Abstract
Maternal care is essential to optimally support survival of the offspring. During evolution of mammalian species, different phenotypes have evolved in relation to gestation length, number, size, and maturation stage of the offspring at parturition, as well as colostrum and milk composition. The aim of the present review is to describe relationships between placental function and colostrum and milk composition in different mammalian species. Species covered in this article include humans, rabbits, rodents (rat and mouse), carnivores (cats and dogs), and a variety of ungulate species (cattle, sheep, goats, pigs, and horses). Species-specific aspects are elucidated with a special focus on the transfer of passive immunity. In this regard, the structure and thus the capability of the placenta to transport immunoglobulins from maternal to fetal circulation in utero dictates the necessity of the passive transfer of immunity via colostrum. Consequently, species with exclusive postpartal transfer of immunity such as in all ungulate species have greater immunoglobulin G concentrations in colostrum than species with a prepartal transfer in utero, where especially immunoglobulin A with its local immune function in the gastrointestinal tract is present in colostrum (e.g., rabbit and human). In terms of the nutritional purpose, suckling frequency is an important factor determining the gross composition of colostrum as well as in the mature milk of these species. Milk of nidicolous animals with long intervals in-between suckling events contains more fat than milk of nidifugous animals with constant access to their mother. However, the importance of colostrum and milk consumption for newborn animals and human babies goes beyond nutrition and the transfer of immunity. Numerous bioactive components such as growth factors, hormones, and oligosaccharides are enriched in colostrum and transition milk, which support the development of the intestinal tract and local immune system.
Keywords: colostrum, mammals, mammary gland, placenta, transfer of passive immunity
The present review describes relationships between placentation type and mammary gland function in different mammalian species. We specifically address the consequences of a differential transfer of passive immunity (placental or colostral) on colostrum and milk properties.
Introduction
Lactation evolved to the characteristic investment in maternal care of mammalian species. However, lactation implies more than only providing milk to nourish the offspring. From an evolutionary point of view, glandular skin secretions with antimicrobial and immune-protective properties co-evolved into their role in nourishing the offspring (Oftedal, 2012). The purpose of colostrum and milk to provide immunological active constituents is still conserved in many mammalian species. Besides humans, the emphasis of this article is laid on various farm, companion, and laboratory animals: humans, rabbits, rodents (rat and mouse), carnivores (cats and dogs), and ungulates (cattle, sheep, goats, pigs, and horses). Considering the manifold phenotypic species differences of mammalian newborns (e.g., birth weight or maturation stage), it is not surprising that composition of colostrum and mature milk is not homogenous. Despite various similarities of dietary habits or body size of mammals, distinct anatomical and functional differences of the placenta determine the necessity of a timely colostrum supply in some species, whereas colostrum is of minor importance for the neonate of other species. Species-specific colostrum traits and the impact of colostrum components on neonatal development and health were subject of numerous scientific papers and reviews (e.g., Blum and Hammon, 2000; Blättler et al., 2001). The emphasis of the present review is to illustrate relationships in various mammalian species regarding the secretory activity of the mammary gland at parturition and consequences for the offspring, for example, manner of transfer of passive immunity or frequency of nursing. We link anatomical traits (i.e., type of placentation) with the contents of individual components in colostrum and milk, and point out further associations of maternal care and offspring development.
Investments During Gestation and the Importance of Lactation in Maternal Care of Mammals
Until parturition, maternal investments focus on the maintenance of gravidity and the development of a viable fetus. In horses and donkeys (precocial and nidifugous neonates), the gestation period takes up half the time or more of the overall maternal investment, whereas in pigs (precocial but nidicolous neonates) the gestation length accounts for less than 50% of the maternal investment (Langer, 2008). The reproductive strategy of metatheria is quite the opposite of precocial mammals, as their offspring are born in a very immature state after a very short gravidity (Brennan et al., 2007; Bradshaw and Bradshaw, 2011; Cheng and Belov, 2017), which in turn requires a long lactation period with steadily increasing milk production in parallel to the growth of the young. Here, the young is completely dependent on the dam and milk as the sole feed source for extended periods of time after birth (Brennan et al., 2007; Bradshaw and Bradshaw, 2011; Cheng and Belov, 2017). In contrast to most eutherians, the mammary gland of metatheria undergoes excessive mammogenesis during an ongoing lactation (Bradshaw and Bradshaw, 2011). In the latter, a pronounced regulation by local factors is necessary, as siblings differ in age and maturation state, thus their claims to the mammary gland are completely different (Forsyth and Hayden, 1977; Brennan et al., 2007; Bradshaw and Bradshaw, 2011).
Concomitantly with advanced gestation, the mammary gland prepares for the onset of lactation. The milk-only period is the most straining phase for the mother. Therefore, the milk-only period accounts for only a small portion in relation to the overall duration of maternal care (Langer, 2008). In horses, the milk-only period takes less than 5% of the total maternal investment. Small mammals and species living in cold environments, for example, arctic whales and seals, have a relatively longer milk-only phase (Lee et al., 1991; Langer, 2008). In contrast to terrestrial species like horses and cattle, their milk has a very high-fat content, which provides an easily digestible and energy-rich feed source supporting the blubber formation to reduce heat losses (Akers, 2002).
Lactation, however, does not only provide milk as a customized nutrient source that fully covers the neonate’s needs during the sole milk feeding period, but serves several additional purposes as well. The first milk secreted after parturition (colostrum) is crucial for certain species to receive passive immunization with immunoglobulins (Ig). A separate chapter later will address this issue in detail. Moreover, lactation can suppress cyclic ovarian activity and consequently the establishment of a new gravidity to spare resources for the current offspring (Schmidt et al., 1983; Chao, 1987; Butler, 2005). Furthermore, lactation and suckling strengthen the mother-offspring bonding (Henry et al., 2020). In parallel to the milk feeding phase, the digestive tract of the offspring gradually adjusts for its later independence from the dam, starting as early as with the stimulatory effects of colostrum on the development of intestinal structures (Blättler et al., 2001; Blum and Baumrucker, 2002). In parallel to the consumption of milk, solid feed further supports gastrointestinal development along with the establishment of a functioning intestinal microbiome, especially, in herbivore species (Blättler et al., 2001; Blum and Baumrucker, 2002).
In some species like horses and cows, lactation continues on a lower level beyond the milk-only phase. Although foals and calves are nutritionally independent of maternal care quite soon after birth, they are still nursing their dam for several months up to more than 1 yr in feral or semi-feral conditions. This slow weaning process plays an important role in the maintenance of the mother-offspring bonding (Reinhardt and Reinhardt, 1981; Henry et al., 2020). The continuous support beyond nourishing further benefits offspring survival, for example, due to better protection against predators when living in a herd.
Placentation Types, Placental Transfer of Immunoglobulins During Gestation, and Consequences for Mammary Gland Function
There are different approaches to distinguish and categorize placentation types depending on morphological or histological structures, and wherefrom extraembryonic membranes and placental blood vessels origin within the fetus (Kressin and Brehm, 2019). We will mainly consider histological typing, as this classification fits best in terms of the permeability of blood components from the dam to the fetus. Based on the origin of the placenta, we can differentiate between the yolk sac (or choriovitelline placenta, resp.) and the chorioallantoic placenta. In most species addressed in this article, the yolk sac placenta represents a transient organ that is completely replaced by an allantoic placenta during pregnancy (Carter and Enders, 2016). However, in rodents and rabbits, parts of the yolk sac placenta persist until the end of pregnancy and share the function of transferring nutrients and Ig with the newly shaped allantoic placenta (Jollie, 1990; Carter and Enders, 2016).
