Abstract
During the complex interaction between an infectious agent and a host organism, the pathogen can interfere with the host cell's programmed death to its own benefit. Induction or prevention of host cell apoptosis appears to be a critical step for determining the infection outcome. Members of the gram-negative bacterial genus Brucella are intracellular pathogens which preferentially invade monocytic cells and develop within these cells. We investigated the effect of Brucella suis infection on apoptosis of human monocytic phagocytes. The present study provides evidence that Brucella infection inhibited spontaneously occurring apoptosis in human monocytes. Prevention of monocyte apoptosis was not mediated by Brucella lipopolysaccharide and required bacterial survival within infected cells. Both invaded and noninvaded cells were protected, indicating that soluble mediators released during infection were involved in the phenomenon. Analysis of Brucella-infected monocytes revealed specific overexpression of the A1 gene, a member of the bcl-2 family implicated in the survival of hematopoietic cells. Brucella infection also rendered macrophage-like cells resistant to Fas ligand- or gamma interferon-induced apoptosis, suggesting that Brucella infection protected host cells from several cytotoxic processes occurring at different steps of the immune response. The present data clearly show that Brucella suis modulated the monocyte/macrophage's apoptotic response to the advantage of the pathogen, thus preventing host cell elimination. This might represent a strategy for Brucella development in infected hosts.
In recent years, it has become obvious that programmed death (or apoptosis) of cells of the monocytic lineage may be relevant for local immune responses against microorganisms (26, 31). Several bacterial organisms, such as Shigella flexneri (47), Legionella pneumophilia (35), Yersinia enterocolitica (41), Bordetella pertussis (20), Actinobacillus actinomycetemcomitans (18), Listeria monocytogenes (40), and Salmonella enterica serovar Typhimurium (27), promote the destruction of monocytic phagocytes by apoptosis, thus circumventing the first line of defense of the immune system. Surviving bacteria infect neighboring cells and disseminate to other tissues, often epithelial cells. Recently, it was reported that some intracellular bacteria that preferentially infect monocytic phagocytes have a totally opposite strategy and protect their host against apoptosis. Mycobacterium tuberculosis, which was reported to promote alveolar macrophage apoptosis (19, 22), and Mycobacterium bovis were shown to inhibit spontaneously occurring apoptosis in human monocytes (9, 24), possibly by inducing tumor necrosis factor alpha (TNF-α) production. Furthermore, HeLa cells infected with the obligate intracellular bacteria chlamydiae are resistant to apoptosis triggered by exogeneous stimuli (12), and Rickettsia rickettsii prevents the programmed cell death of endothelial cells (7). Molloy et al. showed that apoptosis of BCG-infected macrophages kills intracellular mycobacteria (30). It was thus postulated that inhibition of host cell apoptosis benefits the intracellular pathogen because it protects it against immune attacks from the outside. This could favor an optimal multiplication of intracellular bacteria (7, 9, 12, 24).
Brucellae are gram-negative, facultative, intracellular bacteria that induce chronic infections in a wide range of mammals, including humans and domestic ruminants. In humans, after invasion of the reticuloendothelial system, the bacteria develop within mononuclear phagocytes, and the infected monocytes (or macrophages) play an important role in dissemination of the bacteria in specific locations of the body (spleen, brain, heart, and bones) (44). To analyze the strategies adopted by Brucella to survive and multiply within mononuclear cells, we investigated whether in vitro Brucella infection of monocytic phagocytes affected (induced or prevented) the spontaneous or stimulus-triggered apoptosis in host cells. We report here that in vitro Brucella infection inhibits spontaneously occurring apoptosis in human monocytes by a TNF-α-independent mechanism which does not involve bacterial lipopolysaccharide (LPS) and requires Brucella survival within the host cells. Furthermore, Brucella infection also renders monocytic phagocytes resistant to gamma interferon (IFN-γ)- or Fas-mediated apoptosis, suggesting that several cytotoxic processes at different steps of the immune response are impaired in Brucella infection. Together, the data show that by preventing host cell elimination, Brucella suis modulates the monocyte/macrophage's apoptotic response to its advantage.
MATERIALS AND METHODS
Cells and reagents. (i) Human monocytes.
Human peripheral blood was obtained from healthy donors and processed by centrifugation with Ficoll-Hypaque (Sigma, Saint-Quentin, France). Monocytes were purified on the basis of their adherence properties (5, 6) and cultured at 37°C in 5% CO2 in complete medium, i.e., RPMI 1640 supplemented with 5 mM glutamine (Gibco BRL Life Technologies, Cergy, France) and 10% (vol/vol) heat-inactivated fetal calf serum (Sigma Chimie).
(ii) THP-1-derived monocytes.
THP-1 cells (ATCC TIB 202; American Type Culture Collection, Manassas, Va.) were treated with 10−7 M 1,25-dihydroxyvitamin D3 (VD3) (Hoffman-LaRoche, Basel, Switzerland) for 72 h and differentiated into monocyte-like cells (5). Adherent cells were harvested and cultured in complete medium.
(iii) Reagents.
Brucella abortus 99 LPS was obtained from Zygmunt and Dubray (48). B. suis LPS was prepared by the phenol-water method (25). Escherichia coli LPS (serotype 055:B5) was from Sigma. Human recombinant TNF-α and IFN-γ and neutralizing anti-TNF-α antibody (Ab) were from Genzyme (Cambridge, Mass.). Anti-Fas/CD95-PE (PN IM2446) was from Immunotech (Marseille, France). All reagents used in this study were endotoxin free (6).
Infection assay.
Human monocytes were infected as previously described (5, 6) with (i) B. suis 503 (B. suis) (6), (ii) GFP-B. suis, a B. suis mutant producing the green fluorescence protein (GFP) (37), or (iii) a nonvirulent dnaK null mutant of B. suis (dnaK-KO B. suis) (23). Infections were performed in six-well plates (4 × 106 cells/well), in 24-well plates (8 × 105 cells/well), or in 96-well plates (2 × 105 cells/well) (Falcon; Becton Dickinson, Meylan, France) or in eight-chamber culture slides (Lab-Tek; Nunc, Naperville, Ill.) (105 cells/well). Briefly, bacteria from stationary-phase cultures were centrifuged, washed, and suspended in RPMI 1640. Cells were incubated with 100 μl to 1 ml of bacterial suspension (usually with a multiplicity of infection [MOI] of 20 [5]) for 30 min at 37°C. Nonadherent bacteria were extensively washed. Infected cells were reincubated with 50 μg of gentamicin per ml to kill extracellular bacteria and cultured for different times.
