Abstract
Objectives
Scutellaria baicalensis (SB) has been traditionally used to combat a variety of conditions ranging from ischemic heart disease to cancer. The protective effects of SB are due to the action of two main flavonoids baicalin (BA) and baicalein (BE). This paper aimed to provide a narrative review of the protective and antidotal effects of SB and its main constituents against natural toxicities and physical hazards.
Evidence acquisition
Scientific databases Medline, Scopus, and Web of Science were thoroughly searched, based on different keywords for in vivo, in vitro and clinical studies which reported protective or therapeutic effects of SB or its constituents in natural and physical toxicities.
Results
Numerous studies have reported that treatment with BE, BA, or total SB extract prevents or counteracts the detrimental toxic effects of various natural compounds and physical hazards. The toxic agents include mycotoxins, lipopolysaccharide, multiple plants and animal-derived substances as well as physical factors which negatively affected vital organs such as CNS, liver, kidneys, lung and heart. Increasing the expression of radical scavenging enzymes and glutathione content as well as inhibition of pro-inflammatory cytokines and pro-apoptotic mediators were important mechanisms of action.
Conclusion
Different studies on the Chinese skullcap have exhibited that its total root extract, BA or BE can act as potential antidotes or protective agents against the damage induced by natural toxins and physical factors by alleviating oxidative stress and inflammation. However, the scarcity of high-quality clinical evidence means that further clinical studies are required to reach a more definitive conclusion.
Graphical abstract
Keywords: Scutellaria baicalensis, Baicalin, Baicalein, Antidote, Natural toxin, Physical hazards
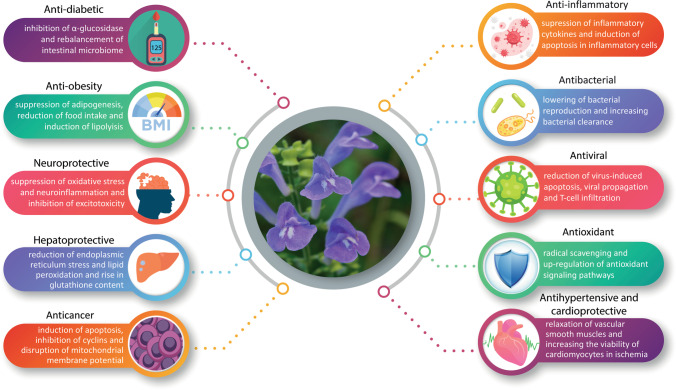
Introduction
Baicalin (BA), a flavone glycoside, and its aglycone form, baicalein (BE) (Fig. 1), are two compounds abundantly found in the root and leaves of Scutellaria baicalensis (SB). Also known as Baikal or Chinese skullcap, SB is a plant that has a long tradition of being used for different purposes in traditional Chinese medicine (TCM) [1]. A set of other flavonoids such as wogonin and oroxylin A can also be isolated from the plant’s root extract. In a review of the pharmacological properties of oroxylin A, Lu et al. reported inhibition of multiple inflammatory pathways can play a possible role in decreasing multidrug resistance in tumors [2]. The compound has also shown efficacy in reversing lipopolysaccharide-induced inflammation [2]. As for wogonin, it can decrease the production of malondialdehyde and the expression of inducible nitric oxide synthase in the brain which can be potentially useful in alleviating neurodegeneration [3]. It has been demonstrated that BA and BE possess the highest level of antioxidant activity among all SB flavonoids, with BE being almost three times stronger in this respect [4]. Nevertheless, others have suggested that BA is the more potent compound in other radical scavenging pathways including the inhibition of xanthine oxidase [5], which make both BA and BE potential candidates for use in cardiovascular and neurodegenerative diseases [6]. BA and BE have neuroprotective [7–9], anti-ischemic [10], anti-cancer [11–14], anti-inflammatory [15–17] and anti-platelet [18] properties. As a comprehensive example, Alsharairi concluded that a host of mechanisms can be responsible for ameliorating nicotine-induced non-small cell lung cancer and asthma: inhibition of cell proliferation and migration and angiogenesis, as well as promotion of apoptosis and autophagy [19]. A variety of other possible therapeutic applications have been reported for S. radix (the root of SB) flavones including ocular diseases [20], obesity [21], bacterial meningitis [22] and attention deficit hyperactivity disorder [23], to name a few. For instance, when obese mice were treated with BA, a significant decrease in food intake and insulin resistance was observed [24–26]. The flavonoid acted via different and even paradoxical mechanisms based on the tissue under study. In skeletal muscles, the upregulation of protein kinase B (Akt) and p38 contributed to a rise in glucose transporter type 4 (GLUT4) which in turn promoted glucose uptake [24, 26]. However, in the liver, a decline in p38 phosphorylation due to BA was responsible for lowering hepatic gluconeogenesis which alleviated hyperglycemia and insulin resistance [25]. A review of the main pharmacological effects of SB and its active ingredients and some of the contributing mechanisms is outlined in Fig. 2. Although varied in nature, many natural and physically induced toxicities occur via similar routes. The generation of reactive oxygen species (ROS) and induction of inflammation are important mechanisms for different fungal and plant toxins, as well as bacterial lipopolysaccharide (LPS) [27, 28]. Exposure to some physical hazards such as radiation and excessive heat may also trigger cellular death in ways comparable to natural toxins [29, 30]. The search for compounds with antidotal and protective properties against this wide range of toxic agents has been conducted for a long time. Multiple plants and their active ingredients have been evaluated for their antitoxic effects and have shown efficacy such as black seed [31], rosemary [32], and curcumin [33]. Considering the multipotent pharmacological properties of BA and BE, they possess great potential as therapeutic and protective agents. Both have been evaluated for their antioxidant and anti-inflammatory effects against the backdrop of natural and physically induced toxicities. The goal and novelty of this paper is to provide a review of the studies that investigated the anti-toxic effects of total SB extract (SBE), BE or BA with their respective cellular and molecular mechanisms, based on the nature and source of the harmful substances.Also, regarding to different studies which are categorized in the current review, SB extract (SBE), BE or BA can be mentioned as potential remedy against natural and physical toxicities in further clinical studies.
Fig. 1.
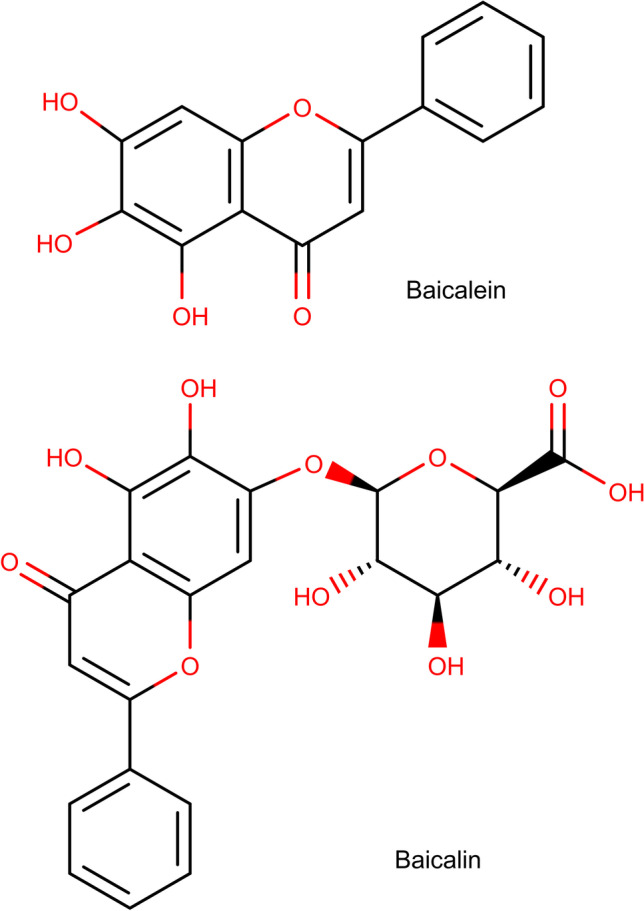
Chemical structure of baicalein and baicalin
Fig. 2.
A summary of main pharmacological effects of Scutellaria baicalensis and its active constituents and some of the mechanisms involved (graphics courtesy of freepik.com)
Method
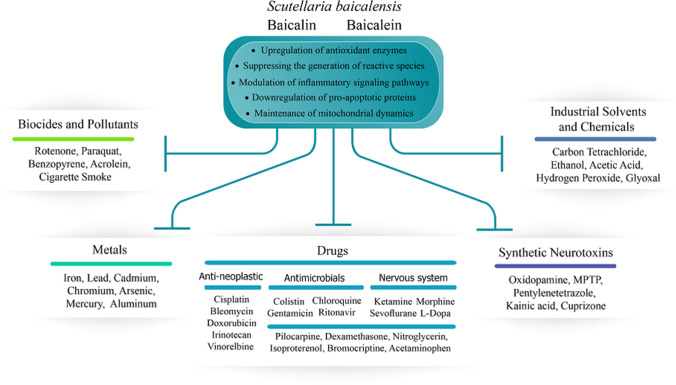
This study aimed to provide a narrative review of the antidotal and protective properties of BA, BE, and total flavonoids from SB root, stem, and leaves against natural and physically induced toxicities. Initially, scientific databases PubMed, Scopus, and Web of Science were screened for studies that included keywords ‘baicalin’, ‘baicalein’, ‘Scutellaria baicalensis’, ‘hepatotoxicity’, ‘nephrotoxicity’, ‘toxin’, ‘mycotoxins’, ‘lipopolysaccharide’ and ‘radiation’ in their titles or abstracts. In vitro, in vivo and clinical studies that reported antitoxic and protective effects of SBE, BA or BA, only in cases of exposure to natural toxins and physical hazards were included in the review. The bibliographies of the selected articles were once again searched for relevant material. The review was conducted on a total of 96 studies from 1998 to 2021. It is important to note that SB, BA and BE have all manifested remarkable protection against the toxicity of numerous chemical compounds, drugs, and heavy metals [34–36]. However, due to the extensiveness of the literature surrounding SB and its active ingredients, the authors decided to solely focus on natural toxins and physical hazards in this paper.
Natural-induced toxicity
The protective effects of SBE and its main constituent, BA, and BE, against natural toxins (except LPS) in both in vivo and in vitro studies are summarized in Table 1.
Table 1.