Ungulates have an epitheliochorial placenta, where up to six layers of maternal and fetal tissue remain present until the end of pregnancy (Enders, 2009). Here, the mother and fetus share only a superficial connection without significant invasion of the maternal tissue (Furukawa et al., 2014; Carter and Enders, 2016). Carnivores have an endotheliochorial placenta type, where maternal uterine epithelium and connective tissue are dissolved and the fetal trophoblast has direct contact with the maternal endothelium (Enders, 2009; Furukawa et al., 2014; Carter and Enders, 2016). Primates, rodents, and rabbits belong to the hemochorial subgroup, where all maternal layers get invaded and dissolved by the fetal membranes (Enders, 2009). Here, the fetal membranes are in direct contact with maternal blood (Furukawa et al., 2014; Carter and Enders, 2016).
The necessity for considering placentation types emanates not least by the fact that the permeability of the placenta during gestation, for example, for Ig, has fundamental implications on the mammary gland function in terms of colostrum formation. Across the different mammalian species, the immune system of neonates is not fully developed at birth (Tsafaras et al., 2020). Therefore, the newborn offspring depends on the passive immunization either via placental Ig transfer during gestation or through colostrum intake immediately after birth.
A prenatal Ig transfer is enabled through the yolk sac placenta in rabbits and rodents, and through the allantoic placenta in humans and potentially other primate species (Peri and Rothberg, 1986; Leach et al., 1996; Carter and Enders, 2016). Hence, a timely colostrum supply immediately after birth is of minor importance in these species. Despite a partial Ig transfer in utero, cats and dogs rely mostly on colostrum (Casal et al., 1996; Stoffel et al., 2000; Claus et al., 2006). Therefore, the uptake of adequate amounts of colostrum of sufficient quality directly postpartum in the latter species is still crucial (Casal et al., 1996; Chastant and Mila, 2019). Domestic ungulates (cattle, sheep, goats, etc.) rely solely on colostrum for transfer of passive immunity as the placenta does not allow an Ig transfer during gestation (Rooke and Bland, 2002; Castro et al., 2011a, 2011b). Here, the immediate availability of colostrum at birth is essential.
In contrast to most eutherian species, metatheria are born in a very immature state without a functioning lymphocytic system. Thus, the transfer of passive immunity happens solely via colostrum/milk consumption as well (Cheng and Belov, 2017). In contrast to eutheria, two separate consecutive phases of Ig transfer with a period of low Ig concentration in milk in-between can be identified while the young grow in the pouch. The first phase begins right after birth and lasts for several weeks, whereas the second phase starts a few months later concomitantly with the young leaving the pouch for the first time and getting in contact with an extensive range of new pathogens (Cheng and Belov, 2017).
In general, the number of layers in between fetal and maternal blood circulation is related to the potential of the intra-uterine Ig transfer and efficiency of nourishing the fetus, but other factors are involved in the efficiency of placental Ig and nutrient transfer to the fetus, too. Most species with a less intertwined placental surface regarding the histological structure of the interhemal barrier have greater Ig concentrations in colostrum than species with a more extensive and closer contact between maternal and fetal tissues (Butler and Kehrli, 2005; Markowska-Daniel and Pomorska-Mól, 2010; Capellini et al., 2011).
The placental transfer of macromolecules depends largely, but not exclusively on the number of placental layers (Furukawa et al., 2014; Tanner et al., 2022). Different mechanisms exist to overcome these barriers, for example, a partial thinning of the existing six placental layers in pigs, or transport mechanisms such as phagocytosis or secretion (Furukawa et al., 2014). In dogs, Ig can pass through additional placental layers by an integrated transport system, where no prenatal Ig transfer was observed in the hemophagic marginal zones (three layers), but in the labyrinth zone characterized by four layers (Stoffel et al., 2000). Although not described in detail, we assume that the neonatal Fc receptor (FcRn) system is involved in Ig transport, though dog-specific evidence has yet to be brought forward.
Capellini et al. (2011) stated that rather than the invasiveness of the fetal membranes it is the complexity of the mother-fetus interface along with a greater surface area, which allows a more efficient transfer of nutrients across the placenta. According to Capellini et al. (2011), species with a larger mother-fetus interface grow more rapidly, but instead of giving birth to larger offspring the duration of gestation is shortened. This observation especially applies to altricial offspring, which are characterized by a low maturation stage at birth but fast growth rate. Cats and dogs have a close, labyrinthine placental interdigitation and give birth to altricial offspring, whereas cow and calf (precocial) share a more distant, villous interdigitation.
Colostrogenesis and Colostrum Composition in Different Species
Immunoglobulins and proteins
Independent from species-specific particularities, an insufficient or lacking placental Ig transfer must be compensated by an Ig-rich secretion of the mammary gland (i.e., colostrum) immediately after parturition. During colostrogenesis maternal Ig appear and accumulate in the mammary gland to ensure a timely and sufficient immunological protection of the neonate immediately after parturition (Barrington et al., 2001). Whereas ungulates rely on the immediate availability of colostrum at birth, an immediate colostrum supply in species with a significant placental Ig transfer during gestation (e.g., rabbit and human) is of minor importance (Peri and Rothberg, 1986; Leach et al., 1996). Humans produce only small amounts of colostrum directly postpartum, while the onset of copious milk production can be delayed for 2 to 4 d (Neville and Morton, 2001; Alekseev, 2021).
In horses, the transfer of Ig into pre-colostrum occurs during the last 2 wk of pregnancy (McCue and Sitters, 2011). Thus colostrogenesis in horses is apparently much shorter than in cows, where colostrogenesis is assumed to start several weeks antepartum (Brandon et al., 1971). As a drop of Ig concentration in the serum of the mare occurs concomitantly to the Ig appearance in the pre-colostrum, the assumption is confirmed that at least part of the Ig in colostrum is derived from the maternal circulation (Peaker et al., 1979). Similarly, colostrum formation in pigs was observed during the last 10 d of pregnancy and the first day postpartum (Quesnel and Farmer, 2019; Farmer and Quesnel, 2020). Whereas the majority of IgG transported into the mammary gland originates from serum, more than half of IgA is produced locally in the mammary gland in pigs (Bourne and Curtis, 1973) and cows (Porter, 1972). No information could be gathered for carnivores and humans.
The exact mode of Ig transfer into mammary secretions is not yet fully elucidated. Most of the available information refers to rodents and cattle since information about other species is scarce. The prepartal Ig transport into the mammary gland is assumed to be mediated by the FcRn system as evidence in rodents and cows suggests (Cianga et al., 1999; Mayer et al., 2005). IgG is transported by a transcellular pathway from the bloodstream into mammary epithelial cells (Wall et al., 2015) and secreted by the FcRn system, which has been first discovered in the gut of newborn rats and mice (Rodewald, 1976), but is also present in mammary epithelial tissue of cattle and sheep (Mayer et al., 2002, 2005).
