In 2022, the obtention of sustained deep molecular response (DMR) with successful subsequent treatment-free remission (TFR) is part of the new paradigm of treatment of chronic phase chronic myeloid leukemia (CP-CML).1 Although generally known as being “feasible and safe”, we observed six cases of blast crises (BC) after tyrosine kinase inhibitor (TKI) cessation, reported here.
Physicians of the French CML Study Group (Fi-LMC) were asked to report any cases of BC occurring in TFR patients; TFR being considered as ≥2 years of 4.5-log reduction (MR4.5) as recommended.2 All patients were informed by the appropriate procedures approved by local ethical committees. Hematologic assessments were performed locally. BCR::ABL1 transcripts were quantified by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and reported as BCR::ABL1/ABL1 in percent international scale (%IS),1 TK domain mutations were screened by next-generation sequencing (NGS) and copy number variation (CNV) analyses were performed on marrow cell DNA at CP (all patients except patient 2) and BC (all patients) diagnoses. Panels included 48 “myeloid” genes (Online Supplementary Table S1) and 62 additional “lymphoid” genes for lymphoid blast crises (LBC) (Online Supplementary Table S2).
Chronic phase chronic myeloid leukemia descriptive analysis (Table 1)
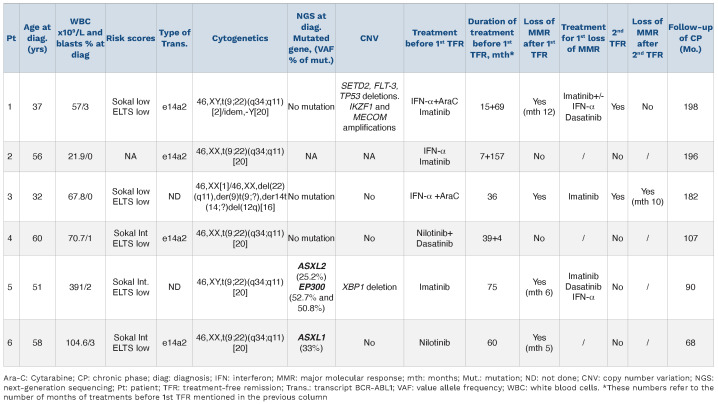
Patient 1 is a 37-year-old male, with CP-CML diagnosed in 2000 treated with IFN-α+AraC from 2000-2001. He was switched to imatinib (IM) following IFN-α failure. After >5 years of IM and 2 years of DMR, he made a first cessation attempt, but lost major molecular response (MMR) at 12 months (M12). After IM resumption he switched to dasatinib 2011-2013 before attempting TFR for a second time. Patient 2 is a 56-year-old female, with CP-CML diagnosed in 2003 treated with IFN-α for 7 months and then with IM from 2003 to 2016. After 10 years of MR4.5 she stopped IM for TFR. Patient 3 is a 32-year-old female, partly reported in,6 diagnosed in CP in 1996, treated with IFN-α+AraC from 1996 to 1999 who became intolerant while in DMR. She started IM in 2006, following molecular recurrence. After 3 years IM and 30 months MR4.5 she stopped IM in 2009 for a second attempt at TFR. Patient 4 is a 60-year-old female with CP-CML diagnosed in 2012, treated with nilotinib from 2012 to 2016. After nilotinib intolerance, while in sustained DMR, she switched to dasatinib that was also withdrawn 4 months later due to intolerance. Patient 5 is a 51-year-old male, CP-CML diagnosed in 2007 and treated with IM until 2013. After 6.25 years and 4.5 years of MR4, he stopped IM in 2013 for TFR. Patient 6 is a 58-year-old, CP-CML diagnosed in 2010 and treated with nilotinib from 2011-2014. Discovery of atherosclerosis resulted in nilotinib dose reduction from 2014 to 2016 and suspension after 5 years and >3 years in sustained MR4.5.
