Abstract
Background
Adequate identification and testing of people at risk for HIV is fundamental for the HIV care continuum. A key strategy to improve timely testing is HIV indicator condition (IC) guided testing.
Aim
To evaluate the uptake of HIV testing recommendations in HIV IC-specific guidelines in European countries.
Methods
Between 2019 and 2021, European HIV experts reviewed guideline databases to identify all national guidelines of 62 HIV ICs. The proportion of HIV IC guidelines recommending HIV testing was reported, stratified by subgroup (HIV IC, country, eastern/western Europe, achievement of 90–90–90 goals and medical specialty).
Results
Of 30 invited European countries, 15 participated. A total of 791 HIV IC guidelines were identified: median 47 (IQR: 38–68) per country. Association with HIV was reported in 69% (545/791) of the guidelines, and 46% (366/791) recommended HIV testing, while 42% (101/242) of the AIDS-defining conditions recommended HIV testing. HIV testing recommendations were observed more frequently in guidelines in eastern (53%) than western (42%) European countries and in countries yet to achieve the 90–90–90 goals (52%) compared to those that had (38%). The medical specialties internal medicine, neurology/neurosurgery, ophthalmology, pulmonology and gynaecology/obstetrics had an HIV testing recommendation uptake below the 46% average. None of the 62 HIV ICs, countries or medical specialties had 100% accurate testing recommendation coverage in all their available HIV IC guidelines.
Conclusion
Fewer than half the HIV IC guidelines recommended HIV testing. This signals an insufficient adoption of this recommendation in non-HIV specialty guidelines across Europe.
Keywords: HIV, indicator conditions, AIDS-defining conditions, HIV testing recommendations, guidelines, Europe
Key public health message.
What did you want to address in this study?
Many people are diagnosed with HIV years after initial infection, often with AIDS. A key strategy for a more timely diagnosis and linking to care is to adequately test people with medical conditions indicative of an underlying HIV infection. This study looked at national clinical practice guidelines throughout Europe to determine what current HIV testing recommendations are given.
What have we learnt from this study?
Fewer than half of the HIV indicator condition specific guidelines in Europe contain HIV testing recommendations. Guidelines for medical conditions known to be AIDS defining perform even worse. This deficiency is visible throughout Europe and across all medical specialties. Disturbingly, none of the 62 HIV indicator conditions, countries, or medical specialties have a fully adequate HIV testing recommendation coverage.
What are the implications of your findings for public health?
Clinical guidelines define medical practice in national healthcare systems. The omissions we found mean that people with HIV indicator conditions, remain untested. This is a missed opportunity to help healthcare professionals to provide optimal care for a broad range of patients, hinders efforts to stop the spread of HIV through a timely HIV diagnosis, and signals the need to improve national guidelines.
Introduction
Adequate identification and testing of people at risk for human immunodeficiency virus (HIV) is fundamental for the HIV care continuum. A timely diagnosis of HIV triggers linking people to care, and access to treatment, thus preventing transmission and improving individual health. In 2014, The Joint United Nations Programme on HIV/AIDS (UNAIDS) set the 90–90–90 goals to end the acquired immunodeficiency syndrome (AIDS) epidemic. This cascade of care represents the proportions of people that, by 2020, should be aware of their HIV status, on treatment, and virally suppressed, respectively. Achieving these goals facilitates the desired target of ending the HIV/AIDS epidemic by 2030 [1].
Unfortunately, as of 2020, the 90–90–90 goals had not been achieved in many European countries [2]. Some hopeful signals are however present. Compared to the period 2012 to 2015, the estimated proportion of the total number of people living with HIV (PLWH) who are undiagnosed were found to decrease in eastern Europe from 37% to 18% in 2020 [2]. In western Europe, the proportion of people with undiagnosed HIV decreased to 10% [3,4]. Consequently, the yearly number of new HIV diagnoses in the World Health Organization (WHO) defined European Region increased ca 10% to 136,449 from 2010 to 2019. The increase in new HIV diagnoses was mostly driven by eastern European cases, which also included more AIDS diagnoses than in western Europe. Overall, more than half of newly diagnosed persons with HIV in Europe present with advanced cellular immunodeficiency (CD4+ T-cells < 350 cells/µL), and 31% of all new HIV diagnoses are in the AIDS clinical stage [5]. Late presentations and delayed diagnoses increase morbidity and mortality, increase healthcare costs and fuel ongoing HIV transmission [6-8].
A key strategy, endorsed by the WHO and European Centre for Disease Prevention and Control (ECDC), to facilitate timely diagnosis is HIV indicator condition (IC) guided testing. It has been firmly established that HIV IC-guided testing is a cost-effective strategy to find HIV in conditions with an undiagnosed HIV prevalence ≥ 0·1% [9,10]. HIV ICs are also defined as those conditions where not identifying the presence of an HIV infection may have significant adverse health implications [11]. The HIV indicator Diseases across Europe Studies (HIDES) showed the effectiveness of this testing strategy in various settings throughout Europe [10,12]. Despite healthcare professionals regularly encountering patients with HIV ICs, HIV testing rates remain low [5]. Interventions increased HIV testing rates only temporarily with frequent regression to pre-interventional testing rates [13]. A more durable strategy is needed to ensure a widespread implementation of HIV IC-guided testing. As illustrated in other fields of medicine [14-16], national guideline recommendations from medical specialty societies are considered the standards of care and can have sustainable impact on clinical practices. Having HIV testing recommendations implemented universally in HIV IC guidelines should therefore have significant impact on clinical practice.
In light of the high rate of late presentation with HIV in Europe, the aim of this study was to determine the uptake of HIV testing recommendations in HIV IC-specific guidelines of all relevant medical specialties across countries in Europe. The identification of significant gaps would provide opportunities to assimilate HIV testing recommendations and change practice across medical disciplines.
Methods
Protocol
A systematic guideline review was conducted to analyse the uptake of HIV testing recommendations in HIV IC guidelines of European countries [17], and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. We developed a methodology [19], which was evaluated in a pilot study [20], using a predefined standard operating procedure (the standard operating procedure can be found in Supplement S1). This procedure was then disseminated to participating countries within the established infrastructure of the Optimising Testing and Linkage to Care for HIV across Europe (OptTEST) project [21].
Data sources and synthesis
The ECDC HIV IC guidance [11] was used to develop a list of 62 HIV ICs, including 25 AIDS-defining conditions (ADCs) and 37 non-AIDS-defining HIV ICs (a list of all ADCs and non-AIDS-defining HIV ICs can be found in Supplementary Table S1). One HIV IC (conditions requiring aggressive immune-suppressive therapy) was excluded from the analysis because it lacked a uniform definition leaving room for subjectivity.
Between 2019 and 2021, we approached HIV expert epidemiologists and medical specialists from 30 European countries affiliated to OptTEST. These experts were invited to participate in the current study via email, and reminders were sent upon non-response. A data lock was set in January 2022, which defined the final dataset used for analysis. The experts were asked to identify all relevant medical specialty guidelines for each ADC and non-AIDS-defining HIV IC by reviewing national guideline databases, national scientific medical specialty society websites, and national primary care physician guidelines. Search engines to identify guidelines used free and medical subject heading (MeSH) search terms, and keywords representing the specific HIV IC and guideline as queries (all databases and references used can be found in Supplement S2). Review instructions were to use all sources and register all identified guidelines, excluding duplicate findings. A negative search result was assumed to reflect a lack of guidelines for this HIV IC. Selected guidelines needed to be endorsed by a medical specialty society. As a reference from outside Europe, available HIV IC guidelines from the WHO on recommending HIV testing were reviewed.
The most up-to-date versions of HIV IC guidelines were used. Each guideline was reviewed by standardised study record forms (the standardised study record form can be found in Supplement S3). Reviewers classified guidelines as: (i) HIV not referenced in the guideline; (ii) association with HIV reported, but HIV testing not recommended; or (iii) association with HIV reported and HIV testing recommended [20]. Inter-observer agreement was checked by two inter-reviewer independent evaluations of all Dutch medical specialty guidelines and appeared sufficient with similar interpretations in 60 of 62 HIV ICs. Each study record form was collected centrally to evaluate missing data, inconsistencies or erroneous entries independently by three investigators (CRa, MLJ, CCEJ). Discrepancies were discussed with three other investigators (AS, CRo, MV). Queries were sent out to reviewers if considered necessary.
To obtain demographics of the HIV epidemic in the participating countries, the most recent available ECDC data were used [5,22-26]. We further stratified the outcomes based on region, UNAIDS 90–90–90 goals and medical specialty. Countries were geographically grouped into two regions according to the WHO European Region definition of western Europe (Belgium, Denmark, France, Germany, Greece, Italy, the Netherlands, Switzerland and the United Kingdom (UK)) and eastern Europe (Belarus, Lithuania, Poland, Romania, Russia and Ukraine). Countries were stratified based on whether they have achieved the UNAIDS 90–90–90 goals (Belgium, Denmark, the Netherlands, Switzerland and the UK) or not (Belarus, France, Germany, Greece, Italy, Lithuania, Poland, Romania, Russia and Ukraine) according to the latest ECDC country report (August 2021). Where no 2020 data were available, the most recent available data were used [2]. Additionally, HIV ICs were categorised according to the following medical specialties: dermatology/venereology, gastroenterology/hepatology, gynaecology/obstetrics, haematology, internal medicine, neurology/neurosurgery, ophthalmology, and pulmonology. Where a condition was treated by multiple specialties or different specialties between countries, a categorisation was performed according to the responsible specialty for the HIV IC in the Netherlands, or, if still unclear, according to the primary organ system affected (e.g. anal carcinoma belongs to gastroenterology) (All HIV ICs grouped per specialty can be found in Supplementary Table S2).
Data analysis
The primary outcomes were the proportion of HIV IC guidelines reporting a general association of the IC with HIV and the proportion explicitly recommending HIV testing. For the secondary outcomes, we evaluated outcomes according to relevant subgroups by country, eastern/western Europe, achievement of 90–90–90 goals, and per HIV IC or medical specialty. Finally, we developed HIV guideline covering cascades per country where we used three pillars: (i) the proportion of HIV ICs with at least one guideline available; (ii) of the HIV ICs with at least one guideline available, the proportion of at least one guideline available that mentions the relationship with HIV; and (iii) of the HIV ICs with at least one guideline available, the proportion with at least one guideline available that recommends HIV testing. Descriptive statistics are reported as number (percent) and median (interquartile range (IQR)). The associations of the HIV testing recommendations uptake and AIDS-defining status, setting and year of guideline publication was evaluated using chi-squared test. Data from the study record forms were tabulated and aggregated in a Microsoft Excel spreadsheet (Microsoft, Redmond, the United States (US)). Statistical analysis was conducted using SPSS version 25 (IBM, Armonk, US). A p value of ≤0.05 was considered statistically significant.
Results
Participating countries and available HIV indicator condition guidelines
Of the 30 European countries invited to take part, 15 participated and sent data before the data lock, including 10 where 90–90–90 goals were not yet achieved (Table 1). In 2020, these 15 countries had an accumulated estimated 1.9 million PLWH (83% of the total within the WHO European Region), of whom 336,000 were estimated to be undiagnosed (18%) [2]. A total of 791 relevant guidelines were identified, with a median number of guidelines per country of 47 (IQR: 38–68). The median number of guidelines per HIV IC was one (range: 0–15), covering ADCs (median 14, IQR: 11–20) and non-AIDS-defining HIV ICs (median 32, IQR: 24–45).
Table 1. Number of identified guidelines, number of reviewers, year of guideline publication, 90–90–90 goals and late HIV diagnosis per participating European country, 2019–2021 (n = 15).
| Country | Number of identified guidelines | Number of reviewers | Year of guideline publication, median (IQR) |
Estimated total people living with HIVa | People diagnosed with HIVa | People diagnosed with HIV on ARTa | People on ART who are virally suppresseda | Number of cases with CD4+ T-cell countb | People with late HIV diagnosis in 2020c
(CD4+ T-cells < 350 cells /µL)d |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||||
| Western Europe | ||||||||||||||
| Belgium | 31 | 1 | 2013 (2012–2017) |
18,335 | 16,594 | 91 | 15,238 | 92 | 14,299 | 94 | 500 | 70 | 203 | 41 |
| Denmark | 83 | 4 | 2019 (2018–2020) |
6,750 | 6,150 | 91 | 5,670 | 92 | 5,550 | 98 | 96 | 89 | 58 | 61 |
| France | 65 | 2 | 2013 (2009–2016) |
172,700 | 148,746 | 86 | 133,400 | 90 | 126,800 | 95 | 1,972 | 58 | 1,025 | 52 |
| Germany | 61 | 7 | 2018 (2014–2019) |
87,900 | 77,300 | 88 | 71,400 | 92 | 68,000 | 88 | 732 | 30 | 383 | 52 |
| Greece | 41 | 2 | 2015 (2015–2016) |
15,980 | 13,345 | 84 | 10,618 | 80 | NA | NA | 423 | 71 | 241 | 51 |
| Italy | 38 | 5 | 2015 (2012–2018) |
130,000 | 124,500 | 96 | 117,000 | 94 | 102,000 | 87 | 1,223 | 94 | 734 | 60 |
| The Netherlands | 71 | 3 | 2017 (2014–2018) |
23,300 | 21,360 | 92 | 19,913 | 93 | 19,046 | 96 | 371 | 94 | 190 | 51 |
| Switzerland | 34 | 1 | 2018 (2016–2019) |
16,700 | 15,500 | 93 | 15,000 | 97 | 14,800 | 99 | 168 | 59 | 88 | 52 |
| United Kingdom | 79 | 3 | 2011 (2009–2013) |
103,800 | 96,142 | 93 | 93,384 | 97 | 90,583 | 97 | 2,408 | 87 | 1,005 | 42 |
| Eastern Europe | ||||||||||||||
| Belarus | 47 | 1 | 2018 (2012–2018) |
26,000 | 22,084 | 85 | 11,714 | 80 | 13,575 | 77 | 1,105 | 78 | 398 | 36 |
| Poland | 37 | 3 | 2016 (2013–2018) |
15,166 | 12,385 | 82 | 10,496 | 85 | 10,052 | 96 | NA | NA | NA | NA |
| Lithuania | 52 | 2 | 2015 (2014–2017) |
3,397 | 2,827 | 77 | 1,223 | 43 | 920 | 75 | NA | NA | NA | NA |
| Romania | 29 | 4 | 2014 (2010–2015) |
18,000 | 16,486 | 92 | 12,644 | 77 | 8,064 | 64 | 407 | 93 | 228 | 56 |
| Russia | 44 | 1 | 2016 (2014–2017) |
998,525 | 808,823 | 81 | 319,613 | 40 | 271,671 | 85 | 57,071 | 96 | 16,150 | 27 |
| Ukraine | 76 | 3 | 2016 (2007–2016) |
251,168 | 169,787 | 58 | 136,105 | 80 | 127,871 | 94 | 13,791 | 89 | 7,513 | 55 |
ART: antiretroviral therapy; IQR: interquartile range; NA: not available.
a Data from Continuum of HIV Care, monitoring implementation Dublin 2020 [2].
b New HIV diagnoses in 2020 among persons > 14 years with CD4+ T-cell count levels reported.
c Completeness of number of cases with CD4+.
d Data from HIV/AIDS surveillance in Europe 2020 [5].
Participating countries were European countries involved in the established infrastructure of the Optimising Testing and Linkage to Care for HIV across Europe (OptTEST) project.
Specific guidelines for the following eight HIV ICs were available in all countries: cervical cancer, cervical dysplasia, hepatitis C, malignant lymphoma/non-Hodgkin’s lymphoma, Mycobacterium tuberculosis, pregnancy, primary lung cancer, and sexually transmitted infections (STIs) (Table 2). Of these, the ICs pregnancy and STIs had at least one guideline available in all participating countries that recommended HIV testing. However, none of the 62 HIV ICs had HIV testing recommendations included in all their available guidelines in any participating country. The ICs cervical cancer, cervical dysplasia, and primary lung cancer had guidelines available that recommended HIV testing in two of the participating countries. None of the countries identified a disease-specific guideline for the ADC disseminated penicilliosis. The ADCs coccidioidomycosis, histoplasmosis, progressive multifocal leukoencephalopathy, reactivation of American trypanosomiasis and the non-AIDS-defining HIV IC unexplained fever were only covered by guidelines in one or two countries. Of these, the disease-specific guideline reactivation of American trypanosomiasis did not mention HIV, half of the available guidelines for the other three ADCs recommended HIV testing and both available guidelines for unexplained fever recommended HIV testing.
Table 2. Number of participating European countries with at least one national guideline available per HIV indicator condition and countries with missing HIV indicator condition guidelines, 2019–2021 (n = 15).
| Speciality and HIV indicator condition | Number of countries with at least one guideline available | Number of countries with at least one guideline available that reports HIV association | Number of countries with at least one guideline available that recommends HIV testing | Countries missing the HIV IC guideline | Countries with guideline available for HIV IC without HIV test recommendation |
|---|---|---|---|---|---|
| Dermatology / venereology | |||||
| Herpes simplex, ulcer(s) > I month/bronchitis/pneumonitis | 10 | 6 | 4 | CH, DE, GR, LT, UK | BE, DK, NL, PL, RO, RU |
| Kaposi's sarcoma | 10 | 9 | 4 | BE, FR, GR, NL, RO | BY, DK, IT, LT, PL, UK |
| Herpes zoster | 13 | 10 | 8 | IT, LT | BE, GR, PL, RO, UK |
| Seborrhoeic dermatitis/exanthema | 6 | 5 | 1 | BE, CH, DE, FR, GR, IT, LT, PL, RO | BY, DK, NL, RU, UA |
| Severe or atypical psoriasis | 14 | 10 | 8 | BE | DK, FR, GR, LT, NL, UK |
| Sexually transmitted infections | 15 | 15 | 15 | None | None |
| Gastroenterology / hepatology | |||||
| Candidiasis, oesophageal | 7 | 6 | 2 | BY, CH, FR, IT, LT, PL, RO, RU | BE, DE, DK, GR, NL |
| Cryptosporidiosis diarrhoea, > 1 month | 10 | 9 | 4 | BE, CH, FR, RO, RU | DK, GR, LT, NL, PL, UK |
| Cystoisosporiasis (formerly known as Isosporiasis) > 1 month | 8 | 6 | 3 | CH, DE, DK, FR, LT, RO, RU | BE, GR, NL, PL, UK |
| Anal cancer/dysplasia | 13 | 12 | 8 | BE, RO | BY, GR, LT, RU, UA |
| Hepatitis A | 10 | 3 | 2 | BE, CH, IT, PL, UA | BY, DE, DK, FR, GR, LT, RO, UK |
| Hepatitis B (acute or chronic) | 13 | 13 | 12 | BE, CH | RU |
| Hepatitis C (acute or chronic) | 15 | 15 | 13 | None | BE, RU |
| Unexplained chronic diarrhoea | 6 | 4 | 1 | BE, CH, DE, DK, FR, IT, LT, PL, RU | BY, GR, NL, UA, UK |
| Gynaecology / obstetrics | |||||
| Cervical cancer | 15 | 7 | 2 | None | BE, CH, DE, DK, FR, GR, IT, LT, NL, PL, RO, RU, UK |
| Cervical dysplasia | 15 | 8 | 2 | None | BE, CH, DE, DK, FR, GR, IT, LT, NL, PL, RO, RU, UK |
| Pregnancy (implications for the unborn child) | 15 | 15 | 15 | None | None |
| Haematology | |||||
| Non-Hodgkin lymphoma | 14 | 14 | 13 | GR | RU |
| Castleman's disease | 4 | 3 | 2 | BE, CH, DE, FR, GR, IT, LT, NL, RO, UA, UK | BY, PL |
| Idiopathic/thrombotic thrombocytopenic purpura | 14 | 10 | 9 | LT | CH, DK, GR, PL, UA |
| Malignant lymphoma/Hodgkin's lymphoma | 15 | 15 | 14 | None | DK |
| Internal medicine | |||||
| Atypical disseminated leishmaniasis | 8 | 6 | 3 | BE, BY, CH, FR, GR, RO, RU | DK, IT, LT, NL, PL |
| Candidiasis, bronchial/tracheal/lungs | 7 | 3 | 1 | BY, CH, IT, LT, PL, RO, RU, UK | BE, DE, DK, F, GR, NL |
| Coccidioidomycosis, disseminated/extrapulmonary | 2 | 2 | 1 | BE, BY, CH, DE, FR, GR, IT, LT, NL, PL, RO, RU, UK | DK |
| Cryptococcosis, extrapulmonary | 5 | 4 | 2 | BY, CH, DE, FR, IT, LT, PL, RO, RU, UK | BE, GR, NL |
| Cytomegalovirus, other (except liver, spleen, glands) | 6 | 6 | 2 | CH, DE, DK, FR, GR, LT, RO, UA, UK | BE, NL, PL, RU |
| Histoplasmosis, disseminated/extrapulmonary | 2 | 1 | 1 | BE, BY, CH, DE, FR, GR, IT, LT, NL, PL, RO, RU, UK | DK |
| Penicilliosis, disseminated | 0 | 0 | 0 | BE, BY, CH, DE, DK, FR, GR, IT, LT, NL, PL, RO, RU, UA, UK | None |
| Reactivation of American trypanosomiasis (meningoencephalitis or myocarditis) | 2 | 0 | 0 | BE, BY, CH, DE, FR, GR, LT, NL, PL, RO, RU, UA, UK | DK, IT |
| Salmonella septicaemia, recurrent | 8 | 5 | 2 | BY, CH, FR, IT, PL, RO, UK | BE, DE, DK, GR, LT, NL |
| Candidaemia | 4 | 1 | 0 | BE, BY, CH, DK, FR, IT, LT, RO, RU, UA, UK | DE, GR, NL, PL |
| Candidiasis | 8 | 4 | 1 | CH, DE, DK, FR, LT, PL, UK | BE, GR, IT, NL, RO, RU, UA |
| Invasive pneumococcal disease | 5 | 3 | 0 | BY, CH, DE, FR, GR, IT, PL, RO, RU, UA | BE, DK, LT, NL, UK |
| Mononucleosis-like illness | 6 | 6 | 4 | BE, CH, DE, FR, GR, IT, LT, PL, RO | DK, NL |
| Oral hairy leukoplakia | 7 | 4 | 3 | BE, CH, FR, GR, LT, NL, PL, RO | DE, DK, IT, UK |
| Unexplained chronic renal impairment | 13 | 5 | 5 | CH, DK | BE, DE, GR, LT, NL, PL, RU, UK |
| Unexplained fever | 2 | 2 | 2 | BE, BY, CH, DE, FR, GR, IT, LT, NL, PL, RO, RU, UA | None |
| Unexplained leukocytopenia/thrombocytopenia lasting > 4 weeks | 8 | 7 | 6 | BE, BY, GR, IT, LT, RO, RU | DK, PL |
| Unexplained lymphadenopathy | 7 | 6 | 6 | BE, BY, CH, FR, GR, LT, NL, RO | PL |
| Unexplained oral candidiasis | 9 | 4 | 3 | BY, CH, DK, F, GR, LT | BE, DE, IT, NL, PL, UA |
| Unexplained weight loss | 4 | 2 | 1 | BE, BY, CH, FR, GR, IT, LT, NL, PL, RU, UA | DE, DK, UK |
| Visceral leishmaniasis | 6 | 5 | 2 | BE, BY, CH, DK, FR, IT, PL, RU, UK | GR, LT, NL, RO |
| Neurology / neurosurgery | |||||
| Cerebral toxoplasmosis | 8 | 8 | 3 | CH, DE, FR, IT, RO, RU, UK | DK, GR, LT, NL, PL |
| Primary cerebral lymphoma | 11 | 11 | 10 | CH, GR, NL, RO | BY |
| Progressive multifocal leukoencephalopathy | 2 | 1 | 1 | BE, BY, CH, DK, FR, GR, IT, LT, NL, PL, RO, RU, UK | DE |
| Cerebral abscess | 8 | 4 | 4 | DK, FR, IT, LT, NL, RO, UK | GR, PL, RU, UA |
| Guillain–Barré́ syndrome | 5 | 5 | 4 | BE, CH, FR, GR, IT, LT, PL, RO, UA, UK | NL |
| Lymphocytic meningitis | 9 | 7 | 4 | CH, IT, LT, PL, RO, RU | BE, BY, DK, NL, UA |
| Mononeuritis | 7 | 5 | 4 | BE, CH, DE, LT, PL, RO, RU, UA | FR, GR, UK |
| Multiple sclerosis-like disease | 8 | 5 | 3 | BE, CH, FR, IT, PL, RU, UA | BY, DK, GR, LT, NL |
| Peripheral neuropathy | 9 | 6 | 2 | BE, CH, LT, PL, RU, UA | BY, DE, FR, GR, IT, NL, UK |
| Primary space occupying lesion of the brain | 3 | 2 | 2 | BE, CH, DE, DK, F, IT, LT, NL, PL, RO, UA, UK | RU |
| Subcortical dementia | 10 | 9 | 6 | BY, FR, LT, PL, RU | BE, NL, UA, UK |
| Ophthalmology | |||||
| Cytomegalovirus retinitis | 7 | 7 | 3 | BE, CH, FR, GR, IT, LT, RO, UK | DK, NL, PL, RU |
| Infective retinal diseases, including herpes viruses and toxoplasma | 5 | 2 | 1 | BE, CH, DE, DK, FR, GR, IT, NL, RO, RU | LT, PL, UA, UK |
| Pulmonology | |||||
| Mycobacterium avium complex or Mycobacterium kansasii, disseminated or extrapulmonary | 7 | 6 | 4 | BE, CH, FR, GR, IT, LT, NL, RU | DE, DK, PL |
| Mycobacterium, other species or unidentified species, disseminated or extrapulmonary | 3 | 2 | 2 | BE, BY, CH, DE, DK, FR, GR, IT, LT, NL, RU, UA | PL |
| Mycobacterium tuberculosis, pulmonary or extrapulmonary | 15 | 14 | 12 | None | PL, RU, UK |
| Pneumocystis carinii pneumonia | 9 | 7 | 4 | CH, DE, FR, IT, PL, UK | BE, GR, LT, NL, RU |
| Pneumonia, recurrent (2 or more episodes in 12 months) | 11 | 4 | 2 | FR, IT, PL, RO | BE, BY, DE, DK, LT, NL, RU, UA, UK |
| Community-acquired pneumonia | 14 | 5 | 2 | RO | BE, BY, CH, FR, GE, IT, LT, NL, PL, RU, UA, UK |
| Primary lung cancer | 15 | 4 | 2 | None | BE, BY, CH, DE, DK, GR, IT, LT, NL, PL, RO, RU, UK |
BE: Belgium, BY: Belarus; CH: Switzerland; DE: Germany; DK: Denmark; FR: France; GR: Greece; IT: Italy; LT: Lithuania; NL: the Netherlands; PL: Poland; RO: Romania; RU: Russia; UA: Ukraine; UK: United Kingdom.
AIDS-defining conditions are written in bold.
Participating countries were European countries involved in the established infrastructure of the Optimising Testing and Linkage to Care for HIV across Europe (OptTEST) project.
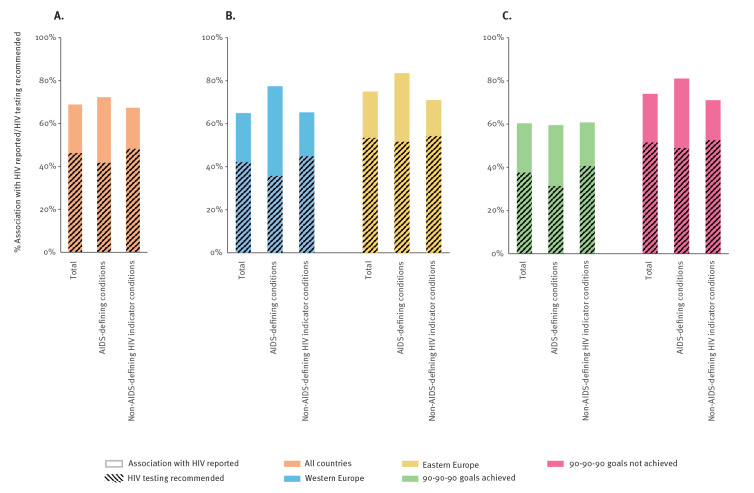
Overall, the participating countries had at least one guideline available for 57% of the HIV ICs, including 56% and 58% for western (n = 9) and eastern (n = 6) European countries, respectively. Overall, 545 of the 791 (69%) identified guidelines reported the association with HIV and 366 of the 791 (46%) guidelines recommended HIV testing (The total number and proportions of identified HIV IC guidelines that report the association with HIV and recommend HIV testing overall, geographically ordered and according to achieved 90-90-90 goals can be found in Supplementary Table S3). Furthermore, 175/242 (72%) ADC guidelines and 370/549 (67%) non-AIDS-defining HIV IC guidelines reported an association with HIV, and 101/242 (42%) ADC guidelines and 265/549 (48%) non-AIDS-defining HIV IC guidelines recommended HIV testing (p = 0.089, Figure 1A). The recommendation to test was not associated with the year of guideline publication (p = 0.13) (The total number and proportions of identified HIV IC guidelines per publication year can be found in Supplementary Table S4). Using WHO guidelines as a reference, we found higher reported HIV association (overall 91%; 100% ADCs vs 88% non-AIDS-defining HIV ICs) and comparable testing recommendation rates (overall 50%; 56% ADCs vs 48% non-AIDS-defining HIV ICs) in the 34 guidelines covering 14 HIV ICs (4 ADCs and 10 non-AIDS-defining HIV ICs) (The total number and proportions of identified HIV IC guidelines in the WHO guideline database can be found in Supplementary Table S5).
Figure 1.
Proportions of identified HIV indicator condition guidelines that report the association with HIV (solid boxes) and recommend HIV testing (dashed boxes) for (A) all countries, (B) western Europe vs eastern Europe, (C) 90-90-90 goals achieved vs not achieved, 2019–2021 (n = 15)
The association with HIV was more frequently reported in HIV IC guidelines from eastern European countries and those countries yet to achieve the 90–90–90 goals (Figure 1B and 1C). The 288 guidelines identified in eastern European countries showed overall higher test recommendations than the 503 guidelines identified in western European countries (53% vs 42%, p = 0.002). This was mostly driven by the more frequent HIV testing recommendations in available ADC guidelines. A higher HIV testing recommendation uptake in guidelines was also observed in the countries that did not achieve the 90–90–90 goals than those that did achieve these goals (52% of 493 guidelines vs 38% of 298 guidelines, p < 0.001).
There was no HIV IC that had HIV testing universally recommended in all identified guidelines and had at least one guideline available in all participating countries (The total number and proportions of HIV IC guidelines that report HIV and recommend HIV testing per HIV IC can be found in Supplementary Table S6). When further evaluating the guidelines that were available in these countries, we found that the disease-specific guidelines for coccidioidomycosis, mononucleosis-like illness, non-Hodgkin lymphoma, and unexplained fever had a 100% coverage of mentioning the relationship with HIV. However, substantial differences were present in the total number of available guidelines per HIV IC, ranging from 0 to 76. Of the 62 HIV ICs, only one HIV IC (1.6%), unexplained fever, had 100% coverage of HIV testing recommendations, and four HIV ICs (6.5%), non-Hodgkin lymphoma, pregnancy, primary cerebral lymphoma and unexplained leukocytopenia/thrombocytopenia had more than 75% coverage of testing recommendations among the available guidelines. HIV testing recommendations were not included in any guidelines of American trypanosomiasis, candidaemia or invasive pneumococcal disease. In western Europe, the guidelines for candidiasis, candidaemia, cervical cancer, cervical dysplasia, oral hairy leukoplakia, Salmonella septicaemia, unexplained chronic diarrhoea and unexplained weight loss lacked any HIV testing recommendation, and in eastern Europe this applied to community-acquired pneumonia, recurrent pneumonia, and seborrhoeic dermatitis/exanthema (The total number and proportions of HIV IC guidelines that report HIV and recommend HIV testing per HIV IC geographically grouped can be found in Supplementary Table S7). In countries not yet achieving the 90–90–90 goals, 56 HIV ICs had at least one guideline with HIV testing recommendations available, although the median overall testing recommendation rates remained at 53% (The total number and proportions of HIV IC guidelines that report HIV and recommend HIV testing per HIV IC grouped according to achieved 90-90-90 goals can be found in Supplementary Table S8).
HIV testing recommendation gaps in medical non-HIV specialty guidelines
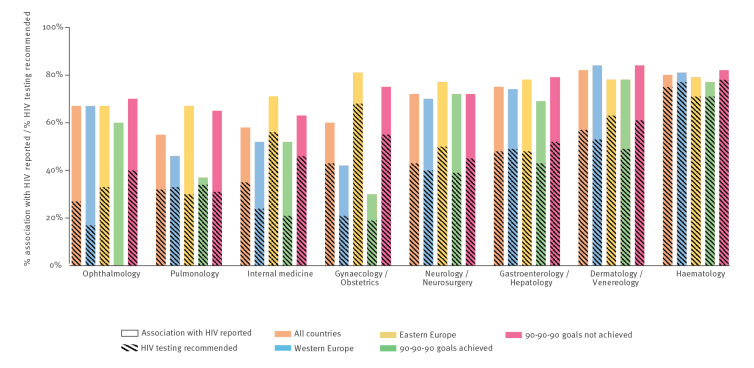
Given the central role of medical specialty societies in national guideline development, we also analysed HIV testing recommendation uptake according to medical specialty (Figure 2) (The total number and proportions of HIV IC guidelines that report HIV and recommend HIV testing per HIV IC grouped according to achieved 90-90-90 goals can be found in Supplementary Table S9). Compared with the overall uptake of HIV testing recommendations in HIV IC guidelines (46%), guidelines for the specialties of dermatology/venereology, gastroenterology/hepatology, and haematology recommended HIV testing more often (range 48–75%). Guidelines on haematological conditions that at least reported the association with HIV (80%) also had the highest HIV testing recommendation rate (75%), whereas the average proportion of guidelines from all medical specialties that mentioned an association with HIV was 69%, with just 46% also recommending testing. The specialties gynaecology, internal medicine, neurology/neurosurgery, ophthalmology and pulmonology reported fewer overall HIV testing recommendation rates (range 27–43%). The largest discrepancies in the HIV testing recommendation uptake of guidelines between western and eastern European countries, and between countries with and without achieved 90–90–90 goals, were found in the specialties gynaecology (21% vs 68% and 19% vs 55%) and internal medicine (24% vs 56% and 21% vs 46%). Ophthalmology and pulmonology had the poorest reporting of HIV testing recommendations both overall (27% and 32%, respectively) and across all variables (western/eastern European countries and 90-90-90 goals achieved or not) (range 0–40% and 30–34%, respectively).
Figure 2.
The proportions of identified HIV indicator condition guidelines that report the association with HIV and recommend HIV testing for all countries, for western and eastern European countries, and according to whether the 90–90–90 goals have been achieved, per specialty, 2019–2021 (n = 15)
HIV indicator condition guideline coverage cascade
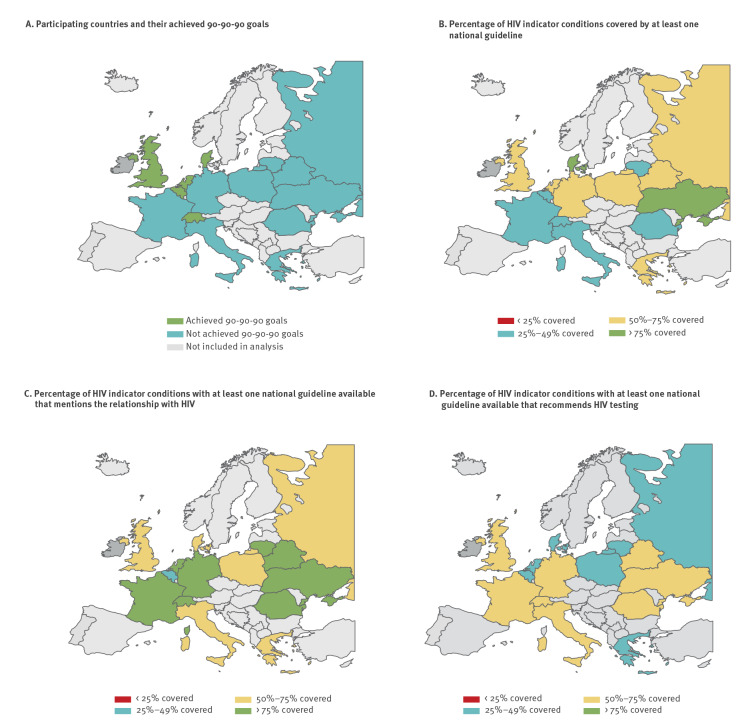
We analysed the HIV guideline coverage cascade in all participating countries and related it to the achievement of the 90–90–90 goals as of December 2021 (Figure 3A). The first pillar of the HIV IC guideline covering cascade (HIV IC guideline availability) was lowest in Switzerland (31%) and France (39%), and highest in Ukraine (76%) and Denmark (77%) (Figure 3B). The second pillar which represented mentioning the relationship with HIV where a guideline was available was lowest in Belgium (47%), and > 75% in Belarus, France, Germany, Lithuania, the Netherlands, Romania, Switzerland, and Ukraine (range 79–98%) (Figure 3C). Relevant gaps in the uptake of HIV testing recommendations where a guideline was available (third pillar) were identified in all countries (Figure 3D). The median coverage of the third pillar was 50% (IQR: 31–68%) with seven countries having HIV testing recommendations included in less than 50% of their available HIV IC guidelines (range 29–46%). The coverage of the third pillar was higher in eastern European countries than in western European countries (55% vs 46%) and in countries that had not yet achieved the 90–90–90 goals (54%) vs countries that had reached these goals (41%).
Figure 3.
HIV indicator condition guideline coverage cascades for countries (A) that have achieved or not achieved the 90–90–90 goals, (B) the proportion of HIV indicator condition covered by at least one national guideline, (C) the proportion of the available guidelines with at least one guideline available that mentions the relationship with HIV, (D) the proportion of the available guidelines with at least one guideline available that recommends HIV testing, 2019–2021 (n = 15)
Discussion
This European guideline review demonstrates that fewer than half of the national guidelines for HIV ICs recommend HIV testing. This observation was consistent across a wide range of HIV ICs, countries and medical specialties. Importantly, many guidelines for those HIV ICs known to be ADCs lacked HIV testing recommendations. These findings highlight clinically relevant gaps throughout Europe in the representation of HIV testing recommendations in disease-specific guidelines for HIV ICs. Since clinical practice guidelines represent a cornerstone of clinical medicine, ensuring guidelines for HIV ICs with a universal uptake of HIV testing recommendations should improve good clinical practice for people with undiagnosed HIV as it has in other fields of HIV medicine [14-16,27]. Ultimately, a timely HIV diagnosis can expedite treatment initiation which prevents disease progression, death and onward HIV transmission [28,29].
We did find some promising signals in our data. Firstly, all countries that did not yet achieve the 90-90-90 goals, and all eastern European countries regardless of whether they had achieved the 90-90-90 goals or not, had a higher uptake of HIV testing recommendations in their guidelines. Accurate HIV testing recommendations in guidelines can be regarded as the first step to better implementation of HIV testing in daily practice. The uptake of HIV testing recommendations in guidelines may be associated with improved identification of PLWH, as illustrated by the decreasing number of those undiagnosed in eastern Europe, although other factors likely determine the accurate testing in clinical practice, including available resources and stigma to test in these settings. Secondly, regarding medical specialties, haematology guidelines had a superior HIV testing recommendation uptake in available guidelines of related HIV ICs, with a homogenous pattern across Europe. Thirdly, two prevalent HIV ICs (pregnancy and STIs) had guidelines available in all European countries with at least one guideline recommending HIV testing in every country. This indicates that a high assimilation of HIV testing recommendations in guidelines across the European continent is possible.
Our study adds evidence to the current knowledge on the uptake of HIV testing recommendations in HIV IC guidelines. Studies conducted in the UK, Greece and Australia found HIV testing recommendations in 26% to 38% of the guidelines [20,30,31]. We introduce the concept of HIV IC guideline coverage cascades which can help monitor the current uptake and future progress of HIV testing recommendations and allow comparisons between settings and across time. In addition, our study highlights a more general omission in incorporating medical guidance from the WHO and ECDC on HIV IC-guided testing into national non-HIV specialty guidelines. This may indicate a possible lack of HIV expertise within these medical specialties’ guideline panels. Although medical specialties responsible for HIV care differ throughout Europe, no specialty comes close to a universal uptake of HIV testing recommendations. In order to begin to address this, we suggest consideration should be given to involving HIV medical specialists in relevant guideline development, better education of healthcare professionals on HIV IC-guided testing and support from national HIV patient associations. Where national HIV testing guidelines exist (or the country has a policy to follow ECDC or WHO guidelines), guideline authors and policymakers should be made aware of, and called on, to correct the lack of consistency across guidelines and policies. Informing and enabling the public to test for HIV upon indication and ensuring destigmatised HIV testing options for everyone remain important components which, if insufficiently ensured, can considerably hinder translation of guidelines into clinical practice and linkage to care [32-34].
This study has a number of potential limitations. Firstly, only half of the 30 countries approached provided data for this analysis. However, the countries included are the main European countries of the continental epidemic, which together cover ca 83% of the undiagnosed PLWH in Europe. Also, the cultures and 90–90–90 goals of the included countries are comparable to the countries that were approached, but did not provide any data for this review, making generalisability likely. Secondly, we acknowledge that subjectivity in data collection may have biased our findings. Therefore, several measures were taken to mitigate this. We used a standard operating procedure for the search strategy that was previously validated in one country. The data collection was focused on three simple key variables to limit the risk of individual interpretation. Thirdly, the level of the reviewers’ HIV expertise varied and the possibility to search for guidelines by online search engines could have introduced reporting bias. A pilot we ran in one country indicated that the same procedural instructions resulted in a high inter-observer guideline review interpretation agreement. This implies that there is no large systematic error in the HIV IC guideline coverage cascades. Fourthly, we cannot exclude the possibility that guidelines have been missed. However, the reviewers were recruited because of their HIV expertise and multiple reviewers were generally available per country, which reduced the risk of missing relevant guidelines. Finally, we focused on the availability of official national guidelines only. By not taking the potential recommended use of international guidelines for HIV ICs into account by physicians, we risk underestimating the actual availability and use of HIV IC-specific guidelines in practice in certain specialities in some countries. However, we felt that the presence of national guidelines in native languages and including country-specific information was optimal for supporting setting-specific HIV IC-guided testing.
Conclusion
Within the European continent, HIV IC guidelines consistently missed opportunities to recommend HIV testing, contributing to the high levels of undiagnosed HIV and late HIV presentations observed across Europe. Given their influence on clinical practice, inclusion of HIV testing recommendations should improve HIV awareness and HIV testing practices among physicians, allowing earlier linkage to care and leading to reductions in late presentations and transmission.
Ethical statement
No ethical approval was required for this study as this study does not contain any involvement of human participants and all data gathered in this article are freely available in the public domain.
Data availability statement
Data are available upon request from the corresponding author.
Funding statement
This submitted work arises partly from the project Optimising testing and linkage to care for HIV across Europe (OptTEST by HiE) and the Joint Action on integrating prevention, testing and linkage to care strategies across HIV, viral hepatitis, TB and STIs in Europe (INTEGRATE), which both have received funding from the European Union under the framework of the 2nd and 3rd Health Programme respectively, and the #aware.hiv project (www.awarehiv.com) that received funding from the Federation of Medical Specialist (FMS) Kwaliteitsgelden Medisch Specialisten (SKMS) and Aidsfonds P-61803.
Acknowledgements
We thank all medical professionals and patient representatives who contributed to the data collection.
Supplementary Data
Supplementary Data
Supplementary Data
Supplementary Data
Conflict of interest: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: This submitted work arises partly from the project Optimising testing and linkage to care for HIV across Europe (OptTEST by HiE) and the Joint Action on integrating prevention, testing and linkage to care strategies across HIV, viral hepatitis, TB and STIs in Europe (INTEGRATE), which both have received funding from the European Union under the framework of the 2nd and 3rd Health Programme respectively.
Outside the submitted work: CRo has received research grants from ViiV Healthcare, ZonMW, AIDSfonds, Erasmus MC, and Health~Holland and honorariums for advisory boards from Gilead and ViiV Healthcare; CDS has received grants, personal fees and non-financial support from AbbVie, Gilead Sciences, Janssen-Cilag, MSD, Cepheid, GSK, and ViiV Healthcare during the conduct of the study; and fees, grants or non-financial support from AstraZeneca, Apeiron, BBraun Melsungen, Eli Lilly, Formycon, Molecular partners, and SOBI; JJM received consulting fees from MAPLE health group, reimbursements for travel expenses from Gilead Sciences, ViiV Healthcare and Correvio Pharma, institutional research funding from the German Center for Infection Research and the US. National Institutes of Health; RM has received consulting fees from ViiV Healthcare, GSK, MSD, payment for lectures from ViiV Healthcare, GSK, MSD, KRKA, “Baltijos idėjų grupė ir partneriai“, institutional research funding from WEEPI foundation, payement for expert testimory from ViiV Healthcare, GSK, support for meeting and/or travel from Abbvie, Johnson and Johnson, MSD; EGK has been supported for attending meetings and travel from Gilead Sciences; KA has received research grants from Gilead, honorariums for lectures and advisory boards from Gilead, Angelini, ViiV Healthcare, Mylan/Viatrix, Pfizer Hellas and travel grants from Gilead, Angelini, ViiV Healthcare, Mylan/Viatrix, Pfizer Hellas and MSD Greece; ND is a postdoctorate clinical master specialist of the F.R.S.-FNRS and has received personal fees from Roche and Boehringer-Ingelheim, and non-financial support from Pfizer, Janssen, and Merck Sharp & Dohme, outside the submitted work; AH has received reimbursement of travel expenses and congress fees from MSD and Gilead and participated in advisory boards from MSD; GMNB received honorariums for advisory boards or lecturing from Gilead, ViiV Healthcare, MSD, Janssen, and Virology Education; AP has received reimbursement of congress fees from MSD and GSK, honoraria for lectures from Gilead Sciences and MSD; KC has received honoraria for presentations and one advisory board from Gilead; AG has received consultancy fees from Mylan and educational and travel support from Gilead Sciences and ViiV Healthcare; TSB is a fellow of the ECDC Fellowship Programme, supported financially by the European Centre for Disease Prevention and Control. The views and opinions expressed herein do not state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
No other relationships or activities that could appear to have influenced the submitted work.
Authors’ contributions: CCEJ, CRa, MLJ, AKS and CRo designed the study. All authors contributed to data acquisition and interpretation. AV reviewed the available guidelines for Belarus, ND for Belgium, MLJ, AG, SFJ and AR for Denmark, KC and EP for France, JJM, TSB, GMNB, HG, AN, CDS and FV for Germany, KA and EGK for Greece, SN, ALG, GL, MM and CT for Italy, RM and EM for Lithuania, CCEJ, BJVW and KSH for the Netherlands, MT, MAB and ASK for Poland, OS, ASC1, ASC2 and VDM for Romania, AP for Russia, AH for Switzerland, MV, AD and MS for Ukraine and CR, EL and AS for the United Kingdom. Country data collection was coordinated by MLJ and CRa. CCEJ structured the data and conducted the analyses. CCEJ, MV and CRo drafted the work. All authors critically reviewed the manuscript for important intellectual content, take responsibility for the work and approved the final version of the manuscript. AKS and CRo supervised the study. Patient involvement occurred extensively within the OptTEST programme with representation of the steering group, working groups and with feedback from representatives of the patient community from different countries on all programme-related tools. In line with the overarching aim of this study, our group established the infrastructure to share datasets with a wide range of policy makers, physicians, patient representatives and the public upon motivated request to the corresponding author. We support data sharing with (inter)national scientific medical specialty associations, HIV patient associations, and physician/public focused initiatives promoting destigmatised testing practices.
References
- 1.The Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90- An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2017. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90
- 2.European Centre for Disease Prevention and Control (ECDC). HIV Continuum of care: Monitoring implementation of the Dublin Declaration on partnership to fight HIV/AIDS in Europe and Central Asia (2020 progress report). Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/hiv-continuum-care-monitoring-implementation-dublin-declaration
- 3.European Centre for Disease Prevention and Control (ECDC). Continuum of HIV care. Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2017 progress report. Stockholm: ECDC; 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Continuum-of-HIV-care-2017.pdf
- 4.European Centre for Disease Prevention and Control (ECDC). Continuum of HIV care. Monitoring implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2018 progress report. Stockholm: ECDC; 2018. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/HIV-continuum-of-care-monitoring-dublin-declaration-progress-report-2018.pdf
- 5.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2020 (2019 data). Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillance-report-2020.pdf
- 6. Fleishman JA, Yehia BR, Moore RD, Gebo KA, HIV Research Network . The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48(12):1071-9. 10.1097/MLR.0b013e3181f81c4a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Late Presentation Working Groups in EuroSIDA and COHERE . Estimating the burden of HIV late presentation and its attributable morbidity and mortality across Europe 2010-2016. BMC Infect Dis. 2020;20(1):728. 10.1186/s12879-020-05261-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grabmeier-Pfistershammer K, Rieger A, Schröck T, Schlag M. Economic burden of late presentation in HIV disease in Austria: a comparison of the initial costs imposed by advanced HIV disease vs. non-late presentation. Wien Klin Wochenschr. 2013;125(13-14):402-7. 10.1007/s00508-013-0392-5 [DOI] [PubMed] [Google Scholar]
- 9. Bert F, Gualano MR, Biancone P, Brescia V, Camussi E, Martorana M, et al. Cost-effectiveness of HIV screening in high-income countries: A systematic review. Health Policy. 2018;122(5):533-47. 10.1016/j.healthpol.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 10. Sullivan AK, Raben D, Reekie J, Rayment M, Mocroft A, Esser S, et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study). PLoS One. 2013;8(1):e52845. 10.1371/journal.pone.0052845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HIV in Europe. HIV indicator conditions: guidance for implementing HIV testing in adults in health care settings. Copenhagen: HIV in Europe. [Accessed: 17 Dec 2021]. Available from: www.eurotest.org/portals/0/Guidance.pdf.pdf
- 12. Raben D, Sullivan AK, Mocroft A, Kutsyna G, Hadžiosmanović V, Vassilenko A, et al. Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study - 2012 - 2015. PLoS One. 2019;14(8):e0220108. 10.1371/journal.pone.0220108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cayuelas Redondo L, Ruíz M, Kostov B, Sequeira E, Noguera P, Herrero MA, et al. Indicator condition-guided HIV testing with an electronic prompt in primary healthcare: a before and after evaluation of an intervention. Sex Transm Infect. 2019;95(4):238-43. 10.1136/sextrans-2018-053792 [DOI] [PubMed] [Google Scholar]
- 14. Schaefer R, Schmidt HA, Ravasi G, Mozalevskis A, Rewari BB, Lule F, et al. Adoption of guidelines on and use of oral pre-exposure prophylaxis: a global summary and forecasting study. Lancet HIV. 2021;8(8):e502-10. 10.1016/S2352-3018(21)00127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogers AJ, Akama E, Weke E, Blackburn J, Owino G, Bukusi EA, et al. Implementation of repeat HIV testing during pregnancy in southwestern Kenya: progress and missed opportunities. J Int AIDS Soc. 2017;20(4):e25036. 10.1002/jia2.25036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Townsend CL, Cliffe S, Tookey PA. Uptake of antenatal HIV testing in the United Kingdom: 2000-2003. J Public Health (Oxf). 2006;28(3):248-52. 10.1093/pubmed/fdl026 [DOI] [PubMed] [Google Scholar]
- 17. Ioannidis JPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181(8):488-93. 10.1503/cmaj.081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European AIDS Clinical Society (EACS). HIV indicator condition guidelines review. A quick guide on how to start. Brussels: EACS; 2018. Available from: https://www.eacsociety.org/media/ic_guideline_review_guide.pdf
- 20. Lord E, Stockdale AJ, Malek R, Rae C, Sperle I, Raben D, et al. British Association of Sexual Health HIV (BASHH)/British HIV Association (BHIVA) guideline review group for the Optimising Testing and Linkage to Care for HIV across Europe (OptTEST) project by HIV in Europeb. Evaluation of HIV testing recommendations in specialty guidelines for the management of HIV indicator conditions. HIV Med. 2017;18(4):300-4. 10.1111/hiv.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OptTest. Optimising testing and linkage to care for HIV. [Accessed: 2 Feb 2022]. Available from: https://www.opttest.eu/
- 22.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2018 (2017 data). Stockholm: ECDC; 2018. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-aids-surveillance-europe-2018.pdf
- 23.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2017 (2016 data). Stockholm: ECDC; 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/20171127-Annual_HIV_Report_Cover%2BInner.pdf
- 24.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2015. Stockholm: ECDC; 2015. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/HIV-AIDS-surveillance-Europe-2015.pdf
- 25.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2019 (2018 data). Stockholm: ECDC; 2019. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillance-report-2019.pdf
- 26.European Centre for Disease Prevention and Control (ECDC) and World Health Organization. (WHO). HIV/AIDS surveillance in Europe 2014. Stockholm: ECDC; 2014. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/hiv-aids-surveillance-in-Europe-2014.pdf
- 27.Hensley KS, Jordans CCE, van Kampen JJA, Mollema FPN, Gisolf EH, El Moussaoui R, et al. Significant impact of COVID-19 on HIV care in hospitals affecting the first pillar of the HIV care continuum. Clin Infect Dis. 2021. [DOI] [PMC free article] [PubMed]
- 28. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428-38. 10.1016/S0140-6736(19)30418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397-404. 10.1097/QAD.0b013e32832b7dca [DOI] [PubMed] [Google Scholar]
- 30. Lin YD, Eades L, Nair A, Korman TM, Woolley I. Review of HIV testing recommendations in Australian specialty guidelines for HIV indicator conditions: a missed opportunity for recommending testing? Intern Med J. 2020;50(3):293-8. 10.1111/imj.14641 [DOI] [PubMed] [Google Scholar]
- 31. Akinosoglou K, Kostaki EG, Paraskevis D, Gogos CA. Assessment of HIV testing recommendations in Greek specialty guidelines: A missed opportunity and room for improvement for recommending testing. AIDS Care. 2021;33(10):1312-5. 10.1080/09540121.2021.1876837 [DOI] [PubMed] [Google Scholar]
- 32. Elgalib A, Fidler S, Sabapathy K. Hospital-based routine HIV testing in high-income countries: a systematic literature review. HIV Med. 2018;19(3):195-205. 10.1111/hiv.12568 [DOI] [PubMed] [Google Scholar]
- 33. Mohajer MA, Lyons M, King E, Pratt J, Fichtenbaum CJ. Internal medicine and emergency medicine physicians lack accurate knowledge of current CDC HIV testing recommendations and infrequently offer HIV testing. J Int Assoc Physicians AIDS Care (Chic). 2012;11(2):101-8. 10.1177/1545109711430165 [DOI] [PubMed] [Google Scholar]
- 34. Power L, Hows J, Jakobsen SF. OptTEST by HIV in Europe. Key findings on legal and regulatory barriers to HIV testing and access to care across Europe. HIV Med. 2018;19(Suppl 1):58-62. 10.1111/hiv.12591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.