The IgG transfer from blood into the mammary gland can be extremely fast after a preceding colostrum removal shortly before calving (Gross et al., 2014). Presumably, blood is not the only source for Ig to be transferred into colostrum as Ig concentrations in blood were not related to the Ig content in colostrum in cows (Baumrucker et al., 2016). Alternative sources could be local production or an Ig pool within or close to the mammary gland (Baumrucker et al., 2016). However, colostrogenesis does not immediately cease at parturition, but may continue for the first hours after calving until the blood-milk barrier (BMB) is completely closed (Gross et al., 2014; Wall et al., 2015). Mechanisms mediating the shift from colostrogenesis to copious milk production are not finally elucidated. We assume that the discontinuation of placental hormone concentrations (e.g., estradiol) are substantially involved.
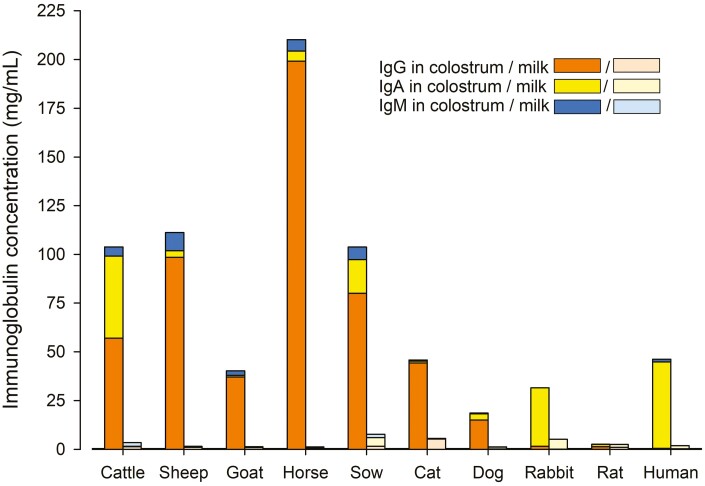
Compared to mature milk, colostrum is characterized by an elevated content of proteins including Ig and albumin (Samarütel et al., 2016; Figures 1 and 2). In ungulates and carnivores, where the transfer of IgG to the offspring happens via colostrum intake, Ig represents the most abundant protein fraction in colostrum. In this article, we will primarily address species differences in the colostral Ig content and refer to the three most relevant isotypes IgA, IgG, and IgM. IgG is the most abundant isotype in blood and colostrum (Figure 2). IgA is primarily active on mucosal surfaces and in secretions, where it neutralizes antigens or prevents their binding to the surface. It is the most abundant Ig in rabbit and human mammary gland secretions (Figure 2). Overall, Ig concentrations in colostrum are characterized by a considerable variation.
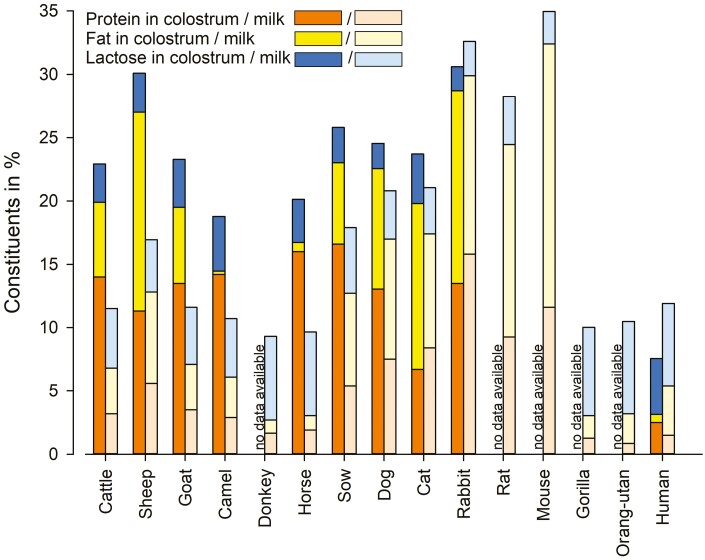
Figure 1.
Average contents of protein, fat, and lactose in colostrum (left bars) and mature milk (right bars) of different mammalian species. Cumulative contents of protein, fat, and lactose indicate DM content of colostrum and milk. Data are derived from (for cattle) Pereira (2014), Kessler et al. (2020), (for sheep) Merlin Junior et al. (2015), Kessler et al. (2019), (for goat) Guo et al. (2001), Kessler et al. (2019), (for camel) Zhang et al. (2005), Mohamed et al. (2021), (for donkey) Guo et al. 2007, (for horse) Salimei et al. (2002), Pecka et al. (2012), Barreto et al. (2020), (for sow) Hurley (2015), (for dog) Oftedal (1984), Mila et al. (2015a), Chastant-Maillard and Mila (2016), (for cat) Dobenecker et al. (1998), Jacobsen et al. (2004), (for rabbit) Anderson et al. (1975), Ludwiczak et al. (2020), (for rat) Cox and Mueller (1937), Keen et al. (1981), Grigor et al. (1986), Nicholas and Hartmann (1991), (for mouse) Görs et al. (2009), (for gorilla) Garcia et al. (2017), (for orangutan) Garcia et al. (2017), (for human) Yuen et al. (2012), Palmeira and Carneiro-Sampaio (2016), and Lima et al. (2018).
Figure 2.
Contents of IgG, IgA, and IgM in colostrum (left bars) and mature milk (right bars) of different mammalian species. Bars show average values of data derived from (for cattle) Guidry et al. (1980), Butler and Kehrli (2005), Hurley and Theil (2011), Wellnitz et al. (2013), Hernández-Castellano et al. (2016), (for sheep) Campbell et al. (1977), Butler and Kehrli (2005), Hurley and Theil (2011), Hernández-Castellano et al. (2016), (for goat) Sánchez-Macías et al. (2014), Hernández-Castellano et al. (2016), (for horse) Kohn et al. (1989), Sheoran et al. (2000), Butler and Kehrli (2005), Hurley and Theil (2011), (for sow) Butler and Kehrli (2005), Markowska-Daniel and Pomorska-Mól (2010), Hurley and Theil (2011), (for cat) Butler and Kehrli (2005), Claus et al. (2006), (for dog) Heddle and Rowley (1975), Butler and Kehrli (2005), Mila et al. (2015b), (for rabbit) Butler and Kehrli (2005), (for rat) McGhee et al. (1975), Michalek et al. (1975), Butler and Kehrli (2005), (for human) Butler and Kehrli (2005), Hurley and Theil (2011), Sousa et al. (2014), and Czosnykowska-Łukacka et al. (2020).
The high protein and Ig contents in colostrum rapidly decline with the closure of the BMB concomitantly to the onset of copious milk production after parturition (Butler and Kehrli, 2005; Wall et al., 2015; Kessler et al., 2019). Along with the general decline of the protein and Ig contents during the transition from colostrum to mature milk (Figures 1 and 2), concentrations of IgA increase relatively to IgG in mature milk. However, in ruminants IgG continues to be the most abundant Ig in mature milk, too (Butler and Kehrli, 2005; Sánchez-Macías et al., 2014).
A less pronounced difference in the protein contents between colostrum and mature milk was observed in human (Yuen et al., 2012) and rabbits (Ludwiczak et al., 2020). In these species, the offspring receives IgG already in utero and is therefore not depending on a colostral IgG supply (Peri and Rothberg, 1986; Leach et al., 1996). In contrast to ungulates and carnivores, the most abundant Ig isotype in colostrum and mature milk is IgA (Butler and Kehrli, 2005), which emphasizes the importance of colostrum for the development of local immune competence in rabbit and human by coating the mucosal surface of the intestine in neonates (Pang and Hartmann, 2007). A noteworthy exception is the cat, whose kittens rely on colostrum for the transfer of passive immunity (Casal et al., 1996), although IgG (Claus et al., 2006) and total milk protein concentrations are similar in colostrum and milk throughout lactation (Jacobsen et al., 2004).