Blast crisis chronic myeloid leukemia descriptive analysis (Table 2)
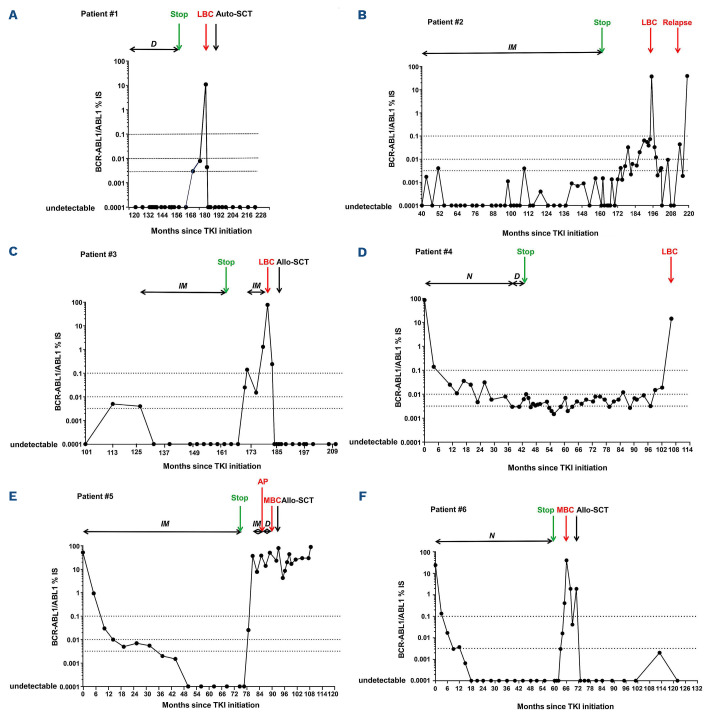
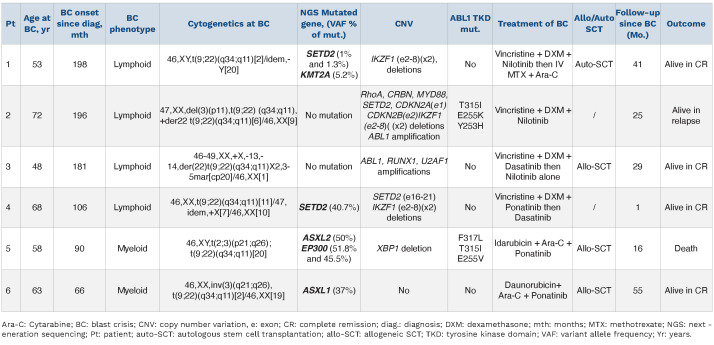
BC occurred during the TFR phase in four of the six patients. MBC was observed in patients 5 and 6 whereas the remaining four patients were in B-lineage LBC. BC occurred in TFR phase for patients 1, 2, 4, and 6 (Figure 1A, B, D and F) whereas patients 3 and 5 experienced BC 9 months after IM resumption following MMR loss (Figure 1C and E). For patient 5, IM was switched to dasatinib after 4 months due to molecular progression and the emergence of ACA in Philadelphia chromosome-positive (Ph+) cells was demonstrated 5 months prior to BC. For patients with transformation during TFR, time to BC from TKI cessation varied from 6 to 63 months but the kinetics of BC followed similar dramatic patterns. The intervals from last MMR to BC were 1, 2, 3 and 6 months for patients 2, 5, 4, and 1 respectively. MMR loss occurred at the same time as BC for three of four patients (patients 1, 2 and 4) and belatedly following TKI cessation for these three patients (M48, M32 and M63 respectively). A Ph duplication was present at BC for patients 2 and 3. For MBC (patients 5 and 6) a 3q26 (involving EVI1), already present in accelerated phase prior to BC for patient 5, was identified again in BC. Patient 3 had a variant t(9;22)(q34;q11) and patient 1 a Y chromosome loss. CNV analysis detected ABL1 amplification for patients 2 and 3. Double deletions of IKZF1 (exons 2-8) were reported for patients 1, 2 and 4. SETD2 deletions (whole gene or exons 16-21) were found for patients 2 and 4. Other CNV were also reported: RhoA, CRBN, MYD88, CDKN2A, CDKN2B deletions for patient 2, or RUNX1, U2AF1 amplification for patient 3, and XBP1 for patient 5. Of note, XBP1 deletion was present in patient 5 whereas deletions of SETD2, FLT3 and TP53 with amplification of IKZF1 and MECOM were reported in patient 1, all at CP diagnosis. Multiple gene mutations were found for MBC patients (patients 1, 4, 5 and 6). For patient 6, a nonsense mutation in ASXL1 with a variant allele frequency (VAF) of 37% in exon 12 was observed. This clone was also present at CP (VAF: 33%) but not on a CCyR sample. For patient 5 a nonsense mutation ASXL2 (VAF: 50%) in exon 12 was found with two EP300 mutations. This clone was also present at CP (VAF: 25.2% for ASXL2 and 52.7% and 50.8% for EP300). Interestingly, mutations were observed in SETD2 for two patients (1 and 4) at BC, not detectable at CP. Patient 1 had two low-level SETD2 variants whereas a stop-gain variant was estimated as 40.7% for patient 4. An additional KMT2A splice-acceptor variant was found for patient 1. In addition, patient 2 showed a T315I (VAF: 2%) + E255K (VAF: 5%) + Y253H (VAF: 5%) ABL1 TK domain mutations at BC relapse (18 months after BC diagnosis). Patient 5 had three mutations at BC: F317L (VAF: 10.6%); T315I (VAF: 4.9%) and E255V (VAF:23.6%), but the patient had been on TKI for 9 months.
