Abstract
Background
Recently, despite the steady decline in the tuberculosis (TB) epidemic globally, school TB outbreaks have been frequently reported in China. This study aimed to quantify the transmissibility of Mycobacterium tuberculosis (MTB) among students and non-students using a mathematical model to determine characteristics of TB transmission.
Methods
We constructed a dataset of reported TB cases from four regions (Jilin Province, Xiamen City, Chuxiong Prefecture, and Wuhan City) in China from 2005 to 2019. We classified the population and the reported cases under student and non-student groups, and developed two mathematical models [nonseasonal model (Model A) and seasonal model (Model B)] based on the natural history and transmission features of TB. The effective reproduction number (Reff) of TB between groups were calculated using the collected data.
Results
During the study period, data on 456,423 TB cases were collected from four regions: students accounted for 6.1% of cases. The goodness-of-fit analysis showed that Model A had a better fitting effect (P < 0.001). The average Reff of TB estimated from Model A was 1.68 [interquartile range (IQR): 1.20–1.96] in Chuxiong Prefecture, 1.67 (IQR: 1.40–1.93) in Xiamen City, 1.75 (IQR: 1.37–2.02) in Jilin Province, and 1.79 (IQR: 1.56–2.02) in Wuhan City. The average Reff of TB in the non-student population was 23.30 times (1.65/0.07) higher than that in the student population.
Conclusions
The transmissibility of MTB remains high in the non-student population of the areas studied, which is still dominant in the spread of TB. TB transmissibility from the non-student-to-student-population had a strong influence on students. Specific interventions, such as TB screening, should be applied rigorously to control and to prevent TB transmission among students.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40249-022-01046-z.
Keywords: Tuberculosis, Transmission, Compartmental model, Occupational-specific dynamics, Student, Non-student, China
Background
Despite widespread implementation of control measures, including pharmaceutical therapy and vaccination, tuberculosis (TB) remains a major cause of disease and death in most high-burden countries. In 2021, most TB cases occurred in the 30 high-burden countries (87%), in which 8 countries account for two-thirds, with China (7.4%) ranking after India (28%) and Indonesia (9.2%) [1]. China is also on the three lists of high-burden countries for TB, HIV-associated TB, and multidrug resistant tuberculosis (MDR-TB) of the World Health Organization (WHO). Despite the difficulties that remain, such as the emergence of drug-resistant strains of Mycobacterium tuberculosis (MTB) and the coinfection of TB with the human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), the global incidence of tuberculosis is estimated to slowly decline by 1.6% per year, far from the 4–5% estimated to be required to reach the objectives of the WHO’s End TB Strategy [2]. Due to the emergence of the COVID-19 pandemic, there is a large global drop in people newly diagnosed with TB and reported in 2020, compared to 2019[3].
In China 2021, the number of reported TB cases is ranked second highest after viral hepatitis, and in terms of death is the second highest after AIDS [4]. There are about 250 million students in China (about 20% of the population). The reported TB cases in students account for about 4–6% of the total reported TB cases [5]. TB cases in the 15–24-year age group accounted for about 85% of the total reported TB cases in students, which means the number of TB cases in high school and college students is higher, especially in the 18-year-old age group [6–8]. When MTB spreads in schools, it can be transmitted rapidly and have a major impact on young people simply because of cluster. Therefore, it is one of the reasons why school TB outbreaks have been reported frequently in China, despite the steady global decline of the TB incidence trend [9–13]. Moreover, MDR-TB outbreaks have also been reported in schools, making TB control in schools much more difficult [14, 15].
Theoretical epidemiology, also known as the mathematical model of epidemiology, uses mathematical formulas to express the law of disease prevalence explicitly and quantitatively between cause, host and environment, and to theoretically explore the effects of different control measures. Mathematical modelling has become a powerful tool for analysing epidemiological characteristics [16], which is used to reveal the characteristics of the internal spread of infectious diseases. Transmission dynamic models are commonly used in infectious disease models, including Susceptible-infectious-recovered model, Susceptible-exposed-symptomatic-recovered (SEIR) model, and Autoregressive integrated moving average model. Some studies use models to analyse TB, such as TB intervention assessments [17], analysis of vaccine control effectiveness [18, 19], and TB treatment [20–23]. Different models have been developed to treat latent TB infections (LTBI) that incorporate certain factors such as drug-resistant strains [24], coinfection with HIV [25], and TB reinfection [26], and to study the epidemiology of TB [27]. Specific targeted sub-populations have been defined, including age-specific subgroups [28], adults and children [29], and smokers and non-smokers [30]. However, only a few studies have used occupational mathematical models to study TB transmission in China. The construction of TB models which are used to explore the dynamics of TB transmission between students and non-students is unclear.
The prevention and control of TB in schools has been improved with the efforts of medical personnel staff at all levels. In the past 10 years, control measures have been continuously strengthened and improved, but the transmission characteristics of TB in schools are still unclear. The aim of this study is to establish a mathematical model of TB between students and non-students, to analyse and explore the transmissibility of MTB in schools, and then to take reasonable and effective measures to control TB in schools.
Methods
Study design
In this study, based on the reported and observed morbidity characteristics, we developed a SEIR model with two occupational groups (students and non-students). We investigated the role of occupation in the transmission process and evaluated feasible control strategies to achieve the objectives outlined in the WHO End TB Strategy [3]. Furthermore, this study classified active TB patients into high or low transmissibility groups according to their pathogenic status [31].
Firstly, in this study, a dataset was constructed, including basic information (sex, age, occupation, and location) and case classification (positive and negative cases of pathogen). Demographic data was obtained from the Chinese Statistical Yearbook [32–35], including the total population, the total student population, birth rate, and the death rate for each city (Additional file 1: Table S1).
Secondly, two mathematical models (Models A and B refer to non-seasonal and seasonal models, respectively) were constructed to simulate the reported TB cases of the four regions in China, based on the natural history and seasonality of TB. In each model, we divided the collected data into four subpopulations of active diseases in two dimensions. The first dimension for all calculations and outputs was the occupation of students (1 subscript) or non-students (2 subscript), while the second dimension was the pathogenic status, including pathogen positive (p subscript) and pathogen negative (n subscript) pulmonary disease. In addition, goodness-of-fit was conducted to evaluate the effectiveness of model fitting.
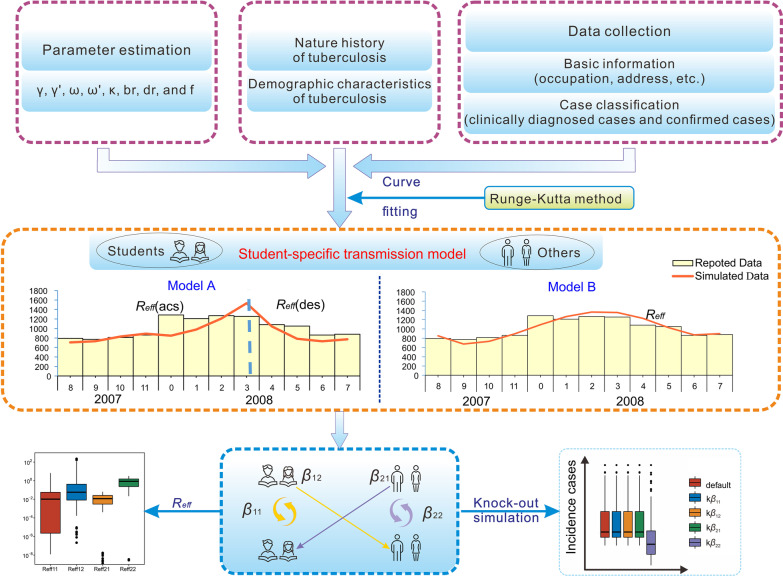
Finally, we simulated the sub-population-to-sub-population transmission process, to determine the combination with the most distinctive impact, via calculating effective reproduction number (Reff) and performing knock-out analysis. This enabled the formulation of effective and targeted control measures for TB transmission in China, in accordance with occupation-specific prevention and control (Fig. 1).
Fig. 1.
Study design for analysing the transmissibility of TB among students and non-students. The four subscripts are denoted as follows: TB transmission in student groups (11 subscript), TB transmission of student-to-non-student groups (12 subscript), TB transmission in non-student groups (22 subscript), TB transmission of non-student-to-student groups (21 subscript)
Data collection
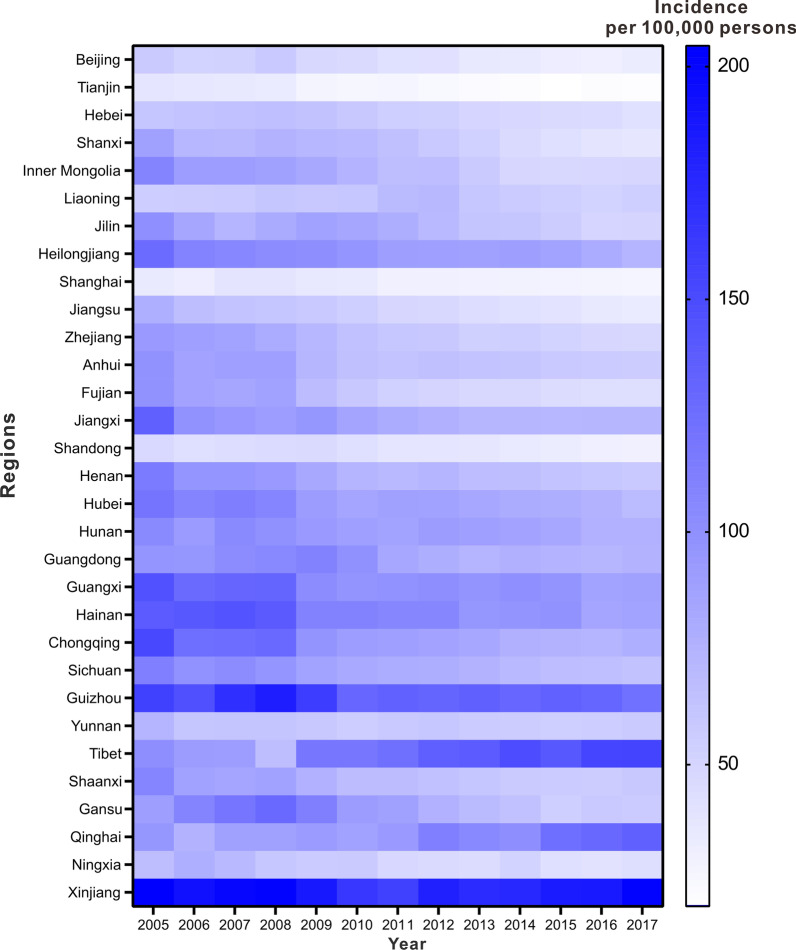
We collected year-based TB incidence data from the China Public Health Science Data Center (http://www.phsciencedata.cn/Share/index.jsp) from January 1, 2005 to December 31, 2017 for each province in China (not included Hong Kong, Macao, and Taiwan) [36]. After we calculated the average annual incidence rate and plotted the incidence map (Fig. 2), we found an inequality in the disease burden.
Fig. 2.
Regional and national distribution of reported TB incidence. Reported TB incidence in different regions (not included Hong Kong, Macao, and Taiwan) in China from 2005 to 2017
Considering the geographic position, the TB incidences in four regions (located in the north, south, southwest, and middle of China) are average incidences compared to those of the other regions in China, which is consistent with the population distribution [37], which means that the selection can better reflect the TB epidemiological characteristics in geographical differences.
This study collected data on reported TB cases, populations, and areas in four regions [Jilin Province, Wuhan City in Hubei Province, Chuxiong Yi Autonomous Prefecture (Chuxiong Prefecture) in Yunnan Province, and Xiamen City in Fujian Province] (Additional file 1: Table S1), which are from the Health Commission of each region, the Statistics Bureau of each region and data mentioned in some researches [38, 39], etc. Therefore, the TB results from these four regional analyses are effectively representative of TB epidemiological characteristics in China.