Fat
A considerable variation in the fat content of colostrum and milk can be observed among different mammalian species (Figure 1). In typical dairy species (cow, sheep, and goat), the fat content in colostrum is higher than in mature milk (Guo et al., 2001; Kessler et al., 2019, 2020). On the other hand, colostrum of pigs has a lower fat content compared with mature milk. As piglets are born without brown body fat (Berg et al., 2006), they rely on fast digestible energy such as carbohydrates (Farmer, 2015) to maintain thermoregulation by shivering (Le Dividich and Noblet, 1984; Berg et al., 2006). Human colostrum has similar fat content, although babies, as opposed to piglets, are born with a fat reserve and are consequently not instantly depending on energy-rich milk (Pang and Hartmann, 2007). Rabbits have a high milk fat content throughout lactation, likely as their offspring is altricial and fed only once daily by their mother. Therefore, kids require a long-lasting and filling energy source (Zarrow et al., 1965). In contrast, horse milk has a low fat content throughout lactation, probably because the foal is precocial and able to follow its mother sucking multiple times per hour (Tyler, 1972). We only found inconsistent data concerning colostrum and milk composition in cats and dogs, and no data for rodent colostrum Figure 1).
Lactose and other carbohydrates
Lactose is the main osmotically active component in milk of most eutherians, whose content is positively related to the amount of water and thus to the volume of milk produced (Fox et al., 2015; Urashima et al., 2022). Milk lactose content rises with the onset of copious milk production (Fox et al., 2015). Newborn and milk-fed eutherians express the enzyme lactase, which is important for lactose digestion (Lebensthal et al., 1975). All species covered in this review have higher amounts of lactose in mature milk than in colostrum (Figure 1). Besides lactose, oligosaccharides (OS) represent an important fraction of carbohydrates. In monotremata and marsupialia, they even represent the vast majority of milk carbohydrates, as lactose appeared in greater amounts in milk only after the evolution of α-lactalbumin from lysozyme in eutheria (Urashima et al., 2022). OS concentrations are greater in colostrum compared to mature milk (Albrecht et al., 2014). From an evolutionary point of view, secretions as precursors of today’s milk had numerous immunological protective properties, whereas nowadays their nutritive purpose in mammals predominates (Urashima et al., 2022). Colostrum might be assumed an intermediate step in the evolution from an anti-infectional to a nutritional liquid.
Humans, and presumably many other eutherian species as well, are only able to digest OS to a lesser extent by endogenous enzymes (Urashima et al., 2022). Instead, OS serve as prebiotics in the large intestine of the newborn that stimulates the growth of beneficial microbes and their colonization of the large intestine (Plaza-Díaz et al., 2018; Quinn et al., 2020). Besides supporting the intestinal microbiota (Fischer et al., 2018), OS-mediated effects are speculated to enhance IgG absorption (Gill et al., 1999), and binding and neutralizing of pathogens (Martín et al., 2002; Quinn et al., 2020). While their effects are probably similar among species, every species seems to have its own pattern with regard to types and concentrations of OS in milk (Albrecht et al., 2014; Quinn et al., 2020). The greatest concentration and diversity of OS is observed in human milk, with about 20 g/L at the fourth day of lactation (Coppa et al., 1993) and over 200 different types discovered so far (Plaza-Díaz et al., 2018). Compared to human milk, bovine colostrum contains much lower amounts of OS (Urashima et al., 2022).
Most likely due to their importance in neonatal health, human milk OS is the most studied, whereas scientific knowledge about OS contents in colostrum and milk of other mammals is rather scarce. Other primates have a high diversity in OS as well, but similarities to humans do not necessarily seem to be due to phylogenetic factors, but rather to different types of antigen exposure of the newborns (Bode, 2012). Albrecht et al. (2014) discussed the importance of colostral and milk OS for the development of the neonatal gastrointestinal system, and observed similarities of OS distribution in colostrum and milk of species with a similar food source and digestive system. No specific data could be acquired for carnivores, rodents, and rabbits.
Non-nutritive bioactive components
Besides nutrients, colostrum is rich in minerals, trace elements, vitamins, and cells (leukocytes, lactocytes from the epithelium, and erythrocytes; Blum, 2000). Furthermore, bioactive components such as hormones and growth factors (growth hormone, insulin-like growth factor or epidermal growth factor, prolactin, insulin, glucagon, releasing factors, and prostaglandins), enzymes, lactoferrin, and transferrin appear at elevated concentrations in colostrum, whereas only traces can be detected in mature milk (Grosvenor et al., 1993; Blum and Baumrucker, 2002; Fischer-Tlustos et al., 2021). The impact of bioactive components in colostrum on the development of the gastrointestinal tract and other organs has been extensively studied in bovines and pigs, whereas data concerning other species are scarce (Grosvenor et al., 1993; Blättler et al., 2001; Blum and Baumrucker, 2002).
Conclusions
The structure and number of layers of the placenta determine the intrauterine transfer of Ig during gravidity, and consequently the necessity of colostrum supply to ensure passive immunization. In particular, many domestic farm animals are ungulates and rely on the timely supply of colostrum immediately after parturition. Colostrum and milk composition further provide a customized liquid feed source meeting the demands of neonates born at different developmental and maturity stages. However, placentation does only partially explain the heterogeneity of lactogenesis and colostrum formation. Despite similarities in placentation, Ig content and milk composition may vary tremendously among and within different species.
Acknowledgments
The financial support of the Hans Sigrist Foundation (University of Bern, Switzerland) by providing a scholarship to N.A.B. is gratefully acknowledged.
Glossary
Abbreviations
- BMB
blood–milk barrier
- FcRn
neonatal Fc receptor
- Ig
immunoglobulin
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgG1
immunoglobulin G type 1
- IgG2
immunoglobulin G type 2
- IgM
immunoglobulin M
- OS
oligosaccharides
Contributor Information
Naomi A Bigler, Veterinary Physiology, Vetsuisse Faculty, University of Bern, Bern, Switzerland.
Rupert M Bruckmaier, Veterinary Physiology, Vetsuisse Faculty, University of Bern, Bern, Switzerland.
Josef J Gross, Veterinary Physiology, Vetsuisse Faculty, University of Bern, Bern, Switzerland.
Conflict of Interest Statement
The authors have declared no conflicts of interests.
