Abstract
The importance of cell-mediated immunity (CMI) and CD4+ T lymphocytes in host resistance against Cryptococcus neoformans is well documented and is exemplified by the high susceptibility to progressive infection with this pathogen of AIDS patients with reduced CD4+ T-cell numbers. Although much has been learned about the role of CMI in the clearance of C. neoformans from the lungs and other internal organs, less is known about the protective mechanisms in the brain, the organ most frequently involved with a fatal outcome of cryptococcosis. We hypothesized that host resistance mechanisms against C. neoformans in the central nervous system (CNS) were similar to those outside the CNS (i.e., gamma interferon [IFN-γ], CD4+ T cells, and others). To test this hypothesis, we used a murine model of cryptococcal meningitis whereby cryptococci are introduced directly into the CNS. In experiments where mice were immunized to mount an anticryptococcal CMI response, our results indicate that immunization induced protective mechanisms that could be detected in the CNS by inhibition of the growth of viable yeast cells. Flow cytometric analyses of leukocytes in brain and spinal cord homogenates revealed that T lymphocytes, macrophages, and neutrophils accumulated in C. neoformans-infected brains of immune mice. In vivo depletion of CD4+ T cells, but not CD8+ T cells, resulted in significantly reduced leukocyte accumulation in the brains of immune mice. Furthermore, depletion of CD4+ T cells or neutralization of IFN-γ exacerbated CNS infection in immune mice, suggesting a critical role for CMI mechanisms in acquired protection in the CNS.
Cryptococcosis is a life-threatening disease caused by the encapsulated fungus Cryptococcus neoformans. The disease is generally thought to be acquired via inhalation and, following dissemination of the organism into the bloodstream, the disease generally is manifested as often fatal meningoencephalitis (27, 33). The incidence of cryptococcosis has increased significantly as a result of the growing number of immunocompromised individuals, due to the expanding use of immunosuppressive drugs and to the spread of human immunodeficiency virus. Loss of CD4+ T cells predisposes individuals to progressive infection with C. neoformans, emphasizing the importance of cell-mediated immunity (CMI) in host resistance to this organism, and may explain the high incidence of cryptococcosis in AIDS patients with reduced CD4+ T-cell numbers. Immunocompromised individuals suffering from cryptococcosis must remain on antifungal therapy for life because the currently available drugs do not completely eradicate the organism from the body (45).
Many reports have described pulmonary infection with C. neoformans and the immune events and interactions taking place in the lungs following infection (19, 20, 22, 32, 35). It is clear that CD4+ T lymphocytes and the development of CMI are required for the clearance of the organism from pulmonary and other non-central nervous system (CNS) sites of infection in animal models (6, 14–17, 20, 22, 23, 31, 35, 41, 46). Certain cytokines, such as gamma interferon (IFN-γ), have also been implicated as critical components of host resistance in pulmonary cryptococcosis and disseminated cryptococcosis (1, 18, 24–26). However, there is less understanding of the host resistance mechanisms that function directly in the brain, the organ most frequently involved with a fatal outcome of cryptococcosis. In light of the fact that the brain has a restrictive blood-brain barrier and unique effector cells (microglial cells and astrocytes) (10), it is not clear whether the same mechanisms (CD4+ T cells, IFN-γ, and so forth) that are important in anticryptococcal resistance in the lungs and other extracerebral tissues play a direct role in host defense in the brain or if they simply lower the C. neoformans burden in extracerebral tissues, resulting in less seeding of the organism into the brain.
In the present study, we wanted to determine whether or not anticryptococcal CMI mechanisms afford protection in the CNS. To ensure that the groups of mice being examined had the same number of organisms in the CNS at the start of the experiment, we used an intracerebral (i.c.) infection with viable C. neoformans cells. Because the same numbers of organisms were deposited directly into the CNS, the brain CFU in different comparative groups of mice (immune versus control) could be monitored to determine whether host responses affected the brain fungal burden. To induce a systemic anticryptococcal CMI response, mice were injected with a nonreplicating cryptococcal antigen (CneF) emulsified in incomplete Freund's adjuvant (IFA). Thus, using this murine model, we were able to induce an anticryptococcal CMI response with a nonreplicating antigen and monitor the effects of this systemically produced response on regional host resistance against C. neoformans in the CNS.
MATERIALS AND METHODS
Mice.
Female inbred CBA/J mice purchased from Jackson Laboratory (Bar Harbor, Maine) were used at 7 to 10 weeks of age.
Maintenance of endotoxin-free conditions.
The experimental conditions were maintained endotoxin free by using purchased endotoxin-free plasticware and heating all glassware for 3 h at 180°C. All reagents used in the experiments contained less than 8 pg of endotoxin/ml (minimal detectable level) when tested with the Limulus amebocyte lysate assay (Whittaker Bioproducts Inc., Walkersville, Md.).
Antigen preparation and analysis.
The cryptococcal culture filtrate antigen (CneF) used for immunization and footpad and sponge injections was prepared from C. neoformans 184-A (38) as previously described (4). Briefly, a defined growth medium consisting of 2% dextrose, 0.4 mM thiamine, 1% trace elements (0.5 mg of CuSO4 · 5H2O, 200 mg of ZnSO4 · 7H2O, 3.2 mg of MnCl2 · 4H2O, 8 g of MgSO4 · 7H2O, 5.4 mg of Na2MoO4 · 2H2O, and 5.7 mg of H3BO3 per liter of endotoxin-free water), 10 g of asparagine, 0.025 g of CaCl2, and 0.4 g of K2HPO4 per liter of endotoxin-free water was prepared, and the pH was adjusted to 5.0. The medium was sterilized by autoclaving and inoculated with 109 yeast cells/liter, and the culture was incubated for 5 days at 30°C. Following incubation, all cultures were examined microscopically for evidence of contamination, and Formalin (2% [vol/vol]) was added to kill the cryptococcal cells.
