Abstract
Digital health technology is receiving increasing attention in cardiology. The rise of accessibility of digital health tools including wearable technologies and smart phone applications used in medical practice has created a new era in healthcare. The coronavirus pandemic has provided a new impetus for changes in delivering medical assistance across the world. This Consensus document discusses the potential implementation of digital health technology in older adults, suggesting a practical approach to general cardiologists working in an ambulatory outpatient clinic, highlighting the potential benefit and challenges of digital health in older patients with, or at risk of, cardiovascular disease. Advancing age may lead to a progressive loss of independence, to frailty, and to increasing degrees of disability. In geriatric cardiology, digital health technology may serve as an additional tool both in cardiovascular prevention and treatment that may help by (i) supporting self‐caring patients with cardiovascular disease to maintain their independence and improve the management of their cardiovascular disease and (ii) improving the prevention, detection, and management of frailty and supporting collaboration with caregivers. Digital health technology has the potential to be useful for every field of cardiology, but notably in an office‐based setting with frequent contact with ambulatory older adults who may be pre‐frail or frail but who are still able to live at home. Cardiologists and other healthcare professionals should increase their digital health skills and learn how best to apply and integrate new technologies into daily practice and how to engage older people and their caregivers in a tailored programme of care.
Keywords: Digital health, Digital technology, eHealth, Older adults, Frailty, Cardiovascular disease, Cardiovascular prevention, Geriatric cardiology
Introduction
Digital health (DH) technology is increasingly adopted in cardiovascular (CV) medicine, although its implementation has been slow, and quality standards focused around improvements in clinical practice are still lacking. 1 , 2 The increased accessibility of wearable technologies and mobile applications (mApps) has created a new era in health tracking, with the coronavirus disease‐2019 (COVID‐19) pandemic providing the impetus for changes in delivering healthcare across the world and in building a more comprehensive picture of a patient during follow‐up. 3 , 4 , 5 , 6
The potential support of DH technology may have a special role in patients living with health status limitations due to ageing. Ageing is a natural process that poses several challenges and threats to the preservation of independence. 7 In healthy older adults the maintenance of healthy ageing, defined as developing and maintaining the functional ability that enables well‐being in older age, has become a principal goal worldwide. 8 , 9 Moreover, advancing age may lead to frailty, which has been increasingly recognized to be central to health and outcomes in an ageing population and, more generally, in patients with CV diseases. 10 , 11 , 12 This is a multisystem/multidomain complex condition characterized by reduced functional reserves and increased vulnerability to adverse stress and health events, often associated with multimorbidity. Frailty can contribute to an accelerated clinical decline (Figure 1 A ) which may lead to progressive loss of independence and disability, defined as difficulty or dependency in carrying out activities essential for daily living, including tasks needed for self‐care and living. 10 , 11 , 12 The frail condition is the result of deficits in various domains: physical, medical, psychological, cognitive, and social. Better consideration of these specific domains allows better identification of specific needs, which can then be targeted (Figure 1B ). 11 , 13 Because frailty may be, at least in part, reversible, early identification and characterization of the frailty, along with interventions on frailty components, together with the general management of frailty (including support for physical activity, nutrition, medical optimization, and social interaction) may improve the degree of frailty, or at least slow down the frailty trajectory. 11 , 14
Figure 1.
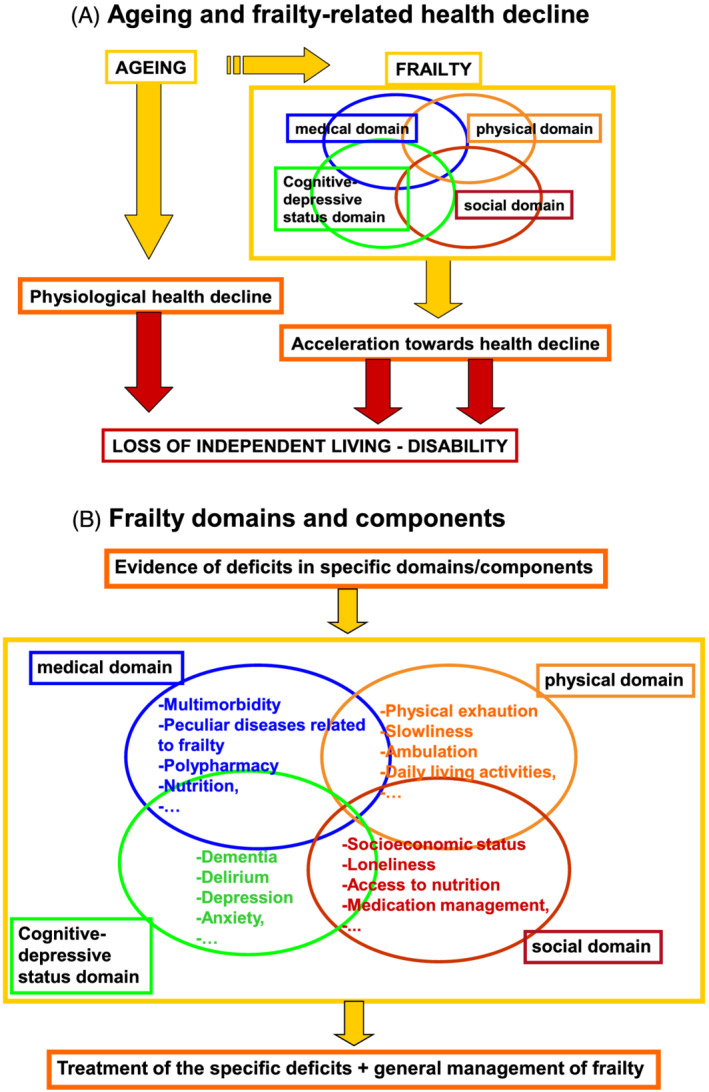
(A) Ageing and frailty‐related health decline. (B) Frailty domains and components.
Figure 2.

DH interventions in older adults with, or at risk of, CV disease.
Although these terms are not synonymous, ageing, frailty, and disability are clearly interconnected. 11 Considering the impact of increasing population ageing on health care systems, the older population is therefore a prime target for new technologies and interventions. 10
This Consensus document highlights the potential benefit and challenges of DH in older adults with, or at risk of, CV disease, providing a practical approach to general cardiologists working in an ambulatory (outpatients) setting by (i) providing suggestions for DH in the management of common age‐associated CV diseases so as to foster self‐care and independence and (ii) providing suggestions for DH in the prevention, detection, and management of frailty.
Technical innovations for the care of older adults
Although DH technologies (Box: Definitions) 15 , 16 , 17 have become increasingly common place, their utility, feasibility, and roles may differ by age group. Gerontology studies on digital technology include applications to physical and mental health, mobility, social connectedness, loneliness, communication, leisure, and safety. 18 In relation to older adults, DH technology holds the potential to improve well‐being, optimize healthcare delivery and monitoring particularly in individuals with limited mobility and to support ageing people in a safe and independent environment. 19 , 20
Box: Definitions.
Electronic‐Health (eHealth). The use of information and communications technology in support of health and health‐related fields, including health care services, health surveillance, health literature, and health education, knowledge and research.
Mobile‐Health (mHealth). The use of mobile and wireless technologies to support health objective; the application of sensors, mobile apps, social media, and location‐tracking technology to obtain data pertinent to wellness and disease diagnosis, prevention, and management. mHealth is a component of eHealth.
Digital Health. An overarching term that comprises eHealth (which includes mHealth), and emerging areas, such as the use of computing sciences in the fields of artificial intelligence, big data and genomics.
Applications (Apps). Computer software programs that operate on computer, tablets and other mobile devices such as smartphones and smartwatches [Mobile‐Apps (mApps)].
Client‐to‐provider telemedicine. Provision of health services at a distance; delivery of health care services where clients/patients and health workers are separated by distance.
Digital biomarkers. Physiological and behavioural measures collected by means of digital devices such as portables, wearables, implantables, or digestibles that characterize, influence, or predict health‐related outcomes.
Digital diagnostics. The application of wearable and ambient sensors, mobile apps, social media, and location‐tracking technology singly or in combination to diagnose medical conditions.
Digital therapeutics. Interventions that use wearable and ambient sensors, mobile apps, social media, and location‐tracking technology independently or in conjunction with medications, devices, or other therapies to improve patient care and health outcomes.