Literature Cited
- Akers, R. M. 2002. Lactation and the mammary gland. 1st ed. Ames (IA): Wiley-Blackwell. [Google Scholar]
- Albrecht, S., Lane J. A., Mariño K., Al Busadah K. A., Carrington S. D., Hickey R. M., and Rudd P. M.. . 2014. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 111:1313–1328. doi: 10.1017/S0007114513003772 [DOI] [PubMed] [Google Scholar]
- Alekseev, N. P. 2021. Physiology of human female lactation. 1st ed. Cham (Switzerland): Springer. doi: 10.1007/978-3-030-66364-3 [DOI] [Google Scholar]
- Anderson, R. R., Sadler K. C., Knauer M. W., Wippler J. P., and Marshall R. T.. . 1975. Composition of cottontail rabbit milk from stomachs of young and directly from gland. J. Dairy Sci. 58:1449–1452. doi: 10.3168/jds.S0022-0302(75)84736-9 [DOI] [PubMed] [Google Scholar]
- Barreto, M. L. G., Urbano S. A., Oliveira C. A. A., Macêdo C. S., Borba L. H. F., Chags B. M. E., and Rangel A. H. N.. . 2020. Chemical composition and lipid profile of mare colostrum and milk of the quarter horse breed. PLoS One 15:1–10. doi: 10.1371/journal.pone.0238921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington, G. M., McFadden T. B., Huyler M. T., and Besser T. E.. . 2001. Regulation of colostrogenesis in cattle. Livest. Prod. Sci. 70:95–104. doi: 10.1016/S0301-6226(01)00201-9 [DOI] [Google Scholar]
- Baumrucker, C. R., Dechow C. D., Macrina A. L., Gross J. J., and Bruckmaier R. M.. . 2016. Mammary immunoglobulin transfer rates following prepartum milking. J. Dairy Sci. 99:9254–9262. doi: 10.3168/jds.2016-11370 [DOI] [PubMed] [Google Scholar]
- Berg, F., Gustafson U., and Andersson L.. . 2006. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2:e1291178–e1291181. doi: 10.1371/journal.pgen.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blättler, U., Hammon H. M., Morel C., Philipona C., Rauprich A., Romé V., Le Huerou I., Guilloteau P., and Blum J. W.. . 2001. Feeding colostrum, its composition and feeding duration variably modify proliferation and morphology of the intestine and digestive enzyme activities of neonatal calves. J. Nutr. 12:1256–1263. doi: 10.1093/jn/131.4.1256 [DOI] [PubMed] [Google Scholar]
- Blum, J. W., and Baumrucker C. R.. . 2002. Colostral and milk insulin-like growth factors and related substances: mammary gland and neonatal (intestinal and systemic) targets. Domest. Anim. Endocrinol. 23:101–110. doi: 10.1016/s0739-7240(02)00149-2 [DOI] [PubMed] [Google Scholar]
- Blum, J. W., and Hammon H.. . 2000. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livest. Prod. Sci. 66:151–159. doi: 10.1016/S0301-6226(00)00222-0 [DOI] [Google Scholar]
- Bode, L. 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. doi: 10.1093/glycob/cws074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, F. J., and Curtis J.. . 1973. The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology 24:157–162. [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, F. J., and Bradshaw D.. . 2011. Progesterone and reproduction in marsupials: a review. Gen. Comp. Endocrinol. 170:18–40. doi: 10.1016/j.ygcen.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Brandon, M. R., Watson D. L., and Lascelles A. K.. . 1971. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust. J. Exp. Biol. Med. Sci. 49:613–623. doi: 10.1038/icb.1971.67 [DOI] [PubMed] [Google Scholar]
- Brennan, A. J., Sharp J. A., Lefevre C., Topcic D., Auguste A., Digby M., and Nicholas K. R.. . 2007. The tammar wallaby and fur seal: models to examine local control of lactation. J. Dairy Sci. 90:E66–E75. doi: 10.3168/jds.2006-483 [DOI] [PubMed] [Google Scholar]
- Butler, W. R. 2005. Inhibition of ovulation in the postpartum cow and the lactating sow. Livest. Prod. Sci. 98:5–12. doi: 10.1016/j.livprodsci.2005.10.007 [DOI] [Google Scholar]
- Butler, J. E., and Kehrli M. E. J.. . 2005. Immunoglobulins and immunocytes in the mammary gland and its secretions. In Mestecky J. F., Beinenstock J., Lamm M. E., Mayer L., McGhee J. R., and Strober W., editors. Mucosal Immunology. 3rd ed. Burlington (VT): Academic Press; pp.1763–1793. [Google Scholar]
- Campbell, S. G., Siegel M. J., and Knowlton B. J.. . 1977. Sheep immunoglobulins and their transmission to the neonatal lamb. N. Z. Vet. J. 25:361–365. doi: 10.1080/00480169.1977.34458 [DOI] [PubMed] [Google Scholar]
- Capellini, I., Venditti C., and Barton R. A.. . 2011. Placentation and maternal investment in mammals. Am. Nat. 177:86–98. doi: 10.1086/657435 [DOI] [PubMed] [Google Scholar]
- Carter, A. M., and Enders A. C.. . 2016. Placentation in mammals: definitive placenta, yolk sac, and paraplacenta. Theriogenology 86:278–287. doi: 10.1016/j.theriogenology.2016.04.041 [DOI] [PubMed] [Google Scholar]
- Casal, M. L., Jezyk P. F., and Urs G.. . 1996. Transfer of colostral antibodies from queens to their kittens. Am. J. Vet. Res. 57:1653–1658. [PubMed] [Google Scholar]
- Castro, N., Capote J., Batista M., Bruckmaier R. M., and Argüello A.. . 2011a. Effects of induced parturition in goats on immunoglobulin G and chitotriosidase activity in colostrum and plasma and on plasma concentrations of prolactin. Domest Anim. Endocrinol. 40:192–196. doi: 10.1016/j.domaniend.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Castro, N., Capote J., Bruckmaier R. M., and Argüello A.. . 2011b. Management effects on colostrogenesis in small ruminants: a review. J. Appl. Anim. Res. 39:85–93. doi: 10.1080/09712119.2011.581625 [DOI] [Google Scholar]
- Chao, S. 1987. The effect of lactation on ovulation and fertility. Clin. Perinatol. 14:39–50. doi: 10.1016/S0095-5108(18)30780-2 [DOI] [PubMed] [Google Scholar]
- Chastant, S., and Mila H.. . 2019. Passive immune transfer in puppies. Anim. Reprod. Sci. 207:162–170. doi: 10.1016/j.anireprosci.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastant-Maillard, S., and Mila H.. . 2016. Canine colostrum. Vet. Focus 26:32–38. [Google Scholar]
- Cheng, Y., and Belov K.. . 2017. Antimicrobial protection of marsupial pouch young. Front. Microbiol. 8:1–8. doi: 10.3389/fmicb.2017.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianga, P., Medesan C., Richardson J. A., Ghetie V., and Ward E. S.. . 1999. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur. J. Immunol. 29:2515–2523. doi: [DOI] [PubMed] [Google Scholar]
- Claus, M. A., Levy J. K., MacDonald K., Tucker S. J., and Crawford P. C.. . 2006. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J. Feline Med. Surg. 8:184–191. doi: 10.1016/j.jfms.2006.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppa, G. V., Gabrielli O., Pierani P., Catassi C., Carlucci A., and Giorgi P. L.. . 1993. Changes in carbohydrate composition in human milk over 4 month of lactation. Pediatrics 91:637–641. doi: 10.1542/peds.91.3.637 [DOI] [PubMed] [Google Scholar]