Classification of TB patients
Reported TB cases included in this study consisted of laboratory confirmed pulmonary tuberculosis (PTB), and clinical diagnostic PTB. Since the Chinese government implemented the National Notifiable Disease Surveillance System (NNDSS) for infectious diseases in 2004, the diagnostic criteria for TB has changed several times (“WS288–2008 Diagnosis of tuberculosis” [40] with the adjusted notice in 2017 [41], and the “WS 288–2017 Diagnosis of Tuberculosis” [42] and the “WS 196–2017 Classification of Tuberculosis” standards [43], with the adjusted notice in 2018 [31]). All TB cases were classified on the base of the following criteria. The confirmed PTB cases were denoted as people with possible PTB symptoms, such as a continuous cough for more than 2 weeks, hemoptysis, and night sweat, and confirmed by a sputum smear and/or a sputum culture with the result of detectable acid-fast bacilli or positive result from a rapid molecular diagnostic instrument (e.g., GeneXpert). The clinical diagnosis of PTB was defined as people with obviously abnormal chest radiography along with no curative effect from anti-inflammatory treatment under the circumstance of negative results from laboratory tests or absence of related results [44–46].
The PTB cases are classified as follows, based on pathogenic findings: sputum smear positive, sputum smear negative, sputum culture positive, sputum culture negative, molecular biology positive, and without sputum PTB [42]. In the latest notice published in 2018, the classification of TB cases, which must be reported in the NNDSS, was adjusted to “pathogen positive (including sputum smear positive and only sputum culture positive PTB)”, “pathogen negative (including sputum smear negative PTB)”, “rifampicin resistant”, “no pathogenic findings (including without sputum PTB and tuberculous pleurisy” [31]. We have reclassified all historical data according to the new classification notice for consistency (see detail in Additional file 2: Table S2).
Diagnosis criteria of PTB patients
The diagnosis of PTB is based on a pathogenic examination (including bacteriology and molecular biology), combined with epidemiological history, clinical manifestations, chest imaging, relevant auxiliary examinations, differential diagnosis, and other comprehensive analyses [47]. Pathogenic and pathological results were used as the basis for diagnosis. Therefore, the following inclusion criteria were TB cases with pathogen positive [“positive cases with MTB detected by sputum smear, culture-confirmed or molecular biology (nucleic acid of MTB)”] and negative [“TB cases without MTB detected (including patients with negative sputum smear and without sputum)”]. The rifampicin resistance category was officially reported in 2019 and represented a small percentage (< 5%) of the total data collected. Therefore, to maintain the consistency of the overall data, we excluded these data from the analysis.
Occupational-specific transmission model of TB
Based on the model, the total population (N) was divided into the following five compartments: susceptible population (S), exposed population (or low-risk latent tuberculosis infection, LTBI) (E), pathogen positive tuberculosis population (Ip), pathogen negative tuberculosis population (In), and recovered or removed population (R).
Susceptible population (S): people who have not been exposed to MTB or those who experienced self-clearance by their own immune system. The latter is a state in which the bacteria in the body cannot replicate to the extent that self-clearance occurs due to the strong immunity of the body after exposure, a state in which the body has a sustained immune response to MTB antigen stimulation.
Exposed population (or low-risk LTBI) (E): A susceptible population is exposed to MTB through contact with a highly infectious or less infectious population and is in an MTB carrier state but is temporarily noninfectious.
Pathogen positive TB population (Ip): positive cases with MTB detected by sputum smear, culture-confirmed, or molecular biology (nucleic acid of MTB).
Pathogen negative TB population (In): TB cases without MTB detected (including patients with negative sputum smear and no sputum), with low infectiousness.
Recovered or removed population (R): This is a state of cure or recovery, noninfectious and asymptomatic, referring to the population undergoing successful treatment, including the treatment success for the “pathogen negative” population and the “pathogen positive” population (both the cured and the treatment success population).
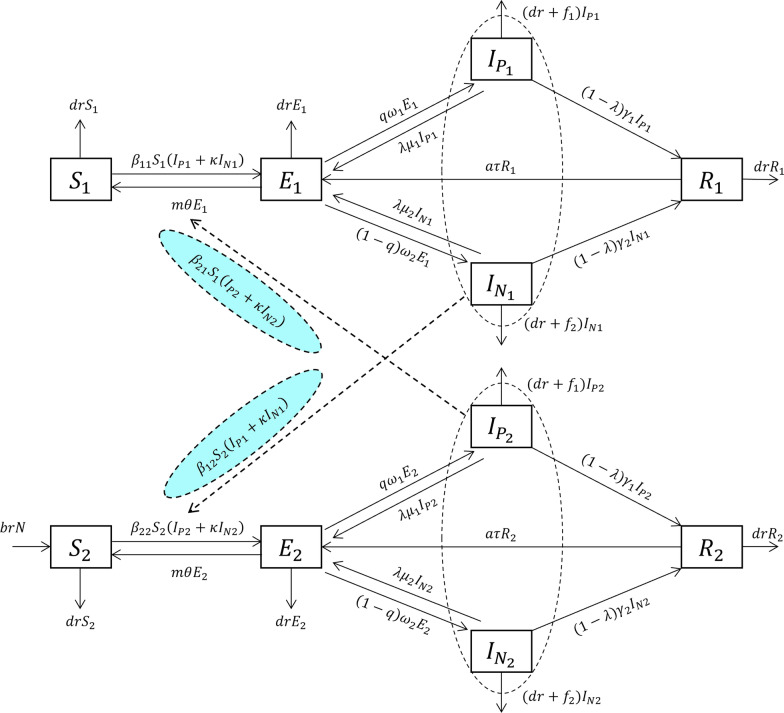
Based on the natural history of TB, we developed a mathematical model Susceptible-exposed-symptomatic (pathogen positive)-symptomatic (pathogen negative)-recovered (SEIpInR) model to investigate the transmission process of TB. The proposed SEIpInR model is based on the following facts and assumptions:
Births and natural deaths change the total population (N); the birth rate and the death rate are br and dr, respectively. The entire birth population enters group 2(the non-student group).
This population is generally susceptible to MTB infection. When an infected individual is exposed, the exposed population (E) progresses to the active TB-infected population (I) at a rate of β. Since the transmissibility of the pathogen positive TB population (Ip) is higher than the pathogen negative TB population (In), the transmissibility of In is set to be a к times (к < 1) compared to Ip.
Approximately 5–10% of the exposed population (E) infected with MTB will develop symptoms and become Ip or In; both belong to the active TB-infected population and will receive treatment. Most exposed people do not develop symptoms, but undergo a process of self-clearance and become a susceptible population (S). If E progresses to Ip at a rate of ω1 (incubation period coefficient) with a scale factor of q, and E progresses to In at a rate of ω2 (latent period coefficient) with a scale factor of (1-q). The progression from E to S occurs at a rate of θ (early self-clearance rate) on a scale factor of m. At time t, the progression from E to Ip, from E to In and from E to S is proportional to the number of exposed populations, which is qω1E, (1-q)ω2E, and mθE, respectively.
Studies have shown that the proportion of patients with TB cured by the directly observed treatment and short course chemotherapy (DOTS) who require retreatment in the next 1–2 years is 2 to 7% [48, 49]. Patients who are retreated can be broadly divided into two categories: those who were not successfully cured following treatment, and those who relapsed after being cured.
There were two outcomes for the Ip compartment: First, a certain proportion of treatment success individuals (1-λ) transform into a recovered or removed population (R), while another proportion of treatment failure individuals (λ) transform into an exposed population (E). At time t, the rate of development from Ip to R, which is proportional to the Ip population, is given as (1-λ)γ1Ip, while the rate of development from Ip to E, which is proportional to the E population, is given as λμ1Ip. γ1 is the removal coefficient, whereas μ1 is the coefficient of development from Ip to E. Similarly, the rate of development to R in the In compartment is given as (1-λ)γ2In, while the rate of development to E is given as λμ2In. γ2 is the removal coefficient and μ2 is the coefficient of development of In to E.
The people in the Ip and In compartments recover or are removed (R) after successful treatment (completion of treatment for In and Ip [50]). Reinfection occurs after the completion of treatment or cured, that is, the active TB-infected population (Ip, In) returns to the exposed population (E).
Reactivation (or relapse) is often associated with immunodeficiency, such as the onset of disease due to HIV/TB coinfection or low resistance, such as severe cold. If people in the R compartment develop into E with a relapse ratio a where τ represents the relapse coefficient, the rate of development from R to E at time t is proportional to R, which is aτR.
The pathogen positive TB population (Ip) and the pathogen negative TB population (In) die of disease, in addition to natural deaths. Suppose the fatality rates for Ip were f1 and that for In were f2; then, at time t, the death rates for Ip and In are f1Ip and f2In, respectively.
The student population was set as S1, E1, Ip1, In1, and R1, whereas the non-student population was set as S2, E2, Ip2, In2, and R2. Interactions were observed between students and non-students. We defined the relative transmission rate of student-to-student as β11, non-student-to-non-student as β22, student-to-non-student as β12, and non-student-to-student as β21. Therefore, the number of newly emerging infections was β11S1(Ip1 + кIn1) from the student-to-student population, β22S2(Ip2 + кIn2) from the non-student-to-non-student population, β12S2(Ip1 + кIn1) from the student-to-non-student population, and β21S1(Ip2 + кIn2) from the non-student-to-student population.
A framework diagram of the SEIpInR model is shown in Fig. 3. The mathematical expression of the differential equation of the SEIpInR model are as follows:
Fig. 3.
Flowchart of the SEIpInR model. The four occupational compartments are denoted as follows: pathogen positive students (Ip1 subscript), pathogen positive non-students (Ip2 subscript), pathogen negative students (In1 subscript) and pathogen negative non-students (In2 subscript)
Parameter estimation
Fourteen parameters were obtained from references or actual data in this model: birth rate (br), death rate (dr), transmission relative rate (β), proportion of early clearance (m), rate of early clearance (θ), transmission relative rate between pathogen negative and positive TB population (к), proportion of exposed to TB population (q), rate of exposed to TB population (ω), proportion of treatment failure (λ), rate from TB population to exposed population (μ), TB population removal coefficient (γ), Case fatality rate of TB population (f), recurrence ratio- proportion of recovered or removed population developing into exposed population (a), and reciprocal of time to recurrence rate at which recovered or removed population progresses to exposed population (τ).
Parameter β was derived from the curve-fitting results. Some parameters (br, dr, and q) were obtained from actual data, while other parameters were obtained from the literature. The description of each variable and parameter in this model is detailed in Table 1.
Early self-clearance (early clear) was defined as a persistent negative interferon-gamma release test (IGRA) (patients with pathogenically positive TB were tested at baseline and after 14 weeks). Studies in Indonesia have shown that early self-clearance is 25% [51]. The time to self-clearance was set at 14 weeks, which is the interval between the two IGRA tests; thus θ = 1/ (14/4) = 0.286.
Treatment failure: The WHO 2021 TB report [3] showed the treatment success rate was 95.9% in 2019 and 95.7% in 2020. Previous reports revealed that this value did not change much between 2000 and 2020. Therefore, we considered the treatment success rate as 95% and set the treatment failure rate (λ) to 5%, that is λ = 0.050. The conventional treatment course was 6 months. Therefore, the time to complete the treatment was set as 6 months, that is, μ1 = μ2 = 1/6 = 0.167 [52].
Relapse: Studies [53–55] in China showed the relapse rate was 5.3–6.9%. Therefore, the median was chosen and the relapse proportion was set at a = 0.062 (recurrence ratio). A domestic study [53] showed that the median time from the first attack to relapse in TB patients was 1.3 years [interquartile range (IQR) 0.6–2.8 years]. Therefore, the relapse rate was established at 1/(1.3*12), i.e., τ = 0.064 (reciprocal of time to recurrence).
The transmissibility coefficient of In relative to Ip was set as κ to 0.2 [56] with reference to the actual data and the relevant literature.
