Abstract
Objectives
This study aims to examine the association between migraine and various psychiatric and somatic comorbidities in Japan.
Design
Cross-sectional study using existing data of the 2017 Japan National Health and Wellness Survey (NHWS).
Setting
Nationally representative sample of persons (in terms of age and gender) living in the general community aged 18 years or older in Japan.
Participants
Out of a sample of 30 001 NHWS respondents, 378 respondents were identified as migraine patients and 25 209 were identified as non-migraine patients. After propensity score (PS) matching (1:4), 1512 matched non-migraine respondents were identified.
Primary and secondary outcome measures
Prevalence and PS-matched prevalence ORs (PORs) were assessed for each psychiatric and somatic comorbidity among migraine patients and matched non-migraine respondents (including migraine patients with less than 15 monthly headache days (MHDs) and migraine patients with more than 15 MHDs).
Results
Migraine patients were predominately female and had significantly higher prevalence than matched non-migraine respondents to have psychiatric and somatic comorbidities. Psychiatric comorbidities with >5% prevalence among migraine patients included depression, post-traumatic stress disorder and anxiety disorders, while gastrointestinal disorders were the most prevalent somatic comorbidity category. Other somatic comorbidities included allergies, insomnia, premenstrual syndrome and anaemia. Migraine patients with more than 15 MHDs tended to have higher point estimates for POR.
Conclusion
Psychiatric and somatic conditions were more prevalent in migraine patients than matched non-migraine respondents, some being novel associations not previously reported in Japan. This study provided insights on comorbidities, which could complicate care, clinical practice and outcomes among migraine patients.
Keywords: Migraine, PUBLIC HEALTH, Quality in health care
STRENGTHS AND LIMITATIONS OF THIS STUDY.
An age-and-gender-stratified sampling frame was imposed during the recruitment to ensure and reflection of the general adult population of Japan in terms of age and gender.
The study identified potential psychiatric and somatic comorbidities from self-reported medical history of migraine patients as well as several covariates including sociodemographic factors, general health characteristics and migraine-specific covariates.
This study uses an online survey and thus respondents without internet access were not included in the study.
This study assessed respondents’ self-declaration of migraine manifestation and comorbidities which may bias the prevalence OR and the causal relationship between migraine and comorbidities cannot be concluded.
Introduction
Migraine is a common debilitating neurological disorder that is highly prevalent globally.1 The global prevalence of migraine was approximately 14.0%, with annual prevalence estimates increasing over the years.2 In Japan, the estimated prevalence of migraine among the general population was 6% in 2004,3 and more recently, 9% among working, socially active individuals in the Tokyo metropolitan area.4
Previous reports demonstrated that approximately 90% of chronic migraine patients have at least one comorbidity.5 A nationwide population-based study in the USA reported an association between migraine comorbidities and the intensity of headache pain and frequency.6 It is important to take into consideration the comorbidities of migraine from diagnosis through to treatment.7 The previously reported comorbidities of migraine include various psychiatric8–11 and somatic conditions such as cardiovascular disorders,6 12 13 gastrointestinal disorders,6 14–16 allergy-related disorders,6 17 18 sleep disorders6 19 20 and gynaecological disorders.21 A meta-analysis showed that the most frequently addressed comorbidities among migraine patients in clinical and population studies were depressive disorders, hypertension and anxiety disorders.22
Studies have shown a strong bidirectional association between migraine and psychiatric conditions such as depression, anxiety, suicide risks,9 10 and may have shared neuropathic mechanisms.11 23 Similarly, somatic comorbidities such as those of cerebrovascular and metaboloendocrine (eg, stroke, insulin sensitivity, hypothyroidism, endometriosis) also reportedly have bidirectional association with migraine.23
In Japan, there is an evidence gap on the comorbidities of migraine. Comorbidities such as hypertension, heart diseases, cerebrovascular diseases, depression, bipolar disorder, anxiety disorder, epilepsy, asthma, allergic diseases and autoimmune diseases had been recognised as common migraine comorbidities in the Clinical Practice Guideline for Chronic Headache.24 Although there were a few studies investigating the comorbidities of migraine in Japan, previous studies primarily focused on one disease or one category of diseases.25 26 However, to our knowledge, no studies have been published that assessed a range of comorbidities associated with migraine referencing non-migraine individuals in Japan.
Therefore, the objectives of this study were to identify a list of potential psychiatric and somatic comorbidities experienced by migraine patient as well as examine the association between migraine and these psychiatric and somatic comorbidities in Japan by assessing the prevalence OR (POR) between migraine and these comorbidities, with reference to non-migraine individuals. The initial findings from this study on the potential comorbidities of migraine may provide insights to learning the aetiology of migraine and help physicians and patients with effective migraine management and treatment.
Methods
This study used existing data collected from a cross-sectional online survey, the 2017 Japan National Health and Wellness Survey (NHWS). Individuals aged 18 years or older were recruited to the NHWS through an existing, general-purpose (ie, not healthcare-specific) web-based consumer panel. All panellists explicitly agreed to join the panel and receive periodic invitations to participate in online surveys. An age-stratified and gender-stratified sampling frame, based on Japan governmental census data, was imposed during the recruitment from the panellists. This was to ensure representativeness of panellists who completed the NHWS to reflect the general adult population of Japan in terms of age and gender.
Study population
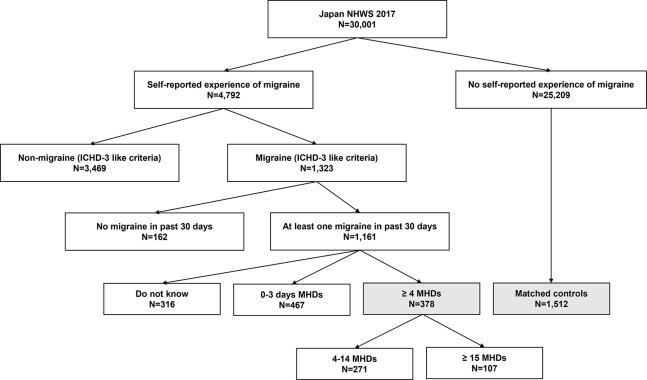
Migraine patients in this study were defined by ICHD-3 (International Classification of Headache Disorders, third Edition)-like criteria which were detailed in a previous publication using the same data,27 among respondents who self-reported migraine in the past 12 months and self-reported having at least 4 monthly headache days (MHDs) (figure 1). The ICHD-3-like criteria were based on the diagnostic criteria from ICHD-3 but modified for the variables available in the existing NHWS data.27 The ICHD-3-like inclusion criteria also define migraine respondents without aura as having at least five migraines in the past 6 months or self-reported physician diagnosis of migraine, migraine lasting for at least 4 hours but not more than 72 hours if untreated, experienced at least two migraine pain symptoms (pain on one side of head; pulsating, throbbing or pounding pain; moderate-to-severe pain or pain made worse by routine), and experienced at least in one migraine-related symptom (nausea and/or vomiting; light hypersensitivity or sound hypersensitivity). Migraine with aura was defined as respondents having at least two migraines in the past 6 months or self-reported physician diagnosis of migraine, and experience migraine-related symptom (see spots, flashing lights or heat waves) before or during the migraine. Respondents having migraine without aura or migraine with aura were also included as migraine patients in this study. Respondents who did not self-report migraine were classified as non-migraine respondents. There were no exclusion criteria.
Figure 1.
Respondent flow chart. Note: Shaded squares indicate the study populations—migraine patients (≥4 MHDs; N=378) and matched non-migraine respondents (matched controls, N=1512). ICHD-3, International Classification of Headache Disorder, third edition; MHDS, Monthly Headache Days; NHWS, National Health and Wellness Survey.
Patient and public involvement
Respondents and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Covariate and outcome assessment
Sociodemographic and general health characteristics variables measured in this study included age, gender, marital status, level of education, household income, region, insurance type, employment status, Charlson Comorbidity Index (CCI),28 body mass index (BMI), smoking status, alcohol use and exercise behaviour. Migraine-specific covariates were assessed using the Headache Impact Test-6 (HIT-6). The HIT-6 measures the impact of headaches on the respondents’ ability to function in social situations, including home and work. Higher HIT-6 depicts greater functional impairment.29
A list of potential psychiatric and somatic comorbidities was created from the self-reported medical history of the NHWS respondents (refer to full list in figures 2 and 3). The list includes diseases for which the associations with migraine have been reported; diseases for which the association may be explained from the pathophysiology; and other conditions of special interest related to treatment choices. All respondents answered the questions ‘Have you ever experienced …’ or ‘Have you experienced … in the past 12 months’ depending on the condition in NHWS.
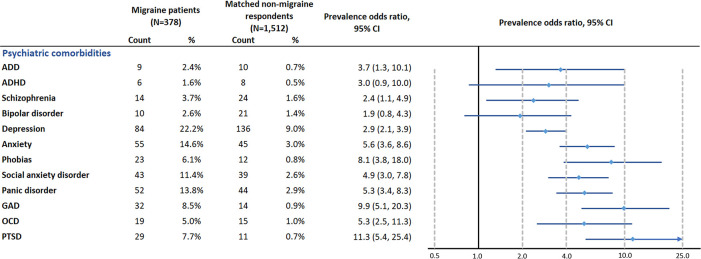
Figure 2.