Table 1.
Patient characteristics at diagnosis of chronic phase chronic myleoid Leukemia.
Allogeneic stem cell transplantation (allo-SCT) was performed for patients 3, 5, 6, and autologous-SCT (auto-SCT) in CR for patient 1. Patient 2 received EWALL induction chemotherapy+nilotinib. A stroke during induction led to dose interruption. Relapse was observed at 18 months from induction with BCR::ABL1 at 0.04% IS in the blood and 9.5% IS in the marrow. She received four injections of vincristine/dexamethasone+ponatinib but acute pancreatitis onset led to a switch to dasatinib. Blasts and transcripts progressed particularly from the T315I clone (VAF: 100%) at latest follow-up. Patient 5 was refractory to induction and received hydroxyurea+6-mercaptopurine+ponatinib, resulting in a partial response, a sibling donor allo-SCT was performed after conditioning with clofarabine+cytarabine+cyclophosphamide+busulfan+ATG. The patient progressed after allo-SCT and received three DLI+dasatinib with no success. Haplo-SCT with his daughter was further proposed. Unfortunately, thrombotic micro-angiopathy and multiple infections resulted in death. At latest follow-up the three other patients are in sustained DMR >12 months after transplant (M37, M25, M49) (Figure 1A, C and F). After 1.5 months follow-up, patient 4 is currently in cytological remission and recovering from induction chemotherapy with dexamethasone+vincristine+ponatinib and has been switched to dasatinib due to liver toxicity.
Figure 1.
Kinetics of transcript aligned on international scale (BCR::ABL1/ABL1IS). (A) Patient 1, (B) patient 2, (C) patient 3, (D) patient 4 (E) patient 4 and (F) patient 6. Months (M) corresponding to the dates from which transcript was aligned on international scale, treatment discontinuation, blast crisis, and last molecular follow-up from chronic phase chronic myeloid leukemia (CP-CML) diagnosis are indicated on the x axis. IM: Imatinib; D: Dasatinib; N: Nilotinib; IFN: interferon; Ara-C: Cytarabine; Allo-SCT: allogeneic stem cell transplantation; auto-SCT: autologous stem cell transplantation; AP: accelerated phase; LBC: lymphoid blast crisis; MBC: myeloid blast crisis; MMR: major molecular response; DMR: deep molecular response (i.e., MR4 and MR4.5).
Table 2.
Patient characteristics at onset of blast crisis.