Protective effects of Scutellaria baicalensis and its main constituents, baicalin and baicalein against natural toxins
| Study type (animal/cell line) | Protective agent (concentration/dosage) | Toxin | Molecular mechanisms | Ref(s) |
|---|---|---|---|---|
|
In vitro (TA1535/pSK1002) In vivo (mouse) |
BE (50–300 μM) BE (5 μM in the liquid diet for 1 week) |
AFB1 |
In vitro: ↓umuC gene expression In vivo: ↓ AFQ1 and AFBO |
[37] |
| In vivo (rats) | Dried SB (10 g/kg orally for 5 weeks) |
↓CYP3A2 expression ↑GST A5 subunit gene expression, sensitivity to apoptosis |
[38] | |
| In vitro (rat liver) | SBE (10–50 μg/ml) |
↓CYP1A1/2 activity ↓CYP2B1, CYP2C11, and CYP3A1/2 activity ↓ AFM1 and AFBO |
[39] | |
| In vivo (piglets) | BA (0.1% basal diet for 14 days) | DON |
↑Final body weight, feed to gain ratio ↑Ileal and jejunal villus height, crypt depth, and villus height/crypt depth ↓ALP, ALT, AST, LDH ↑Alb ↓IL-8, IL-1β, IL-6, IFN-γ, TNF-α serum concentration and intestinal expression ↑SOD, GSH-px, GSH, T-AOC, Intestinal mTOR protein ↓MDA, Intestinal NF-κB p65 protein |
[27] |
|
In vitro (rabbit cell culture) In vivo (mouse) |
BA (2–16 μg/ml) BA (50–100 mg/kg SC doses for 3 days) |
Hla |
In vitro: ↓rabbit erythrocytes hemolysis In vivo: ↑survival after 24–72 h ↓lung cellular infiltrates ↓IL-1β, IFN-γ, and TNF-α in BAL |
[40, 41] |
| In vitro (BMEC) | BA (2.5–10 μg/ml) | PVL | ↓ apoptosis, cleaved caspase-9, RNA III, and LukS-PV expression | [42] |
|
In vitro (HeLa) In vivo (mouse) |
BA (4.5–36 μM) BA (100 mg/kg SC doses for 6 days) |
Stxs |
In vitro: ↑protein synthesis ↓LDH release, cell death In vivo: ↓death rate, weight loss ↓kidney tubular damage, Scr, BUN, hemolysis ↓IL-1β, IL-4, IL-6, TNF-α, IFN-γ |
[43, 44] |
| In vitro (Vero cells) | BE (0.13 μM) | ↓cell death, transcription of stx1 | [45] | |
|
In vitro (HeLa) In vivo (mouse) |
BA (4.5–36 μM) BA (100 mg/kg SC doses for 3 days) |
Ricin |
In vitro: ↑protein synthesis ↓LDH release, cell death In vivo: ↓death rate, weight loss ↑blood glucose ↓IL-1β, IL-6, TNF-α |
[46] |
| In vivo (mouse) | BA (80–160 mg/kg/day i.p. for 3 days) | AA |
↓BUN, Scr ↓plasma AUC of AAI, AAI content in liver and kidney ↓tubular necrosis and dilation ↑CYP1A1/2 expression |
[47] |
|
In vitro (rat hepatocytes, mouse splenocytes) In vivo (mouse) |
BA (10 μg/ml) BA (three doses of 100–200 mg/kg i.p.) |
Con A |
In vitro: ↓ TNF-α, IFN-γ in splenocytes ↓caspase-3/9 activity and SAPK/JNK activation in hepatocytes In vivo: ↓ALT, AST, MPO, MDA, IL-6, TNF-α, IFN-γ ↓ hepatocyte necrosis, lymphocyte infiltration, and cellular swelling ↓caspase-3 expression and activity ↑SOD activity |
[48] |
|
In vitro (mouse splenocytes) In vivo (mouse) |
BE (5–20 μM) BE (100 mg/kg single i.p. dose) |
In vitro: ↑apoptosis in CD3+ and CD19+ splenocytes and Jurkat cells ↓MMPs, Bcl-2 ↑Bax, caspase-3/9 In vivo: ↓ALT, TNF-α, IFN-γ ↓MNC, CD3+ T cell, and CD19+ B cell hepatic infiltration |
[49] | |
| In vitro (rat L6 cells) | BA (50 μg/ml), BE (50 μg/ml) | AMA |
↑cell viability, ATP production, ATP/ADP ↑MMP ↓superoxide level Enhancement of mitochondrial function through increasing the expression of PGC-1α, Sirt1, and phosphorylated AMPK |
[50] |
|
In vitro (RAW 264.7) In vivo (rats) |
SBE (12.5–100 μg/ml) SBE (200–400 mg/kg single oral dose) |
Cantharidin |
In vitro: ↓NO, PGE2 In vivo: ↓hematuria, bladder epithelium necrosis ↑urine volume ↓COX-2 expression, c-FOS signal |
[51] |
| In vivo (rats) | SB (25–100 mg/kg/day orally for 36 days) | OA |
↓neuronal loss and swelling, nuclear pyknosis, neurofibrillary degeneration, neuronophagia, Aβ1–40 positive neurons ↑Nissl bodies density, GFAP-positive astrocytes ↓MDA ↑GSH-px |
[52] |
| In vivo (mouse) | BE (25–100 mg/kg/day i.p. doses for 2 months) | Pristane |
↓proteinuria, inflammatory infiltrates, splenomegaly Glomerulonephritis amelioration ↓anti-dsDNA IgG, IL-1β, IL-6, IL-17A, IL-18, IFN-α ↓NLRP3 inflammasome activation, ROS levels, NF-κB phosphorylation ↑Nrf2 activation |
[53] |
| In vivo (rat) |
BA (100 mg/kg) BE (10 mg/kg/day i.p. for 30 days) BE (50–100 mg/kg/day orally for 14–28 days) |
MCT |
↓RSVP, RV/(LV + S) thickness, PA wall thickness ↓IL-1β, IL-6, TNF-α, ET-1, ETAR mRNA and protein ↓Bax/Bcl-2, cleaved caspase-9 ↓ROS ↓iNOS activity ↓MDA level ↑SOD, GSH-px and eNOS activity ↓NF-κB, ERK1/2 and PI3K/Akt/MAPK signaling ↑IκBα levels, GSK3β activity, BMP signaling |
[54–61] |
AA aristolochic acid; Aβ amyloid β; AFB1 aflatoxin B1; AFBO aflatoxin B1 oxide; AFM1 aflatoxin M1; AFQ1 aflatoxin Q1; Akt protein kinase B; Alb albumin; ALP alkaline phosphatase; ALT alanine transaminase; AMA antimycin A; AMPK AMP-activated protein kinase; AST aspartate transaminase; AUC area under the curve; BA baicalin; BAL bronchoalveolar lavage; BE baicalein; BMP bone morphogenetic protein; BUN blood urea nitrogen; CON A concanavalin A; COX-2 cyclooxygenase-2; DON deoxynivalenol; eNOS endothelial nitric oxide synthase; ERK extracellular signal-regulated kinases; ET-1 endothelin-1; ETAR ETA receptor; GFAP glial fibrillary acidic protein; GSH glutathione; GSH-px glutathione peroxidase; GSK3β glycogen synthase kinase 3 beta; GST glutathione S-transferase; Hla α-hemolysin; IFN interferon; IgG immunoglobulin gamma; IκBα nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; IL interleukin; iNOS inducible nitric oxide synthase; i.p. intraperitoneal; JNK c-jun N-terminal kinase; LDH lactate dehydrogenase; MAPK mitogen-activated protein kinase; MCT monocrotaline; MDA malondialdehyde; MMP mitochondrial membrane potential; MMPs matrix metalloproteinases; MNC mononuclear cells; MPO myeloperoxidase; mTOR mammalian target of rapamycin; NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3 NLR family pyrin domain containing 3; NO nitric oxide; Nrf2 nuclear factor erythroid 2-related factor 2, OA okadaic acid; PA pulmonary artery; PGC-1α peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PGE2 prostaglandin E2; PI3K phosphoinositide 3-kinase; PVL panton-valentine leukocidin; ROS reactive oxygen species; RSVP right ventricular systolic pressure; RV/(LV + S) right ventricle/(left ventricle + septum); SAPK stress-activated protein kinase; SB Scutellaria baicalensis; SBE Scutellaria baicalensis extract; SC subcutaneous; Scr serum creatinine; Sirt1 sirutin 1; SOD superoxide dismutase; Stxs shiga-like toxins; T-AOC total antioxidant capacity; TNF-α tumor necrosis factor-α
Mycotoxins
Aflatoxin B1 (AFB1)
AFB1 is one of the most characterized mycotoxins produced in different fungal genera such as Aspergillus. It is also a proven hepatocarcinogen in animals and possibly in humans [37]. One study reported that the addition of dried SB to a daily diet of rats reduced the mutagenicity of a subsequent AFB1 administration. Researchers suggested an increase in the expression of the glutathione S-transferase (GST) gene as a possible mechanism [38]. It has also been shown that BE can suppress the genotoxicity of AFB1 by inhibiting the formation of metabolites aflatoxin Q1 (AFQ1) and AFB1-epoxide (AFBO), both in vitro and in mice [37]. In another experiment, S. baicalensis extract (SBE) inhibited CYP1A enzyme activity. This led to a remarkable decrease in the production of aflatoxin M1 (AFM1) which is another toxic metabolite of AFB1 [39]. Therefore, SBE can potentially strengthen cellular antioxidant defense to counteract AFB1 toxicity.
Deoxynivalenol (DON)
DON is a secondary metabolite that is most commonly seen in Fusarium spp. It can cause different levels of gastrointestinal toxicity, especially in the small intestine. Adding DON to the diet of piglets significantly reduced their body weight. Cotreatment with BA prevented this change in animals. It also inhibited the elevations of some hepatic markers. DON administration led to an upregulation of gene expression of many inflammatory cytokines in ileum and jejunum. The inflammation process was mitigated after the addition of BA. These beneficial changes were supposedly driven by a modulation in mammalian target of rapamycin (mTOR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) gene expression and protein concentration [27].
α-Hemolysin (Hla)
Hla is a channel-forming toxin that is well characterized in Staphylococcus aureus-induced pneumonia and mammary gland inflammation. Molecular studies suggest that BA does not affect Hla expression. However, it can decrease the hemolytic activity of HIa in rabbit cell culture in a dose-dependent manner [40]. In vivo studies in mice further demonstrated that BA administration can significantly reduce mortality due to S. aureus-induced pneumonia. BA possibly interacted with Hla and prevented it from assembling into the heptameric confirmation that it needs to form pores [40]. Another study showed that a combination of similar concentrations of osthole and BA had a more remarkable protective effect against hemolysis and mortality both in vitro and in vivo than each of the compounds alone. However, it is doubtful if this can be attributed to a synergistic combination since osthole was shown to exert its impact through suppressing Hla expression [41]. To conclude, more data is required in order to attribute a possible antibacterial effect to BA which is mediated by Hla functional inhibition.
