Abstract
Background
Mental health is a critical and neglected public health problem for adolescents in sub-Saharan Africa. In this paper we aim to determine the prevalence of depressive symptoms and the association with HIV risk behaviours in adolescents aged 15–19 years in Zambia and SA.
Methods
We conducted a cross-sectional survey from August-November 2017 in seven control communities of HPTN 071 (PopART) trial (a community-randomised trial of universal HIV testing and treatment), enrolling approximately 1400 eligible adolescents. HIV-status was self-reported. Depressive symptoms were measured with the Short Mood and Feelings Questionnaire (SMFQ), with a positive screen if adolescents scored ≥12. We fitted a logistic regression model to identify correlates of depressive symptoms with subgroup analyses among those who self-reported ever having had sex, by gender and country.
Results
Out of 6997 households approached, 6057 (86.6%) were enumerated. 2546 adolescents were enumerated of whom 2120 (83.3%) consented to participate and were administered the SMFQ. The prevalence of depressive symptoms was 584/2120 (27.6%) [95%CI: 25.7%-29.5%]. Adolescents in SA were less likely to experience depressive symptoms (Adjusted Odds Ratio [AOR] = 0.63 (95% CI: 0.50, 0.79), p-value<0.0001).
Female adolescents (AOR = 1.46 (95% CI: 1.19, 1.81), p-value<0.0001); those who reported ever having sex and being forced into sex (AOR = 1.80 (95% CI: 1.45, 2.23), p-value<0.001) and AOR = 1.67 (95% CI: 0.99, 2.84); p-value = 0.057 respectively) were more likely to experience depressive symptoms. Among 850 (40.1%) adolescents who self-reported to ever having had sex; those who used alcohol/drugs during their last sexual encounter were more likely to experience depressive symptoms (AOR = 2.18 (95% CI: 1.37, 3.47); p-value = 0.001), whereas those who reported using a condom were less likely to experience depressive symptoms (AOR = 0.74 (95% CI: 0.55, 1.00); p-value = 0.053).
Conclusion
The prevalence of depressive symptoms among adolescents ranged from 25–30% and was associated with increased HIV-risk behaviour.
Introduction
Mental health disorders (MHDs) are a critical and neglected public health problem for adolescents aged 10–19 years in sub-Saharan Africa (SSA) [1]. MHDs account for 16% of the global burden of disease and injury in adolescents [2], with half of all MHDs starting by 14 years of age and most being undetected and untreated [3]. These conditions often persist for a long time, severely disrupting adolescents’ access to livelihoods, health care, and education, and exposing them to stigma, isolation, suicidal behaviour, discrimination, and sexual abuse [4–6]. Adolescents’s MHDs also extend to adulthood, limiting opportunities to lead fulfilling lives as adults.
Depression is one of the most common MHDs among adolescents globally [6–8]. A systematic review covering general population studies encompassing 14 409 adolescents from 16 different sub-Sahara Africa (SSA) countries found a prevalence of depression of 26.9% (IQR 20.1–31.1) [9]. Another review on the prevalence of MHDs in SSA adolescents found that one in seven children and adolescents (14.3%) experience significant psychological challenges, and one in ten (9.5%) qualifies for a psychiatric diagnosis [7]. Depression can be attributed to physical, biological, emotional and social changes that form part of adolescent formative period of transition [1, 10]. A systematic review of global MHDs among adolescents showed that risk factors can be categorized into life-long risk factors, such as genetic background, and age-specific risk factors such as substance use, developmental-behavioural disorders such as stress of puberty, and cognitive changes [11].
Research linking HIV-risk behaviour and specific MHDs problems has yielded mixed results. Some studies found no associations between specific diagnoses and HIV-risk behaviours, but other findings support such links. Internalizing problems (low self-esteem, depression, anxiety) are associated with low perceived self-efficacy, decreased assertiveness, and minimal ability to negotiate safe sex with a partner [12, 13]. Depression is also linked to illicit drug use, sexually permissive attitudes, having sexually active friends, sexual behaviour, low contraception use and high risk of pregnancy [14, 15]. Moreover, hopelessness and helplessness may reduce adolescents’ motivation to make health-promoting choices [15].
In other recent studies, it has been widely acknowledged that depression is a marker of increased HIV risk in both adults and adolescents [16]. Research has found depressive symptomatology among youth to be associated with earlier sexual debut, higher numbers of lifetime sexual partners, multiple and casual sexual partnerships, substance abuse, pregnancy, perceived barriers to condom use and having more risky partners [17–21]. However, most of this evidence comes from high-income countries, and these associations have not been well established in SSA where the HIV epidermic is generalized.
In SSA, the link between the HIV risk sexual behaviours and depressive symptomatology in adolescent population remains largely unexplored. A prospective cohort of young people (YP) in Eastern Cape, South Africa (SA) set out to investigate whether depressive symptomatology was associated with risky sexual behaviour [21]. Individuals with depressed symptoms were more likely to report lifetime intimate partner violence. In women, depressive symptomatology was associated with transactional sex and having dated an older partner. However, men with depressive symptoms were more likely to report ever having had transactional sex and perpetration of rape. Men were also less likely to report correct condom use at last sex.
HIV infection among adolescents with MHDs remains an important public health problem, but existing research is very scanty. In SSA, depression is believed to be higher among adolescents living with HIV (ALHIV) compared with those in the general population, with an estimated prevalence of 17–25 percent [22, 23]. Social, physical and psychological stressors associated with living with HIV are key risk factors for depression [10, 24]. For ALHIV, depression is often associated with faster disease progression, poor treatment adherence and earlier death.
In this paper we aim to determine the prevalence of depressive symptoms and the association with HIV risk behaviours in adolescents aged 15–19 years in Zambia and SA. Evaluating potential intersections between depression and HIV risk behaviours among adolescents could inform strategies that concurrently address mental wellbeing and HIV prevention within this group.
Methods
Study design and setting
The HPTN 071 (PopART) trial, was a three-arm community randomized trial in 12 communities in Zambia and 9 in South Africa (SA) evaluating the impact of a combination HIV prevention package, including universal HIV testing and treatment (UTT), on community-level HIV incidence [25, 26]. The PopART trial was conducted between 2013–2018 in 21 urban/peri-urban communities in Zambia and Western Cape Province, SA [25]. The 21 communities were divided into four triplets in Zambia and three in SA; communities in each triplet were randomly assigned to the three study arms: Arm A (PopART intervention with universal ART), Arm B (PopART intervention, ART per local guidelines), and Arm C (standard-of-care). Details of the PopART trial are described elsewhere [25, 27].
Nested within the PopART trial was a sub-study called PopART for Youth (P-ART-Y) whose primary outcome was knowledge of HIV status among 15–19 year-old adolescents [28]. The P-ART-Y study also presented an opportunity to explore other areas of adolescent HIV-related health which had limited data such as mental health, stigma, sex education, HIV risk behaviour and substance abuse. In order to meet these secondary objectives we conducted a cross-sectional survey. The P-ART-Y cross-sectional survey was conducted from August to November 2017 primarily to collect comparative data from the control communities on knowledge of HIV status among adolescents aged 15–19 years, for comparison with the intervention communities in which such data were collected during the third round (R3) of the PopART intervention (August 2016-December 2017) [29]. The cross-sectional survey collected additional data on mental health and formed the basis for the analysis of depression presented in this paper.
Sampling, eligibility criteria and recruitment
The sampling frame for the survey was provided by a census of all households in the clinic catchment area of the study communities in 2013 prior to the beginning of the PopART trial. In the 7 Arm C communities, communities were subdivided into blocks, each block consisted on average of 50 (~ 40–60) households in Zambia and about 80 (~ 70–90) households in SA. Blocks were randomly selected to be part of the study. All households within a sampling block and eligible adolescents residing in these households were invited to participate in the study. To be eligible for the study, participants had to between 15–19 years old, living in a block of houses randomly selected for recruitment and able and willing to provide informed consent. We anticipated to enrol on average ~17 adolescents in each block, from ~12 blocks in each community. We aimed to enroll 200 adolescents aged 15–19 years per community, for a total of 1,400 participants.
The study population was a community sample of adolescents sampled from within the HPTN 071 (Pop ART) trial i.e ALHIV and those HIV-negative.
The Research Assistants (RAs) interviewed all adolescents aged 15–19 years at the time of enrollment and were living in a block of houses randomly selected for recruitment, who gave either a written informed consent (for adolescents 18 years or older) or an informed assent given by a responsible adult/parent (for adolescents less than 18 years old).
Procedures and activities
Community sensitization, using a door to door approach was done to inform residents of the community about the survey prior to enrolment. At each house, RAs asked the responsible adult/parent for permission to enter the home and invite 15-19-year-old household members to participate in the survey. The study was explained to the guardian present and eligible adolescents. For those absent during this household visit, RAs made plans to return to the house at a time when they were expected to be at home. HIV status was self-reported.
All ALHIV who had not enrolled in care were referred to the clinic. Adolescents were also screened for TB using a TB symptoms screen (cough ≥2 weeks, drenching night sweats, unintentional weight loss). Adolescents with symptoms suggestive of TB were referred to the clinic for further management and care.
