10, IFN-γ, and β-actin competitor PCR fragments were distinguishable from native fragments by their smaller size (48, 50). Column-purified, RNA-free plasmids containing cytokine competitors were serially diluted 10-fold with sterile double-distilled water and stored at −20°C until used. Total RNA (0.5 to 1.0 μg) was reverse-transcribed to cDNA in a 20-μl volume by using oligo(dT)16 (Perkin Elmer, Branchburg, N.J.) according to the manufacturer's instructions. For each sample tested, PCRs were performed, with each reaction mixture containing PCR primers; mimic DNA (0.1 to 1000 fg); cDNA (0.05 to 5 ng); a master mixture containing 10× PCR buffer (final concentration, 1×), magnesium (final concentration, 2.5 mM), and deoxynucleoside triphosphates (final concentration, 1 mM); AmpliTaq-Gold (1 U per reaction mixture; Perkin-Elmer); and water in a 50-μl volume. The PCR mixture was preheated to 94°C for 10 min to activate the AmpliTaq-Gold, followed by 35 cycles of amplification under the following conditions: 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min. The reactions were completed by an extension at 72°C for 10 min and stored at 4°C until analyzed. PCR products (20 μl) were electrophoresed on a 1% agarose gel containing ethidium bromide and quantified under UV light using a digital imaging system (IS1000; Alpha Innotech Corporation, San Leandro, Calif.). After correcting for differences in molecular weight between native and competitor DNA, the ratios between the amplified products of the target and competitor sequences at each competitor concentration were calculated. The logs of the ratios were plotted against the input concentrations of competitor DNA, and a regression equation was obtained. At the point of equivalence, where the amplified target/competitor DNA ratio equals 1.0, the amount of cytokine cDNA in the test sample equals the amount of competitor DNA. To compare the amount of cytokine mRNA in different samples, cytokine mRNA levels were normalized to the amount of β-actin. Genomic DNA was not detected for any cytokine or β-actin in the RNA samples. Results are presented in femtograms of cytokine RNA per microgram of total cellular RNA.
Statistical analysis.
A one-tailed, nonpaired Student's t test was used to compare the mean levels of proliferation, IFN-γ production, and steady-state cytokine transcript levels in LNC for the groups of calves. A one-tailed, paired Student's t test was used to compare these parameters for right and left prescapular LN in the different groups. A P value of ≤0.05 was considered significant.
RESULTS
A. marginale-specific proliferation in PBMC of MSP-2-immunized calves.
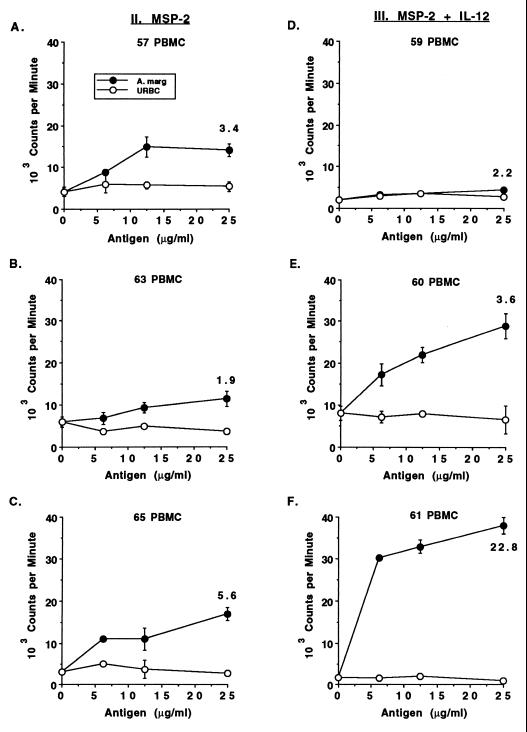
Calves were immunized, as detailed in Table 1, at 3- to 4-week intervals with gel-purified native MSP-2 antigen in alum and with or without IL-12 until specific proliferation was observed. PBMC were tested after each immunization for proliferation against several concentrations of membrane antigen prepared from A. marginale (Florida strain) or control URBC. Calf 61, which received MSP-2 and IL-12, responded after the third inoculation, whereas the remaining calves in both immunized groups did not respond until after the fifth antigen inoculation, at 17 or 18 weeks. The responses were variable, with those of calves 63 and 59 being the weakest (Fig. 2). Control calves that did not receive MSP-2 failed to respond to A. marginale or URBC at any time point (data not shown).
FIG. 2.
Dose-dependent, antigen-specific proliferative responses of PBMC from MSP-2-immunized calves. PBMC were obtained at 2 (calves 57, 60, 61, 63, and 65) or 3 (calf 59) weeks following the fifth antigen inoculation and tested for proliferation against 0 to 25 μg of membrane antigen prepared from A. marginale or URBC per ml. Cells were cultured for 6 days, radiolabeled, harvested, and counted on a scintillation counter. Data are the means ± the SEM of triplicate cultures. For the response to 25 μg of A. marginale per ml, SI values are also indicated.
Once specific proliferation was detected, we attempted to establish short-term T-cell lines from PBMC of MSP-2-immunized cattle. A short-term T-cell line was successfully established from only one animal (calf 57) in the group II calves that received MSP-2 only, whereas T-cell lines were established from all three calves in group III that were immunized with MSP-2 and IL-12. These lines contained a mixture of CD4+ T cells and γ/δ T cells (data not shown). Specific proliferation was observed in all four cell lines after 1 and 2 weeks of culture (Table 2), and responses to URBC were not significant (data not shown). However, repeated attempts to propagate cell lines from PBMC of animals 63 and 65 in group II were unsuccessful.