In some experiments, bacteria were opsonized with heat-inactivated polyclonal human anti-Brucella Abs isolated from serum of an infected patient. The Ab dilution allowed staining of all bacteria in the presence of a secondary fluorescein isothiocyanate-labeled antibody without aggregate formation and enhanced bacterial phagocytosis by at least 10-fold (5, 6). Infection was assayed by CFU determination after cell lysis (5, 6). As for M. bovis infection (24), analysis of GFP-B. suis-infected cells by fluorescence microscopy or cytofluorimetry allowed rapid and easy determinations of infected-cell percentages. The values obtained by this method were not significantly different from those obtained by indirect labeling of intracellular bacteria with specific antibodies after permeabilization of fixed cells.
Analysis of cell viability.
Cells infected with B. suis (or not) in 96-well plates were cultured at 37°C for different periods of time. Cell death was then evaluated with the CellTiter AQ assay (Promega Corp., Madison, Wis.). In this assay, in the presence of phenazine methosulfate, living cells reduce MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] into soluble formazan, which displays absorbance at 490 nm. The percentage of nonviable monocytes in an infected culture (or control) at time t was calculated by the following formula (35): 100 × [1 − (optical density at 490 nm of the infected or control culture at time t/optical density at 490 nm of the infected or control culture 1 h after the onset of the experiment)]. A490 values of four similar wells were averaged to determine the percentage of dead cells.
Assessment of monocytic cell apoptosis.
Monocyte apoptosis was evaluated by DNA fragmentation analysis and fluorescence microscopy, two techniques recently used by our group (41), or by flow cytometry analysis of cell DNA.
(i) DNA fragmentation.
Cells (10 × 106) were lysed with lysis buffer (0.5% Triton X-100, 20 mM EDTA, 5 mM Tris, pH 7.4). After centrifugation, the supernatant containing cytoplasmic low-molecular-weight DNA (41) was treated with proteinase K (150 μg/ml) for 1 h at 60°C and overnight at 37°C. Lysates were extracted once with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol) and once with an equal volume of chloroform-isoamyl alcohol (24:1, vol/vol) to eliminate protein and high-molecular-weight DNA. The samples were left for more than 1 h with continuous shaking after phenol-chloroform-isoamyl alcohol addition. Low-molecular-weight DNA in the aqueous phase was precipitated with ethanol–0.3 M sodium acetate containing 40 μg of glycogen/ml (Boehringer, Mannheim, Germany), washed with 70% ethanol, and resuspended in 10 mM Tris (pH 8)–1 mM EDTA–2 μg of RNase/ml. Samples were incubated overnight at 37°C and subjected to electrophoresis on a 1.2% agarose gel. DNA fragments were visualized by ethidium bromide staining.
(ii) Fluorescence microscopy assessment of apoptosis.
Apoptotic cells were detected and quantified by an assay based on the detection of phosphatidylserine exposed on the outer leaflets of apoptotic cells. Adherent cells were stained with fluorescein-conjugated annexin V, which has high affinity to membrane-exposed phosphatidylserine, according to the manufacturer's instructions (Annexin-V-Fluos; Boehringer). Simultaneous application of a DNA stain, propidium iodide (PI) (Sigma), allowed discrimination of apoptotic cells from necrotic cells, since the nuclei of necrotic cells conserve their shapes but nuclei of apoptotic cells appear to be strongly condensed. Cells in earlier stages of apoptosis thus have an almost normal appearance and display green fluorescence, since they are annexin V positive. Apoptotic cells in later stages shrink substantially, lose membrane integrity, and show additional red fluorescence, as their condensed chromatin is strongly stained with PI. Percentages of apoptotic cells were determined by counting a minimum of 800 cells per sample with a fluorescence microscope (Leica DM IRB).
(iii) DNA analysis by flow cytometry.
Cell DNA content was also assessed by flow cytometry. Infected or noninfected adherent cells were treated with cell dissociation solution (Sigma) and harvested. They were fixed with 75% ethanol for 2 h, treated with 0.1 M citric acid, washed, and stained for 30 min at 37°C with 40 μg of PI per ml in the presence of 100 μg of RNase per ml. They were then analyzed on a FACScalibur (Becton Dickinson, Mountain View, Calif.). When necessary, experiments performed with GFP-B. suis allowed simultaneous determination of the percentages of apoptosis in infected and noninfected monocytes.
(iv) Induction of apoptosis in THP-1-derived monocytes.
Control or infected THP-1-derived monocytes were cultured for 48 h in (i) complete medium plus gentamicin supplemented with 10,000 U of IFN-γ (Genzyme, Cergy, France) per ml or (ii) complete medium plus gentamicin supplemented (after 24 h) with 5 μg of cycloheximide (Sigma) per ml and 250 ng of anti-CD95 monoclonal antibody (CH11 clone; Immunotech) per ml (11). (This agonistic Fas antibody mimics cross-linking of the Fas [CD95] molecule by cells expressing the Fas ligand.) Cells were then harvested, and the percentage of cells with hypodiploid DNA was analyzed by cytometry. To account for differences in apoptotic and/or infected cells depending on donors, percentages of cell death prevention, when so mentioned, were expressed as suggested by Estaquier and Ameisen (11): [(apoptosis in noninfected cells) − (apoptosis in infected cells)/(apoptosis in noninfected cells)] × 100.
Statistical analysis.
P was calculated by using the paired Student t test.
Analysis of bcl-2 and A1 mRNAs by reverse transcription-PCR.
Total RNA from either infected or noninfected cells (2.5 × 107 cells per sample) was extracted with Trizol (Gibco BRL Life Technologies) (5). Reverse transcription, cDNA quantification, and cDNA amplification were performed exactly as described previously (5). The specific primers used and amplicon lengths were as follows: for bcl-2, 5′ primer 5′-CATTATAAGCTGTCGCAGAGGGGCTACGAGT-3′ and 3′ primer 5′-CAAAGGCATCCCAGCCTCCGTTATCCTGGATCC-3′ (537 bp); for A1, 5′ primer 5′-TACAGGCTGGCTCAGGACTATC-3′ and 3′ primer 5′-GGTATCCACATCCGGGGCAAT-3′ (315 bp); and for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, 5′ primer 5′-TCGCCAGCCGAGCCACAT-3′ and 3′-primer 5′-GGAACATGTAAACCATGTAGTTG-3′ (171 bp). The number of cycles (90°C for 1 min, 60°C for 1 min, and 72°C for 1.5 min) was adjusted for each mRNA to be in a linear phase of amplification (15 to 35 cycles). Amplification of GAPDH was used as control. PCR products were run on 1.2% agarose gels supplemented with ethidium bromide, and their sizes were evaluated with a molecular size standard (200-bp ladder; Gibco).