Digital tools may vary from simple text messaging platforms (short message service: SMS) to mApps, or more complex algorithms including information obtained by biological sensors. Text messages are simple, instant, and popular. They offer a widely available medium for delivery of health‐related communication and can be sent remotely to large numbers of people in an unobtrusive manner. Apps are computer software programs that operate on smartphones, tablets, and other mobile devices such as smartwatches. 17 Apps are generally readily available—for those that have a mobile device—and relatively easy to use via touchscreen interfaces. Examples of more complex DH technology based on sensors are algorithms that can detect physical instability and predict the risk of falls. A number of studies have utilized camera and sensor‐based systems to assess gait and developed predictive algorithms with the formation of novel digital fall risk assessment protocols, thus allowing early preventive intervention. 21 Because falls are the main cause of accidental death and disability (and the related healthcare costs) within the European Union in older adults, 22 , 23 the development of new technical solutions is receiving much attention.
Digital health instruments that may be useful in common age‐associated cardiovascular diseases
The use of text message programs has been shown to support the management of chronic disease and CV risk factors. These include smoking cessation, weight loss, physical activity, blood pressure lowering, and diabetes care (see below). 24 Because the prevention, detection and treatment of CV disease [including atrial fibrillation (AF) and heart failure (HF)] are closely related to improving health decline in older subjects 11 DH technology management of these diseases is becoming part of the routine CV care in older adults (Figure 2 ).
Historical underutilization of DH technologies, such as video visits and remote patient monitoring, reflects an incomplete understanding of their value and applications across the chronological and physiological spectrum of older age. This is related to various factors including (i) healthcare workers' incomplete understanding and inertia in old methods of care delivery; (ii) older patients' lower rates of digital device usage and comprehension; (iii) manufacturers' lack of attention to adapted needs of older adults, for example, simple devices with large screens and text sizes.
During the COVID‐19 pandemic, the use of DH technology has been accelerated to preserve and optimize the health of older adults with regard to CV prevention and treatment of CV disease. Restrictions on the use of DH technology have been modified to increase flexibility for clinicians to conduct non–face‐to‐face visits and to improve patient access to healthcare. Several hospitals and commercial insurance companies currently reimburse telehealth similarly to in‐person visits, thus supporting increased telehealth utilization. How this will play out as the pandemic precautions are withdrawn, is unclear and may differ geographically.
In the past decade there has been a proliferation of Web sites and mApps that claim to support secondary prevention of heart disease. A systematic review and meta‐analysis of telehealth (phone, Internet, and videoconference communication between patient and health care provider) found that such interventions were not associated with lower all‐cause mortality but resulted in significantly lower re‐hospitalization or cardiac events compared with non‐intervention groups. 25 , 26
Arterial hypertension and dyslipidaemias
DH technologies may offer various potential improvements in the care of older adult including a closer relationship between patients and medical staff, empowerment of the patients, more frequent measures to tailor therapy, and the chance of avoiding transportation needs, particularly distressing in the oldest and frail patients. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35
Remote monitoring and telehealth models of care are important for older adult patients with chronic diseases because they allow acquisition of physiological data from home locations.
Hypertension is a good target for telemedicine, and in particular, for telemonitoring, as it is the most common and important risk factor for CV disease worldwide. 35 , 36 Although data are highly heterogenous regarding both the DH methodology used for telemonitoring, the clinical setting of the patients studied, and the presence of co‐morbidities, most reviews and meta‐analyses tend to show an improvement in blood pressure control. 35 , 36 , 37 , 38 In addition to the use of telemonitoring, many DH trials include other interventions leading to a comprehensive approach to hypertension, including patient education, behavioural and motivational support, close follow‐up and focus on medication adherence, probably all contributing to optimization of clinical outcomes. Moreover, with a new DH intervention combining self‐monitoring of blood pressure with guided self‐management in patients with poorly controlled hypertension, the drop in blood pressure was larger in the DH‐managed group when compared with the usual care group (follow‐up 12 months: mean difference of −3.4 mm Hg in systolic blood pressure, 95% confidence interval −6.1 to −0.8 mm Hg, and mean difference of −0.5 mm Hg in diastolic blood pressure, −1.9 to 0.9 mm Hg). However, the benefit was observed to be larger in patients aged less than 67 years. 39
Although telemedicine has been shown to improve blood pressure control as compared with standard care, its place in daily clinical practice is not yet clear. While most guidelines refer to it in the context of excluding white coat or masked hypertension, there are no current specific recommendations on the place of telemedicine in general hypertension management, with the partial exception of the 2018 Hypertension Clinical Practice Guidelines, which suggest that telehealth strategies can be useful adjuncts to interventions shown to reduce blood pressure for adults with hypertension. 40
Several barriers still limit the implementation of telemedicine in the routine clinical management of CV risk factors, including the fact that telemedicine is considered as an add‐on to existing care rather than an indispensable tool to be blended in current care delivery. 41
Nevertheless, adopters of DH activity trackers tend to adhere more to hypertension and dyslipidaemia medications, and adherence increases with tracking frequency and smartphone‐associated blood pressure controls. 34 , 38 , 39 , 41 , 42 , 43 , 44 Digital interventions in the presence of multimorbidity, in patients with difficult‐to‐treat hypertension or with poor adherence to medication management seem to be clinically relevant.
Diabetes mellitus
Diabetes mellitus is an important risk factor for the development of CV morbidity and mortality. Self‐management with DH technologies was recommended recently by the European Society of Cardiology (ESC) guidelines on diabetes and CV diseases. 45 mApps can facilitate self‐management by providing reminders for regular measurement of the required parameters and medication adherence, and may support education and motivational support. Improved glycaemic control may improve other aspects of CV health such as reducing AF incidence and recurrence. 46 , 47 Regular transmission of blood glucose levels from patients to their physicians can be based on SMS, e‐mail, or various web‐based services. Bluetooth‐enabled glucose meters are frequently used. 48 , 49 BlueStar™ (Welldoc, Columbia, MD), was the first to receive US FDA clearance for diabetes mellitus management: it comes with an App which requires a physician prescription and enables patients to titrate insulin dosing by using the proprietary insulin calculator. The Freestyle™ LibreLink™ app (Abbott Laboratories, Abbott Park, IL) links with an associated continuous glucose monitoring patch and displays trends. 50
Stand‐alone diabetes management mApps have recently been reviewed. 51 Efficacy for improving glycaemic control in randomized controlled trials has shown varied results. 52 Meta‐analyses indicate that mobile phone interventions for self‐management reduced haemoglobin (Hb)A1c modestly by 0.2–0.5% over a median of 6‐months' follow‐up, with a greater reduction in patients with type 2 compared with type 1 diabetes. 53 A significant impact on clinical outcomes may affect healthcare expenditures by reducing the need for in‐person contact with healthcare providers, preventing hospital admissions, and improving prognosis. In a retrospective study, the use of DH technologies was associated with a 21.9% reduction in medical spending than a control group during the first year. 54 Key determinants to successful uptake of decision‐support mApps will likely be their user‐friendliness, simplicity, delivery of electronic communications, and feedback to the patient.
Atrial fibrillation
Although opportunistic tools for AF diagnosis are widely used, the key to making AF identification clinically valuable is the selection of patients with an increased likelihood of harbouring undiagnosed AF 55 and an increased risk of stroke that may qualify for anticoagulation. Patients with non‐valvular AF and left ventricular hypertrophy are often older and with a higher prevalence of multimorbidity. 56
Different mHealth‐based modalities for arrhythmia monitoring may be used in different settings and to address different questions. Recording electrocardiogram (ECG) tracings (single or multi‐lead, in intermittent or continuous format, of various durations) and non‐ECG technologies such as pulse photoplethysmography are two different modalities to approach mHealth signal monitoring. 57
Mobile recorders are increasingly used to facilitate frequent brief (e.g. 30 s) recordings over prolonged periods of time by the ubiquity of such devices (including smartphone‐based mApps or watches). 57 These devices are particularly well suited to capture intermittent or non‐persistent arrhythmias. However, because snapshot ECG recordings are unable to capture infrequent paroxysmal AF, new algorithms based on more frequent sampling are needed. 58 Such algorithms include repetitive verifications of pulse rate regularity through plethysmography sensors followed by periodic verifications of ECG rhythm when irregularity is detected. According to the Apple Heart Study, notification of irregular pulse is very low in participants without self‐reported arrhythmias. However, once notification was received, the chance to confirm AF after returning an ECG patch was about 84%, thus supporting the ability of the algorithm to correctly identify AF in users whom it notifies of irregular pulses. 59
AF burden is increasingly recognized as a powerful independent predictor of stroke. 60 , 61 Formal screening with mobile ECG recordings has yielded higher incidence of newly diagnosed AF compared with diagnosis relying only on the office ECG. 57 The yield generally is enhanced by the presence of risk factors, such as older age and higher CHA2DS2‐VASc scores. By focusing on older patients (75–76 years of age) at greater risk, Swedish studies identified new AF in 3% of study participants, and up to 7.4% when additional risk factors beyond age were required. 62 , 63 , 64 A recent meta‐analysis found that new AF detection rate increased progressively with age from 0.34% for <60 years to 2.73% ≥ 85 years. 65 Importantly, the number of subjects needed to screen to discover AF meeting indications for anticoagulation was 1089 for subjects < 60 years but only 83 for ≥ 65 years.