TABLE 2.
Proliferative responses of short-term T-cell lines derived from PBMC of A. marginale MSP-2-immunized calves and cultured for 1 or 2 weeks with A. marginale
| Group and calf from which cell line was deriveda | Mean proliferation (cpm ± SEM) against A. marginaleb
|
|
|---|---|---|
| Wk 1 | Wk 2 | |
| II | ||
| 57 | 3,959 ± 507 (2.2)c | 4,595 ± 734 (11.7) |
| 63 | 121 ± 13 (1.1) | NDd |
| 65 | 153 ± 10 (0.4) | ND |
| III | ||
| 59 | 10,425 ± 926 (3.1) | 10,560 ± 327 (2.4) |
| 60 | 8,038 ± 57 (2.0) | 14,876 ± 3,608 (3.1) |
| 61 | 29,670 ± 647 (5.8) | 39,641 ± 2,052 (3.1) |
PBMC were obtained from calves in group II (immunized with MSP-2) or group III (immunized with MSP-2 and IL-12) from 3 to 5 weeks after the fifth inoculation of antigen, cultured for 7 days with A. marginale homogenate, and washed, and T cells (5 × 105 cells per ml) were restimulated with antigen and APC for an additional 7 days.
After 1 or 2 weeks of culture, T cells were washed and proliferation assays were performed for 4 days with 3 × 104 T cells, 2 × 105 APC, and 25 μg of antigen prepared from membranes of A. marginale per ml. Numbers in parentheses show SI values.
Bold type indicates a significant response (SI ≥ 2).
ND, not determined because cell lines did not expand after the second stimulation.
IgG responses in calves immunized with MSP-2 or MSP-2 and IL-12.
Serological responses were monitored throughout the immunization by Western blot analysis. All preimmunization sera and control sera from calves immunized with alum and IL-12 were negative for reactivity on the immunoblots (data not shown). IgG responses in all three calves that received IL-12 and MSP-2 were detected at the first time point tested, 2 weeks following the second antigen inoculation (week 6), whereas only one of three calves inoculated with MSP-2 alone was seropositive (data not shown). The other two calves in the MSP-2 only group seroconverted after the third (week 10) and fourth (week 13) antigen injections. The sera reacted with a 36-kDa band on the immunoblots, which coincides with the molecular mass of MSP-2. Analysis of IgG1- and IgG2-specific titers in sera obtained 2 weeks after the fifth inoculation of antigen showed that the calves immunized with IL-12 made significantly more IgG1 than the calves that received MSP-2 alone (Table 3). Furthermore, one of three calves in the IL-12 group produced IgG2.
TABLE 3.
A. marginale-specific IgG1 and IgG2 titers in the sera of calves immunized with MSP-2
| Groupc and animal | Titer of subclassa:
|
|
|---|---|---|
| IgG1 | IgG2 | |
| I | ||
| 58 | <100 | <100 |
| 56 | <100 | <100 |
| 62 | <100 | <100 |
| II | ||
| 57 | 5,000 | <100 |
| 63 | 1,000 | <100 |
| 65 | 1,000 | <100 |
| III | ||
| 59 | 10,000b | 1,000 |
| 60 | 10,000b | <100 |
| 61 | 5,000b | <100 |
Results are presented for sera obtained 2 weeks after the fifth antigen inoculation. Sera were diluted 1:100 to 1:100,000 and tested for reactivity against A. marginale Florida homogenate on Western blots. The titer is defined as the reciprocal of the dilution giving a positive signal on the blots. A value of <100 indicates no reaction was observed at a dilution of 1:100 or higher.
The IgG1 titers in group III are significantly higher than the IgG1 titers in group II as determined by the one-tailed Student's t test (P = 0.025).
Animals in group I were immunized with IL-12, animals in group II were immunized with MSP-2, and animals in group III were immunized with MSP-2 and IL-12.
Analysis of antigen-induced proliferation and cytokine production by draining LN lymphocytes.
Once A. marginale-specific proliferative and serological responses were detected, the calves were given a final antigen inoculation so that antigen-draining (right) and contralateral (left) lymph nodes could be surgically biopsied for examination of antigen-induced recall responses. The booster inoculation was staggered so that one calf from each group was used for each of three time points (Table 1). The relative percentages of T-cell subsets in the LN were similar in all groups (Table 4).
TABLE 4.