- Cox, W. M., and Mueller A. J.. . 1937. The composition of milk from stock rats and an apparatus for milking small laboratory animals. J. Nutr. 13:249–261. doi: 10.1093/jn/13.3.249 [DOI] [Google Scholar]
- Czosnykowska-Łukacka, M., Lis-Kuberka J., Królak-Olejnik B., and Orczyk-Pawiłowicz M.. . 2020. Changes in human milk immunoglobulin profile during prolonged lactation. Front. Pediatr. 8:428. doi: 10.3389/fped.2020.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobenecker, B., Zottmann B., Kienzle E., Wolf P., and Zentek J.. . 1998. Milk yield and milk composition of lactating queens. J. Anim. Physiol. Anim. Nutr. (Berl) 80:173–178. doi: 10.1111/j.1439-0396.1998.tb00523.x [DOI] [Google Scholar]
- Enders, A. C. 2009. Reasons for diversity of placental structure. Placenta 23:15–18. doi: 10.1016/j.placenta.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Farmer, C. 2015. The gestating and lactating sow. 1st ed. Wageningen (The Netherlands): Wageningen Academic Publishers. [Google Scholar]
- Farmer, C., and Quesnel H.. . 2020. Current knowledge on the control of onset and cessation of colostrogenesis in swine. J. Anim. Sci. 98:S133–S139. doi: 10.1093/jas/skaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. J., Malmuthuge N., Guan L. L., and Steele M. A.. . 2018. Short communication: the effect of heat treatment of bovine colostrum on the concentration of oligosaccharides in colostrum and in the intestine of neonatal male Holstein calves. J. Dairy Sci. 101:401–407. doi: 10.3168/jds.2017-13533 [DOI] [PubMed] [Google Scholar]
- Fischer-Tlustos, A. J., Lopez A., Hare K. S., Wood K. M., and Steele M. A.. . 2021. Effects of colostrum management on transfer of passive immunity and the potential role of colostral bioactive components on neonatal calf development and metabolism. Can. J. Anim. Sci. 101:405–426. doi: 10.1139/cjas-2020-0149 [DOI] [Google Scholar]
- Forsyth, I. A., and Hayden T. J.. . 1977. Comparative endocrinology of mammary growth and lactation. In: Peaker M., editor. Comparative aspects of lactation. London (UK): Academic Press; p. 135–163. [Google Scholar]
- Fox, P. F., Uniacke-Lowe T., McSweeney P. L. H., and O’Mahony J. A.. . 2015. Dairy chemistry and biochemistry. 2nd ed. Berlin Heidelberg (Denmark): Springer. [Google Scholar]
- Furukawa, S., Kuroda Y., and Sugiyama A.. . 2014. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 27:11–18. doi: 10.1293/tox.2013-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M., Power M. L., and Moyes K. M.. . 2017. Immunoglobulin A and nutrients in milk from great apes throughout lactation. Am. J. Primatol. 79:1–11. doi: 10.1002/ajp.22614 [DOI] [PubMed] [Google Scholar]
- Gill, R. K., Mahmood S., Nagpaul J. P., and Mahmood A.. . 1999. Functional role of sialic acid in IgG binding to microvillus membranes in neonatal rat intestine. Biol. Neonate 76:55–64. doi: 10.1159/000014131 [DOI] [PubMed] [Google Scholar]
- Görs, S., Kucia M., Langhammer M., Junghans P., and Metges C. C.. . 2009. Technical note: milk composition in mice—methodological aspects and effects of mouse strain and lactation day. J. Dairy Sci. 92:632–637. doi: 10.3168/jds.2008-1563 [DOI] [PubMed] [Google Scholar]
- Grigor, M. R., Allan J., Carne A., Carrington J. M., and Geursen A.. . 1986. Milk composition of rats feeding restricted litters. Biochem. J. 233:917–919. doi: 10.1042/bj2330917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J., Kessler E. C., Bjerre-Harpoth V., Dechow C., Baumrucker C. R., and Bruckmaier R. M.. . 2014. Peripartal progesterone and prolactin have little effect on the rapid transport of immunoglobulin G into colostrum of dairy cows. J. Dairy Sci. 97:2923–2931. doi: 10.3168/jds.2013-7795 [DOI] [PubMed] [Google Scholar]
- Grosvenor, C. E., Picciano M. F., and Baumrucker C. R.. . 1993. Hormones and growth factors in milk. Endocr Rev. 14:710–728. doi: 10.1210/edrv-14-6-710 [DOI] [PubMed] [Google Scholar]
- Guidry, A. J., Butler J. E., Pearson R. E., and Weinland B. T.. . 1980. IgA, IgG1, IgG2, IgM, and BSA in serum and mammary secretion throughout lactation. Vet. Immunol. Immunopathol. 1:329–341. doi: 10.1016/0165-2427(80)90012-4 [DOI] [PubMed] [Google Scholar]
- Guo, M. R., Dixon P. H., Park Y. W., Gilmore J. A., and Kindstedt P. S.. . 2001. Seasonal changes in the chemical composition of commingled goat milk. J. Dairy Sci. 84:E79–E83. doi: 10.3168/jds.s0022-0302(01)70201-9 [DOI] [Google Scholar]
- Guo, H. Y., Pang K., Zhang X. Y., Zhao L., Chen S. W., Dong M. L., and Ren F. Z.. . 2007. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 90:1635–1643. doi: 10.3168/jds.2006-600 [DOI] [PubMed] [Google Scholar]
- Heddle, R. J., and Rowley D.. . 1975. Dog immunoglobulins. I. Immunochemical characterization of dog serum, parotid saliva, colostrum, milk and small bowel fluid. Immunology 29:185–195. [PMC free article] [PubMed] [Google Scholar]
- Henry, S., Sigurjónsdóttir H., Klapper A., Joubert J., Montier G., and Hausberger M.. . 2020. Domestic foal weaning: need for re-thinking breeding practices? Animals 10:361. doi: 10.3390/ani10020361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Castellano, L. E., Almeida A. M., Renaut J., Argüello A., and Castro N.. . 2016. A proteomics study of colostrum and milk from the two major small ruminant dairy breeds from the Canary Islands: a bovine milk comparison perspective. J. Dairy Res. 83:366–374. doi: 10.1017/S0022029916000273 [DOI] [PubMed] [Google Scholar]
- Hurley, W. L. 2015. Composition of sow colostrum and milk. In: Farmer C., editor. The gestating and lactating sow. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 193–230. doi: 10.3920/978-90-8686-803-2_9 [DOI] [Google Scholar]
- Hurley, W. L., and Theil P. K.. . 2011. Perspectives on immunoglobulins in colostrum and milk. Nutrients 3:442–474. doi: 10.3390/nu3040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, K. L., DePeters E. J., Rogers Q. R., and Taylor S. J.. . 2004. Influences of stage of lactation, teat position and sequential milk sampling on the composition of domestic cat milk (Felis catus). J. Anim. Physiol. Anim. Nutr. (Berl) 88:46–58. doi: 10.1046/j.1439-0396.2003.00459.x [DOI] [PubMed] [Google Scholar]
- Jollie, W. P. 1990. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology 41:361–381. doi: 10.1002/tera.1420410403 [DOI] [PubMed] [Google Scholar]