While subclinical device‐detected AF is associated with heightened stroke risk, there is insufficient data and ongoing debate about the minimum duration which would be associated with heightened risk of stroke warranting anticoagulation therapy. One key study suggested that decisions to anticoagulate should not be based on a single cutoff but rather consideration of AF duration relative to CHA2DS2‐VASc score (i.e. AF ≥ 6 min/day if CHA2DS2‐VASc scores 3 or more or AF ≥ 23.5 hours/day if CHA2DS2‐VASc score is 2). 66
However, according to 2020 AF ESC Guidelines, ECG documentation is recommended to establish the diagnosis of AF. 67 When screening for AF, definite diagnosis in screen‐positive cases is established only after the physician reviews the single‐lead ECG recording of ≥30 s or 12‐lead ECG and confirms that it shows AF. Moreover, systematic ECG screening for those ≥75 years, or those at high risk of stroke should be considered, 67 thus emphasizing the relevance for screening in the older patient.
In addition to the role of ECG for arrhythmia detection, recent studies have shown promising results for detecting undiagnosed left ventricular dysfunction, hypertrophy, and ischaemic heart disease. 68
Heart failure
The prevalence of HF is ≥10% among those 70 years and older. It is associated with co‐morbidities, poor quality of life, high healthcare utilization, and increased mortality. 11 , 12 People living with HF may often be geographically separated from specialized healthcare providers, making symptom monitoring and disease control more difficult.
Telehealth programmes for HF patients at home have been suggested to have positive impacts on both mortality and morbidity. 69 However, adoption is limited by the fact that most often programmes require the patients' ability to use a computer, a tablet or a mobile phone together with other medical equipment. 70 The most recent ESC HF guideline 71 made a ‘may be considered’ recommendation for the use of home‐based telemonitoring, based on a meta‐analysis published in 2017. 72
Clinical guidelines and national organizations have recently recommended the integration of palliative care into standard HF care. 73 The Educate, Nurture, Advise, Before Life Ends (ENABLE) Comprehensive Heartcare For Patient and Caregivers (CHF‐PC) Study 74 has been designed to implement behavioural support in advanced HF patients. The study includes a series of 30 to 60 min, weekly telehealth coaching sessions with a nurse addressing palliative care topics. 74
Remote monitoring in HF patients may monitor symptom and activity levels, sleep disordered breathing, changes in heart rate (as a marker of autonomic activity), arrhythmia, and support dietary and medication adherence. Such monitoring can be achieved using stand‐alone equipment and/or wearables, or cardiac implantable electronic devices (if present). Such systems may enable remote adjustment of medication, and other earlier interventions to reduce the need for emergency department visits and unplanned HF‐related hospitalizations. If scalable, remote monitoring coupled with mobile communication may reduce costs associated with HF, although the evidence to date is not conclusive. 75 , 76 , 77 , 78 Careful patient selection, more rapid and locally‐integrated responses to evidence of deterioration, and reimbursement support are likely success factors. 78
Patients with an implantable cardiac device such as an implantable cardioverter‐defibrillator (ICD) or cardiac resynchronization therapy (CRT) require regular checks to monitor device performance, battery longevity and detection of arrhythmia, but most modern devices can wirelessly connect with home monitors that transmit relevant data and alerts, allowing a device check to be performed remotely. 79 Home monitoring is safe and effective for routine device checks, with earlier detection of arrhythmia and technical issues. 80 Centres using home monitoring of implanted devices have reported reduced face‐to‐face contact. 81 Such devices can also collect physiological data that may correlate with HF status. Multiparametric monitoring, incorporating intrathoracic impedance with other variables such as heart rate, heart rate variability, physical activity and heart sounds, has shown potential, but requires consideration of the workflow issues such as what actions should be taken in response to ‘alerts’ being raised, and the need to persuade patients to change therapy (or be more adherent to lifestyle and drug therapies) despite them feeling well. 82
Implantable haemodynamic monitors have shown promise at preventing HF hospitalization. Pulmonary artery pressure (PAP) increases in response to increasing intracardiac pressure or fluid volume, with rises in pressure typically preceding symptoms by some weeks. 83 A randomized trial showed that remote daily PAP monitoring, (via a CardioMEMSdevice;Abbott) and titration of medications in response to rises in pressure, reduced subsequent HF hospitalization by 30% in NYHA Class III patients who had been admitted for HF in the previous year. 84 Data from NYHA Class III patients outside the US confirm this benefit. 85 , 86 The GUIDE HF study recently reported benefit in a pre‐specified pre‐COVID‐19 subgroup analysis of a broader spectrum of symptomatic severity 87 leading to FDA support of the use of CardioMEMS in patients with HF and a recent hospitalization or raised natriuretic peptides.
ESC HF guidelines make a recommendation only for CardioMEMS and as only a ‘may be considered’ Class 2 B level of evidence. 71
Digital tools able to prevent and manage frailty
DH tools and mApps have been developed to assess and monitor frailty status in patients with CV disease, and are being studied to target therapeutically the various components of frailty. 11 Certain mApps have integrated assessments of frailty alongside CV risk scores to provide global estimates of post‐procedural risk or poor outcomes. One example is the Frailty Tool mApp (frailtytool.com) that integrates the Essential Frailty Toolset (EFT) alongside the Society of Thoracic Surgeons risk score to guide decision making in older adults referred for transcatheter aortic valve replacement (TAVR) or cardiac surgery. In addition to prognostic value, 88 , 89 therapeutic value has been demonstrated by de‐frailing patients with high EFT score using prehabilitation and multicomponent geriatric intervention. 90 , 91
In daily clinical practice, general cardiologists are increasingly using DH tools for the management of deficits associated with older‐age (Figure 2 ). The progression of health decline and frailty may be usefully opposed by DH‐supported management of medication adherence, physical and nutritional needs, and the specific requirements after acute events. Moreover DH assistance may help to enhance the collaboration with family caregivers and health personnel who are supporting older adults living at home (Figure 2 ).
Because major cognitive impairment or poor social support often limit the ambulatory access to an outpatient clinic, DH‐based management focused on the physical and medical domains deficits may be particularly relevant to frail patients with CV disease who are seen in office‐based practice. 11
Drug adherence and persistence
Poor adherence to medical therapy is frequent in older patients 92 , 93 resulting in poorer clinical outcomes and increased healthcare costs 94 ; forgetfulness, communication barriers, socio‐economic factors, and lack of motivation represent the main causes of poor adherence. Interventions to assess and improve medication adherence within a home setting based on mHealth techniques have been investigated. The assessment of drug adherence may rely on self‐report methods, visual confirmation by smartphone, digital pills dispenser or a Quick Response code. 95 , 96 , 97
Drug adherence may be improved by two broad categories of strategies: behavioural (e.g. ‘smart’ pill boxes, follow‐up telephone calls, SMSs, and Apps) and educational (e.g. web‐based e‐learning). A systematic review including 10 trials reported that mHealth interventions improved medication adherence in CV patients, although the magnitude of benefit was not consistently large. 98 A recent review, evaluating drug adherence in older adults identified 50 studies (14 269 participants) comparing interventions versus usual care. 99 Behavioural‐only (RR 1.22, 95% CI 1.07 to 1.38) and mixed interventions (RR 1.22, 95% CI 1.08 to 1.37) may increase adherence, while educational‐only interventions (SMD 0.16, 95% CI −0.12 to 0.43) may have little or no impact. Globally, the quality of evidence is low, due to heterogeneity and methodological limitations of studies included in the review. Further studies are required.
Greater progress is expected with better co‐designed smartphone mApps that take into account age‐related factors that limit optimal use by elderly and/or frail patients, with more optimal user friendliness obtained with appropriate levels of training and support. 100 , 101 Voice and visual interfaces could be useful by recognizing vocal biomarkers of change in neurological or mental health status. 4 , 102 Potential concerns on privacy and security regarding medications may be overcome for instance by using biometrics during authentication. 103 , 104 A patient‐centred approach is encouraged to assist patients construct their own individualized adherence strategies. 105 Machine learning and artificial intelligence may help in personalized patients experiences, taking into account socio‐economic, cultural and personal characteristics. 106 Current evidence suggests that DH tools can improve medication adherence in older patients with, or at risk of, CV disease.