Panton-Valentine leukocidin (PVL)
PVL is another virulence factor in S. aureus that acts in a rather similar mechanism on cells and promotes apoptosis in many tissues. One study reported that both wild-type and recombinant PVL were sources of great damage to bovine mammary epithelial cells (BMEC). BA significantly decreased cell death in a dose-dependent manner. It also helped to reduce cleaved caspase-9 levels. It seemed probable that BA acted via inhibiting the expression or secretion of upstream toxin regulator RNA III and toxin subunit LukS-PV [42].
Shiga-like toxins (Stxs)
Stxs are a group of virulence factors produced by Shiga toxin-producing Escherichia coli (STEC). They can lead to various conditions, ranging from bloody diarrhea to hemolytic uremic syndrome (HUS) [43]. Stxs have two subtypes, of which Stx-2 is the more lethal one [43]. It has been reported that BA can induce the formation of inactive oligomers of the toxin, which is a ribosome-inactivation protein (RIP). Therefore, it inhibits Stx poisonous activity in the gut and kidneys. In vitro and animal studies proved that BA can significantly prevent cell death and restore renal function to a great extent. BA reduced the lethality of recombinant Stx in mice [43]. Furthermore, it protects mice from kidney damage in enterohemorrhagic E. coli infection [44].
Another in vitro study indicated that BE can also remarkably decrease the cytotoxicity of both Stxs. This effect was observed both when BE was premixed with the toxins before addition to cell culture and also when cells were preincubated with the same concentration of BE and then exposed to Stxs. The study also reported that BE had no effect on the secretion of these two toxins but possibly inhibited their action via forming binding structures inside their pentamers [45].
To summarize, BA and BE seemed to have no or limited effects on the expression of bacterial exotoxins, whereas the flavonoids could impair the functionality of toxins.
Ricin
Ricin is a potent toxin found in castor beans. It belongs to the group of RIPs and manifests its toxicity by inhibiting protein synthesis and finally causing cell death [62]. Ricin lethality can be seen in 4 to 5 days and no therapeutic agent is efficacious in preventing it. A study found that pretreatment with BA can effectively inhibit cell death caused by ricin through restoring protein translation. In further in vivo models, a significant percentage of animals that received BA after initial exposure to ricin survived and experienced significantly less severe symptoms. The mechanism through which BA acts was promoting ricin to form oligomers which in turn hindered its active sites and reduced its toxicity [46]. Once again, the ability of BA as a physical antagonist against toxins was emphasized here.
Aristolochic Acid (AA)
Aristolochia species have long been used for the anti-inflammatory properties of their active components including aristolochic acid I and II (AAI and AAII) [47]. A study was performed to investigate the BA effect in the reduction of AAI nephrotoxic effects. BA, a potent CYP1A2 gene expression inducer, significantly reversed histological deterioration. Moreover, pretreatment with BA markedly decreased the area under the curve of AAI in vivo via promoting its metabolization through CYP1A [47].
Concanavalin A (Con A)
Con A is isolated from jack beans and is a mitogen lectin that activates lymphocytes. Con A has the potential to cause acute liver injury in animal models [48]. To establish the hepatoprotective impacts of BA, one study demonstrated that BA pretreatment can prevent hepatic enzyme elevation, tissue necrosis, and apoptosis caused by Con A to a great extent. The enhancement of cellular antioxidant properties and inhibition of tumor necrosis factor (TNF)-α expression were also responsible for hepatoprotection in vitro [48].
Con A-induced hepatitis in a murine model was significantly attenuated by BE administration. BE managed to suppress pro-inflammatory markers and lower tissue necrosis. The induction of apoptosis in mononuclear cells (MNCs) and T and B cells in the liver was considered as the pivotal mechanism of BE as a hepatoprotective agent [49]. To sum up, the modulation of inflammation appears to contribute significantly to the alleviation of Con A-induced liver injury by SBE flavonoids.
Antimycin A (AMA)
Antimycins are a group of secondary metabolites that were first isolated from Streptomyces bacteria. AMA is used to kill invasive fish in fishery management. Its inhibitory effect on mitochondrial cytochrome c and respiratory chain is its main mechanism that leads to the production of ROS and in turn, promotes cell apoptosis [63]. In a study on AMA-induced mitochondrial dysfunction in vitro, pretreatment with water and ethanol extracts of BA and BE was reported to increase total cell ATP and reduce ADP/ATP ratio. Besides, it further prevented mitochondrial membrane potential disruption. All in all, it attributed to fewer oxidative injuries caused by AMA and increased cell viability [50].
Aconitine
Although the plants from Aconitum spp. have some medicinal applications in TCM, they can easily lead to poisoning. The toxicity manifests itself in cardiac and neurological symptoms. There exists no specific antidote for aconitine-induced arrhythmia. In one experiment on arrhythmic patients, the group that was treated with 450 mg of BA for 48 h, reached hemodynamical stability significantly sooner than the control group that used conventional treatment. Normalization of cardiogram was seen after 12 h, and the patients recovered from flutter and sinus bradycardia within 15 min and 12 h respectively. It was suggested that BA interfered with the membrane permeability of cardiomyocytes to ions and possibly moderated the overt excitement in the vagus and cerebral cortex [64]. The results of this study implied that flavonoids may be useful clinically for managing acute cases of poisoning.
Cantharidin
Cantharidin is isolated from Spanish fly Mylabris phalerata and has been used in various conditions in TCM and even in Europe. However, there are numerous cases of intoxication with this compound. One of the major organs susceptible to cantharidin toxicity is the bladder. SB water extract that included BA as an active ingredient, significantly protected against cantharidin-induced hemorrhagic cystitis. It reduced hematuria and increased urine volume. The uroprotective effects of SBE were attributed to an inhibition of cyclooxygenase-2 (COX-2) activity and prostaglandin E2 (PGE2) production [51].
Okadaic Acid (OA)
OA is a shellfish toxin and a known protein phosphatase (PP) inhibitor. If phosphorylated proteins are not degraded by PPs, it can lead to an overgeneration of free radicals and dysfunction in many parts of the brain. In one in vivo model, OA caused a huge amount of neuronal loss and damage in the hippocampus and cerebral cortex. SB flavonoids significantly improved neuronal count. They also reversed the increase in amyloid β-positive cells induced by OA. Besides, the antioxidant status of the tissues was remarkably improved by the flavonoids [52].
Pristane
Pristane is a terpenoid alkane that was originally isolated from shark liver oil. It is known for its ability to cause autoimmune diseases such as arthritis and lupus. Pristane-induced lupus glomerulonephritis in mice was significantly alleviated with the administration of BE for two months. BE improved renal markers and further mitigated the deleterious effects of inflammation in kidney tissue. These changes and the reductions that were observed in proinflammatory markers were explained by two parallel mechanisms: induction of nuclear factor erythroid 2-related factor 2 (Nrf2) that led to upregulation of heme oxygenase-1 (HO-1) expression, and suppression in (NOD)-like receptor protein 3 (NLRP3)/NF-κB pathway. Both of these routes ended in a desirable regulation of myeloid-derived suppressor cells (MDSCs), which play an indisputable role in lupus nephritis pathophysiology [53]. This evidence, however limited, can be a starting point to probe into the possible effects of BE in autoimmune diseases of various etiologies.
Monocrotaline (MCT)
MCT is a pyrrolizidine alkaloid with selective toxicity for lung endothelial that can cause pulmonary artery hypertension (PAH) and vascular inflammation and remodeling [55]. Studies reported that 50–100 mg/kg doses of BA or BE significantly attenuated MCT-induced PAH and vascular remodeling in rats. Furthermore, a similar reduction in pro-inflammatory cytokines was observed due to flavonoid treatment [56–59, 65]. An investigation of pathohistological markers showed a reduced level of ROS and inducible nitric oxide synthase (iNOS), as well as a rise in endothelial nitric oxide synthase (eNOS), are partially responsible for BE effect on PAH [56]. The inhibitory effect of BE and BA on the expression of endothelin-1 (ET-1) and ETA receptor (ETAR) was also important since both proteins are involved in vascular proliferation and constriction processes [56, 59]. The changes were achieved via the modulation of different intracellular signaling pathways. BE decreased glycogen synthase kinase 3 beta (GSK3β) inactivation by Akt and extracellular signal-regulated kinases (ERK1/2). This in turn prevented ET-1 transcription [56, 60].
BE also had liver detoxifying properties in MCT-induced hepatic sinusoidal obstruction syndrome (SOS). It reduced matrix metalloproteinase (MMP)-9 and myeloperoxidase (MPO) activity. Besides, BE downregulated the expression of toll-like receptor (TLR) and transcriptional factors involved in inflammatory regulations in liver tissues. Furthermore, BE decreased in malondialdehyde (MDA) amount caused by MCT. Lastly, it suppressed mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling pathways [54].
Lipopolysaccharide (LPS)
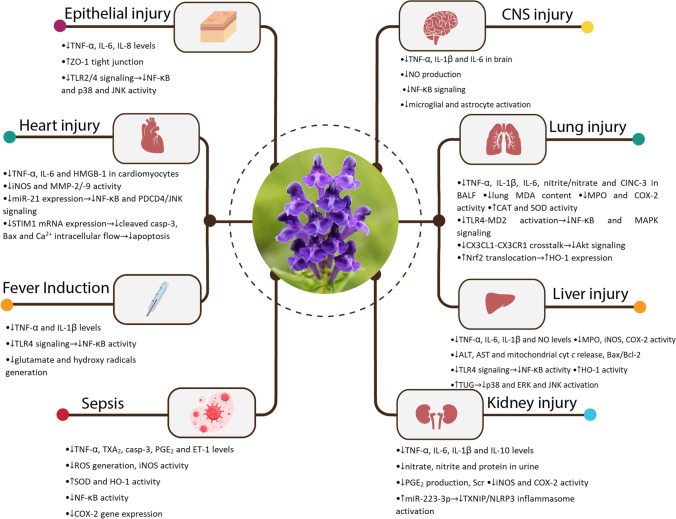
One of the major virulence factors of gram-negative bacteria is endotoxin or LPS. It is capable of causing a wide range of pathological conditions in various organs of the body and can lead to septic shock and death at its extreme. SB and its flavonoids have shown various protective effects against LPS-induced toxicity (Fig. 3).