- Keen, C. L., Lonnerdal B., Clegg L., and Hurley L. S.. . 1981. Developmental changes in composition of rat milk: trace elements, minerals, protein, carbohydrate and fat. J. Nutr. 111:226–236. doi: 10.1093/jn/111.2.226 [DOI] [PubMed] [Google Scholar]
- Kessler, E. C., Bruckmaier R. M., and Gross J. J.. . 2019. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J. Dairy Sci. 102:5542–5549. doi: 10.3168/jds.2018-16235 [DOI] [PubMed] [Google Scholar]
- Kessler, E. C., Bruckmaier R. M., and Gross J. J.. . 2020. Colostrum composition and immunoglobulin G content in dairy and dual-purpose cattle breeds. J. Anim. Sci. 98:1–6. doi: 10.1093/jas/skaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, C. W., Knight D., Hueston W., Jacobs R., and Reed S. M.. . 1989. Colostral and serum IgG, IgA, and IgM concentrations in Standardbred mares and their foals at parturition. J. Am. Vet. Med. Assoc. 195:64–68. [PubMed] [Google Scholar]
- Kressin, M., and Brehm R.. . 2019. Embryologie der Haustiere. 7th ed. Stuttgart (Germany): Georg Thieme Verlag KG. doi: 10.1055/b-006-163266 [DOI] [Google Scholar]
- Langer, P. 2008. The phases of maternal investment in eutherian mammals. Zoology 111:148–162. doi: 10.1016/j.zool.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Leach, J. L., Sedmak D. D., Osborne J. M., Rahill B., Lairmore M. D., and Anderson C. L.. . 1996. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J. Immunol. 157:3317–3322. [PubMed] [Google Scholar]
- Lebensthal, E., Antonowicz I., and Shwachman H.. . 1975. Correlation of lactase activity, lactose tolerance and milk consumption in different age groups. Am. J. Clin. Nutr. 28:595–600. doi: 10.1093/ajcn/28.6.595 [DOI] [PubMed] [Google Scholar]
- Le Dividich, J., and Noblet J.. . 1984. Effect of colostrum intake on metabolic rate and plasma glucose in the neonatal pig in relation to environmental temperature. Biol. Neonate 46:98–104. doi: 10.1159/000242039 [DOI] [PubMed] [Google Scholar]
- Lee, P. C., Majluf P., and Gordon I. J.. . 1991. Growth, weaning and maternal investment from a comparative perspective. J. Zool. 225:99–114. doi: 10.1111/j.1469-7998.1991.tb03804.x [DOI] [Google Scholar]
- Lima, H., Vogel K., Wagner-Gillespie M., Wimer C., Dean L., and Fogleman A.. . 2018. Nutritional comparison of raw, holder pasteurized, and shelf-stable human milk products. J. Pediatr. Gastroenterol. Nutr. 67:649–653. doi: 10.1097/MPG.0000000000002094 [DOI] [PubMed] [Google Scholar]
- Ludwiczak, A., Składanowska-Baryza J., Kuczyńska B., and Stanisz M.. . 2020. Hycole doe milk properties and kit growth. Animals 10:214. doi: 10.3390/ani10020214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska-Daniel, I., and Pomorska-Mól M.. . 2010. Shifts in immunoglobulins levels in the porcine mammary secretions during whole lactation period. Bull. Vet. Inst. Puławy 54:345–349. [Google Scholar]
- Martín, M. J., Martíín-Sosa S., and Hueso P.. . 2002. Binding of milk oligosaccharides by several enterotoxigenic Escherichia coli strains isolated from calves. Glycoconj. J. 19:5–11. doi: 10.1023/A:1022572628891 [DOI] [PubMed] [Google Scholar]
- Mayer, B., Doleschall M., Bender B., Bartyik J., Bosze Z., Frenyó L. V., and Kacskovics I.. . 2005. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J. Dairy Res. 72:107–112. doi: 10.1017/s0022029905001135 [DOI] [PubMed] [Google Scholar]
- Mayer, B., Zolnai A., Frenyó L. V., Jancsik V., Szentirmay Z., Hammarström L., and Kacskovics I.. . 2002. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology 107:288–296. doi: 10.1046/j.1365-2567.2002.01514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue, P. M., and Sitters S.. . 2011. Lactation. In: McKinnon A. O., Squires E. L., Vaala W. E., and Varner D. D., editors. Equine reproduction. Hoboken (NJ): Blackwell Publishing Ltd; p. 2277–2290. [Google Scholar]
- McGhee, J. R., Michalek S. M., and Ghanta V. K.. . 1975. Rat immunoglobulins in serum and secretions: purification of rat IgM, IgA and IgG and their quantitation in serum, colostrum, milk and saliva. Immunochemistry 12:817–823. doi: 10.1016/0019-2791(75)90146-9 [DOI] [PubMed] [Google Scholar]
- Merlin Junior, I. A., Santos J. S., Costa L. G., Costa R. G., Ludovico A., Rego F. C., and Santana E. H.. . 2015. Sheep milk: physical-chemical characteristics and microbiological quality. Arch. Latinoam. Nutr. 65:193–198. [PubMed] [Google Scholar]
- Michalek, S. M., Rahman A. F. R., and McGhee J. R.. . 1975. Rat immunoglobulins in serum and secretions: comparison of IgM, IgA and IgG in serum, colostrum, milk and saliva of protein malnourished and normal rats. Proc. Soc. Exp. Biol. Med. 148:1114–1118. doi: 10.3181/00379727-148-38699 [DOI] [PubMed] [Google Scholar]
- Mila, H., Coinus S., Grellet A., Feugier A., Mariani C., Power M. L., Maslanka M., and Chastant-Maillard S.. . 2015a. Nutritional and immunological composition of canine colostrum. Book of abstracts of the 18th EVSSAR congress on reproduction and pediatrics in dogs, cats and exotics. September 11–12, 2015. Hannover, Germany. Page 109 [Abstract]. [Google Scholar]
- Mila, H., Feugier A., Grellet A., Anne J., Gonnier M., Martin M., Rossig L., and Chastant-Maillard S.. . 2015b. Immunoglobulin G concentration in canine colostrum: evaluation and variability. J. Reprod. Immunol. 112:24–28. doi: 10.1016/j.jri.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Mohamed, H., Nagy P., Agbaba J., and Kamal-Eldin A.. . 2021. Use of near and mid infra-red spectroscopy for analysis of protein, fat, lactose and total solids in raw cow and camel milk. Food Chem. 334:127436. doi: 10.1016/j.foodchem.2020.127436 [DOI] [PubMed] [Google Scholar]
- Neville, M. C., and Morton J.. . 2001. Physiology and endocrine changes underlying human lactogenesis II. J. Nutr. 131:3005S–3008S. doi: 10.1093/jn/131.11.3005S [DOI] [PubMed] [Google Scholar]
- Nicholas, K. R., and Hartmann P. W.. . 1991. Milk secretion in the rat: progressive changes in milk composition during lactation and weaning and the effect of diet. Comp. Biochem. Physiol. A Comp. Physiol. 98:535–542. doi: 10.1016/0300-9629(91)90443-g [DOI] [PubMed] [Google Scholar]
- Oftedal, O. T. 1984. Lactation in the dog: milk composition and intake by puppies. J. Nutr. 114:803–812. doi: 10.1093/jn/114.5.803 [DOI] [PubMed] [Google Scholar]
- Oftedal, O. T. 2012. The evolution of milk secretion and its ancient origins. Animal 6:355–368. doi: 10.1017/S1751731111001935 [DOI] [PubMed] [Google Scholar]
- Palmeira, P., and Carneiro-Sampaio M.. . 2016. Immunology of breast milk. Rev. Assoc. Med. Bras. 62:584–593. doi: 10.1590/1806-9282.62.06.584 [DOI] [PubMed] [Google Scholar]