BC currently is an exceptional event during the course of CML with 0.7-4.5% of CP-CML patients on IM front-line treatment progressing to BC3 especially during early years.4 These progressions occur in patients with secondary resistance or suboptimal response or failure to TKI,1 and are myeloid in 75%, or lymphoid in 25% of the cases.11,13 The prognosis of BC remains poor4,5,13 despite intensification procedures. According to the large number of patients enrolled, France is currently a pioneer in TFR clinical studies (≥600 patients). Based on these studies we estimate the risk of BC in TFR as being very low, below 0.1%. Until now, only two cases have been reported.6,7 We hereby report six cases occurring after 41 (range, 6-124) months median sustained MR4.5 prior to cessation and for four of these six cases after a median of 40 months of cessation, while the two remaining patients went into BC following TKI resumption after 6 and 11 months of TFR. Interestingly, four of six BC were lymphoid which is not the current pattern of BC seen in TKI first-line treatment (75% myeloid8,13), and all of the cases with one exception (patient 5) occurred suddenly as seen in BC cases observed in patients in cytogenetic response on imatinib or IFN-α9,17 and in Al Fayez et al. in TFR.7 The same clone as identified by identical ASXL-1 mutation (patient 6) or a derived sub-clone identified by identical ASXL-2 and EP300 mutations, with clonal evolution (patient 5) were detected again at BC. This suggests that stem cells, had survived the various TKI challenges and although undetectable, had remained unstable and capable of promoting disease transformation. Clearly, the malignant cells or an aggressive subfraction of them had not been eradicated. In addition, these two patients harbored a myeloid phenotype. ABL-1 mutations may be observed in up to 80% of BC cases and while occurring in late-CP patients, are associated with a greater likelihood of progression. Other mutations or CNV are historically known to be associated with progression,10 particularly genes involved in myeloid (~25% TP53 mutated or deleted11) or lymphoid phenotypes (50% p16 deleted). Recently, other genes have been identified as being involved at CP diagnosis as well as at BC diagnosis, such as RUNX-1, IKZF1, ASXL1, DNMT3A, SETBP1, WT1, TET2, IDH1, NRAS, KRAS, CBL.12-14 In this article, mutation(s) and/or CNV were identified in all the BC patients, which is a common finding, however, in two of six patients some mutations/deletions in two genes, EP300 (patients 3 [LBC] and 5 [MBC]) and SETD2 genes (patients 1 and 4, [both LBC] were found to have recurred. These two genes are involved in epigenetic regulations and are rarely reported in de novo lymphoblastic B-ALL (~3.86-10% of cases15,16) or in CML-BC (<5%13). Whether or not these represent BC-TFR-related markers requires further investigation. Complex copy number alterations were found in two of six patients, comparable to that of Ochi et al.13 TKI probably exert sustained therapeutic pressure on residual leukemia stem cells and progeny, thus preventing overt genetic instability for being induced in BCR::ABL1+ stem cells.8
These six cases underline the necessity for sustained long-lasting molecular follow-up for patients in TFR.
Supplementary Material
Acknowledgments
The authors acknowledge Dr Emmanuelle Clappier hôpital Saint Louis, Dr Audrey Bidet Hôpital Haut Lévêque, Pessac, Dr Viviane Dubruille Hôtel Dieu, Nantes, and Dr Emmanuel Beillard, Centre Léon Bérard, Lyon, France for advice and help in data collection. We are grateful for the association Anim’ Montbernier (Ruy-Montceau, France) and its president Mr Armand Glasson for continuous support. We thank Mrs Barbara Meunier-White (Chasselay, France) for proofreading the English.
References
- 1.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rea D, Ame S, Berger M, et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer. 2018;124(14):2956-2963. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolini FE, Alcazer V, Cony-Makhoul P, et al. Long-term follow-up of de novo chronic phase chronic myelogenous leukemia patients on front-line imatinib. Exp Hematol. 2018;64:97-105. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424-430. [DOI] [PubMed] [Google Scholar]
- 7.Alfayez M, Richard-Carpentier G, Jabbour E, et al. Sudden blastic transformation in treatment-free remission chronic myeloid leukaemia. Br J Haematol. 2019;187(4):543-545. [DOI] [PubMed] [Google Scholar]
- 8.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian H, O’Brien S, Cortes J, et al. Sudden onset of the blastic phase of chronic myelogenous leukemia: patterns and implications. Cancer. 2003;98(1):81-85. [DOI] [PubMed] [Google Scholar]
- 10.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103(8):2794-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84(8):2391-2411. [PubMed] [Google Scholar]
- 12.Branford S, Kim DDH, Apperley JF, et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia. 2019;33(8):1835-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochi Y, Yoshida K, Huang Y-J, et al. Clonal evolution and clinical implications of genetic abnormalities in blastic transformation of chronic myeloid leukaemia. Nat Commun. 2021;12(1):2833-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossmann V, Kohlmann A, Zenger M, et al. A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia. 2011;25(3):557-560. [DOI] [PubMed] [Google Scholar]
- 15.Jing Y, Li Y-F, Wan H, Liu D-H. Detection of EP300-ZNF384 fusion in patients with acute lymphoblastic leukemia using RNA fusion gene panel sequencing. Ann Hematol. 2020;99(11):2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Duns G, Westers H, Sijmons R, van den Berg A, Kok K. SETD2: an epigenetic modifier with tumor suppressor functionality. Oncotarget. 2016;7(31):50719-50734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian H, O'Brien S, Cortes J, et al. Sudden onset of the blastic phase of chronic myelogenous leukemia. Patterns and implications. Cancer. 2003;98(1):81-85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.