Fig. 3.
Protective effects of Scutellaria baicalensis and its constituents, baicalin and baicalein against LPS toxicity on various organ systems (graphics courtesy of freepik.com). Abbreviations: Akt, protein kinase B; ALT, alanine transaminase; AST, aspartate transaminase; BALF, bronchoalveolar lavage fluid; casp-3, caspase-3; CAT, catalase; CINC-3, cytokine-induced neutrophil chemoattractant-3; COX-2, cylooxygenase-2; CX3CL1, CX3C chemokine ligand 1; CX3CR1, CX3C chemokine receptor 1; cyt c, cytochrome c; ERK, extracellular signal-regulated kinase; ET-1, endothelin-1; HMGB1, high mobility group box 1; HO-1, heme oxygenase-1; IL, interleukin; iNOS, induced nitric oxide synthase; JNK, c-jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MD-2, myeloid differentiation factor-2; MDA, malondialdehyde; MMP, matrix metalloproteinase; MPO, myeloperoxidase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, nod-like receptor family pyrin domain containing 3; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; PDCD4, programmed cell death 4; PGE2, prostaglandin E2; ROS, radical oxygen species; Scr, serum creatinine; SOD, superoxide dismutase; STIM1, stromal interaction molecule 1; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TUG1, taurine upregulated gene 1; TXA2, thromboxane A2; TXNIP, Thioredoxin-Interacting Protein; ZO-1, zonula occludens-1
Lung injury
Acute lung injury (ALI) is a major result of endotoxin pulmonary toxicity. It is characterized by lung edema and white blood cell infiltration [66]. A single BE or BA (20–100 mg/kg i.p) dose before LPS administration in rats resulted in significant attenuation of lung injury pathological markers [67, 68]. Furthermore, in experiments on mice, a single IV dose of 5–10 mg/kg BE [69] or seven days of oral BA (100–200 mg/kg/day) [70, 71] before LPS exposure showed protective effects. One study reported the therapeutic effect of a single SC dose of 20 mg/kg of BA in rats as a post-treatment against endotoxin-induced ALI [72]. BE decreased the wet to dry weight of the lung. It also reduced multiple inflammatory cytokines in bronchoalveolar lavage fluid (BALF) such as TNF-α, interleukin (IL)-1β, IL-6, and cytokine-induced neutrophil chemoattractant-3 (CINC-3) and inhibited polymorphonuclears (PMNs) infiltration. Furthermore, a higher level of catalase (CAT) and superoxide dismutase () activity was observed in the flavonoid-treated group [66, 70–72]. One study tried to investigate the underlying mechanism of BA reducing lung water content and increasing alveolar fluid clearance. They found that growth in the number of α-epithelial Na channels due to elevation of cAMP in lungs both in vivo and in vitro was responsible for this effect [67]. Blocking of NF-κB and Akt signaling pathways were other important modes of action for the flavonoids. They also upregulated the Nrf2 transcription factor which led to HO-1 enhanced activity against oxidative stress. Another central means of protection was suppressing MAPK pathway mediators ERK, p38, and c-jun N-terminal kinase (JNK). These were some of the suggested mechanisms of action of BA [70, 71, 73], BE [66, 69], and SB total flavonoids [74, 75] against LPS-induced ALI. To summarize the multitude of mechanisms at play in the flavonoids’ protection against ALI, one can consider the promotion of antioxidant defenses and inhibition of inflammation as central.
Cardiac injury
Cardiac hypertrophy is another consequence of endotoxin toxicity that can lead to heart failure. It is primarily attributed to ROS generation in heart tissue that possesses less antioxidant activity than many other organs. BE was found to significantly prevent endotoxin-induced hypertrophy in rat cardiomyocytes that were treated with 1 μM BE before incubation with LPS. Both early inflammatory markers such as TNF-α and IL-6 and late ones such as iNOS and high mobility group box one-1 (HMGB-1) protein that have established roles in endotoxin cardiotoxicity were reduced by treatment with 1 μM of BE in a significant manner. The flavonoid was also able to suppress ROS production and MMPs expression [76]. In other experiments, BA (100 μM) appeared promising in saving H9C2 cardiomyocytes from LPS-induced apoptosis in a concentration of 1 μM. This was achieved by moderating calcium-overload through stromal interaction molecule 1 (STIM1)-mRNA [77] and a down-regulation of miR-21 expression [78].
Liver injury
Mice that were pretreated intraperitoneally with 150–300 mg/kg/day doses of BA for 2 days before administration of LPS/D-galactosamine (GalN) showed significantly less mortality. Furthermore, they exhibited lower elevations in aminotransferases levels and a suppressed level of TNF-α mRNA and protein in the liver and serum. Histopathological data manifested only spotty necrosis and slight inflammation in livers of the BA group compared to diffuse necrosis and acute inflammation of LPS/D-GalN. This could be possibly explained through inhibition of NF-κB translocation into the nucleus [79, 80]. Moreover, BA was able to increase SOD activity and reduce MDA content in LPS-treated mice. Furthermore, it was found out that a solid dispersion of BA is of superior efficacy than BA alone [81]. BA pretreatment also exhibited an ability to inhibit LPS-induced COX-2 expression in chicken with 100–200 mg/kg/day oral doses for 7 days [82]. One recent in vitro study reported that a 20 mM concentration of BA could increase normal human liver cell viability. This effect was probably due to an up-regulation of taurine upregulated gene 1 (TUG1), a long non-coding RNA that acts as an inflammation regulator [83]. Oral BE (100–150 mg/kg) displayed comparable hepatoprotective effects in mice as a single dose before endotoxin treatment. The flavonoid improved hepatic biological markers and microscopic evidence [84]. A substantial reduction of serum nitric oxide (NO) and iNOS activity, and considerable anti-apoptotic characteristics were other reported results [84]. Pretreatment of mice with 200 mg/kg/day doses of SB ethanol extract for 7 days significantly alleviated LPS insult on the liver. The inhibition of COX-2, iNOS, and NF-κB activation are likely to contribute to the above process [85].
Kidney injury
Histological deterioration of murine kidney and a rise in serum creatinine (Scr) due to LPS were remarkably prevented by 74 mg/kg/day doses of oral BA for 7 days. Suggested mechanisms of action were reducing inflammatory markers in both renal tissue and serum, as well as raising citrate synthase and pyruvate kinase expression, both crucial enzymes in the tricarboxylic acid (TCA) cycle [86]. BA rescued HK-2 cells through modulating thioredoxin-interacting protein (TXNIP)/NLRP3 overactivity and promoting miR-223-3p expression [87]. The effectiveness of BE as a therapeutic agent against LPS nephrotoxicity was manifested elsewhere. Oral administration of BE for 14 days in 150 mg/kg/day doses led to suppression of PGE2 and prostaglandin F2α (PGF2α) [88].
CNS injury
It has been suggested that daily administrations of 100 mg/kg of SBE for 32 days after LPS infusion were able to mitigate its detrimental effects on spatial memory and hippocampal microglial activation in rats [89]. Another experiment reported that cotreatment of BA (10 mg/kg/day) with endotoxin for 7 days can also minimize neuroinflammation in mice's cerebral cortex and hippocampus [90, 91]. The involved mechanism was a combination of inhibiting NF-κB phosphorylation and modulating neuroglial activation [90]. Furthermore, a significant diminution of LPS-induced inflammatory markers by BA rescued BV-2 cells in another study [92]. According to in vitro experiments, BE acted through similar mechanisms to protect CNS against LPS. Moreover, it also restrained oxidative stress and COX-2 activity [93–95].
Fever induction
Endotoxin is a known pyrogenic factor and is thought to act through a hypothalamic pathway that involves TNF-α, glutamate, and hydroxyl radicals [96]. Two in vivo studies conducted on fever models showed that pretreatment with a single dose of BA (10–20 mg/kg IV in rabbits or 160 mg/kg by gastric perfusion in rats) could significantly decrease the elevated body temperature due to LPS. Animals in the BA group had remarkably lower levels of both upstream inflammatory factors including TLR4 mRNA and protein and downstream ones such as TNF-α and IL-1β than the endotoxin group. However, the levels were still higher than the control group [96, 97].
Epithelial injury
The generation of oxidative stress and inflammation in epithelial cells in various organs is another deleterious effect of endotoxin. When cultures of human oral keratinocytes were treated with LPS in presence of an 80 μM concentration of BA, up-regulation in IL-6 and IL-8 mRNA and protein was significantly prevented. BA possibly acted by suppressing the TLR2/4 signaling pathway and its downstream adapters in HaCaT cells [98]. BE also showed promise in alleviating oral lichen planus symptoms by inhibiting the inflammatory response and NF-κB activation [99]. Furthermore, oral administration of 50–100 mg/kg/day doses of BA for 3 days was protective against LPS-induced injuries in mice colonic and ileal mucosa [100]. In vitro experiments on IEC-6 cells manifested that pretreatment with 10 μg/ml of BA can decrease LPS-induced cellular death. This protective effect was suggested to be mainly due to an increase in ZO-1 protein which acts as a tight junction [101].
Sepsis
The expression of the iNOS gene, iNOS activity, and the subsequent NO production in various cells is of great importance in the pathophysiology of sepsis. Both BE and BA in 20 and 40 μM concentrations managed to remarkably lower nitrite content in macrophages treated with LPS but did not have a meaningful effect on COX-2 expression [102]. In vivo experiments on endotoxemic rats further elucidated that IV administration of a single dose of BE (10–20 mg/kg) was successful in re-stabilizing the hemodynamic markers. As well as normalizing hypotension and tachycardia, BE reduced pro-inflammatory cytokines in plasma and lungs. This therapeutic effect was attributed to decreased superoxide anion formation in the aorta and reduced plasma nitrite to nitrate ratio. Besides, BE ameliorated heart contractile dysfunction [103, 104]. Pretreatment with BA and BE was found to have meaningful effects on reducing mice mortality in endotoxemia [103, 105].
Miscellaneous
The role of BA and BE in ameliorating LPS-induced mastitis both in vivo and in vitro was investigated in other studies. The inhibition of MAPK and TLR4 signaling pathways was again emphasized as the main mechanism for improving histological evidence and suppressing pro-inflammatory cytokines [106, 107]. Another protective measure of flavonoids was up-regulating heat shock protein 72 (HSP72) that led to a reduction of apoptosis [107].