RESULTS
B. suis infection prevents programmed cell death of resting human monocytes.
When freshly isolated human monocytes were cultured in complete medium, there was a progressive decrease in the absolute number of surviving cells, with 50% of the cells disappearing in 48 h (Fig. 1A). As stated by others (28), we observed that the addition of E. coli LPS (0.5 μg/ml) or TNF-α (100 U/ml) inhibited cell death. After 48 h in the presence of these activators, only 9 and 10% of cells died, respectively. Loss of cell viability occurs mainly through programmed cell death, with monocytes spontaneously entering apoptosis unless they have received signals to induce their differentiation into macrophages (28).
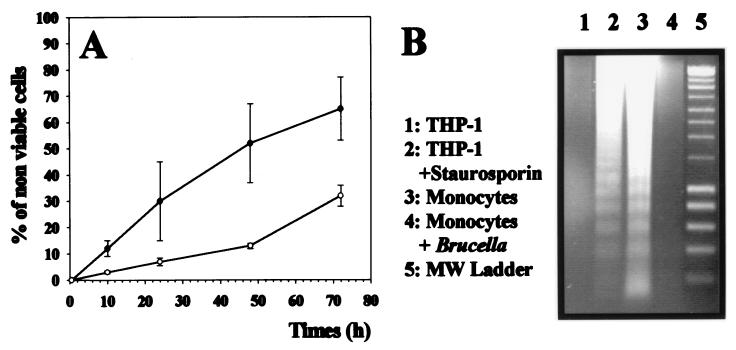
FIG. 1.
(A) B. suis infection prevents monocyte death. Human monocytes were infected with B. suis (○) or not (●) in 96-well plates or stimulated with E. coli LPS or 1,000 U of TNF-α per ml and cultured in complete medium. At different times postinfection, the percentages of nonviable cells in the different cultures were evaluated as described in Materials and Methods. (Values for LPS or TNF-α activation are indicated in the text.) Data are means and standard deviations from quadruplicate determinations. (B) DNA fragmentation assays on agarose gels. Cytosolic low-molecular-weight DNA was isolated from different monocytic phagocytes and electrophoresed on 1.2% agarose gels for 3 h at 100 V. Lanes 1 and 2 (control experiments), DNA isolated from THP-1 cells (lane 1) or from THP-1 cells treated with staurosporine for 7 h (lane 2). Lane 3, DNA isolated from human monocytes cultured for 48 h in complete medium. Lane 4, DNA from a 48-h culture of B. suis-infected monocytes. Lane 5, 200-bp DNA ladder molecular size (MW) markers (Smart ladder; Eurogentec, Seraing, Belgium).
When it is phagocytized by human monocytes, B. suis survives and develops within these cells (5, 6). Comparison of resting and B. suis-infected monocyte cultures showed that cell death was reduced in infected cultures (Fig. 1A). We therefore investigated DNA fragmentation of monocytes in infected or control cultures at 48 h after infection. Figure 1B shows that resting monocytes exhibited DNA degradation characterized by a typical ladder pattern of apoptotic cells (lane 3), whereas B. suis-infected cells showed much less nucleosomal fragmentation (lane 4). Staurosporine-induced fragmentation of THP-1 DNA, typical of apoptosis, was studied as a control (lanes 1 and 2).
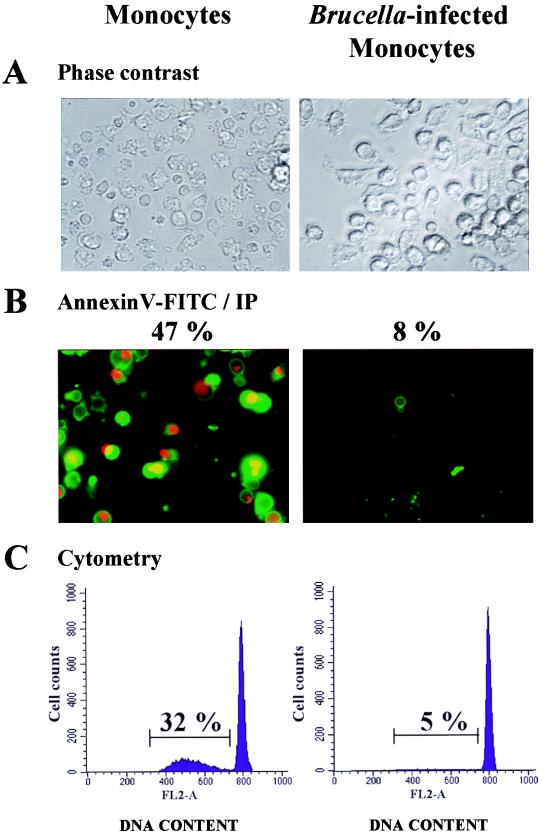
To determine whether Brucella infection interrupted monocyte apoptosis, control and B. suis-infected cells were colabeled with fluorescein-conjugated annexin V and PI, and the percentage of apoptotic cells was evaluated by microscopic analysis at different times postinfection. These observations revealed that 24% of resting monocytes were apoptotic at 24 h after onset of the experiment, whereas B. suis-infected cultures displayed only 5% of cells in apoptosis (not shown). These percentages increased up to 47 and 8%, respectively, at 48 h (Fig. 2A and B). The number of apoptotic cells with hypodiploid DNA was also quantified by flow cytometry after PI staining. At 48 h, the percentages of apoptotic monocytes determined by this method were 32 and 5% in control and B. suis-infected monocyte cultures, respectively (Fig. 2C). The difference between apoptotic cell percentages measured by microscopy or flow cytometry could be explained by the fact that annexin V labeled an early stage of apoptosis not detected in permeabilized-cell DNA analyzed by cytometry after PI staining.
FIG. 2.
B. suis infection prevents spontaneously occurring apoptosis in human monocytes. Monocytes infected (or not) with B. suis (MOI = 20) were cultured for 2 days in complete medium. Cells infected in eight-chamber culture slides were directly stained with PI (IP) and fluorescein (FITC)-conjugated annexin V. They were then concomitantly analyzed by phase-contrast microscopy (A) or fluorescence microscopy (B). Magnification, ×400. For each culture condition, the percentage of apoptotic cells was determined by counting at least 800 cells. Among them, those which displayed green fluorescence and those which simultaneously displayed red fluorescence and condensed chromatin were considered apoptotic cells. (C) In parallel experiments, cells from the same donors were infected in six-well plates, cultured for 2 days, harvested, permeabilized, and stained with PI. Single cells were then gated by classical procedures which exclude doublets and aggregates (FL2-W versus FL2-A) and analyzed with a flow cytometer. The percentages of resting or B. suis-infected monocytes with hypodiploid DNA were calculated and are indicated. Both types of experiment were repeated with at least 10 different donors.
These experiments were repeated with 10 different healthy donors. Microscopy analyses showed that percentages of apoptotic cells were 35% ± 14% and 7.6% ± 2.5% in 48-h cultures of resting monocytes and B. suis-infected monocytes, respectively. Statistical analysis of the data confirmed that B. suis infection inhibited monocyte spontaneous apoptosis (P < 0.003).
