Abstract
Objectives
To examine antibody responses after the second vaccination in healthcare workers (HCWs) with underlying health conditions.
Design
Cohort study.
Setting
Oxford University Hospitals in the United Kingdom.
Participants
Healthcare workers who had SARS-CoV-2 serological data available and received two SARS-CoV- 2 vaccinations.
Primary outcome
Peak SARS-CoV-2 anti-spike IgG responses after the second vaccination and associations with underlying health conditions and the estimated risk of severe COVID-19 using an occupational health risk assessment tool.
Methods
We used univariable and multivariable linear regression models to investigate associations between antibody levels and demographics (age, sex, ethnicity), healthcare role, body mass index, underlying health conditions, vaccination status, prior infection and the Association of Local Authority Medical Advisors COVID-age risk score.
Results
1635 HCWs had anti-spike IgG measurements 14–84 days after second vaccination and data on any underlying health conditions. Only five HCWs (0.3%), all on immunosuppressive treatment, (including four organ transplant recipients), did not seroconvert after second vaccination. Antibody levels were independently lower with older age, diabetes, immunosuppression, respiratory disorders other than asthma and markedly so in organ transplant recipients. Levels were independently lower in ChAdOx1 versus BNT162b2 recipients and higher following previous infection. HCWs with ‘very high’ COVID-age risk scores had lower median antibody levels than those with ‘low’, ‘medium’ or ‘high’ risk scores; 4379 AU/mL, compared with 12 337 AU/mL, 9430 AU/mL and 10 524 AU/mL, respectively.
Conclusions
Two vaccine doses are effective in generating antibody responses among HCWs, including those with a high occupational risk. However, HCWs with underlying health conditions, especially diabetes, immunosuppression and organ transplant, had lower antibody levels, and vaccine response monitoring may be needed.
Keywords: COVID-19, EPIDEMIOLOGY, OCCUPATIONAL & INDUSTRIAL MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study focuses on antibody levels after vaccination in healthcare workers (HCWs) with underlying health conditions.
The study examines the association between the Association of Local Authority Medical Advisors COVID-age tool and vaccine response.
The study only examines the peak anti-spike IgG levels after the second vaccination and does not assess antibody waning longitudinally.
The study may not be widely generalisable given the study population is predominantly working-age HCWs.
Introduction
Healthcare workers (HCWs) have played a central role in the response to the SARS-CoV-2 global pandemic. In many settings, HCWs have been shown to be at an increased risk of SARS-CoV-2 infection, with risks relating both to proximity to infected patients and also to increased contact with colleagues compared with those working from home.1–3
Several interventions have been made to protect HCWs, including risk assessments, improved access to personal protective equipment (PPE) and training, better understanding of effective PPE selection, and modifications to working environments including social distancing and improved ventilation. One widely used UK tool that has been developed to assist in SARS-CoV-2 occupational health risk assessments is the Association of Local Authority Medical Advisors (ALAMA) COVID-age score,4 which uses demographic and medical history factors to estimate the risk of death if an HCW were to become infected. For those at the highest risk, restriction of patient contact or alternative working patterns may be recommended.
Additionally, multiple vaccines have been developed that show good protection against COVID-19 infection, hospitalisation and death,5–8 including in studies specifically looking at HCWs. Vaccination therefore plays an important role in facilitating those HCWs at higher risk of adverse outcomes from infection to remain in their usual work. Some HCWs, however, may not generate protective immunity following vaccination because of underlying medical conditions, especially those at the highest risk of adverse outcomes.
One potential way to assess which HCWs have responded to vaccination is to look at their antibody levels post-vaccination. Various studies have examined antibody responses in HCWs, including looking at the duration, magnitude and response trajectories,9–12 to understand the level of protection induced from vaccination, and its association with age, sex and ethnicity.13 However, few studies have examined the antibody responses in HCWs with underlying comorbidities. In particular, the association between estimated vulnerability of HCWs and antibody response to vaccination has not been studied.
Here we report findings from a retrospective observational study looking at anti-spike IgG antibody responses after second COVID-19 vaccination in HCWs with underlying health conditions, specifically focusing on the association between peak antibody levels with the pre-existing underlying health conditions.
Methods
Participants and settings
Oxford University Hospitals (OUH) consists of four teaching hospitals in Oxfordshire UK, providing acute and specialist services and employing 13 500 staff members. OUH offered vaccination to all HCWs. The programme began on 8 December 2020, initially prioritising those at highest risk of severe COVID-19, starting with the Pfizer-BioNTech BNT162b2 vaccine, with Oxford-AstraZeneca ChAdOx1 nCoV-19 added from 4 January 2021 and predominately provided to all staff at one acute hospital. Some HCWs received the Oxford-AstraZeneca vaccine in clinical trials beginning 23 April 2020 and were included following unblinding if receiving active vaccine.
OUH has offered SARS-CoV-2 testing to all symptomatic and asymptomatic staff. SARS-CoV-2 PCR testing of combined nasal and oropharyngeal swabs for symptomatic staff (those with a new persistent cough, temperature ≥37.8°C, anosmia or ageusia) was offered from 27 March 2020 onwards. Asymptomatic HCWs were invited to participate in voluntary nasal and oropharyngeal swab PCR testing and serological testing from 23 April 2020 to 30 June 2021, as previously described.9 14 All swabbing was performed by trained staff rather than self-administered. Additional serological testing of HCWs was undertaken by the Occupational Health Department based on clinical assessment (results are included from 9 April 2020 onwards).
For occupational health purposes, all HCWs were asked to complete an individual COVID-19 risk assessment and those with underlying health conditions had more detailed risk assessments undertaken by the Occupational Health Department. Staff members completed an online questionnaire about their age, sex, ethnicity, body mass index (BMI), underlying health conditions, smoking and pregnancy status, vaccination details, job role and location. COVID-age risk scores4 were calculated based on this information to enable an appropriate risk assessment to be made by the Occupational Health Team.