The anti-abortive properties of BA were tested on an endotoxin-induced abortion model. The flavonoid augmented the survival rate of isolated decidual cells in presence of endotoxin in vitro. When administered by oral gavage in days 7–8 of gestation (12.5–25 mg/kg/day), BA further managed to increase mice uteri weight and lower fetal resorption rate, basically by suppression of TNF-α, interferon (IFN)-γ and NO, while maintaining progesterone levels [108].
In one unique study performed on Caenorhabditis elegans, the inhibition of apoptosis and ROS production and reduction in IL-1, IL-6 and TNF-α were found as essential mechanisms that underlined BA protection against LPS toxicity in worms [109, 110].
As it could be seen in Fig. 3, suppression of TLR4/ NF-κB signaling and its downstream inflammatory mediators TNF-α, IL-1β and IL-6 are the main mechanisms behind protective effects of Scutellaria baicalensis and its constituents against LPS toxicity in different organs. Moreover, the upregulation of HO-1 expression and decreasing the activity of iNOS were other important modes of action for the flavonoids.
Physical hazards
Protective effects of BA and BE against radiation toxicity
It is a fact that prolonged or repetitive exposure to different kinds of high-energy radiation can lead to organ damage and carcinogenesis. BA and to a lesser extent BE, have shown significant protective effects against ultraviolet (UV) A, B, or C radiation-induced aging and apoptosis. Treatment of human fibroblasts and keratinocytes with BA (25–50 μg/ml) before or after UV exposure increased cell viability and reduced apoptosis remarkably [111–119]. Topical application of BA (0.5–1.5 mg per cm2 of skin) resulted in less dermal hyperplasia and collagen loss in mice [113, 120, 121]. BA protected against tumor induction and mutagenesis by preventing abnormal DNA adduct formation [111, 112, 118, 120] and suppressing excessive cell proliferation [121]. Moreover, BA lowered the level of tumor suppressor proteins to normal levels and thereby attenuated the apoptosis rate [111, 113, 120]. Other mechanisms for the flavonoids’ protection were upregulating AMP-activated protein kinase (AMPK) signaling [115], enhancing radical scavenging [114, 117, 122], absorbing UV [122], and blocking mTOR [115] and TLR4 [29] pathways.
Elsewhere, BA and BE showed protection against other types of high-energy ionizing radiation. BA (50 μM) significantly prevented the γ-ray irradiated alveolar epithelial cells from transitioning to mesenchymal tissue [123]. Besides, IV administration of BA mitigated the laser-induced retinal lesions due to neovascularization [124]. BE in daily 5–10 mg/kg doses was successful in alleviating X-ray injury in intestinal tissue [125] and kidneys [126] of mice. In another murine model, rebalancement of gut microbiota was cited as a possible mechanism by which BE (100 mg/kg i.p before and after radiation) alleviated radiation-induced intestinal toxicity [127]. Finally, a notable reduction of DNA damage due to γ-rays was achieved by administering BE both in vivo and in vitro [128].
To summarize, BE and BA prevent radiation-induced DNA injury or facilitate its repair, which can be a direct consequence of their radical scavenging properties.
Protective effects of BA and BE against thermal stress
Spermatogenesis is a temperature-regulated process. Heat stress can impair this reproductive procedure in testes. It was shown that BA significantly rescued Sertoli cells from apoptosis due to heat. This in vitro experiment reported a fall in Fas and FasL and a rise in HSP72 levels due to pretreatment with BA (10–20 μg/ml) [129]. These protective effects were also observed after BA treatment (50 mg/kg/day i.p. for 7 days) in mice testes [30].
Protective effects of BA and BE against noise-induced injury
Chronic noise can impair normal cochlear cell homeostasis and reduce auditory function. Mice that were pretreated with 300–500 mg/kg/day doses of SBE before being exposed to noise, showed a significantly lower hearing threshold. Furthermore, using 30 mg/kg daily doses of BE exhibited markedly better protective properties than BA or SBE [130, 131].
Conclusion and future perspective
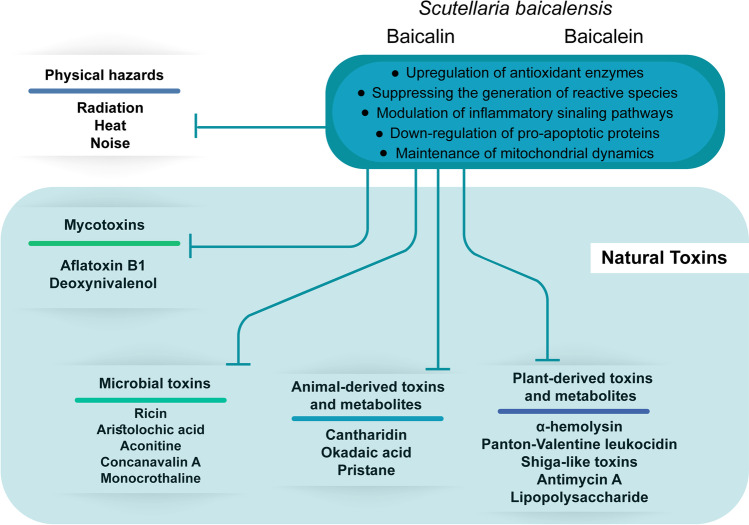
The body of research conducted on the Chinese skullcap has exhibited that both its root extract and the isolated flavonoids display a wide range of potential roles as antidotes or protective agents against natural toxins and physical factors’ damages (Fig. 4). The paper tried to present for the first time, a comprehensive review of the evidence surrounding the molecular mechanisms of these antitoxic effects that cytoprotective properties of BA and BE in vivo and in vitro are most potent when they are introduced into the culture medium or animal as a pretreatment of the toxic compounds. Nevertheless, co- and post-treatment of this plant and its constituents have also shown promise in alleviating toxicants in many studies.
Fig. 4.
A summary of natural toxicities and physical hazards which Scutellaria baicalensis, baicalin or baicalein can prevent or counter
Administration of BA, BE or SBE is efficient in preventing or counteracting the morphological and functional impairments that are induced by mycotoxins (AFB1 and DON), bacterial toxins (Hla, PVL, Stxs, and LPS), plant-derived substances (ricin, AA, Con A, AMA, aconitine, and MCT) and animal-derived compounds (cantharidin, OA and pristane) in the liver, kidney, heart and other organs. Moreover, flavonoids are effective in their protection against damage due to physical factors such as radiation, heat, and noise.
The anti-inflammatory and radical scavenging properties make them able to prevent the formation of ROS in cells. Thus, oxidative damages to biological membranes, proteins, and DNA are alleviated, either directly or by modulation of immune system cytokines. Strengthening the endogenous antioxidant enzymes such as CAT and SOD via Nrf2/HO-1 pathway as well as increasing intracellular glutathione supplies are contributed to this process. On the other hand, the down-regulation of many intracellular adapters including FasL and Bax that trigger apoptosis by different pathways is another mode of action. The inhibition of PI3K/MAPK and TLR4/NF-κB pathways and their subsequent involvement in inflammatory cascades are some of the most important molecular mechanisms that played a role in flavonoids’ protection. BA and BE also modulate the rate and extent of metabolization in the liver. Some toxic agents are prevented by BE from being transformed into more dangerous metabolites and others are more readily excreted after supplementation of BA.
Although all the evidence supports the possible effectiveness of BA and BE as antidotes, the lack of reports on the effects of BA and BE against some important natural toxins including snake venoms is notable. Besides, the scarcity of clinical evidence surrounding the issue means that further clinical studies are required to reach a more definitive conclusion.
Abbreviations
- AA
aristolochic acid
- AFB1
aflatoxin B1
- Akt
protein kinase B
- AMA
antimycin A
- BA
baicalin
- BE
baicalein
- CAT
catalase
- Con A
concanavalin A
- COX-2
cyclooxygenase-2
- DON
deoxynivalenol
- ERK
extracellular signal-regulated kinase
- Hla
α-hemolysin
- HO-1
heme oxygenase-1
- HSP
heat shock protein
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide;
- MAPK
mitogen-activated protein kinase
- MCT
monocrotaline
- MDA
malondialdehyde
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
(NOD)-like receptor protein 3
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- OA
okadaic acid
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- PP
protein phosphatase
- PVL
Panton-Valentine leukocidin
- RIP
ribosome-inactivation protein
- ROS
radical oxygen species
- SB
Scutellatia baicalensis
- SBE
Scutellatia baicalensis extract
- SOD
superoxide dismutase
- Stxs
shiga-like toxins
- TCM
traditional chinese medicine
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Authors contributions
Conceptualization: Hossein Hosseinzadeh; Literature research and data analysis: Ali Ahmadi, Zoha Mortazavi; Writing (original draft preparation and graphic design): Ali Ahamdi; Writing (review and editing): Hossein Hosseinzadeh, Soghra Mehri, Ali Ahamdi.