- Pang, W. W., and Hartmann P. E.. . 2007. Initiation of human lactation: secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 12:211–221. doi: 10.1007/s10911-007-9054-4 [DOI] [PubMed] [Google Scholar]
- Peaker, M., Rossdale P. D., Forsyth I. A., and Falk M.. . 1979. Changes in mammary development and the composition of secretion during late pregnancy in the mare. J. Reprod. Fertil 27:555–561. [PubMed] [Google Scholar]
- Pecka, E., Dobrzański Z., Zachwieja A., Szulc T., and Czyz K.. . 2012. Studies of composition and major protein level in milk and colostrum of mares. Anim. Sci. J. 83:162–168. doi: 10.1111/j.1740-0929.2011.00930.x [DOI] [PubMed] [Google Scholar]
- Pereira, P. C. 2014. Milk nutritional composition and its role in human health. Nutrition 30:619–627. doi: 10.1016/j.nut.2013.10.011 [DOI] [PubMed] [Google Scholar]
- Peri, B. A., and Rothberg R. M.. . 1986. Transmission of maternal antibody prenatally and from milk into serum of neonatal rabbits. Immunology 57:49–53. [PMC free article] [PubMed] [Google Scholar]
- Plaza-Díaz, J., Fontana L., and Gil A.. . 2018. Human milk oligosaccharides and immune system development. Nutrients 10:1038. doi: 10.3390/nu10081038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, P. 1972. Immunoglobulins in bovine mammary secretions. Quantitative changes in early lactation and absorption by the neonatal calf. Immunology 23:225–238. [PMC free article] [PubMed] [Google Scholar]
- Quesnel, H., and Farmer C.. . 2019. Review: nutritional and endocrine control of colostrogenesis in swine. Animal 13:s26S26–s26s34. doi: 10.1017/s1751731118003555 [DOI] [PubMed] [Google Scholar]
- Quinn, E. M., Joshi L., and Hickey R. M.. . 2020. Symposium review: dairy-derived oligosaccharides their influence on host–microbe interactions in the gastrointestinal tract of infants. J. Dairy Sci. 103:3816–3827. doi: 10.3168/jds.2019-17645 [DOI] [PubMed] [Google Scholar]
- Reinhardt, V., and Reinhardt A.. . 1981. Natural sucking performance and age of weaning in zebu cattle (Bos indicus). J. Agric. Sci 96:309–312. doi: 10.1017/s0021859600066089 [DOI] [Google Scholar]
- Rodewald, R. 1976. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J. Cell Biol. 71:666–669. doi: 10.1083/jcb.71.2.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke, J. A., and Bland I. M.. . 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78:13–23. doi: 10.1016/S0301-6226(02)00182-3 [DOI] [Google Scholar]
- Salimei, E., Varisco G., and Rosi F.. . 2002. Major constituents, leptin, and non-protein nitrogen compounds in mares’ colostrum and milk. Reprod. Nutr. Dev. 42:65–72. doi: 10.1051/rnd:2002007 [DOI] [PubMed] [Google Scholar]
- Samarütel, J., Baumrucker C. R., Gross J. J., Dechow C. D., and Bruckmaier R. M.. . 2016. Quarter variation and correlations of colostrum albumin, immunoglobulin G1 and G2 in dairy cows. J. Dairy Res. 83:209–218. doi: 10.1017/S0022029916000091 [DOI] [PubMed] [Google Scholar]
- Sánchez-Macías, D., Moreno-Indias I., Castro N., Morales-delaNuez A., and Argüello A.. . 2014. From goat colostrum to milk: physical, chemical, and immune evolution from partum to 90 days postpartum. J. Dairy Sci. 97:10–16. doi: 10.3168/jds.2013-6811 [DOI] [PubMed] [Google Scholar]
- Schmidt, P. M., Chakraborty P. K., and Wildt D. E.. . 1983. Ovarian activity, circulating hormones and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biol. Reprod. 28:657–671. doi: 10.1095/biolreprod28.3.657 [DOI] [PubMed] [Google Scholar]
- Sheoran, A. S., Timoney J. F., Holmes M. A., Karzenski S. S., and Crisman M. V.. . 2000. Immunoglobulin isotypes in sera and nasal mucosal secretions and their neonatal transfer and distribution in horses. Am. J. Vet. Res. 61:1099–1105. doi: 10.2460/ajvr.2000.61.1099 [DOI] [PubMed] [Google Scholar]
- Sousa, S. G., Delgadillo I., and Saraiva J. A.. . 2014. Effect of thermal pasteurisation and high-pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chem. 151:79–85. doi: 10.1016/j.foodchem.2013.11.024 [DOI] [PubMed] [Google Scholar]
- Stoffel, M. H., Friess A. E., and Hartmann S. H.. . 2000. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. J. Reprod. Fertil 118:315–326. doi: 10.1530/jrf.0.1180315 [DOI] [PubMed] [Google Scholar]
- Tanner, A. R., Kennedy V. C., Lynch C. S., Hord T. K., Winger Q. A., Rozance P. J., and Anthony R. V.. . 2022. In vivo investigation of ruminant placenta function and physiology—a review. J. Anim. Sci. 100:skac045. doi: 10.1093/jas/skac045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsafaras, G. P., Ntontsi P., and Xanthou G.. . 2020. Advantages and limitations of the neonatal immune system. Front. Pediatr. 8:5. doi: 10.3389/fped.2020.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, S. J. 1972. The behaviour and social organization of the New Forest Ponies. Anim. Behav. Monogr 5:87–196. doi: 10.1016/0003-3472(72)90003-6 [DOI] [Google Scholar]
- Urashima, T., Katayama T., Sakanaka M., Fukuda K., and Messer M.. . 2022. Evolution of milk oligosaccharides: origin and selectivity of the ratio of milk oligosaccharides to lactose among mammals. Biochim. Biophys. Acta, Gen. Subj. 1866:130012. doi: 10.1016/j.bbagen.2021.130012 [DOI] [PubMed] [Google Scholar]
- Wall, S. K., Gross J. J., Kessler E. C., Villez K., and Bruckmaier R. M.. . 2015. Blood-derived proteins in milk at start of lactation: indicators of active or passive transfer. J. Dairy Sci. 98:7748–7756. doi: 10.3168/jds.2015-9440 [DOI] [PubMed] [Google Scholar]
- Wellnitz, O., Arnold E. T., Lehmann M., and Bruckmaier R. M.. . 2013. Short communication: differential immunoglobulin transfer during mastitis challenge by pathogen-specific components. J. Dairy Sci. 96:1681–1684. doi: 10.3168/jds.2012-6150 [DOI] [PubMed] [Google Scholar]
- Yuen, J. W. M., Loke A. Y., and Gohel M. D. I.. . 2012. Nutritional and immunological characteristics of fresh and refrigerated stored human milk in Hong Kong: a pilot study. Clin. Chim. Acta 413:1549–1554. doi: 10.1016/j.cca.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Zarrow, M. X., Denenberg V. H., and Anderson C. O.. . 1965. Rabbit: frequency of suckling in the pup. Science 150:1835–1836. doi: 10.1126/science.150.3705.1835 [DOI] [PubMed] [Google Scholar]
- Zhang, H., Yao J., Zhao D., Liu H., Li J., and Guo M.. . 2005. Changes in chemical composition of Alxa bactrian camel milk during lactation. J. Dairy Sci. 88:3402–3410. doi: 10.3168/jds.S0022-0302(05)73024-1 [DOI] [PubMed] [Google Scholar]