After approximately two weeks of effective treatment, TB cases with a nondrug-resistant active infection usually do not remain infectious to others and become low in infection status [57]. Short-course (3-to 4-month) rifamycin-based treatment regimens are preferred over longer-course (6 to 9 months) isoniazid monotherapy for the treatment of low-infection cases of TB [8]. Therefore, we set the duration of the illness at 14 weeks (average value 3–4 month), γ1 = γ2 = 1/(14/4) = 0.286.
The birth rate (br) and the death rate (dr) for each year in each region were obtained from the statistical offices of each study area.
Table 1.
The description and features of estimated parameters
| Parameter | Description | Unit | Value | Source |
|---|---|---|---|---|
| br | Birth rate | 1 | Null | Reported data |
| dr | Death rate | 1 | Null | Reported data |
| β | Transmission relative rate | Per person. per month | Null | Curve fitting |
| β11 | Transmission relative rate among students | Per person. per month | Null | Curve fitting |
| β22 | Transmission relative rate among non-students | Per person. per month | Null | Curve fitting |
| β12 | Transmission relative rate from students to non-students | Per person. per month | Null | Curve fitting |
| β21 | Transmission relative rate from non-students to students | Per person. per month | Null | Curve fitting |
| κ | Transmission relative rate between population In and population Ip | 1 | 0.2 | Reference [56] |
| m | Proportion of early clearance | 1 | 0.25 | Reference [51] |
| θ | Rate of early clearance | Per month | 0.286 | Reference [51] |
| q | Proportion from E to Ip | 1 | Null | Reported data |
| 1-q | Proportion from E to In | 1 | Null | Reported data |
| ω1 | Rate from E to I1 | Per month | 0.667 | Reference [93] |
| ω2 | Rate from E to I2 | Per month | 0.667 | Reference [93] |
| λ | Proportion of treatment failure | 1 | 0.05 | Reference [3] |
| μ1 | Rate from I1 to E (reciprocal time to retreatment) | Per month | 0.167 | Reference [52] |
| μ2 | Rate from I2 to E (reciprocal time to retreatment) | Per month | 0.167 | Reference [52] |
| γ1 | I1 removal coefficient | Per month | 0.286 | Reference [57] |
| γ2 | I2 removal coefficient | Per month | 0.286 | Reference [57] |
| f1 | Case fatality rate of I1 | 1 | 0.1284 | References [94, 95] |
| f2 | Case fatality rate of I2 | 1 | 0.1284 | Reference [94, 95] |
| a | Proportion of R developing into E (recurrence ratio) | 1 | 0.062 | References [53–55] |
| τ | Rate at which R progresses to E (reciprocal of time to recurrence) | Per month | 0.064 | Reference [53] |
E for the exposed population (or low-risk latent tuberculosis infection, LTBI), Ip for pathogen positive tuberculosis population, In for pathogen negative tuberculosis population, I1 for student tuberculosis population, I2 for non-student tuberculosis population, and R for recovered or removed population
Transmissibility index
The basic reproduction number (R0) is an important parameter for determining the infectiousness of a disease. R0 refers to the number of new cases expected from an infected case in a susceptible population during an average infectious period. We set Reff as the evaluation index, which denotes R0 after intervention measures were taken, to evaluate the impact of intervention measures on the relative transmissibility of MTB in the population.
In this study, Reff was calculated using the next-generation matrix method, and all source codes are accessible at GitHub (https://github.com/rorschachkwok/tb_reff). In this study, Reff1 represents the transmissibility of the population of students with active TB [sum of transmissibility from student cases to student cases (Reff11) and from student cases to non-student cases (Reff12)], while Reff2 represents the transmissibility of the population of non-student active TB cases [sum of transmissibility from non-student cases to non-student cases (Reff22) and from non-student cases to student cases (Reff21)].
Simulation methods and statistical analysis
Berkeley Madonna 8.3.18 (developed by Robert Macey and George Oster of the University of California in Berkeley. Copyright ©1993–2001 Robert I. Macey & George F. Oster) was used to fit the curves of the incidence data. The estimated model coefficients and the simulation of the intervention effects were also generated using this software. The curving fit was performed using the fourth order Runge–Kutta method to obtain the key parameter values: student-to-student (β11), non-student-to-non-student (β22), student-to-non-student (β12), and non-student-to-student (β21) transmission rates.
To consider the potential seasonality transmission of TB, although seasonality remains unclear, we developed two models in this study, which are described as follows:
Model A: seasonality excluded.
In Model A, the epidemic curve for each year was divided into ascending and descending periods according to the characteristics of the reported number of TB cases (Fig. 1). The SEIpInR model without seasonality was adopted to fit the data in each period, and the corresponding transmission relative rates (β, β11, β12, β22, and β21), the ascending and descending Reff (Reff(asc) and Reff(des), respectively) were calculated.
Model B: seasonality included.
In Model B, we used a seasonality function in the SEIpInR model to fit the reported TB epidemic curve (Fig. 1), which is shown as follows:
In this equation, βt, β0, c, and T refer to the transmission rate at time t, the transmission rate at time = 0, the correcting value of time (month) and the potential seasonality cycle, respectively.
The goodness-of-fit test was performed between the fitted results and the collected data by calculating the R2 and P values. Key parameters (β11, β12, β22, β21) were knocked out, and the cumulative number of cases was calculated to assess the main parameter affecting transmissibility. SPSS Statistics for Windows, version 13.0 (SPSS Inc., Chicago, Ill, USA) was used to perform statistical analyses. The coefficient of determination (R2) was used to evaluate the curve fitness.
Results
Epidemiology of tuberculosis in the four regions
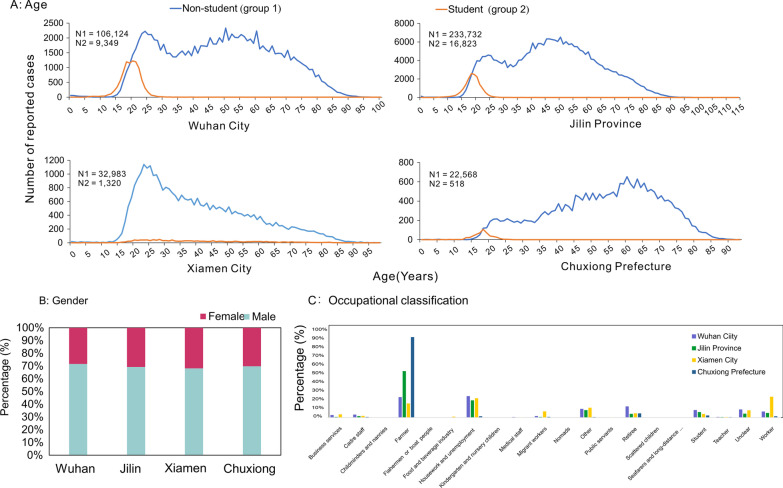
The age range of the TB patients was between 15 and 90 years, with two peaks in the incidence of TB: a large and a small peak in the age groups 35–90 and 15–35, respectively. In Wuhan City, Jilin Province and Chuxiong Prefecture, there were two age distribution peaks of non-student TB patients (15–35 and 45–60 years group), while in Xiamen City, there was only one peak (15–35 years group). Student patients with TB were among 15–25 year group (Fig. 4A). Most patients with TB in the four regions were male, with a male-to-female ratio of 7∶3 (Fig. 4B). The Chinese Infectious Disease Report Card categorises cases into 18 categories, and the top six occupations (88.4% of the total cases) in the four regions were: farmers, housework and unemployment, others, workers, students, and retirees. Among these four regions, the top three occupations of TB patients in Wuhan were domestic and unemployed (23.2%), farmers (22.2%), and retirees (12.1%). The top three occupations of TB patients were farmers (50.3%), domestic and unemployed (18.6%), and others (8.0%) in Jilin Province; workers (22.5%), farmers (15.3%), and others (10.6%) in Xiamen; farmers (87.2%), retirees (4.5%), and students (2.2%) in Chuxiong Prefecture (Fig. 4C). The ranking of students with TB was sixth, sixth, eighth and third in Wuhan, Jilin, Xiamen, and Chuxiong Prefecture, respectively.
Fig. 4.
Age, gender, and occupation distributions: A Wuhan City, B Jilin Province, C Xiamen City, and D Chuxiong Prefecture
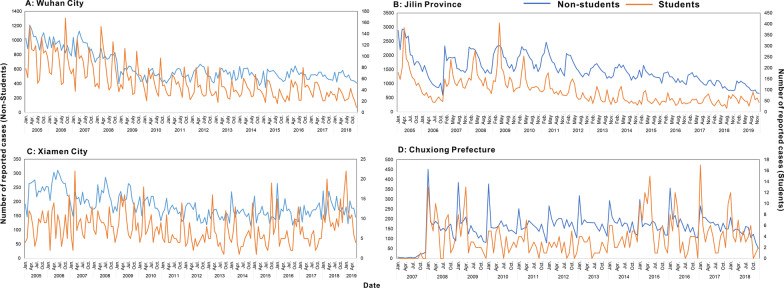
The number of reported TB cases in Wuhan City and Jilin Province showed a decreasing annual trend, while the number of reported TB cases in Xiamen City and Chuxiong Prefecture showed a slight fluctuation trend (Fig. 5). The incidence in the student population was distinctly low during the winter holidays (January–February, approximately 30 days) and summer vacation (July–August, approximately 60 days), with one or two distinct peaks after returning to school (the remaining months of the year). There were slight differences between regions in the time of occurrence of these peaks: Wuhan (March and September–October), Xiamen (March and October), Jilin (April and September), and Chuxiong (April and October). However, for the non-student population, there were no clear lows or peaks.
Fig. 5.
Temporal distribution by month: A Wuhan City, B Jilin Province, C Xiamen City, and D Chuxiong Prefecture
Most cases had either positive or negative pathogen results (87.3%), and the ratio was 1∶1.13. The proportion of cases without pathogenic findings was 12.6%; rifampicin resistant results accounted for 0.1%. The number of pathogen positive and negative cases was essentially the same in Jilin Province, while the other three regions reported more pathogen negative cases than positive. The proportion of patients without pathogenic findings was the lowest in Xiamen City and the highest in Chuxiong Prefecture. Very few cases of resistance to rifampicin were reported in any region (Fig. 6).
Fig. 6.