Composition of T-cell subsets in the lymph nodes of immunized calves
| Group and LNa | Percentage of LNCb | Percentage of CD3-positive T cellsc
|
||
|---|---|---|---|---|
| CD4 | CD8 | γ/δ TcR | ||
| I | ||||
| Left | 59.3 ± 10.0 | 50.0 ± 5.3 | 18.3 ± 4.5 | 23.0 ± 15.4 |
| Right | 59.7 ± 6.4 | 52.0 ± 1.7 | 17.3 ± 4.0 | 22.3 ± 12.2 |
| II | ||||
| Left | 64.6 ± 4.0 | 52.6 ± 9.3 | 22.0 ± 5.2 | 17.3 ± 3.0 |
| Right | 63.0 ± 16.5 | 54.6 ± 7.5 | 22.3 ± 3.8 | 14.0 ± 4.6 |
| III | ||||
| Left | 61.6 ± 17.8 | 53.3 ± 8.6 | 18.0 ± 2.6 | 19.3 ± 4.5 |
| Right | 49.0 ± 22.0 | 51.3 ± 5.1 | 20.6 ± 2.9 | 13.6 ± 9.8 |
Adjuvant control (left) or antigen-draining (right) LN were removed 3 days after the last inoculations of antigen and cell suspensions were prepared. Calves of group I were immunized with IL-12, calves of group II were immunized with MSP-2, and calves of group III were immunized with MSP-2 and IL-12.
Cells were stained with anti-CD3 MAb and analyzed by flow cytometry, and the results indicate the percentage of cells in the total LNC population that expressed CD3. Values are the means ± the SEM of the results for the three calves of each group.
Cells were stained with MAb against bovine CD4, CD8, or the γ/δ T-cell receptor and analyzed by flow cytometry. Values are the means ± the SEM for each group of calves.
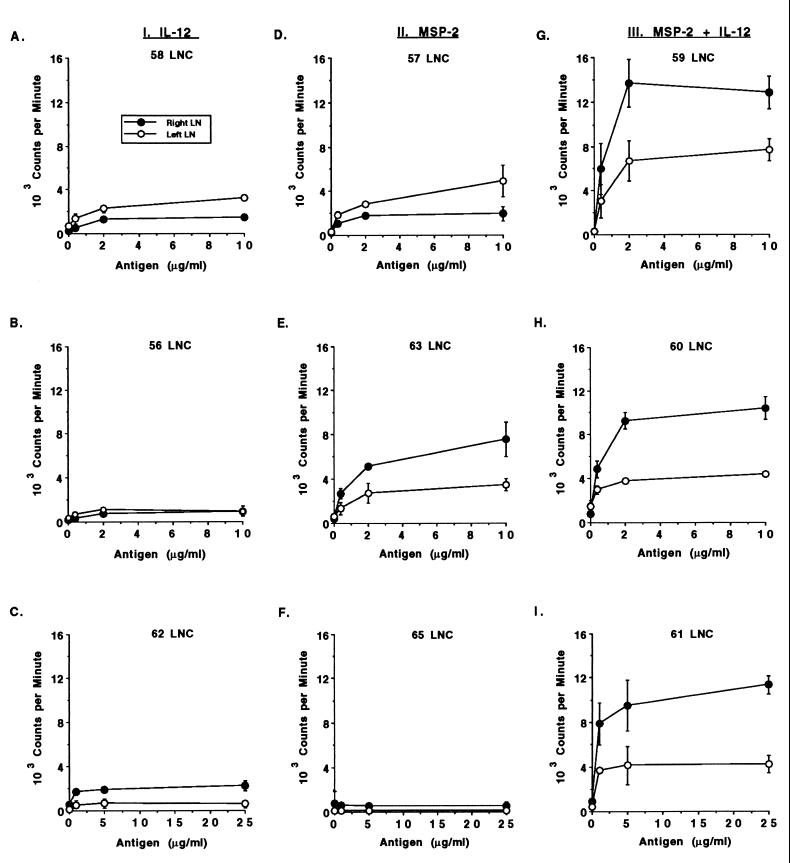
Antigen-induced proliferation of LNC in the different groups was then compared. Proliferation against URBC antigen was not detected (data not shown), whereas A. marginale-specific and dose-dependent responses were evident in two of the three calves given MSP-2 alone and in all calves given MSP-2 plus IL-12 (Fig. 3). Antigen-draining (right) LNC from calves immunized with MSP-2 and IL-12 had significantly higher levels of proliferation than calves inoculated with either IL-12 or MSP-2 alone. Furthermore, the proliferative response of the antigen-draining LNC in calves given MSP-2 and IL-12 was significantly higher than that of the contralateral LNC.
FIG. 3.
Comparison of the A. marginale-specific proliferative responses of antigen-draining (right) and adjuvant control (left) LNC from control and MSP-2-immunized calves. Cell suspensions were obtained from left and right prescapular LN biopsies of one calf per group at three different times. Freshly obtained (A, B, D, E, G, and H) or cryopreserved (C, F, and I) LNC from the antigen-draining right LN or adjuvant control left LN were assayed for proliferation against 0 to 25 μg of A. marginale or control URBC per ml (data not shown). LNC (3 × 105) were cultured for 4 days, radiolabeled, harvested, and counted. Data are the means ± the SEM of triplicate cultures and are representative of three separate assays performed with fresh or cryopreserved LNC.
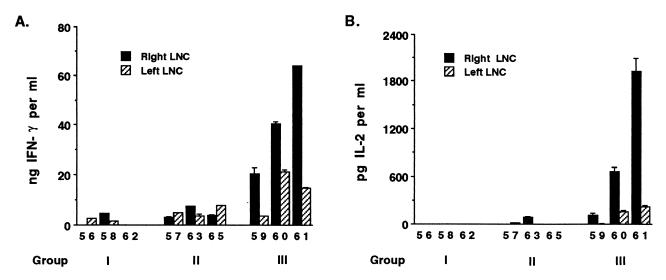
Freshly obtained LNC from all calves were cultured with A. marginale antigen, and supernatants were tested for IFN-γ and IL-2 production (Fig. 4). Significantly more IFN-γ was produced by the antigen-draining (right) LNC of group III calves that received IL-12 and MSP-2 than by group I control calves or group II calves immunized with MSP-2 alone. Furthermore, for group III calves, the antigen-draining right LNC secreted higher levels of IFN-γ than the contralateral LNC. Higher levels of IL-2 were also produced by LNC from group III calves immunized with MSP-2 and IL-12. Increased IL-2 production by group III calves is consistent with their stronger proliferative responses to antigen (Fig. 3).