Primary outcome measure
The primary outcome of the study was prevalence of depressive symptoms. Depressive symptoms were measured using the self-administered 13-item Short Moods and Feelings questionnaire (SMFQ) captured on a tablet (S1 Appendix), The SMFQ is designed for examining the presence of depressive symptoms in epidemiological studies; has been shown to be a strong predictor of depression; is validated in clinical and non-clinical settings and is recommended as a screening tool [30–32]. The SMFQ summaries 13 items to give a score ranging between 0–26, where greater scores represent higher depression. Many studies have used the SMFQ for exploring the nature of depression during adolescence [30, 31].
A study in New Zealand sought to validate the SMFQ among adolescents and used an optimal cut-off of ≥12 [33]. Due to lack of SMFQ validated data in SSA region, including Zambia, we adopted the same cut-off as the New Zealand study for our analysis. We therefore defined the presence of depressive symptoms if an individual’s SMFQ score was ≥12 [33]. We further grouped the scale response (0–26) into 3-equal width categories (i.e. low = 0–8, medium = 9–17 and high = 18–26) and defined the high-score category as the presence of underlying depression tendencies and the low/medium, otherwise. The second definition was then used for sensitivity analysis.
Other measures
Stigma measurement
The survey presented adolescents with five statements about judgments towards PLHIV (stigmatizing attitudes towards people living with HIV (PLHIV)). We used standardized statements that had previously been used in different populations of the HPTN071 (PopART) trial [34, 35]. The statements related solely to judgmental attitudes that were held by participants: “I would be ashamed if someone in my family was living with HIV; I would not like to sit close to someone living with HIV; young people (YP) living with HIV should not share cups; YP living with HIV should not have sex; YP living with HIV should not get pregnant/have children”. (S2 Appendix) The stigma tool was captured electronical and offered to everybody, including ALHIV.
Adolescents were asked to respond to the statements using a 4-point Likert scale (strongly agree, agree, disagree, strongly disagree) [Cronbach’s alpha: 0.65(overall), 0.61(Zambia) and 0.71(SA)]. Strongly agree and agree were collapsed into one group (scored as 1) and strongly disagree + disagree into another (scored as 0). Those who were scored as 1 were viewed as exhibiting stigmatizing attitudes towards PLHIV.
Alcohol and substance use measurement
Questions on drug and alcohol use were extracted from standard questions used in other studies within the HPTN071 (PopART) study.
Ethical considerations
In Zambia, we obtained written informed consent from adolescents aged 18–19 years and written assent for adolescents aged 15–17 years [36]. A waiver of parental consent was obtained for those aged under 18 years as the survey was considered to be low risk, only involving completion of a questionnaire. In SA, all participants (regardless of age) signed informed consent, with only verbal parental permission to enter the home required. Ethical approval was obtained from the ethics committees of the Universities of Zambia, Stellenbosch and London School of Hygiene and Tropical Medicine. Permission to conduct the study was obtained from Ministries of Health.
Data collection
An electronic device was used to collect data. The questionnaire was administered by the RAs and self-administered for sensitive sections such as mental health, sex education/ HIV risk behavior, stigmatizing attitudes towards PLHIV, drug and alcohol use. RAs were available to guide adolescents who chose to self-administer the questionnaire.
Data analysis
The main analyses combined Zambia and SA data. Age was the only continuous variable which was categorized into 2 categories (i.e. 15-17-year-olds and 18-19-year-olds). Frequencies (n) and percentages (%) were used to summarize categorical data and a two-sample proportion test was used for comparisons, with 95% confidence limits based on the binomial distribution. Missing data for risk factors collected across both countries was below 5% and thus a complete case analysis approach was used.
A logistic regression model was used to investigate the association between experiencing depressive symptoms and a set of potential risk factors collected during the survey. A likelihood ratio test comparing a model with clustering by block and a logistic model, showed no evidence of clustering by block, inference made by a mixture of chi-square tests, (p-value = 0.21), and therefore a logistic model was fitted.
Age, sex, country and community were selected as priori confounders and fitted as the baseline model. In a forward stepwise manner, potential risk factors were added to the baseline model and a p-value of ≤0.1 was used to identify which risk factors were associated with depressive symptoms. Variables that were conceptually on a causal pathway were not adjusted for in the analysis and variables with a p-value > 0.1 were dropped in the forward stepwise model fitting procedure. The final model in the main analysis was used as the baseline model in the consequent subgroup analysis. Subgroup analyses was carried out among adolescents who self-reported ever having had sex, separately by sex and country.
A sensitivity analysis to investigate the change in risk factors associated with depressive symptoms once a different cut-off of the scale response was used, was also carried out. Data were analysed using Stata version 15.1.
Results
Descriptive analysis
Participation
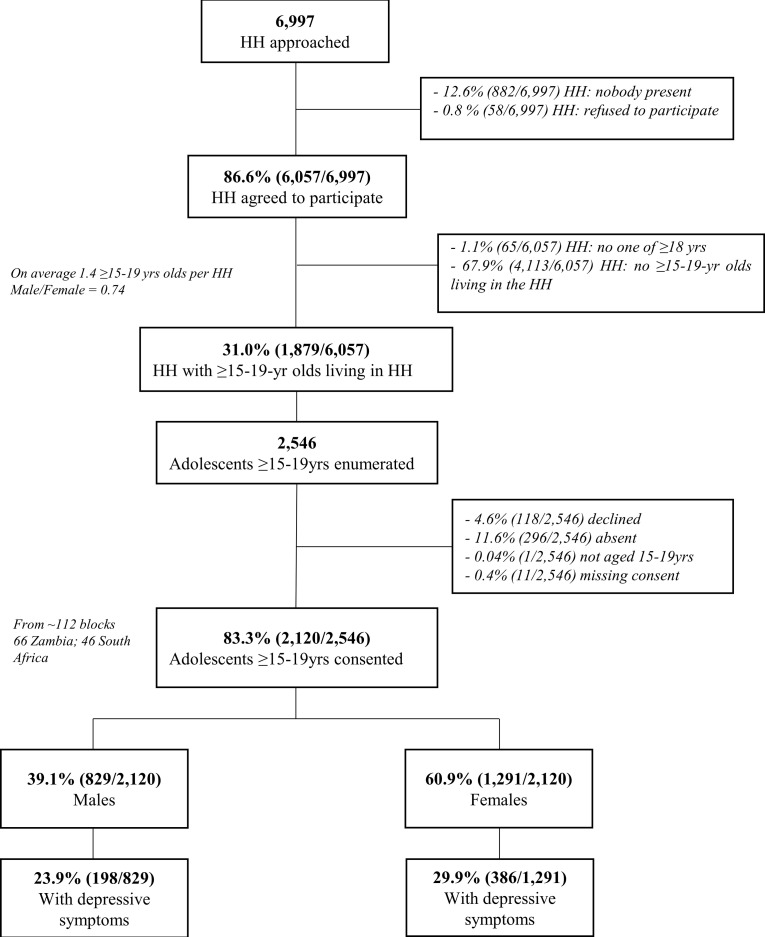
Across Zambia and SA, a total of 6997 households were approached and 6057(86.6%) consented to enumeration. Only 1879 (31%) of these households had a 15–19 year old living there at the time of the household visit. A total of 2546 adolescents aged between15-19 years were enumerated and of these 2120 (83.3%) consented to participate in the study and were administered the questionnaire (Figs 1 and S1)
Fig 1. Study enumeration and participation.
Note: HH = Household; Yrs = Years.
Study population characteristics
Out of a total of 2120 adolescents who agreed to be part of the study, the majority were aged 15–17 years, 1335 (63.0%), female, 1291(60.9%), and residents in Zambia,1453 (68.5%) (Table 1). Over three-quarters, 1719 (81.1%), had not finished secondary education. A small proportion, 14 (0.7%), self-reported to be HIV positive while a majority, 1040 (49.1%), self-reported to never have tested for HIV.
Table 1. Distribution of the potential risk factors for depressive symptoms stratified by sex and country and combined Zambia and South Africa.