The supernatant from the culture was collected with a Millipore OM-141 Pellicon Tangential Flow System and a 0.45-μm-pore-size cassette (Millipore, Bedford, Mass.) to remove all cryptococcal yeast cells. The resulting supernatant was washed extensively with sterile endotoxin-free physiological saline and concentrated 10-fold by use of the Pellicon System with a 30,000-molecular-weight-cutoff cassette. Thus, the supernatant was composed of only C. neoformans-produced molecules with a molecular weight of greater than 30,000 and suspended in saline. The concentrated supernatant, designated CneF, was filter sterilized and stored at −20°C until used. The CneF preparation used in these studies had a protein concentration of 0.243 mg/ml, as determined by the bicinchoninic acid assay (BCA Protein Assay; Pierce Chemical Co., Rockford, Ill.), and a carbohydrate concentration of 3.2 mg/ml, as determined by the phenol-sulfuric acid assay (9). This lot of CneF gave a reaction equivalent to 32 pg of endotoxin/ml in the Limulus assay. CneF contains high concentrations of glucuronoxylomannan, which consists of a mannan backbone with side chains of glucuronic acid and xylose (7). Because glucuronic acid gives a positive reaction in the Limulus assay (42) and endotoxin-free reagents and glassware were used, the reactivity demonstrated by CneF was considered to be due to the glucuronic acid rather than to endotoxin contamination.
Induction and elicitation of the anticryptococcal delayed-type hypersensitivity (DTH) response.
To induce an anticryptococcal CMI response, mice were injected subcutaneously at two sites at the base of the tail with 0.1 ml of a 1:1 emulsion of CneF in IFA. Control animals were injected in a similar manner with sterile endotoxin-free physiological saline emulsified 1:1 with IFA (saline-IFA).
The level of anticryptococcal CMI reactivity induced by the immunization protocol was determined as previously described (4). At 6 days after immunization, the hind footpads of the mice were measured and then injected with 30 μl of saline in the left footpad and 30 μl of CneF in the right footpad. After 24 h, the footpads were measured, and the increase in footpad thickness (in 10−3 in.) was calculated with the formula (CneF24h − CneF0h) − (saline24h − saline0h) = swelling, where CneF24h is equal to the measurement of the CneF-injected footpad 24 h after injection, CneF0h is the preinjection measurement of the same footpad, and so forth. Negative controls were naive mice and mice given saline-IFA in place of CneF-IFA at 6 days before the footpads were injected with saline or CneF.
i.v. and i.c. infections with C. neoformans.
C. neoformans yeast cells taken from a 2-day culture on modified Sabouraud's agar were transferred to RPMI 1640 medium (Gibco, Gaithersburg, Md.) containing 10% heat-inactivated fetal bovine serum and incubated for 18 h at 37°C in 7% CO2. The overnight culture was washed with sterile endotoxin-free physiological saline, counts were determined on a hemacytometer, and the culture was adjusted to the appropriate concentration for intravenous (i.v.) or i.c. injection. The numbers of viable C. neoformans cells injected were confirmed by culturing dilutions of the inoculum on modified Sabouraud's agar plates. i.v. infection was accomplished by injecting 0.2 ml of a prewarmed yeast cell suspension (2.5 × 105 yeast cells/ml) through a 27-gauge needle into the tail vein.
For i.c. infections, mice were anesthetized with xylazine and ketamine (5 mg of xylazine and 50 mg of ketamine per kg of body weight) as previously described (4). The area between the ear and the eye of anesthetized mice was disinfected with 70% ethanol, and 30 μl of a prewarmed solution of cryptococci (3 × 104 yeast cells/ml) was injected through the temple with a tuberculin syringe and a 27-gauge needle. In each experiment, five mice from each i.c. infected group were sacrificed 1 h after infection to determine the numbers of organisms introduced into the brain.
Clearance of cryptococci from tissues.
At designated times after infection, mice were sacrificed, and their brains, spleens, and lungs were removed and placed individually in sterile stomacher bags. The organs were homogenized in sterile saline with a Stomacher Lab 80 Lab Blender (Seward Medical, London, England). Serial 10-fold dilutions of each sample were plated in duplicate on modified Sabouraud's agar plates and incubated at room temperature for 2 to 3 days, and counts were determined.
Isolation of leukocytes from the CNS.
Prior to staining for flow cytometry, leukocytes were separated from brain myelin debris by modifications of a previously published protocol (47). Mice were sacrificed and perfused with 40 ml of cold phosphate-buffered saline (PBS). Brains and spinal cords were pressed through 70-μm nylon mesh to form single-cell suspensions and were washed with cold Hanks balanced salt solution (HBSS). The cells were resuspended in 4 ml of 70% Percoll diluted in HBSS and placed in a 15-ml centrifuge tube. Four milliliters of 37% Percoll in HBSS was gently layered onto the cell suspension, followed by 4 ml of 30% Percoll. The Percoll gradients were centrifuged for 20 min at 500 × g and room temperature. The myelin debris was retained on the top of each gradient, and the leukocytes formed a band at the 37%–70% Percoll interface. The myelin debris was removed prior to collection of the leukocytes. The leukocytes were washed free of Percoll by centrifugation and counted on a hemacytometer.
Flow cytometric analysis.