FIG. 4.
Comparison of IFN-γ and IL-2 production by LNC from calves immunized with MSP-2 or MSP-2 and IL-12. Freshly obtained LNC were cultured for 24 to 96 h at a concentration of 2.7 × 106 cells per ml with 5 μg of A. marginale membrane antigen per ml. Supernatants were collected, centrifuged, and tested for IFN-γ by ELISA (A) or for IL-2 by bioassay and an IL-2-dependent T-cell clone (B). Results for individual cattle are indicated by the shaded bars. Results for IFN-γ are the amounts of IFN-γ produced in 72-h (calves 61, 62, and 65) or 96-h (all other calves) supernatants (data are means + SEM [error bars] of the results of duplicate assays per sample) and are based on a standard curve obtained with bovine IFN-γ. Results for IL-2 are the amounts of IL-2 produced in 48 h supernatants (values are means ± SEM) based on assays of triplicate wells per sample and a standard curve obtained with human IL-2.
Cytokine transcript expression by antigen-draining and contralateral LNC.
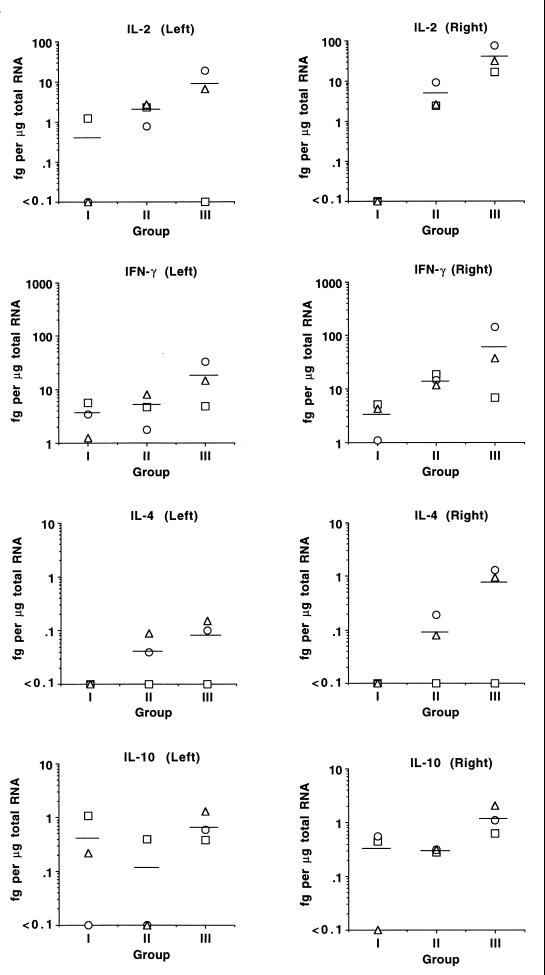
To verify the adjuvant effect of IL-12 on enhancing IL-2 and IFN-γ production and to examine other cytokines important for priming and immune regulation, QC-RT-PCR analysis of IL-2, IFN-γ, IL-4, and IL-10 transcript expression was performed with RNA obtained from LNC of all calves. RNA was obtained from freshly collected LNC or following stimulation with A. marginale for 6 or 12 h. In MSP-2-immunized calves, the amount of cytokine transcript determined by RT-PCR increased following antigen activation (data not shown), so RNA from the 12-h time point was selected for use in QC-RT-PCR. For both MSP-2-immunized groups, comparison of the mean transcript levels in antigen-draining (right) LNC and contralateral (left) LNC revealed higher transcript levels in the antigen-draining LNC (Fig. 5). Calves given MSP-2 and IL-12 (group III) had the highest mean levels of transcripts in both antigen-draining (right) and contralateral (left) LNC. When the mean levels of transcripts were compared, group III calves had approximately ninefold more IL-2 and fourfold more IFN-γ than the calves immunized with MSP-2 alone (group II). Although the difference in IFN-γ transcript levels between group II and III calves was not significant due to the low response of calf 59, calves 60 and 61 had IFN-γ transcript levels well above 95% confidence limits of the mean IFN-γ values for group II calves. IL-4 and IL-10 transcripts were absent or were present at levels that were relatively low in all calves but higher in the group III calves than in group II calves. Overall, the relative steady-state levels of IL-2 and IFN-γ transcripts analyzed using QC-RT-PCR were consistent with the relative amount of cytokine product determined by bioassay or ELISA. Collectively, these data demonstrate that immunization with IL-12 augments the production of IL-2 and IFN-γ during a recall response to antigen.
FIG. 5.
QC-RT-PCR analysis of cytokine mRNA levels following antigen stimulation of antigen-draining (right) and control contralateral (left) prescapular LNC. LNC were obtained from one animal per group 3 days following the final MSP-2 inoculation. Freshly obtained LNC (2.7 × 106 per ml) were cultured for 12 h with 5 μg of A. marginale membrane antigen per ml, and RNA was prepared. QC-RT-PCR analysis was performed for β-actin and cytokines IL-2, IFN-γ, IL-4, and IL-10, and the amount of transcript was calculated, as described in the text, based on the known amount of cytokine competitor gene added. The data are plotted for individual animals in groups I (IL-12 and alum), II (MSP-2 and alum), and III (MSP-2, IL-12, and alum), respectively: calves 56, 57, and 59 (squares); calves 62, 63, and 60 (circles); and calves 58, 65, and 61 (triangles). The mean LN cytokine transcript levels for each group are indicated by the horizontal lines.
Neutralizing antibody to human IL-12.
The variable cytokine responses among calves immunized with MSP-2 and IL-12 (Fig. 4 and 5) raised the possibility that the differences might correlate with the presence of neutralizing antibodies to human IL-12. By an IL-12 bioassay consisting of costimulation of bovine PBMC with 0.02 ng of IL-12 and 1 μg of PHA, which stimulated production of approximately 150 U of IFN-γ per ml (data not shown), serially diluted bovine sera had ND50 titers of 2,506 (calf 59), 2,512 (calf 60), and 1,932 (calf 61). Thus, although three immunizations of human IL-12 did elicit neutralizing antibodies to human IL-12, the ND50 titers did not correlate with the different levels of cytokine expression among group III calves.
Antigen-induced proliferation and IFN-γ production by short-term T-cell lines established from antigen-draining LNC.
We next wished to determine if effector cells cultured from the antigen-draining LN maintained the type 1 cytokine phenotype for several weeks. Short-term T-cell lines were established from all three group III calves and from group II calf 63 by weekly stimulation of LNC with A. marginale membrane antigen and APC. We were unable to establish vigorously growing cell lines from group II calves 57 and 65, so after the 3-week culture period, only enough cells were available to test for IFN-γ production. The inability to propagate cells from these animals is consistent with the low or absent proliferation of these LNC (Fig. 3). All four cell lines that were expanded were comprised mainly of CD4+ T cells (93 to 95%). In proliferation assays these four cell lines responded similarly to A. marginale, after 1 to 3 weeks of culture (data not shown). However, cell lines from MSP-2- and IL-12-immunized calves produced significantly more IFN-γ (Table 5).
TABLE 5.
IFN-γ production by T-cell lines established from calves immunized with A. marginale MSP-2 without or with IL-12
| Group and animal | IFN-γ (U/ml) produced by T-cell lines stimulated witha:
|
||
|---|---|---|---|
| Medium | URBC | A. marginale | |
| II | |||
| 57 | NDb | ND | <10 |
| 63 | ND | ND | 236 |
| 65 | ND | ND | <10 |
| III | |||
| 59 | 694 | 654 | 1,325 |
| 60 | 1,400 | 1,413 | 2,884 |
| 61 | 691 | 948 | 1,642 |
T-cell lines were established from antigen-draining (right) LNC and stimulated weekly with antigen and APC for 3 weeks. After being washed, 2 × 106 T cells were cultured in duplicate for 48 h with 2 × 106 APC and either medium or 3 μg of membrane antigen prepared from URBC or A. marginale per ml. Supernatants were analyzed for IFN-γ production by ELISA with bovine IFN-γ as a standard. Values are the mean titers of IFN-γ production for each cell line. The mean titer of IFN-γ produced by cell lines from group III calves (immunized with MSP-2 and IL-12) is significantly greater (P < 0.05) than that produced by cell lines from group II calves (immunized with MSP-2), as analyzed by the one-tailed, nonpaired Student's t test for samples with unequal variance. APC cultured with antigen produced <1 U of IFN-γ per ml.
ND, not determined.
Duration of the memory response to A. marginale.
To determine if immunization with MSP-2 and IL-12 elicited long-term immunological memory, PBMC obtained from all six MSP-2-immunized calves 9 months after the last group was boosted with MSP-2 were tested for antigen-specific proliferation. Dose-dependent and specific responses to A. marginale homogenate were observed in two MSP-2-immunized and all three MSP-2- and IL-12-immunized calves, with the highest responses observed in calves that received IL-12. The SI values in response to the optimal antigen concentration (5 or 25 μg of protein per ml) for group II calves 57, 63, and 65 were 2.6, 3.1, and 1.3, and those for group III calves 59, 60, and 61 were 4.6, 3.6, and 27.7, respectively, which values are representative of two experiments. The lack of response by PBMC from calf 65 is consistent with the lack of a recall response in the antigen-draining LNC (Fig. 3).
DISCUSSION
This study is the first to examine CD4+ T-cell responses in cattle immunized with native MSP-2 antigen. We demonstrated specific and long-lived recognition of purified A. marginale membranes or organism homogenate from the homologous Florida strain by CD4+ T cells from PBMC and LNC, showing that MSP-2 is capable of priming for an anamnestic T-cell response to the organism. These data are consistent with earlier studies which showed that PBMC and several CD4+ T cell clones obtained from calves immunized with purified A. marginale membranes responded to organism homogenate, purified outer membranes, and MSP-2 (7, 9). MSP-2 and related proteins, including the OMP of C. ruminantium (MAP 1), E. chaffeensis (OMP-1), and the agent of HGE (GE MSP-2 and HGE-44), are candidate vaccine antigens (28, 30, 31). Our results are thus directly relevant for the potential use of ehrlichial OMP as immunogens for ehrlichial pathogens of humans and domestic animals (14, 18, 27, 28, 30, 36, 51, 53).