| Sex | Country | Combined Zambia and South Africa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Zambia | South Africa | |||||||
| n | % | n | % | n | % | n | % | n | % | |
| (N = 829) | (N = 1291) | (N = 1453) | (N = 667) | (N = 2120) | ||||||
| Socio-demographic variables | ||||||||||
| Country | ||||||||||
| Zambia | 559 | 67.4 | 894 | 69.2 | - | - | - | - | 1453 | 68.5 |
| South-Africa | 270 | 32.6 | 397 | 30.8 | - | - | - | - | 667 | 31.5 |
| Community | ||||||||||
| Z1 | 161 | 19.4 | 248 | 19.2 | 409 | 28.1 | - | - | 409 | 19.3 |
| Z2 | 160 | 19.3 | 241 | 18.7 | 401 | 27.6 | - | - | 401 | 18.9 |
| Z3 | 81 | 9.8 | 153 | 11.9 | 234 | 16.1 | - | - | 234 | 11.0 |
| Z4 | 157 | 18.9 | 252 | 19.5 | 409 | 28.1 | - | - | 409 | 19.3 |
| SA1 | 84 | 10.1 | 146 | 11.3 | - | - | 230 | 34.5 | 230 | 10.8 |
| SA2 | 72 | 8.7 | 136 | 10.5 | - | - | 208 | 31.2 | 208 | 9.8 |
| SA3 | 114 | 13.8 | 115 | 8.9 | - | - | 229 | 34.3 | 229 | 10.8 |
| Sex | ||||||||||
| Male | - | - | - | - | 559 | 38.5 | 270 | 40.5 | 829 | 39.1 |
| Female | - | - | - | - | 894 | 61.5 | 397 | 59.5 | 1291 | 60.9 |
| Age | ||||||||||
| 15–17 years | 518 | 62.5 | 817 | 63.3 | 915 | 63.0 | 420 | 63.0 | 1335 | 63.0 |
| 18–19 years | 311 | 37.5 | 474 | 36.7 | 538 | 37.0 | 247 | 37.0 | 785 | 37.0 |
| Education level | ||||||||||
| None + Incomplete primary | 151 | 18.2 | 207 | 16 | 308 | 21.2 | 50 | 7.5 | 358 | 16.9 |
| Complete primary | 218 | 26.3 | 325 | 25.2 | 376 | 25.9 | 167 | 25 | 543 | 25.6 |
| Incomplete secondary | 329 | 39.7 | 489 | 37.9 | 513 | 35.3 | 305 | 45.7 | 818 | 38.6 |
| Complete secondary +Higher | 129 | 15.6 | 269 | 20.8 | 256 | 17.6 | 142 | 21.3 | 398 | 18.8 |
| missing | 2 | 0.2 | 1 | 0.1 | 0 | 0 | 3 | 0.4 | 3 | 0.1 |
| HIV-Related Risk factors | ||||||||||
| HIV Test Status | ||||||||||
| Never tested | 458 | 55.2 | 586 | 45.4 | 750 | 51.6 | 294 | 44.1 | 1044 | 49.2 |
| Tested>12 Months | 154 | 18.6 | 239 | 18.5 | 278 | 19.1 | 115 | 17.2 | 393 | 18.5 |
| Tested≤12 Months | 217 | 26.2 | 466 | 36.1 | 425 | 29.2 | 258 | 38.7 | 683 | 32.2 |
| HIV Status | ||||||||||
| Never tested | 456 | 55.0 | 584 | 45.2 | 749 | 51.5 | 291 | 43.6 | 1040 | 49.1 |
| HIV negative | 366 | 44.1 | 696 | 53.9 | 693 | 47.7 | 369 | 55.3 | 1062 | 50.1 |
| HIV positive | 5 | 0.6 | 9 | 0.7 | 10 | 0.7 | 4 | 0.6 | 14 | 0.7 |
| missing | 2 | 0.2 | 2 | 0.2 | 1 | 0.1 | 3 | 0.4 | 4 | 0.2 |
| TB Status | ||||||||||
| Asymptomatic | 547 | 66.0 | 906 | 70.2 | 967 | 66.6 | 486 | 72.9 | 1453 | 68.5 |
| Symptomatic | 279 | 33.7 | 380 | 29.4 | 481 | 33.1 | 178 | 26.7 | 659 | 31.1 |
| On treatment | 3 | 0.4 | 5 | 0.4 | 5 | 0.3 | 3 | 0.4 | 8 | 0.4 |
| Staying with a HIV positive adult or child | ||||||||||
| no | 757 | 91.3 | 1144 | 88.6 | 1307 | 90.0 | 594 | 89.1 | 1901 | 89.7 |
| yes | 69 | 8.3 | 144 | 11.2 | 146 | 10.0 | 67 | 10.0 | 213 | 10.0 |
| missing | 3 | 0.4 | 3 | 0.2 | 0.0 | 6 | 0.9 | 6 | 0.3 | |
| Stigmatizing attitude towards others | ||||||||||
| no | 501 | 60.4 | 951 | 73.7 | 1027 | 70.7 | 425 | 63.7 | 1452 | 68.5 |
| yes | 314 | 37.9 | 322 | 24.9 | 412 | 28.4 | 224 | 33.6 | 636 | 30.0 |
| missing | 14 | 1.7 | 18 | 1.4 | 14 | 1.0 | 18 | 2.7 | 32 | 1.5 |
| Sexual risk behaviour | ||||||||||
| Ever had sex | ||||||||||
| no | 457 | 55.1 | 810 | 62.7 | 914 | 62.9 | 353 | 52.9 | 1267 | 59.8 |
| yes | 370 | 44.6 | 480 | 37.2 | 539 | 37.1 | 311 | 46.6 | 850 | 40.1 |
| missing | 2 | 0.2 | 1 | 0.1 | 0 | 0.0 | 3 | 0.4 | 3 | 0.1 |
| Forced into sex during last sexual encounter * | ||||||||||
| No | 355 | 95.9 | 425 | 88.5 | 473 | 87.8 | 307 | 98.7 | 780 | 91.8 |
| Yes | 15 | 4.1 | 55 | 11.5 | 66 | 12.2 | 4 | 1.3 | 70 | 8.2 |
| Age difference between last sexual partner and participant* | ||||||||||
| within ±5 years | 305 | 82.4 | 380 | 79.2 | 419 | 77.7 | 266 | 85.5 | 685 | 80.6 |
| >5 years older | 5 | 1.4 | 82 | 17.1 | 67 | 12.4 | 20 | 6.4 | 87 | 10.2 |
| ≤5 years younger | 52 | 14.1 | 7 | 1.5 | 53 | 9.8 | 6 | 1.9 | 59 | 6.9 |
| Missing | 8 | 2.2 | 11 | 2.3 | 0 | 0.0 | 19 | 6.1 | 19 | 2.2 |
| Number of sexual partners in the last 1 year * a | ||||||||||
| 0 | 68 | 18.4 | 43 | 9.0 | 111 | 20.6 | - | - | 111 | 13.1 |
| 1 | 94 | 25.4 | 196 | 40.8 | 290 | 53.8 | - | - | 290 | 34.1 |
| ≥2 | 72 | 19.5 | 66 | 13.8 | 138 | 25.6 | - | - | 138 | 16.2 |
| Missing | 136 | 36.8 | 175 | 36.5 | 0 | 0.0 | - | - | - | - |
| Condom use during last sexual intercourse* | ||||||||||
| Not used | 160 | 43.2 | 186 | 38.8 | 238 | 44.2 | 108 | 34.7 | 346 | 40.7 |
| Used | 210 | 56.8 | 294 | 61.3 | 301 | 55.8 | 203 | 65.3 | 504 | 59.3 |
| Alcohol/drug use during last sexual encounter * | ||||||||||
| no | 311 | 84.1 | 444 | 92.5% | 488 | 90.5 | 267 | 85.9 | 755 | 88.8 |
| yes | 59 | 15.9 | 36 | 7.5% | 51 | 9.5 | 44 | 14.1 | 95 | 11.2 |
| HIV Test Status* | ||||||||||
| Never tested | 176 | 47.6 | 107 | 22.3% | 191 | 35.4 | 92 | 29.6 | 283 | 33.3 |
| Tested>12 Months | 70 | 18.9 | 108 | 22.5% | 120 | 22.3 | 58 | 18.6 | 178 | 20.9 |
| Tested< = 12 Months | 124 | 33.5 | 265 | 55.2% | 228 | 42.3 | 161 | 51.8 | 389 | 45.8 |
| Circumcised** | ||||||||||
| no | 415 | 50.1 | - | - | 245 | 43.8 | 170 | 63.0 | 415 | 50.1 |
| Medical circumcision | 312 | 37.6 | - | - | 276 | 49.4 | 36 | 13.3 | 312 | 37.6 |
| Traditional circumcision | 50 | 6.0 | - | - | 26 | 4.7 | 24 | 8.9 | 50 | 6.0 |
| Declined to answer | 50 | 6.0 | - | - | 12 | 2.1 | 38 | 14.1 | 50 | 6.0 |
| Missing | 2 | 0.2 | - | - | 0 | 0.0 | 2 | 0.7 | 2 | 0.2 |
| Currently Pregnant*** | ||||||||||
| no | - | - | 1254 | 97.1 | 870 | 97.3 | 384 | 96.7 | 1254 | 97.1 |
| yes | - | - | 37 | 2.9 | 24 | 2.7 | 13 | 3.3 | 37 | 2.9 |
Note:
*Among those who self-reported to ever had sex
**Among males
***Among females
“-”missing information
N = denominator
Z1-Z4 = Community 1 to community 4 in Zambia; SA1-SA3 = Community 1 to community 3 in South Africa.
%(n/N) = proportion of participants at each level of the potential risk factors expressed as a percentage for the totals in Zambia, South Africa and both countries combined.
a Collected in Zambia only
A total of 659 (31.1%) adolescents reported having one or more TB symptoms and 8 (0.4%) self-reported to be currently on TB treatment. Furthermore, 213 (10.0%) reported to have been staying with an HIV-positive adult or child and 636(30.0%) exhibited stigmatizing attitudes towards PLHIV. Overall, 850 (40.1%) self-reported to have ever had sex. Among those who self-reported to ever had sex, 70 (8.2%) reported having been forced into sex, and during the last sexual encounter, 346 (40.7%) reported not using a condom and 95 (11.2%) reported using alcohol or a drug. More than half of the male participants reported not to be circumcised with a majority of those circumcised having undergone medical circumcision and 37 (2.9%) of the female participants, reported to be pregnant. (Table 1).