Data availability
Data sharing not applicable – no new data generated.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Srinivas NR. Baicalin, an emerging multi-therapeutic agent: Pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica. 2010;40:357–367. doi: 10.3109/00498251003663724. [DOI] [PubMed] [Google Scholar]
- 2.Lu L, Guo Q, Zhao L. Overview of Oroxylin A: A Promising Flavonoid Compound. Phytother Res. 2016;30:1765–1774. doi: 10.1002/ptr.5694. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi-Rad J, Herrera-Bravo J, Salazar LA, Shaheen S, Abdulmajid Ayatollahi S, Kobarfard F, et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Tan S, editor. Evidence-Based Complement Altern Med. 2021;2021:1–9. [DOI] [PMC free article] [PubMed] [Retracted]
- 4.Wozniak D, Drys A, Matkowski A. Antiradical and antioxidant activity of flavones from Scutellariae baicalensis radix. Nat Prod Res. 2015;29:1567–1570. doi: 10.1080/14786419.2014.983920. [DOI] [PubMed] [Google Scholar]
- 5.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res Greece. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 6.Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets - Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- 7.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017;95:1021–32. [DOI] [PubMed]
- 8.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018;8:104. doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Zhao J, Hölscher C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs. 2017;31:639–52. [DOI] [PubMed]
- 10.Liang W, Huang X, Chen W. The effects of Baicalin and Baicalein on cerebral ischemia: A review. Aging Dis. 2017;8:850–67. [DOI] [PMC free article] [PubMed]
- 11.Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng P, et al. The fascinating effects of baicalein on cancer: A review. Int J Mol. Sci. 2016;17:1681. [DOI] [PMC free article] [PubMed]
- 12.Donald G, Hertzer K, Eibl G. Baicalein - An Intriguing Therapeutic Phytochemical in Pancreatic Cancer. Curr Drug Targets. 2012;13:1772–1776. doi: 10.2174/138945012804545470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. [DOI] [PubMed]
- 14.Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014;354:5–11. [DOI] [PMC free article] [PubMed]
- 15.Zhang XP, Zhang L, He JX, Zhang RP, Cheng QH, Zhou YF, et al. Experimental study of therapeutic efficacy of Baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:717–24. [DOI] [PMC free article] [PubMed]
- 16.Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Wei Y, Luo Q, Xu F, Zhao Z, Zhang H, et al. Baicalin attenuates inflammation in mice with OVA-induced asthma by inhibiting NF-kappaB and suppressing CCR7/CCL19/CCL21. Int J Mol Med Greece. 2016;38:1541–1548. doi: 10.3892/ijmm.2016.2743. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Matsushita N, Yokoi-Hayashi K, Okuda H. Effects of baicalein isolated from Scutellaria baicalensis Radix on adhesion molecule expression induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. Planta Med. 2001;67:331–334. doi: 10.1055/s-2001-14328. [DOI] [PubMed] [Google Scholar]
- 19.Alsharairi NA. Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. Int J Environ Res Public Health. 2021;18:5243. doi: 10.3390/ijerph18105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao J-R, Do C-W, To C-H. Potential Therapeutic Effects of Baicalein, Baicalin, and Wogonin in Ocular Disorders. J Ocul Pharmacol Ther. 2014;30:605–614. doi: 10.1089/jop.2014.0074. [DOI] [PubMed] [Google Scholar]
- 21.Baradaran Rahimi V, Askari VR, Hosseinzadeh H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother Res. 2021;35:3558–74. [DOI] [PubMed]
- 22.Tang Y-J, Zhou F-W, Luo Z-Q, Li X-Z, Yan H-M, Wang M-J, et al. Multiple Therapeutic Effects of Adjunctive Baicalin Therapy in Experimental Bacterial Meningitis. Inflammation. 2010;33:180–188. doi: 10.1007/s10753-009-9172-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhou R, Han X, Wang J, Sun J. Baicalin may have a therapeutic effect in attention deficit hyperactivity disorder. Med. Hypotheses. 2015;85:761–4. [DOI] [PubMed]
- 24.Fang P, Yu M, Zhang L, Wan D, Shi M, Zhu Y, et al. Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol Cell Endocrinol. 2017;448:77–86. doi: 10.1016/j.mce.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Fang P, Sun Y, Gu X, Shi M, Bo P, Zhang Z, et al. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine. 2019;64:153074. doi: 10.1016/j.phymed.2019.153074. [DOI] [PubMed] [Google Scholar]
- 26.Yu M, Han S, Wang M, Han L, Huang Y, Bo P, et al. Baicalin protects against insulin resistance and metabolic dysfunction through activation of GALR2/GLUT4 signaling. Phytomedicine. 2022;95:153869. doi: 10.1016/j.phymed.2021.153869. [DOI] [PubMed] [Google Scholar]
- 27.Liao P, Li Y, Li M, Chen X, Yuan D, Tang M, et al. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-κB and increasing mTOR signaling pathways in piglets. Food Chem Toxicol. 2020;140:111326. doi: 10.1016/j.fct.2020.111326. [DOI] [PubMed] [Google Scholar]
- 28.Xue D, Zhang W, Zhang Y, Wang H, Zheng B, Shi X. Adjusting Effects of Baicalin for Nuclear Factor-κB and Tumor Necrosis Factor-α on Rats With Caerulein-Induced Acute Pancreatitis. Mediators Inflamm. 2006;2006:1–6. doi: 10.1155/MI/2006/26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherwani MA, Yang K, Jani A, Abed RA, Taufique AK, Dosunmu TG, et al. Protective Effect of Baicalin Against TLR4-mediated UVA-induced Skin Inflammation. Photochem Photobiol. 2019;95:605–611. doi: 10.1111/php.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui J, Feng Y, Li H, Cao R, Tian W, Jiang Z. Baicalin protects mouse testis from injury induced by heat stress. J Therm Biol. 2019;82:63–69. doi: 10.1016/j.jtherbio.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res. 2017;16:2–23. [PMC free article] [PubMed]
- 32.Alavi MS, Fanoudi S, Ghasemzadeh Rahbardar M, Mehri S, Hosseinzadeh H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother Res. 2021;35:1313–1328. doi: 10.1002/ptr.6894. [DOI] [PubMed] [Google Scholar]
- 33.Hosseini A, Hosseinzadeh H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed Pharmacother. 2018;99:411–421. doi: 10.1016/j.biopha.2018.01.072. [DOI] [PubMed] [Google Scholar]
- 34.Fouad AEA, Fouad AA, Al-Melhim WN. Protective effect of baicalin in rats exposed to arsenic-induced testicular toxicity. Indian J Forensic Med Toxicol. 2018;12:256–261. doi: 10.5958/0973-9130.2018.00112.3. [DOI] [Google Scholar]
- 35.Li XX, He GR, Mu X, Xu B, Tian S, Yu X, et al. Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur J Pharmacol. 2012;674:227–33. [DOI] [PubMed]
- 36.Liu W, Chen X, Liu J, Chen C, Ai J. The effect of baicalein on bleomycin-induced fibrosis in lungs of rats. Chinese J Appl Physiol China. 2009;25:145–149. [PubMed] [Google Scholar]
- 37.Ueng YF, Shyu CC, Liu TY, Oda Y, Lin YL, Liao JF, et al. Protective effects of baicalein and wogonin against benzo[a]pyrene- and aflatoxin B1-induced genotoxicities. Biochem Pharmacol. 2001;62:1653–60. [DOI] [PubMed]
- 38.De Boer JG, Quiney B, Walter PB, Thomas C, Hodgson K, Murch SJ, et al. Protection against aflatoxin-B 1 -induced liver mutagenesis by Scutellaria baicalensis. Mutat Res- Fundam Mol Mech Mutagen. 2005;578:15–22. [DOI] [PubMed]
- 39.Kim BR, Kim DH, Park R, Kwon KB, Ryu DG, Kim YC, et al. Effect of an extract of the root of Scutellaria baicalensis and its flavonoids on aflatoxin B1 oxidizing cytochrome P450 enzymes. Planta Med. 2001;67:396–399. doi: 10.1055/s-2001-15810. [DOI] [PubMed] [Google Scholar]
- 40.Qiu J, Niu X, Dong J, Wang D, Wang J, Li H, et al. Baicalin protects mice from staphylococcus aureus pneumonia via inhibition of the cytolytic activity of-hemolysin. J Infect Dis. 2012;206:292–301. [DOI] [PubMed]
- 41.Liu S, Liu B, Luo Z-Q, Qiu J, Zhou X, Li G, et al. The combination of osthole with baicalin protects mice from Staphylococcus aureus pneumonia. World J Microbiol Biotechnol. 2017;33:11. doi: 10.1007/s11274-016-2162-9. [DOI] [PubMed] [Google Scholar]
- 42.Jia F, Ma W, Zhang X, Wang D, Zhou X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J Dairy Sci. 2020;103:2731–2742. doi: 10.3168/jds.2019-17619. [DOI] [PubMed] [Google Scholar]
- 43.Dong J, Zhang Y, Chen Y, Niu X, Zhang Y, Yang C, et al. Baicalin inhibits the lethality of Shiga-like toxin 2 in mice. Antimicrob. Agents Chemother. 2015;59:7054–60. [DOI] [PMC free article] [PubMed]
- 44.Zhang Y, Qi Z, Liu Y, He W, Yang C, Wang Q, et al. Baicalin Protects Mice from Lethal Infection by Enterohemorrhagic Escherichia coli. Front Microbiol. 2017;8:395. [DOI] [PMC free article] [PubMed]
- 45.Vinh, Shinohara, Yamada, Duc, Nakayama, Ozawa, et al. Baicalein Inhibits Stx1 and 2 of EHE: Effects of Baicalein on the Cytotoxicity, Production, and Secretion of Shiga Toxins of Enterohaemorrhagic Escherichia coli. Toxins (Basel). 2019;11:505. [DOI] [PMC free article] [PubMed]
- 46.Dong J, Zhang Y, Chen Y, Niu X, Zhang Y, Li R, et al. Baicalin inhibits the lethality of ricin in mice by inducing protein oligomerization. J Biol Chem. 2015;290:12899–12907. doi: 10.1074/jbc.M114.632828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Feng C, Li C, Yao J, Xie X, Gong L, et al. Baicalin protects mice from aristolochic acid I-Induced kidney injury by induction of CYP1A through the aromatic hydrocarbon receptor. Int J Mol Sci. 2015;16:16454–16468. doi: 10.3390/ijms160716454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu LL, Gong LK, Wang H, Xiao Y, Wu XF, Zhang YH, et al. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007;27:582–91. [DOI] [PubMed]
- 49.Zhang Y, Shan L, Hua Y, Wang D, Zeng H, Liu R, et al. Baicalein Selectively Induces Apoptosis in Activated Lymphocytes and Ameliorates Concanavalin A-Induced Hepatitis in Mice. PLoS One. 2013;8:e69592. [DOI] [PMC free article] [PubMed]