A total of 5968 HCWs had serological data available between 9 April 2020 and 26 August 2021, among which 2878 received two vaccinations. A total of 1635 HCWs had anti-spike IgG measurements after second vaccination and provided data on any underlying health conditions; these HCWs were included in the study (online supplemental figure S1).
bmjopen-2022-066766supp001.pdf (142KB, pdf)
Laboratory tests
Post-vaccination anti-trimeric spike IgG antibody levels were measured using the Abbott SARS-CoV-2 IgG II Quant antibody test (Abbott, Maidenhead, UK) targeting the spike receptor-binding domain, with the cut-off of ≥50 AU/mL reported as positive and a linear quantification of detected results from 50 to 40 000 AU/mL.15 Anti-spike IgG levels above 40 000 AU/mL were truncated at 40 000 AU/mL. The conversion between AU/mL and BAU/mL provided by the manufacturer is: 7 AU/mL=1 BAU/mL. Pre-vaccination anti-nucleocapsid IgG levels were measured using the Abbott Architect i2000 chemiluminescent microparticle immunoassay (Abbott, Maidenhead, UK), with antibody levels ≥1.40 manufacturer’s arbitrary units reported as positive. Pre-vaccination anti-trimeric-spike IgG levels were measured using an ELISA developed by the University of Oxford,16 with ≥8 million units reported as positive.
PCR was performed using the Public Health England SARS-CoV-2 assay (targeting the RdRp gene) or one of five commercial assays: Abbott RealTime (targeting RdRp and N genes; Abbott, Maidenhead, UK), Altona RealStar (targeting E and S genes; Altona Diagnostics, Liverpool, UK), Cepheid Xpert Xpress SARS-CoV-2 (targeting N2 and E; Cepheid, California, USA), BioFire Respiratory 2.1 (RP2.1) panel with SARS-CoV-2 (targeting ORF1ab and ORF8; Biofire diagnostics, Utah, USA), Thermo Fisher TaqPath assay (targeting S and N genes, and ORF1ab; Thermo Fisher, Abingdon, UK) or using the ABI 7500 platform (Thermo Fisher, Abingdon, UK) with the US Centers for Disease Control and Prevention Diagnostic Panel of two probes targeting the N gene.
Outcome
We included HCWs aged 17–77 years who completed an occupational health risk assessment, received a two-vaccination course and had antibody measurements after their second vaccination. The vaccination type was divided into a homologous ChAdOx1 course, homologous BNT162b2 course, and other vaccine types or mixed vaccination.
We used the peak antibody level 14–84 days after second vaccination as the outcome. Antibody response was divided into three groups: high response (peak anti-spike IgG level >700 AU/mL, converted from 100 BAU/mL which is associated with 67% protection against Delta infection17), low response (50–700 AU/mL) and no response (<50 AU/mL).
Antibody measurements after breakthrough infections after first vaccination were excluded from the analysis: 26 HCWs had evidence of infection at least 14 days after their first vaccination but prior to their second vaccination, and 37 HCWs had evidence of infection at least 14 days after their second vaccination.
Covariates
HCWs’ sex (grouped into male, female and non-disclosed), ethnicity (white, Asian, black, mixed, other and not stated) and BMI (<16, 16–24.9, 25–29.9, 30–34.9, 35–39.9 and 40+) were included. Job role was grouped into nurse or healthcare assistant, doctor, administrative staff, physical, occupational or speech therapist, laboratory staff, porter or domestic worker, medical or nursing student, or ‘other’, which included security, estates, catering staff, pharmacists, midwives and other allied healthcare professionals.
Medical conditions and other potential risk factors included in the analysis were smoking and pregnancy status, and whether each HCW had asthma, hypertension, a thyroid disorder excluding malignancy, diabetes, immunosuppression, psoriasis, heart disease, a non-haematological malignancy, a rheumatological disorder, a respiratory disease other than asthma, a haematological disease excluding malignancy, liver disease, a neurological disorder, chronic kidney disease stage 3, 4 or 5, lupus, a splenic disorder excluding traumatic splenectomy, a haematological malignancy and an organ transplant. Prior infection was defined as having had a positive PCR result or a positive anti-spike antibody result or a positive anti-nucleocapsid antibody result before the first vaccination dose.
The ALAMA COVID-age risk score was calculated based on age, sex, ethnicity and presence of comorbidities. It estimates the probability of death should infection occur in the absence of vaccination or previous infection. We used the COVID-age risk score as a proxy for HCWs’ vulnerability to a poor outcome following SARS-CoV-2 infection and examined its association with antibody levels. A score ≥85 indicates very high vulnerability, 70–84 high vulnerability, 50–69 moderate vulnerability and <50 low vulnerability. Details of the calculation formula and methods can be found online.4
Statistical analysis
We first used the Kruskal-Wallis rank test to compare the outcome by different covariate groups. We then built univariable and multivariable linear regression models to examine the association between the outcome on the log10 scale and demographics (age, sex, ethnicity), healthcare role, BMI, underlying health conditions, vaccination status, prior infection and COVID-age risk score. Age was truncated at the 2.5th and 97.5th percentile to avoid undue outlier influence and modelled with and without natural cubic splines to test for the non-linear effects. For the multivariable model, backward elimination was used and the model with the lowest Akaike information criteria was selected. COVID-age score was not included in the multivariable model as it is based on other factors already included in the model.
All analyses were performed in R (V.4.1), using the following packages: tidyverse (V.1.3.1), splines (V.4.0.5) and stats (V.4.0.5).
Patient and public involvement
The oversight committee of the research database used by the study has patient and public representation who participated in reviewing and approving a study summary and analysis plan.
Results
Among 1635 HCWs, the median (IQR) (range) age was 46 (33–56) (17–77) years. A total of 1344 (82.2%) were female, 1169 (71.5%) were of white ethnicity, and 779 (47.6%) worked in a nursing or healthcare assistant role. Eight hundred seventy-two (53.3%) did not have any underlying medical condition. The proportion reporting each condition ranged from 0.2% to 19.3%, with asthma being the most common comorbidity. Two hundred eighty-six (17.5%) HCWs had evidence of infection prior to their first vaccination. The median (IQR) (range) COVID-age score was 50 (35–59) (16–124), with 120 (7.5%) and 22 (1.4%) HCWs falling in the ‘high’ and ‘very high’ risk groups, respectively. A total of 1234 (75.5%) and 387 (23.7%) HCWs received two BNT162b2 and ChAdOx1 vaccinations, respectively, and 13 HCWs (0.8%) received other combinations, including mRNA-1273 (tables 1 and 2). The characteristics were generally similar to the larger group of 5968 HCWs with serological data, so the cohort included in the analysis should be representative (online supplemental table S1).
Table 1.
Characteristics of the study population according to the peak anti-spike IgG levels post-second vaccination
| Total (n=1635) | Peak antibody levels >700 AU/mL (n=1555) | Peak antibody levels 50–700 AU/mL (n=75) | Peak antibody levels <50 AU/mL (n=5) | Peak antibody levels 14–84 days post-second vaccination (AU/mL) | P value | ||
| Median | IQR | ||||||
| Age (years) | |||||||
| Median | 46 | 46 | 49 | 34 | (Overall) 10 763 | 3925–22 017 | |
| Q1, Q3 | 33, 56 | 33, 56 | 36, 56 | 33, 43 | |||
| Age group | |||||||
| 17–34 | 457 (100.0%) | 439 (96.1%) | 15 (3.3%) | 3 (0.7%) | 14 668 | 5359–25 801 | <0.001 |
| 35–54 | 723 (100.0%) | 682 (94.3%) | 39 (5.4%) | 2 (0.3%) | 10 153 | 3773–20 578 | |
| 55–77 | 455 (100.0%) | 434 (95.4%) | 21 (4.6%) | 0 (0.0%) | 9328 | 3461–19 046 | |
| Sex | |||||||
| Female | 1344 (100.0%) | 1271 (94.6%) | 68 (5.1%) | 5 (0.4%) | 10 779 | 3856–22 541 | 0.7 |
| Male | 290 (100.0%) | 283 (97.6%) | 7 (2.4%) | 0 (0.0%) | 10 710 | 4524–19 026 | |
| Non-disclosed | 1 (100.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 5438 | ||
| Ethnicity | |||||||
| White | 1169 (100.0%) | 1110 (95.0%) | 56 (4.8%) | 3 (0.3%) | 10 971 | 3907–22 009 | 0.3 |
| Asian | 304 (100.0%) | 290 (95.4%) | 14 (4.6%) | 0 (0.0%) | 10 433 | 4180–21 829 | |
| Black | 50 (100.0%) | 45 (90.0%) | 4 (8.0%) | 1 (2.0%) | 7081 | 2104–14 388 | |
| Mixed | 36 (100.0%) | 36 (100.0%) | 0 (0.0%) | 0 (0.0%) | 10 725 | 4573–29 932 | |
| Other | 55 (100.0%) | 53 (96.4%) | 1 (1.8%) | 1 (1.8%) | 8581 | 4703–20 589 | |
| Not stated | 21 (100.0%) | 21 (100.0%) | 0 (0.0%) | 0 (0.0%) | 15 786 | 5686–25 064 | |
| Role | <0.001 | ||||||
| Administrative staff | 245 (100.0%) | 236 (96.3%) | 8 (3.3%) | 1 (0.4%) | 11 653 | 5660–15 013 | |
| Doctor | 106 (100.0%) | 102 (96.2%) | 4 (3.8%) | 0 (0.0%) | 12 003 | 6677–22 113 | |
| Laboratory staff | 61 (100.0%) | 58 (95.1%) | 2 (3.3%) | 1 (1.6%) | 20 279 | 8790–31 137 | |
| Medical or nursing student | 12 (100.0%) | 12 (100.0%) | 0 (0.0%) | 0 (0.0%) | 7933 | 6061–20 103 | |
| Nurse/HCA | 779 (100.0%) | 737 (94.6%) | 39 (5.0%) | 3 (0.4%) | 10 844 | 3897–21 515 | |
| Other | 331 (100.0%) | 314 (94.9%) | 17 (5.1%) | 0 (0.0%) | 9208 | 2533–20 284 | |
| Porter or domestic worker | 31 (100.0%) | 27 (87.1%) | 4 (12.9%) | 0 (0.0%) | 8476 | 1533–15 644 | |
| OT/PT/SLT | 70 (100.0%) | 69 (98.6%) | 1 (1.4%) | 0 (0.0%) | 11 849 | 6021–24 371 | |
| BMI | 0.3 | ||||||
| <16 | 3 (100.0%) | 2 (66.7%) | 1 (33.3%) | 0 (0.0%) | 17 716 | 9195–18 471 | |
| 16–24.9 | 698 (100.0%) | 667 (95.6%) | 28 (4.0%) | 3 (0.4%) | 11 587 | 4186–22 134 | |
| 25–29.9 | 517 (100.0%) | 491 (95.0%) | 24 (4.6%) | 2 (0.4%) | 10 169 | 3404–19 046 | |
| 30–34.9 | 261 (100.0%) | 247 (94.6%) | 14 (5.4%) | 0 (0.0%) | 10 149 | 4318–24 585 | |
| 35–39.9 | 85 (100.0%) | 81 (95.3%) | 4 (4.7%) | 0 (0.0%) | 10 743 | 4534–27 281 | |
| >40 | 71 (100.0%) | 67 (94.4%) | 4 (5.6%) | 0 (0.0%) | 11 199 | 5877–28 260 | |
| Vaccine combination | |||||||
| ChAdOx1/ChAdOx1 | 387 (100.0%) | 315 (81.4%) | 70 (18.1%) | 2 (0.5%) | 1603 | 879–3521 | |
| BNT162b2/BNT162b2 | 1234 (100.0%) | 1227 (99.4%) | 4 (0.3%) | 3 (0.2%) | 14 824 | 8432–25 853 | |
| Other | 13 (100.0%) | 13 (100.0%) | 1 (0.0%) | 0 (0.0%) | 6993 | 4693–13 212 | |
| Evidence of COVID-19 infection at baseline | <0.001 | ||||||
| No | 1349 (100.0%) | 1269 (94.1%) | 75 (5.6%) | 5 (0.4%) | 9960 | 3302–20 056 | |
| Yes | 286 (100.0%) | 286 (100.0%) | 0 (0.0%) | 0 (0.0%) | 17 227 | 7111–28 277 | |
| COVID-age score groups | <0.001 | ||||||
| Low | 769 (100.0%) | 737 (95.8%) | 32 (4.2%) | 0 (0.0%) | 12 337 | 4634–23 170 | |
| Medium | 696 (100.0%) | 659 (94.7%) | 35 (5.0%) | 2 (0.3%) | 9430 | 3693–19 302 | |
| High | 120 (100.0%) | 114 (95.0%) | 5 (4.2%) | 1 (0.8%) | 10 524 | 3106–23 939 | |
| Very high | 22 (100.0%) | 17 (77.3%) | 3 (13.6%) | 2 (9.1%) | 4379 | 889–11 214 | |
| COVID-age score | |||||||
| Median | 50 | 50 | 53 | 76 | |||
| Q1, Q3 | 35, 59 | 35, 60 | 36, 57 | 54, 85 | |||
Other for vaccine included mRNA-1273 and other vaccine combinations.
Bold indicates a significant p value of <0.05.
BMI, body mass index; HCA, healthcare assistant; OT/PT/SLT, occupational therapist, physiotherapist, and speech and language therapist.
Table 2.
Comorbidity status of the study population according to the peak anti-spike IgG levels post-second vaccination
| Total (n=1635) | Peak antibody levels >700 AU/mL (n=1555) | Peak antibody levels 50–700 AU/mL (n=75) | Peak antibody levels <50 AU/mL (n=5) | Peak antibody levels 14–84 days post-second vaccination (AU/mL) | P value | ||
| Median | IQR | ||||||
| Comorbidity | 0.009 | ||||||
| No | 872 (100.0%) | 830 (95.2%) | 42 (4.8%) | 0 (0.0%) | 11 681 | 4362–23 299 | |
| Yes | 763 (100.0%) | 725 (95.0%) | 33 (4.3%) | 5 (0.7%) | 9637 | 3493–19 750 | |
| Smoking | 106 (100.0%) | 99 (93.4%) | 7 (6.6%) | 0 (0.0%) | 7588 | 1828–19 639 | 0.003 |
| Pregnant | 23 (100.0%) | 23 (100.0%) | 0 (0.0%) | 0 (0.0%) | 14 684 | 8199–19 453 | 0.6 |
| Asthma | 316 (100.0%) | 300 (94.9%) | 16 (5.1%) | 0 (0.0%) | 10 161 | 4296–19 559 | 0.2 |
| Hypertension | 176 (100.0%) | 168 (95.5%) | 8 (4.5%) | 0 (0.0%) | 8770 | 3272–18 746 | 0.01 |
| Thyroid disorder (excluding malignancy) | 137 (100.0%) | 128 (93.4%) | 9 (6.6%) | 0 (0.0%) | 10 395 | 3280–25 672 | 0.9 |
| Diabetes | 95 (100.0%) | 89 (93.7%) | 5 (5.3%) | 1 (1.1%) | 8748 | 2950–19 346 | 0.04 |
| Immunosuppression | 80 (100.0%) | 68 (85.0%) | 7 (8.8%) | 5 (6.2%) | 7451 | 1503–17 695 | 0.002 |
| Psoriasis | 48 (100.0%) | 43 (89.6%) | 5 (10.4%) | 0 (0.0%) | 7435 | 2573–13 850 | 0.06 |
| Heart disease | 34 (100.0%) | 32 (94.1%) | 2 (5.9%) | 0 (0.0%) | 13 925 | 4999–22 430 | 0.7 |
| Non-haematological malignancy | 41 (100.0%) | 40 (97.6%) | 1 (2.4%) | 0 (0.0%) | 13 159 | 9261–23 955 | 0.3 |
| Rheumatological disorder | 27 (100.0%) | 24 (88.9%) | 2 (7.4%) | 1 (3.7%) | 5691 | 1770–15 567 | 0.05 |
| Respiratory disease (excluding asthma) | 37 (100.0%) | 33 (89.2%) | 4 (10.8%) | 0 (0.0%) | 6993 | 2302–12 927 | 0.01 |
| Haematological disease (excluding malignancy) | 36 (100.0%) | 35 (97.2%) | 1 (2.8%) | 0 (0.0%) | 12 236 | 6738–20 060 | 1 |
| Liver disease | 11 (100.0%) | 11 (100.0%) | 0 (0.0%) | 0 (0.0%) | 11 419 | 5818–13 705 | 0.4 |
| Neurological disorder | 12 (100.0%) | 12 (100.0%) | 0 (0.0%) | 0 (0.0%) | 6035 | 5258–12 886 | 0.3 |
| CKD stage 3, 4 or 5 | 6 (100.0%) | 5 (83.3%) | 0 (0.0%) | 1 (16.7%) | 10 129 | 5672–17 965 | 0.7 |
| Lupus | 7 (100.0%) | 5 (71.4%) | 1 (14.3%) | 1 (14.3%) | 1478 | 695–13 582 | 0.1 |
| Splenic disorder (excluding traumatic splenectomy) | 4 (100.0%) | 4 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1796 | 1040–9695 | 0.2 |
| Haematological malignancy | 3 (100.0%) | 3 (100.0%) | 0 (0.0%) | 0 (0.0%) | 7731 | 7230–16 186 | 0.9 |
| Organ transplant | 5 (100.0%) | 1 (20.0%) | 0 (0.0%) | 4 (80.0%) | 11 | 6–21 | <0.001 |
Bold indicates a significant p value of <0.05.
CKD, chronic kidney disease.
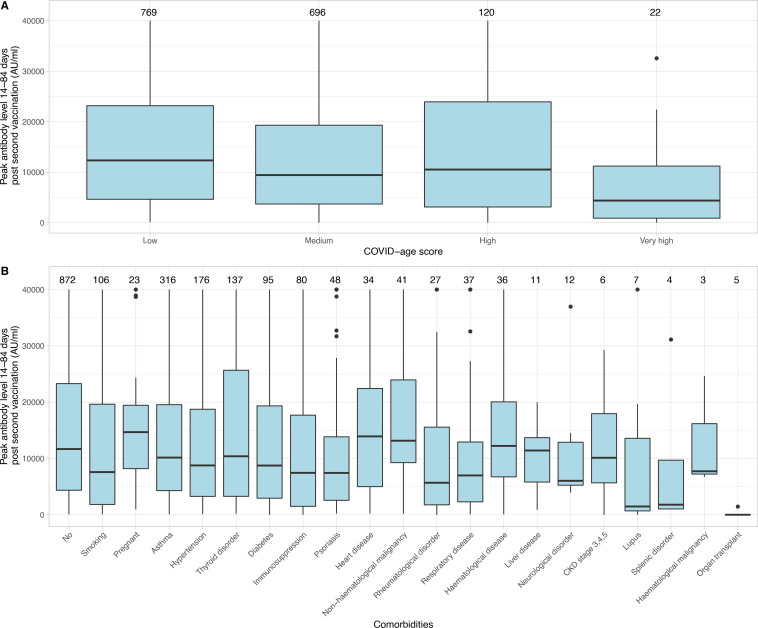
Among 1635 HCWs, the median (IQR) peak anti-spike binding antibody level 14–84 days after second vaccination was 10 763 (3925–22 017) AU/mL. The distribution of peak antibody levels is shown in online supplemental figure S2. Observed antibody levels were different across age groups, healthcare roles, vaccination types, with or without evidence of prior infection, and COVID-age scores (p<0.001). HCWs with ‘very high’ vulnerability according to COVID-age scores had the lowest median level of 4379 AU/mL, compared with 12 337 AU/mL, 9430 AU/mL, and 10 524 AU/mL in the ‘low’, ‘moderate’, and ‘high’ vulnerability groups (table 1 and figure 1A). HCWs with medical conditions and other risk factors had lower median antibody levels than those without (9637 AU/mL vs 11 681 AU/mL, p=0.009); specifically, antibody levels were lower in smokers (7588 AU/mL, p=0.003), those with hypertension (8770 AU/mL, p=0.01), diabetes (8748 AU/mL, p=0.04), immunosuppression (7451 AU/mL, p=0.002), a respiratory disease other than asthma (6993 AU/mL, p=0.01) and those who had an organ transplant (11 AU/mL, p<0.001) (table 2 and figure 1B). There was no evidence of an association between pregnancy and antibody levels (14 684 AU/mL, p=0.6).
Figure 1.
Box and whisker plot of peak anti-spike IgG levels 14–84 days post-second vaccination according to COVID-age score (A) and comorbidities (B). The number on top of each panel indicates the total number of HCWs in each group. Thyroid disorder excludes malignancy, respiratory disease excludes asthma, haematological disease excludes malignancy, splenic disorder excludes traumatic splenectomy. CKD, chronic kidney disease; HCWs, healthcare workers.
A total of 1555 (95.1%) HCWs had a peak anti-spike IgG level >700 AU/mL, that is, a level associated with >67% protection from infection (see the Methods section). Seventy-five (4.6%) HCWs had a suboptimal antibody level between 50 and 700 AU/mL, and five (0.3%) HCWs did not generate a positive antibody response (<50 AU/mL) after the second vaccination. Of the 75 and 5 HCWs with low or no antibody response, the median COVID-age risk score was 53 (IQR 36–57) and 76 (IQR 54–85), respectively—higher than in the high response group (50, IQR 35–60) (p=0.03), but not sufficiently different for the low response group for COVID-age score alone to identify those likely to be in this group. Among the 80 HCWs with low or no antibody response, 72 received two ChAdOx1 vaccinations, accounting for 18.6% of all the ChAdOx1 recipients, while the proportion having a low response was only 0.5% of all the BNT162b2 recipients (table 1). HCWs with specific medical conditions were more likely to be in the low or no response groups including 15% of those reporting taking immunosuppression and several other conditions that may also be treated with immunosuppression, including low/no antibody responses in 10% with psoriasis, 11% with rheumatological disorders, 11% with other (non-asthma) respiratory disorders, 29% with lupus and 80% with an organ transplant. Of the five HCWs with no detectable serological response, all were female and immunosuppressed, four HCWs had had organ transplants and the other HCW having an autoimmune disease for which they received rituximab (table 2).
Associations between log10 antibody levels and covariates in univariable linear regression models are shown in table 3. Older age, black ethnicity, working as a porter or domestic worker, and receiving two ChAdOx1 vaccines were associated with lower peak anti-spike antibody levels 14–84 days after second vaccination. Smoking, diabetes, a respiratory disease other than asthma, chronic kidney disease stage 3, 4 or 5, a rheumatological disorder, lupus, being immunosuppressed or having had an organ transplant were all associated with lower antibody levels. A higher COVID-age score, which indicated higher risk of mortality from infection, was also associated with lower antibody levels (p<0.001). Having evidence of COVID-19 infection prior to vaccination, as well as being a laboratory staff worker, were both associated with having higher antibody levels. No evidence of an association was found between antibody levels and sex or BMI.
Table 3.
Association between the peak anti-spike IgG antibody levels 14–84 days post-second vaccination with each characteristic from the univariable model and multivariable model
| Univariable | Multivariable | |||||||
| Coefficient | P value | 95% CI | Coefficient | P value | 95% CI | |||
| Age | ||||||||
| Per 10-year older | −0.034 | 0.002 | −0.06 | −0.01 | −0.03 | <0.001 | −0.05 | −0.01 |
| Sex | ||||||||
| Female | 1 (ref) | |||||||
| Male | 0.02 | 0.6 | −0.06 | 0.09 | −0.04 | 0.14 | −0.09 | 0.01 |
| Ethnicity | ||||||||
| White | 1 (ref) | |||||||
| Asian | 0.01 | 0.7 | −0.06 | 0.09 | ||||
| Black | −0.22 | 0.009 | −0.38 | −0.05 | ||||
| Mixed | 0.07 | 0.5 | −0.13 | 0.26 | ||||
| Other | −0.07 | 0.4 | −0.23 | 0.09 | ||||
| Role | ||||||||
| Nurse/HCA | 1 (ref) | |||||||
| Doctor | 0.1 | 0.1 | −0.02 | 0.22 | ||||
| Administrative staff | 0.07 | 0.1 | −0.01 | 0.15 | ||||
| OT/PT/SLT | 0.11 | 0.1 | −0.03 | 0.26 | ||||
| Laboratory staff | 0.24 | 0.002 | 0.09 | 0.39 | ||||
| Porter or domestic worker | −0.23 | 0.03 | −0.44 | −0.03 | ||||
| Medical or nursing student | 0.07 | 0.7 | −0.26 | 0.4 | ||||
| Other | −0.06 | 0.1 | −0.14 | 0.01 | ||||
| BMI | ||||||||
| <16 | −0.13 | 0.7 | −0.8 | 0.53 | ||||
| 16–24.9 | 1 (ref) | |||||||
| 25–29.9 | −0.06 | 0.1 | −0.12 | 0.01 | ||||
| 30–34.9 | 0.01 | 0.9 | −0.08 | 0.09 | ||||
| 35–39.9 | 0.01 | 0.9 | −0.12 | 0.14 | ||||
| 40+ | 0.05 | 0.5 | −0.09 | 0.19 | ||||
| Comorbidity | ||||||||
| Yes vs no | ||||||||
| Smoking | −0.17 | 0.003 | −0.29 | −0.06 | −0.06 | 0.12 | −0.14 | 0.02 |
| Pregnant | 0.13 | 0.3 | −0.11 | 0.37 | ||||
| Asthma | 0.005 | 0.9 | −0.15 | 0.04 | ||||
| Hypertension | −0.06 | 0.2 | −0.15 | 0.03 | −0.06 | 0.09 | −0.12 | 0.01 |
| Thyroid disorder (excluding malignancy) | 0.01 | 0.9 | −0.09 | 0.11 | 0.05 | 0.13 | −0.02 | 0.12 |
| Diabetes | −0.14 | 0.03 | −0.26 | −0.01 | −0.14 | <0.001 | −0.22 | −0.05 |
| Immunosuppression | −0.38 | <0.001 | −0.51 | −0.25 | −0.22 | <0.001 | −0.31 | −0.13 |
| Psoriasis | −0.13 | 0.1 | −0.3 | 0.04 | ||||
| Heart disease | 0.05 | 0.6 | −0.14 | 0.25 | ||||
| Non-haematological malignancy | 0.14 | 0.1 | −0.04 | 0.32 | 0.13 | 0.04 | 0.01 | 0.25 |
| Rheumatological disorder | −0.28 | 0.01 | −0.5 | −0.06 | ||||
| Respiratory disease (excluding asthma) | −0.22 | 0.02 | −0.41 | −0.03 | −0.17 | 0.01 | −0.3 | −0.04 |
| Haematological disease (excluding malignancy) | 0.06 | 0.6 | −0.14 | 0.25 | ||||
| Liver disease | −0.03 | 0.9 | −0.38 | 0.32 | ||||
| Neurological disorder | −0.001 | 0.9 | −0.33 | 0.33 | ||||
| CKD stage 3, 4 or 5 | −0.84 | <0.001 | −1.3 | −0.37 | ||||
| Lupus | −0.68 | 0.002 | −1.1 | −0.24 | ||||
| Splenic disorder (excluding traumatic splenectomy) | −0.42 | 0.2 | –1 | 0.15 | ||||
| Haematological malignancy | 0.13 | 0.7 | −0.53 | 0.79 | ||||
| Organ transplant | −3.06 | <0.001 | −3.55 | −2.57 | −2.66 | <0.001 | −3.01 | −2.31 |
| Vaccination | ||||||||
| BNT162b2/BNT162b2 | ||||||||
| ChAdOx1/ChAdOx1 | −0.9 | <0.001 | −0.95 | −0.85 | −0.91 | <0.001 | −0.96 | −0.87 |
| Evidence of COVID-19 infection at baseline (yes vs no) | 0.25 | <0.001 | 0.18 | 0.33 | 0.29 | <0.001 | 0.24 | 0.34 |
| COVID-age score | ||||||||
| Per 10 score higher | −0.05 | <0.001 | −0.06 | −0.03 | ||||
Bold indicates a significant p value of <0.05. The outcome was modelled in log10 scale. Variables in the multivariable model were selected using backward selection by Akaike information criteria.
BMI, body mass index; CKD, chronic kidney disease; HCA, healthcare assistant; OT/PT/SLT, occupational therapist, physiotherapist, and speech and language therapist.
A total of 1593 HCWs with complete information on all variables were included in the multivariable model. The baseline intercept in log10 scale was 4.25. Older age (−0.03 per 10 years older, 95% CI: −0.05 to –0.01), diabetes (−0.14, 95% CI: −0.22 to –0.05), a respiratory condition other than asthma (−0.17, 95% CI: −0.3 to –0.04), an organ transplant (−2.66, 95% CI: −3.01 to –2.31), being immunosuppressed (−0.22, 95% CI: −0.31 to –0.13) and receiving two ChAdOx1 vaccinations (−0.91, 95% CI: −0.96 to –0.87) were all independently associated with lower peak spike antibody levels 14–84 days after second vaccination. Having evidence of prior infection was associated with having higher antibody levels (0.29, 95% CI: 0.24 to 0.34) (table 3).
Discussion
While SARS-CoV-2 vaccination generates antibody responses for most HCWs, we found several risk factors associated with lower antibody levels after vaccination, including older age, diabetes, respiratory diseases other than asthma, being immunosuppressed and having had an organ transplant. Given antibody levels are associated with vaccine efficacy and protection against SARS-CoV-2 infection,18 19 HCWs with these risk factors could have a higher risk of infection. Infection before the first vaccination led to higher antibody levels post-vaccination.
Only five (0.3%) HCWs did not seroconvert after second vaccination, which is a smaller proportion than the approximately 1% of the general population who do not seroconvert after two vaccinations,17 and reflects the effectiveness of two vaccine doses in generating antibody responses in this population of predominantly healthy adults of working age.
Receiving two ChAdOx1 vaccine doses yielded lower antibody levels than receiving two BNT162b2 vaccine doses. Although this has been previously reported17 and may not reflect overall vaccine effectiveness, it was potentially an important factor in many of the 75 (4.6%) HCWs with low antibody responses. These HCWs had peak antibody levels lower than the level associated with 67% protection against the Delta variant infection in a previous study (100 BAU/mL, 700 AU/mL).17 Further, with new variants circulating, such as Omicron, with higher antibody levels required for the same level of protection,20 21 two doses of vaccination may not provide good levels of protection for this group.
Among the 80 HCWs who had no or low antibody response, most had underlying medical conditions, including immunosuppression or organ transplant, and 72 had received ChAdOx1 vaccination. These were also identified as the main risk factors for having lower antibody levels in the multivariable regression model, similar to previous studies reporting lower anti-spike IgG levels in HCWs with any comorbidity compared with healthy HCWs,22 and low antibody levels or seroconversion rates in organ transplant or immunosuppressed patients,23 24 leading to a higher risk of mortality following SARS-CoV-2 infection.25 26 Therefore, it may be helpful to routinely assess post-vaccination antibody levels in HCWs with comorbidities, especially immunosuppression or organ transplantation. Booster mRNA vaccine doses should be prioritised for this population, in particular those with two prior ChAdOx1 doses, as evidence has shown that a third or fourth dose could significantly improve the suboptimal immune response in organ transplant recipients.27–29
Other comorbidities independently associated with lower antibody levels post-vaccination were diabetes and respiratory diseases other than asthma. Antibody response and seropositivity rates in patients with diabetes were also found to be lower than in the healthy population after vaccination in a recent systematic review.30 However, adequate glycaemic control after vaccination improved immunological responses and may even restore the protection against SARS-CoV-2 infection.31 We did not find an association between peak antibody levels with BMI, but a study in Scotland suggested that obesity could lead to faster waning of immunity after vaccination, which may explain increased disease severity from breakthrough infections in people with obesity.32 Previous studies also found that hypertension33 and smoking34 35 were associated with lower antibody responses post-vaccination; there was marginal evidence for a similar effect in our population (p=0.09; 0.12).
We also examined the relationship between a COVID-age risk stratification score and vaccine response. The ALAMA COVID-age score is based on OpenSAFELY data4 36 and assesses demographic and health-related risk factors to calculate personal vulnerability to COVID-19, which can be quantified as the probability of death should infection occur in the absence of vaccination or previous infection. In our cohort, 6%–7% of HCWs had a high risk, and 1%–2% of HCWs had a very high risk based on the scoring system. Overall, higher risk groups had lower antibody levels after second vaccination. The COVID-age score can thus potentially be used to identify HCWs at risk of lower antibody levels. However, in most instances, these were still at levels associated with high levels of protection against infection, with a median peak level of around 10 000 for the low to high-risk groups. The peak level was lower in the ‘very high’ risk group, but more than 75% of HCWs in this group still generated peak levels >700 AU/mL (associated with 67% protection against the Delta variant infection).17 Therefore, vaccination (or previous infection) could provide good immunity and potentially reduce the personal vulnerability to COVID-19 for most HCWs. However, a small minority of HCWs may not be well protected by vaccination and these individuals are also potentially at higher risk of adverse outcomes if infected. Therefore, HCWs assessed as at ‘very high’ risk of more severe outcome from COVID-19 infection who do not have a history of COVID-19 infection should have further vaccine outcome assessment as part of their occupational risk assessment. In those with limited antibody responses, if these remain after booster vaccinations, it may be appropriate to put in place enhanced additional risk mitigation for those HCWs wishing to remain in their current role.
Limitations of this study include that we only examined the peak anti-spike IgG levels after the second vaccination and did not assess antibody waning longitudinally. We therefore did not assess antibody responses after a third booster dose, and this requires further study. We only measured anti-spike IgG levels using a single assay and did not measure neutralising antibodies or T cell responses. Vaccine induces a broad range of both B and T cell responses and measure of quantitative IgG antibody is only a surrogate for a broad range of immune response.37 However, the assay is commercially available and well-calibrated as previously described,15 and neutralising antibodies are strongly correlated with anti-spike antibodies.17 The wider generalisability of the analyses is limited given the cohort included in this analysis was predominantly working-age HCWs with 82% being female and 72% of white ethnicity. However, this cohort had diverse healthcare roles and comorbidities and provides useful data for decision-making related to HCWs. Future work with HCWs could focus on creating risk models that adjust for vaccination status, and ideally markers of vaccination response such as antibody levels.
The study has several implications for occupational health assessment. Most HCWs seroconverted after their second vaccination including those who had a high risk of adverse outcomes from COVID-19, indicating that two vaccinations are generally effective in generating antibody responses among HCWs, such that large-scale antibody testing is not necessary. However, HCWs with ‘very high’ COVID-age risk score had lower antibody levels, suggesting the COVID-age tool may help to identify HCWs at risk of lower antibody levels and prioritise which HCWs require further assessment of vaccine responses. Multiple factors are associated with whether an HCW mounts a sufficient response to COVID-19 vaccination. Assessment of these may be pertinent to decisions regarding workplace controls to support HCWs at high risk working safely. Given the high exposure to SARS-CoV-2, routine antibody assessments among high-risk HCWs, such as immunosuppressed patients or organ transplant recipients, could be important, and further booster vaccinations should be prioritised for these groups to improve their immune response alongside careful use of other protective measures.
Supplementary Material
Acknowledgments
This work uses data provided by healthcare workers and collected by the UK’s National Health Service as part of their care and support. We thank all the people of Oxfordshire who contribute to the Infections in Oxfordshire Research Database. Research Database Team: L Butcher, H Boseley, C Crichton, DW Crook, D Eyre, O Freeman, J Gearing (community), R Harrington, K Jeffery, M Landray, A Pal, TEA Peto, TP Quan, J Robinson (community), J Sellors, B Shine, AS Walker, D Waller. Patient and Public Panel: G Blower, C Mancey, P McLoughlin, B Nichols.
Footnotes
Contributors: The study was designed and planned by WG, EB, SD, KJ, DE and A-MO. This specific analysis was designed by VW, JW and DE. VW and JW contributed to the statistical analysis of the data. VW, JW, DE and A-MO drafted the manuscript. DE is the guarantor of the manuscript and accept full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish. All authors contributed to interpretation of the data and results and revised the manuscript. All authors approved the final version of the manuscript.
Funding: This work was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Oxford University in partnership with the UK Health Security Agency (NIHR200915), and the NIHR Biomedical Research Centre, Oxford. EB is an NIHR Senior Investigator. SD is funded by an NIHR Global Research Professorship (NIHR300791). DE is a Big Data Institute Robertson Fellow.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or the UK Health Security Agency. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: DE declares lecture fees from Gilead, outside the submitted work. No other author has a conflict of interest to declare.
Patient and public involvement: The oversight committee of the research database used by the study has patient and public representation who participated in reviewing and approving a study summary and analysis plan.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data analysed during the current study are not publicly available as they contain personal data but are available from the Infections in Oxfordshire Research Database (https://oxfordbrc.nihr.ac.uk/research-themes-overview/antimicrobial-resistance-and-modernising-microbiology/infections-in-oxfordshire-research-database-iord/), subject to an application and research proposal meeting the ethical and governance requirements of the Database.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants. Following approval from the OUH’s Caldicott guardian, deidentified data were obtained from the Infections in Oxfordshire Research Database (IORD), which has approvals from the National Research Ethics Service South Central–Oxford C Research Ethics Committee (19/SC/0403), the Health Research Authority and the National Confidentiality Advisory Group (19/CAG/0144). Participants gave informed consent to participate in the study before taking part.
References
- 1.Eyre DW, Lumley SF, O'Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020;9:1–37. 10.7554/eLife.60675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platten M, Nienhaus A, Peters C, et al. Cumulative incidence of SARS-CoV-2 in healthcare workers at a general Hospital in Germany during the Pandemic-A longitudinal analysis. Int J Environ Res Public Health 2022;19. doi: 10.3390/ijerph19042429. [Epub ahead of print: 19 02 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szajek K, Fleisch F, Hutter S, et al. Healthcare institutions' recommendation regarding the use of FFP-2 masks and SARS-CoV-2 seropositivity among healthcare workers: a multicenter longitudinal cohort study. Antimicrob Resist Infect Control 2022;11:6. 10.1186/s13756-021-01047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alama . Covid-19 medical risk assessment. Available: https://alama.org.uk/covid-19-medical-risk-assessment/
- 5.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis 2021;21:1529–38. 10.1016/S1473-3099(21)00289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399:924–44. 10.1016/S0140-6736(22)00152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis 2021;73:e699–709. 10.1093/cid/ciab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Çağlayan D, Süner AF, Şiyve N, et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol 2022;94:2212–21. 10.1002/jmv.27620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonfrate D, Piubelli C, Gobbi F, et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: a prospective study. Clin Microbiol Infect 2021;27:1845–50. 10.1016/j.cmi.2021.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021;325:1467–9. 10.1001/jama.2021.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021;26:2100096. 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021;384:533–40. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyre DW, Lumley SF, Wei J, et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect 2021;27:1516.e7–1516.e14. 10.1016/j.cmi.2021.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National SARS-CoV-2 Serology Assay Evaluation Group . Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390–400. 10.1016/S1473-3099(20)30634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Pouwels KB, Stoesser N, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med 2022;28:1072–82. 10.1038/s41591-022-01721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43–50. 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021;27:2032–40. 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 omicron. Nature 2022;602:682–8. 10.1038/s41586-022-04399-5 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell 2022;185:457–66. 10.1016/j.cell.2021.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JH, Kim YR, Heo ST, et al. Healthcare workers in South Korea maintain a SARS-CoV-2 antibody response six months after receiving a second dose of the BNT162b2 mRNA vaccine. Front Immunol 2022;13:238. 10.3389/fimmu.2022.827306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertram S, Blazquez-Navarro A, Seidel M, et al. Predictors of impaired SARS-CoV-2 immunity in healthcare workers after vaccination with BNT162b2. Sci Rep 2022;12:6243. 10.1038/s41598-022-10307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. 10.1001/jama.2021.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields AM, Anantharachagan A, Arumugakani G, et al. Outcomes following SARS-CoV-2 infection in patients with primary and secondary immunodeficiency in the UK. Clin Exp Immunol 2022;209:247–58. 10.1093/cei/uxac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turtle L, Thorpe M, Drake TM, et al. Outcome of COVID-19 in hospitalised immunocompromised patients: an analysis of the who ISARIC CCP-UK prospective cohort study. medRxiv 2022. 10.1101/2022.08.08.22278576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidov Y, Indenbaum V, Tsaraf K, et al. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J Hepatol 2022;77:702–9. 10.1016/j.jhep.2022.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hod T, Ben-David A, Olmer L, et al. BNT162b2 third booster dose significantly increases the humoral response assessed by both RBD IgG and neutralizing antibodies in renal transplant recipients. Transpl Int 2022;35:10239. 10.3389/ti.2022.10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schimpf J, Davidovic T, Abbassi-Nik A, et al. Enhanced SARS-CoV-2 antibody response after a third heterologous vector vaccine Ad26COVS1 dose in mRNA Vaccine-Primed kidney transplant recipients. Transpl Int 2022;36:10357. 10.3389/ti.2022.10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soetedjo NNM, Iryaningrum MR, Lawrensia S, et al. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr 2022;16:102406. 10.1016/j.dsx.2022.102406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marfella R, D'Onofrio N, Sardu C, et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the caveat study. Diabetes Obes Metab 2022;24:160–5. 10.1111/dom.14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Klaauw AA, Horner EC, Pereyra-Gerber P, et al. Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity. medRxiv 2022;7. 10.1101/2022.06.09.22276196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soegiarto G, Wulandari L, Purnomosari D, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine 2022;40:4046–56. 10.1016/j.vaccine.2022.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moncunill G, Aguilar R, Ribes M, et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers. EBioMedicine 2022;75:103805. 10.1016/j.ebiom.2021.103805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herzberg J, Vollmer T, Fischer B, et al. SARS-CoV-2-antibody response in health care workers after vaccination or natural infection in a longitudinal observational study. Vaccine 2022;40:206–12. 10.1016/j.vaccine.2021.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore S, Kronsteiner B, Longet S, et al. Evolution of long-term hybrid immunity in healthcare workers after different COVID-19 vaccination regimens: a longitudinal observational cohort study. medRxiv 2022;28. 10.2139/ssrn.4180810 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066766supp001.pdf (142KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data analysed during the current study are not publicly available as they contain personal data but are available from the Infections in Oxfordshire Research Database (https://oxfordbrc.nihr.ac.uk/research-themes-overview/antimicrobial-resistance-and-modernising-microbiology/infections-in-oxfordshire-research-database-iord/), subject to an application and research proposal meeting the ethical and governance requirements of the Database.