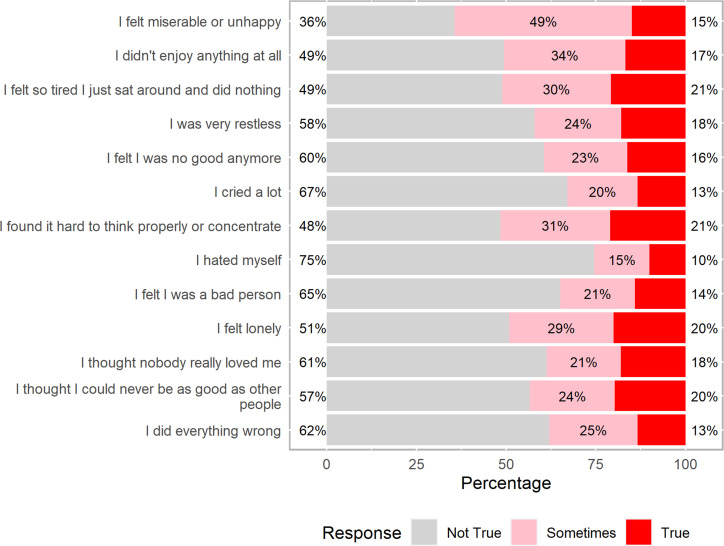
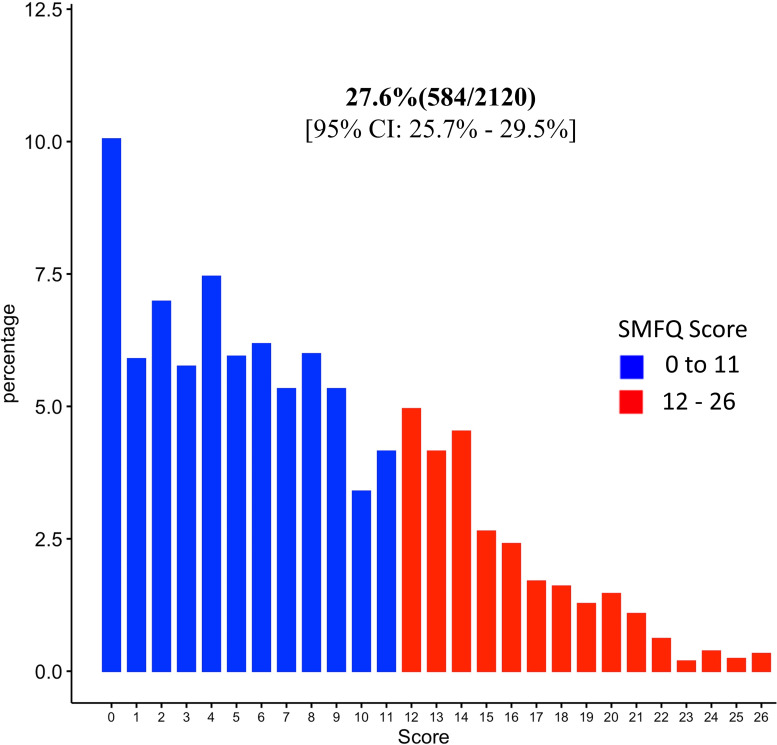
By combining those who answered sometimes and those who answered true to the SMFQ questions, more than 50% reported to have felt miserable or unhappy, did not enjoy anything at all, felt so tired that they just sat around and did nothing, found it hard to think properly or concentrate and 49% reported to have felt lonely (Figs 2 and S2). Using a cut-off of ≥12, the overall prevalence of depressive symptoms was 584 (27.6%) [95% Confidence-Interval [CI]: 25.7%-29.5%] (Figs 3 and S3).
Fig 2. Frequency distribution of the 13 SMFQ items responses (in percentage).
Fig 3. Prevalence of depressive symptoms using a ≥12 cut-off value of the SMFQ “0–26” scale response.
The prevalence of depressive symptoms was higher among adolescents residing in Zambia 432 (29.7%); females 386 (29.9%); ALHIV 7 (50.0%); those who tested for HIV in the previous year, 216 (31.6%); those currently on TB treatment, 3 (37.5%); those who reported staying with a HIV positive adult or child, 75/213 (35.2%); those who exhibited stigmatizing attitudes towards others 192 (30.2%) and those who self-reported to have ever had sex 289(34.0%).
Among those who self-reported to have had sex before, the prevalence of depressive symptoms was higher among those who reported to have been forced into sex, 37(52.9%); those who did not used a condom during their last sexual encounter, 135 (39.0%) and those who used alcohol or drugs during their last sexual encounter 46 (48.4%). Prevalence was also high among those currently pregnant, 20 (54.1%).
Risk factors associated with depressive symptoms
Results from a logistic regression model adjusting for country, sex, age, TB status, staying with a HIV positive adult or child, holding stigmatizing attitude towards PLHIV, ever had sex and HIV test status, showed that; adolescents in SA were less likely to experience depressive symptoms (Adjusted Odds Ratio [AOR] = 0.63 (95% CI: 0.50, 0.79), p-value<0.0001). Female adolescents were more likely to experience depressive symptoms (AOR = 1.46 (95% CI: 1.19, 1.81), p-value<0.0001). Presumptive TB cases and those on TB treatment (AOR = 1.41(95% CI: 1.15, 1.74), p-value = 0.001) as well as adolescents who exhibited stigmatizing attitudes towards PLHIV (AOR = 1.33(95% CI: 1.07, 1.65), p-value = 0.01) were more likely to experience depressive symptoms. There was strong evidence that adolescents who reported ever having had sex were more likely to experience depressive symptoms (AOR = 1.80 (95% CI: 1.45, 2.23), p-value<0.001). Furthermore, there was of an association between depression and HIV test status (p-value = 0.02).
Country, sex, TB status, stigmatizing attitude towards others, ever had sex and HIV test status were identified as the risk factors associated with depressive symptoms (Table 2).
Table 2. Potential risk factors associated with depressive symptoms using the ≥12 cut-off.
| Descriptive analysis | Unadjusted model | Adjusted model 1 | Adjusted model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Potential risk factors | N | n | % | OR (95%CI) | P-value† | AOR (95%CI) | P-value† | AOR (95%CI) | P-value† |
| Country | |||||||||
| Zambia | 1453 | 432 | 29.7 | Reference | 0.001 | - | - | Reference | <0.001 |
| South-Africa | 667 | 152 | 22.8 | 0.70(0.56–0.86) | - | 0.63(0.50–0.79) | |||
| Sex | |||||||||
| Male | 829 | 198 | 23.9 | Reference | 0.003 | - | - | Reference | <0.001 |
| Female | 1291 | 386 | 29.9 | 1.36(1.11–1.66) | - | 1.46(1.19–1.81) | |||
| Age | |||||||||
| 15–17 years | 1335 | 365 | 27.3 | Reference | 0.8 | - | - | Reference | 0.3 |
| 18–19 years | 785 | 219 | 27.9 | 1.03(0.84–1.25) | - | 0.88(0.71–1.10) | |||
| TB Status* | |||||||||
| Asymptomatic | 1453 | 365 | 25.1 | Reference | 0.0003 | Reference | 0.0003 | Reference | 0.001 |
| On TB treatment/Symptomatic | 667 | 219 | 32.8 | 1.46(1.19–1.78) | 1.46(1.19–1.78) | 1.41(1.15–1.74) | |||
| Staying with a HIV positive adult or child | |||||||||
| No | 1901 | 509 | 26.8 | Reference | 0.009 | Reference | 0.013 | Reference | 0.1 |
| Yes | 213 | 75 | 35.2 | 1.49(1.10–2.00) | 1.46(1.08–1.97) | 1.31(0.95–1.80) | |||
| Missing | 6 | 0 | 0.0 | - | - | - | |||
| Stigmatizing attitude towards others | |||||||||
| No | 1452 | 380 | 26.2 | Reference | 0.058 | Reference | 0.009 | Reference | 0.01 |
| Yes | 636 | 192 | 30.2 | 1.22(0.99–1.50) | 1.32(1.07–1.63) | 1.33(1.07–1.65) | |||
| Missing | 32 | 12 | 37.5 | - | - | - | |||
| Ever had sex | |||||||||
| No | 1267 | 295 | 23.3 | Reference | <0.0001 | Reference | <0.0001 | Reference | <0.001 |
| Yes | 850 | 289 | 34.0 | 1.70(1.40–2.06) | 1.91(1.55–2.35) | 1.80(1.45–2.23) | |||
| Missing | 3 | 0 | 0.0 | - | - | - | |||
| HIV Test Status | |||||||||
| Never tested | 1044 | 273 | 26.1 | Reference | 0.0119 | Reference | 0.01 | Reference | |
| Tested>12 Months | 393 | 95 | 24.2 | 0.90(0.69–1.18) | 0.90(0.68–1.18) | 0.78(0.58–1.04) | |||
| Tested≤12 Months | 683 | 216 | 31.6 | 1.31(1.06–1.62) | 1.32(1.06–1.65) | 1.18(0.94–1.50) | 0.02 | ||
| Amongst those who self-reported to ever had sex¶ | |||||||||
| Forced into sex during last sexual encounter | |||||||||
| No | 780 | 252 | 32.3 | Reference | 0.001 | Reference | 0.017 | Reference | 0.057 |
| Yes | 70 | 37 | 52.9 | 2.35(1.44–3.85) | 1.86(1.12–3.10) | 1.67(0.99–2.84) | |||
| Condom use during last sexual intercourse | |||||||||
| Not used | 346 | 135 | 39.0 | Reference | 0.011 | Reference | 0.02 | Reference | 0.053 |
| Used | 504 | 154 | 30.6 | 0.69(0.52–0.92) | 0.80(0.60–1.07) | 0.74(0.55–1.00) | |||
| Alcohol/drug use during last sexual encounter | |||||||||
| No | 755 | 243 | 32.2 | Reference | 0.002 | Reference | Reference | 0.001 | |
| Yes | 95 | 46 | 48.4 | 1.98(1.29–3.04) | 2.35(1.50–3.67) | 0.0002 | 2.18(1.37–3.47) | ||
| Amongst females¶¶ | |||||||||
| Currently Pregnant | |||||||||
| No | 1254 | 366 | 29.2 | Reference | 0.002 | Reference | Reference | 0.024 | |
| Yes | 37 | 20 | 54.1 | 2.85(1.48–5.51) | 2.94(1.51–5.70) | 0.001 | 2.22(1.11–4.45) | ||
Note:
† P-values from Likelihood ratio test
%(n/N) = proportion with depressive symptoms expressed as a percentage (Number with depressive symptoms/denominator)
“-” Information missing
OR = Odds Ratio; AOR = Adjusted Odds Ratio; CI = Confidence Interval;
* For TB status; the symptomatic and on treatment (i.e. 3/8) are collapsed into one category for the analysis at this stage
Adjusted model 1 = Adjusted for country, age and sex
Adjusted model 2 = Final model for the main analysis
Among those who reported to have sex, the baseline model is the final model in the main analysis excluding HIV test status
Among females, the baseline model is the final model in the main analysis
Subgroup analysis
Among adolescents who self-reported ever having had sex; there was strong evidence that those who used alcohol/drugs during their last sexual encounter were more likely to experience depressive symptoms (AOR = 2.18 (95% CI: 1.37, 3.47); p-value = 0.001) (Table 2). However, there was moderate evidence for those who reported to have been forced into sex during their last sexual encounter (AOR = 1.67 (95% CI: 0.99, 2.84); p-value = 0.057). Adolescents who reported to have used a condom during their last sexual encounter were less likely to experience depressive symptoms (AOR = 0.74(95% CI: 0.55, 1.00); p-value = 0.053).