- 50.Im AR, Kim YH, Uddin MR, Lee HW, Chae SW, Kim YH, et al. Scutellaria baicalensis extracts and flavonoids protect rat l6 cells from antimycin a-induced mitochondrial dysfunction. Evidence-based Complement Altern Med. 2012;2012:517965. [DOI] [PMC free article] [PubMed]
- 51.Huan SK-H, Wang K-T, Yeh S-D, Lee C-J, Lin L-C, Liu D-Z, et al. Scutellaria baicalensis Alleviates Cantharidin-Induced Rat Hemorrhagic Cystitis through Inhibition of Cyclooxygenase-2 Overexpression. Molecules. 2012;17:6277–89. [DOI] [PMC free article] [PubMed]
- 52.Zhang SF, Dong YC, Zhang XF, Wu XG, Cheng JJ, Guan LH, et al. Flavonoids from Scutellaria attenuate okadaic acid-induced neuronal damage in rats. Brain Inj. 2015;29:1376–1382. doi: 10.3109/02699052.2015.1042053. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Shi G, Wang J, Zhang D, Pan Y, Dou H, et al. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res Ther. 2019;21:1–14. doi: 10.1186/s13075-019-1876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Sheng Y, Shi L, Zheng Z, Chen M, Lu B, et al. Quercetin and baicalein suppress monocrotaline-induced hepatic sinusoidal obstruction syndrome in rats. Eur J Pharmacol Netherlands. 2017;795:160–168. doi: 10.1016/j.ejphar.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Schultze AE, Roth RA. Chronic pulmonary hypertension-the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Heal Part B. 1998;1:271–346. doi: 10.1080/10937409809524557. [DOI] [PubMed] [Google Scholar]
- 56.Hsu WL, Lin YC, Jeng JR, Chang HY, Chou TC. Baicalein Ameliorates Pulmonary Arterial Hypertension Caused by Monocrotaline through Downregulation of ET-1 and ET AR in Pneumonectomized Rats. Am J Chin Med. 2018;46:769–783. doi: 10.1142/S0192415X18500404. [DOI] [PubMed] [Google Scholar]
- 57.Shi R, Zhu D, Wei Z, Fu N, Wang C, Liu L, et al. Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition. Life Sci. 2018;207:442–450. doi: 10.1016/j.lfs.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 58.Shi R, Wei Z, Zhu D, Fu N, Wang C, Yin S, et al. Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting vascular remodeling in rats. Pulm Pharmacol Ther England. 2018;48:124–135. doi: 10.1016/j.pupt.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, Zhang L, Sun C, Kong F, Wang J, Xin Q, et al. Baicalin attenuates monocrotaline-induced pulmonary hypertension through bone morphogenetic protein signaling pathway. Oncotarget. 2017;8:63430–41. [DOI] [PMC free article] [PubMed]
- 60.Yan G, Wang J, Yi T, Cheng J, Guo H, He Y, et al. Baicalin prevents pulmonary arterial remodeling in vivo via the AKT/ERK/NF-kappaB signaling pathways. Pulm Circ. United States; 2019;9:2045894019878599. [DOI] [PMC free article] [PubMed]
- 61.Luan Y, Chao S, Ju ZY, Wang J, Xue X, Qi TG, et al. Therapeutic effects of baicalin on monocrotaline-induced pulmonary arterial hypertension by inhibiting inflammatory response. Int. Immunopharmacol. 2015;26:188–93. [DOI] [PubMed]
- 62.Gal Y, Mazor O, Falach R, Sapoznikov A, Kronman C, Sabo T. Treatments for pulmonary ricin intoxication: Current aspects and future prospects. Toxins (Basel). 2017;9:311. [DOI] [PMC free article] [PubMed]
- 63.Liu J, Zhu X, Kim SJ, Zhang W. Antimycin-type depsipeptides: discovery, biosynthesis, chemical synthesis, and bioactivities. Nat Prod Rep. 2016;33:1146–1165. doi: 10.1039/C6NP00004E. [DOI] [PubMed] [Google Scholar]
- 64.Xiao GL, Zhang CH, Liu GD, Liu FY, Liu ZY, Hu SY, et al. Clinical study of the effects of baicalin on arrhythmia induced by aconitine poisoning. J Med Plants Res. 2011;5:88–92. [Google Scholar]
- 65.Xue X, Zhang S, Jiang W, Wang J, Xin Q, Sun C, et al. Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF-α signaling pathway. Pharmacol Res Perspect. 2021;9:e00703. doi: 10.1002/prp2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai C, Lin Y, Wang H, Chou T. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014;153:197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Deng J, Wang DX, Liang AL, Tang J, Xiang DK. Effects of baicalin on alveolar fluid clearance and α-ENaC expression in rats with LPS-induced acute lung injury. Can J Physiol Pharmacol. 2017;95:122–128. doi: 10.1139/cjpp-2016-0212. [DOI] [PubMed] [Google Scholar]
- 68.Long Y, Xiang Y, Liu S, Zhang Y, Wan J, Yang Q, et al. Baicalin Liposome Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice via Inhibiting TLR4/JNK/ERK/NF-κB Pathway. Mediators Inflamm. 2020;2020:8414062. doi: 10.1155/2020/8414062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Zhang Y, Zhang W, Liu H, Sun C, Zhang B, et al. Inhibition of myeloid differentiation factor 2 by baicalein protects against acute lung injury. Phytomedicine. 2019;63:152997. [DOI] [PubMed]
- 70.Ding XM, Pan L, Wang Y, Xu QZ. Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-B pathway in CX3CL1-knockout mice. Int J Mol Med. 2016;37:703–715. doi: 10.3892/ijmm.2016.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng X, Hu L, Li W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1421–1433. doi: 10.1007/s00210-019-01680-9. [DOI] [PubMed] [Google Scholar]
- 72.Huang KL, Chen CS, Hsu CW, Li MH, Chang H, Tsai SH, et al. Therapeutice effects of baicalin on lipopolysaccharide-induced acute lung injury in rats. Am J Chin Med. 2008;36:301–311. doi: 10.1142/S0192415X08005783. [DOI] [PubMed] [Google Scholar]
- 73.Dong S, Zhong Y, Lu W, Li G, Jiang H, Mao B. Baicalin Inhibits Lipopolysaccharide-Induced Inflammation Through Signaling NF-kappaB Pathway in HBE16 Airway Epithelial Cells. Inflammation. 2015;38:1493–1501. doi: 10.1007/s10753-015-0124-2. [DOI] [PubMed] [Google Scholar]
- 74.Feng T, Zhou L, Gai S, Zhai Y, Gou N, Wang X, et al. Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS‐induced pro‐inflammatory responses through NF‐кB, MAPK, and PI3K‐Akt signaling pathways in alveolar epithelial type II cells. Phytother Res. 2019;33:3251–60. [DOI] [PubMed]
- 75.Chen JJ, Huang CC, Chang HY, Li PY, Liang YC, Deng JS, et al. Scutellaria baicalensis Ameliorates Acute Lung Injury by Suppressing Inflammation in Vitro and in Vivo. Am J Chin Med. 2017;45:137–157. doi: 10.1142/S0192415X17500100. [DOI] [PubMed] [Google Scholar]
- 76.Chen HM, Liou SF, Hsu JH, Chen TJ, Cheng TL, Chiu CC, et al. Baicalein inhibits HMGB1 release and MMP-2/-9 expression in lipopolysaccharide-induced cardiac hypertrophy. Am J Chin Med. 2014;42:785–797. doi: 10.1142/S0192415X14500505. [DOI] [PubMed] [Google Scholar]
- 77.Li MF, Hu XY, Chen LW, Lian J, Zhao GJ, Hong GL, et al. Baicalin regulates STIM1-mediated calcium overload and reduces apoptosis of cardiomyocytes induced by lipopolysaccharide. Chin Med J (Engl). China; 2019;99:3176–82. [DOI] [PubMed]
- 78.Liu X, Wang S, Zhao G. Baicalin relieves lipopolysaccharide-evoked inflammatory injury through regulation of miR-21 in H9c2 cells. Phytother Res. 2020;34:1134–41. [DOI] [PubMed]
- 79.Wan JY, Gong X, Zhang L, Li HZ, Zhou YF, Zhou QX. Protective effect of baicalin against Lipopolysaccharide/d-galactosamine-induced liver injury in mice by up-regulation of Heme oxygenase-1. Eur J Pharmacol. 2008;587:302–308. doi: 10.1016/j.ejphar.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 80.Liu A, Wang W, Fang H, Yang Y, Jiang X, Liu S, et al. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur J Pharmacol. 2015;748:45–53. doi: 10.1016/j.ejphar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 81.Wang C, Nie H, Li K, Zhang Y-X, Shu K-G, Chen X-J. Protective effect of baicalin solid dispersion on D-galactosamine induced acute hepatic injury in mice. Chinese J Integr Tradit West Med. 2014;34:71–74. [PubMed] [Google Scholar]
- 82.Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun X, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. 2017;8:1–12. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y, Sun M, Yang X, Ma A, Ma Y, Zhao A. Baicalin relieves inflammation stimulated by lipopolysaccharide via upregulating TUG1 in liver cells. J Physiol Biochem. 2019;75:463–73. [DOI] [PubMed]
- 84.Wu Y, Lian L, Wan Y, Nan J. Chemico-Biological Interactions Baicalein inhibits nuclear factor- B and apoptosis via c-FLIP and MAPK in d -GalN / LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–534. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Thanh HN, Minh HPT, Le TA, Ly HDT, Huu TN, Duc LV, et al. Ethanol extracts of Scutellaria baicalensis protect against lipopolysaccharideinduced acute liver injury in mice. Asian Pac J Trop Biomed. 2015;5:761–767. doi: 10.1016/j.apjtb.2015.07.007. [DOI] [Google Scholar]
- 86.Liao S, Li P, Wang J, Zhang Q, Xu D, Yang M, et al. Protection of baicalin against lipopolysaccharide induced liver and kidney injuries based on 1H NMR metabolomic profiling. Toxicol Res (Camb) 2016;5:1148–1159. doi: 10.1039/C6TX00082G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Y, Liu MW, Zhao YH, Lu YX, Wang YA, Tong CW. Baicalin attenuates lipopolysaccharide-induced renal tubular epithelial cell injury by inhibiting the TXNIP/NLRP3 signalling pathway via increasing miR-223–3p expression. J Biol Regul Homeost Agents. 34:69–82. [DOI] [PubMed]
- 88.Yeh J-H, Chiu H-F, Wang J-S, Lee J-K, Chou T-C. Protective Effect of Baicalein Extracted from Scutellaria baicalensis against Lipopolysaccharide-Induced Glomerulonephritis in Mice. Int J Pharmacol. 2010;6:81–88. doi: 10.3923/ijp.2010.81.88. [DOI] [Google Scholar]
- 89.Hwang YK, Jinhua M, Choi BR, Cui CA, Jeon WK, Kim H, et al. Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion- induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments. J Ethnopharmacol. 2011;137:681–689. doi: 10.1016/j.jep.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 90.Shah M-A, Park D-J, Kang J-B, Kim M-O, Koh P-O. Baicalin attenuates lipopolysaccharide-induced neuroinflammation in cerebral cortex of mice via inhibiting nuclear factor kappa B (NF-κB) activation. J Vet Med Sci. 2019;81:1359–67. [DOI] [PMC free article] [PubMed]