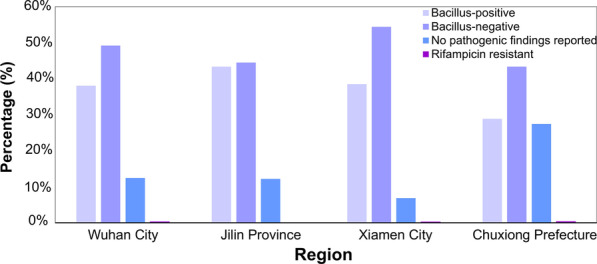
Proportions of patients reporting pulmonary tuberculosis in the four study areas
Curve fitting
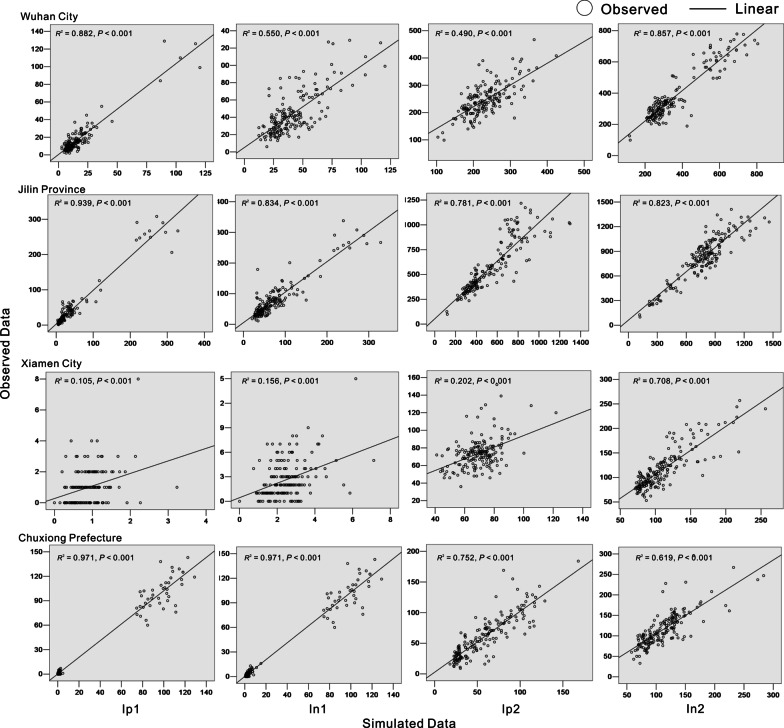
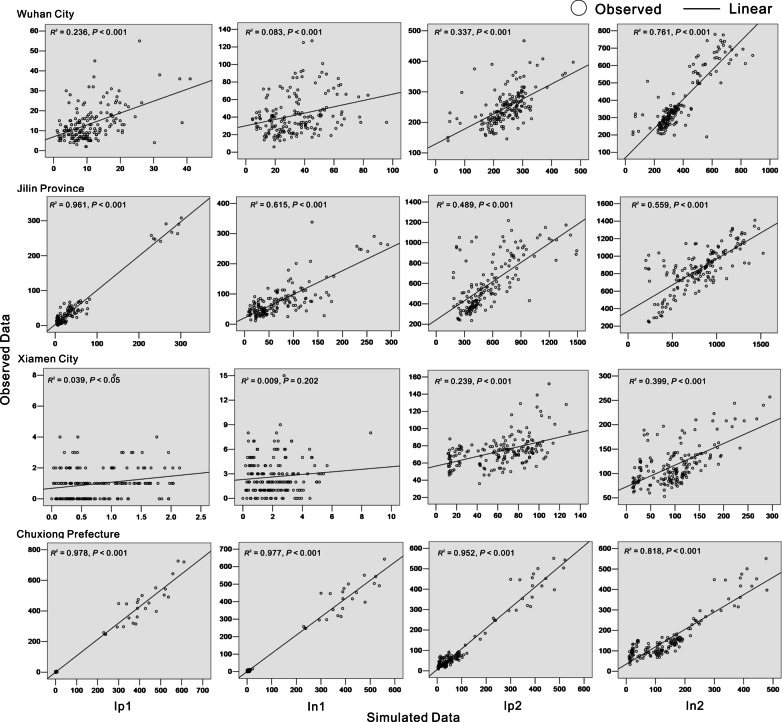
We conducted goodness-of-fit tests for the two models based on the case datasets from the four regions (Wuhan City, Jilin Province, Chuxiong Prefecture, and Xiamen City) (Figs. 7 and 8). R2 values were calculated for the four model groups (pathogen positive cases in the student group, Ip1; pathogen negative cases in the student group, In1; pathogen positive cases in the non-student group, Ip2; and pathogen negative cases in the non-student group, In2). The values showed that, although the two established TB models fitted well with the trend of TB incidence rates (Table 2), Model A had better fitting results than Model B.
Fig. 7.
Plot of goodness-of-fit results for the non-seasonal model (Model A)
Fig. 8.
Plot of goodness-of-fit results for the seasonal model (Model B)
Table 2.
Goodness-of-fit test results of the two models (Models A and B) in the study areas
| Region | Ip1 | In1 | Ip2 | In2 | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | |
| Model A | ||||||||
| Wuhan City | 0.882 | < 0.001 | 0.55 | < 0.001 | 0.49 | < 0.001 | 0.857 | < 0.001 |
| Jilin Province | 0.939 | < 0.001 | 0.834 | < 0.001 | 0.781 | < 0.001 | 0.823 | < 0.001 |
| Xiamen City | 0.105 | < 0.001 | 0.156 | < 0.001 | 0.202 | < 0.001 | 0.708 | < 0.001 |
| Chuxiong Prefecture | 0.971 | < 0.001 | 0.971 | < 0.001 | 0.752 | < 0.001 | 0.619 | < 0.001 |
| Model B | ||||||||
| Wuhan City | 0.236 | < 0.001 | 0.083 | < 0.001 | 0.337 | < 0.001 | 0.761 | < 0.001 |
| Jilin Province | 0.961 | < 0.001 | 0.615 | < 0.001 | 0.489 | < 0.001 | 0.559 | < 0.001 |
| Xiamen City | 0.039 | 0.009 | 0.009 | 0.202 | 0.239 | < 0.001 | 0.399 | < 0.001 |
| Chuxiong Prefecture | 0.978 | < 0.001 | 0.977 | < 0.001 | 0.952 | < 0.001 | 0.818 | < 0.001 |
Correlation between the simulated and observed data was tested using R2 and p values. We divided all the compartments representing active diseases (I) into four occupational compartments: pathogen positive students (Ip1 subscript), pathogen positive non-students (Ip2 subscript), pathogen negative students (In1 subscript) and pathogen negative non-students (In2 subscript)
Transmissibility for interactions among the four groups
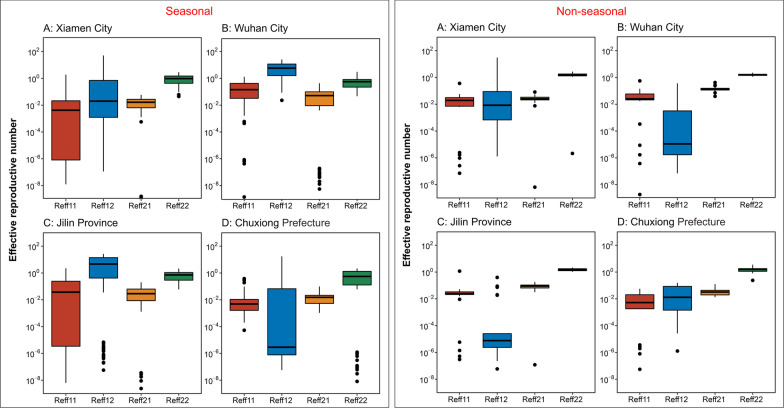
The results of Reff among and between the different populations in each region are shown in Fig. 9.
Fig. 9.
The chart of effective regeneration number plotted according to the two models
In Wuhan City, the median Reff for TB among the mixed population was 1.79 (IQR: 1.56–2.02). Most TB transmissions occurred due to the high transmission in non-student populations, including among non-student populations [median Reff22 was 1.57 (IQR: 1.41–1.72)] and non-student-to-student populations [median Reff21 was 0.14 (IQR: 0.11–0.15)], with a median Reff2 of 1.71(IQR: 1.54–1.87). The values of Reff22 and Reff21 slowly descended from 2005 to 2018. The values of Reff12 and Reff11 were nearly zero excluding in 2006 (Reff12 was 6.39, Reff11 was 0.19) and 2013 (Reff11 was 0.58) (Table 3).
Table 3.
Reff of Model A between students and non-students (Wuhan City)
| Year | Reff11 | Reff 12 | Reff 22 | Reff 21 | Reff1 | Reff2 |
|---|---|---|---|---|---|---|
| 2005 (asc) | 0.04 | 0.00 | 2.12 | 0.24 | 0.04 | 2.37 |
| 2005 (des) | 0.08 | 0.00 | 1.73 | 0.13 | 0.08 | 1.87 |
| 2006 (asc) | 0.05 | 0.36 | 1.62 | 0.16 | 0.41 | 1.79 |
| 2006 (des) | 0.14 | 0.00 | 1.75 | 0.16 | 0.14 | 1.91 |
| 2007 (asc) | 0.06 | 0.00 | 1.86 | 0.14 | 0.07 | 2.00 |
| 2007(des) | 0.04 | 0.00 | 1.49 | 0.13 | 0.04 | 1.62 |
| 2008 (asc) | 0.02 | 0.00 | 2.27 | 0.42 | 0.02 | 2.68 |
| 2008 (des) | 0.02 | 0.00 | 1.41 | 0.14 | 0.02 | 1.55 |
| 2009 (asc) | 0.10 | 0.00 | 1.79 | 0.27 | 0.10 | 2.05 |
| 2009 (des) | 0.00 | 0.00 | 1.12 | 0.14 | 0.00 | 1.26 |
| 2010 (asc) | 0.06 | 0.00 | 1.75 | 0.20 | 0.06 | 1.94 |
| 2010 (des) | 0.03 | 0.00 | 1.20 | 0.12 | 0.03 | 1.32 |
| 2011 (asc) | 0.03 | 0.00 | 1.62 | 0.15 | 0.03 | 1.77 |
| 2011(des) | 0.02 | 0.00 | 1.43 | 0.11 | 0.02 | 1.54 |
| 2012 (asc) | 0.02 | 0.00 | 1.57 | 0.10 | 0.03 | 1.67 |
| 2012 (des) | 0.02 | 0.00 | 1.45 | 0.08 | 0.02 | 1.53 |
| 2013 (asc) | 0.55 | 0.01 | 1.61 | 0.08 | 0.56 | 1.69 |
| 2013 (des) | 0.03 | 0.00 | 1.57 | 0.12 | 0.03 | 1.69 |
| 2014 (asc) | 0.02 | 0.00 | 1.59 | 0.12 | 0.02 | 1.71 |
| 2014 (des) | 0.02 | 0.00 | 1.25 | 0.12 | 0.02 | 1.37 |
| 2015 (asc) | 0.00 | 0.02 | 1.57 | 0.15 | 0.02 | 1.72 |
| 2015 (des) | 0.02 | 0.00 | 1.29 | 0.09 | 0.02 | 1.38 |
| 2016 (asc) | 0.00 | 0.00 | 1.62 | 0.13 | 0.00 | 1.75 |
| 2016 (des) | 0.06 | 0.00 | 1.41 | 0.15 | 0.06 | 1.56 |
| 2017 (asc) | 0.11 | 0.01 | 1.71 | 0.16 | 0.11 | 1.87 |
| 2017 (des) | 0.00 | 0.03 | 1.36 | 0.12 | 0.03 | 1.47 |
| 2018 (asc) | 0.00 | 0.07 | 1.56 | 0.04 | 0.07 | 1.60 |
| 2018 (des) | 0.02 | 0.00 | 1.21 | 0.07 | 0.02 | 1.28 |
| Median | 0.06 | 0.02 | 1.57 | 0.14 | 0.07 | 1.71 |
| P25 | 0.02 | 0.00 | 1.41 | 0.11 | 0.02 | 1.54 |
| P75 | 0.06 | 0.00 | 1.72 | 0.15 | 0.07 | 1.87 |
Reff11 denotes the transmissibility of MTB from student cases to student cases. Reff 12 denotes the transmissibility of MTB from student cases to non-student cases. Reff 21 denotes the transmissibility of MTB from non-student cases to student cases. Reff 22 denotes the transmissibility of MTB from the non-student cases to non-student cases. Reff1 represents the transmissibility of the population of students with active TB cases (sum of Reff11 and Reff 12), whereas Reff 2 represents the transmissibility of the population of non-student active TB cases (sum of Reff 22 and Reff 21)
asc denotes the ascending Reff (Reff(asc)). des denotes the descending Reff (Reff(des))
In Jilin Province, the median Reff for TB among the mixed population was 1.75 (IQR: 1.37–2.02). Most TB transmission occurred due to the high transmission in the non-student population, including among non-student populations [median Reff22 was 1.57, (IQR: 1.27–1.77)] and from non-student-to-student populations [median Reff21 was 0.09, (IQR: 0.07–0.11)], with a median Reff2 of 1.66 (IQR: 1.35–1.89). The Reff21 and Reff12 values maintained stable fluctuations at values lower than 1 from 2007 to 2019. The value of Reff11 was nearly zero excluding in 2009, when it reached 1.19 (Reff(asc) 1.17, Reff(des) 0.02) (Table 4).
Table 4.