There was strong evidence that those who reported to be currently pregnant were more likely to experience depressive symptoms (AOR = 2.22 (95% CI: 1.11, 4.45); p-value = 0.02. For both countries, community, TB status, ever had sex and the use of alcohol/drug during the last sexual encounter were associated with depressive symptoms (S2 Table). Furthermore, in Zambia, sex, exhibiting stigmatizing attitudes towards others and HIV test status were identified as risk factors. S3 Table shows risk factors by sex.
Sensitivity analysis to outcome definition
A fitted logistic regression model with this new cut off of ≥18, identified country, sex education level, TB status, ever had sex, forced into sex during their last sexual encounter and alcohol/drug use during last sexual encounter as risk factors for depressive symptoms (S4 Table).
Discussion
Our study aimed at examining the association between adolescents’ mental health status, their HIV serostatus and the associated risk behaviours. It highlights the magnitude of depressive symptoms among adolescents in the general population and at-risk adolescents living in SSA. This study adds to literature in this area and to our knowledge by looking not only at prevalence of depression but also the associations between depression and several HIV risk factors reflecting the fact that if we want to address HIV prevention, we also need to address adolescent depression. HIV prevention programs can be more effective when they include a mental health treatment component [15]. High numbers of AYP seek mental health services, which makes them particularly accessible for HIV prevention because they are already in a mental health service system. By identifying the unique risk mechanisms associated with adolescents, customized prevention programs for youths with mental health problems can be designed. Because risk factors of specific MHDs may vary, prevention programs need to be tailored accordingly.
Adolescents in Zambia and South Africa exhibited very high depressive symptoms ranging between 25–30%, similar to other studies in SSA [9, 37, 38]. Other comparable prevalence estimates for depressive symptoms ranging from 28.8 to 32.5% were found in adolescent populations from Ghana, Nigeria, Tanzania, Uganda and Ethiopia [9]. One study conducted among adolescents (aged 15–17) in SA reported a prevalence of 2.6% (males: 3.1% vs. females: 2.0%) for depressive symptoms which was much lower than our finding [39]. Variability in prevalence across studies may be due to differences in sampled populations, study designs, sample sizes, and different screening tools used.
In Zambia, we found that adolescent girls were more likely to report depressive symptoms compared to boys while in SA results were comparable across the two groups. Previous literature has shown an association between female gender and depression across Europe and SSA [40, 41]. Depressive symptomatology has been shown to be more common in young women, while stable or decreasing prevalence has been observed in young men [42]. Adolescent girls are considered to be more at risk of mood disorders including depression; the risk being probably a result of biological, social and psychological dynamics, gender discrimination, exposure to violence and sexual abuse [43, 44]. A study in Cape Town administered a SMFQ to 1034 Grade 8 learners (mean age 14.2) and found a 41.2% prevalence of clinically significant depressive symptoms, with more females than males scoring positive [45]. However, contrary to our findings, a study in Kenya found that adolescent boys had higher chances of experiencing depressive symptoms compared to girls [46].
In this study we used the SMFQ tool to measure depressive symptoms. The SMFQ has been shown to be a strong predictor of depression in adolescents [30]. Several studies in high income countries have shown that the SMFQ is sufficient to be used as a screening tool, with gender-based cut-offs [42, 47]. Although the SMFQ tool has not yet been validated in SSA (including Zambia and SA) before, it was been validated in adolescent populations elsewhere and our reported prevalence rates were comparable to others [48, 49]. There is still lack of consensus as to which are the most valid and reliable tools to measure depressive symptoms [50]. One review surveyed 160 studies of adolescent depression and identified 33 different diagnostic and symptom measurement instruments being used by investigators globally [50].
The significant comorbidity between HIV and MHDs has been widely acknowledged [51]. The prevalence of depression symptoms among PLHIV is estimated to range between 12% and 60%, but most studies involve adult populations [41, 52]. HIV infection among adolescents with MHDs remains an important public health problem, but existing research is very scanty.
In our study, almost half of ALHIV screened positive for depressive symptoms, similar to findings elsewhere although the numbers in our study were quite small [10, 37, 53, 54]. In a systematic review looking at prevalence of depressive symptoms across 14 studies in SSA among ALHIV, the median point prevalence for depression was 22.2% (IQR 15,5–41,1) [9]. In a study conducted in Zambia among 200 ALHIV aged 15–19 years, prevalence of depressive symptoms was 25.3% [38]. In another study conducted in Choma district in Zambia in 2017, among 103 ALHIV, more than three quarters had MH problems [37]. In Uganda among 336 adolescents, 154 (~46%, [95% CI: 40.5–51.2]) had depressive symptoms; the risk was higher among those ≥ 15 years, had disclosed HIV status and had risky sexual practices [10]. The high prevalence of depression among ALHIV is probably due to the direct effect of HIV on the brain, the long-term effects of antiretroviral therapies and various biological and social stressors [7, 41, 55, 56].
Our study contributes to the overlapping burden of depressive symptoms and HIV risk behaviours among adolescents in SSA. Symptoms of depression should be considered as potential markers of increased HIV risk and this association can be causal [21]. We found that adolescents with high depressive symptomatology were more likely to report behaviours that placed them at risk for HIV infection compared to those who reported no symptoms. This finding suggests that HIV risk reduction strategies among adolescents should take into consideration the level of distress they experience. Depression may interfere with the motivation necessary for appropriate HIV risk reducing behaviours. The presence of depressive symptoms may also contribute to a higher degree of isolation and less accessibility for prevention efforts.
A study in Western Kenya assessed prevalence of HIV risk behaviours and depressive symptoms among adolescent girls and young women (AGYW) aged 15–24 years attending 4 public family planning clinics [57]. Among 487 AGYW 59 (12%) AGYW reported moderate-to-severe depression (MSD). MSD was associated with HIV risk factors including partner ≥10 years older, recent transactional sex, forced sex, intimate partner violence, and alcohol use (each p≤0.005). Thirty-four percent of AGYW with MSD had a high HIV risk score corresponding to 5 to 15 incident HIV cases per 100 person-years [57]. The findings in this study in Kenya that demonstrated that frequency of multiple HIV risk factors was higher among AGYW with depression was consistent with other studies in Uganda and SA [21, 58, 59]. Youths in Uganda who had high scores on depression were more likely to report having high numbers of sexual partners [60].
Co-morbidity of depression and substance use disorders are common among adolescents and outcomes are linked with each other. Depressed adolescents are at higher risk of developing substance use disorders especially if the onset of substance use is at an early age [61]. In our study, there was strong evidence that adolescents who reported using alcohol/drugs during their last sexual encounter were more likely to experience depressive symptoms. These findings are consistent with those observed in Zimbabwe and Uganda [10, 62].
Adolescents exposed to sexual violence in different settings are at risk of negative health outcomes, including greater likelihood of depression, substance use, suicidal ideation, anti-social behaviour, and risky sexual behaviour [63, 64]. These health consequences persist into adulthood. In a nationally representative cross-sectional study of sexual abuse of individuals aged 5–17 years in SA, 9·99% (95% CI 8·65–11·47) of boys and 14·61% (95% CI 12·83–16·56) of girls reported some lifetime sexual victimisation [39]. Adolescent ‘s own substance misuse (4·72, 3·73–5·98) and high-risk sexual behaviour (3·71, 2·99–4·61) were the behaviours most frequently associated with sexual abuse, with MHDs strongly associated with sexual victimisation (post-traumatic stress disorder 2·81, 1·65–4·78; depression 3·43, 2·26–5·19; anxiety 2·48, 1·61–3·81) [39].
The violence against children surveys were conducted in Nigeria, Uganda, and Zambia in 2014 and 2015 to examine the prevalence of coerced and forced sexual initiation (FSI) and its consequences among YP aged 13–24 years [65]. Over one in ten YP aged 13–24 years who had ever had sex experienced FSI [65]. In multivariable logistic regression, FSI was significantly associated with infrequent condom use (OR = 1.4, 95%CI = 1.1–2.1), recent experiences of sexual violence (OR = 1.6, 95%CI: 1.1–2.3), physical violence (OR = 2.2, 95%CI: 1.6–3.0), and emotional violence (OR = 2.0, 95%CI: 1.3–2.9), moderate/serious mental distress (OR = 1.5, 95%CI: 1.1–2.0), hurting oneself (OR = 2.0, 95% CI: 1.3–3.1), and thoughts of suicide (OR = 1.5, 95%CI: 1.1–2.3) [65].
We found weak evidence of an association between holding stigmatizing attitudes towards PLHIV and having depressive symptoms, similar to other studies conducted in Zambia [38, 66]. The stigma questions we asked in this study were about the attitudes of survey participants toward PLHIV i.e. negative attitudes that are about fear and judgment. They were not AYP experiences of stigma rather about what these AYP thought of others (people living with HIV). The majority of the survey participants were HIV- negative, so they would not have experienced HIV-related stigma.
Studies have shown that HIV stigma is prevalent in both Zambia and SA, be it in terms of the stigmatizing attitudes of individuals not living with HIV or as measured by stigmatizing experiences of those living with HIV [67]. For PLHIV in a highly stigmatized context such as that of our study population, the knowledge that their HIV status serves as a social blemish and leads to devaluation of their person is experienced in a variety of ways, including being the object of prejudice and discrimination, anticipation of prejudice and discrimination and internalization of negative beliefs and feelings about themselves [68] all of which are associated with higher levels of mental distress [69].
In this study, TB status was associated with depressive symptoms although evidence was quite weak. Due to the small numbers, those on TB treatment and those with at least one TB symptom were collapsed into one category for analysis. Depression is one of the most common psychiatric conditions that TB patients experience due to reduced quality of life brought about by morbidity, side effects of treatment, social stigma, fear of transmitting the disease to others, and other comorbidities associated with TB (especially HIV) [69–77]. TB may also trigger depression through an inflammatory response and or dysregulation of the hypothalamic-pituitary-adrenal axis [75].
Strengths and limitations
Our study had a large sample size and high participation rate by adolescents in both countries. However, we acknowledge that our inclusion of risk factors was not exhaustive as the study was nested within an already ongoing large trial. The screening tool used in this study had an option to be self-administered, this was a strength as adolescents were likely to be truthful on sensitive issues, such as risk-behaviour related questions.
A major limitation of the study is that there are few culturally sensitive, standardized, and validated depression screening tools for use in adolescent populations in SSA [48, 49, 78]. However, the tool used in this study has been used in both countries [45]. Most depression tools were developed for adults and imported from high income countries [48]. Furthermore, studies have used different cut-off points for the same tool making comparisons difficult. We also cannot infer causation from our findings, having a cross-sectional study design, and therefore, for instance, it is hard to tell if a participant had depressive symptoms before or after using alcohol or drugs in their last sexual encounter.
Conclusion
The study highlights the high prevalence of depressive symptoms among adolescents’ in Zambia and SA of approximately 25–30%. Our study shows the link between depressive symptomatology and HIV risk behaviours among adolescents. Adolescent depressive symptoms are associated with increased HIV-risk behaviour. For HIV prevention programs to be more effective they need to include a mental health treatment component. We believe that a greater understanding of the psychological factors that affect AYP is an important precursor to the design of effective HIV prevention strategies.
Supporting information
(DOCX)
(DOCX)
Study Participation (a) In Zambia (b) In South Africa.
(PDF)
(a) Stratified by country (b) Stratified by sex.
(PDF)
(a)stratified by country (b) stratified by Sex.
(PDF)
(a) Overall (b) stratified by the outcome i.e. those who have depressive symptoms and those who don’t.
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to all members of the HPTN 071 (PopART) Study Team, and to the study participants and their communities, for their contributions to the research.
The HPTN 071 (PopART) Study Team: James Hargreaves (London School of Hygiene & Tropical Medicine, London, UK), Deborah Watson-Jones (London School of Hygiene & Tropical Medicine, London, UK), Peter Godfrey-Faussett (London School of Hygiene & Tropical Medicine, London, UK), Kalpana Sabapathy (London School of Hygiene & Tropical Medicine), Katharina Hauck (Imperial College London, London, UK), Peter C. Smith (Imperial College London, London, UK), Anne Cori (Imperial College London, London, UK), Mike Pickles (Imperial College London, London, UK), Nomtha Mandla (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Blia Yang (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Anelet James (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Redwaan Vermaak (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Nozizwe Makola (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Graeme Hoddinott (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Vikesh Naidoo (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Virginia Bond (London School of Hygiene & Tropical Medicine, London, United Kingdom, and Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Musonda Simwinga (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Alwyn Mwinga (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Barry Kosloff (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Mohammed Limbada (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Justin Bwalya (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Chepela Ngulube (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Christophe Fraser (Nuffield Department of Medicine, Oxford University, Oxford, UK), Susan Eshleman (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, US), Yaw Agyei (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, United States), Vanessa Cummings (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, US), Denni Catalano (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, US), Estelle Piwowar-Manning (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, US), Deborah Donnell (HIV Prevention Trials Network Statistical and Data Management Center, Statistical Center for HIV/AIDS Research and Prevention, Seattle, Washington, United States of America), Lynda Emel (Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, US), Lisa Bunts (Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, US), Heather Noble (Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, US), David Burns (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, US), Alain Kouda (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, US), Niru Sista (FHI 360, Durham, North Carolina, US), Ayana Moore (FHI 360, Durham, North Carolina, US), Rhonda White (FHI 360, Durham, North Carolina, US), Tanette Headen (FHI 360, Durham, North Carolina, US), Eric Miller (FHI 360, Durham, North Carolina, US), Kathy Hinson (FHI 360, Durham, North Carolina, US), Sten Vermund (Yale University, New Haven, Connecticut, US), Mark Barnes (Ropes & Gray, Boston, Massachusetts, US), Lyn Horn (Desmond Tutu TB Centre, Stellenbosch University, Stellenbosch, South Africa), Albert Mwango (Zambart, University of Zambia School of Medicine, Lusaka, Zambia), Megan Baldwin (Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, US), Shauna Wolf (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, US), Erin Hughes (Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, US), and Wafaa el-Sadr (Mailman School of Public Health, Columbia University, New York, New York, United States of America).
The content herein is solely the responsibility of the authors and does not necessarily represent the official views of our funders, i.e. The National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), The US President’s Emergency Plan for AIDS Relief (PEPFAR), The International Initiative for Impact Evaluation (3ie), The Bill & Melinda Gates Foundation or the Evidence for HIV Prevention in Southern Africa (EHPSA).
Data Availability
All relevant data are within the paper and its Supporting Information files. In the supporting information files, the data that would be used to replicate our findings is shared in aggregate form (i.e. aggregated at block level separate for Zambia and South Africa).
Funding Statement
The National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), The US President’s Emergency Plan for AIDS Relief (PEPFAR), The International Initiative for Impact Evaluation (3ie), The Bill & Melinda Gates Foundation or the Evidence for HIV Prevention in Southern Africa (EHPSA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNICEF. Adolescent demographics 2019 [Available from: https://data.unicef.org/topic/adolescents/demographics/.
- 2.Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377(9783):2093–102. doi: 10.1016/S0140-6736(11)60512-6 [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC AM, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007; 6: 168–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Üstün TB. Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry. 2007;20(4):359–64. doi: 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juma K, Wekesah FM, Kabiru CW, Izugbara CO. Burden, Drivers, and Impacts of Poor Mental Health in Young People of West and Central Africa: Implications for Research and Programming. In: McLean ML, editor. West African Youth Challenges and Opportunity Pathways. Cham: Springer International Publishing; 2020. p. 233–65. [Google Scholar]
- 6.World Health Organisation. Mental Health Action Plan 2013–2020 2013 [Available from: https://www.who.int/mental_health/publications/action_plan/en/.
- 7.Cortina MA, Sodha A, Fazel M, Ramchandani PG. Prevalence of Child Mental Health Problems in Sub-Saharan Africa: A Systematic Review. Archives of Pediatrics & Adolescent Medicine. 2012;166(3):276–81. doi: 10.1001/archpediatrics.2011.592 [DOI] [PubMed] [Google Scholar]
- 8.WHO. DEPRESSION: A Global CrisisWorld Mental Health Day, October 10 2012. 2012.
- 9.Jörns-Presentati A, Napp A-K, Dessauvagie AS, Stein DJ, Jonker D, Breet E, et al. The prevalence of mental health problems in sub-Saharan adolescents: A systematic review. PLoS One. 2021;16(5):e0251689-e. doi: 10.1371/journal.pone.0251689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemigisha E, Zanoni B, Bruce K, Menjivar R, Kadengye D, Atwine D, et al. Prevalence of depressive symptoms and associated factors among adolescents living with HIV/AIDS in South Western Uganda. AIDS Care. 2019;31(10):1297–303. doi: 10.1080/09540121.2019.1566511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, et al. Child and adolescent mental health worldwide: evidence for action. The Lancet. 2011;378(9801):1515–25. doi: 10.1016/S0140-6736(11)60827-1 [DOI] [PubMed] [Google Scholar]
- 12.McFarlane AH, Bellissimo A, Norman GR. The role of family and peers in social self-efficacy: links to depression in adolescence. Am J Orthopsychiatry. 1995;65(3):402–10. doi: 10.1037/h0079655 [DOI] [PubMed] [Google Scholar]
- 13.Brown LK, Danovsky MB, Lourie KJ, DiClemente RJ, Ponton LE. Adolescents with psychiatric disorders and the risk of HIV. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1609–17. doi: 10.1016/S0890-8567(09)66573-4 [DOI] [PubMed] [Google Scholar]
- 14.Dolcini MM, Adler NE. Perceived competencies, peer group affiliation, and risk behavior among early adolescents. Health Psychol. 1994;13(6):496–506. doi: 10.1037//0278-6133.13.6.496 [DOI] [PubMed] [Google Scholar]
- 15.Donenberg GR, Emerson E, Bryant FB, Wilson H, Weber-Shifrin E. Understanding AIDS-risk behavior among adolescents in psychiatric care: links to psychopathology and peer relationships. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(6):642–53. doi: 10.1097/00004583-200106000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiClemente RJ, Wingood GM, Crosby RA, Sionean C, Brown LK, Rothbaum B, et al. A prospective study of psychological distress and sexual risk behavior among black adolescent females. Pediatrics. 2001;108(5):E85. doi: 10.1542/peds.108.5.e85 [DOI] [PubMed] [Google Scholar]
- 17.Garrison CZ, Schluchter MD, Schoenbach VJ, Kaplan BK. Epidemiology of depressive symptoms in young adolescents. J Am Acad Child Adolesc Psychiatry. 1989;28(3):343–51. doi: 10.1097/00004583-198905000-00007 [DOI] [PubMed] [Google Scholar]
- 18.Rubin AG, Gold MA, Primack BA. Associations between depressive symptoms and sexual risk behavior in a diverse sample of female adolescents. J Pediatr Adolesc Gynecol. 2009;22(5):306–12. doi: 10.1016/j.jpag.2008.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LK, Tolou-Shams M, Lescano C, Houck C, Zeidman J, Pugatch D, et al. Depressive symptoms as a predictor of sexual risk among African American adolescents and young adults. J Adolesc Health. 2006;39(3):444 e1-8. doi: 10.1016/j.jadohealth.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 20.Foley JD, Vanable PA, Brown LK, Carey MP, DiClemente RJ, Romer D, et al. Depressive symptoms as a longitudinal predictor of sexual risk behaviors among African-American adolescents. Health Psychol. 2019;38(11):1001–9. doi: 10.1037/hea0000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nduna M, Jewkes RK, Dunkle KL, Shai NPJ, Colman I. Associations between depressive symptoms, sexual behaviour and relationship characteristics: a prospective cohort study of young women and men in the Eastern Cape, South Africa. Journal of the International AIDS Society. 2010;13:44-. doi: 10.1186/1758-2652-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutumba M, Resnicow K, Bauermeister JA, Harper GW, Musiime V, Snow RC, et al. Development of a Psychosocial Distress Measure for Ugandan Adolescents Living with HIV. AIDS and Behavior. 2015;19(2):380–92. doi: 10.1007/s10461-014-0973-y [DOI] [PubMed] [Google Scholar]
- 23.Betancourt T, Scorza P, Kanyanganzi F, Fawzi MCS, Sezibera V, Cyamatare F, et al. HIV and Child Mental Health: A Case-Control Study in Rwanda. Pediatrics. 2014;134(2):e464–e72. doi: 10.1542/peds.2013-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambaw F, Mayston R, Hanlon C, Alem A. Depression among patients with tuberculosis: determinants, course and impact on pathways to care and treatment outcomes in a primary care setting in southern Ethiopia—a study protocol. BMJ Open. 2015;5(7):e007653. doi: 10.1136/bmjopen-2015-007653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014;15:57. doi: 10.1186/1745-6215-15-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bond V, Chiti B, Hoddinott G, Reynolds L, Schaap A, Simuyaba M, et al. "The difference that makes a difference": highlighting the role of variable contexts within an HIV Prevention Community Randomised Trial (HPTN 071/PopART) in 21 study communities in Zambia and South Africa. AIDS care. 2016;28 Suppl 3:99–107. doi: 10.1080/09540121.2016.1178958 [DOI] [PubMed] [Google Scholar]
- 27.Hayes R, Floyd S, Schaap A, Shanaube K, Bock P, Sabapathy K, et al. A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLOS Medicine. 2017;14(5):e1002292. doi: 10.1371/journal.pmed.1002292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanaube K, Schaap A, Chaila MJ, Floyd S, Mackworth-Young C, Hoddinott G, et al. Community intervention improves knowledge of HIV status of adolescents in Zambia: findings from HPTN 071-PopART for youth study. AIDS. 2017;31 Suppl 3(Suppl 3):S221–S32. doi: 10.1097/QAD.0000000000001530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floyd S, Shanaube K, Yang B, Schaap A, Griffith S, Phiri M, et al. HIV testing and treatment coverage achieved after 4 years across 14 urban and peri-urban communities in Zambia and South Africa: An analysis of findings from the HPTN 071 (PopART) trial. PLoS Med. 2020;17(4):e1003067-e. doi: 10.1371/journal.pmed.1003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angold A, Costello E, Messer S, Pickles A, Winder F, Silver D. The Development of a Questionnaire for Use in Epidemiological Studies of Depression in Children and Adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–49. [Google Scholar]
- 31.Kwong A. Examining the longitudinal nature of depressive symptoms in the Avon Longitudinal Study of Parents and Children (ALSPAC) [version 2; peer review: 3 approved]. Wellcome Open Research. 2019;4(126). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins K, Crosland P, Elliott N, Bewley S. Diagnosis and management of depression in children and young people: summary of updated NICE guidance. BMJ. 2015;350:h824. doi: 10.1136/bmj.h824 [DOI] [PubMed] [Google Scholar]
- 33.Thabrew H, Stasiak K, Bavin L-M, Frampton C, Merry S. Validation of the Mood and Feelings Questionnaire (MFQ) and Short Mood and Feelings Questionnaire (SMFQ) in New Zealand help-seeking adolescents. Int J Methods Psychiatr Res. 2018;27(3):e1610-e. doi: 10.1002/mpr.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargreaves JR, Stangl A, Bond V, Hoddinott G, Krishnaratne S, Mathema H, et al. HIV-related stigma and universal testing and treatment for HIV prevention and care: design of an implementation science evaluation nested in the HPTN 071 (PopART) cluster-randomized trial in Zambia and South Africa. Health Policy and Planning. 2016;31(10):1342–54. doi: 10.1093/heapol/czw071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnaratne S, Bond V, Stangl A, Pliakas T, Mathema H, Lilleston P, et al. Stigma and Judgment Toward People Living with HIV and Key Population Groups Among Three Cadres of Health Workers in South Africa and Zambia: Analysis of Data from the HPTN 071 (PopART) Trial. AIDS Patient Care STDS. 2020;34(1):38–50. doi: 10.1089/apc.2019.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(MOH) MoH. National Assembly. National Health Research Act No. 2. Lusaka: Zambia Government Printers. 2013.