For analysis of surface markers, 105 to 106 cells isolated from brains were treated with Fc block (rat anti-mouse Fc-γ receptor; ATCC clone HB197) for 20 min on ice. The cells were pelleted, stained by resuspension in 100 μl (1 μg) of fluorochrome-labeled antibodies in staining buffer (PBS, 0.1% NaN3, 0.1% bovine serum albumin), and incubated on ice for 30 min. The antibodies were phycoerythrin (PE)–anti-CD4, fluorescein isothiocyanate (FITC)–anti-CD8, FITC–anti-Ly-6G, and PE–anti-Mac-1 (CD11b) (all from Caltag, San Francisco, Calif.) and FITC–anti-CD45 (30F-11; leukocyte common antigen) (PharMingen, San Diego, Calif.). Appropriate fluorochrome-labeled isotype-matched control antibodies (PharMingen) were used to quantify the levels of nonspecific staining. After being washed in staining buffer, the cells were fixed with cold 1% paraformaldehyde in PBS containing 0.1% NaN3 and analyzed on a FacStar+ flow cytometer. The samples were gated for cells of the subset of interest and then analyzed for staining with the specific fluorochrome-labeled antibody. In our experiments with the CBA/J strain of mice, Mac-1+ CD45hi Ly-6G− cells were macrophages, while Mac-1+ CD45lo cells were considered microglial cells (43).
In vivo depletion of lymphocytes.
Four groups of five mice were immunized with CneF-IFA 6 days prior to i.c. infection. CD4+ T lymphocytes were depleted from one group of mice by injecting 100 μg of purified anti-CD4 monoclonal antibody (clone GK1.5) intraperitoneally (i.p.). CD8+ T lymphocytes were depleted from another group with anti-CD8 monoclonal antibody (clone YTS 169.4). Mice were injected with monoclonal antibody 2 days prior to infection and again every other day thereafter. Additional groups of immunized mice received 100 μg of purified rat immunoglobulin G (Cappel, Aurora, Ohio) i.p. as an antibody control or no antibody treatment prior to i.c. infection. We found that administration of the isotype-matched control antibody did not affect the percentage and numbers of leukocytes in the animals, so to conserve animals and reagents, the isotype-matched control antibody-treated group was omitted from some experiments. The efficacy of depletion of a specific T-cell type was monitored by staining spleen cells from each group of mice with PE-conjugated rat anti-CD4, FITC-labeled rat anti-CD8, or appropriate fluorochrome-labeled isotype-matched control antibodies, followed by analysis on a FacStar+ flow cytometer. In every experiment, anti-CD4 and anti-CD8 treatments depleted CD4+ T lymphocytes and CD8+ T lymphocytes, respectively, without affecting the numbers of the other cell populations.
In vivo neutralization of IFN-γ.
Three groups of mice (10 mice/group) were immunized with CneF-IFA. Six days after immunization, the mice were infected with 103 C. neoformans i.c. To neutralize IFN-γ in vivo, one group of mice was injected i.p. with 0.5 ml of rabbit anti-murine IFN-γ serum on the same day as infection and every other day thereafter. Another group was injected i.p. with 0.5 ml of normal rabbit serum at the same times. The third group was not treated. On days 3 and 6 after infection, brains were removed and analyzed for CFU.
Statistical analysis.
Means, standard errors of the means (SEM), and unpaired Student's t test and analysis of variance results were used to analyze the data. P values of 0.05 or less indicate statistical significance.
RESULTS
Immunization with CneF-IFA induces anticryptococcal clearance mechanisms that reduce the C. neoformans burden in the brain.
Immunization with CneF-IFA induced an anticryptococcal CMI response, as measured by DTH reactivity to CneF. Mice immunized with CneF-IFA had mean ± SEM footpad swelling of (22.6 ± 2.8) × 10−3 in, whereas the mean ± SEM increase in footpad thickness for control mice (saline-IFA treated) was (2.2 ± 0.37) × 10−3 in. To assess the effects of immunization on disseminated infection, treated mice were infected i.v., and the brain fungal burden was monitored for 14 days (Table 1). CneF-IFA-immunized (immune) and saline-IFA-treated (control) mice had increasing brain fungal burdens through 7 days, with significantly fewer brain CFU in immune mice than in control mice. Brain CFU in immune mice declined thereafter, and the mice appeared healthy; however, control mouse brain CFU continued to increase, with some mortality of the mice.
TABLE 1.
CFU present in brains of mice on days 1, 3, 7, and 14 after i.v. infection with C. neoformans
| Day after infection | Mean ± SEM CFU/brain in mice treated witha:
|
P | |
|---|---|---|---|
| Saline-IFA | CneF-IFA | ||
| 1 | 1,004 ± 23 | 692 ± 46 | <0.006 |
| 3 | 7.4 × 104 ± 3.6 × 103 | 2.0 × 104 ± 2.2 × 103 | <0.001 |
| 7 | 22.4 × 106 ± 1.4 × 106 | 2.6 × 106 ± 0.95 × 106 | <0.002 |
| 14 | 48.5 × 106 ± 12.0 × 106 | 1.1 × 106 ± 0.51 × 106 | <0.007 |
Three mice from each group were analyzed for brain CFU at each time. The data are representative of one experiment that was performed twice, with similar results.
Immunization with CneF-IFA induces protective mechanisms that function in the clearance of C. neoformans in the brain.
The data in Table 1 suggest that the immunization protocol induced an anticryptococcal response capable of reducing the fungal burden in the brain. However, i.v. infection cannot address whether the lower brain CFU in immune mice was due to a regional immune response in the brain or the effect of reduced seeding of the brain with cryptococci in immune mice. To examine whether host resistance mechanisms participated in the clearance of C. neoformans in the CNS, equal numbers of cryptococci were introduced into the CNS of control and immune mice by i.c. injection. To ensure that the control and immune mice received the same numbers of cryptococci i.c., the numbers of organisms were cultured from the brains of five mice from each group at 1 h after infection. In every experiment, similar numbers were cultured from immune and control animals.
The results from a representative i.c. infection are shown in Table 2. Three days after i.c. infection, brains from immune mice contained significantly fewer cryptococci than infected brains from saline-IFA-treated mice (P < 0.003) or untreated mice (P < 0.0003). At 5 days after infection, the difference in CFU in the brains of immune versus control mice was even greater (P < 0.00002, compared to saline-IFA-treated and untreated mice). Brain CFU in immune mice decreased slightly at day 7 after infection, whereas CFU increased in saline-IFA-treated mice (P < 0.006, compared to immune mice) and untreated mice (P < 0.003). In some experiments, significantly fewer brain CFU were observed in immune mice at 24 h after infection than in control mice. No significant differences in brain CFU were detected between saline-IFA-treated mice and untreated mice at any time examined.
TABLE 2.
CFU present in brains, spleens, and lungs of mice on days 3, 5, and 7 after i.c. infection with C. neoformans
| Day | Treatment | Mean ± SEM CFU/organa
|
||
|---|---|---|---|---|
| Brain | Spleen | Lungs | ||
| 3 | None (untreated) | 9.6 × 104 ± 2.0 × 104bc | 40 ± 15 | 120 ± 46 |
| Saline-IFA | 10.0 × 104 ± 3.8 × 104d | 119 ± 42 | 375 ± 159 | |
| CneF-IFA | 1.4 × 104 ± 0.9 × 104 | 19 ± 13 | 3 ± 3 | |
| 5 | None (untreated) | 5.2 × 106 ± 0.4 × 106bc | 4.5 × 103 ± 968 | 14.6 × 103 ± 4.9 × 103 |
| Saline-IFA | 4.1 × 106 ± 0.7 × 106e | 2.3 × 103 ± 555 | 3.4 × 103 ± 0.5 × 103 | |
| CneF-IFA | 0.14 × 106 ± 0.08 × 106 | 90 ± 30 | 6 ± 4 | |
| 7 | None (untreated) | 11.7 × 106 ± 2.2 × 106bd | 12.8 × 104 ± 0.4 × 104 | 2.1 × 104 ± 1.3 × 104 |
| Saline-IFA | 12.0 × 106 ± 4.6 × 106f | 4.1 × 104 ± 1.5 × 104 | 0.5 × 104 ± 0.1 × 104 | |
| CneF-IFA | 0.06 × 106 ± 0.02 × 106 | 44 ± 18 | 703 ± 284 | |
Five mice from each group were examined at each time. The data are representative of one of three experiments with similar results. Statistical comparisons were made by an analysis of variance.
Not significant compared to saline-IFA group.
P < 0.0003 compared to CneF-IFA group.
P < 0.005 compared to CneF-IFA group.
P < 0.0005 compared to CneF-IFA group.
P < 0.007 compared to CneF-IFA group.
Concomitant with the brain CFU analysis, we examined spleens and lungs of i.c. infected mice for fungal burdens to determine the extent of dissemination of the organism from the CNS. At 24 h after infection, no organisms were isolated from spleens or lungs of untreated, saline-IFA-treated, or immune mice. By day 3 postinfection, limited numbers of CFU were cultured from spleens and lungs of all three groups of mice, with fewer CFU being observed in tissues from immune mice (Table 2). The numbers of CFU cultured from spleens and lungs increased at day 5 after infection in all three groups, with significantly fewer CFU being observed in immune mice than in control groups. This trend continued at day 7 after infection, with the exception of spleen CFU from immune mice, which decreased slightly. Although the cryptococci disseminated to extracerebral tissues following i.c. infection, the limited CFU numbers in spleens and lungs early after infection (first 3 days) and the small numbers at all times in comparison to brain CFU numbers suggest that limited spread of the organism to extracerebral sites occurred following i.c. infection and that reseeding of the brain with cryptococci from extracerebral tissues contributed very little if any to the fungal burden in the brain.
CD4+ T lymphocytes are present in C. neoformans-infected brains of immunoprotected mice.
Once we had established that immune mice were able to limit the growth of C. neoformans in the CNS, we next examined what types of leukocytes were present in the CNS during infection. The results of a phenotypic analysis of leukocytes in the CNS of naive and infected mice are shown in Table 3. CneF-IFA-immunized mice injected i.c. with saline (mock infected) had about the same leukocyte numbers in the brain (data not shown) as naive mice (Table 3), indicating that immunization alone did not significantly affect the cellular makeup of the brain leukocyte population. Furthermore, mock-infected brains at 3 days after injection were not different from naive brains (data not shown), indicating that the i.c. injection itself did not induce an observable inflammatory response. In contrast, infected brains of CneF-IFA-immunized mice had increased numbers of neutrophils, CD4+ and CD8+ cells, and macrophages compared to infected brains of nonimmune control mice at both 3 and 7 days after infection. Leukocyte numbers continued to increase from day 3 to day 7 in both immune and nonimmune mice infected with C. neoformans. Microglial cell numbers at day 3 were larger in the brains of nonimmune mice, but by day 7, larger numbers of microglial cells were obtained from the brains of immune mice than from those of control mice. The predominant leukocyte types in the brains of infected immune mice at 3 and 7 days after infection were CD4+ T lymphocytes and macrophages, two cell types most closely associated with a CMI response.
TABLE 3.
Numbers of leukocytes present in brains of naive mice and control and immune mice on days 3 and 7 after infection with C. neoformans
| Cells | No. of leukocytes per brain of the following mice on the indicated daya:
|
||||
|---|---|---|---|---|---|
| Naive | i.c. infected
|
||||
| Saline-IFA treated
|
CneF-IFA treated
|
||||
| 3 | 7 | 3 | 7 | ||
| Neutrophils | 51 | 20 | 44,908 | 138 | 121,992 |
| CD4+ lymphocytes | 1,760 | 1,240 | 110,526 | 2,645 | 365,364 |
| CD8+ lymphocytes | 548 | 420 | 19,075 | 782 | 57,120 |
| Macrophages | 2,882 | 3,280 | 300,295 | 9,246 | 537,540 |
| Microglial | 16,958 | 39,600 | 64,201 | 27,393 | 95,064 |
Pooled leukocytes isolated from the brains of naive or treated mice were counted and stained for flow cytometric analysis. To obtain the total number of each cell type present in each brain, the total number of leukocytes per mouse from each group was multiplied by the percentage of cells staining positive for the cell marker. Leukocytes were pooled from 10 mice per group. The experiment was performed twice, with similar results.
To obtain the phenotypic data, we had to combine the leukocytes from 10 identically treated mice; thus, we were unable to perform statistical analyses on the leukocyte populations from the different groups. However, in a separate experiment, individual mouse brain leukocyte populations were counted on days 3 and 6 after i.c. infection. Infected brains of immune mice contained (1.3 ± 0.17) × 105 leukocytes per brain on day 3 after infection, compared to (0.9 ± 0.05) × 103 leukocytes per brain of i.c. infected control mice (P < 0.04). By day 6 postinfection, the leukocyte numbers per brain were (8.9 ± 0.87) × 105 in immune mice and (4.9 ± 0.75) × 105 in control mice (P < 0.006).
Depletion of CD4+ T lymphocytes abrogates protection in the brain.
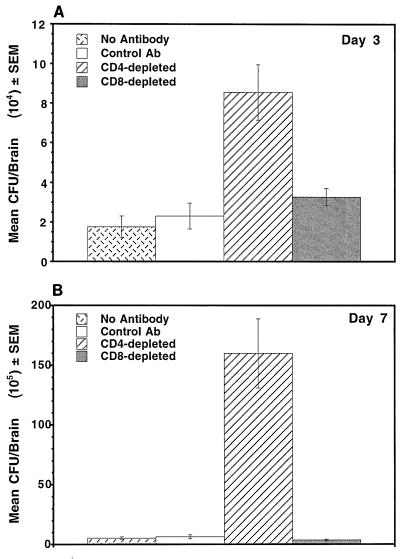
CD4+ T lymphocytes are critical components of CMI responses and have been implicated in mediating cellular infiltration into C. neoformans-infected lungs (20, 22) and into anticryptococcal DTH reaction sites (4). Because we had observed increases in CD4+ and CD8+ T lymphocytes in infected brains of immune mice and both cell types have been shown to play some role in anticryptococcal CMI responses (20, 22, 34, 36), we examined whether or not depletion of CD4+ or CD8+ T cells affected the clearance of C. neoformans from the CNS of immune mice. Results from a representative experiment are shown in Fig. 1. Three days after infection, CD4-depleted immune mice had significantly larger numbers of cryptococcal CFU in their brains than immune mice given either no antibody or isotype-matched control antibody (P < 0.0005) (Fig. 1A). Depletion of CD8+ cells from immune mice had no effect on brain fungal burden. Seven days after infection, CD4-depleted immune mice had significantly larger numbers of cryptococci in their brains than immune mice treated with no antibody, isotype-matched control antibody, or anti-CD8 antibody (P < 0.0001) (Fig. 1B). There was no significant difference in the CFU numbers in the brains of immune mice treated with no antibody, isotype-matched control antibody, or anti-CD8 antibody on day 3 or 7 after infection.
FIG. 1.
CD4+ but not CD8+ T lymphocytes are required for growth inhibition of C. neoformans in the brains of immune mice. Brains of immune mice depleted of CD4+ T lymphocytes contained significantly more cryptococcal CFU than those of immune mice and CD8-depleted immune mice. Depletion of specific T-cell subsets by antibodies was confirmed by flow cytometric analysis of spleen cells from the treated mice. The data are representative of one of three experiments that yielded similar results. Ab, antibody.
Depletion of CD4+ T lymphocytes decreases leukocyte accumulation in the CNS of i.c. infected immune mice.
The depletion data indicated that CD4+ T lymphocytes were critical for protective immunity against C. neoformans in the CNS; thus, we next asked whether CD4+ T lymphocytes were required for leukocyte accumulation in C. neoformans-infected brains of immune mice. The results from a CD4+ and CD8+ T-cell depletion experiment are shown in Fig. 2. Infected brains of immune mice depleted of CD4+ T lymphocytes contained significantly fewer leukocytes on day 6 after infection than infected brains of CD8-depleted immune mice or immune mice that had intact CD4+ and CD8+ T-lymphocyte populations (P < 0.02). The numbers of leukocytes isolated from C. neoformans-infected brains of CD8-depleted immune mice were no different from those in infected brains of immune mice not treated with antibody (Fig. 2).
FIG. 2.
CD4+ T lymphocytes are required for optimal leukocyte accumulation in C. neoformans-infected brains of immune mice. Brains and spinal cords of immune mice depleted of CD4+ T cells contained significantly fewer leukocytes 6 days after i.c. infection with C. neoformans than those of CD8+ T-cell-depleted immune mice and immune mice with both CD4+ and CD8+ T cells. Leukocytes from five or six mice from each group were obtained as described in Materials and Methods and counted. Depletion of specific T-cell subsets by antibodies was confirmed by flow cytometric analysis of spleen cells from treated mice. The experiment was repeated once, with similar results.
We further analyzed the types of cells recruited into infected brains of CD4-depleted or CD8-depleted mice. C. neoformans-infected brains of immune mice depleted of CD4+ cells contained (1.2 ± 0.30) × 104 macrophages, compared to (8.6 ± 1.66) × 104 macrophages in infected brains of immune mice given no antibody and (7.4 ± 1.36) × 104 macrophages in infected brains of immune mice depleted of CD8+ cells. This result represents a six- to sevenfold reduction in the numbers of macrophages present in infected brains of CD4-depleted immune mice compared to CD8-depleted immune mice and immune mice that had intact CD4+ and CD8+ cells. Neutrophil numbers were decreased in infected brains of CD4-depleted immune mice [(2.0 ± 0.49) × 104] compared to infected brains of CD8-depleted immune mice [(6.4 ± 1.20) × 104] and immune mice with intact CD4+ and CD8+ T cells [(4.6 ± 0.88) × 104].
IFN-γ is required for optimal host resistance in the CNS of immune mice.
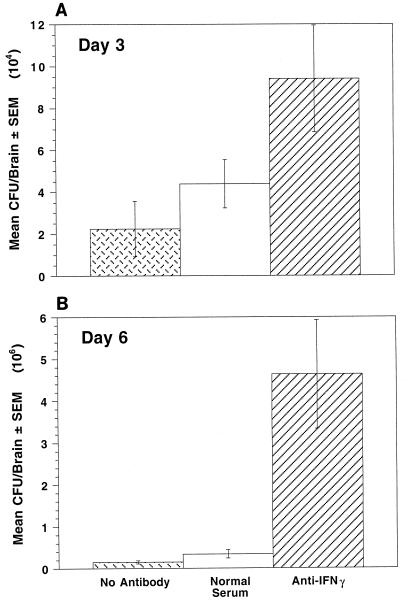
Because CD4+ T lymphocytes are major sources of IFN-γ and IFN-γ has been shown to be important in host defense against C. neoformans (1, 18, 24–26), we next asked whether depletion of IFN-γ affected clearance in the CNS of immune mice. Brains of immune mice given neutralizing antibody to IFN-γ (Fig. 3) had significantly more cryptococcal CFU at 3 days after i.c. infection than brains of immune mice given either no antibody (P, <0.04) or control serum (P, <0.05) (Fig. 3A). At day 6 after i.c. infection, the difference in brain CFU between immune mice given anti-IFN-γ serum and immune mice given normal serum (P, <0.009) or no serum (P, <0.007) was more pronounced.
FIG. 3.
IFN-γ is required for optimal host resistance in the CNS of immune mice. Cryptococcal CFU in brains of i.c. infected immune mice given neutralizing antibodies to IFN-γ were significantly higher than those in i.c. infected immune mice given no antibody or control antibody. Five mice were used per group.
DISCUSSION
CMI has been shown to be important in host protection in pulmonary cryptococcosis and systemic cryptococcosis (6, 14, 15, 17, 20, 22, 23, 31, 32, 35, 41, 46). However, it has been unclear whether a protective anticryptococcal response can function directly in the clearance of C. neoformans once the organism is in the brain or whether it only reduces the organism burden in tissues outside the CNS, thereby reducing the number of cryptococci that reach the brain. Although infection with C. neoformans is generally thought to be acquired by inhalation, the majority of cryptococcosis patients are not diagnosed until signs of CNS involvement are present. The current antifungal therapies are not completely effective in eradicating the organism; thus, a better understanding of the host response in the CNS would be beneficial in developing new immune-based therapies.
The present study describes a cryptococcal i.c. infection model involving immunocompetent mice immunized with a cryptococcal antigen, CneF. The advantage of this model over other infection models is that i.c. infection introduces the organisms directly into the CNS so that comparative groups begin with the same numbers of organisms in the CNS. In addition, immunization with CneF induces a systemic protective CMI response without introducing replicating cryptococci. Thus, clearance of the organisms from the CNS can be studied under conditions where activated T lymphocytes specific for cryptococcal antigens are present systemically (39) and all organisms present in tissues initiated from a regional CNS infection.
The data presented here demonstrate that infection by i.c. injection of organisms resulted in a regional CNS infection with limited dissemination to extracerebral tissues. Immune mice contained fewer brain CFU than control mice, indicating that a protective anticryptococcal CMI response induced systemically can function in inhibiting the growth of C. neoformans in the CNS. Protective immunity correlated with leukocyte recruitment into the CNS. Others have observed cellular infiltration in the brains of mice (1, 2, 21), rats (13), and humans (28, 29) infected with C. neoformans; thus, the host can mount an inflammatory response against the organism in the brain. We extended these observations by demonstrating that cellular infiltration into infected brains of immune mice included the accumulation of T lymphocytes, macrophages, and neutrophils, presumably by migration from the peripheral blood into the CNS. The pattern of cellular infiltration into infected brains of immune mice was reminiscent of anticryptococcal CMI responses in other tissues (4, 5). Furthermore, CD4+ T lymphocytes were required for optimal recruitment or intracerebral expansion of leukocytes in the CNS.
Hill and Aguirre (16) reported that the presence of CD4+ T lymphocytes in mice correlates with reduced fungal burden in the brains of mice infected either intratracheally or i.v. However, the issue of whether clearance of the cryptococci occurred in the CNS or extracerebrally, leading to reduced seeding of the brain from the bloodstream, was not addressed. In our study, the observation that significantly fewer cryptococci were present in the brains of immune mice within 1 day after i.v. infection than in those of control mice (Fig. 1) suggests that at least some clearance occurs extracerebrally. Our data obtained with the i.c. infection model unequivocally demonstrate that CD4+ cells are required for inhibiting the growth of C. neoformans in the CNS of mice immunized with a cryptococcal antigen.
We also observed increases in the numbers of CD8+ T lymphocytes in infected brains of immune mice, although the numbers were considerably lower than those of CD4+ T lymphocytes. Others have reported CD8+ T lymphocytes present along with CD4+ T lymphocytes in rat brains infected with C. neoformans (13). CD8+ T lymphocytes have been shown to play a role in the development of an anticryptococcal DTH response following immunization with heat-killed C. neoformans or intratracheal infection with viable C. neoformans (34, 36). CD8+ T lymphocytes have also been shown to be important in host resistance against C. neoformans following pulmonary infection (17, 20, 22, 34). The role of CD8+ T lymphocytes in host protection against C. neoformans in human cryptococcal meningoencephalitis is unknown; however, our results indicate that CD8+ T lymphocytes are not required for optimal leukocyte recruitment and early growth inhibition of C. neoformans in the CNS of mice immunized with CneF. Immunization with CneF in complete Freund adjuvant does not induce CD8+ T lymphocytes, and CD8+ T lymphocytes are not needed for a DTH response or host resistance in mice immunized with CneF in complete Freund adjuvant (40). Presumably, CD8+ T lymphocytes are not induced by CneF-IFA as well. Thus, at this time, it is unclear whether the lack of requirement for CD8+ T lymphocytes in the CNS resistance that we observed is due to the type of immune response generated by immunization with a cryptococcal antigen or is a general phenomenon associated with host resistance against C. neoformans in the CNS.
The numbers of neutrophils and microglial cells were also increased in infected brains of immune mice. Neutrophils are commonly found in CMI reactions in mice (8), and we have observed abundant neutrophils at sites of anticryptococcal CMI responses (4, 5). Others have reported neutrophils present in a rat model of cryptococcal meningitis (13) and in brain sections from non-AIDS patients with cryptococcal meningoencephalitis (28, 29). In AIDS-associated cryptococcal meningoencephalitis, the primary leukocytes observed in the brain were macrophages, with few neutrophils (28, 29). Our data on immune mice depleted of CD4+ T lymphocytes having reduced numbers of neutrophils during infection are in agreement with the observations of leukocyte types in AIDS patients reported by Lee and colleagues (28, 29). Thus, T lymphocytes may be required for recruitment of neutrophils into the CNS in cryptococcosis. The role of neutrophils in CNS protection against C. neoformans, as well as the detrimental effects of the liberation of neutrophil products on host tissue during infection, has yet to be determined.
Microglial cells are resident macrophage-like cells of the CNS (12) that have the ability to kill cryptococci in vitro (3, 30). Because microglial cells are resident cells of the CNS, one might not expect to see changes in their numbers in the CNS as a result of infection; however, we observed a dramatic increase after i.c. introduction of C. neoformans. The increase in microglial cell numbers in infected brains of immune mice might have been due to either proliferation or changes in the cellular environment during the immune response that allowed them to be isolated in larger numbers from the CNS. Recently, Sedgwick and colleagues (44) reported a threefold increase in the number of isolated microglial cells over a 3-day period in a rat model of CNS graft-versus-host disease. They proposed that this increase was due to T-cell-induced microglial cell proliferation in the CNS (44). It is unclear at this time what mechanism is responsible for increased microglial cell numbers in our model.
Cellular infiltration was also evident in infected brains of control mice, although the magnitude of the inflammatory response was considerably lower and delayed in comparison to that in infected immune mouse brains. The cellular infiltration observed in infected control mice likely was due in part to the generation of an anticryptococcal CMI response and the subsequent recruitment of leukocytes to the site of infection by components of this response. In fact, control mice exhibited significant DTH reactivity by 7 days after i.c. infection (data not shown).
IFN-γ has been shown to be important in protection against C. neoformans in animal models (1, 18, 24–26). We observed that IFN-γ was essential for optimal growth inhibition when C. neoformans was introduced directly into the CNS of immune mice. Because IFN-γ activates macrophages to better kill cryptococci (11, 37), protection mediated via IFN-γ is presumably due to the activation of effector cells already present at the site of infection or recruited to the site. Thus, the cryptococcal growth inhibition that we observed in the brains of immune mice likely is due in part to the production of IFN-γ, which activates macrophages recruited to the CNS or endogenous effector cells, such as microglial cells or astrocytes, to kill the cryptococci.
In summary, our data demonstrate that CD4+ T lymphocytes induced by immunization with a cryptococcal antigen are present in the CNS of C. neoformans-infected mice. These CD4+ T lymphocytes are required for optimal accumulation of macrophages and neutrophils in the CNS, and the inflammatory response generated results in a reduction in the cryptococcal burden in the CNS, potentially by an IFN-γ-dependent mechanism.
ACKNOWLEDGMENTS
We thank Juneann W. Murphy and Paul L. Fidel for helpful discussions and critical reading of the manuscript.
This work was supported by a Burroughs Wellcome Fund new investigator award in molecular pathogenic mycology to K.L.B.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi E, Barluzzi R, Mazzolla R, Pitzurra L, Puliti M, Saleppico S, Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasi E, Barluzzi R, Mazzolla R, Tancini B, Saleppico S, Puliti M, Pitzurra L, Bistoni F. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglial cell line. J Neuroimmunol. 1995;58:111–116. doi: 10.1016/0165-5728(95)00016-u. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan K L, Murphy J W. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1993;61:2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan K L, Murphy J W. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology. 1997;90:189–197. doi: 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauley L K, Murphy J W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979;23:644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherniak R. Soluble polysaccharides of Cryptococcus neoformans. Curr Top Med Mycol. 1988;2:40–54. doi: 10.1007/978-1-4612-3730-3_2. [DOI] [PubMed] [Google Scholar]
- 8.Crowle A J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- 9.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 10.Fabry Z, Raine C S, Hart M N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 11.Flesch I E, Schwamberger G, Kaufmann S H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989;142:3219–3224. [PubMed] [Google Scholar]
- 12.Gehrmann J, Matsumoto Y, Kreutzberg G W. Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 13.Goldman D L, Casadevall A, Cho Y, Lee S C. Cryptococcus neoformans meningitis in the rat. Lab Investig. 1996;75:759–770. [PubMed] [Google Scholar]
- 14.Graybill J R, Drutz D J. Host defense in cryptococcosis. II. Cryptococcosis in the nude mouse. Cell Immunol. 1978;40:263–274. doi: 10.1016/0008-8749(78)90334-9. [DOI] [PubMed] [Google Scholar]
- 15.Hill J O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992;175:1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2350. [PubMed] [Google Scholar]
- 17.Hill J O, Harmsen A G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 19.Hoag K A, Street N E, Huffnagle G B, Lipscomb M F. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagle G B, Lipscomb M F, Lovchik J A, Hoag K A, Street N E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Huffnagle G B, McNeil L K. Dissemination of C. neoformans to the central nervous system: role of chemokines, Th1 immunity and leukocyte recruitment. J Neurovirol. 1999;5:76–81. doi: 10.3109/13550289909029748. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle G B, Yates J L, Lipscomb M F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huffnagle G B, Yates J L, Lipscomb M F. T-cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Imun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami K, Kohno S, Kadota J, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami K, Qureshi M H, Zhang T, Koguchi Y, Shibuya K, Naoe S, Saito A. Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans. Clin Exp Immunol. 1999;117:113–122. doi: 10.1046/j.1365-2249.1999.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol Med Microbiol. 1996;13:123–130. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 27.Kwon-Chung K J, Varma A, Howard D H. Ecology of Cryptococcus neoformans and prevalence of its two varieties in AIDS and non-AIDS associated cryptococcosis. In: Vanden Bosche H, Mackenzie D W R, Cauwenberg G, Van Cutsem J, Drouhet E, Dupont B, editors. Mycoses in AIDS patients. New York, N.Y: Plenum Press; 1990. pp. 103–113. [Google Scholar]
- 28.Lee S C, Casadevall A, Dickson D W. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol. 1996;148:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S C, Dickson D W, Casadevall A. Pathology of cryptococcal meningitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee S C, Kress Y, Dickson D W, Casadevall A. Human microglia mediate anti-Cryptococcus neoformans activity in the presence of specific antibody. J Neuroimmunol. 1995;62:43–52. doi: 10.1016/0165-5728(95)00097-l. [DOI] [PubMed] [Google Scholar]
- 31.Lim T S, Murphy J W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980;30:5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim T S, Murphy J W, Cauley L K. Host-etiological agent interactions in intranasally and intraperitoneally induced cryptococcosis in mice. Infect Immun. 1980;29:633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mody C H, Chen G, Jackson C, Curtis J L, Toews G B. Depletion of murine CD8+ T cells in vivo decreases pulmonary clearance of a moderately virulent strain of Cryptococcus neoformans. J Lab Clin Med. 1993;121:765–773. [PubMed] [Google Scholar]
- 35.Mody C H, Lipscomb M F, Street N E, Toews G B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense of Cryptococcus neoformans. J Immunol. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 36.Mody C H, Paine R I, Jackson C, Chen G-H, Toews G B. CD8 cells play a critical role in delayed type hypersensitivity to intact Cryptococcus neoformans. J Immunol. 1994;152:3970–3979. [PubMed] [Google Scholar]
- 37.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 38.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy J W, Schafer F, Casadevall A, Adesina A. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infect Immun. 1998;66:2632–2639. doi: 10.1128/iai.66.6.2632-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muth S M, Murphy J W. Effects of immunization with Cryptococcus neoformans cells or cryptococcal culture filtrate antigen on the direct anticryptococcal activities of murine T lymphocytes. Infect Immun. 1995;63:1645–1651. doi: 10.1128/iai.63.5.1645-1651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura K, Miyaji M. Histopathological studies on experimental cryptococcosis in nude mice. Mycopathologia. 1979;68:145–153. doi: 10.1007/BF00578522. [DOI] [PubMed] [Google Scholar]
- 42.Nowak T P, Barondes S H. Agglutinin from Limulus polyphemus. Purification with formalinized horse erythrocytes as the affinity adsorbent. Biochim Biophys Acta. 1975;393:115–123. [PubMed] [Google Scholar]
- 43.Renno T, Krakowski M, Piccirillo C, Lin J, Owens T. TNF-α expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 44.Sedgwick J D, Ford A L, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol. 1998;160:5320–5330. [PubMed] [Google Scholar]
- 45.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of the initial infection in recurrent cryptococcal meningitis. Lancet. 1993;341:595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- 46.Yuan R R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeine R, Owens T. Direct demonstration of the infiltration of murine nervous system by Pgp-1/CD44high CD45low CD4+ T cells that induce experimental allergic encephalomyelitis. J Neuroimmunol. 1992;40:57–69. doi: 10.1016/0165-5728(92)90213-5. [DOI] [PubMed] [Google Scholar]