Reff of Model A between students and non-students (Jilin Province)
| Year | Reff11 | Reff 12 | Reff 22 | Reff 21 | Reff1 | Reff2 |
|---|---|---|---|---|---|---|
| 2007 (asc) | 0.00 | 0.00 | 1.27 | 0.03 | 0.00 | 1.30 |
| 2007 (des) | 0.01 | 0.00 | 1.22 | 0.11 | 0.01 | 1.33 |
| 2008 (asc) | 0.05 | 0.00 | 1.80 | 0.18 | 0.05 | 1.98 |
| 2008 (des) | 0.00 | 0.00 | 1.23 | 0.11 | 0.00 | 1.35 |
| 2009 (asc) | 1.17 | 0.02 | 1.60 | 0.00 | 1.19 | 1.60 |
| 2009 (des) | 0.02 | 0.09 | 1.16 | 0.09 | 0.10 | 1.26 |
| 2010 (asc) | 0.02 | 0.00 | 1.53 | 0.12 | 0.02 | 1.65 |
| 2010 (des) | 0.00 | 0.02 | 1.25 | 0.09 | 0.02 | 1.34 |
| 2011 (asc) | 0.05 | 0.00 | 1.99 | 0.15 | 0.05 | 2.14 |
| 2011 (des) | 0.02 | 0.00 | 1.28 | 0.08 | 0.02 | 1.36 |
| 2012 (asc) | 0.03 | 0.00 | 1.68 | 0.10 | 0.03 | 1.78 |
| 2012 (des) | 0.02 | 0.00 | 1.25 | 0.06 | 0.02 | 1.31 |
| 2013 (asc) | 0.03 | 0.00 | 1.84 | 0.09 | 0.03 | 1.93 |
| 2013 (des) | 0.03 | 0.00 | 1.68 | 0.09 | 0.03 | 1.76 |
| 2014 (asc) | 0.04 | 0.00 | 2.02 | 0.11 | 0.04 | 2.12 |
| 2014 (des) | 0.02 | 0.07 | 1.54 | 0.05 | 0.10 | 1.59 |
| 2015 (asc) | 0.04 | 0.00 | 2.04 | 0.10 | 0.04 | 2.13 |
| 2015 (des) | 0.02 | 0.00 | 1.56 | 0.06 | 0.02 | 1.62 |
| 2016 (asc) | 0.04 | 0.00 | 2.16 | 0.13 | 0.04 | 2.30 |
| 2016 (des) | 0.03 | 0.00 | 1.58 | 0.06 | 0.03 | 1.64 |
| 2017 (asc) | 0.03 | 0.00 | 2.02 | 0.09 | 0.03 | 2.11 |
| 2017 (des) | 0.03 | 0.00 | 1.54 | 0.09 | 0.03 | 1.63 |
| 2018 (asc) | 0.02 | 0.08 | 1.60 | 0.06 | 0.10 | 1.66 |
| 2018 (des) | 0.02 | 0.00 | 1.48 | 0.11 | 0.02 | 1.59 |
| 2019 (asc) | 0.00 | 0.40 | 1.40 | 0.09 | 0.40 | 1.49 |
| 2019 (des) | 0.02 | 0.00 | 1.04 | 0.09 | 0.02 | 1.13 |
| Median | 0.07 | 0.03 | 1.57 | 0.09 | 0.09 | 1.66 |
| P25 | 0.02 | 0.00 | 1.27 | 0.07 | 0.02 | 1.35 |
| P75 | 0.03 | 0.00 | 1.77 | 0.11 | 0.05 | 1.89 |
Reff11 denotes the transmissibility of MTB from student cases to student cases. Reff 12 denotes the transmissibility of MTB from student cases to non-student cases. Reff 21 denotes the transmissibility of MTB from non-student cases to student cases. Reff 22 denotes the transmissibility of MTB from the non-student cases to non-student cases. Reff1 represents the transmissibility of the population of students with active TB cases (sum of Reff11 and Reff 12), whereas Reff 2 represents the transmissibility of the population of non-student active TB cases (sum of Reff 22 and Reff 21)
asc denotes the ascending Reff (Reff(asc)). des denotes the descending Reff (Reff(des))
In Chuxiong Prefecture, the median Reff of TB among the mixed population was 1.68 (IQR: 1.20–1.96). Most TB transmissions occurred due to the high transmission in non-student populations with a median Reff22 1.59 (IQR: 1.14–1.80), and the other three values (Reff11, Reff21, Reff12) were nearly zero each year. The values of Reff2 and Reff1 fluctuated smoothly from 2008 to 2018, with a median Reff2 of 1.63 (IQR: 1.17–1.82) and a median Reff1 of 0.05 (IQR: 0.02–0.09), respectively (Table 5).
Table 5.
Reff of Model A between students and non-students (Chuxiong Prefecture)
| Year | Reff11 | Reff 12 | Reff 22 | Reff 21 | Reff1 | Reff2 |
|---|---|---|---|---|---|---|
| 2008 (des) | 0.01 | 0.00 | 0.80 | 0.02 | 0.01 | 0.82 |
| 2009 (asc) | 0.02 | 0.01 | 1.96 | 0.02 | 0.03 | 1.98 |
| 2009 (des) | 0.00 | 0.04 | 0.87 | 0.02 | 0.05 | 0.89 |
| 2010 (asc) | 0.02 | 0.00 | 1.97 | 0.03 | 0.02 | 2.01 |
| 2010 (des) | 0.00 | 0.00 | 1.04 | 0.02 | 0.01 | 1.06 |
| 2011 (asc) | 0.01 | 0.00 | 1.52 | 0.04 | 0.01 | 1.56 |
| 2011 (des) | 0.00 | 0.08 | 1.11 | 0.01 | 0.08 | 1.12 |
| 2012 (asc) | 0.02 | 0.00 | 1.26 | 0.03 | 0.02 | 1.29 |
| 2012 (des) | 0.01 | 0.05 | 1.72 | 0.02 | 0.06 | 1.74 |
| 2013 (asc) | 0.00 | 0.01 | 0.24 | 0.05 | 0.02 | 0.29 |
| 2013 (des) | 0.00 | 0.09 | 1.71 | 0.02 | 0.09 | 1.73 |
| 2014 (asc) | 0.02 | 0.00 | 1.14 | 0.04 | 0.02 | 1.17 |
| 2014 (des) | 0.00 | 0.09 | 1.78 | 0.04 | 0.09 | 1.82 |
| 2015 (asc) | 0.03 | 0.02 | 2.82 | 0.13 | 0.05 | 2.95 |
| 2015 (des) | 0.03 | 0.00 | 1.73 | 0.05 | 0.03 | 1.78 |
| 2016 (asc) | 0.06 | 0.00 | 3.58 | 0.09 | 0.06 | 3.66 |
| 2016 (des) | 0.00 | 0.13 | 1.52 | 0.02 | 0.13 | 1.54 |
| 2017 (asc) | 0.00 | 0.16 | 1.80 | 0.00 | 0.16 | 1.80 |
| 2017 (des) | 0.00 | 0.09 | 1.58 | 0.03 | 0.09 | 1.61 |
| 2018 (asc) | 0.03 | 0.00 | 2.01 | 0.12 | 0.04 | 2.12 |
| 2018 (des) | 0.00 | 0.09 | 1.26 | 0.04 | 0.09 | 1.30 |
| Median | 0.01 | 0.04 | 1.59 | 0.04 | 0.05 | 1.63 |
| P25 | 0.00 | 0.00 | 1.14 | 0.02 | 0.02 | 1.17 |
| P75 | 0.02 | 0.09 | 1.80 | 0.04 | 0.09 | 1.82 |
Reff11 denotes the transmissibility of MTB from student cases to student cases. Reff 12 denotes the transmissibility of MTB from student cases to non-student cases. Reff 21 denotes the transmissibility of MTB from non-student cases to student cases. Reff 22 denotes the transmissibility of MTB from the non-student cases to non-student cases. Reff1 represents the transmissibility of the population of students with active TB cases (sum of Reff11 and Reff 12), whereas Reff 2 represents the transmissibility of the population of non-student active TB cases (sum of Reff 22 and Reff 21)
asc denotes the ascending Reff (Reff(asc)). des denotes the descending Reff (Reff(des))
In Xiamen City, we excluded data analysis in 2019 for only 3 months data collection from January to March, which was not a valid TB representation for the entire year. Except that the median Reff for TB among the mixed population was 1.67 (IQR: 1.40–1.93). Most TB transmissions of occurred due to the high transmission in non-student populations, with a median Reff22 of 1.58 (IQR: 1.32–1.80). Reff2 values slowly decreased between 2005 and 2018, with a median Reff2 of 1.61 (IQR: 1.35–1.85). Although there were several values of Reff12 higher than 0.10 in student-non-student transmission (Reff (des) in 2005, 2012, 2016, and Reff (asc) in 2006, 2008, 2010), the overall transmissibility was annual decreasing with a median Reff12 of 0.04 (IQR: 0.00–0.07) (Table 6).
Table 6.
Reff of Model A between students and non-students (Xiamen City)
| Year | Reff11 | Reff 12 | Reff 22 | Reff 21 | Reff1 | Reff2 |
|---|---|---|---|---|---|---|
| 2005 (asc) | 0.04 | 0.00 | 2.21 | 0.03 | 0.04 | 2.24 |
| 2005 (des) | 0.00 | 0.22 | 2.03 | 0.03 | 0.22 | 2.06 |
| 2006 (asc) | 0.02 | 0.11 | 2.71 | 0.01 | 0.13 | 2.72 |
| 2006 (des) | 0.02 | 0.00 | 1.77 | 0.05 | 0.02 | 1.83 |
| 2007 (asc) | 0.04 | 0.00 | 1.81 | 0.04 | 0.04 | 1.84 |
| 2007 (des) | 0.03 | 0.01 | 2.00 | 0.04 | 0.04 | 2.04 |
| 2008 (asc) | 0.00 | 0.15 | 1.86 | 0.03 | 0.15 | 1.89 |
| 2008 (des) | 0.03 | 0.00 | 1.54 | 0.03 | 0.03 | 1.57 |
| 2009 (asc) | 0.00 | 0.09 | 1.75 | 0.02 | 0.09 | 1.77 |
| 2009 (des) | 0.04 | 0.01 | 1.69 | 0.05 | 0.05 | 1.73 |
| 2010 (asc) | 0.04 | 0.15 | 1.60 | 0.03 | 0.19 | 1.62 |
| 2010 (des) | 0.03 | 0.01 | 1.33 | 0.02 | 0.04 | 1.36 |
| 2011 (asc) | 0.03 | 0.00 | 2.01 | 0.05 | 0.03 | 2.06 |
| 2011 (des) | 0.01 | 0.00 | 1.39 | 0.03 | 0.01 | 1.42 |
| 2012 (asc) | 0.02 | 0.00 | 1.36 | 0.02 | 0.02 | 1.39 |
| 2012 (des) | 0.00 | 0.12 | 1.31 | 0.03 | 0.12 | 1.34 |
| 2013 (asc) | 0.06 | 0.00 | 1.79 | 0.08 | 0.06 | 1.87 |
| 2013 (des) | 0.02 | 0.00 | 1.28 | 0.02 | 0.02 | 1.29 |
| 2014 (asc) | 0.01 | 0.04 | 1.16 | 0.01 | 0.05 | 1.18 |
| 2014 (des) | 0.01 | 0.02 | 1.19 | 0.03 | 0.03 | 1.22 |
| 2015 (asc) | 0.02 | 0.00 | 1.48 | 0.02 | 0.02 | 1.50 |
| 2015 (des) | 0.01 | 0.02 | 1.21 | 0.05 | 0.03 | 1.27 |
| 2016 (asc) | 0.02 | 0.01 | 1.34 | 0.02 | 0.03 | 1.37 |
| 2016 (des) | 0.00 | 0.10 | 1.11 | 0.01 | 0.10 | 1.12 |
| 2017 (asc) | 0.02 | 0.00 | 1.38 | 0.02 | 0.02 | 1.40 |
| 2017 (des) | 0.01 | 0.06 | 1.43 | 0.01 | 0.07 | 1.45 |
| 2018 (asc) | 0.02 | 0.01 | 1.32 | 0.02 | 0.03 | 1.35 |
| 2018 (des) | 0.02 | 0.00 | 1.22 | 0.02 | 0.02 | 1.25 |
| Median | 0.02 | 0.04 | 1.58 | 0.03 | 0.06 | 1.61 |
| P25 | 0.01 | 0.00 | 1.32 | 0.02 | 0.03 | 1.35 |
| P75 | 0.03 | 0.07 | 1.80 | 0.03 | 0.07 | 1.85 |
Reff11 denotes the transmissibility of MTB from student cases to student cases. Reff 12 denotes the transmissibility of MTB from student cases to non-student cases. Reff 21 denotes the transmissibility of MTB from non-student cases to student cases. Reff 22 denotes the transmissibility of MTB from the non-student cases to non-student cases. Reff1 represents the transmissibility of the population of students with active TB cases (sum of Reff11 and Reff 12), whereas Reff 2 represents the transmissibility of the population of non-student active TB cases (sum of Reff 22 and Reff 21)
asc denotes the ascending Reff (Reff(asc)). des denotes the descending Reff (Reff(des))
A similar transmission relationship among and between student and non-student populations was calculated in Model B. However, the model revealed exceedingly high values of Reff over many years in the four regions, which indicates that the findings of Model B may be unsuitable to show the characteristics of TB. Additional details of the results are provided in Additional file 3: Tables S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6.
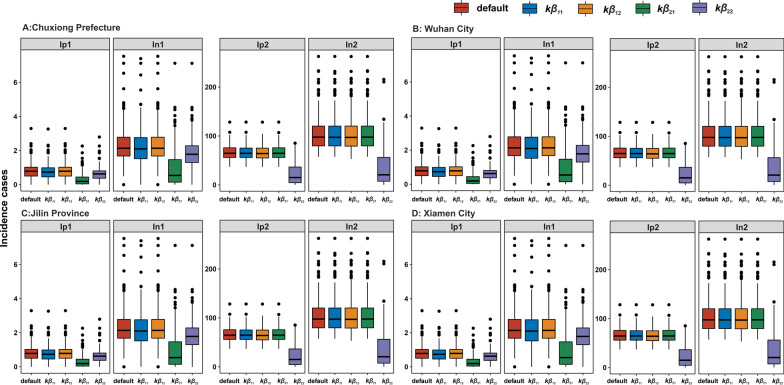
Cumulative incidence rate after the knock-out-pathways, β11, β12, β22, and β21
According to the knock-out results (Fig. 10), the number of TB cases among students was significantly reduced by more than half (60–70%) when the transmissibility of non-student-to-student populations (β21) was knocked out. When TB transmission among non-students (β22) was blocked, the number of TB cases was reduced by approximately 67% (65–70%) among non-students and by approximately 28% (25–30%) among students. There was only a 5% reduction (2–12%) among students when TB transmission among students (β11) was blocked, and TB reported cases had barely changed (less than 1%) while TB transmission from non-student-to-students (β12) was blocked.
Fig. 10.
Knockout analysis in the four study areas: A Wuhan City, B Jilin Province, C Xiamen City, and D Chuxiong Prefecture. The subscripts refer to the occupation of the students (1 subscript) or non-students (2 subscript). The initial state is denoted as the default. The performance of the knockout for each of the transmission pathway was determined by setting the beta value to zero. For example, kb11 stands for getting rid of the student-to-students’ transmission pathway
Discussion
This study is the first to address the occupational-specific transmission dynamics of TB and emphasise the importance of control between student groups, which can increase our understanding of the characteristics of TB transmission in different occupational groups.
Analysis of epidemiological characteristics
The incidence rate of TB decreased in the study regions, which is in good agreement with previous global reports [3], but was unevenly distributed between these four regions. This phenomenon may be attributed to several reasons. First, the inclusion of previously untreated patients in the management after several years of continuous active screening led to a certain decline in the number of subsequent patient detection cases. Second, since 2017, a nationwide survey of underreporting and under-registration of TB [58] and a diagnostic review [59] were carried out, which improved the quality of TB reporting and diagnosis in each region. However, the reported incidence rate of TB is still higher in areas less economically developed than in the west, such as Chuxiong Prefecture, although the attention and support of governments and health administrations, as well as the support for precise health poverty alleviation have been undertaken at all levels [9].
Remarkably, the reported incidence of TB in the student population has increased. The reported data also confirmed that the proportion of student patients has increased from 4.0% in 2015 to 6.2% in 2019, with a difference of 2.2% [9]. This is mainly due to the fact that early warning of individual cases of TB in schools has been included in the National Automatic Early Warning System for Infectious Diseases since July 2018 [60]. Furthermore, disease control agencies at all levels have strengthened the information verification process of school-age patients and improved the sensitivity of the surveillance of student patients [61]. Schools have also strengthened medical examinations and handled clusters of TB outbreaks [62]. Our results highlight an obvious incidence peak among students at the beginning of the semester. There are several explanations for this observation. Students are in close contact with social residents and are exposed to TB patients in the community during holidays. Considering LTBI [8, 63], students infected with MTB on vacation do not become ill immediately, but become ill after returning to school when they are exposed to several inducements, including high pressure and cold, among others. Farmers always had the highest reported incidence rates. However, this is not surprising if we consider that the rural population represents most of the total population of China, and the allocation of medical and health resources in rural areas is inadequate, resulting in unequal access to medical resources for urban and rural residents [13]. Furthermore, it may be related to the lower level of education of farmers, poorer living conditions, and lack of awareness of health protection [64].
The diagnosis results in the study areas, which had a low pathogen positive rate of less than 50%. A previous report showed the pathogen positive rate for PTB reported in China in 2020 was 57%, up from 45.03% in 2019 [3]. However, a gap still exists when this is compared with surveillance results based on laboratory pathogenic diagnostic evidence in other countries worldwide. Both the TB laboratory diagnostic and the TB imaging detection capacity need to be improved in primary care institutions in China, which is consistent with the outcomes of one diagnostic and therapeutic survey on TB sentinel medical institutions [65] and on the current status of TB diagnostic capacity at county-level TB sentinel medical institutions in China [66]. To achieve the goal of "reaching a pathogenic positivity rate of more than 50% by 2022" as required by the Action Plan to Stop TB (2019–2022) [67], it is still necessary to continue to strengthen the quality of laboratory work [68].
Analysis of TB transmission dynamics characteristics
In this study, two mathematical models of TB were constructed according to the transmission characteristics of TB: Models A and B. Although there may be seasonal fluctuations in the actual incidence of TB in some areas, Model A fitted better than Model B. Therefore, we believe that the analysis results of Model A can better reflect the real situation of TB incidence.
Therefore, the following interpretations were made according to the results of the Reff calculation of Model A and results of a knock-out analysis:
A) Overall, the average values of Reff in the four regions showed that a single TB case could effectively spread to one or two people. TB transmissibility among non-students (Reff2) was 23.30 times (IQR: 1.94–7.24) higher than among students (Reff1). TB transmission remained dominant in the non-student population. This finding also existed in the knock-out analysis. Transmission among non-students increased the number of reported TB cases in all four groups (67% in non-students and 28% in students). The non-student population was large, and included 17 occupations, different locations with active cases, and a wide range of age groups. In high-burden areas, such as China, most TB transmission occurs outside the home (< 20% of household transmission) which is not necessarily attributable to known close contacts [69, 70]. The probability of TB transmission to others by a TB patient is determined by many factors, including socioeconomic, environmental, high or low regional disease burden, infectiousness of the case, MTB strain, and host susceptibility. Determining the specific site of TB transmission outside the home is difficult. The potential for airborne transmission even during brief contact, combined with variable incubation periods, makes it exceptionally difficult to establish a specific TB transmission link. Despite these challenges, certain specific settings have been identified as important contributors to TB risk, such as nasal transmission [71–74], hospital-associated transmission [75], homeless shelters [76], prisons [77, 78], public transportation [79], churches [80], schools [69, 81] and slums [82–84]. This is precisely because the places where students study and live are close, providing good conditions for the spread of TB; therefore, the implementation of TB control policies in schools is especially important. The presence of these factors has contributed to the high rates of acquired TB in this group over the years.
Furthermore, the concentration of TB transmission in certain settings and subpopulations also leads to heterogeneity of transmission, which can serve to increase Reff and may make it more difficult to control transmission [85]. Moreover, adults in their most active age groups are more likely to be infected with TB due to their close contact with each other [86]. To explain why transmission among the non-student populations increased the number of infected patients among non-students, it may be assumed that household and unnoticed transmissions in the community contribute simultaneously [87].
B) The results from knock-out analysis indicated that non-student-to-student transmission increased the number of reported TB cases in the student group (either pathogen positive or negative), and transmission among non-students increased the number of reported TB cases in all the four groups. There may be several reasons for this. First, the home–school transmission route may be one of the reasons. TB is actively transmitted by household exposure [88], and a prospective case–control study found that previous exposure to TB in a household could cause an infected student to spread TB to their classmates [89]. Second, we believe that the school community transmission route is important due to increased exposure to other occupations during vacations.
C) Although TB transmission is spread mainly by non-students, the transmissibility of student-to-non-students in some years and in some regions, is particularly high, such as the Reff12 of Chuxiong Prefecture in 2016 (Reff12: 0.13), 2017 (Reff12: 0.16), and that of Wuhan City in 2006 (Reff12: 0.36), etc. This could be due to TB outbreaks in schools [90]. Once TB transmission occurs in schools, the spread of TB will exceed beyond the public due to the frequent contact between students and cause widespread TB in schools. Due to this particularity of TB school transmission, the TB reporting system of China is more sensitive to the population of student occupation. A national single-case warning system is used to identify the student tuberculosis patients. When a student is diagnosed, close contacts screening, isolation and treatment of the TB patients are implemented in the shortest time. These measures make the control of student TB outbreaks much more effective, and then reduce the tuberculosis cases of this outbreak. But in the real world, if the epidemic was not dealt with promptly, a widespread TB outbreak in schools will be inevitable.
Prevention and control of TB among students
The relevant authorities must continue to strengthen the prevention and control of TB in student populations in the future [91]. There are shortcomings at all levels, including schools, medical institutions and TB control institutions, and improvements are needed. For schools, the implementation of a system to trace the causes of absence from school to detect patients in a timely and proactive manner is effective. Medical institutions should keep the epidemic information channels open with schools and TB control institutions, and provide timely information about confirmed students to schools and TB control institutions. TB prevention and control institutions should perform timely information verification and close contact follow-up after the detection of the infected student.
In addition, we suggest that more attention should be paid to men, farmers, and young and middle-aged people; and the bacteriological diagnosis of TB should be strengthened. More data collection from social contact surveys is required to provide information on how individual behaviors drive disease dynamics at the population level.
In particularly, several limitations may have influenced the results obtained. The first is selection bias due to inconsistency at the administrative levels in our study areas, which includes three cities and one province. The second is that we only included cases that were diagnosed as “bacteriologically confirmed positive or negative” and excluded those that were diagnosed as “rifampicin resistant” when processing the initial data. The latter could also contribute to TB transmission. Furthermore, complete immunity does not occur in patients with TB after recovery. However, partial immunity has been observed in previously infected individuals, which can prevent reinfection (risk ratio = 0.5) [92]. The last limitation of our methodology is that it was not possible to subdivide the 17 non-student occupations to better articulate the mechanisms of transmission between different occupations and quantify the impact of different non-student occupations on the student population.
Conclusions
This study has the potential to improve our understanding of the features of TB transmission in different occupational groups. The transmission of MTB was high in non-student populations, and that in the non-student population was 23.30 times higher than in the student population. It had the strongest influence among non-student groups. It not only increases the incidence of TB among non-students, but also among students. The incidence of TB among students has been on the rise and is the fourth highest in occupational distribution (especially in economically developed areas with a high number of students), despite the incidence of TB in China showing a downward trend annually. The TB outbreak among students can rapidly improve the transmissibility of TB in a short time, which will affect the prevalence of TB in other groups. TB screening should be performed rigorously at the beginning of the school semesters, when returning to school, to detect patients with LTBI. This implies the need for the implementation of more control measures such as strengthening the school TB management efforts and timely management of identified TB-infected students, after the academic year begins.
Supplementary Information
Additional file 1: Table S1. The basic information of the four regions.
Additional file 2: Table S2. Two different classifications of tuberculosis in National Notifiable Disease Surveillance System (NNDSS).
Additional file 3: Table S3. Estimation of transmissibility between students and non-students (Wuhan City).
Additional file 4: Table S4. Estimation of transmissibility between students and non-students (Jilin Province).
Additional file 5: Table S5. Estimation of transmissibility between students and non-students (Chuxiong Perfecture).
Additional file 6: Table S6. Estimation of transmissibility between students and non-students (Xiamen City).
Acknowledgements
The authors thank Miss Qiao Liu for her helpful comments.
Abbreviations
- SEIR
Susceptible-exposed-symptomatic-recovered
- Reff
Effective reproduction number
- MTB
Mycobacterium tuberculosis
- TB
Tuberculosis
- PTB
Pulmonary tuberculosis
- MDR-TB
Multidrug resistant tuberculosis
- HIV
Human immunodeficiency virus
- AIDS
Acquired immune deficiency syndrome
- LTBI
Latent TB infections
- NNDSS
National Notifiable Disease Surveillance System
- IGRA
Interferon-gamma release assays
- R0
Basic reproduction number
- DOTS
Directly observed treatment and short course chemotherapy
Author contributions
QC, SY, JR, YG, SY, GA, ZY, CL, LL, MW, ZL, QZ, LG, YN, RF, and TC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YN, RF, and TC were responsible for study conception and design. MW, and QZ collected the data. QC, SY, JR, YG, SY, GA, ZY, CL, LL, LG, and ZL were responsible for data analysis and interpretation. QC, SY and JR drafted the manuscript. YN, RF, and TC critically revised the manuscript for intellectual content. YN, RF, and TC contributed equally to this study. All authors read and approved the final manuscript.
Funding
This study was supported by the Bill and Melinda Gates Foundation (Grant Number: INV-005834). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the School of Medicine, Xiamen University. Consent requirement, either verbal or written, was waived by the thics Committee of the School of Medicine on the following grounds: (1) only anonymized records were used without the need for direct involvement nor active participation of patients; (2) neither medical intervention nor biological samples were involved; (3) study procedures and results would not affect clinical management of patients in any form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Qiuping Chen, Shanshan Yu and Jia Rui contributed equally to this study
Contributor Information
Yan Niu, Email: niuyan@chinacdc.cn.
Roger Frutos, Email: frutossmt@gmail.com.
Tianmu Chen, Email: 13698665@qq.com.
References
- 1.Global tuberculosis report 2022. https://www.who.int/publications/i/item/9789240061729. Accessed 10 Nov 2022.
- 2.Collaborators GBDT. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18(3):261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Tuberculosis Report 2021 https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021. Accessed 21 Oct 2021.
- 4.Overview of notifiable infectious diseases in China in 2021 [in Chinese]. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml. Accessed 10 Nov 2022.
- 5.China Statistical Yearbook in 2021 [in Chinese]. http://www.stats.gov.cn/tjsj/ndsj/2021/indexch.htm. Accessed 6 Oct 2021.
- 6.Hui C, Hui Z, Jun C. Interpretation of the guidelines for prevention and control of tuberculosis in Chinese schools. Chin J Autituberc. 2021;43(6):4. [Google Scholar]
- 7.Circular on issuing guidelines on Tuberculosis Prevention and Control in Chinese Schools [in Chinese]. http://www.gov.cn/zhengce/zhengceku/2020-12/05/content_5567137.htm. Accessed 21 Feb 2022.
- 8.Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian W, Tao L, Xin D, Ni N, Yanlin Z, Hui Z. National analysis of reported incidence of tuberculosis, 2015–2019. Chin J Antitubercul. 2021;43(02):107–112. [Google Scholar]
- 10.Fang Y, Ma Y, Lu Q, Sun J, Pei Y. An outbreak of pulmonary tuberculosis and a follow-up investigation of latent tuberculosis in a high school in an eastern city in China, 2016–2019. PLoS ONE. 2021;16(2):e0247564. doi: 10.1371/journal.pone.0247564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Pang Y, Yang X, Chen T, Yang H, Yang R, et al. Outbreak of Mycobacterium tuberculosis Beijing strain in a high school in Yunnan, China. Am J Trop Med Hyg. 2020;102(4):728–730. doi: 10.4269/ajtmh.19-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stosic MB, Plavsa D, Mavroeidi N, Jovanovic D, Vucinic V, Stevanovic G, et al. Tuberculosis outbreak among high school students in Novi Pazar, Serbia 2016: a retrospective-cohort study. J Infect Dev Ctries. 2019;13(2):101–110. doi: 10.3855/jidc.10952. [DOI] [PubMed] [Google Scholar]
- 13.Senlu W, Yan C, Nianqiang L, Xijiang W, Xinqi W, Weidong W, et al. A tuberculosis outbreak at a school-Xinjiang Uygur autonomous region, China. China CDC Weekly. 2020;2(46):881–883. doi: 10.46234/ccdcw2020.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Pérez-Vélez CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383(9928):1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Wang T, Hou S, Ye J, Li S. An outbreak of multidrug-resistant tuberculosis in a secondary school—Hubei Province, 2019, China. CDC Weekly. 2019;1(5):3. [PMC free article] [PubMed] [Google Scholar]
- 16.Houben RMGJ, Menzies NA, Sumner T, Huynh GH, Arinaminpathy N, Goldhaber-Fiebert JD, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health. 2016;4(11):e806–e815. doi: 10.1016/S2214-109X(16)30199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gammaitoni L, Nucci MC. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg Infect Dis. 1997;3(3):335–342. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams A, Hatch GJ, Clark SO, Gooch KE, Hatch KA, Hall GA, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis. 2005;85(1–2):29–38. doi: 10.1016/j.tube.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Castillo-Chavez C, Feng Z. Global stability of an age-structure model for TB and its applications to optimal vaccination strategies. Math Biosci. 1998;151(2):135–154. doi: 10.1016/s0025-5564(98)10016-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Wang Y. A mathematical study of a TB model with treatment interruptions and two latent periods. Comput Math Methods Med. 2014;2014:932186. doi: 10.1155/2014/932186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alani A, Barton IE, Seymour MJ, Wrobel LC. Application of Lagrangian particle transport model to tuberculosis (TB) bacteria UV dosing in a ventilated isolation room. Int J Environ Health Res. 2001;11(3):219–228. doi: 10.1080/09603120020047000. [DOI] [PubMed] [Google Scholar]
- 22.Beste DJ, Hooper T, Stewart G, Bonde B, Avignone-Rossa C, Bushell ME, et al. GSMN-TB: a web-based genome-scale network model of Mycobacterium tuberculosis metabolism. Genome Biol. 2007;8(5):R89. doi: 10.1186/gb-2007-8-5-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine. 2007;25(16):2990–2993. doi: 10.1016/j.vaccine.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–1201. doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez MS, Lloyd-Smith JO, Williams BG, Getz WM. Using mathematical models to monitor and evaluate the impact of public health interventions on epidemics: the case of the TB/HIV co-pandemic in Africa. In: Gumel AB, Lenhart S Eds. Modeling Paradigms and Analysis of Disease Transmission Models. Volume 75. Providence: Amer Mathematical Soc; 2010:135.
- 26.Feng Z, Castillo-Chavez C, Capurro AF. A model for tuberculosis with exogenous reinfection. Theor Popul Biol. 2000;57:235–247. doi: 10.1006/tpbi.2000.1451. [DOI] [PubMed] [Google Scholar]
- 27.Waaler H, Geser A, Andersen S. The use of mathematical models in the study of the epidemiology of tuberculosis. Am J Public Health Nations Health. 1962;52(6):1002–1013. doi: 10.2105/ajph.52.6.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Li M, Yuan S. Analysis of transmission and control of tuberculosis in mainland China, 2005–2016, based on the age-structure mathematical model. Int J Environ Res Public Health. 2017;14(10):1192. doi: 10.3390/ijerph14101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatmawati, Khan MA, Bonyah E, Hammouch Z, Shaiful EM. A mathematical model of tuberculosis (TB) transmission with children and adults groups: a fractional model. AIMS Math. 2020;5(4):2813–2842. [Google Scholar]
- 30.Faniran T, Ali A, Adewole MO, Adebo B, Akanni OO. Asymptotic behavior of tuberculosis between smokers and non-smokers. Partial Differ Equ Appl Math. 2022;5:100244. [Google Scholar]
- 31.National Health and Wellness Commission of the People's Republic of China, National Health and Wellness Commission General Office on adjusting the classification of tuberculosis infectious disease reporting, National Health Office Disease Control Letter (2019) 296. 2019–03–21 [in Chinese].
- 32.Jilin Statistical Yearbook [in Chinese]. http://tjj.jl.gov.cn/tjsj/tjnj/. Accessed 10 Sep 2021.
- 33.2021 National Economic and Social Development Statistical Bulletin of Chuxiong Prefecture [in Chinese]. http://www.tjcn.org/tjgb/25yn/37038.html. Accessed 10 Sep 2021.
- 34.Yearbook of Xiamen Special Economic Zone [in Chinese]. http://www.xm.gov.cn/zfxxgk/xxgkznml/gmzgan/tjnj/index.htm. Accessed 10 Sep 2021.
- 35.National Economic and Social Development Statistical Communique of Wuhan City [in Chinese]. http://tjj.wuhan.gov.cn/tjfw/tjgb/index.shtml. Accessed 14 Oct 2021.
- 36.DCEPH National notifiable infectious disease database [in Chinese]. http://www.phsciencedata.cn/Share/ky_sjml.jsp. Accessed Dec 2021.
- 37.Bulletin of the Seventh National Census (No.3) -- Regional population [in Chinese]. http://www.stats.gov.cn/tjsj/tjgb/rkpcgb/qgrkpcgb/202106/t20210628_1818822.html. Accessed 16 Mar 2022.
- 38.Yang Y, Lin L, Tang N, Ma J, Pang Y. Epidemic characteristics of student pulmonary tuberculosis analysis in Yuman province, 2013. Chin J Epidemiol. 2015;36(6):663–664. [PubMed] [Google Scholar]
- 39.Jianjun MY, Yuan, Tiejuan Z, Ying G, Wang Y. Epidemiological characteristics and medical treatment of floating population tuberculosis patients in Jilin province. Sanitary Eng China. 2018; 17(1).
- 40.Circular of the Ministry of Health of the People's Republic of China [in Chinese]. http://www.nhc.gov.cn/bgt/pw10803/200805/e6ed8929f7de4afc829b14ce00a794d7.shtml. Accessed 24 Aug 2022.
- 41.National Health and Family Planning Commission of the People's Republic of China / Notice of the General Office of the National Health and Family Planning Commission on the Adjustment of the Reporting Classification of Tuberculosis Infectious Diseases/National Health Office Disease Control Letter [2017] No. 600. 2017–06–15 [in Chinese].
- 42.National Health and Family Planning Commission of the People's Republic of China. WS 196–2017 Classification of tuberculosis. 2017–11–09 [in Chinese].
- 43.National Health and Family Planning Commission of the People's Republic of China. ws 288–2017 tuberculosis diagnosis. 2017–11–09 [in Chinese]. In.