The requirement for five immunizations to detect antigen-specific proliferation is likely due to the use of alum, which is a poor adjuvant. When either RIBI or complete Freund's adjuvant was used with a comparable amount of protein antigen from Babesia bigemina (37) or A. marginale (MSP-1) (W. C. Brown, T. C. McGuire, and G. H. Palmer, unpublished observations), specific proliferative responses were detected after three antigen inoculations. Additionally, when cattle were immunized with A. marginale membranes in saponin, responses were detected after two or three inoculations (7).
This study also demonstrates the adjuvant effect of IL-12 in priming for enhanced memory IgG and type 1 cytokine responses to A. marginale MSP-2. Alum was used with IL-12, since in mice alum fails to elicit a type-1 response, permitting an evaluation of the effects of IL-12, and because it appeared to prolong the in vivo effect of IL-12 (19). IL-12 promotes type-1 responses in several ways (reviewed in reference 16). First, IL-12 produced by the major APC, the dendritic cell, acts on naïve Th cells during antigen priming, causing them to differentiate into Th1 cells that secrete high levels of IFN-γ upon stimulation. Second, as a costimulator, IL-12 potentiates the secretion of IFN-γ by differentiated T cells in response to specific antigen. Finally, IL-12 stimulates the differentiation of IFN-γ-producing effector cells from memory cells when cultured with the priming antigen. Our studies with bovine CD4+ T cells have also shown the ability of IL-12 to enhance IFN-γ production by antigen-stimulated Th0 and Th1 clones, and to promote the differentiation of IFN-γ-producing effector cells from resting PBMC-derived lymphocytes that were subsequently cultured for several weeks with antigen (4, 49). However, the results presented here are the first to demonstrate the in vivo effects of administering IL-12 to cattle as an adjuvant for a soluble protein antigen.
The effects of using IL-12 as an adjuvant during immunization with MSP-2 were evaluated only after multiple immunizations, so that immediate effects on priming were not determined. Instead, our experiments examined the effect of IL-12 on a recall response. As expected, IL-12 stimulated significantly higher production of IFN-γ by LNC draining the MSP-2 injection site and by short-term cultured CD4+ T-cell lines established from these cells. The production of IFN-γ by the CD4+ T-cell lines indicates that CD4+ T cells are a source of IFN-γ in the LNC. Five- to tenfold more IFN-γ was produced by these cell lines than by the CD4+ T-cell line established from calf 63 immunized with MSP-2 alone, or by CD4+ T cell lines from two calves immunized with A. marginale outer membranes in saponin (7). These results are consistent with the previously observed effects of IL-12 on differentiated T-cell clones and on memory T cells cultured with IL-12 and antigen (4, 49).
Elevated expression of IL-2, IL-4, and IL-10 was also observed in the IL-12 and MSP-2-immunized calves, demonstrating that IL-12 did not polarize the response to a strictly Th1 response. IL-10 was similarly induced by IL-12 following priming in mice or in vitro priming of human T cells, presumably as a negative-feedback response (16). Furthermore, immunization with IL-12 supported the development of both Th1 and Th2 recall responses to keyhole limpet hemocyanin in mice (3). These mixed Th1 and Th2 cytokine responses were accompanied by mixed IgG2a and IgG1 responses. Additionally, the response to a soluble protein antigen adsorbed with alum was shifted from a Th2-biased response, characterized by predominant IL-4, IL-5, and IL-10 cytokine and IgG1 antibody responses, to a Th1 response, marked by increased IFN-γ, decreased IL-4, IL-5, and IL-10 responses, and induction of IgG2a, IgG2b, and IgG3 (19). However, significantly increased IgG1 titers were observed in the IL-12-treated mice, suggesting that the regulation of IgG1 in this system was independent of IL-4.
In cattle, IFN-γ enhanced IgG2 production and IL-4 enhanced IgG1 production by B cells activated through T-independent type 2 stimulation (10, 11). Furthermore, the production of IFN-γ by CD4+ T-cell clones cultured with antigen and B cells correlated with IgG2 production by the B cells (6). Based on this information, we expected to stimulate only IgG1 production with MSP-2 and alum, and both IgG1 and IgG2 antibody responses with the combined IL-12-MSP-2-alum formulation. Although a restricted IgG1 response in calves given MSP-2 alone occurred and addition of IL-12 enhanced this response, IL-12 had no consistent effect on IgG2 production. There are several possible explanations for this result. First, the amount of IL-12 used (three inoculations of 10 μg) may have been too little in this large animal species to induce sufficient IFN-γ in vivo to facilitate isotype switching. Second, the development of neutralizing antibodies against human IL-12 may have prevented the optimal induction of bovine IFN-γ and suggests that it would be preferable to use homologous IL-12. Finally, in cattle, T-cell-dependent IgG2 production may not be solely dependent on IFN-γ. In support of the latter possibility, Estes et al. (12) recently demonstrated that when bovine B cells were costimulated with IL-2, CD40 ligand and anti-surface IgM to mimic T-cell-dependent activation, the addition of IFN-γ enhanced IgG2 production only twofold, whereas type-1 IFN had a greater effect. Furthermore, in our study the single calf (calf 59) that produced IgG2 was the lowest responder in terms of PBMC proliferation and LNC IL-2 and IFN-γ production, again suggesting that factors other than or in addition to IFN-γ are important for IgG2 production.
Enhanced IL-2 production, which was associated with higher levels of transcript expression and significantly greater proliferation by antigen-draining LNC, was demonstrated in calves that received IL-12. This result contrasts that of Jankovic et al. (19), who observed an IFN-γ- and NO-dependent suppression of IL-2 production and antigen-induced proliferation of splenocytes from mice immunized with IL-12 and antigen coadsorbed to alum. The suppression was dependent on the dose of IL-12, which supports the possibility that the amount of IL-12 given in our experiments was not sufficient to induce suppressive levels of IFN-γ. Toxicity with high doses of IL-12 has been observed in mice and humans (22), but the dosage and timing of IL-12 administration used in our experiments did not cause inhibition of bovine lymphocyte responses.
The choice of IL-12 as an adjuvant to induce protection against acute infection with ehrlichial pathogens, such as A. marginale, is supported by our results. Although the calves in this study were not challenged, the strong IFN-γ response by T-cell lines from calves protectively immunized with A. marginale outer membranes is consistent with our hypothesis that protection is in part dependent on IFN-γ production (7, 34). Furthermore, experiments in mice with related rickettsial organisms demonstrated the importance of IFN-γ and NO in the protective immune response (13, 23, 35). Protection against ehrlichial pathogens may also be mediated by cytophilic antibodies that can facilitate phagocytosis and killing of the organisms. In cattle both IgG1 and IgG2 subclasses are capable of in vitro binding to activated macrophages, but only IgG2 had opsonizing activity for monocytes and neutrophils, suggesting that this subclass may be more important for phagocytosis in vivo (25). Although it is still uncertain how bovine IgG2 responses to T-cell-dependent antigens are regulated in vivo, IL-12 should enhance macrophage activation through the induction of IFN-γ and aid in clearance of the infection caused by A. marginale and related organisms.
ACKNOWLEDGMENTS
We thank Teresa Harkins, Bev Hunter, Emma Karel, and Kim Kegerreis for excellent technical assistance and the Genetics Institute for providing recombinant human IL-12.
This research was supported by research grant no. US-2799-96C from BARD, the United States-Israel Binational Agricultural Research and Development Fund, USDA-NRICGP grant 95-37204-2347, USDA agreement 58-5348-8-004, and NIH NIAID grants R01-AI30136 and R01-AI44005.
REFERENCES
- 1.Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Beyer J C, Stich R W, Brown W C, Cheevers W P. Cloning and expression of caprine interferon-gamma. Gene. 1998;210:103–108. doi: 10.1016/s0378-1119(98)00046-8. [DOI] [PubMed] [Google Scholar]
- 3.Bliss J, Van Cleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas A K, Wolf S F. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 4.Brown W C, Davis W C, Tuo W. Human IL-12 upregulates proliferation and IFN-γ production by parasite antigen-stimulated Th cell clones and γ/δ T cells of cattle. Ann N Y Acad Sci. 1996;795:321–324. doi: 10.1111/j.1749-6632.1996.tb52682.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Logan K S, Wagner G G, Tetzlaff C L. Cell-mediated immune responses to Babesia bovis antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991;59:2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown W C, McElwain T F, Palmer G H, Chantler S E, Estes D M. Bovine CD4+ T-lymphocyte clones specific for rhoptry-associated protein 1 of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect Immun. 1999;67:155–164. doi: 10.1128/iai.67.1.155-164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown W C, Shkap V, Zhu D, McGuire T C, Tuo W, McElwain T F, Palmer G H. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect Immun. 1998;66:5406–5413. doi: 10.1128/iai.66.11.5406-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown W C, Zhao S, Rice-Ficht A C, Logan K S, Woods V M. Bovine helper T cell clones recognize five distinct epitopes on Babesia bovis merozoite antigens. Infect Immun. 1992;60:4364–4372. doi: 10.1128/iai.60.10.4364-4372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown W C, Zhu D, Shkap V, McGuire T C, Blouin E F, Kocan K M, Palmer G H. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect Immun. 1998;66:5414–5422. doi: 10.1128/iai.66.11.5414-5422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 11.Estes D M, Hirano A, Heussler V T, Dobbelaere D A E, Brown W C. Expression and biological activities of bovine interleukin 4: effects of recombinant bovine interleukin 4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol. 1995;163:268–279. doi: 10.1006/cimm.1995.1126. [DOI] [PubMed] [Google Scholar]
- 12.Estes D M, Tuo W, Brown W C, Goin J. Effects of type I/type II interferons and transforming growth factor-β on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology. 1998;95:604–611. doi: 10.1046/j.1365-2567.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng H M, Popov V L, Walker D H. Depletion of IFN-γ and TNF-α in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun. 1994;62:1952–1960. doi: 10.1128/iai.62.5.1952-1960.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French D F, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French D F, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. The interleukin-12/interleukin-12 receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh C, Macatonia S E, Tripp C S, Wolf S E, O'Garra A, Murphy K M. Listeria-induced Th1 development in αβ-TCR transgenic CD4+ T cells occurs through macrophage production of IL-12. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 18.Ijdo J W, Un W, Zhang Y, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic D, Caspar P, Zweig M, Garcia-Moll M, Showalter S D, Vogel F R, Sher A. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV gp120. J Immunol. 1997;159:2409–2417. [PubMed] [Google Scholar]
- 20.Johnson W C, Cluff C W, Goff W L, Wyatt C R. Reactive oxygen and nitrogen intermediates and products from polyamine degradation are babesiacidal in vitro. Ann N Y Acad Sci. 1996;791:136–147. doi: 10.1111/j.1749-6632.1996.tb53520.x. [DOI] [PubMed] [Google Scholar]
- 21.Knowles D, Torioni de Echaide S, Palmer G, McGuire T, Stiller D, McElwain T. Antibody against Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996;34:2225–2230. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Jerrels T R, Spitalny G L, Walker D H. Gamma interferon as a crucial defense against Rickettsia conorii in vivo. Infect Immun. 1987;55:1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C-S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naïve CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 25.McGuire T C, Musoke A J, Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils, and skin. Immunology. 1979;38:249–256. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller M A, Skeen M J, Ziegler H K. Long-lived protective immunity to Listeria is conferred by immunization with particulate or soluble listerial antigen preparations coadministered with IL-12. Cell Immunol. 1998;184:92–104. doi: 10.1006/cimm.1998.1270. [DOI] [PubMed] [Google Scholar]
- 27.Murphy C I, Storey J R, Recchia J, Doros-Richert L A, Gingrich-Baker C, Munroe K, Bakken J S, Coughlin R T, Beltz G A. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 1998;66:3711–3718. doi: 10.1128/iai.66.8.3711-3718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyika A, Mahan S M, Burridge M J, McGuire T C, Rurangirwa F, Barbet A F. A DNA vaccine protects mice against the rickettsial agent Cowdria ruminantium. Parasite Immunol. 1998;20:111–119. doi: 10.1046/j.1365-3024.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 29.Oberle S M, Palmer G H, Barbet A F, McGuire T C. Molecular size variation in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988;56:1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic gene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 (MSP-2) is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3803–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer G H, McElwain T F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 33.Palmer G H, McGuire T C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984;133:1010–1015. [PubMed] [Google Scholar]
- 34.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 35.Park J, Rikihisa Y. l-Arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992;60:3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez S D, Palmer G H, McElwain T F, McGuire T C, Ruef B J, Chitko-McKown C G, Brown W C. CD4+ T-helper lymphocyte responses against Babesia bigemina rhoptry-associated protein 1. Infect Immun. 1996;64:2079–2087. doi: 10.1128/iai.64.6.2079-2087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruef B J, Tuo W, Rodriguez S D, Roussel A J, Chitko-McKown C G, Palmer G H, McElwain T F, Canals A, Zarlenga D S, Gasbarre L C, Brown W C. Immunization with Babesia bigemina rhoptry-associated protein 1 induces a type 1 cytokine response. J Interferon Cytokine Res. 1997;17:45–54. doi: 10.1089/jir.1997.17.45. [DOI] [PubMed] [Google Scholar]
- 39.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 41.Seder R A, Gazzinelli R, Sher A, Paul W E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for IFNγ production and diminishes IL-4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoda L K M, Zarlenga D S, Hirano A, Brown W C. Cloning of a cDNA encoding bovine interleukin 18 (IL-18) and analysis of IL-18 expression in macrophages and its IFN-γ-inducing activity. J Interferon Cytokine Res. 1999;19:1169–1177. doi: 10.1089/107999099313118. [DOI] [PubMed] [Google Scholar]
- 43.Sousa C R, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin-12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1799–1802. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stich R W, Shoda L K M, Dreewes M, Adler B, Jungi T W, Brown W C. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect Immun. 1998;66:4130–4136. doi: 10.1128/iai.66.9.4130-4136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk A H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 48.Tuo W, Bazer F W, Davis W C, Zhu D, Brown W C. Differential effects of type I IFNs on the growth of WC1− CD8+ γδ T cells and WC1+ CD8− γδ T cells in vitro. J Immunol. 1999;162:245–253. [PubMed] [Google Scholar]
- 49.Tuo W, Estes D M, Brown W C. Comparative effects of IL-12 and IL-4 on cytokine responses by antigen-stimulated memory CD4+ T cells of cattle: IL-12 enhances IFN-γ production, whereas IL-4 has marginal effects on cytokine expression. J Interferon Cytokine Res. 1999;19:743–751. doi: 10.1089/107999099313587. [DOI] [PubMed] [Google Scholar]
- 50.Tuo W, Macmillan H, Günter N, Bazer F W, Brown W C. Upregulation of IL-4 and IFN-γ expression by interferon-τ, a member of the type I IFN family. J Interferon Cytokine Res. 1999;19:179–187. doi: 10.1089/107999099314324. [DOI] [PubMed] [Google Scholar]
- 51.van Vliet A H M, Jongejan F, van Kleef M, van der Zeijst B A M. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect Immun. 1994;62:1451–1456. doi: 10.1128/iai.62.4.1451-1456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarlenga D S, Canals A, Gasbarre L. Method for constructing internal standards for use in competitive PCR. BioTechniques. 1995;19:324–326. [PubMed] [Google Scholar]
- 53.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]