- 37.Lyambai K and Mwape L. Mental Health Problems Experienced by HIV Positive Adolescents; A Case of Choma District, Zambia. Open Journal of Psychiatry. 2018;8:97–114. [Google Scholar]
- 38.Okawa S, Mwanza Kabaghe S, Mwiya M, Kikuchi K, Jimba M, Kankasa C, et al. Psychological well-being and adherence to antiretroviral therapy among adolescents living with HIV in Zambia. AIDS Care. 2018;30(5):634–42. doi: 10.1080/09540121.2018.1425364 [DOI] [PubMed] [Google Scholar]
- 39.Ward CL, Artz L, Leoschut L, Kassanjee R, Burton P. Sexual violence against children in South Africa: a nationally representative cross-sectional study of prevalence and correlates. Lancet Glob Health. 2018;6(4):e460–e8. doi: 10.1016/S2214-109X(18)30060-3 [DOI] [PubMed] [Google Scholar]
- 40.Claude Mellins and Malee Kathleen. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. JIAS. 2013;16(18593). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MH, Mazenga AC, Yu X, Devandra A, Nguyen C, Ahmed S, et al. Factors associated with depression among adolescents living with HIV in Malawi. BMC psychiatry. 2015;15:264-. doi: 10.1186/s12888-015-0649-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoare E, Millar L, Fuller-Tyszkiewicz M, Skouteris H, Nichols M, Malakellis M, et al. Depressive symptomatology, weight status and obesogenic risk among Australian adolescents: a prospective cohort study. BMJ Open. 2016;6(3):e010072. doi: 10.1136/bmjopen-2015-010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, et al. The Influence of Genetic Factors and Life Stress on Depression Among Adolescent Girls. Archives of General Psychiatry. 1999;56(3):225–32. doi: 10.1001/archpsyc.56.3.225 [DOI] [PubMed] [Google Scholar]
- 44.AP. M, Salvador MC, Costa JJ, Pinheiro MR, Arnarson E, Craighead WE. The relationship between internalizing and externalizing problemsin adolescence: does gen der make a difference?. Can Int J Soc Sci Educ. 2017;66:45–63. [Google Scholar]
- 45.Stansfeld SA, Rothon C, Das-Munshi J, Mathews C, Adams A, Clark C, et al. Exposure to violence and mental health of adolescents: South African Health and Well-being Study. BJPsych Open. 2018;3(5):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamau JW, Kuria W, Mathai M, Atwoli L, Kangethe R. Psychiatric morbidity among HIV-infected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24(7):836–42. doi: 10.1080/09540121.2011.644234 [DOI] [PubMed] [Google Scholar]
- 47.Jarbin H, Ivarsson T, Andersson M, Bergman H, Skarphedinsson G. Screening efficiency of the Mood and Feelings Questionnaire (MFQ) and Short Mood and Feelings Questionnaire (SMFQ) in Swedish help seeking outpatients. PLOS ONE. 2020;15(3):e0230623. doi: 10.1371/journal.pone.0230623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashaba S, Cooper-Vince C, Vořechovská D, Maling S, Rukundo GZ, Akena D, et al. Development and validation of a 20-item screening scale to detect major depressive disorder among adolescents with HIV in rural Uganda: A mixed-methods study. SSM—Population Health. 2019;7:100332. doi: 10.1016/j.ssmph.2018.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweetland A, Belkin G, Verdeli H. Measuring depression and anxiety in sub-Saharan Africa. Depression and anxiety. 2014;31. doi: 10.1002/da.22142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks SJ, Kutcher S. Diagnosis and Measurement of Adolescent Depression: A Review of Commonly Utilized Instruments. Journal of Child and Adolescent Psychopharmacology. 2001;11(4):341–76. doi: 10.1089/104454601317261546 [DOI] [PubMed] [Google Scholar]
- 51.WHO. Depression and Other Common Mental Disorders, global health estmates. 2017.
- 52.Nakasujja N, L Skolasky R, Musisi S, Allebeck P, Robertson K, Ronald A, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10(1):44. doi: 10.1186/1471-244X-10-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon et al. Mental Health of HIV Positive Adolescents in Zambia 2009.
- 54.Degun S, Menon A, McPherson A, Ngoma M, Nair R, Andren J, et al. Factors Influencing Mental Health in Zambian Adolescents with HIV and AIDS. Pediatric Research. 2011;70(5):444-. [Google Scholar]
- 55.Agwu AL FL. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. 2013. p. 18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naar-King S, Templin T, Wright K, Frey M, Parsons JT, Lam P. Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care STDS. 2006;20(1):44–7. doi: 10.1089/apc.2006.20.44 [DOI] [PubMed] [Google Scholar]
- 57.Larsen A, Kinuthia J, Lagat H, Sila J, Abuna F, Kohler P, et al. Depression and HIV risk behaviors among adolescent girls and young women seeking family planning services in Western Kenya. Int J STD AIDS. 2020;31(7):652–64. doi: 10.1177/0956462420920423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundberg P, Rukundo G, Ashaba S, Thorson A, Allebeck P, Ostergren PO, et al. Poor mental health and sexual risk behaviours in Uganda: a cross-sectional population-based study. BMC Public Health. 2011;11:125. doi: 10.1186/1471-2458-11-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jewkes R, Dunkle K, Nduna M, Levin J, Jama N, Khuzwayo N, et al. Factors associated with HIV sero-status in young rural South African women: connections between intimate partner violence and HIV. Int J Epidemiol. 2006;35(6):1461–8. doi: 10.1093/ije/dyl218 [DOI] [PubMed] [Google Scholar]
- 60.Lehrer JA, Shrier LA, Gortmaker S, Buka S. Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics. 2006;118(1):189–200. doi: 10.1542/peds.2005-1320 [DOI] [PubMed] [Google Scholar]
- 61.Whitesell M, Bachand A, Peel J, Brown M. Familial, Social, and Individual Factors Contributing to Risk for Adolescent Substance Use. Journal of Addiction. 2013;2013:579310. doi: 10.1155/2013/579310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langhaug LF, Pascoe SJ, Mavhu W, Woelk G, Sherr L, Hayes RJ, et al. High prevalence of affective disorders among adolescents living in Rural Zimbabwe. Journal of community health. 2010;35(4):355–64. doi: 10.1007/s10900-010-9261-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merrill KG, Campbell JC, Decker MR, McGready J, Burke VM, Mwansa JK, et al. Prevalence of physical and sexual violence and psychological abuse among adolescents and young adults living with HIV in Zambia. PLoS One. 2020;15(6):e0235203. doi: 10.1371/journal.pone.0235203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goin DE, Pearson RM, Craske MG, Stein A, Pettifor A, Lippman SA, et al. Depression and Incident HIV in Adolescent Girls and Young Women in HIV Prevention Trials Network 068: Targets for Prevention and Mediating Factors. American Journal of Epidemiology. 2019;189(5):422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen KH, Padilla M, Villaveces A, Patel P, Atuchukwu V, Onotu D, et al. Coerced and forced sexual initiation and its association with negative health outcomes among youth: Results from the Nigeria, Uganda, and Zambia Violence Against Children Surveys. Child Abuse & Neglect. 2019;96:104074. doi: 10.1016/j.chiabu.2019.104074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munakampe MN. Strengthening mental health systems in Zambia. Int J Ment Health Syst. 2020;14:28. doi: 10.1186/s13033-020-00360-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stangl AL, Pliakas T, Mainga T, Steinhaus M, Mubekapi-Musadaidzwa C, Viljoen L, et al. The effect of universal testing and treatment on HIV stigma in 21 communities in Zambia and South Africa. AIDS. 2020;34(14):2125–35. doi: 10.1097/QAD.0000000000002658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS and behavior. 2009;13(6):1160–77. doi: 10.1007/s10461-009-9593-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turan B, Budhwani H, Fazeli PL, Browning WR, Raper JL, Mugavero MJ, et al. How Does Stigma Affect People Living with HIV? The Mediating Roles of Internalized and Anticipated HIV Stigma in the Effects of Perceived Community Stigma on Health and Psychosocial Outcomes. AIDS Behav. 2017;21(1):283–91. doi: 10.1007/s10461-016-1451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deribew A, Tesfaye M, Hailmichael Y, Apers L, Abebe G, Duchateau L, et al. Common mental disorders in TB/HIV co-infected patients in Ethiopia. BMC Infect Dis. 2010;10:201. doi: 10.1186/1471-2334-10-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauer M, Ahmed S, Benedetti A, Greenaway C, Lalli M, Leavens A, et al. Health-related quality of life and tuberculosis: a longitudinal cohort study. Health Qual Life Outcomes. 2015;13:65. doi: 10.1186/s12955-015-0250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alene KA, Clements ACA, McBryde ES, Jaramillo E, Lonnroth K, Shaweno D, et al. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis. J Infect. 2018;77(5):357–67. doi: 10.1016/j.jinf.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 73.Bond V, Floyd S, Fenty J, Schaap A, Godfrey-Faussett P, Claassens M, et al. Secondary analysis of tuberculosis stigma data from a cluster randomised trial in Zambia and South Africa (ZAMSTAR). Int J Tuberc Lung Dis. 2017;21(11):49–59. doi: 10.5588/ijtld.16.0920 [DOI] [PubMed] [Google Scholar]
- 74.Sweetland A, Oquendo M, Wickramaratne P, Weissman M, Wainberg M. Depression: a silent driver of the global tuberculosis epidemic. World Psychiatry. 2014;13(3):325–6. doi: 10.1002/wps.20134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweetland AC, Kritski A, Oquendo MA, Sublette ME, Norcini Pala A, Silva LRB, et al. Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. Int J Tuberc Lung Dis. 2017;21(8):852–61. doi: 10.5588/ijtld.16.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doherty AM, Kelly J, McDonald C, O’Dywer AM, Keane J, Cooney J. A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry. 2013;35(4):398–406. doi: 10.1016/j.genhosppsych.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 77.Pachi A, Bratis D, Moussas G, Tselebis A. Psychiatric morbidity and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat. 2013;2013:489865. doi: 10.1155/2013/489865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teivaanmäki T, Cheung YB, Maleta K, Gandhi M, Ashorn P. Depressive symptoms are common among rural Malawian adolescents. Child: Care, Health and Development. 2018;44(4):531–8. doi: 10.1111/cch.12567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Study Participation (a) In Zambia (b) In South Africa.
(PDF)
(a) Stratified by country (b) Stratified by sex.
(PDF)
(a)stratified by country (b) stratified by Sex.
(PDF)
(a) Overall (b) stratified by the outcome i.e. those who have depressive symptoms and those who don’t.
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. In the supporting information files, the data that would be used to replicate our findings is shared in aggregate form (i.e. aggregated at block level separate for Zambia and South Africa).