- 91.Shah M-A, Park D-J, Kang J-B, Kim M-O, Koh P-O. Baicalin alleviates lipopolysaccharide-induced neuroglial activation and inflammatory factors activation in hippocampus of adult mice. Lab Anim Res. 2020;36:32. doi: 10.1186/s42826-020-00058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang G, Wu J, Wang J. Effects of baicalin from traditional Chinese medicine against lipopolysaccharide-induced inflammation in BV2 cells in vitro. Lat Am J Pharm. 2019;38:204–208. [Google Scholar]
- 93.Yan J-J, Du G-H, Qin X-M, Gao L. Baicalein attenuates the neuroinflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-kappaB expressions and regulation of metabolic abnormality. Int Immunopharmacol. Netherlands; 2020;79:106092. [DOI] [PubMed]
- 94.Li F-Q, Wang T, Pei Z, Liu B, Hong J-S. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm Austria. 2005;112:331–347. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- 95.Chen C-J, Raung S-L, Liao S-L, Chen S-Y. Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem Pharmacol. 2004;67:957–965. doi: 10.1016/j.bcp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Tsai CC, Lin MT, Wang JJ, Liao JF, Huang WT. The antipyretic effects of baicalin in lipopolysaccharide-evoked fever in rabbits. Neuropharmacology. 2006;51:709–717. doi: 10.1016/j.neuropharm.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Ye L, Tao Y, Wang Y, Feng T, Li H. The effects of baicalin on the TLR2/4 signaling pathway in the peripheral blood mononuclear cells of a lipopolysaccharide-induced rat fever model. Int Immunopharmacol. 2015;25:106–111. doi: 10.1016/j.intimp.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 98.Luo W, Wang CY, Jin L. Baicalin Downregulates Porphyromonas gingivalis Lipopolysaccharide-Upregulated IL-6 and IL-8 Expression in Human Oral Keratinocytes by Negative Regulation of TLR Signaling. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Luo H, Yang L, Li Y. Baicalein induces apoptosis and reduces inflammation in LPS-stimulated keratinocytes by blocking the activation of NF-κB: implications for alleviating oral lichen planus. Cell Mol Biol (Noisy-le-grand). 2016;62:55–60. [PubMed]
- 100.Wu Q, Ye H, Zhu Y-Z, Guo M, He X-X, Zheng X-B. Protective effect of baicalin against LPS-induced intestinal injury. China J Chinese Mater medica. 2013;38:2854–2858. [PubMed] [Google Scholar]
- 101.Chen J, Zhang R, Wang J, Yu P, Liu Q, Zeng D, et al. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can J Physiol Pharmacol. 2015;93:233–237. doi: 10.1139/cjpp-2014-0262. [DOI] [PubMed] [Google Scholar]
- 102.Chen YC, Shen SC, Chen LG, Lee TJF, Yang LL. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem Pharmacol. 2001;61:1417–1427. doi: 10.1016/S0006-2952(01)00594-9. [DOI] [PubMed] [Google Scholar]
- 103.Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem Pharmacol. 2007;73:793–804. doi: 10.1016/j.bcp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 104.Lee YM, Cheng PY, Chim LS, Kung CW, Ka SM, Chung MT, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J Ethnopharmacol. 2011;135:179–185. doi: 10.1016/j.jep.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 105.Liu LL, Gong LK, Wang H, Xiao Y, Wu XF, Zhang YH, et al. Baicalin inhibits macrophage activation by lipopolysaccharide and protects mice from endotoxin shock. Biochem Pharmacol. 2008;75:914–22. doi: 10.1016/j.bcp.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 106.He X, Wei Z, Zhou E, Chen L, Kou J, Wang J, et al. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int Immunopharmacol. 2015;28:470–476. doi: 10.1016/j.intimp.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Li H, Cong X, Wang X, Jiang Z, Zhang Q, et al. Baicalin attenuates lipopolysaccharide induced inflammation and apoptosis of cow mammary epithelial cells by regulating NF-κB and HSP72. Int Immunopharmacol. 2016;40:139–145. doi: 10.1016/j.intimp.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Zhao Y, Zhong X. Protective effects of Baicalin on decidua cells of LPS-induced mice abortion. J Immunol Res. 2014;2014:1–7. doi: 10.1155/2014/387950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma J, Wang R, Yan H, Xu R, Xu A, Zhang J. Protective Effects of Baicalin on Lipopolysaccharide-Induced Injury in Caenorhabditis elegans. Pharmacology Switzerland. 2020;105:109–117. doi: 10.1159/000503238. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y, Bao Y, Shi W, Wang X, Zhong X. Protective effects of baicalin on lipopolysaccharide (LPS)-induced implantation failure and the uterine endometrium in mice. African J Pharm Pharmacol. 2011;5:1661–1668. doi: 10.5897/AJPP11.108. [DOI] [Google Scholar]
- 111.Min W, Lin XF, Miao X, Wang BT, Yang ZL, Luo D. Inhibitory effects of Baicalin on ultraviolet B-induced photo-damage in keratinocyte cell line. Am J Chin Med. 2008;36:745–760. doi: 10.1142/S0192415X0800620X. [DOI] [PubMed] [Google Scholar]
- 112.Zhou B-R, Luo D, Wei F-D, Chen X-E, Gao J. Baicalin protects human fibroblasts against ultraviolet B-induced cyclobutane pyrimidine dimers formation. Arch Dermatol Res. 2008;300:331–334. doi: 10.1007/s00403-008-0851-4. [DOI] [PubMed] [Google Scholar]
- 113.Zhang JA, Yin Z, Ma LW, Yin ZQ, Hu YY, Xu Y, et al. The protective effect of baicalin against UVB irradiation induced photoaging: An in vitro and in vivo study. PLoS One. 2014;9:e99703. [DOI] [PMC free article] [PubMed]
- 114.Chang W-S, Lin E-Y, Hsu S-W, Hu P-S, Chuang C-L, Liao C-H, et al. Baicalin Scavenged Reactive Oxygen Species and Protected Human Keratinocytes Against UVB-induced Cytotoxicity. In Vivo (Brooklyn) 2016;30:605–610. [PubMed] [Google Scholar]
- 115.Zhang JA, Luan C, Huang D, Ju M, Chen K, Gu H. Induction of autophagy by baicalin through the AMPK-mTOR pathway protects human skin fibroblasts from ultraviolet B radiation-induced apoptosis. Drug Des Devel Ther. 2020;14:417–428. doi: 10.2147/DDDT.S228047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Min W, Liu X, Qian Q, Lin B, Wu D, Wang M, et al. The effects of baicalin against UVA-induced photoaging in skin fibroblasts. Am J Chin Med. 2014;42:709–727. doi: 10.1142/S0192415X14500463. [DOI] [PubMed] [Google Scholar]
- 117.Zhou BR, Yin H Bin, Xu Y, Wu D, Zhang ZH, Zhi-Qiang Yin, et al. Baicalin protects human skin fi broblasts from ultraviolet A radiation-induced oxidative damage and apoptosis. Free Radic Res. 2012;46:1458–71. [DOI] [PubMed]
- 118.Wang S-C, Chen S-F, Lee Y-M, Chuang C-L, Bau D-T, Lin S-S. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo (Brooklyn) 2013;27:707–714. [PubMed] [Google Scholar]
- 119.Manca ML, Mir-Palomo S, Caddeo C, Nacher A, Díez-Sales O, Peris JE, et al. Sorbitol-penetration enhancer containing vesicles loaded with baicalin for the protection and regeneration of skin injured by oxidative stress and UV radiation. Int J Pharm. 2019;555:175–183. doi: 10.1016/j.ijpharm.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 120.Bing-Rong Z, Song-Liang J, Xiao-E C, Xiang-Fei L, Bao-Xiang C, Jie G, et al. Protective effect of the Baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. Photodermatol Photoimmunol Photomed. 2008;24:175–182. doi: 10.1111/j.1600-0781.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 121.Zhou BR, Liu WL, Luo D. Protective effect of baicalin against multiple ultraviolet b exposure-mediated injuries in C57BL/6 mouse skin. Arch Pharm Res. 2011;34:261–268. doi: 10.1007/s12272-011-0212-2. [DOI] [PubMed] [Google Scholar]
- 122.Oh MC, Piao MJ, Fernando PMDJ, Han X, Hewage SRKM, Park JE, et al. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol Ther. 2016;24:616–622. doi: 10.4062/biomolther.2016.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu J, Zhong Y, Lin Z, Lin X, Chen Z, Wu X, et al. Baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type II alveolar epithelial cells via TGF-β and ERK/GSK3β signaling pathways. Biomed Pharmacother. 2017;95:1219–1224. doi: 10.1016/j.biopha.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 124.Yang SJ, Jo H, Kim JG, Jung SH. Baicalin attenuates laser-induced choroidal neovascularization. Curr Eye Res. 2014;39:745–751. doi: 10.3109/02713683.2013.868908. [DOI] [PubMed] [Google Scholar]
- 125.Jang H, Lee J, Park S, Kim JS, Shim S, Lee S, et al. Baicalein mitigates radiation-induced enteritis by improving endothelial dysfunction. Front Pharmacol. 2019;10:1–13. doi: 10.3389/fphar.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee EK, Kim JM, Choi J, Jung KJ, Kim DH, Chung SW, et al. Modulation of NF-κB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic Res. 2011;45:507–517. doi: 10.3109/10715762.2011.555479. [DOI] [PubMed] [Google Scholar]
- 127.Wang M, Dong Y, Wu J, Li H, Zhang Y, Fan S, et al. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. Netherlands; 2020;261:118463. [DOI] [PubMed]
- 128.Gandhi NM. Baicalein protects mice against radiation-induced DNA damages and genotoxicity. Mol Cell Biochem. 2013;379:277–281. doi: 10.1007/s11010-013-1649-z. [DOI] [PubMed] [Google Scholar]
- 129.Guo X, Chi S, Cong X, Li H, Jiang Z, Cao R, et al. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod Toxicol. 2015;57:196–203. doi: 10.1016/j.reprotox.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 130.Kang TH, Hong BN, Park C, Kim SY, Park R. Effect of baicalein from Scutellaria baicalensis on prevention of noise-induced hearing loss. Neurosci Lett. 2010;469:298–302. doi: 10.1016/j.neulet.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez I, Hong BN, Nam YH, Kim EY, Park GH, Ji MG, et al. Bioconversion of Scutellaria baicalensis extract can increase recovery of auditory function in a mouse model of noise-induced hearing loss. Biomed Pharmacother. 2017;93:1303–1309. doi: 10.1016/j.biopha.2017.07.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated.