Abstract
At present, no European recommendations for the management of pediatric thyroid nodules and differentiated thyroid carcinoma (DTC) exist. Differences in clinical, molecular, and pathological characteristics between pediatric and adult DTC emphasize the need for specific recommendations for the pediatric population. An expert panel was instituted by the executive committee of the European Thyroid Association including an international community of experts from a variety of disciplines including pediatric and adult endocrinology, pathology, endocrine surgery, nuclear medicine, clinical genetics, and oncology. The 2015 American Thyroid Association Pediatric Guideline was used as framework for the present guideline. Areas of discordance were identified, and clinical questions were formulated. The expert panel members discussed the evidence and formulated recommendations based on the latest evidence and expert opinion. Children with a thyroid nodule or DTC require expert care in an experienced center. The present guideline provides guidance for healthcare professionals to make well-considered decisions together with patients and parents regarding diagnosis, treatment, and follow-up of pediatric thyroid nodules and DTC.
Keywords: pediatric, thyroid cancer, thyroid nodule, recommendation, European
Introduction
Pediatric differentiated thyroid carcinoma (DTC) is a rare disease; however, its worldwide incidence is rising (1, 2). DTC comprises several histological subtypes, with papillary thyroid carcinoma (PTC) accounting for the vast majority of thyroid carcinoma cases. Other tumor subtypes such as follicular thyroid cancer and non-invasive follicular thyroid neoplasm with papillary-like nuclear features are extremely rare in the pediatric population and will therefore not be discussed separately.
There are important differences between adult and pediatric DTC regarding clinical, molecular, and pathological characteristics. Compared to adults with DTC, pediatric patients more often present with advanced disease at diagnosis, including more lymph node involvement, distant metastasis, and multifocal disease (3). Despite this more aggressive presentation, pediatric DTC has an excellent prognosis (1, 2). Also, the most common genetic alterations in pediatric DTC are RET-PTC and NTRK fusions, while mutations in BRAF, V600E, and RAS point mutations are less frequent (4, 5). Due to these genomic differences, the utility of molecular testing on biopsies of thyroid nodules and on thyroid tissue in children may be different from that in adults. In addition, the consequences of possible adverse effects of treatment may be different for children because of their longer life expectancy.
These differences emphasize the need for specific recommendations for the pediatric population (6, 7, 8). The American Thyroid Association (ATA) has developed recommendations for pediatric nodules and thyroid carcinoma (8); however, in Europe, such recommendations are not yet available. Regulations for medical care differ between the United States of America and Europe, and there are potential cultural differences. Therefore, specific European recommendations are required.
The present guideline will provide guidance for healthcare professionals to make well-considered decisions together with patients and parents regarding diagnostics, treatment, and follow-up of DTC in children.
Methods
The expert panel for this guideline was instituted by the executive committee of the European Thyroid Association (ETA). The panel represents an international community of experts from a variety of disciplines including pediatric and adult endocrinology, pathology, endocrine surgery, nuclear medicine, clinical genetics, and oncology. All experts were divided into three panels: (i) diagnostics and staging, (ii) treatment, and (iii) follow-up. All experts represented their own discipline in the panel. The three panels were chaired by a pediatric endocrinologist (HvS), and the project was coordinated by a PhD student (CL).
Consensus was achieved to use the 2015 ATA Pediatric Guideline 2015 as framework for the 2022 ETA Pediatric Guideline (8). Based on the 2015 ATA Pediatric Guideline, the expert panel identified areas of discordance and clinical questions were formulated. For each clinical question, a systematic literature search was performed using Pubmed (last search date: May 2020) (Appendix B, see section on supplementary materials given at the end of this article).
In total, 3251 studies were identified. All abstracts were screened by two reviewers following the general inclusion criteria: (i) English language, (ii) children and adolescents (<21 years of age), and (iii) study population of at least n = 20. All studies with an age limit of <21 years were included, to avoid excluding important pediatric literature in which the age limit of <21 years was used instead of <18 years. For some clinical questions, specific inclusion criteria were defined (shown in each section). After abstract selection, 45 full papers were included. Each full paper was summarized and graded by two independent reviewers. The modified Grading of Recommendations Assessment, Development, and Evaluation system was used to grade the quality of evidence (9, 10). Quality of evidence was scored as level 1: high (randomized controlled trial (RCT) evidence/meta-analysis – high-quality evidence (⊕⊕⊕⊕)); level 2: moderate (intervention short of RCT or large observational studies –moderate-quality (⊕⊕⊕⊖)); level 3: low quality (case–control studies, case series – low quality (⊕⊕⊖⊖)); level 4: very low quality (case reports, expert opinion – very low quality (⊕⊖⊖⊖)) (9).
If all expert panel members agreed on a recommendation of the 2015 ATA Pediatric Guideline (8), no specific search was performed. The grade of quality of evidence, as had been assigned by the ATA working group, was assumed. The statements based on recommendations of the 2015 ATA Pediatric Guideline are considered as ‘expert opinion’ (level 4).
The expert panel members discussed the evidence and formulated statements based on the best available evidence and expert opinion (Table 1). The expert panel identified several significant gaps in current knowledge that require further research to improve management of pediatric thyroid nodules and DTC (Table 2). The final statements were formed by consensus of the expert panel members. The strength of each statement was scored as strong (S, a recommendation) or weak (W, a suggestion – not a recommendation), depending on the clinical significance and weight of opinion favoring the statement. Strong recommendations are clinically important best practice and should be applied to most patients in most circumstances. In contrast, weak statements should be considered by the clinician and will be applicable to best practice only to certain patients or under certain circumstances. Strong statements are associated with the phrase ‘we recommend’, and weak statements are associated with the phrase ‘we suggest’ (10).
Table 1.
Research questions and conclusions of evidence
| Research questions, conclusions of evidence | Quality of evidence | |
|---|---|---|
| Diagnostics and staging | ||
| 1. What is the sensitivity and specificity of thyroid ultrasound for distinction of thyroid cancer from a benign thyroid nodule of a child? | ||
| Conclusion | The expert panel concludes that specificity and sensitivity of thyroid ultrasound for distinction of thyroid cancer from a benign thyroid nodule in children depends on multiple ultrasound characteristics. | ⊕⊕⊕⊖ |
| 2. What is the sensitivity/specificity of different suspicious US findings for presence of DTC metastasis to a lymph node? | ||
| Conclusion |
No evidence was found on suspicious US findings specific to DTC in childhood.
The expert panel concludes that the sensitivity/specificity of different suspicious US findings for presence of DTC metastasis to a lymph node may be referred to adult literature. |
⊕⊖⊖⊖ |
| 3. Will molecular testing in an FNB specimen of a thyroid nodule in a child help you to distinguish it from a benign nodule? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that prospective studies are needed to determine if molecular testing in an FNB specimen of a thyroid nodule in a child helps to distinguish DTC from a benign nodule |
⊕⊖⊖⊖ |
| 4. Does molecular testing in thyroid carcinoma tissue in a child alter its management? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient to conclude that molecular testing in pediatric thyroid carcinoma tissue has consequences for pediatric DTC management and prospective studies are needed. |
⊕⊖⊖⊖ |
| 5. What is sensitivity of the different imaging modalities for presence of pre-operative metastasis? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that the sensitivity for neck palpation, comprehensive neck ultrasonography, or laboratory work-up to predict DTC, could not be determined. |
⊕⊖⊖⊖ |
| 6. Are histopathological criteria related to distant/any metastases? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient to relate histopathological criteria to distant/any metastases and prospective studies are needed. |
⊕⊖⊖⊖ |
| 7. Which imaging modality is most sensitive for the presence of DTC, post-operatively? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient to state which imaging modality is most sensitive for the presence of DTC, post-operatively. |
⊕⊖⊖⊖ |
| 8. What is the diagnostic value of serum calcitonin in a child with a thyroid nodule? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient to determine the diagnostic value of serum calcitonin in a child with a thyroid nodule |
⊕⊖⊖⊖ |
| 9. What is the prevalence of non-clinically relevant thyroid nodules in a child? | ||
| Conclusion | The prevalence non-clinically relevant thyroid nodules in a non-childhood cancer survivor cohort of children seem to vary between 0.6 and 2%. | ⊕⊕⊕⊖ |
| Treatment | ||
| 10. What is the difference in outcome of DTC in children treated with a total thyroidectomy vs hemithyroidectomy or vs subtotal thyroidectomy? | ||
| Conclusion | Total thyroidectomy may be associated with more recurrence-free survival and disease-free survival. | ⊕⊕⊖⊖ |
| 11. What is the difference in outcome of DTC in children with microcarcinoma (<1 cm) treated with nodule excision/resection vs subtotal resection or vs hemithyroidectomy? | ||
| Conclusion | No studies investigated differences in outcome of patients with TMC treated with total thyroidectomy vs hemi or subtotal thyroidectomy. No differences in disease-specific survival and overall survival between patients with TMC and patients with DTC > 1 cm, although patients with TMC were more often treated with partial thyroidectomy/ lobectomies/isthmusectomies and not followed by RAI. | ⊕⊖⊖⊖ |
| 12. What is the difference in outcome of DTC in children treated with a (prophylactic) central lymph node dissection vs no central lymph node dissection? | ||
| Conclusion | Conflicting results were found. One study suggests that an aggressive surgical approach may both simultaneously decrease the risk of recurrence and improve prognostication in patients with more advanced or aggressive disease. Another study showed no difference in recurrence-free survival between patients treated with LND compared to limited node excision of no LND. However, location of LND was not specified. It remains unclear if these patients underwent prophylactic central lymph node dissection. | ⊕⊖⊖⊖ |
| 13. Is outcome of microcarcinoma worse in children treated with I-131 vs those not treated with I-131? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient and prospective studies are needed to evaluate outcome of small pediatric DTC not treated with I-131 vs those treated with I-131. |
⊕⊖⊖⊖ |
| 14. Is the most optimal dose-effect curve of radioiodine with least side effects calculated by body weight/fixed-dose dosimetry? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient and agreed that individual patient-based approach should be used to calculate the most optimal activity of I-131 taking into account the potential side effects of I-131 with an increasing activity. The preferred individual administered activity should be discussed in the multidisciplinary tumor board taking the individuality of the patient into account. |
⊕⊖⊖⊖ |
| 15. Is rhTSH effective and safe in children during treatment with I-131? | ||
| Conclusion | All studies reported TSH levels after rhTSH stimulation of >50mIU. No significant side effects were reported. No studies reported on iodine uptake after rhTSH injection. | ⊕⊕⊖⊖ |
| 16. What is the difference in outcome in children with measurable but not rising Tg after treatment for DTC? (incomplete biochemical response with I-131 vs a wait-and-see approach) | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient and prospective studies are needed to evaluate outcome in children with an incomplete biochemical response treated with I-131 compared to a wait-and-see approach. |
⊕⊖⊖⊖ |
| 17. What is the difference in outcome in children with recurrent disease/progressive thyroid cancer treated with additional I-131/surgery/other vs a wait-and-see approach? | ||
| Conclusion |
No evidence was found.
The expert panel concludes that current evidence is insufficient and prospective studies are needed to evaluate outcome in children with recurrent disease/progressive thyroid cancer treated with additional I-131/surgery/other vs a wait-and-see approach. |
⊕⊖⊖⊖ |
| 18. What is the difference in outcome of DTC in children treated with different treatment than surgery and I-131? | ||
| Conclusion | Based on case reports, targeted therapy may play a role in the management of disease in very rare cases of the pediatric patient with progressive I-131-refractory PTC, for which no standard therapy exists. | ⊕⊖⊖⊖ |
| Follow-up | ||
| 19. What is the sensitivity/specificity of neck ultrasound for recurrent DTC in follow-up of children who have been treated for DTC? | ||
| Conclusion | The sensitivity and specificity of thyroid ultrasound for recurrent DTC in follow-up of children who have been treated with total thyroidectomy and radioiodine therapy for DTC are 85.7 and 89.4% respectively. | ⊕⊕⊕⊖ |
| 20. What is the sensitivity of I-124, I-123, as well as FDG PET/CT for DTC/thyroid rest or recurrent disease in follow-up of children who have been treated for DTC? | ||
| Conclusion |
No evidence was found.
Prospective studies are needed to determine the sensitivity of radioiodine imaging and FDG PET/CT for the detection of persistent or recurrent disease in children who have been treated for DTC. |
⊕⊖⊖⊖ |
| 21. What are the late effects of treatment of DTC? (cardiac late effects, salivary glands, psychosocial, bone, female fertility) | ||
| Conclusion | Cardiac dysfunction: in 21.2% of asymptomatic survivors, diastolic dysfunction was found. Salivary gland dysfunction: in 1.9–47.6 and 35.5% of the DTC survivors, salivary dysfunction and xerostomia were found, respectively. Quality of life: no differences were found in the course of life questionnaire between DTC survivors and two non-affected groups (non-affected with cancer and other CCS). Also, on most quality-of-life subscales, score of DTC survivors and controls did not differ significantly. However, more physical problems, more role limitations due to physical problems, and more mental fatigue were described by DTC survivors. Bone mineral density: no differences were found with respect to BMD and Z scores at any site evaluated by DXA and in bone microstructure parameters between DTC survivors and controls. However, calcium-D3 medication has a beneficial effect on BMD. TSH-suppressive therapy does not affect BMD in women treated for DTC at young age, at least after 10 years of follow-up. Female fertility: no major abnormalities in reproductive characteristics and in predictors of ovarian failure in female survivors of DTC who received I-131 treatment during childhood were reported. |
⊕⊕⊕⊖ |
| 22. Is presentation, outcome, and/or disease course of DTC in children with genetic syndromes different than in children without genetic syndromes for which treatment and/or follow-up should be adjusted? | ||
| Conclusion | In children DICER1 or PTHS, DTC does not seem to have a more aggressive presentation, outcome, and disease course. | ⊕⊖⊖⊖ |
| 23. Is presentation, outcome, and/or disease course of DTC in children with a history of radiation exposure different than in children without a history of radiation exposure for which treatment and/or follow-up should be adjusted? | ||
| Conclusion | Presentation: CCS with subsequent DTC tended to have on average smaller tumors and might have more often bilateral disease. Disease course: inconsistent findings about difference in tumor characteristics (ETE and LNM) were reported. ETE and LMN might be more frequently found in radiation-induced thyroid tumors in children diagnosed in the Chernobyl region. Outcome: no significant differences were found between CCS with subsequent DTC and controls in the occurrence of surgical complications, recurrence rate or disease-related death. |
⊕⊖⊖⊖ |
CCS, childhood cancer survivors; DTC, differentiated thyroid carcinoma; LND, lymph node dissection; PTC, papillary thyroid carcinoma; PTHS, PTEN hamartoma tumor syndrome; rhTSH, recombinant TSH; TSH, thyroid-stimulating hormone.
The modified GRADE system was used to grade the quality of evidence: high (RCT evidence/meta-analysis –high-quality evidence (⊕⊕⊕⊕)); level 2: moderate (intervention short of RCT or large observational studies – moderate-quality (⊕⊕⊕⊖)); level 3: low quality (case–control studies, case series – low-quality (⊕⊕⊖⊖)); levels 4: very-low quality (case reports, expert opinion – very-low-quality (⊕⊖⊖⊖)) (9).
Table 2.
Research goals for future studies on pediatric DTC based on current gaps in literature
| Research goals in pediatric DTC |
|---|
Pediatric thyroid nodules
|
Pre-operative management
|
Post-operative management
|
Follow-up
|
The recommendations and suggestions are listed in Table 3.
Table 3.
Overview of recommendations and suggestions in the 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma.
| Location | # | Recommendation or suggestion |
|---|---|---|
| [A] Organization of care and goals for pediatric thyroid nodules and thyroid carcinoma | ||
| [A2] | 1 |
Thyroid expert team We recommend that a child with suspicion of thyroid cancer and proven DTC or MTC should be referred to an experienced multidisciplinary thyroid team, specifically with experience in pediatric thyroid cancer (4S). |
| [A3] | 2 |
Goals of therapy for DTC in children We recommend that children with DTC are stratified according to those who may benefit from higher-intensity treatment vs those for whom lower-intensity treatment will suffice. By stratification, the goals of therapy for pediatric DTC (to maintain the high survival rate with low recurrence rate and to minimize adverse effects of treatment) will be reached (4S). |
| [B] Recommendations and suggestions for the pediatric thyroid nodule | ||
| [B2] | 3A 3B |
Risk of malignancy in thyroid nodule during childhood We recommend thyroid ultrasound to assess the risk of cancer in a thyroid nodule, based on multiple ultrasound characteristics. However, ultrasound alone cannot definitively distinguish a benign thyroid nodule from thyroid cancer. For this reason, in suspect nodules, FNB is recommended (Fig. 1) (2S). The expert panel recommends, in children with thyroid nodule(s), a complete neck ultrasound to evaluate all cervical levels for the presence of lymph node enlargement (4S). |
| [B5] | 4A 4B |
Children at high risk for developing DTC We recommend that patients with a high risk of developing DTC (history of radiation exposure to the thyroid or a thyroid cancer predisposition syndromes) should be counseled for surveillance (4S). We suggest that initiation of surveillance and the decision regarding which surveillance modality (neck palpation and optionally neck ultrasound) to use are the result of shared decision-making between the physician and the high-risk patient (4W). |
| [B6] | 5 |
Diagnostic value of serum calcitonin in a child with a thyroid nodule We suggest that, in selected cases (conditions which suggest MEN2, a positive family history of MEN2 or in case of bulky thyroid disease), measurement of calcitonin may be of additional value for early diagnosis of MTC (4W). |
| [B8] | 6 |
Molecular testing in FNB specimen We suggest that molecular gene analysis for presence of BRAF V600E mutation in a FNB specimen may be helpful for diagnosis of PTC and therefore may be considered in the diagnostic work-up. The presence of PTC however must be confirmed cytologically or histologically before total thyroidectomy is performed (4W). |
| [B9] | 7A 7B |
Role of surgery for benign thyroid lesions We recommend that benign nodules should be followed by serial ultrasound and undergo repeat FNB if suspicious features develop (4S). We suggest hemithyroidectomy for benign nodules, performed by an experienced high-volume pediatric thyroid cancer surgeon, in patients with compressive symptoms, cosmetic concerns, or according to patient/parent preference after counseling of the possible benefits and risks of thyroid surgery (4W). |
| [B10] | 8A 8B |
Autonomous thyroid nodules in children We suggest hemithyroidectomy for autonomous nodules during childhood, which must always be performed by an experienced high-volume pediatric thyroid cancer surgeon (4W). We recommend discussion of the advantages and disadvantages of surgery vs radioiodine treatment using shared decision-making in each individual case (4S). |
| [C] Recommendations and suggestions for the management of pediatric differentiated thyroid carcinoma | ||
| [C1] | 9A 9B 9C 9D |
Pre-operative evaluation We recommend neck palpation, comprehensive neck ultrasonography, and laboratory work-up as minimal pre-operative evaluation measures in the pediatric population. The expert panel suggests further genetic or imaging diagnostics in case of suspicion of familial or extensive disease (4S). We suggest additional pre-operative investigations using MRI or low-dose non-contrast CT in case of bulky disease or suspicion of lung metastases (4W). We recommend confirmation with FNB of suspicious lateral lymph nodes (size, aspect, or ultrasound characteristics) (4S). We suggest assessment of vocal cord function in children with bulky disease pre-operatively (4W). |
| [C2] | 10A 10B 10C |
Surgical approach for DTC (Fig. 2) We suggest total thyroidectomy as treatment for children with DTC (3W). See Recommendation 10C for exceptions. We recommend that future studies are conducted that evaluate the impact of limited surgery for pediatric DTC with respect to recurrence and remission rates (4S). We suggest that, in pediatric patients with incidentally found, very small thyroid carcinoma, and non-aggressive histological features, hemithyroidectomy may be considered as therapeutic option (4W). |
| [C3] | 11A 11B 11C |
Therapeutic central and lateral lymph node dissection We suggest that prophylactic central lymph node dissection should only be performed in advanced pediatric thyroid cancer (extracapsular extension, vascular invasion, distant metastases). It can be avoided or limited to ipsilateral lymphadenectomy in patients without suspicious features for advanced thyroid cancer on neck ultrasound (4W). We suggest that therapeutic central lymph node dissection is always recommended in pediatric DTC in case of suspicious central lymph nodes based on neck ultrasound or intraoperative assessment, or perioperative visible extracapsular tumor growth (4W). We recommend that therapeutic lateral lymph node dissection is performed in all children with pre-operatively proven lymph node metastases or in case of evident (pathological) lateral lymph node(s). The expert panel does not recommend prophylactic lateral lymph node dissection (4S). |
| [C4] | 12 |
Surgical complications of thyroidectomy and neck dissection We recommend that all children with DTC should be operated on by high-volume pediatric thyroid cancer surgeons with experience in pediatric thyroid cancer and who are embedded in a center with expertise in the management of DTC (4S). |
| [C5] | 13A 13B |
Post-operative staging We recommend that post-operative staging is done using the surgical report, histological report, measurement of Tg, and I-131 post-therapy scintigraphy (4S). We suggest that the AJCC TNM classification system is used to describe the extent of disease in pediatric DTC (4W). |
| [C6] | 14A 14B 14C |
I-131 therapy We suggest that I-131 therapy is indicated for all children following total thyroidectomy, for the treatment of persistent locoregional, or nodal disease that cannot be resected for as well as iodine avid distant metastases (M1) (4W). We suggest that, for patients with persistent disease following post-operative I-131 therapy, the decision to pursue an additional course of I-131 therapy should be individualized according to previous response (4W). We suggest that the minimal interval between I-131 treatment in childhood for DTC be recommended to be around 1 year (4W). |
| [C7] | 15 |
I-131 activity We suggest that an individual patient-based approach is used to calculate the optimal activity of I-131 taking into account the potential side effects of I-131 with increasing activity. The preferred individual administered activity should be discussed in the multidisciplinary tumor board taking the individual characteristics of the patient into account (4W). |
| [C8] | 16A 16B 16C |
Preparation of the patient for treatment with I-131 We recommend that TSH stimulation (>30 mI/L) is induced before I-131 therapy in order to facilitate I-131 uptake (4S).We suggest that stimulated TSH can be achieved either using thyroid hormone withdrawal or rhTSH. The expert panel did not reach consensus on the optimal way of preparation. The decision for one against the other is up to the clinical experience of the treating team (3W). We suggest that a low iodine diet for at least 4 days before I-131 therapy may be favorable for iodine uptake (4W). |
| [C9] | 17 |
Targeted therapy for pediatric DTC We suggest that, in specific cases, treatment with targeted therapy may be considered, but this should preferably only be given in the setting of clinical trials (4W). |
| [C10] | 18A 18B |
Somatic molecular testing (in thyroid carcinoma tissue) We suggest that molecular testing in pediatric thyroid carcinoma tissue be recommended in research setting but that the result has currently no consequences for pediatric DTC management (4W). We suggest that for cases with I-131 refractory DTC, molecular testing in pediatric thyroid carcinoma tissue be recommended as the result may have consequences for pediatric DTC management (4W). |
| [C11] | 19A 19B |
Treatment for pediatric radiation-induced DTC We suggest that children with radiation-induced DTC undergo total thyroidectomy because of the increased risk for bilateral disease (3W). We suggest that for CCS with DTC, specific medical and psychosocial considerations should be taken into account, requiring an individual treatment and follow-up plan (4W). |
| [C12] | 20 |
Treatment for DTC in children with genetic syndromes We do not suggest adjustment of treatment or follow-up for children with DTC and DICER1 or PHTS or any other tumor predisposition syndrome (3W). |
| [D] Surveillance and follow-up of pediatric differentiated thyroid carcinoma | ||
| [D1] | 21A 21B |
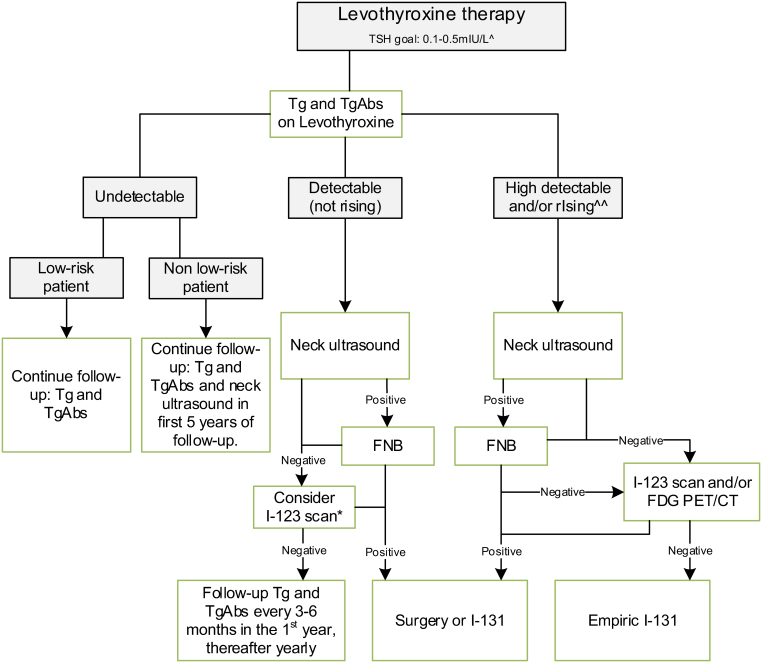
TSH levels during follow-up (Fig. 3) We suggest that TSH levels should be kept suppressed with concomitant high-normal values of FT4 until full clinical remission, while a low-normal value of TSH (between 0.3 and 1.0 mU/L)) should be advised thereafter (4W). We suggest measurement of TSH and FT4 to monitor the level of suppression or substitution of the LT4 therapy every 3–6 months during growth and puberty and thereafter once a year (4W). |
| [D2] | 22A 22B 22C |
Tg measurement during follow-up We recommend that serum Tg is a reliable marker in the follow-up after treatment for DTC in childhood. The expert panel suggests that serum Tg should be assessed every 6 months during the first 3 years and annually thereafter (4S). We suggest that, in case of circulating TgAbs, these may be measured as ‘alternative’ tumor marker (4W). We suggest that a highly sensitive Tg assay should preferably be used in the follow-up of pediatric DTC patients (4W). |
| [D3] | 23A 23B 23C |
Ultrasound during follow-up We recommend follow-up with neck ultrasound in combination with serum Tg measurement for detection of recurrent DTC (2S). We recommend that neck ultrasound is performed by a professional with experience in neck ultrasound in childhood (4S). We suggest that annual neck ultrasound is performed in the first 5 years of follow-up. In low-risk patients, the expert panel suggests, after the first year of follow-up, to only perform neck ultrasound in cases with rising Tg or TgAbs or suspicion of recurrence of disease to avoid false-positive findings (4W). |
| [D4] | 24A 24B |
Other imaging modalities (I-131, I-124, I-123, or FDG PET/CT scans) during follow-up We suggest that children with undetectable Tg on LT4 during follow-up after treatment for DTC should not undergo other imaging modalities (I-131, I-124, I-123, or FDG PET/CT scans) (4W). We suggest that, in children with detectable (but not rising) Tg on LT4 and no focus on neck ultrasound, in individual cases, I-123 scanning may be considered. If no source of Tg is found, serum Tg and serum TgAbs must be followed every 3–6 months. In case of further rising Tg or TgAbs, further imaging is indicated (4W) |
| [D5] | 25A 25B |
Persistent/recurrent cervical disease We suggest performing neck ultrasound in children with consistently rising Tg on LT4 or TgAbs. In these cases, additional I-123 and/or FDG PET scanning may be considered. Surgery or I-131 therapy is indicated depending on the size, tumor load, and degree of progression (4W). We suggest that empiric I-131 iodine treatment be only recommended if the abovementioned diagnostic modalities have failed to identify a source of rising Tg on LT4 or rising TgAbs (4W). |
| [D6] | 26A 26B 26C |
Pulmonary metastases and follow-up We recommend that I-131 is the first-line therapy for patients with pulmonary metastases (4S). We suggest that a pulmonary function test should be performed, before repeated I-131 treatment of patients with diffuse lung metastases (4W). We recommend that in children with a previous history of drugs causing pulmonary toxicity such as bleomycin, I-131 treatment must be given with extra caution given the risk for pulmonary fibrosis (4S). |
| [D7] | 27A 27B |
Radioiodine refractory disease We suggest that, when radioiodine refractory disease is suspected, its presence should be thoroughly investigated and confirmed before considering systemic targeted therapy. An observation or wait-and-see strategy may be appropriate (4W) We suggest that targeted therapy should be reserved only for patients with large-volume disease which is significantly progressing on TSH-suppressive therapy and not amenable to surgical approach and should preferably be given in a research setting (4W). |
| [D8] | 28A 28B 28C 28D 28E 28F |
Late effects of treatment of DTC We suggest counseling pediatric DTC patients about the risk of developing recurrent laryngeal nerve injury or hypoparathyroidism after thyroid surgery and salivary gland dysfunction after exposure to I-131. In addition, the potential risk of subsequent primary neoplasms after I-131 treatment related to I-131 activity and possible risk for cardiac dysfunction after prolonged TSH suppression should be mentioned (3W) We recommend that the recurrent laryngeal nerve and parathyroid gland function is monitored post-operatively (3S). We suggest that all post-pubertal males who receive I-131 may be counseled upon the possibility of (transient) decreased fertility and semen preservation could be offered (3W). We suggest that all pediatric DTC patients receive additional calcium and vitamin D supplementation therapy for optimal bone mineralization during follow-up (4W). We suggest that all patients with pediatric DTC should be offered psychosocial support (4W). We suggest that future studies should further evaluate the prevalence and clinical significance of diastolic dysfunction in survivors of pediatric DTC after prolonged TSH suppressive therapy (4W). |
| [D9] | 29 |
Follow-up scheme and transition to adult care We suggest to continue follow-up of children with DTC for at least 10 years; thereafter, the follow-up strategy should be the result of shared decision-making between the physician and the patient (4W). |
(S) Strong recommendations are clinically important best practice and should be applied to most patients in most circumstances. (W) Weak statements should be considered by the clinician and will be an applicable best practice only to certain patients or under certain circumstances.
CCS, childhood cancer survivor; DTC, differentiated thyroid carcinoma; FDG PET/CT, [18F] fluorodeoxyglucose positron emissive tomography computed tomography; FNB, fine needle biopsy; I-123, iodine-123; I-124, iodine-124; I-131, iodine-131/radioactive iodine; PHTS, PTEN hamartoma syndrome; Tg, thyroglobulin; TSH, thyroid stimulating hormone
[A] Organization of care and goals for treatment of pediatric thyroid nodules and differentiated thyroid carcinoma
A1. Target population: pediatric patients
The expert panel has formulated this guideline specifically for children <18 years of age presenting with a thyroid nodule or DTC. Special recommendations for the management of DTC in this age group are necessary because of differences in presentation and genetics of DTC and the relevance of treatment-related late effects of DTC in young individuals. Moreover, monitoring changes in thyroid nodules in children is not straightforward due to the dynamic changes during development in childhood with a progressive increase in thyroid volume. There is a paucity of data to guide the interpretation of such changes and adult data have limited relevance.
The cut-off age of 18 years may be considered arbitrary, as the behavior, natural history, and characteristics of DTC do not suddenly change at this age. It must be taken into account that behavior and characteristics of DTC change with increasing age. In particular, patients in the age group of 16–25 years may either have DTC that behaves as ‘typical’ childhood DTC or may have a more ‘adult’-like behavior. Such patients, presenting with ‘typical’ childhood DTC may benefit from treatment along the pediatric guidelines, and it is clear that future clinical trials to provide more data to guide, age-appropriate management of thyroid cancer in children and adolescents, are needed.
Due to these differences and the fact that adult recommendations have been developed for individuals aged ≥18 years, this recommendation is intended for individuals <18 years of age.
A2. Thyroid expert team
Due to its rarity, centralization of care to expert centers is an important step for improving the management and outcome of children with DTC (11). For this reason, it is recommended that children with DTC are treated in a center with an experienced team of thyroid cancer experts, including a pediatric and adult endocrinologist, pediatric radiologist, (‘high volume’) pediatric thyroid cancer surgeon, (‘high volume’) pediatric surgeon experienced in thyroid surgery, pathologist, nuclear medicine physician, clinical geneticist, pediatric psychologist, and a pediatric oncologist. Centers with a higher volume of pediatric thyroid cancer cases can provide experience at each stage of the management pathway, with, for example, specific expertise in diagnostics and age-appropriate specialist nursing support. For thyroid surgery specifically, previous studies have shown that the higher number of thyroidectomies per surgeon correlates with improved quality of oncologic surgery as well minimizing rates of surgical morbidity such as hypoparathyroidism and recurrent laryngeal nerve injury (12, 13, 14).
We strongly recommend that patients are discussed within the setting of a multidisciplinary expert team in order to benefit from combined expertise and to optimize outcomes while minimizing treatment-related morbidity.
Recommendation 1:
We recommend that a child with suspicion of thyroid cancer, proven DTC or medullary thyroid carcinoma (MTC), should be referred to an experienced multidisciplinary thyroid team, specifically with experience in pediatric thyroid cancer (4S).
A3. Goals of therapy for DTC in children
The management of pediatric DTC is challenging given that children present more often with extensive and aggressive disease which has, however, little impact on life expectancy. This emphasizes the importance of considering and minimizing the long-term side effects of treatment. The optimal treatment approach to pediatric DTC may be complex and cannot be generalized due to variation in the individual presentation, risk factors, and prognosis. In the management of pediatric DTC, defining several goals of therapy may contribute to the improvement of outcomes.
The survival rates of children with DTC are generally excellent (10-year survival >98%) (2, 15). The first important goal of management is to maintain this excellent prognosis of pediatric DTC.
Unfortunately, life-long treatment-related complications are frequently seen, of which the most common are hypoparathyroidism, recurrent laryngeal nerve injury, and salivary gland dysfunction (sections ‘I-131 therapy’ and ‘Late effects of treatment’). Therefore, the second important goal of DTC management is to minimize short- and long-term adverse effects (14, 16).
In order to achieve these two goals, children with DTC should be stratified according to risk, to determine individualized treatment plans. Successful risk stratification may prospectively identify children who will benefit from higher-intensity treatment vs those in whom lower-intensity treatment will suffice.
Recommendation 2:
We recommend that children with DTC are stratified according to those who may benefit from higher-intensity treatment vs those for whom lower-intensity treatment will suffice.
By stratification, the goals of therapy for pediatric DTC (to maintain a high survival rate with low recurrence rate and to minimize adverse effects of treatment) will be reached (4S).
[B] Recommendations for pediatric thyroid nodules
The management of thyroid nodules in children is challenging with the obvious goal to identify children with a malignant nodule, because a benign nodule does not always require treatment. Thyroid cancer is very rare in childhood with a reported prevalence of 1:1,000,000 in children < 10 years, and up to 1:75,000 in children of 15–19 years of age when diagnosed based on clinical signs and symptoms (17). When populations are screened, ultrasound (US) may detect small, clinically unapparent DTCs at higher incidences, without evidence that treatment of such nodules will decrease mortality rates or improve patient health outcomes (18).
The prevalence of benign thyroid nodules in childhood has been described to be around 0.5–2% dependent on the screening method, either by palpation (19) or US (20), and on the definition of size that is documented (>5 mm or >10 mm). When offering thyroidectomy for benign disease, the possible lifelong adverse events of surgery must be borne in mind, starting with the lifelong need for levothyroxine (LT4) replacement therapy after thyroidectomy and, in a small but significant percentage of cases, permanent hypoparathyroidism that will also require a lifelong need for calcium and vitamin D replacement therapy (21).
B1. Prevalence of incidental (non-clinically relevant) thyroid nodules during childhood
In adults, asymptomatic small thyroid nodules are very common (increasing with age) and are often found incidentally (22). The majority of these remain asymptomatic for the rest of their lives.
The expert panel questioned the prevalence of non-clinically relevant thyroid nodules during childhood (Appendix A, [Q9]). A literature search was performed (Appendix B). The largest data resource was found in the surveillance programs in Fukushima and other parts of Japan that were not contaminated (Aomori, Yamanashi, and Nagasaki). These data revealed that the prevalence of US-detected thyroid nodules of >5 mm or cysts of >20 mm in Japanese children is around 1.0% (20). The prevalence of non-clinically relevant thyroid nodules in childhood was found here to vary between 0.6 and 2% (Appendix C) (23, 24). Based on these results, the expert panel suggests that prospective studies should be performed to increase the level of evidence to provide more certainty in determining the prevalence of non-clinically relevant thyroid nodules in childhood in different populations. However, when conducting such a study, the potential harm caused by association of over-diagnosis should be outweighed for the benefit of detection (21).
B2. Risk of malignancy in a thyroid nodule during childhood
Thyroid nodules in children are reported to be at two- to three-fold increased risk of being malignant when compared to thyroid nodules in adults. Dependent on the background iodine status of the country (due to the fact that in iodine-deficient countries, thyroid nodules are more prevalent), the risk for children of a clinically relevant thyroid nodule (>1 cm) being malignant is 20–25% compared to in 5–10% for a thyroid nodule in adults, respectively (25, 26, 27).
The expert panel questioned the state of evidence of using neck US to distinguish a benign thyroid nodule from thyroid cancer in a child (Appendix A [Q1]). A literature search was performed (Appendix B). For this question, studies were only included when the respective investigators were blinded for the outcome of the assessment modality.
The specificity and sensitivity of thyroid US to distinguish a benign thyroid nodule from thyroid cancer in children were found to vary depending on which US characteristic was assessed and on the use of combinations of such characteristics (Appendix C). The sensitivity for the following US characteristics was: hypoechogenicity: 52.2–63.0% (28, 29),calcifications: 5.3–63.6% (28, 29, 30),taller-than-wide shape: 21.2–26.4% (28, 30),irregular margin: 51.9–73.3% (29, 30, 31), and increased vascularization: 69.6–90.9% (29, 30).When characteristics were combined, the sensitivity of combined radiographic features increased to 28.1–93.2% (30, 32, 33). The specificity of the US characteristics was reported as follows: hypoechogenicity: 50.2–84.0% (28, 29), calcifications: 89.2–98.5% (28, 29, 30), taller-than-wide shape: 89.7–92.3% (28, 30), irregular margin: 80.2–94.4% (29, 30, 31), and increased vascularization: 25.9–97.8% (29, 30).When features were combined (depending on study), specificity increased and varied between 41.4 and 100% (30, 32, 33).
There are several US scoring systems to help stratify for which nodules fine-needle biopsy (FNB) (including fine-needle cytology) is indicated (34, 35, 36). The performance of the US scoring systems is comparable; however, their limitations should be recognized, as these scoring systems are based on adult populations and have not been validated in children. For example, the adult dimensional criteria should not be applied to children who often have smaller thyroid dimensions (37, 38). As mentioned, several specific individual US features may increase the likelihood of a nodule being malignant. It is important that investigators recognize and identify these features so they can guide appropriate management. A US scoring system should be used to systematically report these (suspicious) features of the nodule.
Recommendation 3A:
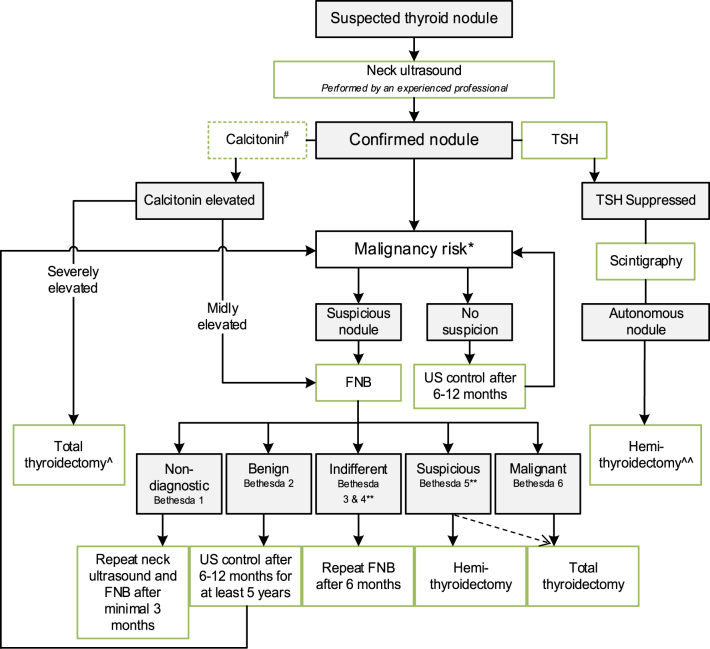
We recommend to undergo thyroid US to assess the risk of cancer in a thyroid nodule, based on multiple US characteristics. However, US alone cannot definitively distinguish a benign thyroid nodule from thyroid cancer. For this reason, in suspect nodules, FNB is recommended (2S) (Fig. 1).
Recommendation 3B:
The expert panel recommends, in children with thyroid nodule(s), a complete neck US to evaluate all cervical levels for the presence of lymph node enlargement (4S).
B3. Presence of lymph node metastases
The expert panel questioned (1) the sensitivity and specificity of suspicious US findings in a lymph node for predicting the presence of DTC metastasis and (2) whether imaging modalities other than neck US could contribute to evaluating the presence of lymph node and/or distant metastases pre-operatively (Appendix A [Q2, Q5]). Literature searches were performed (Appendix B); however, for both questions, no studies were found with evidence regarding the sensitivity and specificity of different suspicious US characteristics in a lymph node predicting DTC presence in childhood. Therefore, the expert panel referred to adult literature, in which the sensitivity of the US characteristics predictive of malignant lymph node involvement was reported as follows: microcalcifications: 5–69%, cystic aspect: 10–34%, peripheral vascularity: 40–86%, hypoechogenicity: 30–87%, and round shape: 37% (40, 41). The specificity was reported as follows: microcalcifications: 93–100%, cystic aspect: 91–100%, peripheral vascularity: 57–93%, hypoechogenicity: 43–95%, and round shape: 70% (40, 41).
At the time of review of these guidelines, a first study including children had been published. In this study including 52 children and adolescents with proven DTC, a significant association was seen between abnormal lymph node histology and round shape (P = 0.0002) and abnormal echotexture (P ≤ 0.0001) or vascularity (P ≤ 0.0001) (42). Sonographic findings correctly predicted whether the nodes were histologically involved with metastatic disease in 42/52 (81%). Sensitivity of sonography was 79%, specificity was 84%, positive predictive value was 90%, negative predictive value was 70%, and accuracy was 81%. These new results may be used to guide the investigator and emphasize the importance of performing neck US by an experienced thyroid ultrasonographer.
B4. Histopathologic characteristics of DTC
PTC is the dominant variant of DTC. Several low- and high-risk (of extent of disease) subtypes of PTC are described, such as the follicular variant (low risk) and tall cell and diffuse sclerosing variants (high risk) (Table 4) (43).
Table 4.
High- and low-risk histological subtypes of DTC and high-risk pathologic characteristics for extent of disease
| High risk | Low risk |
|---|---|
| A. Histological subtypes | |
| Tall cell variant of PTC | Follicular variant of PTC |
| Diffuse sclerosing variant of PTC | Classic variant of PTC |
| Solid/trabecular variant of PTC | Encapsulated PTC |
| Poorly differentiated carcinoma | |
| B. High-risk characteristics found at pathological examination | |
| Multifocal disease | |
| Bilateral disease | |
| Extracapsular invasion | |
| Extra thyroidal extension | |
Although the expert panel was aware of the fact that, during development of these guidelines, the 5th World Health Organization (WHO) classification system of thyroid carcinoma would be developed, and for this current ETA Guideline, the committee agreed to refer to the most recent published thyroid classification system, the 4th WHO classification system (44).
Low-risk subtypes, such as classic and follicular variants of PTC, account for the majority of DTC and are found in 63–85% (43 ,45). High-risk histologic subtypes of PTC are reported to occur in 15–37% of PTC in children, including the tall-cell variant (7–13%), diffuse sclerosing variant (7–16%), and the solid/trabecular variant (1–4%) (43). The coexistence of foci of poorly differentiated carcinoma (PDTC) occurs in 2–6% cases of high-risk PTC in children (43).
The diffuse sclerosing variant of PTC occurs in relatively young patients and has been associated with lymphocytic thyroiditis and circulating antibodies. These patients tend to have lymph node and lung metastases at the time of initial diagnosis (46). The survival rate is not significantly different from classic PTC. Cribriform-morular thyroid carcinoma is an entity independent from PTC and is classically associated with familial adenomatous polyposis and also occurs due to somatic mutations (47). The prognosis of cribriform-morular thyroid carcinoma is good, mainly because these tumors tend to be encapsulated/well-circumscribed (48).
Follicular thyroid carcinoma (is very rare during childhood and is usually minimally invasive (49). Anaplastic carcinomas are extremely rare in children (50).
Histopathology is the cornerstone in post-operative staging of DTC. Particularly, extrathyroidal extension is reported to be predictive for regional lymph node metastasis in pediatric DTC (51, 52).
In the new 5th WHO classification, micro-PTC is no longer considered as variant of PTC, which may be even more applicable in children than in adults (53). Also, the term ‘poorly differentiated carcinoma’ is combined for prognostic purpose with ‘follicular-cell derived carcinomas with high-grade features’ encompassing both the old PDC (‘insular carcinoma’) and PTC and FTC with many mitoses and/or foci of necrosis are introduced.
B5. Children at high risk for developing DTC
Children with a history of exposure to neck irradiation, I-131-MIBG or due to radioactive fallout (defined as exposure to a thyroid dose of 100–500 mGy or more (54)), with a positive family history for thyroid cancer or known to have a thyroid cancer predisposition syndrome (Table 5) may be considered as high risk of developing thyroid nodules and DTC. When such children present with a thyroid nodule, the risk of the nodule being malignant is increased (55). Surveillance programs for these patients aim to identify thyroid nodules suspicious of DTC at an earlier stage so that overall morbidity and mortality can be decreased. As with surveillance of general populations, the potential benefits of surveillance should outweigh any potential harm.
Table 5.
Genetic syndromes associated with thyroid neoplasia
| Inherited tumor syndrome | Germline pathogenic variant and mode of inheritance | Type of thyroid neoplasia | Syndromic features noted on clinical examination (listed in approximate order of appearance; some features only appear in adulthood) |
Additional clinical features |
|---|---|---|---|---|
| Familial adenomatous polyposis (FAP) (includes Gardner syndrome and Turcot syndrome) |
APC
Autosomal dominant 20% cases arise de novo |
Cribriform–morular cancer | Congenital hypertrophy of the retinal pigment epithelium (CHRPE), congenital absence of teeth, delayed eruption of teeth, dentigerous cysts, supernumerary teeth, odontomas, epidermoid cysts, fibrous dysplasia of the skull, mandibular osteomas, fibromas, desmoid tumors, and pilomatricomas. | Hepatoblastoma, medulloblastoma, multiple adenomatous polyps throughout the gastrointestinal tract, principally affecting the colon with high likelihood of malignant transformation, as well as upper GI tract adenomas and adrenal adenomas. |
| Carney complex |
PRKAR1A
Autosomal dominant 30% cases arise de novo |
Papillary thyroid cancer, follicular adenoma, and follicular thyroid cancer | Pale brown to black lentigines of skin, lips, and oral mucosa, soft tissue myxomas, Schwannomas, and epithelioid-type blue nevi. | Benign adrenal tumors (primary pigmented nodular adrenocortical disease), pituitary tumors (often somatotropinomas), large cell calcifying Sertoli cell tumors, breast ductal adenoma, osteochondromyxoma, and psammomatous melanotic Schwannoma of the nerve sheath. |
| DICER1 syndrome |
DICER1
Autosomal dominant |
Multinodular goiter, papillary thyroid cancer, and poorly differentiated carcinoma | Macrocephaly (OFC > 97th centile). | Pleuropulmonary blastoma, ovarian Sertoli–Leydig cell tumors, cystic nephroma, ciliary body medulloepithelioma, botryoid-type embryonal rhabdomyosarcoma, nasal chondromesenchymal hamartoma, pituitary blastoma, pineoblastoma, Wilms tumor, and juvenile intestinal hamartomas. |
|
PTEN Hamartoma tumor syndrome (PHTS) (includes Cowden syndrome, Bannayan–Riley–Ruvalcaba syndrome, and PTEN-related Proteus syndrome) |
PTEN
Autosomal dominant Over 10% of cases arise de novo |
Multinodular goiter, follicular adenoma, papillary thyroid cancer (classical and follicular variant) Follicular thyroid cancer (FTC cases are more common than PTC) |
Macrocephaly (OFC > 97th centile) and dolichocephaly, learning difficulties, autism and developmental delay, lipomas, vascular features including hemangiomas and arteriovenous malformations, gingival hypertrophy, oral papillomas, facial papules, acral keratoses, palmoplantar keratosis, trichilemmomas, pigmented macules of the glans penis, and overgrowth of tissues. | Benign and malignant tumors of the breast, colon, endometrium, and kidney, adult Lhermitte–Duclos disease due to cerebellar dysplastic gangliocytoma. |
| Werner syndrome |
WRN
Autosomal recessive |
Papillary thyroid cancer, follicular thyroid cancer, and anaplastic thyroid cancer |
Short stature (lack of pubertal growth spurt), cataracts, premature aging, tight atrophic skin, ulceration, hyperkeratosis, pigmentary alterations, regional subcutaneous atrophy, and characteristic ‘bird-like facies’, hypogonadism, secondary sexual underdevelopment, premature greying and thinning of scalp hair, pes planus, and abnormal voice. | Malignant melanoma, meningioma, soft tissue sarcomas, leukemia and pre-leukemic conditions of the bone marrow, primary bone neoplasms, osteoporosis, soft tissue calcification, evidence of premature atherosclerosis, and diabetes mellitus. |
Table 4 in the 2015 ATA Pediatric Guidelines (8) formed the basis for this table.
Several surveillance recommendations are available, such as the 2018 International Guideline Harmonization Group (IGHG) recommendations on thyroid cancer surveillance in survivors of childhood, adolescent, and young adult cancer (56), for children with thyroid cancer predisposition syndrome such as DICER-1 and PTEN hamartoma syndrome (PHTS) (57, 58, 59) and for children after nuclear accidents (54).
The advantage of surveillance of patients at risk is the detection of DTC at an earlier stage, which possibly reduces the extent of surgery or additional radioiodine therapy and, with that, morbidity. Furthermore, if no cancer is found with surveillance, its intensity or frequency may be reduced in these patients, which may decrease fear of cancer.
Disadvantages of surveillance include the uncertainty of the benefit of early treatment for DTC (since most DTC can be cured) and false-positive results leading to unnecessary interventions such as neck US and even FNB. This may not only cause unnecessary anxiety, stress, or inconvenience for the patient but also higher healthcare costs and may represent a risk for complications following unnecessary surgery. In addition, surveillance could lead to detection of small nonaggressive DTC, which would never have caused clinical problems and thus may lead to overtreatment. Lastly, false-negative results of surveillance will lead to inappropriate reassurance of the high-risk patient (56).
For this reason, the IGHG has recommended to aim for shared decision-making, to discuss the optimal surveillance strategy together with the patient and family taking into account individual patient circumstances (56). There are both advantages and disadvantages for screening with neck US or clinical neck palpation (Table 6).
Table 6.
Arguments for and against DTC surveillance modalities
|
Arguments for and against DTC surveillance with neck palpation
Advantages:
|
Disadvantages:
|
|
Arguments for and against DTC surveillance with neck ultrasound
Advantages:
|
Disadvantages:
|
DTC, differentiated thyroid carcinoma.
Adapted from Cancer Treatment Reviews, Vol 63, Clement SC, Kremer LCM, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, Alexander EK, Bardi E, Brignardello E, Constine LS, et al., Balancing the benefits and harms of thyroid cancer surveillance in survivors of childhood, adolescent and young adult cancer: recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium, pages 28–39, Copyright (2018), with permission from Elsevier (56).
Initiation of surveillance and the decision regarding which surveillance modality to use should be the result of shared decision-making between the physician and the high-risk patient.
Recommendation 4A:
We recommend that patients with a high risk of developing DTC (history of radiation exposure to the thyroid or a thyroid cancer predisposition syndromes) should be counseled for surveillance (4S).
Suggestion 4B:
We suggest that the initiation of surveillance and the decision regarding which surveillance modality (neck palpation and optionally neck US) to use should be the results of shared decision-making between the physician and the high-risk patient (4W).
B6. Diagnostic value of serum calcitonin in a child with a thyroid nodule
MTC arises from calcitonin-secreting parafollicular C cells. MTC occurs as sporadic and/or hereditary disease (70–75% and 25–30%, respectively). Adults have mostly sporadic disease, caused by somatic RET or RAS pathogenic variants, while the vast majority of children have the hereditary form, due to dominantly transmitted or de novo germline RET proto-oncogene pathogenic variants associated with multiple endocrine neoplasia (MEN) 2A or 2B. MTC accounts for approximately 5% of all pediatric thyroid cancers, with an annual incidence of 0.03/100,000. In children affected with MEN2 syndrome, the age of presentation of MTC depends on the nature of the RET pathogenic variant that is present, and prophylactic thyroid surgery is recommended to prevent MTC accordingly (60).
MTC secretes calcitonin; its serum level begins to rise with the development of C-cell hyperplasia and further increases with progression to carcinoma. The expert panel questioned what the state of evidence is to measure calcitonin in the diagnostic work-up of a thyroid nodule (Appendix A [Q8]). A literature search was performed; however, no studies were found (Appendix B).
Although serum calcitonin is a sensitive test for MTC when calcitonin is highly elevated, mildly elevated levels are not specific and can be caused by various drugs, non-thyroid non-malignant conditions, and assay interference (61). Specificity can be improved by measuring calcitonin after calcium stimulation (62). For children presenting with a thyroid nodule who have a family history of MTC or other MEN 2A or 2B-associated conditions, serum calcitonin measurement is advised. However, no clear evidence exists on the potential benefit of measuring calcitonin on a routine basis in children presenting with a thyroid nodule. However, since MTC > 1 cm will cause severe elevation of calcitonin, a low calcitonin level in a thyroid nodule of >1 cm excludes MTC. Therefore, the decision for measuring calcitonin in a child presenting with a thyroid nodule is based on individual conditions and the preference of the physician. If a thyroid nodule in a child is confirmed to be MTC, RET genetic screening should be performed, since in the majority of cases of MTC in children, a ‘de novo’ hereditary form or a ‘hidden’ hereditary form with an unknown family history has been found (63).
The focus of these current guidelines is the management and treatment of children with DTC; therefore, the expert panel refers to previous studies regarding the management and treatment of MTC in children (64).
Suggestion 5:
We suggest that, in selected cases (conditions that suggest MEN2, a positive family history of MEN2 or in case of bulky thyroid disease), the measurement of calcitonin may be of additional value for early diagnosis of MTC (4W).
B7. Evaluation of the child with a thyroid nodule
See Fig. 1.
Figure 1.
Flowchart of initial evaluation, treatment, and follow-up of the pediatric thyroid nodule. #The expert panel suggests considering the measurement of serum calcitonin in children suspect of MTC based on individual conditions and the preference of the physician (Recommendation 5A). The expert panel suggests that, in selected cases (conditions which suggest MEN2, a positive family history of MEN2, or in case of bulky thyroid disease), measurement of calcitonin may be of additional value for early diagnosis of MTC (Recommendation 5B). *Malignancy risk (suspicious vs no suspicion) is based on neck ultrasound characteristics (37), section ‘B2. Risk of malignancy in a thyroid nodule during childhood’), history of radiation, and (signs of a) pre-disposition syndrome. If there is a significant increase in nodule size or the ultrasound characteristics change over time, (repeated) FNB should be performed. **Analysis of the presence of other oncogenic drivers and gene fusions (e.g. RET/PTC and NTRK-fusions) may be considered in Bethesda 3, 4, or 5 due to the fact of increasing awareness that these are also associated with the presence of PTC (39). In case a BRAF V600E mutation is found, the risk of the thyroid nodule being malignant is high but needs to be confirmed, for example, by frozen section during thyroid surgery. ^Total thyroidectomy after proven presence of MTC. ^^Alternatively, FNB can be performed; in case of DTC, a total thyroidectomy should be performed.
B8. Molecular testing in FNB specimen
The evidence for the benefit of molecular testing in thyroid nodules, in terms of clear clinical implications, is limited. However, this is a rapidly changing field. The expert panel questioned whether currently available evidence supports performing molecular testing in the FNB specimen of a thyroid nodule in a child to determine the likelihood of it being malignant (Appendix A [Q3]). No evidence was found for children (Appendix B, C).
Molecular alterations are found in 77–92% of pediatric DTC (39, 65, 66, 67). In pediatric PTC, BRAF mutations are less common compared to adults. BRAF V600E mutations have not been found in benign thyroid neoplasms (65). For this reason, molecular analysis of a BRAF V600E mutation in FNB specimens classified as Bethesda 5 could be helpful for the diagnosis of PTC. In case where a BRAF V600E mutation is found, the risk of the thyroid nodule being malignant is high but needs to be confirmed, for example, by frozen section during thyroid surgery.
Analysis of the presence of other oncogenic drivers and gene fusions (e.g. RET/PTC and NTRK fusions) may be considered in Bethesda 3, 4, or 5 due to the fact of increasing awareness that these are also associated with the presence of PTC (39, 68). A shift toward the evaluation and management of pediatric DTC by identifying oncogenic drivers and gene fusions in the diagnostic work-up may be expected because knowledge of these molecular alterations may increase the accuracy of cytology results currently classified as indeterminate (39). However, the expert panel agreed that the current evidence is not sufficient to incorporate this as a standard of care for all children with thyroid nodules suspicious of DTC. Analysis of other oncogenic drivers and gene fusions could be performed in a research setting.
Suggestion 6:
We suggest that molecular gene analysis for the presence of BRAF V600E mutation in an FNB specimen may be helpful for the diagnosis of PTC and therefore may be considered in the diagnostic work-up. The presence of PTC however must be confirmed cytologically or histologically (preoperative FNB or intraoperative frozen section) before total thyroidectomy is performed (4W).
B9. Role of surgery for benign thyroid lesions
For benign thyroid lesions (thyroid cysts or nodules with Bethesda II on FNB), follow-up is recommended with US after 6–12 months. For a stable lesion, subsequent US follow-up is recommended every 12–24 months, for at least 5 years (8).
When there is a significant change in palpable or US characteristics, repeated FNB should be considered (69, 70). When benign nodules cause clinical symptoms (e.g. compression symptoms and cosmetic concerns), surgery may be the preferred choice of treatment (8, 71).
When a thyroid nodule is found in a child with a background of Graves’ disease, there is a slightly increased risk for malignancy (72). Indications for FNB however do not change. When surgery is indicated in such children, total thyroidectomy is recommended above hemithyroidectomy.
In children, tumors of uncertain potential of malignancy (oncocytic lesion and follicular neoplasia) are diagnosed through FNB up to 35% (8). Studies found that 28% of AUS/FLUS lesions and 58% of those suggestive of follicular or Hurthle cell neoplasm are malignant (73, 74), which prompts surgical treatment. If FNB of the thyroid nodule shows indifferent results (Bethesda 3 or 4), repeated FNB is suggested after 6 months (Table 3). If FNB again is indifferent, it is suggested to discuss about the patient in the multidisciplinary board regarding the subsequent appropriate diagnostic approach (e.g. molecular imaging or diagnostic surgery). Diagnostic hemithyroidectomy is the recommended surgical approach for unifocal lateralized benign lesions. Total or near-total thyroidectomy is recommended in case of lesions in both lobes (e.g. symptomatic nodular goiter).
Recommendation 7A:
We recommend that benign nodules are followed by serial US and should undergo repeat FNB if suspicious features develop (4S).
Suggestion 7B:
We suggest hemithyroidectomy or total thyroidectomy for benign nodules, performed by an experienced high-volume pediatric thyroid cancer surgeon, in patients with compressive symptoms, cosmetic concerns, or according to patient/parent preference after counseling of the possible benefits and risks of thyroid surgery (4W).
B10. Autonomous thyroid nodules in children
Autonomous thyroid nodules are diagnosed as autonomous nodule when thyroid-stimulating homone (TSH) is suppressed, and scintigraphy confirms the functional hyperactivity of the nodule. Autonomous nodules are usually found in post-pubertal girls; however, they are very rare in children, and (large) cohort studies are lacking (75). As in adults, autonomous thyroid nodules in children are mostly benign (76), but malignancy may be present. Unlike Graves’ disease, the autonomous thyroid nodule is usually progressive and does not regress spontaneously. In children, such nodules are most often caused by somatic mutations that increase the constitutive activity of the TSH receptor (TSHR). There are two treatment options for a permanent cure of a hyperactive nodule: surgery or administration of I-131 therapy. Due to the risk of malignancy present in an autonomous nodule during childhood (77), the expert panel agreed to recommend surgery as preferred treatment for the autonomous nodule in a child to obtain definitive histological diagnosis. The administration of I-131 may be considered for small nodules. An argument in favor of I-131 treatment is the avoidance of adverse events due to thyroid surgery such as hypoparathyroidism or recurrent laryngeal nerve injury (known complications of thyroidectomy).
Suggestion 8A:
We suggest hemithyroidectomy for autonomous nodules during childhood, which must always be performed by an experienced high-volume pediatric thyroid cancer surgeon (4W).
Recommendation 8B:
We recommend discussion of the advantages and disadvantages of surgery vs radioiodine treatment using shared decision-making in each individual case (4S).
[C] Thyroid carcinoma management guideline
C1. Pre-operative evaluation
Pre-operative evaluation of the child with DTC must comprise a clinical and comprehensive neck US investigation, laboratory testing, and FNB, flanked by genetic testing when family history is suggestive of familial disease (78, 79, 80). Palpation of the neck may identify pathological thyroid nodules or lymph nodes; however, US examination including all six cervical lymph node levels is more sensitive and well tolerated. US can be useful to guide FNB allowing cytology and/or molecular work-up to guide broader examination. Where there is suspicion of extrathyroidal, extensive neck nodal, or infiltrative disease, anatomic imaging modalities, for example MRI or CT, may be valuable to direct surgery (78, 79, 80).
Vocal cord exam can be of additional value in children with bulky disease to be optimally informed pre-operatively. The expert panel questioned the sensitivity of different imaging modalities for the presence of pre-operative metastasis (Appendix A [Q5]). A literature search was performed; however, no literature was found (Appendix B).
The expert panel agreed that in children with large or fixed thyroid masses, vocal cord paralysis, bulky metastatic lymphadenopathy, or (suspected) tumor invasion in the esophagus or trachea determined by physical examination or extensive neck US, a pre-operative MRI of the neck is recommended.
Local advanced disease, with the exception of metastatic lymphadenopathy, is rare in children. In case of extensive cervical lymphadenopathy, the expert panel suggests considering a low-dose CT of the thorax without contrast medium to assess the presence of pulmonary metastases; however, these metastases will also become visible at the moment of I-131 scanning. A contrast-enhanced CT is best avoided unless explicitly desired for surgical planning. If contrast-enhanced CT is performed, there should be an interval of at least 6 weeks to several months before I-131 treatment is given to optimize the uptake of I-131 in the benign or malignant thyroid cells.
In case of suspicious lateral neck lymph nodes (size, aspect, or US characteristics), FNB is recommended to confirm metastases. In addition, thyroglobulin (Tg) measurement on needle-washing fluid could be considered to confirm metastases.
Recommendation 9A:
We recommend neck palpation, comprehensive neck ultrasonography, and laboratory work-up as minimal pre-operative evaluation measures in the pediatric population. The expert panel suggests further genetic or imaging diagnostics in case of suspicion of familial or extensive disease (4S).
Suggestion 9B:
We suggest additional pre-operative investigations using MRI of the neck and/or low-dose non-contrast CT of the thorax in case of bulky disease or suspicion of lung metastases (4W).
Recommendation 9C:
We recommend confirmation of suspicious lateral lymph nodes (size, aspect, or US characteristics) with FNB (4S).
Suggestion 9D:
We suggest the assessment of vocal cord function in children with bulky disease pre-operatively (4W).
C2. Surgical approach for DTC
Surgical approach for DTC (in general)
In the majority of cases, pediatric thyroid cancer presents with locally advanced tumor growth and early cervical lymph node metastases, which impact surgical approach and distinguish the management of pediatric DTC from adult DTC.
In the discussion of whether subtotal thyroid resection or lobectomy should be considered instead of total thyroidectomy in the treatment of pediatric DTC; the associated complication risks (i.e. hypoparathyroidism and recurrent laryngeal nerve palsy) need to be weighed against the likelihood of persistent and recurrent DTC (81, 82). Total thyroidectomy is required to enable radioiodine therapy (78, 81, 82). Adequate primary surgery is the premise to avoid neck recurrence and defines the ongoing course of the disease (78, 81, 82).
The expert panel questioned the difference in the outcome of pediatric DTC after total thyroidectomy vs hemithyroidectomy or subtotal thyroidectomy (Appendix A [Q10]). A literature search was performed (Appendix B, C).
One study reported a possible superiority of total thyroidectomy to subtotal thyroidectomy in pediatric DTC from a perspective of disease/recurrence-free survival (univariate analysis) (83). However, in a multivariate analysis, no difference in outcome was found between total thyroidectomy and subtotal thyroidectomy (83, 84). Bal et al. (2015) found total thyroidectomy to be a significant prognostic factor for remission (univariate analysis and multivariate analysis) (85).
Disease-free survival in children with low-risk disease without clinically apparent nodal disease (by pre-operative physical examination, US, FNB, and intraoperative inspection) and without gross extrathyroidal extension (based on imaging/clinical features) treated by lobectomy was shown not to be inferior to that in children treated by total thyroidectomy (86). With these results in mind, the excellent prognosis of childhood DTC and the necessity to minimize the risk of complications and to maintain quality of life, less extensive surgery may be considered more frequently in low-risk pediatric patients (78). However, studies about the impact of limited surgery on recurrence and remission rates in children are lacking. For this reason, prospective studies are needed before such recommendations can be made.
Surgical approach for co-incidentally found, very small DTC (exception)
Due to the fact that the diameter of thyroid cancer is not related to the presence of cervical lymph node metastases (87), total thyroidectomy is recommended in all children with DTC regardless of the size of the nodule. However, as mentioned above, considering the excellent prognosis of DTC, the expert panel questioned whether children with very small lesions (found coincidentally) with no clinical signs could be treated differently (Appendix A [Q11]). A literature search was performed. No studies were found to evaluate differences in outcome between patients with DTC < 1 cm (also defined in literature as thyroid microcarcinoma (TMC)) treated with total thyroidectomy vs hemithyroidectomy or subtotal thyroidectomy.
Two studies were found that reported no differences in disease-specific survival and overall survival between patients with TMC and patients with DTC > 1 cm, although patients with TMC were more often treated with subtotal thyroidectomy/lobectomy/isthmusectomy, with no additional I-131 treatment (88, 89) (Appendix B, C).
Prospective studies are necessary to evaluate if pediatric patients with small thyroid carcinoma may be treated with less extensive surgery in case of non-aggressive disease. For such studies, it may be considered to offer limited surgery to those with determinants for excellent recurrence-free and overall survival, such as classical PTC or intrathyroidal tumor localization with intact capsule (83, 88, 90).
The expert panel discussed the approach to a child with ‘incidental’ DTC found on post-surgical pathology for another presumed benign thyroid condition, for example, hemithyroidectomy, for autonomous nodule or multinodular goiter. A small case series (26 patients, all adults) was found that showed no difference in outcome (i.e. disease-free at median follow-up of 4 years) in patients who underwent total thyroidectomy vs active surveillance (91). Similar to the 2015 ATA Pediatric Guideline, the expert panel recommends extensive neck US in these cases to detect contralateral disease/regional lymph node spread (8). Patients with no disease in US may be stratified as low risk and regular surveillance screening can be undertaken. In patients who are found to have contralateral disease/regional lymph node involvement in US, cytological confirmation of the node should be performed.
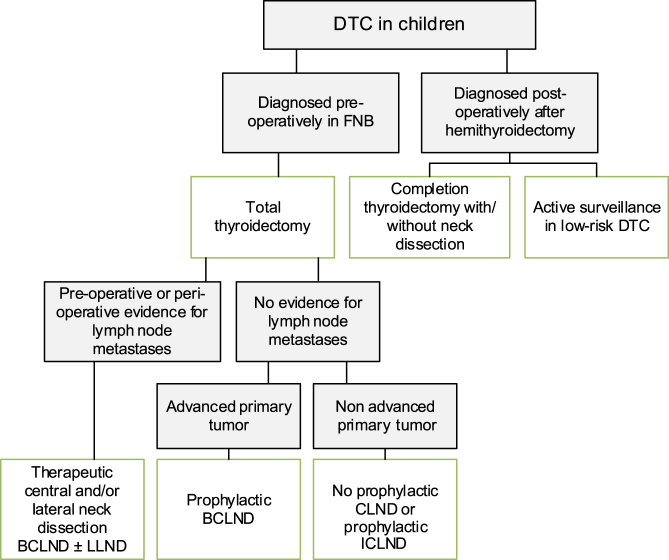
Suggestion 10A:
We suggest total thyroidectomy as treatment for children with DTC (3W). See Recommendation 10C for exceptions. See Fig. 2
Figure 2.
Flowchart of surgical approach for DTC in children. BCLND, bilateral central lymph node dissection; CLND, central lymph node dissection; DTC, differentiated thyroid carcinoma; FNB, fine needle biopsy; ICLND, ipsilateral central lymph node dissection. ‘Active surveillance’ in low-risk DTC implies ultrasound of the leftover thyroid tissue, including the evaluation of the cervical lymph nodes every 6–12 months by neck palpation and ultrasound.
.
Recommendation 10B:
We recommend that future studies be conducted that evaluate the impact of limited surgery for pediatric DTC with respect to recurrence and remission rates (4S).
Suggestion 10C:
We suggest that, in pediatric patients with incidentally found, very small thyroid carcinoma and non-aggressive histological features, hemithyroidectomy may be considered as therapeutic option (4W).
C3. Therapeutic central and lateral neck dissection
The expert panel agreed that the indication for central and/or lateral lymph node dissection (LLND) is based on pre-operative clinical assessment with neck palpation and extensive US or other imaging modalities suggesting nodal neck disease. The indication for compartment-oriented LLND is lymph node metastasis identified by neck US and diagnosed in FNB and/or Tg measurement on needle-washing fluid. In children, the risk of lymph node metastasis is higher than in adults.
However, often, the predictors of nodal neck disease of DTC such as tumor size of T3/T4, tumor multifocality, thyroid capsule infiltration, diffuse sclerosing variant, lymphatic and vascular invasion can only be assessed post-operatively (87, 92). Frozen section histopathology may help to identify all or some of these features. The incidence of positive lymph nodes can only be assessed in routine systematic neck dissection, which results in further patient qualification for radioiodine therapy. Therefore, attempts have been made to generate risk factors and prediction models for lateral lymph node metastasis, as reported by Liang et al. (93). The authors assessed 102 children and adolescents with PTC and found that independent risk factors for lateral lymph node metastasis were multifocality, tumor size, and the number of central lymph node metastases.
Prophylactic central lymph node dissection (CLND) aims at elimination of micrometastases of DTC to optimize outcome, improving cure rates and minimizing lymph node relapses. However, especially the CLND exposes the parathyroid glands and their vascularization, and to a lesser extent risks, the recurrent laryngeal nerves. The main complication of CLND is post-operative transient and permanent hypoparathyroidism, even more so in the pediatric population, followed by transient and permanent recurrent laryngeal nerve palsy. Therefore, indications for prophylactic CLND in children should be limited and reserved for identified risk factors (14, 78).
A literature search was performed for the differences in the outcome of DTC in children treated with a (prophylactic) CLND vs no (prophylactic) CLND (Appendix A [Q12], B, C). Conflicting results were found. Rubinstein et al. suggested that an aggressive surgical approach may simultaneously decrease the risk of recurrence and improve prognosis in patients with more advanced or aggressive disease (94). Olmsted et al. showed no difference in recurrence-free survival among patients treated with LND compared to limited node excision of no LND (95). However, location of LND was not specified. It remains unclear if these patients underwent prophylactic CLND.
Prophylactic CLND is debatable in children as well as in adults. It should be borne in mind, however, that the risk for lymph node metastases is significantly higher in children. The benefits of prophylactic CLND must be balanced with the low risk of missing clinically significant disease during pre-operative (US) or intraoperative assessment and the risk of post-operative complications. The expert panel agreed that prophylactic CLND should only be performed in advanced thyroid cancer (extracapsular extension, vascular invasion, and distant metastases). However, bearing the above in mind, ipsilateral prophylactic CLND may be considered in patients without suspicious features for advanced thyroid cancer on neck US to possibly reduce the risk of reoperation and the necessity of I-131 therapy. Of note, surgical experience is crucial for preventing life-long complications. Prophylactic neck dissection is still the best therapeutic option for patients with advanced thyroid primary disease when performed by high-volume surgeons (78).
Prospective studies are needed to compare the difference in outcome of DTC in children treated with prophylactic CLND vs no prophylactic CLND.
Suggestion 11A:
We suggest that prophylactic central lymph node dissection should only be performed in advanced thyroid cancer (extracapsular extension, vascular invasion, and distant metastases). It can be avoided or limited to ipsilateral lymphadenectomy in patients without suspicious features for advanced thyroid cancer on neck US (4W).
Suggestion 11B:
We suggest that therapeutic central lymph node dissection should always be recommended in pediatric DTC in case of suspicious central lymph nodes based on neck US or intraoperative assessment, or perioperative visible extracapsular tumor growth (4W).
Recommendation 11C:
We recommend that therapeutic lateral lymph node dissection is performed in all children with pre-operatively proven lymph node metastases or in case of evident (pathological) lateral lymph node(s). The expert panel does not recommend prophylactic lateral lymph node dissection (4S).
C4. Surgical complications of thyroidectomy and neck dissection
As in the case of adults, pediatric DTC surgery carries specific risks, clearly correlated with the extent of surgery and the surgical expertise. The most frequent complications are post-operative transient and permanent hypoparathyroidism, transient and permanent recurrent laryngeal nerve palsy, bleeding, and thoracic duct fistula and nerve damage following LLND (96, 97, 98). Due to the close anatomical relation of the thyroid gland with the recurrent laryngeal nerve, damage can occur leading to a hoarse voice, and in case of bilateral damage, respiratory problems. The parathyroid glands may be difficult to identify leading to transient or permanent hypoparathyroidism post-operatively. The number of surgical adverse events decreases in experienced teams (99).
A clear volume–outcome relationship is observed with favorable outcomes and minimal complication rates in expert centers dedicated to pediatric endocrine surgery (98). Surgical diligence, application of magnification loupes, bipolar forceps, and technical innovations such as intraoperative nerve monitoring, parathyroid fluorescence and parathyroid hormone assessment, and access to frozen section histopathology may improve surgical outcome. In the management of apparent complications, these need to be specifically addressed, for example with early calcium- and/or vitamin D supplementation, immediate evacuation of hematoma, or logopedic training for recurrent nerve palsy (96, 97, 98)).
Recommendation 12:
We recommend that all children with DTC should be operated on by high-volume surgeons with experience in pediatric thyroid cancer and who are embedded in a center with expertise in the management of DTC (4S).
C5. Post-operative staging
The expert panel searched for the most sensitive imaging modality for the presence of DTC, post-operatively (Appendix A [Q7], B), but the literature search did not yield any studies comparing various possible modalities. Therefore, reverting to general medical and empirical principles to select those procedures that are both sensitive and sensible was necessary.
In general, especially in children, radiation exposure due to medical procedures must be minimized. For post-operative staging, measurement of Tg, a specific thyroid cell marker, can be used. There is however lack of pediatric literature on cut-off values for low Tg levels (on levothyroxine) and this may be assay-dependent; for this reason, cut-off levels should be determined by the local expert team.
The expert panel agreed that in pediatric DTC patients with low Tg levels, additional staging is unlikely to be necessary, especially when I-131 therapy is planned as post-therapy, scintigraphy will yield highly sensitive staging information. In those patients not deemed to need I-131 (non-advanced DTC), additional staging will be superfluous.
Furthermore, US and MRI appear to be preferable over CT per se if, for example, staging of the neck is warranted. In cases where CT may provide distinct advantages, for example where pulmonary metastases are suspected, it should be considered.
The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control TNM staging system is widely used for predicting the prognosis of DTC in adults (100). However, the TNM staging system comes with limitations regarding the assessment of the prognosis of DTC in pediatric patients: only two disease stages can be identified (stage I: no distant metastasis; stage II: distant metastasis), since all pediatric patients are <45 years of age and secondly, the disease-specific mortality is extremely low. Despite this, the TNM staging system seems to be the most preferred system to be used for staging pediatric DTC.
Recommendation 13A:
We recommend that post-operative staging is done using the surgical report, histological report, measurement of Tg, and I-131 post-therapy scintigraphy (4S).
Suggestion 13B:
We suggest that the AJCC TNM classification system should be used to describe the extent of disease in pediatric DTC (4W).
C6. I-131 therapy
I-131 therapy for benign and malignant thyroid disease has been successfully used for 80 years. Over this time, it has been applied extensively in adult and in pediatric patients alike and has contributed to a normalization of life expectancy in DTC.
In pediatric patients, in particular, I-131 therapy can be extremely helpful; even in case of widely disseminated pulmonary metastases, patients may be cured by one or more courses of I-131.
Yet, the indications for adjuvant post-operative I-131 therapy are a subject of debate, both in terms of which patients should receive it and which therapeutic goal (remnant ablation, adjuvant treatment, or treatment of known disease) should be strived for (101).
Especially for patients without lymph node or distant metastases, it has not been cleared beyond a doubt whether I-131 can improve survival, reduces recurrence rates, or both in these patients. The expert panel searched for specific histopathological criteria related to distant/any metastases (Appendix A [Q6], B). No evidence of specific histopathological criteria predicting lymph node metastases and/or distant metastases was found. Liu et al. reported T3 and T4 tumors and lymph node metastases as factors associated with distant metastases in children (102). The expert panel also questioned whether the outcomes of microcarcinoma treated with I-131 differ from that of microcarcinoma not treated with I-131 (Appendix A [Q13], B), but no literature was found to answer this question.
Considering the possibility of a generally increased genetic susceptibility of patients who develop cancer at such a young age, it remains questionable whether the indiscriminate administration of ionizing radiation during childhood might not do more harm than good. Thus, a risk-benefit evaluation and shared decision-making are key components of pediatric DTC management.
Response to I-131 may be observed up to 15–18 months after therapy (103); therefore, long intervals of at least 12 months are suggested before retreatment.
For I-131 therapy, in pediatric patients, special considerations apply because of differences in biological behavior and differences in dosing (see section ‘Targeted therapy in the management of pediatric DTC’) related to patients’ lower bodyweight and distinct metabolism in children (see section ‘Targeted therapy in the management of pediatric DTC’). Taking into account the inherently good prognosis in most children with DTC, potential adverse effects in patients with a long life expectancy (see section ‘Late effects of treatment’) need to be carefully considered (see section ‘Late effects of treatment’). Studies specifically examining the potential benefits of I-131 in children are difficult to perform because the number of patients is small. As especially the distinction between remnant ablation and adjuvant treatment in I-131 therapy has only been introduced in past few years, this small number of patients also precludes us from making any evidence-based recommendation on the precise goal of I-131 therapy. However, in adults, the advent of novel Tg assays has made remnant ablation as a goal per se more and more superfluous.
If administered correctly, I-131 therapy in the hands of specialists is a highly effective oncologic therapy, which has greatly contributed to the generally favorable outcome of patients with pediatric DTC. After so many years of experience, harmonization, and standardization, (international) registries could be a crucial step forward to personalized treatment strategies.
Suggestion 14A:
We suggest that I-131 therapy is indicated for all children following total thyroidectomy, for the treatment of persistent locoregional disease, remnant thyroid cells, or nodal disease that cannot be resected for as well as iodine avid distant metastases (M1) (4W).
Suggestion 14B:
We suggest that, for patients with persistent disease following post-operative I-131 therapy, the decision to pursue an additional course of I-131 therapy should be individualized according to previous response (4W).
Suggestion 14C:
We suggest that the minimal interval between I-131 treatments for DTC in childhood should be recommended to be around 1 year (4W).
C7. I-131 activity
The question of I-131 activity selection, that is the ‘optimal’ dosage, is of ongoing relevance since very limited evidence is available in the literature. The expert panel questioned what the most optimal dose effect curve is of radioiodine with least side effects calculated by body weight/fixed activity or dosimetry; however, no literature was found (Appendix A [Q14], B).
As a theoretical construct, the activity which delivers an absorbed dose of radiation is sufficient to destroy a lesion/metastasis while low side effects should be chosen. As a standard of care empirically derived, fixed activities are used, and therapy is repeated as deemed necessary. Several parameters are considered equally or even more important than the administered activity, such as I-131 avidity of tumor tissue, residence time of I-131, the effective I-131 half-life, and tumor size and shape. A practical approach was successfully established in one institution in the management of young patients following radiation-induced thyroid cancer (104). This protocol included the administration of 50 MBq/kg bodyweight for remnant ablation/adjuvant therapy and 100 MBq/kg for the treatment of known metastases (104). However, using a fixed activity approach does not take into account the individuality of the patient. Alternatively, dosimetric strategies have been introduced over the last decades. Here, the patients’ iodine biokinetics are determined to calculate the activity as high as safely administrable, thus targeting the safety of the procedure (i.e. 2 Gy to the blood and limit of 3 gigabecquerel (GBq) I-131 retained whole body activity 48 h after I-131 in the presence of (pulmonary) metastases) in an attempt to avoid damage to the hematopoietic system as well as pneumonitis or pulmonary fibrosis, respectively (105). These limits likely must be adapted to account for the lower total mass of the lungs of pediatric patients. The objective of lesion dosimetry is to determine the radioiodine activity that delivers the intended absorbed dose to ablate thyroid remnant or to treat metastatic disease. More recent studies based on measurements with improved equipment (positron emission tomography (PET)/CT) and more suitable tracers for lesion dosimetry (I-124) support the hypothesis that therapeutic outcome correlates with the absorbed dose administered to the target tissue (106, 107, 108, 109).
Suggestion 15:
We suggest that an individual patient-based approach should be used to calculate the optimal activity of I-131 taking into account the potential side effects of I-131 with increasing activity. The preferred administered activity for an individual should be discussed in the multidisciplinary tumor board taking the individual characteristics of the patient into account (4W).
C8. Preparation of the patient for treatment with I-131
It is generally accepted that adequate TSH stimulation (usually indicated by achieving a level of TSH > 30 mU/L (110)) is necessary for optimal I-131 therapy of DTC. This can be achieved by two methods: either by thyroid hormone withdrawal (THW) or by administration of recombinant TSH (rhTSH). Although rhTSH administration has been studied extensively in adults, experience with rhTSH in children is limited at best, and consequently, this drug is not registered or licensed for use in the pediatric population. The expert panel searched for studies on rhTSH effectiveness and safety in children (Appendix A, [Q15]) and retrieved three (Appendix B, C) (111, 112, 113).
All three studies reported TSH levels after rhTSH (2 × 0.9 mg) stimulation of >50 mU/L, and no significant side effects were reported. No studies were found reporting on iodine uptake after rhTSH injection in children. Although rhTSH from this limited data appears to be safe and able to achieve adequate TSH levels, it cannot be conclusively stated that rhTSH is equivalent to the conventional method of THW regarding therapeutic efficacy of I-131 treatment based on this small body of evidence. Furthermore, the considerable costs and potential reimbursements by local health insurance of rhTSH may also affect the decision regarding the use of this preparation method.
A second cornerstone of preparation for I-131 treatment generally consists of a low-iodine diet for a period of 1–2 weeks before administration; however, a recent study showed that a 4-day low-iodine diet seems to be sufficient in areas with iodine-sufficient intake (40, 114, 115). Although in use for many years, its effect on therapy outcome is not uniformly accepted; here, local iodine sufficiency status might also play a role. Although its merit is still subject to some scientific debate, a low-iodine diet before I-131 therapy may favorably influence the uptake of I-131 in DTC during therapy and will do no harm. Therefore, the expert panel agreed to recommend a low-iodine diet for at least 4 days before the administration of I-131 treatment (115).
Recommendation 16A:
We recommend that TSH stimulation (>30 mI/L) is induced before I-131 therapy in order to facilitate I-131 uptake (4S).
Suggestion 16B:
We suggest that stimulated TSH can be achieved either using thyroid hormone withdrawal or rhTSH. The expert panel did not reach consensus on the optimal way of preparation. The decision for one against the other is up to the clinical experience of the treating team (3W).
Suggestion 16C:
We suggest that a low iodine diet for at least 4 days before I-131 therapy may be favorable for iodine uptake (4W).
C9. Targeted therapy in the management of pediatric DTC
Pediatric DTC commonly presents with advanced disease at diagnosis with a high prevalence of cervical lymph node metastases and lung metastases, usually identified on the whole-body scan performed after I-131 treatment. However, the outcome of these cases is good, and death-related events are very rare. The major reason for this good outcome is the responsiveness of the metastatic lesions to I-131 therapy. Very rarely, children with metastatic thyroid cancer require therapies other than I-131. It is however interesting to recall that childhood PTC has a high prevalence of RET/PTC rearrangements as well as NTRK fusions (116). This information is particularly relevant since new tyrosine kinase inhibitors directed against either RET or TRK alterations (117, 118) are under development and have already reached the approval of FDA and EMA for adult patients. The expert panel searched the outcome of DTC in children treated with surgery and I-131 vs those treated with different treatment modalities (Appendix A [Q18], B).
Mahajan et al. reported three cases for whom lenvatinib was given (119). Two patients remained clinically stable on lenvatinib 11 and 23 months after initiation of therapy. The third patient transitioned to a tumor-specific targeted therapy after 1 month. Waguespack et al. have reported one 14-year-old female treated with sorafenib who showed significant improvement in lung metastases 67 days after start of treatment (120).
Based on these case reports, the expert panel agreed that targeted therapy may play a role in the management of disease in very rare cases of pediatric progressive I-131-refractory PTC, for whom no standard therapy exists (Appendix C). There is currently no consensus on the absolute definition or criterion that defines that a patient has I-131-refractory DTC. Each patient should be managed individually with a thorough understanding of the many factors that enter the appraisal of the likelihood that a tumor will be refractory to I-131, as well as weighing the patient's specific clinical scenario and the risks and benefits of I-131 therapy. Pediatric progressive I-131-refractory PTC may be suspected in cases with presence of more than one metastatic lesion with at least one lesion without I-131 uptake in the post-therapy scan, structural progression of tumors after I-131 therapy despite the presence of iodine uptake in the post-therapy scan, or significant uptake on FDG PET/CT (121, 122).
Suggestion 17:
We suggest that, in specific cases, treatment with targeted therapy may be considered, but this should preferably only be given in the setting of clinical trials (4W).
C10. Somatic molecular testing (in thyroid carcinoma tissue)
Molecular testing may be useful to understand the tumor etiology, behavior, predict prognosis, and possibly guide the development of novel treatment strategies.
As pediatric PTCs exhibit a distinct genetic background, it is not usually classified into BRAF V600E -like and RAS-like tumors. In pediatric DTC, BRAF V600E mutation has been described to be present in 25–30%, dependent on age (123). In the studies performed after the Fukushima accident, however, about 60–70% of patients with minuscule PTCs detected in screening projects display BRAF V600E mutations (124, 125). The frequency of BRAF V600E mutation is low in sporadic pediatric DTC cases as well as in radiation-exposed cases (126).
RET gene fusion together with other fusion types is detected in 60–70% of pediatric DTC patients in comparison to 15% in adults. RAS gene family mutations are much rarer in pediatric PTCs (<5%) than in adult PTCs. NTRK fusions occur approximately in 10% (range: 0–26%) of all pediatric PTCs (43).
The expert panel questioned whether molecular testing in thyroid carcinoma tissue in a child alters its management (Appendix A [Q4]). No evidence was found on altering the management after specific genetic findings such as rearrangements in thyroid carcinoma tissue (Appendix C).
The expert panel is aware that this is a rapidly changing field. In line with the recommendations regarding molecular testing in FNB specimen, the expert panel recommends analyzing the presence of oncogenic drivers and gene fusions in thyroid carcinoma tissue in a research setting. Molecular testing may also be performed in rare, advanced cases with I-131 refractory DTC who could benefit from systemic therapy since systemic therapy may re-express the sodium/iodide symporter (NIS) in tumor cells, for example in DTC (119, 127).
Suggestion 18A:
We suggest that molecular testing in pediatric thyroid carcinoma tissue should be recommended in research setting but the result has currently no consequences for pediatric DTC management (4W).
Suggestion 18B:
We suggest that for cases with I-131 refractory DTC, molecular testing in pediatric thyroid carcinoma tissue should be recommended as the result may have consequences for pediatric DTC management (4W).
C11. Treatment for pediatric radiation-induced DTC
Exposure to radiation (external beam and accidental or therapeutic I-131) is a well-known risk factor for developing DTC, especially when exposed during childhood (128, 129). In childhood cancer survivors (CCS) who received radiation to the neck, the risk of DTC was found to increase linearly with increasing estimated radiation dose to the thyroid gland, with a plateau around 10–30 Gy and declining thereafter, consistent with the cell-killing effect (130, 131). The latency time between radiation exposure and the development of DTC is broad, with a minimum latency time of approximately 5–10 years. The risk of developing DTC is elevated up to 50 years after radiation exposure (17). This underscores the importance of long-term follow-up of CCS at risk. The expert panel questioned if presentation, outcome, or disease course of DTC in children with a history of radiation exposure are different than in children without a history of radiation exposure for which treatment or follow-up should be adjusted (Appendix A [Q23]). A literature search was performed (Appendix B, C) (132, 133, 134, 135).
CCS with subsequent DTC tended to have on average smaller tumors and presented more often with bilateral disease (132, 133). Pacini et al. reported more frequent extra-thyroidal extension (ETE) and lymph node metastases (LNM) in radiation-induced thyroid tumors in children diagnosed in the Chernobyl region (134). No significant differences were found between CCS with subsequent DTC and patients with sporadic DTC in the occurrence of surgical complications, recurrence rate, or disease-related death (132, 133).
It may be questioned whether treatment and follow-up of DTC in patients after exposure to I-131 or with a history of radiation should be different compared to patients with sporadic DTC, since no differences were found in outcome. However, CCS were found to have more frequent bilateral disease. For this reason, the expert panel agreed that subsequent DTC should minimally be treated with total thyroidectomy. In addition, several medical and psychosocial considerations may be taken into account for CCS (Table 7) (136). Each patient with a history of radiation will have unique characteristics. Caregivers should individually design the optimal treatment and follow-up plan and include the patient and their parents in the decision-making.
Suggestion 19A:
We suggest that children with radiation-induced DTC should undergo total thyroidectomy because of the increased risk for bilateral disease (3W).
Suggestion 19B:
We suggest that for CCS with DTC, specific medical and psychosocial considerations should be taken into account, requiring an individual treatment and follow-up plan (4W).
Table 7.
Issues specific for childhood cancer survivors developing subsequent differentiated thyroid cancer
| Issue | Example | Possible consequence |
|---|---|---|
| Previous radiation dose from prior diagnostics and treatment | High cumulative radiation dose | Avoidance, when possible, of CT scan or I-131 in the evaluation and treatment of DTC |
| Previous exposure to toxic agents for the childhood cancer | Bleomycin increases the risk of pulmonary dysfunction Alkylating agents and abdominal irradiation increase the risk of gonadal dysfunction Total body irradiation or 131I-MIBG treatment increases the risk of bone marrow toxicity and tertiary malignancies Chest irradiation increases the risk for breast cancer |
May increase the risk for adverse effects of I-131 in the treatment for DTC |
| Possibility of the presence of a genetic predisposition syndrome | Possible underlying genetic mutation may be present, both causing the childhood malignancy and the thyroid malignancy; the fact that an individual has already had cancer during childhood and subsequently develops thyroid cancer may indicate a germline genetic susceptibility to develop cancer | May influence the decision to use adjuvant treatment with I-131 with regard to the risk of developing a third malignancy |
| Risk of cardiotoxicity and prescribing levothyroxine therapy | Anthracycline chemotherapy agents or chest irradiation may increase the risk of cardiotoxicity | Consider keeping TSH levels in the lower normal but not in suppressed range |
| Psychological aspects | Fear of unfavorable prognosis similar to the previous cancer | The psychological impact of DTC diagnosis as a second primary malignancy may be higher than the diagnosis of sporadic DTC |
DTC, differentiated thyroid carcinoma; MIBG, meta-iodobenzylguanidine; TSH, thyroid-stimulating hormone.
Adapted, with permission, from van Santen et al. (136).
C12. Treatment for DTC in children with genetic syndromes
DTC may occur in children with a pathogenic variant in a tumor predisposition syndrome known to result in benign thyroid lesions and DTC (Table 5, section B5) (80, 137, 138). As an increased head circumference may point toward the diagnosis of PHTS or DICER1 (139, 140), this should always be part of the physical examination of the child with a thyroid nodule or DTC.
DTC cancer predisposition syndromes include pathogenic variants in the following genes: APC, DICER1, PRKAR1A, PTEN, and WRN. The appearance of DTC may be the presenting tumor in a hitherto unrecognized syndromic diagnosis in a child or may develop in a child who is at risk of and being monitored for their high-risk thyroid disease, as part of surveillance specific to their syndromic diagnosis. This question is important, because if children with an inherited DTC-prone genetic condition do have a poorer outcome, the 'watch and wait' policy for children at high risk for thyroid neoplasia may need to be reconsidered, with prophylactic thyroidectomy as a possible risk-reducing intervention, to avoid DTC that can be contemplated. Additionally, if a DTC-predisposing pathogenic variant would require more aggressive treatment, then genomic testing at presentation should be recommended for all pediatric cases with possible DTC, to provide best possible oncological management and care.
The expert panel questioned during presentation, outcome and/or disease course of DTC in children with genetic syndromes is different than in children without genetic syndromes for which treatment and/or follow-up should be adjusted (Appendix A [Q22]). A literature search was performed (Appendix B, C) (58, 141).
Van der Tuin et al. have shown a cohort of ten children with DICER1-related DTCs (141). Thyroid specimens of all patients showed diffuse nodular hyperplasia with multiple, discrete, well-circumscribed, and occasionally encapsulated nodules. No infiltrative growth, extrathyroidal extension, vascular invasion, or lymph node metastasis were seen. The authors concluded, based on clinical, histological, and molecular data, that most DICER1-related DTCs could be considered as a low-risk subgroup. Another review reported on disease behavior and outcome of DTC in children with PHTS (58). In this review, five cohort studies were reported, and the incidence of DTC in childhood ranged from 4 to 12%. In addition, in total, 27 cases were identified. FTC was diagnosed in 52% of pediatric DTC patients. No evidence was found for a different clinical behavior of DTC in PHTS patients compared to sporadic DTC. DTC in pediatric PHTS patients does not seem to be more aggressive than sporadic DTC.
However, detection of DICER-1 or PHTS in children with DTC will affect the counseling and follow-up of families regarding the surveillance of organs at risk for malignancies.
Suggestion 20:
We do not suggest the adjustment of treatment or follow-up of DTC for children with DICER1 or PHTS or any other thyroid cancer predisposition syndrome (3W).
[D] Surveillance and follow-up
D1. TSH levels during follow-up
The expert panel did not consider it necessary to perform a new literature search on the TSH level issue during follow-up. The expert panel agreed that LT4 therapy is indicated in all children with DTC after total thyroidectomy to suppress TSH at least until clinical remission of the disease (i.e. undetectable levels of serum Tg on LT4, undetectable levels of Tg antibodies, negative neck US, and, if performed, negative whole-body scan 1 year after last treatment).
While the TSH level should be suppressed, FT4 should be maintained in the normal range to prevent symptoms and signs of thyrotoxicosis. It is important to know that children, especially young children, commonly require a greater amount of LT4 per kg of body weight with respect to adults, and considering that they are growing, frequent monitoring to confirm that the daily dose is appropriate is warranted. Periodic monitoring of the body weight and height to confirm a correct normal growth is also indicated. When, after 1 year, clinical remission is reached (undetectable levels of serum Tg on LT4, undetectable levels of Tg antibodies, negative neck US, and, if performed, negative whole-body scan), and slightly higher TSH levels may be accepted (low-normal values with substitutive LT4 treatment).
Because of lack of evidence on this topic for children with low-risk DTC not treated with I-131, the expert panel suggests keeping TSH in the low-normal range.
Suggestion 21A:
We suggest that TSH levels should be kept suppressed with concomitant high-normal values of FT4 until full clinical remission, while a low-normal value of TSH (between 0.3 and 1.0 mU/L)) should be advised thereafter (4W). See Fig. 3
Figure 3.
Flowchart of follow-up of children with DTC having received complete remission after initial treatment with total thyroidectomy and I-131. This flowchart is developed for children with DTC having received complete remission defined as: undetectable levels of serum Tg on LT4, undetectable levels of Tg antibodies, negative neck ultrasound, and if performed, negative whole-body scan 1 year after last treatment. ^In the first year until clinical remission, TSH levels should be suppressed, while a normal low value of TSH (between 0.5 and 1.0 mU/L) will be advisable thereafter. ^^The definition of consistent rising Tg on LT4 is debatable; the levels of Tg as well as the doubling time should be taken into account and weighted in the individual patient. *The expert panel suggests that, in children with detectable (but not rising) Tg and no focus on neck ultrasound, in individual cases, I-123 scanning may be considered. When both ultrasound and radioiodine imaging did not yield a focus, FDG PET/CT may be considered.
Suggestion 21B:
We suggest the measurement of TSH and FT4 to monitor the level of suppression or substitution of the LT4 therapy every 3–6 months during growth and puberty and thereafter once a year (4W).
D2. Tg measurement during follow-up
Tg is a tumor marker for DTC, but interpretation of Tg needs to be in the context of previous therapy and TSH levels and can also be raised due to thyroid trauma (e.g. FNB/surgery/I-131). Historically, treatment of DTC in children has followed adult guidelines where the goal of treatment was elimination of evidence of disease (e.g. undetectable Tg levels). Given the excellent prognosis, the slow growth of DTC, and highly sensitive Tg measurement, one may question whether it is necessary to aim for undetectable Tg levels during follow-up at cost of potential adverse effects of additional I-131 after surgery. The goal of treatment is to tailor therapy in order to limit overtreatment. For patients with less-differentiated tumors, post-operative Tg may be a less-sensitive marker; however, this is very rare in childhood. Current guidelines suggest the measurement of Tg dependent on risk stratification (3–6 monthly for either 2 (low risk) or 3 years (intermediate/high risk) and then annually (8, 142).
Highly sensitive Tg (hs-Tg) assays have improved sensitivity and precision for Tg and allow the detection of very-low Tg levels, reflecting minimal amounts of thyroid tissue, even without TSH stimulation (143, 144). For this reason, Tg measurement using hs-Tg assays is preferred in follow-up of pediatric DTC.
Circulating Tg antibodies (TgAbs) can affect Tg measurement, and guidelines suggest measuring Tg and TgAbs during follow-up. The expert panel suggests that, in case of circulating TgAbs, these may be measured as ‘alternative’ tumor marker especially when newly occurring or rising. It must be taken into account, however, that TgAbs appear to be more common in pediatric DTC and in those with lymph node metastasis. In almost 50%, TgAbs resolve in 2 years and there is no associated long-term prognostic significance to date (145).
Recommendation 22A:
We recommend that serum Tg is a reliable marker in the follow-up after treatment for DTC in childhood. The expert panel suggests that serum Tg should be assessed every 6 months during the first 3 years, and annually thereafter (4S).
Suggestion 22B:
We suggest that, in case of circulating TgAbs, these may be measured as ‘alternative’ tumor marker (4W).
Suggestion 22C:
We suggest that a highly sensitive Tg assay should preferably be used in the follow-up of pediatric DTC patients (4W).
D3. Ultrasound during follow-up
Neck ultrasonography is a pivotal imaging procedure for detecting DTC locoregional relapse/metastases in adult patients (146). Although in children with DTC, the high prevalence of large inflammatory lymph nodes may reduce specificity (147), its ubiquitous availability together with absence of radiation exposure makes this procedure of particular value in pediatric DTC patients (147).
The expert panel searched the value in terms of sensitivity and specificity of neck US for recurrent DTC in the follow-up of children with DTC (Appendix A [Q19], Appendix B, C) (148). Vali et al. have reported the sensitivity and specificity of neck US for recurrent/persistent disease in the follow-up of DTC in 40 patients (median age, 14.3 years) (148). Suspicious characteristics were defined as: hypoechoic appearance, hyperechoic foci, peripheral vascularization, and round-shape node without hyperechoic hilum. Histopathology was considered as the gold standard to assess the results of neck US, in cases where histopathology was not available, a combination of stimulated Tg levels >10 ng/ml and post-therapy whole-body radioiodine scan was used as the gold standard. The sensitivity and specificity were 85.7 and 89.4%, respectively.
In the case of detectable Tg levels after initial treatment (i.e. total thyroidectomy followed by I-131 therapy), neck US can disclose even small locoregional disease persistence/relapse amenable for further surgical treatment. Furthermore, this procedure can guide FNB of lymph nodes to obtain cytological or biochemical (i.e. Tg measurement in needle wash-out) confirmation before surgery (147). Conventionally, routine neck US at regular intervals has been the standard of care for many years. However, recently in adult patients, it was shown by several groups that its use during the follow-up could be reserved for patients with biochemically incomplete response after initial treatment (i.e. Tg levels ≥1 ng/mL) (149), as in patients with lower Tg levels, the number of false-positive lesions, and consequently unnecessary diagnostics, far outweighs the number of true-positive ones. Considering the low burden of neck US and the possibility of this modality to detect lymph node metastases that are unable to secrete Tg, the expert panel agreed that a neck US once a year in the first 5 years after diagnosis of DTC may be helpful. However, this decision should be made by the clinician who is taking care of the child, depending on the stage of disease at presentation and the individual preferences of the patient and their parents. Considering the abovementioned possible risk for false-positive findings on neck US, especially in children, an option could be to perform neck US during the first year of follow-up but thereafter, only in cases with rising Tg or TgAbs or suspicion of recurrence of disease. In all cases, neck US should always be performed by a professional with expertise in neck US in childhood.
Recommendation 23A:
We recommend follow-up with neck US in combination with serum Tg measurement for the detection of recurrent DTC (2S).
Recommendation 23B:
We recommend that neck US is performed by a professional with experience in neck US in childhood (4S).
Suggestion 23C:
We suggest that annual neck US be performed in the first 5 years of follow-up. In low-risk patients, the expert panel suggests, after the first year of follow-up, to only perform neck US in cases with rising Tg or TgAbs or suspicion of recurrence of disease to avoid false-positive findings (4W).
D4. Other imaging modalities (I-131, I-124, I-123, or FDG PET/CT scans) during follow-up
Although in adults, diagnostic radioiodine scintigraphy with I-123, I-124, or I-131 as well as PET/CT with FDG is commonly used in patients with suspected persistent or recurrent disease, there is little evidence and no prospective studies as to their precise efficacy and indication (40). In the pediatric DTC population, a specific search to this extent search yielded no evidence of the sensitivity of these techniques in this population (Appendix A [Q20], B, C). Therefore, the expert panel concluded that prospective studies are needed to determine the sensitivity of radioiodine imaging and FDG PET/CT for the detection of persistent or recurrent disease in children who have been treated for DTC.
Until sufficient prospective evidence for evidence-based recommendations is available, the expert panel agreed that only experience-based recommendations can be formulated. Certainly, in pediatric patients, neck US is preferable to radioiodine imaging and FDG PET/CT. If neck US however did not detect thyroid tissue, the next step may be radioiodine imaging (under TSH stimulation, Recommendation 16B), as radioiodine avid disease in children is common, whereas radioiodine negative disease is rare (104). When both US and radioiodine imaging are negative, FDG PET/CT may be considered.
Suggestion 24A:
We suggest that children with undetectable Tg on LT4 during follow-up after treatment for DTC should not undergo other imaging modalities (I-131, I-124, I-123, or FDG PET/CT scans) (4W).
Suggestion 24B:
We suggest that, in children with detectable (but not rising) Tg on LT4 and no focus on neck US, in individual cases, I-123 scanning may be considered. If no source of Tg is found, serum Tg and serum TgAbs must be followed every 3–6 months. In case of further rising Tg or TgAbs, further imaging is indicated (4W).
D5. Persistent/recurrent cervical disease
The expert panel searched for differences in outcomes between children with measurable but not rising Tg on LT4 after initial treatment for DTC (incomplete biochemical response) with I-131 compared to a wait-and-see approach (incomplete biochemical response) (Appendix A [Q16]) but no literature was found (Appendix B). In addition, the expert panel performed a literature search on the difference in outcome between patients with recurrent disease/progressive thyroid cancer treated with additional I-131/surgery/other vs a wait-and-see approach (Appendix A [Q17], B). However, again, no studies were found.
Based on these searches and expert opinion, the expert panel agreed to recommend that in children with persistent but not rising Tg on LT4, primarily neck US is recommended, and if negative, I-123 scanning may be considered (under TSH stimulation, Recommendation 16B). If no residual or recurrent disease is found, serum Tg on LT4 and serum TgAbs must be followed every 3–6 months (wait-and-see). In case of consistent rising Tg on LT4 or TgAbs, neck US is recommended, and a I-123 scan and/or FDG PET/CT may be considered to locate the origin of persistent/recurrent disease. The expert panel agreed to not define an exact value for high or rising Tg, as this is dependent on the assay and the course over time. The definition of consistent rising Tg on LT4 is debatable; the levels of Tg as well as the doubling time should be taken into account and weighted in the individual patient. Considering the excellent outcome of childhood DTC and the risk for adverse late effects of I-131, empiric treatment may only be considered after the abovementioned diagnostic modalities have failed to identify a source of rising Tg on LT4 or rising TgAbs.
When a source for the consistent rising Tg on LT4 or TgAbs is found, surgical or I-131 therapy is indicated, dependent on the risk–benefit ratio of both treatment options, bearing in mind the patient’s medical history and previous I-131 exposure. Also, in case of small lymph nodes metastases and a history of repeated I-131 treatments, a wait-and-see strategy can also be advocated. No evidence is available to support that earlier treatment of small lymph node metastases will result in better outcome.
The expert panel suggests that prospective studies are needed to evaluate the outcome of a wait-and-see approach of children with measurable but not rising Tg during follow-up. Also, studies are needed to assess the best approach for children with recurrent disease or progressive thyroid cancer with regard to treatment possibilities (additional I-131 vs surgery vs wait-and-see approach).
Suggestion 25A:
We suggest to perform neck US in children with consistently rising Tg on LT4 or TgAbs. In these cases, additional I-123 and/or FDG PET scanning may be considered. Surgery or I-131 therapy is indicated depending on the size, tumor load, and degree of progression (4W).
Suggestion 25B:
We suggest that empiric I-131 iodine treatment be only recommended if the abovementioned diagnostic modalities have failed to identify a source of rising Tg on LT4 or rising TgAbs (4W).
D6. Pulmonary metastases
Distant metastases in pediatric DTC patients are mainly found in the lung. Overall, these can be detected in up to 20% of DTC patients and are observed particularly in those with extensive regional lymph node metastases (150). In the vast majority of cases, these lesions are micrometastases, and being well-differentiated and iodine avid, respond to I-131 therapy (i.e. 85% of patients) (151).
However, most patients are not cured of their disease, but even in these patients, after treatment, metastatic stability is observed over time and the disease-specific mortality rate is low (<2%) (151, 152, 153).
In patients with previous positive post-therapeutic I-131 whole-body scan (WBS), an I-123 diagnostic (DxWBS) may be of value in the case of detectable Tg levels suggestive of persistent or recurrent disease to identify iodine avid disease amenable to further I-131 therapy. However, pulmonary metastases may not be visualized on I-123 DxWBS (154).
In this setting, considering that structural and biochemical response to first I-131 may be observed up to 15–18 months after therapy (103), long intervals of at least 12 months are suggested before retreatment, even in the rare case of disease progression. Although complete remission may be achieved after therapy, repeated I-131 administrations should always be evaluated with caution and proposed after adequate interval.
In any case, a pulmonary function test is recommended before retreating patients with diffuse lung metastases. In children with a previous history of drugs causing pulmonary toxicity such as bleomycin, I-131 treatment must be given with extra caution given the risk for lung fibrosis (155).
Recommendations 26A:
We recommend that I-131 is the first-line therapy for patients with pulmonary metastases (4S).
Suggestion 26B:
We suggest that a pulmonary function test should be performed, before repeated I-131 treatment of patients with diffuse lung metastases (4W).
Recommendations 26C:
We recommend that in children with a previous history of drugs causing pulmonary toxicity such as bleomycin, I-131 treatment must be given with extra caution given the risk for pulmonary fibrosis (4S).
D7. Radioiodine refractory disease
In pediatric DTC patients, metastatic disease is well differentiated and often characterized by intense iodine uptake on post-therapeutic I-131 WBS. Responses to I-131 in this setting are good and patients often achieve complete remission after repeated I-131 therapeutic courses (103, 105). In the pediatric population, I-131 refractory disease is rare (109). In the setting of radioiodine refractory thyroid cancer not amenable to surgical resection, systemic therapy with TKIs may be considered. However, although TKIs have been largely and successfully used in adult patients, molecularly targeted therapy has not been applied in a large cohort of DTC pediatric patients and only few case report or series are available in literature (119, 120). Although encouraging results have been reported, a long duration of treatment with TKI could significantly influence the quality of life and should be reserved only for specific patients as I-131 refractory pediatric DTC patients usually do well on TSH-suppressive levothyroxine therapy alone (156). In this clinical setting, the definition of I-131 refractory disease is of primary importance considering that very few pediatric patients will not respond to I-131 and even in this setting, may remain stable or without symptoms over the years (122).
Suggestion 27A:
We suggest that, when radioiodine refractory disease is suspected, its presence should be thoroughly investigated and confirmed before considering systemic targeted therapy. An observation or wait-and-see strategy may be appropriate (4W).
Suggestion 27B:
We suggest that targeted therapy should be reserved only for patients with large-volume disease which is significantly progressing on TSH-suppressive therapy and not amenable to surgical approach and should preferably be given in a research setting (4W).
D8. Late effects of treatment
Due to the excellent prognosis of pediatric DTC, it is of great importance to be aware of and to minimize the adverse effects of treatment. Of the adverse effects of treatment, hypoparathyroidism and salivary gland dysfunction are most prevalent. The prevalence of hypoparathyroidism has been shown to be related to the experience of the endocrine surgeon (99).
TSH-suppressive therapy in adult DTC survivors has been shown to be related to the risk of cardiovascular and all-cause mortality (157). Also, in children, diastolic dysfunction has been shown to be present after treatment for pediatric DTC (158). Another possible late effect of TSH suppression in combination with hypoparathyroidism is loss of bone mineral density (16, 158, 159). I-131 treatment has been associated with salivary and lacrimal gland dysfunction and secondary primary malignancies (SPM) and may possibly affect male fertility (160, 161).
Several studies have shown an increased risk to develop SPM after treatment for DTC and it has been shown to be related to the cumulative I-131 activity (162, 163, 164, 165). However, a cohort described by Verkooijen et al. showed an overall increased standardized incidence rate for second primary tumors but not for second primary tumors following I-131 therapy (162). The findings of this latter study suggest a genetic mechanism instead of a causal relation. Several different reports on the risk of SPM in relation to RAI have been made on this subject; however, most studies have limitations and more long follow-up studies are necessary. In one study, the risk to develop SPM in young patients (<25 years) receiving I-131 was found to be comparable to that in adults (163). However, in another study, the overall relative risk (RR) for developing SPM in adults with DTC, dependent on age at DTC treatment and latency time, varies between 0.98 (0.58–1.65) and 1.37 (1.13–1.66) for neurologic and hematologic SPMs, respectively (164). Also, Marti et al. reported an increased risk of SPM in young adult patients (standard incidence ratio (SIR) 1.42) after treatment with I-131 for DTC (163). In this study, mainly salivary carcinoma was reported (SIR, 34.12; P = 0.0007). Mei et al. reported that 4.4% of adult patients treated for DTC developed a SPM after 2 years (169). A recent review on the risk of SPMs, especially secondary hematologic malignancies (SHMs), attributable to RAI therapy was published, concluding (based on low-quality evidence) that an excess risk for the development of SPM cannot be excluded but is expected to be small (166). An even more recent study by Pasqual et al. however pooled nine US Surveillance, Epidemiology, and End Results (SEER) cancer registries including 27,050 ≥ 5-year survivors and found that 6% of solid and 14% of hematologic malignancies in pediatric and young adult DTC survivors may be attributable to RAI (165).
Damage to testicular cells induced by the beta and gamma radiation could cause transient subfertility in male adults which could cause decreased semen quality, elevated levels of luteinizing hormone (LH), and follicle-stimulating hormone and decreased levels of testosterone (167, 168, 169). On the other hand, long-term data have shown that men treated with I-131 had normal semen quality and were able to conceive healthy children (161, 168, 170). However, data on male fertility in boys treated with I-131 is scarce; therefore, the expert panel suggests that post-pubertal males who receive I-131 may be counseled about the possibility of (transient) decreased fertility and semen preservation could be offered.
Next to physical late events, psychosocial effects of having had thyroid cancer in childhood may be present during adulthood.
The expert panel questioned which late effects should be screened for after treatment for pediatric DTC. Consensus was achieved to counsel and screen for hypoparathyroidism and recurrent nerve injury and to counsel on the risk of male fertility and SPM after I-131. A literature search was performed on the frequency and risk for other adverse effects of treatment for DTC (Appendix A [Q21], B, C) (16, 158, 171, 172, 173, 174, 175, 176).
Studies were identified that the investigated presence and risk factors of cardiac dysfunction, salivary gland dysfunction, influence on long-term quality of life, bone mineral density, and female fertility in survivors of DTC (16, 158, 171, 172, 173, 174, 175, 176).
Diastolic dysfunction was found in 21.2% of asymptomatic pediatric DTC survivors (158). In these survivors, the systolic function was unaffected. Also, neither atrial fibrillation nor an association with biomarkers such as NT-proBNP, hs-troponin-T, or galectin was described. The clinical significance of these findings will have to be studied in future cohorts.
Two studies described the prevalence of salivary gland dysfunction after radioiodine treatment in survivors of DTC.Salivary dysfunction and xerostomia were found in 1.9–47.6% and 35.5% of the DTC survivors, respectively (16, 171). Selvakumar et al. found a relationship between salivary gland dysfunction with increasing I-131 activity; a lower salivary secretion and more xerostomia complaints were found in patients treated with a higher cumulative I-131 activity (171).
Quality of life and course of life studies have been reassuring, and scores of survivors did not seem to differ significantly from controls (173). However, more physical problems, more role limitations due to physical problems, and more mental fatigue were described by DTC survivors (173, 176).
Few studies have investigated bone mineral density in survivors of DTC. No differences were found with respect to BMD and Z scores at any site evaluated by DXA and in bone microstructure parameters between survivors of DTC and controls (174, 175). However, calcium–vitamin D3 medication had a beneficial effect on BMD. TSH-suppressive therapy does not seem to affect BMD in women treated for DTC at young age, at least after 10 years of follow-up.
Only one study was found that reported on female fertility in survivors of pediatric DTC (172). In this study, neither major abnormalities in reproductive characteristics nor in predictors of ovarian failure in female survivors of DTC, who received I-131 treatment during childhood, were found.
In conclusion, hypoparathyroidism, cardiac dysfunction, and salivary gland dysfunction occur in 23.8, 21.2, and 1.9-47.6% respectively. No significant effect of treatment for pediatric DTC was found on overall quality of life, bone mineral density, or female fertility.
Suggestion 28A:
We suggest counseling pediatric DTC patients about the risk of developing recurrent laryngeal nerve injury or hypoparathyroidism after thyroid surgery and salivary gland dysfunction after exposure to I-131. In addition, the potential risk of subsequent primary neoplasms after I-131 treatment related to I-131 activity and possible risk for cardiac dysfunction after prolonged TSH suppression should be mentioned (3W).
Recommendation 28B:
We recommend that the recurrent laryngeal nerve and parathyroid gland function is monitored post-operatively (3S).
Suggestion 28C:
We suggest that all post-pubertal males who receive I-131 may be counseled on the possibility of (transient) decreased fertility and semen preservation could be offered (3W).
Suggestion 28D:
We suggest that all pediatric DTC patients receive additional calcium and vitamin D supplementation therapy for optimal bone mineralization during follow-up (4W).
Suggestion 28E:
We suggest that all patients with pediatric DTC are offered psychosocial support (4W).
Suggestion 28F:
We suggest that future studies should further evaluate the prevalence and clinical significance of diastolic dysfunction in survivors of pediatric DTC after prolonged TSH suppressive therapy (4W).
D9. Follow-up scheme and transition to adult care
Most recurrences of DTC occur within the first 5 years after diagnosis (177), and also late recurrences occurring >20 years after initial diagnosis have been described, especially in older studies (177). These patients were, however, treated before hsTg assays were available, and these late recurrences may therefore become unusual in the future (109). In addition, also the psychological impact of prolonged follow-up of DTC and continuous fear of recurrence while in fact, this risk decreases with prolonged follow-up time, must be taken into account, especially in children, despite the fact that this risk decreases with prolonged follow-up time. With this in mind, the expert panel suggests to continue follow-up for least 10 years; thereafter, the follow-up strategy should be the result of shared decision-making between the physician and the patient.
The medical specialties involved in the treatment and follow-up of pediatric DTC vary between European countries (11). The expert panel agreed that the follow-up of children with DTC should be performed by a pediatric thyroid carcinoma expert, within a thyroid expert team, whereby the type of expert is based on the organization of care in each country. Furthermore, as most children are diagnosed between ages of 15 and 18 years, a close cooperation between pediatric and adult clinical teams is needed to enable good transitional clinical care.
Suggestion 29:
We suggest to continue follow-up of children with DTC for at least 10 years; thereafter, the follow-up strategy should be the result of shared decision-making between the physician and the patient (4W).
Supplementary Material
Declaration of interest
The authors have no conflicts of interest to disclose. Frederik A Verburg received speaker honoraria from Sanofi, AstraZeneca, Bayer. Member of advisory board at General Electric Healthcare. All honoraria paid to employer.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Bernier MO, Withrow DR, Berrington de Gonzalez A, Lam CJK, Linet MS, Kitahara CM, Shiels MS. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 20191252497–2505. ( 10.1002/cncr.32125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebbink CA, van den Broek MFM, Kwast ABG, Derikx JPM, Dierselhuis MP, Kruijff S, Links TP, van Trotsenburg ASP, Valk GD, Vriens MRet al. Opposite incidence trends for differentiated and medullary thyroid cancer in young Dutch patients over a 30‐year time span. Cancers 2021135104. ( 10.3390/cancers13205104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Current Opinion in Oncology 20082059–65. ( 10.1097/CCO.0b013e3282f30220) [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Kotch C, Fox E, Surrey LF, Wertheim GB, Baloch ZW, Lin F, Pillai V, Luo M, Kreiger PAet al. NTRK fusions identified in pediatric tumors: the frequency, fusion partners, and clinical outcome. JCO Precision Oncology 20211204–214. ( 10.1200/PO.20.00250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, Astl J, Vlcek P, Kodetova D, Vcelak Jet al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid 2020301771–1780. ( 10.1089/thy.2019.0802) [DOI] [PubMed] [Google Scholar]
- 6.Danese D, Gardini A, Farsetti A, Sciacchitano S, Andreoli M, Pontecorvi A. Thyroid carcinoma in children and adolescents. European Journal of Pediatrics 1997156190–194. ( 10.1007/s004310050580) [DOI] [PubMed] [Google Scholar]
- 7.Guille JT, Opoku‐Boateng A, Thibeault SL, Chen H. Evaluation and management of the pediatric thyroid nodule. Oncologist 20152019–27. ( 10.1634/theoncologist.2014-0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster Met al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 201525716–759. ( 10.1089/thy.2014.0460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. & GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008336924–926. ( 10.1136/bmj.39489.470347.AD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O’Connell D, Oxman AD, Phillips Bet al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Services Research 2004438. ( 10.1186/1472-6963-4-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekker BL, Newbold KL, Führer D, Waguespack SG, Handkiewicz-Junak D, Links TP. & European Initiative on Collaboration on Paediatric Thyroid Cancer. Survey on paediatric differentiated thyroid cancer care in Europe. Hormone Research in Paediatrics 20188958–62. ( 10.1159/000484170) [DOI] [PubMed] [Google Scholar]
- 12.Efremidou EI, Papageorgiou MS, Liratzopoulos N, Manolas KJ. The efficacy and safety of total thyroidectomy in the management of benign thyroid disease: a review of 932 cases. Canadian Journal of Surgery 20095239–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Gough IR.Total thyroidectomy: indications, technique and training. Australian and New Zealand Journal of Surgery 19926287–89. ( 10.1111/j.1445-2197.1992.tb00001.x) [DOI] [PubMed] [Google Scholar]
- 14.Baumgarten HD, Bauer AJ, Isaza A, Mostoufi-Moab S, Kazahaya K, Adzick NS. Surgical management of pediatric thyroid disease: complication rates after thyroidectomy at the Children’s Hospital of Philadelphia high-volume Pediatric Thyroid Center. Journal of Pediatric Surgery 2019541969–1975. ( 10.1016/j.jpedsurg.2019.02.009) [DOI] [PubMed] [Google Scholar]
- 15.Schmidt Jensen J, Grønhøj C, Mirian C, Jensen DH, Friborg J, Hahn CH, Agander TK, Hjuler T. Incidence and survival of thyroid cancer in children, adolescents, and young adults in Denmark: a nationwide study from 1980 to 2014. Thyroid 2018281128–1133. ( 10.1089/thy.2018.0067) [DOI] [PubMed] [Google Scholar]
- 16.Albano D, Bertagna F, Panarotto MB, Giubbini R. Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatric Blood and Cancer 201764e26595. ( 10.1002/pbc.26595) [DOI] [PubMed] [Google Scholar]
- 17.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, Dinauer CA, Udelsman R. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocrine Reviews 201132798–826. ( 10.1210/er.2011-0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JS, Aiello Bowles EJA, Williams SB, Morrison CC. Screening for thyroid cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 20173171888–1903. ( 10.1001/jama.2017.0562) [DOI] [PubMed] [Google Scholar]
- 19.Rallison ML, Dobyns BM, Keating FR, Rall JE, Tyler FH. Thyroid nodularity in children. JAMA 19752331069–1072. [PubMed] [Google Scholar]
- 20.Suzuki S, Suzuki S, Fukushima T, Midorikawa S, Shimura H, Matsuzuka T, Ishikawa T, Takahashi H, Ohtsuru A, Sakai Aet al. Comprehensive Survey Results of Childhood thyroid ultrasound Examinations in Fukushima in the first four years after the Fukushima Daiichi Nuclear Power Plant Accident. Thyroid 201626843–851. ( 10.1089/thy.2015.0564) [DOI] [PubMed] [Google Scholar]
- 21.Cléro E, Ostroumova E, Demoury C, Grosche B, Kesminiene A, Liutsko L, Motreff Y, Oughton D, Pirard P, Rogel Aet al. Lessons learned from Chernobyl and Fukushima on thyroid cancer screening and recommendations in case of a future nuclear accident. Environment International 2021146106230. ( 10.1016/j.envint.2020.106230) [DOI] [PubMed] [Google Scholar]
- 22.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. European Journal of Clinical Investigation 200939699–706. ( 10.1111/j.1365-2362.2009.02162.x) [DOI] [PubMed] [Google Scholar]
- 23.Baez JC, Zurakowski D, Vargas SO, Lee EY. Incidental thyroid nodules detected on thoracic contrast-enhanced ct in the pediatric population: prevalence and outcomes. American Journal of Roentgenology 2015205W360–W365. ( 10.2214/AJR.14.13895) [DOI] [PubMed] [Google Scholar]
- 24.Calle-Toro JS, Kelly A, Ford EJ, Zemel BS, Schall JI, Adgent MA, Umbach DM, Rogan WJ, Stallings VA, Darge Ket al. Incidental findings during ultrasound of thyroid, breast, testis, uterus and ovary in healthy term neonates. Journal of Ultrasound 201922395–400. ( 10.1007/s40477-019-00365-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer AJ.Thyroid nodules in children and adolescents. Current Opinion in Endocrinology, Diabetes, and Obesity 201926266–274. ( 10.1097/MED.0000000000000495) [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, Wassner AJ, Smith JR, Marqusee E, Alexander EKet al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. Journal of Clinical Endocrinology and Metabolism 2013983238–3245. ( 10.1210/jc.2013-1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niedziela M.Pathogenesis, diagnosis and management of thyroid nodules in children. Endocrine-Related Cancer 200613427–453. ( 10.1677/erc.1.00882) [DOI] [PubMed] [Google Scholar]
- 28.Richman DM, Benson CB, Doubilet PM, Peters HE, Huang SA, Asch E, Wassner AJ, Smith JR, Cherella CE, Frates MC. Thyroid nodules in pediatric patients: sonographic characteristics and likelihood of cancer. Radiology 2018288591–599. ( 10.1148/radiol.2018171170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyshchik A, Drozd V, Demidchik Y, Reiners C. Diagnosis of thyroid cancer in children: value of gray-scale and power Doppler US. Radiology 2005235604–613. ( 10.1148/radiol.2352031942) [DOI] [PubMed] [Google Scholar]
- 30.Gannon AW, Langer JE, Bellah R, Ratcliffe S, Pizza J, Mostoufi-Moab S, Cappola AR, Bauer AJ. Diagnostic accuracy of ultrasound with color flow Doppler in children with thyroid nodules. Journal of Clinical Endocrinology and Metabolism 20181031958–1965. ( 10.1210/jc.2017-02464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richman DM, Benson CB, Doubilet PM, Wassner AJ, Asch E, Cherella CE, Smith JR, Frates MC. Assessment of American College of Radiology thyroid imaging reporting and data system (TI-RADS) for pediatric thyroid nodules. Radiology 2020294415–420. ( 10.1148/radiol.2019191326) [DOI] [PubMed] [Google Scholar]
- 32.Creo A, Alahdab F, al Nofal A, Thomas K, Kolbe A, Pittock S. Diagnostic accuracy of the McGill thyroid nodule score in paediatric patients. Clinical Endocrinology 201990200–207. ( 10.1111/cen.13878) [DOI] [PubMed] [Google Scholar]
- 33.Koltin D, O’Gorman CS, Murphy A, Ngan B, Daneman A, Navarro OM, Garcia C, Atenafu EG, Wasserman JD, Hamilton Jet al. Pediatric thyroid nodules: ultrasonographic characteristics and inter-observer variability in prediction of malignancy. Journal of Pediatric Endocrinology and Metabolism 201629789–794. ( 10.1515/jpem-2015-0242) [DOI] [PubMed] [Google Scholar]
- 34.Tessler FN, Middleton WD, Grant EG. Thyroid imaging reporting and data system (TI-RADS): a user’s guide. Radiology 201828729–36. ( 10.1148/radiol.2017171240) [DOI] [PubMed] [Google Scholar]
- 35.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. European Thyroid Journal 20176225–237. ( 10.1159/000478927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, Lim HK, Moon WJ, Na DG, Park JSet al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean Journal of Radiology 201617370–395. ( 10.3348/kjr.2016.17.3.370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Rios C, Daneman A, Bajno L, van der Kaay DCM, Moineddin R, Wasserman JD. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatric Radiology 20184874–84. ( 10.1007/s00247-017-3974-y) [DOI] [PubMed] [Google Scholar]
- 38.Kim PH, Yoon HM, Hwang J, Lee JS, Jung AY, Cho YA, Baek JH. Diagnostic performance of adult-based ATA and ACR-TIRADS ultrasound risk stratification systems in pediatric thyroid nodules: a systematic review and meta-analysis. European Radiology 2021317450–7463. ( 10.1007/s00330-021-07908-8) [DOI] [PubMed] [Google Scholar]
- 39.Franco AT, Ricarte-Filho JC, Isaza A, Jones Z, Jain N, Mostoufi-Moab S, Surrey L, Laetsch TW, Li MM, Clague DeHart JCet al. Fusion oncogenes are associated With increased metastatic capacity and persistent disease in pediatric thyroid cancers. Journal of Clinical Oncology 2022401081–1090. ( 10.1200/JCO.21.01861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger Met al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, Russ G. 2013 European Thyroid Association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. European Thyroid Journal 20132147–159. ( 10.1159/000354537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navallas M, Daneman A, Amirabadi A, Ngan BY, Wasserman J. Utility of sonography for identifying metastatic cervical adenopathy in children with differentiated thyroid carcinoma at presentation. Pediatric Radiology 202151273–281. ( 10.1007/s00247-020-04804-z) [DOI] [PubMed] [Google Scholar]
- 43.Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes 201910. ( 10.3390/genes10090723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd RV, Osamura RY, Klöppel G. WHO Classification of Tumours of Endocrine Organs, 4th ed., p. 355. Lyon, France: IARC, 2017. [Google Scholar]
- 45.Balachandar S, la Quaglia M, Tuttle RM, Heller G, Ghossein RA, Sklar CA. Pediatric differentiated thyroid carcinoma of follicular cell origin: prognostic significance of histologic subtypes. Thyroid 201626219–226. ( 10.1089/thy.2015.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid 2009191225–1231. ( 10.1089/thy.2009.0073) [DOI] [PubMed] [Google Scholar]
- 47.Cameselle-Teijeiro JM, Peteiro-González D, Caneiro-Gómez J, Sánchez-Ares M, Abdulkader I, Eloy C, Melo M, Amendoeira I, Soares P, Sobrinho-Simões M. Cribriform-morular variant of thyroid carcinoma: a neoplasm with distinctive phenotype associated with the activation of the WNT/β-catenin pathway. Modern Pathology 2018311168–1179. ( 10.1038/s41379-018-0070-2) [DOI] [PubMed] [Google Scholar]
- 48.Ozolek JA.Selected topics in the pathology of the thyroid and parathyroid glands in children and adolescents. Head and Neck Pathology 20211585–106. ( 10.1007/s12105-020-01274-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enomoto K, Enomoto Y, Uchino S, Yamashita H, Noguchi S. Follicular thyroid cancer in children and adolescents: clinicopathologic features, long-term survival, and risk factors for recurrence. Endocrine Journal 201360629–635. ( 10.1507/endocrj.ej12-0372) [DOI] [PubMed] [Google Scholar]
- 50.Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology 20187240–52. ( 10.1111/his.13348) [DOI] [PubMed] [Google Scholar]
- 51.Jain NK, Mostoufi-Moab S, Hawkes CP, Nelson ND, Surrey LF, Jones ZS, Adzick NS, Kazahaya K, Bauer AJ. Extrathyroidal extension is an important predictor of regional lymph node metastasis in pediatric differentiated thyroid cancer. Thyroid 2020301037–1043. ( 10.1089/thy.2019.0229) [DOI] [PubMed] [Google Scholar]
- 52.Baumgarten H, Jenks CM, Isaza A, Bhatti T, Mostoufi-Moab S, Kazahaya K, Adzick NS, Bauer AJ. Bilateral papillary thyroid cancer in children: risk factors and frequency of postoperative diagnosis. Journal of Pediatric Surgery 2020551117–1122. ( 10.1016/j.jpedsurg.2020.02.040) [DOI] [PubMed] [Google Scholar]
- 53.Aliyev E, Ladra-González MJ, Sánchez-Ares M, Abdulkader-Nallib I, Piso-Neira M, Rodríguez-Carnero G, Vieiro-Balo P, Pérez-Becerra R, Gude-Sampedro F, Barreiro-Morandeira Fet al. Thyroid papillary microtumor: validation of the (updated) porto proposal assessing sex hormone receptor expression and mutational BRAF gene status. American Journal of Surgical Pathology 2020441161–1172. ( 10.1097/PAS.0000000000001522) [DOI] [PubMed] [Google Scholar]
- 54.Togawa K, Ahn HS, Auvinen A, Bauer AJ, Brito JP, Davies L, Kesminiene A, Laurier D, Ostroumova E, Pacini Fet al. Long-term strategies for thyroid health monitoring after nuclear accidents: recommendations from an Expert Group convened by IARC. Lancet Oncology 2018191280–1283. ( 10.1016/S1470-2045(1830680-6) [DOI] [PubMed] [Google Scholar]
- 55.Lamartina L, Grani G, Durante C, Filetti S, Cooper DS. Screening for differentiated thyroid cancer in selected populations. Lancet. Diabetes and Endocrinology 2020881–88. ( 10.1016/S2213-8587(1930324-9) [DOI] [PubMed] [Google Scholar]
- 56.Clement SC, Kremer LCM, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, Alexander EK, Bardi E, Brignardello E, Constine LSet al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of Childhood, adolescent and young adult cancer: recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treatment Reviews 20186328–39. ( 10.1016/j.ctrv.2017.11.005) [DOI] [PubMed] [Google Scholar]
- 57.Schultz KAP, Stewart DR, Kamihara J, Bauer AJ, Merideth MA, Stratton P, Huryn LA, Harris AK, Doros L, Field Aet al. DICER1 tumor predisposition. In GeneReviews. Seattle, WA, USA: University of Washington, 2020. (available at: https://pubmed.ncbi.nlm.nih.gov/24761742/) [Google Scholar]
- 58.Jonker LA, Lebbink CA, Jongmans MCJ, Nievelstein RAJ, Merks JHM, Nieveen Van Dijkum EJM, Links TP, Hoogerbrugge N, van Trotsenburg ASP, van Santen HM. Recommendations on surveillance for differentiated thyroid carcinoma in children with PTEN hamartoma tumor syndrome. European Thyroid Journal 20209234–242. ( 10.1159/000508872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kratz CP, Jongmans MC, Cavé H, Wimmer K, Behjati S, Guerrini-Rousseau L, Milde T, Pajtler KW, Golmard L, Gauthier-Villars Met al. Predisposition to cancer in children and adolescents. Lancet. Child and Adolescent Health 20215142–154. ( 10.1016/S2352-4642(2030275-3) [DOI] [PubMed] [Google Scholar]
- 60.Ledbetter DJ.Thyroid cancer in children. In Endocrine Surgery in Children, 1st ed., pp. 39–50. Berlin, Germany: Springer Nature, 2017. ( 10.1007/978-3-662-54256-9) [DOI] [Google Scholar]
- 61.Verbeek HHG, de Groot JWB, Sluiter WJ, Muller Kobold AC, van den Heuvel ER, Plukker JTM, Links TP. Calcitonin testing for detection of medullary thyroid cancer in people with thyroid nodules. Cochrane Database of Systematic Reviews 20203CD010159. ( 10.1002/14651858.CD010159.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fugazzola L, di Stefano M, Censi S, Repaci A, Colombo C, Grimaldi F, Magri F, Pagotto U, Iacobone M, Persani Let al. Basal and stimulated calcitonin for the diagnosis of medullary thyroid cancer: updated thresholds and safety assessment. Journal of Endocrinological Investigation 202144587–597. ( 10.1007/s40618-020-01356-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elisei R, Alevizaki M, Conte-Devolx B, Frank-Raue K, Leite V, Williams GR. 2012 European Thyroid Association guidelines for genetic testing and its clinical consequences in medullary thyroid cancer. European Thyroid Journal 20131216–231. ( 10.1159/000346174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, MacHens A, Moley JF, Pacini Fet al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 201525567–610. ( 10.1089/thy.2014.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer AJ.Molecular Genetics of thyroid cancer in children and adolescents. Endocrinology and Metabolism Clinics of North America 201746389–403. ( 10.1016/j.ecl.2017.01.014) [DOI] [PubMed] [Google Scholar]
- 66.Mollen KP, Shaffer AD, Yip L, Monaco SE, Huyett P, Viswanathan P, Witchel SF, Duvvuri U, Simons JP. Unique molecular signatures are associated with aggressive histology in pediatric differentiated thyroid cancer. Thyroid 202232236–244. ( 10.1089/thy.2021.0317) [DOI] [PubMed] [Google Scholar]
- 67.Macerola E, Proietti A, Poma AM, Ugolini C, Torregrossa L, Vignali P, Basolo A, Materazzi G, Elisei R, Santini Fet al. Molecular alterations in relation to histopathological characteristics in a large series of pediatric papillary thyroid carcinoma from a single institution. Cancers (Basel) 202113. ( 10.3390/cancers13133123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stenman A, Backman S, Johansson K, Paulsson JO, Stålberg P, Zedenius J, Christofer Juhlin CC. Pan-genomic characterization of high-risk pediatric papillary thyroid carcinoma. Endocrine-Related Cancer 202128337–351. ( 10.1530/ERC-20-0464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan L, Tan YS, Tan S. Diagnostic accuracy and ability to reduce unnecessary FNAC: a comparison between four Thyroid Imaging Reporting Data System (TI-RADS) versions. Clinical Imaging 202065133–137. ( 10.1016/j.clinimag.2020.04.029) [DOI] [PubMed] [Google Scholar]
- 70.Polat YD, Öztürk VS, Ersoz N, Anik A, Karaman CZ. Is thyroid imaging reporting and data system useful as an adult ultrasonographic malignancy risk stratification method in pediatric thyroid nodules? Journal of Medical Ultrasound 201927141–145. ( 10.4103/JMU.JMU_35_19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zobel MJ, Padilla BE. Surgical management of benign thyroid disease in children. Seminars in Pediatric Surgery 202029150922. ( 10.1016/j.sempedsurg.2020.150922) [DOI] [PubMed] [Google Scholar]
- 72.Kovatch KJ, Bauer AJ, Isaacoff EJ, Prickett KK, Adzick NS, Kazahaya K, Sullivan LM, Mostoufi-Moab S. Pediatric thyroid carcinoma in patients with Graves’ disease: the role of ultrasound in selecting patients for definitive therapy. In Hormone Research in Paediatrics 2015408–413. ( 10.1159/000381185) [DOI] [PubMed] [Google Scholar]
- 73.Monaco SE, Pantanowitz L, Khalbuss WE, Benkovich VA, Ozolek J, Nikiforova MN, Simons JP, Nikiforov YE. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathology 2012120342–350. ( 10.1002/cncy.21199) [DOI] [PubMed] [Google Scholar]
- 74.Smith M, Pantanowitz L, Khalbuss WE, Benkovich VA, Monaco SE. Indeterminate pediatric thyroid fine needle aspirations: a study of 68 cases. Acta Cytologica 201357341–348. ( 10.1159/000351029) [DOI] [PubMed] [Google Scholar]
- 75.Grob F, Deladoëy J, Legault L, Spigelblatt L, Fournier A, Vassart G, van Vliet G. Autonomous adenomas caused by somatic mutations of the thyroid-stimulating hormone receptor in children. Hormone Research in Paediatrics 20148173–79. ( 10.1159/000357143) [DOI] [PubMed] [Google Scholar]
- 76.Ly S, Frates MC, Benson CB, Peters HE, Grant FD, Drubach LA, Voss SD, Feldman HA, Smith JR, Barletta Jet al. Features and outcome of autonomous thyroid nodules in children: 31 consecutive patients seen at a single center. Journal of Clinical Endocrinology and Metabolism 20161013856–3862. ( 10.1210/jc.2016-1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niedziela M, Breborowicz D, Trejster E, Korman E. Hot nodules in children and adolescents in Western Poland from 1996 to 2000: clinical analysis of 31 patients. Journal of Pediatric Endocrinology and Metabolism 200215823–830. ( 10.1515/jpem.2002.15.6.823) [DOI] [PubMed] [Google Scholar]
- 78.Stack BC, Twining C, Rastatter J, Angelos P, Baloch Z, Diercks G, Faquin W, Kazahaya K, Rivkees S, Sheyn Tet al. Consensus statement by the American Association of Clinical Endocrinology (AACE) and the American Head and Neck Society Endocrine Surgery Section (AHNS-ES) on pediatric benign and malignant thyroid surgery. Head and Neck 2021431027–1042. ( 10.1002/hed.26586) [DOI] [PubMed] [Google Scholar]
- 79.Lim-Dunham JE, Toslak IE, Reiter MP, Martin B. Assessment of the American College of Radiology thyroid imaging reporting and data system for thyroid nodule malignancy risk stratification in a pediatric population. American Journal of Roentgenology 2019212188–194. ( 10.2214/AJR.18.20099) [DOI] [PubMed] [Google Scholar]
- 80.Richards ML.Familial syndromes associated with thyroid cancer in the era of personalized medicine. Thyroid 201020707–713. ( 10.1089/thy.2010.1641) [DOI] [PubMed] [Google Scholar]
- 81.Machens A, Elwerr M, Schneider R, Lorenz K, Dralle H. Disease impacts more than age on operative morbidity in children with Graves’ disease after total thyroidectomy. Surgery 2018164993–997. ( 10.1016/j.surg.2018.07.021) [DOI] [PubMed] [Google Scholar]
- 82.Staubitz JI, Bode J, Poplawski A, Watzka F, Pohlenz J, Lang H, Musholt TJ. Thyroid surgery in children and young adults: potential overtreatment and complications. Langenbeck’s Archives of Surgery 2020405451–460. ( 10.1007/s00423-020-01896-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugino K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Suzuki A, Akaishi J, Masaki C, Matsuzu KIet al. Papillary thyroid carcinoma in children and adolescents: long-term follow-up and clinical characteristics. World Journal of Surgery 2015392259–2265. ( 10.1007/s00268-015-3042-4) [DOI] [PubMed] [Google Scholar]
- 84.Qu N, Zhang L, Lu ZW, Ji QH, Yang SW, Wei WJ, Zhang Y. Predictive factors for recurrence of differentiated thyroid cancer in patients under 21 years of age and a meta-analysis of the current literature. Tumour Biology 2016377797–7808. ( 10.1007/s13277-015-4532-6) [DOI] [PubMed] [Google Scholar]
- 85.Bal CS, Garg A, Chopra S, Ballal S, Soundararajan R. Prognostic factors in pediatric differentiated thyroid cancer patients with pulmonary metastases. Journal of Pediatric Endocrinology and Metabolism 201528745–751. ( 10.1515/jpem-2014-0247) [DOI] [PubMed] [Google Scholar]
- 86.Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, Matsuzu K, Suzuki A, Tomoda C, Hames KY, Akaishi Jet al. Risk stratification of pediatric patients with differentiated thyroid cancer: is total thyroidectomy necessary for patients at any risk? Thyroid 202030548–556. ( 10.1089/thy.2019.0231) [DOI] [PubMed] [Google Scholar]
- 87.Spinelli C, Tognetti F, Strambi S, Morganti R, Massimino M, Collini P. Cervical lymph node metastases of papillary thyroid carcinoma, in the central and lateral compartments, in children and adolescents: predictive factors. World Journal of Surgery 2018422444–2453. ( 10.1007/s00268-018-4487-z) [DOI] [PubMed] [Google Scholar]
- 88.Lerner J, Goldfarb M. Pediatric thyroid microcarcinoma. Annals of Surgical Oncology 2015224187–4192. ( 10.1245/s10434-015-4546-8) [DOI] [PubMed] [Google Scholar]
- 89.Golpanian S, Tashiro J, Sola JE, Allen C, Lew JI, Hogan AR, Neville HL, Perez EA. Surgically treated pediatric nonpapillary thyroid carcinoma. European Journal of Pediatric Surgery 201626524–532. ( 10.1055/s-0035-1569150) [DOI] [PubMed] [Google Scholar]
- 90.MacHens A, Lorenz K, Nguyen Thanh P, Brauckhoff M, Dralle H. Papillary thyroid cancer in children and adolescents does not differ in growth pattern and metastatic behavior. Journal of Pediatrics 2010157648–652. ( 10.1016/j.jpeds.2010.04.026) [DOI] [PubMed] [Google Scholar]
- 91.Mandapathil M, Lennon P, Ganly I, Patel SG, Shah JP. Significance and management of incidentally diagnosed metastatic papillary thyroid carcinoma in cervical lymph nodes in neck dissection specimens. Head and Neck 2019413783–3787. ( 10.1002/hed.25905) [DOI] [PubMed] [Google Scholar]
- 92.Propst EJ, Wasserman JD, Gorodensky J, Ngan BY, Wolter NE. Patterns and predictors of metastatic spread to the neck in pediatric thyroid carcinoma. Laryngoscope 2021131E1002–E1009. ( 10.1002/lary.28937) [DOI] [PubMed] [Google Scholar]
- 93.Liang W, Sheng L, Zhou L, Ding C, Yao Z, Gao C, Zeng Q, Chen B. Risk factors and prediction model for lateral lymph node metastasis of papillary thyroid carcinoma in children and adolescents. Cancer Management and Research 2021131551–1558. ( 10.2147/CMAR.S295420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubinstein JC, Dinauer C, Herrick-Reynolds K, Morotti R, Callender GG, Christison-Lagay ER. Lymph node ratio predicts recurrence in pediatric papillary thyroid cancer. Journal of Pediatric Surgery 201954129–132. ( 10.1016/j.jpedsurg.2018.10.010) [DOI] [PubMed] [Google Scholar]
- 95.Olmsted C, Arunachalam R, Gao X, Pesce L, Lal G. Pediatric differentiated thyroid carcinoma: trends in practice and outcomes over 40 years at a single tertiary care institution. Journal of Pediatric Endocrinology and Metabolism 2017301067–1074. ( 10.1515/jpem-2016-0327) [DOI] [PubMed] [Google Scholar]
- 96.Tuggle CT, Roman SA, Wang TS, Boudourakis L, Thomas DC, Udelsman R, Ann Sosa J. Pediatric endocrine surgery: who is operating on our children? Surgery 2008144869–877. ( 10.1016/j.surg.2008.08.033) [DOI] [PubMed] [Google Scholar]
- 97.Kao KT, Ferguson EC, Blair G, Chadha NK, Chanoine JP. Risk factors for the development of hypocalcemia in pediatric patients after total thyroidectomy – a systematic review. International Journal of Pediatric Otorhinolaryngology 2021143110666. ( 10.1016/j.ijporl.2021.110666) [DOI] [PubMed] [Google Scholar]
- 98.Schneider R, Machens A, Lorenz K, Dralle H. Intraoperative nerve monitoring in thyroid surgery - Shifting current paradigms. Gland Surgery 20209S120–S128. ( 10.21037/gs.2019.11.04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lorenz K, Raffaeli M, Barczyński M, Lorente-Poch L, Sancho J. Volume, outcomes, and quality standards in thyroid surgery: an evidence-based analysis—European Society of Endocrine Surgeons (ESES) positional statement. Langenbeck’s Archives of Surgery 2020405401–425. ( 10.1007/s00423-020-01907-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amin M, Edge SB, Greene FL, Byrd D, Brookland R, Washington M, Gershenwald J, Hess K, Sullivan Det al. AJCC Cancer Staging Manual. Berlin, Germany: Springer, 2017. [Google Scholar]
- 101.Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, Dillehay G, Draganescu C, Flux G, Führer Det al. Controversies, consensus, and collaboration in the use of 131 I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association. Thyroid 201929461–470. ( 10.1089/thy.2018.0597) [DOI] [PubMed] [Google Scholar]
- 102.Liu Z, Hu D, Huang Y, Chen S, Zeng W, Zhou L, Zhou W, Wang M, Feng H, Wei Wet al. Factors associated with distant metastasis in pediatric thyroid cancer: evaluation of the seer database. Endocrine Connections 2019878–85. ( 10.1530/EC-18-0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid 201222778–783. ( 10.1089/thy.2011.0522) [DOI] [PubMed] [Google Scholar]
- 104.Reiners C, Biko J, Haenscheid H, Hebestreit H, Kirinjuk S, Baranowski O, Marlowe RJ, Demidchik E, Drozd V, Demidchik Y. Twenty-five years after Chernobyl: outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2013983039–3048. ( 10.1210/jc.2013-1059) [DOI] [PubMed] [Google Scholar]
- 105.Verburg FA, Biko J, Diessl S, Demidchik Y, Drozd V, Rivkees SA, Reiners C, Hänscheid H. I-131 activities as high as safely administrable (AHASA) for the treatment of children and adolescents with advanced differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 201196E1268–E1271. ( 10.1210/jc.2011-0520) [DOI] [PubMed] [Google Scholar]
- 106.Chiesa C, Castellani MR, Vellani C, Orunesu E, Negri A, Azzeroni R, Botta F, Maccauro M, Aliberti G, Seregni Eet al. Individualized dosimetry in the management of metastatic differentiated thyroid cancer. Quarterly Journal of Nuclear Medicine and Molecular Imaging 200953546–561. [PubMed] [Google Scholar]
- 107.Flux GD, Haq M, Chittenden SJ, Buckley S, Hindorf C, Newbold K, Harmer CL. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging 201037270–275. ( 10.1007/s00259-009-1261-3) [DOI] [PubMed] [Google Scholar]
- 108.Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. Journal of Nuclear Medicine 200344451–456. [PubMed] [Google Scholar]
- 109.Verburg FA, van Santen HM, Luster M. Pediatric papillary thyroid cancer: current management challenges. OncoTargets and Therapy 201710165–175. ( 10.2147/OTT.S100512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. British Journal of Radiology 197750799–807. ( 10.1259/0007-1285-50-599-799) [DOI] [PubMed] [Google Scholar]
- 111.Iorcansky S, Herzovich V, Qualey RR, Tuttle RM. Serum thyrotropin (TSH) levels after recombinant human TSH injections in children and teenagers with papillary thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2005906553–6555. ( 10.1210/jc.2005-1550) [DOI] [PubMed] [Google Scholar]
- 112.Rosario PW, Filho AFCM, Lacerda RX, Calsolari MR. Recombinant human TSH for thyroid remnant ablation with 131 I in children and adolescents with papillary carcinoma. Hormone Research in Paediatrics 20127759–62. ( 10.1159/000335088) [DOI] [PubMed] [Google Scholar]
- 113.Hoe FM, Charron M, Moshang TJ. Use of the recombinant human TSH stimulated thyroglobulin level and diagnostic whole body scan in children with differentiated thyroid carcinoma. Journal of Pediatric Endocrinology and Metabolism 20061925–30. ( 10.1515/jpem.2006.19.1.25) [DOI] [PubMed] [Google Scholar]
- 114.Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJG, Tennvall J, Bombardieri E. & European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging 2008351941–1959. ( 10.1007/s00259-008-0883-1) [DOI] [PubMed] [Google Scholar]
- 115.Dekker BL, Links MH, Kobold ACM, Swart-Busscher LG, Kars M, Bons JAP, Brouwers AH, Links TP, van der Horst-Schrivers ANA. Low-iodine diet of 4 days is sufficient preparation for 131 I therapy in differentiated thyroid cancer patients. Journal of Clinical Endocrinology and Metabolism 2022107e604–e611. ( 10.1210/clinem/dgab691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Picarsic JL, Buryk MA, Ozolek J, Ranganathan S, Monaco SE, Simons JP, Witchel SF, Gurtunca N, Joyce J, Zhong Set al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatric and Developmental Pathology 201619115–122. ( 10.2350/15-07-1667-OA.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux Set al. Efficacy of selpercatinib in RET-altered thyroid cancers . New England Journal of Medicine 2020383825–835. ( 10.1056/NEJMoa2005651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL, Rudzinski E, Feraco AMet al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet. Oncology 201819705–714. ( 10.1016/S1470-2045(1830119-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahajan P, Dawrant J, Kheradpour A, Quintanilla NM, Lopez ME, Orth RC, Athanassaki I, Venkatramani R. Response to lenvatinib in children with papillary thyroid carcinoma. Thyroid 2018281450–1454. ( 10.1089/thy.2018.0064) [DOI] [PubMed] [Google Scholar]
- 120.Waguespack SG, Sherman SI, Williams MD, Clayman GL, Herzog CE. The successful use of sorafenib to treat pediatric papillary thyroid carcinoma. Thyroid 200919407–412. ( 10.1089/thy.2008.0429) [DOI] [PubMed] [Google Scholar]
- 121.Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, Dillehay G, Draganescu C, Flux G, Führer Det al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid201929461–470 ( 10.1089/thy.2018.0597) [DOI] [PubMed] [Google Scholar]
- 122.Aashiq M, Silverman DA, Na’ara S, Takahashi H, Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers 2019111382. ( 10.3390/cancers11091382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rangel-Pozzo A, Sisdelli L, Cordioli MIV, Vaisman F, Caria P, Mai S, Cerutti JM. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: an overview comparing pediatric and adult populations. Cancers 2020121–26. ( 10.3390/cancers12113146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mitsutake N, Fukushima T, Matsuse M, Rogounovitch T, Saenko V, Uchino S, Ito M, Suzuki K, Suzuki S, Yamashita Set al. BRAFV600E mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Scientific Reports 2015516976. ( 10.1038/srep16976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohtsuru A, Midorikawa S, Ohira T, Suzuki S, Takahashi H, Murakami M, Shimura H, Matsuzuka T, Yasumura S, Suzuki SIet al. Incidence of thyroid cancer among children and young adults in Fukushima, Japan, screened with 2 rounds of ultrasonography Within 5 years of the 2011 Fukushima daiichi nuclear power station accident. JAMA Otolaryngology– Head and Neck Surgery 20191454–11. ( 10.1001/jamaoto.2018.3121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitsutake N, Saenko V. Molecular pathogenesis of pediatric thyroid carcinoma. Journal of Radiation Research 202162i71–i77. ( 10.1093/jrr/rraa096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lamartina L, Anizan N, Dupuy C, Leboulleux S, Schlumberger M. Redifferentiation-facilitated radioiodine therapy in thyroid cancer. Endocrine-Related Cancer 202128T179–T191. ( 10.1530/ERC-21-0024) [DOI] [PubMed] [Google Scholar]
- 128.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiation Research 1995141259–277. ( 10.2307/3579003) [DOI] [PubMed] [Google Scholar]
- 129.Veiga LHS, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, Sakata R, Schneider AB, Inskip P, Bhatti Pet al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiation Research 2016185473–484. ( 10.1667/RR14213.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Veiga LHS, Lubin JH, Anderson H, de Vathaire F, Tucker M, Bhatti P, Schneider A, Johansson R, Inskip P, Kleinerman Ret al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiation Research 2012178365–376. ( 10.1667/rr2889.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, Berkow RL, Hammond S, Neglia JP, Meadows ATet al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet 20053652014–2023. ( 10.1016/S0140-6736(0566695-0) [DOI] [PubMed] [Google Scholar]
- 132.Clement SC, Lebbink CA, Klein Hesselink MS, Teepen JC, Links TP, Ronckers CM, van Santen HM. Presentation and outcome of subsequent thyroid cancer among childhood cancer survivors compared to sporadic thyroid cancer: a matched national study. European Journal of Endocrinology 2020183169–180. ( 10.1530/EJE-20-0153) [DOI] [PubMed] [Google Scholar]
- 133.Sassolas G, Hafdi-Nejjari Z, Casagranda L, Berger C, Bournaud C, Decaussin-Petrucci M, Berger N, Borson-Chazot F. Thyroid cancers in children, adolescents, and young adults with and without a history of childhood exposure to therapeutic radiation for other cancers. Thyroid 201323805–810. ( 10.1089/thy.2011.0370) [DOI] [PubMed] [Google Scholar]
- 134.Pacini F, Vorontsova T, Demidchik EP, Molinaro E, Agate L, Romei C, Shavrova E, Cherstvoy ED, Ivashkevitch Y, Kuchinskaya Eet al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. Journal of Clinical Endocrinology and Metabolism 1997823563–3569. ( 10.1210/jcem.82.11.4367) [DOI] [PubMed] [Google Scholar]
- 135.Bogdanova T, Zurnadzhy L, Masiuk S, Burko S, Degtyaryova T, Kovalenko A, Bolgov M, Chernyshov S, Gulevatyi S, Thomas Get al. Histopathological characteristics and post-operative follow-up of patients with potentially radiogenic papillary thyroid carcinoma depending on oncocytic changes availability in the tumor cells. Experimental Oncology 201941235–241. ( 10.32471/exp-oncology.2312-8852.vol-41-no-3.13554) [DOI] [PubMed] [Google Scholar]
- 136.van Santen HM, Alexander EK, Rivkees SA, Frey E, Clement SC, Dierselhuis MP, Lebbink CA, Links TP, Lorenz K, Peeters RPet al. Clinical considerations for the treatment of secondary differentiated thyroid carcinoma in childhood cancer survivors. European Journal of Endocrinology 2020183P1–P10. ( 10.1530/EJE-20-0237) [DOI] [PubMed] [Google Scholar]
- 137.Stewart DR, Best AF, Williams GM, Harney LA, Carr AG, Harris AK, Kratz CP, Dehner LP, Messinger YH, Rosenberg PSet al. Neoplasm risk among individuals with a pathogenic germline variant in DICER1. Journal of Clinical Oncology 201937668–676. ( 10.1200/JCO.2018.78.4678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamashita S, Saenko V. Mechanisms of disease: molecular genetics of childhood thyroid cancers. Nature Clinical Practice. Endocrinology and Metabolism 20073422–429. ( 10.1038/ncpendmet0499) [DOI] [PubMed] [Google Scholar]
- 139.Khan NE, Bauer AJ, Doros L, Schultz KAP, Decastro RM, Harney LA, Kase RG, Carr AG, Harris AK, Williams GMet al. Macrocephaly associated with the DICER1 syndrome. Genetics in Medicine 201719244–248. ( 10.1038/gim.2016.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Macken WL, Tischkowitz M, Lachlan KL. PTEN hamartoma tumor syndrome in childhood: a review of the clinical literature. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics 2019181591–610. ( 10.1002/ajmg.c.31743) [DOI] [PubMed] [Google Scholar]
- 141.van der Tuin K, de Kock L, Kamping EJ, Hannema SE, Pouwels MM, Niedziela M, van Wezel T, Hes FJ, Jongmans MC, Foulkes WDet al. Clinical and molecular characteristics may alter treatment strategies of thyroid malignancies in DICER1 syndrome. Journal of Clinical Endocrinology and Metabolism 2019104277–284. ( 10.1210/jc.2018-00774) [DOI] [PubMed] [Google Scholar]
- 142.Wassner AJ, Vecchia della M, Jarolim P, Feldman HA, Huang SA. Prevalence and significance of thyroglobulin antibodies in pediatric thyroid cancer. Journal of Clinical Endocrinology and Metabolism 20171023146–3153. ( 10.1210/jc.2017-00286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clinical Chemistry and Laboratory Medicine 2015531301–1314. ( 10.1515/cclm-2014-0813) [DOI] [PubMed] [Google Scholar]
- 144.Groen AH, Klein Hesselink MS, Plukker JTM, Sluiter WJ, van der Horst-Schrivers ANA, Brouwers AH, Lentjes EGWM, Muller Kobold AC, Links TP. Additional value of a high sensitive thyroglobulin assay in the follow-up of patients with differentiated thyroid carcinoma. Clinical Endocrinology 201786419–424. ( 10.1111/cen.13180) [DOI] [PubMed] [Google Scholar]
- 145.Kuo SF, Chao TC, Chang HY, Hsueh C, Lin CL, Chiang KC, Chuang WY, Chen YC, Lin JD. Prognosis of papillary thyroid cancers with positive serum thyroglobulin antibody after total thyroidectomy. Asian Journal of Surgery 201740186–192. ( 10.1016/j.asjsur.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 146.Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2007923590–3594. ( 10.1210/jc.2007-0444) [DOI] [PubMed] [Google Scholar]
- 147.Antonelli A, Miccoli P, Fallahi P, Grosso M, Nesti C, Spinelli C, Ferrannini E. Role of neck ultrasonography in the follow-up of children operated on for thyroid papillary cancer. Thyroid 200313479–484. ( 10.1089/105072503322021142) [DOI] [PubMed] [Google Scholar]
- 148.Vali R, Rachmiel M, Hamilton J, el Zein M, Wasserman J, Costantini DL, Charron M, Daneman A. The role of ultrasound in the follow-up of children with differentiated thyroid cancer. Pediatric Radiology 2015451039–1045. ( 10.1007/s00247-014-3261-0) [DOI] [PubMed] [Google Scholar]
- 149.Verburg FA, Mäder U, Giovanella L, Luster M, Reiners C. Low or undetectable basal thyroglobulin levels obviate the need for neck ultrasound in differentiated thyroid cancer patients after total thyroidectomy and 131 I ablation. Thyroid 201828722–728. ( 10.1089/thy.2017.0352) [DOI] [PubMed] [Google Scholar]
- 150.Parisi MT, Eslamy H, Mankoff D. Management of differentiated thyroid cancer in children: focus on the American Thyroid Association pediatric guidelines. Seminars in Nuclear Medicine 201646147–164. ( 10.1053/j.semnuclmed.2015.10.006) [DOI] [PubMed] [Google Scholar]
- 151.Pawelczak M, David R, Franklin B, Kessler M, Lam L, Shah B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following 131I treatment: a systematic review. Thyroid 2010201095–1101. ( 10.1089/thy.2009.0446) [DOI] [PubMed] [Google Scholar]
- 152.Vassilopoulou‐Sellin R, Klein MJ, Smith TH, Samaan NA, Frankenthaler RA, Goepfert H, Cangir A, Haynie TP. Pulmonary metastases in children and young adults with differentiated thyroid cancer. Cancer 1993711348–1352. () [DOI] [PubMed] [Google Scholar]
- 153.Nies M, Vassilopoulou-Sellin R, Bassett RL, Yedururi S, Zafereo ME, Cabanillas ME, Sherman SI, Links TP, Waguespack SG. Distant metastases from childhood differentiated thyroid carcinoma: clinical course and mutational landscape. Journal of Clinical Endocrinology and Metabolism 2021106e1683–e1697. ( 10.1210/clinem/dgaa935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schoelwer MJ, Zimmerman D, Shore RM, Josefson JL. The use of 123I in diagnostic radioactive iodine scans in children with differentiated thyroid carcinoma. Thyroid 201525935–941. ( 10.1089/thy.2014.0521) [DOI] [PubMed] [Google Scholar]
- 155.Hebestreit H, Biko J, Drozd V, Demidchik Y, Burkhardt A, Trusen A, Beer M, Reiners C. Pulmonary fibrosis in youth treated with radioiodine for juvenile thyroid cancer and lung metastases after Chernobyl. European Journal of Nuclear Medicine and Molecular Imaging 2011381683–1690. ( 10.1007/s00259-011-1841-x) [DOI] [PubMed] [Google Scholar]
- 156.Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. European Journal of Nuclear Medicine and Molecular Imaging 201138651–655. ( 10.1007/s00259-010-1669-9) [DOI] [PubMed] [Google Scholar]
- 157.Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJL, Vredeveld EJ, van der Horst-Schrivers ANA, van der Horst ICC, Kamphuisen PW, Plukker JTMet al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. Journal of Clinical Oncology 2013314046–4053. ( 10.1200/JCO.2013.49.1043) [DOI] [PubMed] [Google Scholar]
- 158.Klein Hesselink MS, Bocca G, Hummel YM, Brouwers AH, Burgerhof JGM, van Dam EWCM, Gietema JA, Havekes B, van den Heuvel-Eibrink MM, Corssmit EPMet al. Diastolic dysfunction is common in survivors of pediatric differentiated thyroid carcinoma. Thyroid 2017271481–1489. ( 10.1089/thy.2017.0383) [DOI] [PubMed] [Google Scholar]
- 159.Dekker BL, Muller Kobold AC, Brouwers AH, Williams GR, Nies M, Klein Hesselink MS, van der Horst-Schrivers ANA, Havekes B, van den Heuvel-Eibrink MM, van der Pal HJHet al. Bone mineral density in adult survivors of pediatric differentiated thyroid carcinoma: A longitudinal follow-up study. Thyroid 2021311707–1714. ( 10.1089/thy.2021.0179) [DOI] [PubMed] [Google Scholar]
- 160.Clement SC, Peeters RP, Ronckers CM, Links TP, van den Heuvel-Eibrink MM, Nieveen Van Dijkum EJM, van Rijn RR, van der Pal HJH, Neggers SJ, Kremer LCMet al. Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma - a systematic review. Cancer Treatment Reviews 201541925–934. ( 10.1016/j.ctrv.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 161.Nies M, Arts EGJM, van Velsen EFS, Burgerhof JGM, Muller Kobold AC, Corssmit EPM, Netea-Maier RT, Peeters RP, van der Horst-Schrivers ANA, Cantineau AEPet al. Long-term male fertility after treatment with radioactive iodine for differentiated thyroid carcinoma. European Journal of Endocrinology 2021185775–782. ( 10.1530/EJE-21-0315) [DOI] [PubMed] [Google Scholar]
- 162.Verkooijen RBT, Smit JWA, Romijn JA, Stokkel MPM. The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. European Journal of Endocrinology 2006155801–806. ( 10.1530/eje.1.02300) [DOI] [PubMed] [Google Scholar]
- 163.Marti JL, Jain KS, Morris LGT. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid 201525681–687. ( 10.1089/thy.2015.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mei X, Yao X, Feng F, Cheng W, Wang H. Risk and outcome of subsequent malignancies after radioactive iodine treatment in differentiated thyroid cancer patients. BMC Cancer 202121543. ( 10.1186/s12885-021-08292-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pasqual E, Schonfeld S, Morton LM, Villoing D, Lee C, Berrington de Gonzalez A, Kitahara CM. Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. Journal of Clinical Oncology 2022401439–1449. ( 10.1200/JCO.21.01841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reinecke MJ, Ahlers G, Burchert A, Eilsberger F, Flux GD, Marlowe RJ, Mueller HH, Reiners C, Rohde F, van Santen HM, Luster M. Second primary malignancies induced by radioactive iodine treatment of differentiated thyroid carcinoma — a critical review and evaluation of the existing evidence. European Journal of Nuclear Medicine and Molecular Imaging 2022493247–3256. ( 10.1007/s00259-022-05762-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Canale D, Ceccarelli C, Caglieresi C, Moscatelli A, Gavioli S, Santini P, Elisei R, Vitti P. Effects of radioiodine treatment for differentiated thyroid cancer on testis function. Clinical Endocrinology 201582295–299. ( 10.1111/cen.12514) [DOI] [PubMed] [Google Scholar]
- 168.Hyer S, Vini L, O’Connell M, Pratt B, Harmer C. Testicular dose and fertility in men following L131 therapy for thyroid cancer. Clinical Endocrinology 200256755–758. ( 10.1046/j.1365-2265.2002.t01-1-01545.x) [DOI] [PubMed] [Google Scholar]
- 169.Bourcigaux N, Rubino C, Berthaud I, Toubert ME, Donadille B, Leenhardt L, Petrot-Keller I, Brailly-Tabard S, Fromigué J, de Vathaire Fet al. Impact on testicular function of a single ablative activity of 3.7 GBq radioactive iodine for differentiated thyroid carcinoma. Human Reproduction 2018331408–1416. ( 10.1093/humrep/dey222) [DOI] [PubMed] [Google Scholar]
- 170.Sarkar SD, Beierwaltes WH, Gill SP, Cowley BJ. Subsequent fertility and birth histories of children and adolescents treated with 131I for thyroid cancer. Journal of Nuclear Medicine 197617460–464. [PubMed] [Google Scholar]
- 171.Selvakumar T, Nies M, Klein Hesselink MS, Brouwers AH, Van Der Horst-Schrivers ANA, Klein Hesselink EN, Tissing WJE, Vissink A, Links TP, Bocca Get al. Long-term effects of radioiodine treatment on salivary gland function in adult survivors of pediatric differentiated thyroid carcinoma. Journal of Nuclear Medicine 201860172–177. ( 10.2967/jnumed.118.212449) [DOI] [PubMed] [Google Scholar]
- 172.Nies M, Cantineau AEP, Arts EGJM, van den Berg MH, van Leeuwen FE, Muller Kobold AC, Klein Hesselink MS, Burgerhof JGM, Brouwers AH, van Dam EWCMet al. Long-term effects of radioiodine treatment on female fertility in survivors of childhood differentiated thyroid carcinoma. Thyroid 2020301169–1176. ( 10.1089/thy.2019.0560) [DOI] [PubMed] [Google Scholar]
- 173.Nies M, Dekker BL, Sulkers E, Huizinga GA, Klein Hesselink MS, Maurice-Stam H, Grootenhuis MA, Brouwers AH, Burgerhof JGM, van Dam EWCMet al. Psychosocial development in survivors of childhood differentiated thyroid carcinoma: a cross-sectional study. European Journal of Endocrinology 2018178215–223. ( 10.1530/EJE-17-0741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mendonça Monteiro de Barros G, Madeira M, Vieira Neto L, de Paula Paranhos Neto F, Carvalho Mendonça LM, Corrêa Barbosa Lima I, Corbo R, Fleiuss Farias ML. Bone mineral density and bone microarchitecture after long-term suppressive levothyroxine treatment of differentiated thyroid carcinoma in young adult patients. Journal of Bone and Mineral Metabolism 201634417–421. ( 10.1007/s00774-015-0680-4) [DOI] [PubMed] [Google Scholar]
- 175.Leonova TA, Drozd VM, Saenko VA, Mine M, Biko J, Rogounovitch TI, Takamura N, Reiners C, Yamashita S. Bone mineral density in treated at a young age for differentiated thyroid cancer after Chernobyl female patients on TSH-suppressive therapy receiving or not calcium-D3 supplementation. Endocrine Journal 201562173–182. ( 10.1507/endocrj.EJ14-0408) [DOI] [PubMed] [Google Scholar]
- 176.Nies M, Hesselink MSK, Huizinga GA, Sulkers E, Brouwers AH, Burgerhof JGM, van Dam EWCM, Havekes B, van den Heuvel-Eibrink MM, Corssmit EPMet al. Long-term quality of life in adult survivors of pediatric differentiated thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 20171021218–1226. ( 10.1210/jc.2016-2246) [DOI] [PubMed] [Google Scholar]
- 177.Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World Journal of Surgery 2010341192–1202. ( 10.1007/s00268-009-0364-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a