B. suis infection of monocyte cultures protects both invaded and noninvaded cells against apoptosis.
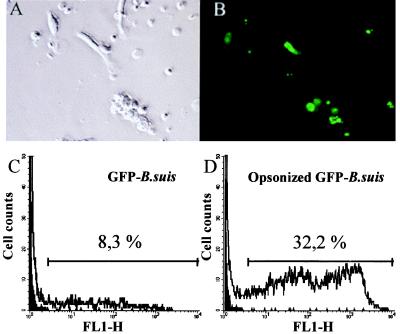
In infections performed with GFP-B. suis, the percentages of infected cells were easily determined by cytofluorimetry or fluorescence microscopy. Figure 3 shows a typical experiment with an MOI of 20 GFP-B. suis organisms/cell: 8% of total monocytes were infected, and this percentage rose to 32% in infection performed with opsonized bacteria.
FIG. 3.
Determination of percentage of B. suis-infected monocytes. Human monocytes were infected with GFP-B. suis or opsonized GFP-B. suis (MOI = 20). Twenty-four hours later, the percentage of infected cells was determined. (A and B) Determination by counting the numbers of total and fluorescent cells. A culture of monocytes infected with opsonized GFP-B. suis visualized by phase-contrast microscopy (A) and by fluorescence microscopy (B) is shown. For each determination, 800 cells were analyzed on four different slides. Magnification, ×400. (C and D) Determination by cytometry. Percentages of infected cells were determined by gating monocytes under forward- and side-scatter parameters and measuring the number of cells expressing green fluorescence (GFP protein); 10,000 cells were analyzed. Cytofluorographs of 24-h monocyte cultures infected with GFP-B. suis (C) or opsonized GFP-B. suis (D) are shown. The dark surface is resting monocytes.
Visualization of GFP-B. suis-infected cells and parallel determination of apoptotic cells with annexin V indicated (i) that the percentage of infected cells increased up from 0.5 to 15% when the MOI increased from 1 to 100 and then remained unchanged (even for an MOI of 200), (ii) that the cell protection was practically optimal for an MOI of 10 bacteria/cell, and (iii) that neither increasing the MOI from 10 to 100 (which enhanced the number of invaded cells) nor B. suis opsonization significantly modified the percentage of apoptotic cells (Table 1). These findings showed that the percentage of protected cells could be far higher than that of infected cells and that B. suis infection protected both invaded and noninvaded cells against apoptosis when a minimal number of cells were infected.
TABLE 1.
Percentages of infected and apoptotic cells in resting or B. suis-infected cultures of human monocytesa
| Monocytes | MOI | %b of:
|
|
|---|---|---|---|
| Infected cells | Apoptotic cells | ||
| Resting | NAc | 35 ± 4 | |
| B. suis infected | 10 | 4 ± 1 | 5.6 ± 2.5 |
| 20 | 7.9 ± 2.4 | 5.8 ± 1.7 | |
| 200 | 15.3 ± 3.3 | 5.1 ± 2.1 | |
| Opsonized B. suis infected | 20 | 42 ± 13 | 6.5 ± 2.2 |
Monocytes were infected (or not) with GFP-B. suis or opsonized GFP-B. suis in eight-chamber culture slides and cultured for 2 days before the percentage of infected cells was determined by microscopy, as described in Materials and Methods. Parallel experiments were performed to quantify the percentage of apoptotic cells by microscopy with fluorescein-labeled annexin V. Infections were performed with MOIs ranging from 1 to 200.
Data are means and standard deviations from three different experiments.
NA, not applicable.
B. suis-induced protection of monocytes against apoptosis does not involve bacterial LPS.
Since the protective effect induced by live Brucella was somewhat similar to the E. coli LPS effect, we examined whether the antiapoptotic properties of B. suis were due to its LPS. Compared to E. coli LPS, B. suis LPS (or B. abortus LPS [not shown]) was a weak protector of monocyte apoptosis (Table 2). Moreover, a neutralizing anti-TNF-α Ab, which inhibited the antiapoptotic activity of TNF-α, also suppressed E. coli LPS- and Brucella LPS-induced inhibition of apoptosis, demonstrating an involvement of TNF-α in LPS-mediated protective effects. In contrast, in line with the absence of TNF-α production in B. suis infection (5, 6), the anti-TNF-α Ab did not affect live Brucella-triggered monocyte protection. These data ruled out direct participation of bacterial LPS in B. suis-induced prevention of apoptosis during phagocytosis, as well as possible involvement of LPS released in culture during the experiment. We effectively could not exclude the death of some infected cells and the presence of killed bacteria in the gentamicin-supplemented medium.
TABLE 2.
Effect of a neutralizing anti-TNF-α antibody on the percentage of apoptotic cells in monocyte cultures infected with B. suis or activated with E. coli LPS, B. suis LPS, or TNF-αa
| Anti-TNF-α monoclonal Ab treatment | %b of apoptotic cells with:
|
||||
|---|---|---|---|---|---|
| Control | B. suis infection | E. coli LPS | B. suis LPS | TNF-α | |
| − | 35 ± 8 | 7 ± 3 | 9 ± 3 | 21 ± 7* | 9 ± 4 |
| + | 34 ± 9 | 6 ± 4 | 25 ± 7 | 33 ± 5* | 31 ± 8 |
Monocytes were cultured in complete medium in the presence (or not) of 0.5 μg of E. coli LPS per ml, 0.5 μg of B. suis LPS per ml, or 1,000 U of TNF-α per ml, or after B. suis infection, in RPMI 1640 as described in Materials and Methods. Neutralizing anti-TNF-α antibody (2.5 μg/ml) was added (or not) to the different cultures. Forty-eight hours later, percentages of apoptotic cells in the different cell cultures were measured by microscopic techniques with fluorescein-labeled annexin V.
Values are means and standard deviations from four different experiments. ∗, significantly different (P < 0.05) as shown by a paired t test.
Prevention of monocyte apoptosis by live Brucella requires intracellular bacteria survival.
We then examined whether phagocytosis of bacteria and/or their survival within the cells was essential for monocyte protection against apoptosis. Infections were performed with the nonvirulent dnaK-KO mutant of B. suis recently characterized in our laboratory (23). These bacteria, which survived in RPMI without multiplication at 37°C, were phagocytized similarly to wild-type B. suis, but they were rapidly killed once they had entered the cells (Table 3). In four independent experiments, these bacteria failed to affect spontaneous apoptosis of monocytes (Table 3), thus demonstrating that the antiapoptotic effect of Brucella required the intracellular survival and multiplication of the bacteria. Furthermore, the findings confirmed that Brucella phagocytosis was necessary but not sufficient and that bacterial adherence to the cell surface did not regulate apoptosis inhibition.
TABLE 3.
Effect of nonvirulent dnaK-KO B. suis on monocyte apoptosisa
| Monocytes | % of apoptotic cells | No. of viable bacteria/well at h of infection:
|
||
|---|---|---|---|---|
| 1.5 | 6 | 24 | ||
| Resting | 42 ± 9 | |||
| B. suis infected | 9 ± 3b | 85,000 ± 1,800 | 43,000 ± 1,200 | 625,000 ± 9,000 |
| dnaK-KO B. suis infected | 43 ± 12b | 48,000 ± 1,200 | 12,500 ± 100 | <5 |
Human monocytes were infected (or not) with B. suis or dnaK-KO B. suis (MOI = 20) in eight-chamber culture slides. Forty-eight hours later, the percentages of apoptotic cells in the different eight-chamber culture slides were measured by microscopy with fluorescein-labeled annexin V. Parallel experiments were performed to measure phagocytosis and development of B. suis or dnaK-KO B. suis within cells. Human monocytes (5 × 105/well) were infected with B. suis or with dnaK-KO B. suis (MOI = 20) in 24-well microplates. At different times postinfection (1.5, 6, and 24 h), infected cells were lysed after gentamicin had killed extracellular bacteria. The number of intracellular viable bacteria was then determined on agarose plates and expressed as CFU per well as previously described (2, 3). Values are means and standard deviations from four different experiments.
Significantly different (P < 0.02) as shown by a paired t test.
A1 but not bcl-2 is upregulated during B. suis infection of human monocytes.
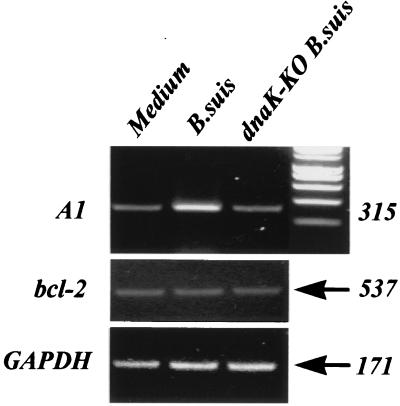
A1 is a hematopoiesis-specific early-inducible gene and a member of the bcl-2 gene family (10). As its product has been found to protect against apoptosis and provide prolonged survival in LPS- or BCG-activated monocytes (24), we investigated a possible change in A1 mRNA expression between resting and B. suis-infected monocytes. Figure 4 shows that B. suis-infected monocytes displayed an overexpressed level of A1 mRNA. In contrast, no A1 mRNA overexpression was seen in dnaK-KO B. suis-infected monocytes, suggesting that A1 induction necessitated bacterial survival within their host. Moreover, as in LPS- or BCG-activated monocytes (24), no change in bcl-2 mRNA expression was observed in B. suis (or dnaK-KO B. suis)-infected monocytes, with this transcript being poorly expressed under all conditions.
FIG. 4.
Reverse transcription-PCR detection of A1, bcl-2, and GAPDH mRNAs in B. suis- and dnaK-KO B. suis-infected monocytes. Monocytes were infected (or not) with B. suis or dnaK-KO B. suis (MOI = 20). Four hours later, total mRNAs were isolated, and after reverse transcription, PCR was performed with 2 ng of cDNA for each sample. PCR products obtained after 25 cycles (A1), 19 cycles (GAPDH), or 35 cycles (bcl-2) were then analyzed on 1.2% agarose gels supplemented with ethidium bromide. Lane 1, uninfected cells; lane 2, B. suis-infected cells; lane 3, dnaK-KO B. suis-infected monocytes. Numbers on the right indicate amplicon length (in base pairs).
B. suis infection renders macrophagic phagocytes resistant to the effect of immunological apoptotic factors.
VD3-differentiated THP-1 cells (VD3-THP-1 cells) display macrophagic cell properties and can be infected by Brucella (6). We used this model to examine whether B. suis-infected cells could become resistant to immunological apoptotic factors which induce macrophage suicide.
When cultured for 2 days in the presence of 250 ng of anti-Fas antibody per ml, VD3-THP-1 cells became apoptotic, with approximately 60% of the cells being labeled with annexin V. In four similar experiments involving cells infected with opsonized bacteria, the anti-Fas-triggered suicide of VD3-THP-1 cells was potently inhibited: in B. suis-infected cell cultures, prevention of anti-Fas-induced apoptosis was 43% ± 1% (P = 0.03). As recently reported for mycobacterium-infected THP-1 cells (3), we noticed that VD3-THP-1 cells did not alter their Fas antigen expression upon Brucella infection (data not shown), an observation which suggested that Brucella negatively modulated Fas-triggered signalling.
IFN-γ exerts a biphasic effect on macrophages: it either stimulates their microbicidal activity or triggers their apoptosis, depending on the concentration (11, 36). At concentrations ranging from 100 to 1,000 U/ml, IFN-γ induced major histocompatibility complex class II expression in VD3-THP-1 cells without promoting any apoptotic phenomenon (data not shown). By contrast, at higher concentrations (1,000 to 10,000 U/ml), IFN-γ induced cell apoptosis. A fluorescence microscopy study with fluorescein-labeled annexin V showed that 65% of VD3-THP-1 cells were apoptotic after 2 days of treatment with 10,000 U of IFN-γ per ml. In this experiment, B. suis infection rendered cells resistant to stimulus-induced apoptosis: in opsonized B. suis-infected cultures of VD3-THP-1 cells, the percentage of IFN-γ-induced apoptotic cells decreased to 25%. In four similar experiments, prevention of IFN-γ-mediated apoptosis was 60% ± 16% (P = 0.008).
Using GFP-B. suis and phycoerythrin-labeled annexin V, we simultaneously visualized infected cells (green fluorescence) and apoptotic cells (red fluorescence) (Fig. 5). We could thus determine the percentages of apoptotic cells, invaded cells, and invaded cells in apoptosis (yellow arrow). Apoptotic infected cells generally displayed weak green fluorescence, with most of intracellular brucellae being killed because of gentamicin which penetrated these cells. In contrast, viable infected cells showed intense diffuse green fluorescence due to the high proliferation of bacteria over the first 48 h. The results of seven similar experiments (Table 4) indicate (i) that the addition of IFN-γ (10,000 U/ml) after infection did not significantly affect the percentage of infected cells measured after 2 days of culture, (ii) that Brucella infection prevented IFN-γ-triggered apoptosis in VD3-THP-1 cell cultures (23% of cells in apoptosis for Brucella-infected cells compared to 66% for control cells), and (iii) that IFN-γ-triggered apoptosis occurred in both invaded (Fig. 5, yellow arrow) and noninvaded cells, with the proportion of apoptotic cells being similar or slightly higher for noninfected cells than for infected cells (25% compared to 15%). Finally, the data demonstrated the survival of B. suis-invaded cells despite their treatment with a high IFN-γ concentration. Comparison of bacterial development in untreated and IFN-γ-treated VD3-THP-1 cells confirmed that IFN-γ did not suppress bacterial development. The observed differences could be explained by infected VD3-THP-1 cells which died in cell cultures treated with 10,000 U of IFN-γ per ml (Fig. 6). Figure 6 also shows that between 100 and 1,000 U of IFN-γ per ml of this cytokine did not significantly affect Brucella development in infected VD3-THP-1 cells. A similar result was already obtained with B. suis-infected human monocytes (4a).
FIG. 5.
B. suis infection of VD3-THP-1 cell cultures renders both invaded and noninvaded cells resistant to IFN-γ-induced apoptosis. VD3-THP-1 cells were infected (or not) with opsonized GFP-B. suis in eight-chamber culture slides and after gentamicin addition were cultured with (or without) IFN-γ (10,000 U/ml). Two days later, cells were stained with phycoerythrin (PE)-labeled annexin V (red fluorescence). The red and green fluorescences of the cells visualized by phase-contrast microscopy were analyzed by fluorescence microscopy to determine the number of GFP-B. suis-infected cells (green fluorescence), the number of apoptotic cells (red fluorescence), and the number of infected cells in apoptosis (green plus red fluorescence; yellow arrow). This figure is representative of seven identical experiments reported in Table 4.
TABLE 4.
Effect of IFN-γ treatment on B. suis-infected VD3-THP-1 cellsa
| Cell treatment | %b of:
|
||
|---|---|---|---|
| Apoptotic VD3-THP-1 cells | Infected VD3-THP-1 cells | Apoptotic cells in the infected-cell population | |
| Medium | 4 ± 1 | ||
| IFN-γ | 66 ± 10 | ||
| GFP-B. suis infection | 3 ± 2 | 14 ± 2 | 1 ± 1 |
| GFP-B. suis infection + IFN-γ | 23 ± 5 | 16 ± 3 | 15 ± 4 |
VD3-THP-1 cells were infected (or not) with opsonized GFP-B. suis in eight-chamber culture slides (as described in Materials and Methods) and then cultured (or not) with 10,000 U of IFN-γ per ml after gentamicin had killed extracellular bacteria. Forty-eight hours later for each condition, the percentage of apoptotic cells (red fluorescence), infected cells (green fluorescence), or apoptotic cells in the infected-cell population (green plus red fluorescence) was determined by fluorescence microscopy with phycoerythrin-labeled annexin V as described in the legend to Fig. 5.
Percentages were determined by analyzing at least 800 cells; values are means and standard deviations from seven different experiments.
FIG. 6.
Intracellular behavior of B. suis within IFN-γ-treated VD3-THP-1 cells. VD3-THP-1 cells (106 cells/well) were infected with opsonized B. suis (MOI = 20) in 24-well plates, as described in Materials and Methods, in the presence of IFN-γ at the indicated concentrations or in RPMI 1640 alone (control). The cells were then cultured in the presence of corresponding concentrations of IFN-γ. At different time periods, they were lysed and the number of viable intracellular bacteria was determined and expressed in CFU per well as described previously (2, 3). All results are means and standard deviations from four separate experiments performed in duplicate.
DISCUSSION
To analyze the strategy adopted by Brucella to develop within mononuclear cells, we examined whether Brucella infection was able to positively or negatively modulate apoptosis in human monocytic phagocytes. The results of various analyses (DNA fragmentation, microscopic analysis, and flow cytometry analysis) showed that Brucella infection inhibited apoptosis which spontaneously occurs in human monocytes in the absence of an activation signal and rendered macrophagic cells resistant to apoptosis induced by immunological factors.
Although the Brucella-mediated effect on monocyte apoptosis was somewhat similar to the E. coli LPS effect (references 24 and 28 and our results), convergent data demonstrated that Brucella LPS cannot account for the antiapoptotic properties of the live bacteria. (i) Experiments performed in the presence of neutralizing anti-TNF-α Ab showed that TNF-α, which plays a central role in Brucella (or E. coli) LPS-induced protection of monocytes, did not participate in the antiapoptotic effect induced by viable B. suis. (ii) dnaK-KO B. suis, which is isogenic to B. suis with respect to LPS and is phagocytized in a manner similar to that of the wild-bacteria, did not protect monocytes against apoptosis. (iii) The antiapoptotic effect of live Brucella was observed in serum-free medium-infected monocyte cultures, i.e., in the absence of lipopolysaccharide-binding protein (LBP), which is necessary for LPS-induced monocyte activation during phagocytosis (43). In fact, Brucella LPS displayed moderate antiapoptotic properties. This could be explained by the fact that Brucella LPS lacks some of the most conspicuous biological properties of the classical LPS that causes phagocyte activation (13, 32–34) and is 103-fold less potent than E. coli LPS (15). In any case, live Brucella and isolated Brucella LPS (or E. coli LPS) inhibit monocyte apoptosis by different mechanisms.
Infection with dnaK-KO B. suis demonstrated that the intracellular survival and proliferation of Brucella, rather than its adherence to the cell surface or phagocytosis, were positively correlated with monocyte protection. Receptors regulating Brucella adherence and phagocytosis have not been clearly identified, even though molecules of the integrin family and mannose-binding receptors, both of which are signal-transducing molecules, could be involved (4). Compared to other intracellular gram-bacteria, only a few Brucella organisms bind to the phagocyte surface and are internalized (39, 42). It is likely that the nature and/or number of receptors committed in Brucella infection is not adequate to allow activation of pathways necessary to inhibit monocyte apoptosis. The antiapoptotic signalling mediated by live Brucella thus occurs downstream from phagocytosis and requires that brucellae counteract stress induced by their internalization within phagosomes, reorient maturation of their phagosomes towards autophagosome-like compartments (14, 39), and impose conditions which allow their survival. Bacterial survival is also an essential condition in Chlamydia-induced prevention of host apoptosis (12). In contrast, dead M. bovis, rendering monocytes resistant to apoptosis (24), bacterium-cell contact, and/or phagocytosis, was claimed to promote stimuli preventing apoptosis in live M. bovis-infected cells. These differences suggest that Brucella, Chlamydia, and M. bovis inhibit monocyte apoptosis in several ways. However, it remains possible that live and dead mycobacteria stimulate monocytes differently.
Both invaded and noninvaded monocytes were protected from apoptosis, which indicates that protection of a given monocyte does not necessitate its invasion by Brucella, which demonstrates participation of soluble mediators released during infection. These mediators could be produced by the bacteria themselves or by the invaded cells. The first possibility seems unlikely, as the bacterial LPS is not involved, and neither dnaK-KO bacteria nor B. suis supernatant (not shown) exerts an antiapoptotic effect on monocytes. By contrast, cytokines released from host cells during infection are putative candidates for the prevention of monocyte apoptosis (11, 29, 36). TNF-α, which is correlated with the protection against monocyte apoptosis in Mycobacterium infection (9, 24), is not the sole cytokine regulating the phenomenon (9). Furthermore, if M. bovis primes monocytes to secrete TNF-α, it does not induce TNF-α production under conditions that protect the cells from apoptosis (24). Other inflammatory cytokines (interleukin-1 [IL-1], granulocyte-macrophage colony-stimulating factor, colony-stimulating factor, and IFN-γ at low concentrations) and the Th1 cytokine IL-12 also prevent monocyte/macrophage suicide (11, 24, 29). Although they do not secrete TNF-α (6), B. suis-infected monocytic cells produce IL-1 and IL-6 (5), IL-12 (45), and probably many other cytokines. One or several of these cytokines might be involved in monocyte activation and play a crucial role in Brucella-mediated monocyte protection.
Brucella-mediated protection did not involve all the cells. Once monocytes are differentiated into macrophages, the nature of factors required for further suppression or induction of death by apoptosis changes in a developmentally regulated fashion (36). The ability of monocytes to evade apoptosis in response to B. suis infection might thus be linked to the cell activation and/or differentiation stage at the onset of infection.
The family of Bcl-2-related proteins includes members that function as either positive or negative regulator of apoptosis. The ratio of death antagonists (Bcl-2, Bcl-xl, Mcl-1, and A1) to agonists (Bax, Bak, and Bik) regulates different steps of the molecular cascade of apoptosis, including cytochrome c release (1, 21), and in turn leads to cell survival. The Epstein-Barr virus thus suppresses apoptosis by inducing expression of the host bcl-2 gene (17). In B. suis-infected monocytes, we did not observe any bcl-2 mRNA overexpression, but we did observe an induction of A1 mRNA. Although the possible participation of other cellular genes cannot be excluded, as already noted for M. bovis infection (24), upregulation of the antiapoptotic gene A1 could play a role in the protection of B. suis-infected monocytes, whereas bcl-2 is not involved. This hypothesis agrees with the absence of A1 mRNA induction in dnaK-KO B. suis-infected monocytes which were not protected. It provides a potential mechanistic basis for Brucella-induced resistance against apoptosis.
The findings with VD3-THP-1 cells highlighted that Brucella-infected human monocytic phagocytes became more resistant than control cells to apoptotic death stimuli triggered by molecules of immunological origin. This shows that B. suis infection protects host cells from several cytotoxic processes induced at different steps of the immune response. Infected monocytic phagocytes might therefore evade apoptosis promoted by activated T cells which express Fas ligand and delete infected macrophages through a Fas-mediated effect (2).
Sensitivity to stimulus-triggered apoptosis is upregulated in IFN-γ-exposed macrophages, and at high concentrations IFN-γ induces monocyte apoptosis (11, 36). To explain these effects, it is speculated (i) that IFN-γ-mediated apoptosis regulates the destructive potential of IFN-γ-activated macrophages and (ii) that IFN-γ-stimulated macrophages which do not succeed in destroying intracellular pathogens use this suicide mechanism to keep microorganisms from developing within their intended sanctuary site (8, 36). Although IFN-γ activates anti-Brucella functions in murine macrophages (16, 46), this cytokine does not affect (or only moderately affects) infection in human monocytic phagocytes, as Brucella evades IFN-γ-induced microbicidal activity. We showed that Brucella also impairs cell apoptosis induced by elevated IFN-γ concentrations. The persistent intramacrophagic multiplication of bacteria observed in these experiments indicates that the Brucella antiapoptotic strategy is crucial for bacterial development. The bacterium triggers a cell signalling which interrupts the IFN-γ apoptotic pathway. This signal might block a central step of apoptosis in invaded cells, as recently observed in Chlamydia infection, where the bacterium suppresses the mitochondrial cytochrome c release necessary for caspase activation in cell cytoplasm and thus inhibits several apoptotic pathways affecting host cells (12, 38). Such a general mechanism would explain the fact that Brucella infection impairs macrophage apoptosis induced by IFN-γ, Fas ligand, or possibly other proapoptotic immunological factors like Th2 cytokines (11). It would also account for the protection against apoptosis in resting monocyte infection. Besides their own antiapoptotic effects, Brucella-induced signals must also encode a factor(s), probably cytokines (as suggested above), which protects neighboring noninvaded cells and thus prepares future habitats for bacteria which have multiplied in infected cells. Moreover, these factors could also upregulate protection in invaded cells in an autocrine manner.
Finally, inhibition of monocyte/macrophage apoptosis at two levels, both in primary infection and after induction of proapoptotic immunological processes of acquired immunity, might set the conditions for persistent Brucella infection. Furthermore, the present study shows that B. suis belongs to a family of intracellular bacteria which protect monocytic cells from apoptosis. Previous studies and our results suggest that intracellular bacteria could be divided into two main groups on the basis of their effects on monocyte apoptosis. The first group consists of intracellular bacteria like Mycobacterium and Brucella, whose preferred hosts are monocytes and which protect the properly differentiated cells from apoptosis. The bacteria which survive the phagocytosis step can thus replicate inside their favored localization site, with the host cell remaining a site of infection. The second group consists of intracellular bacteria like Listeria, Salmonella, Shigella, Legionella, Yersinia, and A. actinomycetemcomitans, for which monocytic cells simply represent a transient passage and which induce phagocyte apoptosis. These bacteria could thus evade the microbicidal activity of monocytes, invade the surrounding cells, and disseminate in the organism until they reach their preferred localization site (often in epithelial cells). In fact, bacterium-induced prevention of apoptosis of the favored host seems to be a more general feature. Chlamydia and R. rickettsii prevent apoptosis of epithelial and endothelial cells, respectively, i.e., their definitive habitats.
ACKNOWLEDGMENTS
This work was supported by grants from INSERM, IREB, and the Human Capital and Mobility program of the European Union. A.G. was supported by a fellowship from ARC.
REFERENCES
- 1.Adachi S, Cross A R, Babior B M, Gottlieb R A. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. doi: 10.1074/jbc.272.35.21878. [DOI] [PubMed] [Google Scholar]
- 2.Ashany D, Song X, Lacy E, Nikolic-Zugic J, Friedman S, Elkon K. Th1 CD4+ lymphocytes delete activated macrophages through Fas/Apo-1 antigen pathway. Proc Natl Acad Sci USA. 1995;92:11255–11261. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr-Perst S I, Munk M E, Schaberg T, Ulrichs T, Schulz R J, Kaufmann S H E. Phenotypically activated γδ T lymphocytes in the peripheral blood of patients with tuberculosis. J Infect Dis. 1999;180:141–149. doi: 10.1086/314844. [DOI] [PubMed] [Google Scholar]
- 4.Campbell G A, Adams L G, Sowa B A. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet Immunol Immunopathol. 1994;41:295–306. doi: 10.1016/0165-2427(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 4a.Caron E. Thesis. Montpellier, France: Université de Montpellier II; 1994. [Google Scholar]
- 5.Caron E, Gross A, Liautard J-P, Dornand J. Brucella species release a specific, protease-sensitive inhibitor of TNF-α expression active on human macrophage-like cells. J Immunol. 1996;257:2885–2893. [PubMed] [Google Scholar]
- 6.Caron E, Peyrard T, Köhler S, Cabane S, Liautard J-P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellacasagrande J, Capo C, Raoult D, Mege J L. IFNγ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol. 1999;162:2259–2265. [PubMed] [Google Scholar]
- 9.Dürrbaum-Landmann I, Gercken J, Flad H D, Ernst M. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect Immun. 1996;64:5384–5389. doi: 10.1128/iai.64.12.5384-5389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elaine Y L, Orlofsky A, Wang H-G, Redd J C, Prytowsky M B. A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood. 1996;87:983–992. [PubMed] [Google Scholar]
- 11.Estaquier J, Ameisen J C. A role for T-helper Type-1 and Type-2 cytokines in the regulation of human monocytes apoptosis. Blood. 1997;90:1618–1625. [PubMed] [Google Scholar]
- 12.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochrondial cytochrome c release and caspace activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freer E, Moreno E, Moriyon I, Pizarro-Cerdà J, Weintraub A, Gorvel J P. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterpart. J Bacteriol. 1996;178:5867–5876. doi: 10.1128/jb.178.20.5867-5876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenchick P J, Markam R J F, Cochrane A H. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am J Vet Res. 1985;46:332–335. [PubMed] [Google Scholar]
- 15.Goldstein J, Hoffman T, Frasch C, Lizzio E F, Beining P R, Hochstein D, Lee Y L, Angus R D, Golding B. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting a possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect Immun. 1992;60:1385–1389. doi: 10.1128/iai.60.4.1385-1389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross A, Spiesser S, Terraza A, Rouot B, Caron E, Dornand J. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect Immun. 1998;66:1309–1316. doi: 10.1128/iai.66.4.1309-1316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Keiff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 18.Kato S, Muro M, Akifusa S, Hanada N, Semba I, Fujii T, Kowashi Y, Nishihara T. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infect Immun. 1995;63:3914–3919. doi: 10.1128/iai.63.10.3914-3919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keane J, Balcewicz-Sablinska M K, Remold H G, Chupp G L, Meek B B, Fenton M J, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C N, Wang X, Huang Y, Ibrado A M, Liu L, Fang G, Bhalla K. Overexpression of Bcl-X(L) inhibits Ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 1997;57:3115–3120. [PubMed] [Google Scholar]
- 22.Klingler K, Tchou-Wong K M, Brändli O, Aston C, Kim R, Chi C, Rom W N. Effects of mycobacteria on the regulation of apoptosis in mononuclear phagocytes. Infect Immun. 1997;65:5272–5278. doi: 10.1128/iai.65.12.5272-5278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler S, Teyssier J, Cloeckaert A, Rouot B, Liautard J P. Participation of the molecular chaperone DNAK in intracellular growth of Brucella suis within U937-derived phagocytes. Mol Microbiol. 1996;16:701–712. doi: 10.1111/j.1365-2958.1996.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 24.Kremer L, Estaquier J, Brandt E, Ameisen J C, Locht C. Mycobacterium bovis Bacillus Calmette Guerin infection prevents apoptosis of resting human monocytes. Eur J Immunol. 1997;27:2450–2456. doi: 10.1002/eji.1830270945. [DOI] [PubMed] [Google Scholar]
- 25.Leong D, Diaz R, Milner K, Rudbach J, Wilson J B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970;1:174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liles W C. Apoptosis-role in infection and inflammation. Curr Opin Infect Dis. 1997;10:165–170. [Google Scholar]
- 27.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangan D F, Welch G R, Whal S M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 29.Mangan D F, Whal S M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and proinflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 30.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore K J, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage infection. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 32.Moreno E. Evolution of Brucella. In: Plommet M, editor. Prevention of brucellosis in mediterranean countries. Wageningen, The Netherlands: Pudoc; 1992. pp. 198–218. [Google Scholar]
- 33.Moreno E, Berman D T, Boettcher L A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981;31:362–369. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno E, Stackebrandt E, Dorsh M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophilia infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn D H, Beall A C, Song D, Wrenn R W, Throckmorton D C. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon-γ. J Exp Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouahrani-Bettache S, Porte F, Teyssier J, Liautard J P, Köhler S. PBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. BioTechniques. 1999;26:620–622. doi: 10.2144/99264bm05. [DOI] [PubMed] [Google Scholar]
- 38.Perera L P, Waldmann T A. Activation of human monocytes induces differential resistance to apoptosis with rapid down regulation of capase-8/FLICE. Proc Natl Acad Sci USA. 1998;95:14308–14313. doi: 10.1073/pnas.95.24.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizarro-Cerdà J, Moreno E, Sanguedolce V, Mège J L, Gorvel J P. Virulent Brucella abortus avoids lysosome fusion and replicates within autophagosome-like compartments. Infect Immun. 1998;66:2687–2692. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 41.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sola-Landa A, Pizarro-Cerdà J, Grillo M, Moreno E, Moriyon I, Blasco J M, Gorvel J P, Lopez-Goñi I. A two component regulatory system conserved in animal pathogenic Brucella and plant pathogenic Agrobacterium is required for host cell invasion and virulence. Mol Microbiol. 1998;29:125–128. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 43.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 44.Young E J. Human brucellosis. Rev Infect Dis. 1983;5:821–842. doi: 10.1093/clinids/5.5.821. [DOI] [PubMed] [Google Scholar]
- 45.Zaitseva M, Golding H, Manischewitz J, Webb D, Golding B. Brucella abortus as a potential vaccine candidate: induction of interleukin-12 secretion and enhanced B7.1 and B7.2 and intercellular adhesion molecule 1 surface expression in elutriated human monocytes stimulated by heat-inactivated B. abortus. Infect Immun. 1996;64:3109–3117. doi: 10.1128/iai.64.8.3109-3117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan Y, Cheers C. Control of IL-12 and IFN-gamma production in response to live or dead bacteria by TNF and other factors. J Immunol. 1998;161:1447–1453. [PubMed] [Google Scholar]
- 47.Zychlinsky A, Prévost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 48.Zygmunt M S, Dubray G. Preparation by ultrafiltration and control by high-performance liquid chromatography of the native hapten of Brucella abortus for use in immunodiffusion diagnostic test. J Clin Biol. 1987;25:1860–1863. doi: 10.1128/jcm.25.10.1860-1863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]