Nutrition
Malnutrition is one of the important determinants of physical frailty and sarcopenia—defined as the progressive loss of muscle mass and strength associated with ageing—and an actionable target for its improvement. Several studies have demonstrated the high prevalence and negative impact of malnutrition in older adults with CV diseases. 11 , 107 , 108 , 109 , 110 Most mApps for multidomain assessment of frailty include malnutrition screening 11 , 111 and many DH technologies have been employed to identify hospital malnutrition. 112
Nutritional therapy should be individualized for each patient according to their needs and consider both food sources and pharmacological supplements such as vitamin D and calcium. 107 A systematic review on nutrition in older adults reported a negative association between lower intake of specific micronutrients and frailty, and a protective association between higher intake of protein and dietary antioxidant and frailty. 108
Calculation of energy, protein, micronutrient needs should be performed in consultation with qualified nutritionists in line with the appropriate guidelines, while taking into account the specific requirements of the older adult given their relevant co‐morbidities such as cancer, gastroenterologic, neurologic, and renal diseases. 113
Telemedicine can be used in the monitoring of patients with parenteral nutrition at home, even though there is limited literature that has focussed in this space. 114 , 115 Elderly and frail patients must have access to nutritional care as a part of primary and secondary healthcare services. 115 Recently, Krznaric and colleagues proposed a simple remote nutritional screening tool and practical guidance for nutritional care in primary care, along with their implementation into telemedicine processes and digital platforms suitable for healthcare providers. 116 The intervention consisted of practical guidance on nutritional interventions for family physicians after identification of nutritional risk and loss of muscle mass and function by validated tools.
Movement and fitness
Physical frailty is characterized by diminished strength and endurance. 11 , 107 Early detection of health transitions towards a frail condition is often challenging, particularly in the pre‐frailty state where changes may be subtle. Screening to diagnose frailty syndrome in older adults with subtle or no overt clinical manifestations of frailty can be achieved by employing DH technologies that incorporate physical performance‐based screening, such as gait speed and assessments of gait, ‘sit‐to‐stand’ tests, grip strength, or using recently developed Apps to quickly perform a multidomain screening for frailty. 11 , 117 Digital biomarkers when applied to the identification of the frailty phenotype are objective, quantifiable, physiological and behavioural data that are collected and measured by means of digital devices such as sensors or wearables, enabling remote data collection and processing of large amounts of real‐life, continuous and long‐term health‐related data. 118 Examples of such digital biomarkers include waist‐worn accelerometer sensors that allow digital monitoring of walking speed—they have been shown to be able to accurately measure continuous gait speed in frail, older patients. 119 Wrist‐worn sensor‐derived frailty indices have also been validated in comparison with other established measures of frailty such as gait, timed ‘up and go’ and ‘sit‐to‐stand’ assessments. 120 Sit‐to‐stand tests can be undertaken remotely through measures of hip and knee angular velocity range, weakness, and exhaustion (coefficient of variation of angular velocity range of hip and knee, and vertical power range) from sensors attached to the trunk and thighs thereby providing remote assessment of this traditional measure of physical frailty. 121
Other sensor based digital solutions provide data to populate risk scores for frailty. For example, wrist‐worn fitness trackers used before TAVR have been used to develop a Fitness‐tracker assisted Frailty‐Assessment Score (FIFA score) that has greater predictive performance for in‐hospital mortality compared with that of the 6‐minute walk test and the Edmonton Frail Scale classification. 122
Physical support and rehabilitation
Telerehabilitation is the supervision and performance of comprehensive cardiac rehabilitation at a distance, typically including video‐consulting, tele‐monitoring, tele‐assessment (active remote assessment), tele‐support (supportive tele‐visits by nurses, psychological support), tele‐therapy (actual interactive therapy), tele‐coaching (support and instruction for therapy), and teleconsulting and tele‐supervision of exercise training. 25 , 26 , 123
Home‐based tele‐rehabilitation has been demonstrated to be safe and effective, with high adherence among people living with HF. It improves physical and psychological status, 6‐minute walk distance, and Quality of Life. 123 , 124 , 125 The recent Scientific Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology highlights that home‐based rehabilitation using telemedicine is a promising new service model. 126
Moreover, such technologies may provide support focused on nutrition and fitness before scheduled procedures, and DH technology have been used after interventional TAVR procedures and to guide rehabilitation after lower limbs revascularization. 11 , 127 , 128
Home stay and interaction with health personnel
The collaboration between caregivers (family members, nurses, and health personnel for home support) and medical personnel is central in the management of older people who have lost some degree of independence but are still living at home. DH technology has the potential to improve the connection and exchange of medical information.
Older adults are considered to be at the highest risk for poor communication with healthcare providers. 129 This is due to the presence of a multiple co‐morbidities, poly‐pharmacy, functional impairment, affective disorders, cognitive decline and sensory impairment. 130 Compared with younger patients, older adults in general are frequently less proactive and ask fewer and less in‐depth questions which may result in poor memory retention of medical information. 131 This is problematic because adequate information recall positively contributes to patient treatment adherence, disease management, quality of life and health outcomes. 129 , 132 , 133 To increase the likelihood that health information will be understood, processed and applied by older adults, it is critical to provide instructions in a variety of ways. 129 Both interpersonal communication (e.g. patient‐provider communication through video consulting) and digital communication (e.g. mHealth Apps) provide opportunities to improve information processing, self‐management and health outcomes in older adults. One important reason to use interpersonal communication during consultations with health communication technologies is their synergistic effect. 134 The combination of multiple communication media exceeds the sum of their individual effects. 135 Moreover, use of online interventions among older adults can be associated with increased social activity, decreased loneliness, increased perceived social support, improved self‐competence, and enhanced wellbeing. 136 , 137 As people with frailty have distinct informational, social and health‐management needs, they might derive unique benefit from accessing relevant health information and from interacting with others with similar health issues through online group interventions or through social media. 138
Limitations faced by older adults in using digital health technology
Although the familiarity of older adults with technology is increasing, barriers to wider adoption include very old age, lower disposable income, and higher co‐morbidity. 139 , 140 , 141 Although patients with cognitive impairment and high degree of frailty are probably less likely to direct benefit from DH approaches to disease management and care, DH technology may provide support to their caregivers. Whilst it has been shown that older adults are less likely to use new technology compared with younger adults, there is ample evidence that they also desire interaction with new technologies to remain active and engaged with society. 24 Several challenges that older adults face when adopting digital technology include the poor confidence in their ability to learn and use technology devices, in part because of their perception that the technology is too complicated, and physical or functional barriers in using technology devices not designed with their needs in mind. 24 , 139 For example, touchscreen devices may be challenging in the case of visual impairment or when hearing defects may impair verbal tele‐communications. Larger font sizes, bigger icons, magnification and volume amplification or earphones might be helpful in this population. Furthermore, elderly patients with cognitive decline may struggle to use technology that requires active interaction rather than more passive monitoring functionality such as the wearing of a sensor or a smart watch. While DH developments may increase access to care for older adults we should be aware of the need to avoid increasing inequality by ageism or geographical or socioeconomic biases. 142 Co‐designing the new technical supports taking into account the specific ageing‐associated needs of those who will use the technology would appear essential.
Conclusions
DH is changing daily practice in general cardiology. Although recent results from an ESC survey developed to assess the knowledge of cardiologists about DH technologies showed interest from cardiologists, the experience of (and knowledge about) DH tools were lower in ‘general’ office‐based cardiologists compared with hospital‐based cardiologist. 143 DH technology has potential to be useful for every field of cardiology, but notably in an office‐based setting with frequent contact with ambulatory older adults who may be pre‐frail or frail but who are still able to live at home.
DH technology may enhance the characterization of older adults' health status and increase the personalisation of clinical follow‐up, and help in the prevention and the general management of frailty, while supporting specific age‐related CV diseases with dedicated tools. However health personnel should not consider DH as a replacement for face‐to‐face clinic visits, but rather as an additional tool to help support better outcome and experience of care. To fully benefit from the potential of DH, cardiologists and other healthcare professionals should increase their DH skills and learn how best to apply and integrate new technologies. The ESC actively supports improved multi‐stakeholder interaction, co‐design and education in DH as a key element of its mission to reduce the burden of CV disease.
Conflict of interest
L Guasti, P Dilaveris, MA Mamas, D Richter, R Christodorescu, J Lumens, M J Schuuring, S Carugo, J Afilalo, M Ferrini, R Asteggiano, and MR Cowie declare no conflict of interest and declare that the submitted work is original and has not been published before (neither in English nor in any other language) and that the work is not under consideration for publication elsewhere.
Guasti, L. , Dilaveris, P. , Mamas, M. A. , Richter, D. , Christodorescu, R. , Lumens, J. , Schuuring, M. J. , Carugo, S. , Afilalo, J. , Ferrini, M. , Asteggiano, R. , and Cowie, M. R. (2022) Digital health in older adults for the prevention and management of cardiovascular diseases and frailty. A clinical consensus statement from the ESC Council for Cardiology Practice/Taskforce on Geriatric Cardiology, the ESC Digital Health Committee and the ESC Working Group on e‐Cardiology . ESC Heart Failure, 9: 2808–2822. 10.1002/ehf2.14022.