- 44.Liu K, Li T, Vongpradith A, Wang F, Peng Y, Wang W, et al. Identification and prediction of tuberculosis in Eastern China: analyses from 10-year population-based notification data in Zhejiang Province, China. Sci Rep. 2020;10(1):7425. doi: 10.1038/s41598-020-64387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K, Peng Y, Zhou Q, Cheng J, Yu H, Tang L, et al. Assessment of active tuberculosis findings in the eastern area of China: a 3-year sequential screening study. Int J Infect Dis. 2019;88:34–40. doi: 10.1016/j.ijid.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Cheng Q, Li C, Stokes E, Collender P, Ohringer A, et al. Evidence for heterogeneity in China's progress against pulmonary tuberculosis: uneven reductions in a major center of ongoing transmission, 2005–2017. BMC Infect Dis. 2019;19(1):615. doi: 10.1186/s12879-019-4262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.General Office of the National Health and Wellness Commission on the issuance of technical specifications for the prevention and control of tuberculosis in China (2020 version) notice.
- 48.Combs DL, O’Brien RJ, Geiter LJ. USPHS tuberculosis short-course chemotherapy Trial 21: effectiveness, toxicity, and acceptability. The report of final results. Ann Intern Med. 1990;112(6):397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 49.Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Results at 30 months. Hong Kong Chest Service/British Medical Research Council. Am Rev Respir Dis. 1991; 143(4 Pt 1):700–6. [DOI] [PubMed]
- 50.Song; Y, Jie; L, Fangjin; B, Aimin; W, Yongzhong; Z, Fang X: Interpretation and reflection on the Technical Specification for Tuberculosis Prevention and Control in China (2020 Edition) [in Chinese]. In: The 33rd National Academic Conference of China Anti-TB Association and China Anti-TB Science and Technology Award Presentation Conference: 2021; Qingdao, Shandong, China; 2021: 10–13.
- 51.Verrall AJ, Alisjahbana B, Apriani L, Novianty N, Nurani AC, van Laarhoven A, et al. Early clearance of Mycobacterium tuberculosis: the INFECT case contact cohort study in Indonesia. J Infect Dis. 2020;221(8):1351–1360. doi: 10.1093/infdis/jiz168. [DOI] [PubMed] [Google Scholar]
- 52.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378(9785):57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 53.Shen X, Yang C, Wu J, Lin S, Gao X, Wu Z, et al. Recurrent tuberculosis in an urban area in China: relapse or exogenous reinfection? Tuberculosis. 2017;103:97–104. doi: 10.1016/j.tube.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Zhang XX, Yu JJ, Liang C, Xing Q, Yao C, et al. Tuberculosis relapse is more common than reinfection in Beijing, China. Infect Dis. 2020;52(12):858–865. doi: 10.1080/23744235.2020.1794027. [DOI] [PubMed] [Google Scholar]
- 55.Du J, Zhang L, Ma Y, Chen XY, Ge QP, Tian XZ, et al. Treatment and recurrence on re-treatment tuberculosis patients: a randomized clinical trial and 7-year perspective cohort study in China. Eur J Clin Microbiol Infect Dis. 2020;39(1):93–101. doi: 10.1007/s10096-019-03696-8. [DOI] [PubMed] [Google Scholar]
- 56.Ebenezer A. Mathematical model of tuberculosis [in Chinese]. PhD. Huazhong Normal University; 2020.
- 57.Ahmed N, Hasnain SE. Molecular epidemiology of tuberculosis in India: moving forward with a systems biology approach. Tuberculosis. 2011;91(5):407–413. doi: 10.1016/j.tube.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Li T, Chen W, Zhao Y, Wang L, Chen M, Du X, et al. Underreporting of notifiable pulmonary tuberculosis cases to the national tuberculosis information management system—China, 2015. China CDC Weekly. 2020;2(12):185–189. [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Z, Eryong L, Qinglin M, Mingting C, Xinhua Z, Weiwei G, et al. Analysis of the quality assessment of pulmonary tuberculosis diagnosis after the implementation of the standard "WS 288–2017 Diagnosis of pulmonary tuberculosis. Chin J Antitubercul. 2020;42(09):910–915. [Google Scholar]
- 60.Law of the People's Republic of China on the Prevention and Treatment of Infectious Diseases [in Chinese]. http://www.nhc.gov.cn/fzs/s3576/201808/6d00c158844f42c5bcf94993bffa665a.shtml. Accessed 2 Sep 2022.
- 61.Jun C, Jianjun L. Status and progress of tuberculosis epidemic surveillance and early warning in schools in China. Chin J Antitubercul. 2020;42(05):436–441. [Google Scholar]
- 62.Hui C, Solemnly X, Cangyou Z, Jun C, Hui Z. Trends and characteristics of the national student tuberculosis epidemic from 2014–2018. Chin J Antitubercul. 2019;41(6):7. [Google Scholar]
- 63.Yates TA, Khan PY, Knight GM, Taylor JG, McHugh TD, Lipman M, et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis. 2016;16(2):227–238. doi: 10.1016/S1473-3099(15)00499-5. [DOI] [PubMed] [Google Scholar]
- 64.Wenjing Z, Hongyan M, Jianjun L, Xiangzheng L, Chang J. Interpretation of the group standard "Health Examination Guide for Rural Residents". Chin J Prev Med. 2020;54(01):18–20. doi: 10.3760/cma.j.issn.0253-9624.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Eryong L, Qian W, Lin Z, Guoqin Z, Xiulei Z, Yongcheng M, et al. Analysis of the current quality of diagnosis of pathogenically tested negative pulmonary tuberculosis in some regions of China. Chin J Antitubercul. 2020;42(09):916–920. [Google Scholar]
- 66.Qian W, Lin Z, Eryong L, Yanlin Z, Tao L, Mingting C, et al. A study on the current status of tuberculosis diagnostic capacity of county-level tuberculosis sentinel medical institutions in China. Chin J Antitubercul. 2020;42(09):926–930. [Google Scholar]
- 67.National Health and Health Commission, National Development and Reform Commission, Ministry of Education, etc., on the issuance of the action plan to curb tuberculosis (2019–2022) Notice of National Health Disease Control [2019] No. 41.2019–05–31. In.
- 68.van’t Hoog AH, Bergval I, Tukvadze N, Sengstake S, Aspindzelashvili R, Anthony RM, et al. The potential of a multiplex high-throughput molecular assay for early detection of first and second line tuberculosis drug resistance mutations to improve infection control and reduce costs: a decision analytical modeling study. BMC Infect Dis. 2015;15:473. doi: 10.1186/s12879-015-1205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis. 2014;210(4):597–603. doi: 10.1093/infdis/jiu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verver S, Warren RM, Munch Z, Richardson M, van der Spuy GD, Borgdorff MW, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363(9404):212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 71.CfD C. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons–Florida and New York, 1988–1991. MMWR Morb Mortal Wkly Rep. 1991;40(34):585–591. [PubMed] [Google Scholar]
- 72.Frieden TR, Sherman LF, Maw KL, Fujiwara PI, Crawford JT, Nivin B, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA. 1996;276(15):1229–1235. [PubMed] [Google Scholar]
- 73.Rullán JV, Herrera D, Cano R, Moreno V, Godoy P, Peiró EF, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis in Spain. Emerg Infect Dis. 1996;2(2):125. doi: 10.3201/eid0202.960208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moro ML, Gori A, Errante I, Infuso A, Franzetti F, Sodano L, et al. An outbreak of multidrug-resistant tuberculosis involving HIV-infected patients of two hospitals in Milan, Italy. AIDS. 1998;12(9):1095–1102. [PubMed] [Google Scholar]
- 75.Gandhi NR, Weissman D, Moodley P, Ramathal M, Elson I, Kreiswirth BN, et al. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis. 2013;207(1):9–17. doi: 10.1093/infdis/jis631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nardell E, McInnis B, Thomas B, Weidhaas S. Exogenous reinfection with tuberculosis in a shelter for the homeless. N Engl J Med. 1986;315(25):1570–1575. doi: 10.1056/NEJM198612183152502. [DOI] [PubMed] [Google Scholar]
- 77.Valway SE, Greifinger RB, Papania M, Kilburn JO, Woodley C, DiFerdinando GT, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. J Infect Dis. 1994;170(1):151–156. doi: 10.1093/infdis/170.1.151. [DOI] [PubMed] [Google Scholar]
- 78.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7(12):e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrews JR, Morrow C, Wood R. Modeling the role of public transportation in sustaining tuberculosis transmission in South Africa. Am J Epidemiol. 2013;177:556–561. doi: 10.1093/aje/kws331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murray E, Marais B, Mans G, Beyers N, Ayles H, Godfrey-Faussett P, et al. A multidisciplinary method to map potential tuberculosis transmission ‘hot spots’ in high-burden communities. Int J Tuberc Lung Dis. 2009;13(6):767–774. [PubMed] [Google Scholar]
- 81.Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191(5):584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wobudeya E, Lukoye D, Lubega IR, Mugabe F, Sekadde M, Musoke P. Epidemiology of tuberculosis in children in Kampala district, Uganda, 2009–2010; a retrospective cross-sectional study. BMC Public Health. 2015;15(1):1–8. doi: 10.1186/s12889-015-2312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira AGL, Medronho RA, Escosteguy CC, Magalhães MAFM, Pereira AGL. Spatial distribution and socioeconomic context of tuberculosis in Rio de Janeiro, Brazil. Rev Saude Publica. 2015;49:1. doi: 10.1590/S0034-8910.2015049005470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koenig SP, Rouzier V, Vilbrun SC, Morose W, Collins SE, Joseph P, et al. Tuberculosis in the aftermath of the 2010 earthquake in Haiti. Bull World Health Organ. 2015;93:498–502. doi: 10.2471/BLT.14.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrews JR, Basu S, Dowdy DW, Murray MB. The epidemiological advantage of preferential targeting of tuberculosis control at the poor. Int J Tuberc Lung Dis. 2015;19(4):375–380. doi: 10.5588/ijtld.14.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tuberculosis. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed by May 2022
- 87.Middelkoop K, Bekker LG, Morrow C, Lee N, Wood R. Decreasing household contribution to TB transmission with age: a retrospective geographic analysis of young people in a South African township. BMC Infect Dis. 2014;14:221. doi: 10.1186/1471-2334-14-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Augustynowicz-Kopeć E, Jagielski T, Kozińska M, Kremer K, van Soolingen D, Bielecki J, et al. Transmission of tuberculosis within family-households. J Infect. 2012;64(6):596–608. doi: 10.1016/j.jinf.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 89.Pan D, Lin M, Lan R, Graviss EA, Lin D, Liang D, et al. Tuberculosis transmission in households and classrooms of adolescent cases compared to the community in China. Int J Environ Res Public Health. 2018;15(12):2803. doi: 10.3390/ijerph15122803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei C, Qiulan C, Solemnly X, Shiming C. Analysis of the characteristics of the national student tuberculosis epidemic from 2008 to 2012. Chin J Antitubercul. 2013;35(12):949–954. [Google Scholar]
- 91.Li Y, Xiaowei J, Wei X, Haixia D, Hongjian J. Epidemiological investigation and management of a school tuberculosis cluster outbreak. J Tuberc Lung Dis. 2020;1(02):89–92. [Google Scholar]
- 92.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54(6):784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swaminathan N, Perloff SR, Zuckerman JM. Prevention of Mycobacterium tuberculosis transmission in health care settings. Infect Dis Clin North Am. 2021;5(4):1013–1025. doi: 10.1016/j.idc.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Collaborators GBDT Global, regional, and national burden of tuberculosis, 1990–2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect Dis. 2018;18(12):1329–1349. doi: 10.1016/S1473-3099(18)30625-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The basic information of the four regions.
Additional file 2: Table S2. Two different classifications of tuberculosis in National Notifiable Disease Surveillance System (NNDSS).
Additional file 3: Table S3. Estimation of transmissibility between students and non-students (Wuhan City).
Additional file 4: Table S4. Estimation of transmissibility between students and non-students (Jilin Province).
Additional file 5: Table S5. Estimation of transmissibility between students and non-students (Chuxiong Perfecture).
Additional file 6: Table S6. Estimation of transmissibility between students and non-students (Xiamen City).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.