References
- 1. Frederix I, Caiani EG, Dendale P, Anker S, Bax J, Böhm A, Cowie M, Crawford J, de Groot N, Dilaveris P, Hansen T, Koehler F, Krstačić G, Lambrinou E, Lancellotti P, Meier P, Neubeck L, Parati G, Piotrowicz E, Tubaro M, van der Velde E. ESC e‐cardiology working group position paper: Overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol. 2019; 26: 1166–1177. [DOI] [PubMed] [Google Scholar]
- 2. Lyles CR, Wachter RM, Sarkar U. Focusing on digital health equity. JAMA. 2021; 326: 1795–1796. [DOI] [PubMed] [Google Scholar]
- 3. Whitelaw S, Mamas MA, Topol E, Van Spall HGC. Applications of digital technology in COVID‐19 pandemic planning and response. Lancet Digit Health. 2020; 2: e435–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richter D, Guasti L, Koehler F, Squizzato A, Nistri S, Christodorescu R, Dievart F, Gaudio G, Asteggiano R, Ferrini M. Late phase of COVID‐19 pandemic in general cardiology. A position paper of the ESC Council for cardiology practice. ESC Heart Fail. 2021; 8: 3483–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuuring MJ, Kauw D, Bouma BJ. COVID‐19 pandemic: Practical considerations on rapid initiation of remote care in chronic cardiac patients. Eur Heart J Dig Health. 2020; 1: 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barsom EZ, Feenstra TM, Bemelman WA, Bonjer JH, Schijven MP. Coping with COVID‐19: Scaling up virtual care to standard practice. Nat Med. 2020; 26: 632–634. [DOI] [PubMed] [Google Scholar]
- 7. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019; 571: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization ; 2017. Decade of Healthy Ageing 2020–2030.
- 9. Fried LP, Rowe JW. Health in aging ‐ past, present, and future. N Engl J Med. 2020; 383: 1293–1296. [DOI] [PubMed] [Google Scholar]
- 10. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: Implications for clinical practice and public health. Lancet. 2019; 394: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 11. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, Savelieva I, Fumagalli S, Bo M, Rocca B, Jensen MT, Pierard L, Sudano I, Aboyans V, Asteggiano R. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), acute cardiovascular care association (ACCA), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European heart rhythm association (EHRA), council on Valvular heart diseases (VHD), council on hypertension (CHT), Council of Cardio‐Oncology (CCO), working group (WG) aorta and peripheral vascular diseases, WG e‐cardiology, WG thrombosis, of the European Society of Cardiology, European primary care cardiology society (EPCCS). Eur J Prev Cardiol. 2021: zwaa167. [DOI] [PubMed] [Google Scholar]
- 12. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart failure association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 13. Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP, Geriatric Cardiology Section Leadership Council, American College of Cardiology . Domain management approach to heart failure in the geriatric patient: Present and future. J Am Coll Cardiol. 2018; 71: 1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community‐dwelling older people: A systematic review and meta‐analysis. Ageing Res Rev. 2019; 50: 81–88. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . WHO guideline: recommendations on digital interventions for health system strengthening. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 16. Sim I. Mobile devices and health. N Engl J Med. 2019; 381: 956–968. [DOI] [PubMed] [Google Scholar]
- 17. Redfern J. Can older adults benefit from Smart devices, wearables, and other digital health options to enhance cardiac rehabilitation? Clin Geriatr Med. 2019; 35: 489–497. [DOI] [PubMed] [Google Scholar]
- 18. Schulz R, Wahl HW, Matthews JT, De Vito DA, Beach SR, Czaja SJ. Advancing the aging and technology agenda in gerontology. Gerontologist. 2015; 55: 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mannheim I, Schwartz E, Xi W, Buttigieg SC, McDonnell‐Naughton M, Wouters EJM, van Zaalen Y. Inclusion of older adults in the research and Design of Digital Technology. Int J Environ Res Public Health. 2019; 16: 3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Hoof J, Kort HS, Rutten PG, Duijnstee MS. Ageing‐in‐place with the use of ambient intelligence technology: Perspectives of older users. Int J Med Inform. 2011; 80: 310–331. [DOI] [PubMed] [Google Scholar]
- 21. Greene BR, McManus K, Redmond SJ, Caulfield B, Quinn CC. Digital assessment of falls risk, frailty, and mobility impairment using wearable sensors. NPJ Digit Med. 2019; 2: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartholt KA, van Beeck EF, Polinder S, van der Velde N, van Lieshout EM, Panneman MJ, van der Cammen TJ, Patka P. Societal consequences of falls in the older population: Injuries, healthcare costs, and long‐term reduced quality of life. J Trauma. 2011; 71: 748–753. [DOI] [PubMed] [Google Scholar]
- 23. Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012; 2012: CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Czaja SJ, Charness N, Fisk AD, Hertzog C, Nair SN, Rogers WA, Sharit J. Factors predicting the use of technology: Findings from the Center for Research and Education on aging and technology enhancement (CREATE). Psychol Aging. 2006; 21: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review. Eur J Cardiovasc Prev Rehabil. 2009; 16: 281–289. [DOI] [PubMed] [Google Scholar]
- 26. Jin K, Khonsari S, Gallagher R, Gallagher P, Clark AM, Freedman B, Briffa T, Bauman A, Redfern J, Neubeck L. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta‐analysis. Eur J Cardiovasc Nurs. 2019; 18: 260–271. [DOI] [PubMed] [Google Scholar]
- 27. Milani RV, Lavie CJ, Ventura HO. New aspects in the management of hypertension in the digital era. Curr Opin Cardiol. 2021; 36: 398–404. [DOI] [PubMed] [Google Scholar]
- 28. Tsoi K, Yiu K, Lee H, Cheng HM, Wang TD, Tay JC, Teo BW, Turana Y, Soenarta AA, Sogunuru GP, Siddique S, Chia YC, Shin J, Chen CH, Wang JG, Kario K, HOPE Asia Network . Applications of artificial intelligence for hypertension management. J Clin Hypertens (Greenwich). 2021; 23: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowie MR, Lam CSP. Remote monitoring and digital health tools in CVD management. Nat Rev Cardiol. 2021; 18: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yatabe J, Yatabe MS, Ichihara A. The current state and future of internet technology‐based hypertension management in Japan. Hypertens Res. 2021; 44: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Padwal R, Wood PW. Digital health approaches for the assessment and optimisation of hypertension care provision. Can J Cardiol. 2021; 37: 711–721. [DOI] [PubMed] [Google Scholar]
- 32. Myers KD, Knowles JW, Staszak D, Shapiro MD, Howard W, Yadava M, Zuzick D, Williamson L, Shah NH, Banda JM, Leader J, Cromwell WC, Trautman E, Murray MF, Baum SJ, Myers S, Gidding SS, Wilemon K, Rader DJ. Precision screening for familial hypercholesterolaemia: A machine learning study applied to electronic health encounter data. Lancet Digit Health. 2019; 1: e393–e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnaswami A, Beavers C, Dorsch MP, Dodson JA, Masterson Creber R, Kitsiou S, Goyal P, Maurer MS, Wenger NK, Croy DS, Alexander KP, Batsis JA, Turakhia MP, Forman DE, Bernacki GM, Kirkpatrick JN, Orr NM, Peterson ED, Rich MW, Freeman AM, Bhavnani SP, Innovations, Cardiovascular Team and the Geriatric Cardiology Councils, American College of Cardiology . Gerotechnology for older adults with cardiovascular diseases: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020; 76: 2650–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kario K. Management of Hypertension in the digital era: Small wearable monitoring devices for remote blood pressure monitoring. Hypertension. 2020; 76: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Omboni S, McManus RJ, Bosworth HB, Chappell LC, Green BB, Kario K, Logan AG, Magid DJ, Mckinstry B, Margolis KL, Parati G, Wakefield BJ. Evidence and recommendations on the use of telemedicine for the Management of Arterial Hypertension: An international expert position paper. Hypertension. 2020; 76: 1368–1383. [DOI] [PubMed] [Google Scholar]
- 36. Kraef C, van der Meirschen M, Free C. Digital telemedicine interventions for patients with multimorbidity: A systematic review and meta‐analysis. BMJ Open. 2020; 10: e036904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brunetti ND, Molinari G, Acquistapace F, Zimotti T, Parati G, Indolfi C, Fedele F, Carugo S. 2019 Italian Society of Cardiology Census on telemedicine in cardiovascular disease: A report from the working group on telecardiology and informatics. Open Heart. 2020; 7: e001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu H, Long H. The effect of smartphone app‐based interventions for patients with hypertension: Systematic review and meta‐analysis. JMIR MhealthUhealth. 2020; 8: e21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McManus RJ, Little P, Stuart B, Morton K, Raftery J, Kelly J, Bradbury K, Zhang J, Zhu S, Murray E, May CR, Mair FS, Michie S, Smith P, Band R, Ogburn E, Allen J, Rice C, Nuttall J, Williams B, Yardley L, HOME BP investigators . Home and online management and evaluation of blood pressure (HOME BP) using a digital intervention in poorly controlled hypertension: Randomised controlled trial. BMJ. 2021; 372: m4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams B, Mancia G, Spiering W, AgabitiRosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I, List of authors/Task Force members . 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the Management of Arterial Hypertension. J Hypertens. 2018; 36: 2284–2309. [DOI] [PubMed] [Google Scholar]
- 41. Quisel T, Foschini L, Zbikowski SM, Juusola JL. The association between medication adherence for chronic conditions and digital health activity tracking: Retrospective analysis. J Med Internet Res. 2019; 21: e11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steinberg DM, Kay MC, Svetkey LP, Askew S, Christy J, Burroughs J, Ahmed H, Bennett GG. Feasibility of a digital health intervention to improve diet quality among women with high blood pressure: Randomized controlled feasibility trial. JMIR MhealthUhealth. 2020; 8: e17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennett GG, Steinberg D, Askew S, Levine E, Foley P, Batch BC, Svetkey LP, Bosworth HB, Puleo EM, Brewer A, DeVries A, Miranda H. Effectiveness of an app and provider counseling for obesity treatment in primary care. Am J Prev Med. 2018; 55: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu S, Feng W, Chhatbar PY, Liu Y, Ji X, Ovbiagele B. Mobile health as a viable strategy to enhance stroke risk factor control: A systematic review and meta‐analysis. J Neurol Sci. 2017; 378: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa‐Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J; 2020: 255–323. [DOI] [PubMed] [Google Scholar]
- 46. Chao TF, Leu HB, Huang CC, Chen JW, Chan WL, Lin SJ, Chen SA. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non‐insulin dependent diabetes. Int J Cardiol. 2012; 156: 199–202. [DOI] [PubMed] [Google Scholar]
- 47. Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: A population‐based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014; 13: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andrès E, Meyer L, Zulfiqar AA, Hajjam M, Talha S, Bahougne T, Ervé S, Hajjam J, Doucet J, Jeandidier N, Hajjam El Hassani A. Telemonitoring in diabetes: Evolution of concepts and technologies, with a focus on results of the more recent studies. J Med Life. 2019; 12: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garabedian LF, Ross‐Degnan D, Wharam JF. Mobile phone and smartphone Technologies for Diabetes Care and Self‐Management. Curr Diab Rep. 2015; 15: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fokkert M, van Dijk P, Edens M, Barents E, Mollema J, Slingerland R, Gans R, Bilo H. Improved well‐being and decreased disease burden after 1‐year use of flash glucose monitoring (FLARE‐NL4). BMJ Open Diabetes Res Care. 2019; 7: e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L. Diabetes digital app technology: Benefits, challenges, and recommendations. A consensus report by the European Association for the Study of diabetes (EASD) and the American Diabetes Association (ADA) diabetes technology working group. Diabetes Care. 2020; 43: 250–260. [DOI] [PubMed] [Google Scholar]
- 52. Agarwal P, Mukerji G, Desveaux L, Ivers NM, Bhattacharyya O, Hensel JM, Shaw J, Bouck Z, Jamieson T, Onabajo N, Cooper M, Marani H, Jeffs L, Bhatia RS. Mobile app for improved self‐Management of Type 2 diabetes: Multicenter pragmatic randomized controlled trial. JMIR MhealthUhealth. 2019; 7: e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pal K, Eastwood SV, Michie S, Farmer A, Barnard ML, Peacock R, Wood B, Edwards P, Murray E. Computer‐based interventions to improve self‐management in adults with type 2 diabetes: A systematic review and meta‐analysis. Diabetes Care. 2014; 37: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 54. Whaley CM, Bollyky JB, Lu W, Painter S, Schneider J, Zhao Z, He X, Johnson J, Meadows ES. Reduced medical spending associated with increased use of a remote diabetes management program and lower mean blood glucose values. J Med Econ. 2019; 22: 869–877. [DOI] [PubMed] [Google Scholar]
- 55. Jensen MT, Treskes RW, Caiani EG, Casado‐Arroyo R, Cowie MR, Dilaveris P, Duncker D, Di Rienzo M, Frederix I, De Groot N, Kolh PH, Kemps H, Mamas M, McGreavy P, Neubeck L, Parati G, Platonov PG, Schmidt‐Trucksäss A, Schuuring MJ, Simova I, Svennberg E, Verstrael A, Lumens J. ESC working group on e‐cardiology position paper: Use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention—In collaboration with the European heart rhythm association, European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professionals, patient forum, and the digital health committee. Eur Heart J Dig Health. 2021; 2: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Proietti M, Marra AM, Tassone EJ, De Vuono S, Corrao S, Gobbi P, Perticone F, Corazza GR, Basili S, Lip GY, Violi F, Raparelli V, ARAPACIS Study Investigators , GIS Group . Frequency of left ventricular hypertrophy in non‐Valvular atrial fibrillation. Am J Cardiol. 2015; 116: 877–882. [DOI] [PubMed] [Google Scholar]
- 57. Varma N, Cygankiewicz I, Turakhia M, Heidbuchel H, Hu Y, Chen LY, Couderc JP, Cronin EM, Estep JD, Grieten L, Lane DA, Mehra R, Page A, Passman R, Piccini J, Piotrowicz E, Piotrowicz R, Platonov PG, Ribeiro AL, Rich RE, Russo AM, Slotwiner D, Steinberg JS, Svennberg E. 2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in arrhythmia management: Digital medical tools for heart rhythm professionals: From the International Society for Holter and NoninvasiveElectrocardiology/Heart Rhythm Society/European heart rhythm association/Asia Pacific Heart Rhythm Society. Ann Noninvasive Electrocardiol. 2021; 26: e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH, Hanke T. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012; 126: 806–814. [DOI] [PubMed] [Google Scholar]
- 59. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP, Apple Heart Study Investigators . Large‐scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019; 381: 1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP, American Heart Association Council on Clinical Cardiology , Council on Cardiovascular and Stroke Nursing , Council on Quality of Care and Outcomes Research , Stroke Council . Atrial fibrillation burden: Moving beyond atrial fibrillation as a binary entity: A scientific statement from the American Heart Association. Circulation. 2018; 137: e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol. 2005; 28: S133–S136. [DOI] [PubMed] [Google Scholar]
- 62. Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75‐year‐old population: Implications for stroke prevention. Circulation. 2013; 127: 930–937. [DOI] [PubMed] [Google Scholar]
- 63. Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al‐Khalili F, Friberg L, Frykman V, Hijazi Z, Rosenqvist M, Engdahl J. Stepwise mass screening for atrial fibrillation using N‐terminal B‐type natriuretic peptide: The STROKESTOP II study. Europace. 2020; 22: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svennberg E, Engdahl J, Al‐Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation. 2015; 131: 2176–2184. [DOI] [PubMed] [Google Scholar]
- 65. Lowres N, Olivier J, Chao TF, Chen SA, Chen Y, Diederichsen A, Fitzmaurice DA, Gomez‐Doblas JJ, Harbison J, Healey JS, Hobbs FDR, Kaasenbrood F, Keen W, Lee VW, Lindholt JS, Lip GYH, Mairesse GH, Mant J, Martin JW, Martín‐Rioboó E, McManus DD, Muñiz J, Münzel T, Nakamya J, Neubeck L, Orchard JJ, Pérula de Torres LÁ, Proietti M, Quinn FR, Roalfe AK, Sandhu RK, Schnabel RB, Smyth B, Soni A, Tieleman R, Wang J, Wild PS, Yan BP, Freedman B. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: A multi country patient‐level meta‐analysis of 141,220 screened individuals. PLoS Med. 2019; 16: e1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2‐VASc score. Circulation. 2019; 140: 1639–1646. [DOI] [PubMed] [Google Scholar]
- 67. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J; 2021: 373–498. [DOI] [PubMed] [Google Scholar]
- 68. Al Hinai G, Jammoul S, Vajihi Z, Afilalo J. Deep learning analysis of resting electrocardiograms for the detection of myocardial dysfunction, hypertrophy, and ischaemia: A systematic review. Eur Heart J Digit Health. 2021; 2: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Inglis SC, Clark RA, Dierckx R, Prieto‐Merino D, Cleland JG. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015; 2015: CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lind L, Karlsson D. Telehealth for "the digital illiterate"‐‐elderly heart failure patients experiences. Stud Health Technol Inform. 2014; 205: 353–357. [PubMed] [Google Scholar]
- 71. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J; 2021: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 72. Dierckx R, Inglis SC, Clark RA, Prieto‐Merino D, Cleland JG. Telemedicine in heart failure: New insights from the Cochrane meta‐analyses. Eur J Heart Fail. 2017; 19: 304–306. [DOI] [PubMed] [Google Scholar]
- 73. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 74. Akyar I, Dionne‐Odom JN, Bakitas MA. Using patients and their caregivers feedback to develop ENABLE CHF‐PC: An early palliative care intervention for advanced heart failure. J Palliat Care. 2019; 34: 103–110. [DOI] [PubMed] [Google Scholar]
- 75. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP, TEHAF investigators . Tailored telemonitoring in patients with heart failure: Results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012; 14: 791–801. [DOI] [PubMed] [Google Scholar]
- 77. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC, Better Effectiveness After Transition–Heart Failure (BEAT‐HF) Research Group . Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: The better effectiveness after transition ‐‐ heart failure (BEAT‐HF) randomized clinical trial. JAMA Intern Med. 2016; 176: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): A randomised, controlled, parallel‐group, unmasked trial. Lancet. 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 79. Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE, Heart Rhythm Society , European Heart Rhythm Association , American College of Cardiology , American Heart Association , European Society of Cardiology , Heart Failure Association of ESC , Heart Failure Society of America . HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008; 5: 907–925. [DOI] [PubMed] [Google Scholar]
- 80. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, TRUST Investigators . Efficacy and safety of automatic remote monitoring for implantable cardioverter‐defibrillator follow‐up: The Lumos‐T safely reduces routine office device follow‐up (TRUST) trial. Circulation. 2010; 122: 325–332. [DOI] [PubMed] [Google Scholar]
- 81. Hernández‐Madrid A, Lewalter T, Proclemer A, Pison L, Lip GY, Blomstrom‐Lundqvist C, Scientific Initiatives Committee , European Heart Rhythm Association . Remote monitoring of cardiac implantable electronic devices in Europe: Results of the European heart rhythm association survey. Europace. 2014; 16: 129–132. [DOI] [PubMed] [Google Scholar]
- 82. Singhal A, Cowie MR. Digital health: Implications for heart failure management. Card Fail Rev. 2021; 7: e08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, Stromberg K, Wilkoff BL, Costanzo MR, Luby A, Aranda JM, Heywood JT, Baldwin HA, Aaron M, Smith A, Zile M. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: Results of the reducing decompensation events utilizing Intracardiac pressures in patients with chronic heart failure (REDUCEhf) trial. Congest Heart Fail. 2011; 17: 248–254. [DOI] [PubMed] [Google Scholar]
- 84. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 85. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European monitoring study for heart failure (MEMS‐HF). Eur J Heart Fail. 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 86. Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, Critoph C, Gardner RS, Guha K, Betts TR, Carr‐White G, Zaidi A, Lim HS, Hayward C, Patwala A, Rogers D, Pettit S, Gazzola C, Henderson J, Adamson PB. Real‐world evidence in a national health service: Results of the UK CardioMEMS HF system post‐market study. ESC Heart Fail. 2022; 9: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB, Costanzo MR. Haemodynamic‐guided management of heart failure (GUIDE‐HF): A randomised controlled trial. Lancet. 2021; 398: 991–1001. [DOI] [PubMed] [Google Scholar]
- 88. Solomon J, Moss E, Morin JF, Langlois Y, Cecere R, de Varennes B, Lachapelle K, Piazza N, Martucci G, Bendayan M, Piankova P, Hayman V, Ouimet MC, Rudski LG, Afilalo J. The essential frailty toolset in older adults undergoing coronary artery bypass surgery. J Am Heart Assoc. 2021; 10: e020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY‐AVR Study. J Am Coll Cardiol. 2017; 70: 689–700. [DOI] [PubMed] [Google Scholar]
- 90. Tumscitz C, Di Cesare A, Balducelli M, Piva T, Santarelli A, Saia F, Tarantino F, Preti G, Picchi A, Rolfo C, Attisano T, Colonna G, De Iaco G, Parodi G, Di Marco M, Cerrato E, Pierini S, Fileti L, Cavazza C, Dall'Ara G, Govoni B, Mantovani G, Serenelli M, Penzo C, Tebaldi M, Campo G, Biscaglia S. Safety, efficacy and impact on frailty of mini‐invasive radial balloon aortic valvuloplasty. Heart. 2021; 107: 874–880. [DOI] [PubMed] [Google Scholar]
- 91. Fountotos R, Munir H, Goldfarb M, Lauck S, Kim D, Perrault L, Arora R, Moss E, Rudski LG, Bendayan M, Piankova P, Hayman V, Rodighiero J, Ouimet MC, Lantagne S, Piazza N, Afilalo J. Prognostic value of handgrip strength in older adults undergoing cardiac surgery. Can J Cardiol. 2021: S0828‐282X(21)00661‐9. [DOI] [PubMed] [Google Scholar]
- 92. Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication acquisition and self‐reported adherence in veterans with hypertension. Med Care. 2009; 47: 474–481. [DOI] [PubMed] [Google Scholar]
- 93. Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko‐Slack L, Maxwell CJ. Medication nonadherence and subsequent risk of hospitalization and mortality among older adults. Drugs Aging. 2006; 23: 345–356. [DOI] [PubMed] [Google Scholar]
- 94. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: Its importance in cardiovascular outcomes. Circulation. 2009; 119: 3028–3035. [DOI] [PubMed] [Google Scholar]
- 95. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017; 48: 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. DiCarlo L, Moon G, Intondi A, Duck R, Frank J, Hafazi H, Behzadi Y, Robertson T, Costello B, Savage G, Zdeblick M. A digital health solution for using and managing medications: Wirelessly observed therapy. IEEE Pulse. 2012; 3: 23–26. [DOI] [PubMed] [Google Scholar]
- 97. Capranzano P, Francaviglia B, Sardone A, Agnello F, Valenti N, Frazzetto M, Legnazzi M, Occhipinti G, Scalia L, Calvi V, Capodanno D, Tamburino C. Suitability for elderly with heart disease of a QR code‐based feedback of drug intake: Overcoming limitations of current medication adherence telemonitoring systems. Int J Cardiol. 2021; 327: 209–216. [DOI] [PubMed] [Google Scholar]
- 98. Gandapur Y, Kianoush S, Kelli HM, Misra S, Urrea B, Blaha MJ, Graham G, Marvel FA, Martin SS. The role of mHealth for improving medication adherence in patientswith cardiovascular disease: A systematic review. Eur Heart J. 2016; 2: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cross AJ, Elliott RA, Petrie K, Kuruvilla L, George J. Interventions for improving medication‐taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020; 2020: CD012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Russell AM, Smith SG, Bailey SC, Belter LT, Pandit AU, Hedlund LA, Bojarski EA, Rush SR, Wolf MS. Older adult preferences of Mobile application functionality supporting medication self‐management. J Health Commun. 2018; 23: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 101. Nahm ES, Son H. Older Adults' use of patient portals: Experiences, challenges, and suggestions shared through discussion board forums. Geriatr Nurs. 2020; 41: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Evangelista L, Steinhubl SR, Topol EJ. Digital health care for older adults. Lancet. 2019; 393: 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Park LG, Ng F, Shim JK, Elnaggar A, Villero O. Perceptions and experiences of using mobile technology for medication adherence among older adults with coronary heart disease: A qualitative study. Digit Health. 2020; 6: 2055207620926844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wildenbos GA, Maasri K, Jaspers M, Peute L. Older adults using a patient portal: Registration and experiences, one year after implementation. Digit Health. 2018; 4: 2055207618797883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hincapie AL, Gupta V, Brown SA, Metzger AH. Exploring perceived barriers to medication adherence and the use of mobile technology in underserved patients with chronic conditions. J Pharm Pract. 2019; 32: 147–153. [DOI] [PubMed] [Google Scholar]
- 106. Treskes RW, Van der Velde ET, Schoones JW, Schalij MJ. Implementation of smart technology to improve medication adherence in patients with cardiovascular disease: Is it effective? Expert Rev Med Devices. 2018; 15: 119–126. [DOI] [PubMed] [Google Scholar]
- 107. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013; 14: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lorenzo‐López L, Maseda A, de Labra C, Regueiro‐Folgueira L, Rodríguez‐Villamil JL, Millán‐Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017; 17: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morante JJH, Martínez CG, Morillas‐Ruiz JM. Dietary factors associated with frailty in old adults: A review of nutritional interventions to prevent frailty development. Nutrients. 2019; 11: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, Volkert D. Dietary quality is related to frailty in community‐dwelling older adults. J Gerontol Ser A Biol Sci Med Sci. 2013; 68: 483–489. [DOI] [PubMed] [Google Scholar]
- 111. Piankova P, Afilalo J. Prevalence and prognostic implications of frailty in Transcatheter aortic valve replacement. Cardiol Clin. 2020; 38: 75–87. [DOI] [PubMed] [Google Scholar]
- 112. Trtovac D, Lee J. The use of Technology in Identifying Hospital Malnutrition: Scoping review. JMIR Med Inform. 2018; 6: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: Effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015; 2015: CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Saqui O, Chang A, McGonigle S, Purdy B, Fairholm L, Baun M. Telehealth videoconferencing: Improving home parenteral nutrition patient care to rural areas of Ontario, Canada. J Parenter Enteral Nutr. 2007; 31: 234–239. [DOI] [PubMed] [Google Scholar]
- 115. Nelson EL, Yadrich DM, Thompson N, Wright S, Stone K, Adams N. Telemedicine support groups for home parenteral nutrition users. Nutr Clin Pract. 2017; 32: 789–798. [DOI] [PubMed] [Google Scholar]
- 116. Krznarić Ž, Bender DV, Laviano A, Cuerda C, Landi F, Monteiro R, Pirlich M, Barazzoni R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID‐19 pandemic. Clin Nutr. 2020; 39: 1983–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006; 54: 1674–1681. [DOI] [PubMed] [Google Scholar]
- 118. Piau A, Steinmeyer Z, Cesari M, Kornfeld J, Beattie Z, Kaye J, Vellas B, Nourhashemi F. Intrinsic Capacitiy monitoring by digital biomarkers in integrated Care for Older People (ICOPE). J Frailty Aging. 2021; 10: 132–138. [DOI] [PubMed] [Google Scholar]
- 119. Mueller A, Hoefling HA, Muaremi A, Praestgaard J, Walsh LC, Bunte O, Huber RM, Fürmetz J, Keppler AM, Schieker M, Böcker W, Roubenoff R, Brachat S, Rooks DS, Clay I. Continuous digital monitoring of walking speed in frail elderly patients: Noninterventional validation study and longitudinal clinical trial. JMIR MhealthUhealth. 2019; 7: e15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kang GE, Naik AD, Ghanta RK, Rosengart TK, Najafi B. A wrist‐worn sensor‐derived frailty index based on an upper‐extremity functional test in predicting functional mobility in older adults. Gerontology. 2021; 67: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Park C, Sharafkhaneh A, Bryant MS, Nguyen C, Torres I, Najafi B. Toward remote assessment of physical frailty using sensor‐based sit‐to‐stand test. J Surg Res. 2021; 263: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mach M, Watzal V, Hasan W, Andreas M, Winkler B, Weiss G, Strouhal A, Adlbrecht C, Delle Karth G, Grabenwöger M. Fitness‐tracker assisted frailty‐assessment before Transcatheter aortic valve implantation: Proof‐of‐concept study. JMIR MhealthUhealth. 2020; 8: e19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Piotrowicz E, Piepoli MF, Jaarsma T, Lambrinou E, Coats AJ, Schmid JP, Corrà U, Agostoni P, Dickstein K, Seferović PM, Adamopoulos S, Ponikowski PP. Telerehabilitation in heart failure patients: The evidence and the pitfalls. Int J Cardiol. 2016; 220: 408–413. [DOI] [PubMed] [Google Scholar]
- 124. Piotrowicz E, Stepnowska M, Leszczyńska‐Iwanicka K, Piotrowska D, Kowalska M, Tylka J, Piotrowski W, Piotrowicz R. Quality of life in heart failure patients undergoing home‐based telerehabilitation versus outpatient rehabilitation—A randomized controlled study. Eur J Cardiovasc Nurs. 2015; 14: 256–263. [DOI] [PubMed] [Google Scholar]
- 125. Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, Orzechowski P, Szalewska D, Pluta S, Glówczynska R, Irzmanski R, Oreziak A, Kalarus Z, Lewicka E, Cacko A, Mierzynska A, Piotrowicz R. Effects of a 9‐week hybrid comprehensive Telerehabilitation program on Long‐term outcomes in patients with heart failure: The Telerehabilitation in heart failure patients (TELEREH‐HF) randomized clinical trial. JAMA Cardiol. 2020; 5: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, Sanderson BK, Whooley MA. Home‐based cardiac rehabilitation: A scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019; 140: e69–e89. [DOI] [PubMed] [Google Scholar]
- 127. Lindman BR, Gillam LD, Coylewright M, Welt FGP, Elmariah S, Smith SA, McKeel DA, Jackson N, Mukerjee K, Cloud H, Hanna N, Purpura J, Ellis H, Martinez V, Selberg AM, Huang S, Harrell FE Jr. Effect of a pragmatic home‐based mobile health exercise intervention after transcatheter aortic valve replacement: A randomized pilot trial. Eur Heart J Digit Health. 2021; 2: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Najafi B, Veranyan N, Zulbaran‐Rojas A, Park C, Nguyen H, Nakahara QK, Elizondo‐Adamchik H, Chung J, Mills JL, Montero‐Baker M, Armstrong DG, Rowe V. Association between wearable device‐based measures of physical frailty and major adverse events following lower extremity revascularization. JAMA Netw Open. 2020; 3: e2020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sparks L, Nussbaum JF. Health literacy and cancer communication with older adults. Patient Educ Couns. 2008; 71: 345–350. [DOI] [PubMed] [Google Scholar]
- 130. van Weert JCM. Facing frailty by effective digital and patient‐provider communication? Patient Educ Couns. 2020; 103: 433–435. [DOI] [PubMed] [Google Scholar]
- 131. Jansen J, van Weert J, van der Meulen N, van Dulmen S, Heeren T, Bensing J. Recall in older cancer patients: Measuring memory for medical information. Gerontologist. 2008; 48: 149–157. [DOI] [PubMed] [Google Scholar]
- 132. McGuire LC. Remembering what the doctor said: Organization and adults' memory for medical information. Exp Aging Res. 1996; 22: 403–428. [DOI] [PubMed] [Google Scholar]
- 133. Flocke SA, Stange KC. Direct observation and patient recall of health behavior advice. Prev Med. 2004; 38: 343–349. [DOI] [PubMed] [Google Scholar]
- 134. Kreps GL. Online information and communication systems to enhance health outcomes through communication convergence. Hum Commun Res. 2017; 43: 518–530. [Google Scholar]
- 135. Nguyen MH, Smets EM, Bol N, Loos EF, van Laarhoven HW, Geijsen D, van Berge Henegouwen MI, Tytgat KM, van Weert JC. Tailored web‐based information for younger and older patients with cancer: Randomized controlled trial of a preparatory educational intervention on patient outcomes. J Med Internet Res. 2019; 21: e14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Czaja SJ, Boot WR, Charness N, Rogers WA, Sharit J. Improving social support for older adults through technology: Findings from the PRISM randomized controlled trial. Gerontologist. 2018; 58: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Morton TA, Wilson N, Haslam C, Birney M, Kingston R, McCloskey LG. Activating and guiding the engagement of seniors with online social networking: Experimental findings from the AGES 2.0 project. J Aging Health. 2018; 30: 27–51. [DOI] [PubMed] [Google Scholar]
- 138. Chen AT, Chu F, Teng AK, Han S, Lin SY, Demiris G, Zaslavsky O. Promoting problem solving about health management: A mixed‐methods pilot evaluation of a digital health intervention for older adults with pre‐frailty and frailty. Gerontol Geriatr Med. 2021; 7: 2333721420985684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vaportzis E, Clausen MG, Gow AJ. Older adults perceptions of technology and barriers to interacting with tablet computers: A focus group study. Front Psychol. 2017; 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shahrokni A, Loh KP, Wood WA. Toward modernization of geriatric oncology by digital health technologies. Am Soc Clin Oncol Educ Book. 2020; 40: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rampioni M, Moșoi AA, Rossi L, Moraru SA, Rosenberg D, Stara V. A qualitative study toward Technologies for Active and Healthy Aging: A thematic analysis of perspectives among primary, secondary, and tertiary end users. Int J Environ Res Public Health. 2021; 18: 7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kilaru AS, Gee RE. Structural ageism and the health of older adults. JAMA Health Forum. 2020; 1: e201249. [DOI] [PubMed] [Google Scholar]
- 143. Asteggiano R, Cowie MR, Richter D, Christodorescu R, Guasti L, Ferrini M. Survey on e‐health knowledge and usage in general cardiology of the Council of Cardiology Practice and the digital health committee. Eur Heart J Digit Health. 2021; 2: ztab032. [DOI] [PMC free article] [PubMed] [Google Scholar]