Propensity score-matched prevalence OR for psychiatric comorbidities. Comorbidities with prevalence between 5% and 10% among migraine patients: phobias, GAD, OCD and PTSD. Comorbidities with more than 10% prevalence among migraine patients: depression, anxiety, social anxiety disorder and panic disorder. ADD, attention-deficit disorder; ADHD, attention-deficit/hyperactivity disorder; GAD, Generalised Anxiety Disorder; OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
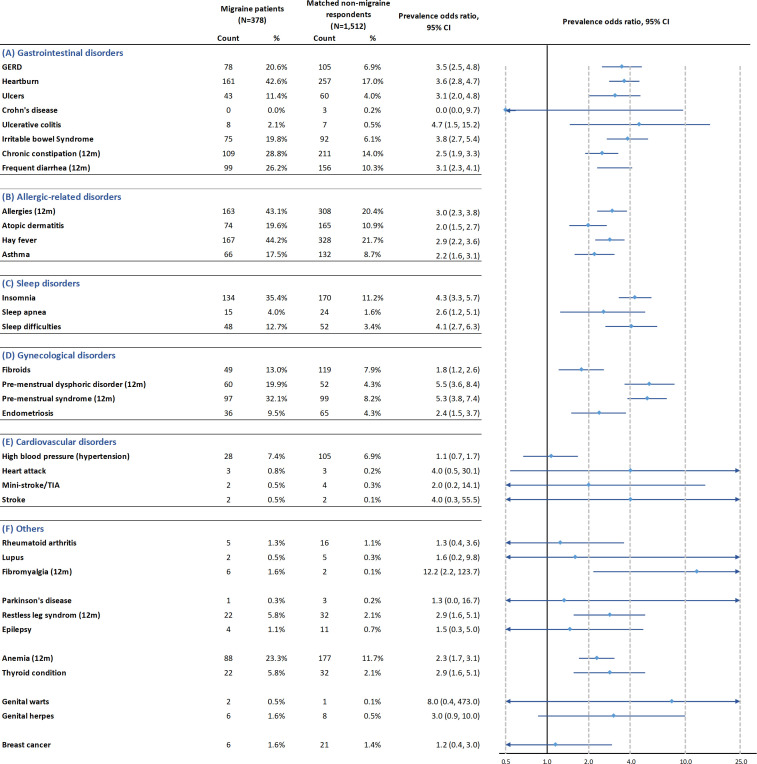
Figure 3.
Propensity score-matched prevalence OR for somatic comorbidities. Comorbidities with prevalence between 5% and 10% among migraine patients: endometriosis, high blood pressure, restless leg syndrome and thyroid condition. Comorbidities with more than 10% prevalence among migraine patients: irritable bowel syndrome, heartbum, GERD, ulcers, frequent diarrhoea, chronic constipation, allergies, hay lever, asthma, atopic dermatitis, insomnia, sleep difficulties, premenstrual dysphonc disorder, premenstrual syndrome, fibroids and anaemia. GERD, gastro-oesophageal reflux disease; TIA, transient ischaemia attack.
Statistical analysis
Sociodemographic and health characteristics were summarised using counts and percentages for categorical variables and means and SDs for continuous variables for migraine patients and non-migraine respondents.
Propensity score (PS) matching with 1:4 ratio of migraine patients to non-migraine respondents, using a greedy matching algorithm, was conducted to create a matched comparison group of non-migraine respondents. Sociodemographic (age, gender, marital status, education, household income, region, insurance and employment status) and general health characteristics (BMI, smoking status, alcohol use, and exercise behaviour) were used in the matching. Balance of matching was assessed using standardised mean differences (SMDs) between migraine patients and matched non-migraine respondents. SMDs greater than 0.1 were considered unbalanced after matching.
Prevalence of the selected psychiatric and somatic comorbidities among migraine patients and matched non-migraine respondents were calculated. Then, PS-matched POR and its 95% CIs for each comorbidity were calculated to evaluate the potential associations of these conditions with migraine.
A post hoc subgroup analysis was conducted among migraine patients with less than 15 MHDs (episodic migraine) and their respective matched non-migraine respondents, as well as among migraine patients with at least 15 MHDs (chronic migraine) and their respective matched non-migraine respondents.
All statistical analyses were performed using IBM SPSS V.25 and R V.3.5.1. No correction for multiple testing was conducted as the study was out of exploratory nature and no formal hypothesis testing was planned. P values were provided as an indication of the difference between migraine patients and non-migraine respondents.
Results
Participant demographics and health characteristics
A total of 30 001 respondents aged 18 years and older provided informed consent and participated in the 2017 Japan NHWS. Among these respondents, 378 respondents were identified as migraine patients and 25 209 respondents were identified as non-migraine respondents (figure 1).
After PS matching, a total of 1512 non-migraine respondents were identified and matching was found to be balanced between matched non-migraine respondents and migraine patients, with all SMDs less than 0.1 (table 1).
Table 1.
Sociodemographic and general health characteristics among migraine patients and matched non-migraine respondents
| Migraine patients (N=378) |
Matched non-migraine respondents (N=1512) | SMD | |||
| % | Count | % | Count | ||
| Age (mean (SD)) | 41.6 (12.7) | 41.2 (15.2) | 0.026 | ||
| Gender | |||||
| Male | 20.1 | 76 | 20.0 | 303 | 0.002 |
| Female | 79.9 | 302 | 80.0 | 1209 | |
| Marital status | |||||
| Married or living with partner | 48.9 | 185 | 49.0 | 741 | 0.001 |
| Divorced/separated/widowed/decline to answer | 51.1 | 193 | 51.0 | 771 | |
| Level of education | |||||
| Completed university education | 41.3 | 156 | 43.2 | 653 | 0.042 |
| Not completed | 46.6 | 176 | 44.6 | 674 | |
| Decline to answer | 12.2 | 46 | 12.2 | 185 | |
| Household income | |||||
| <¥3 000 000 | 19.6 | 74 | 18.9 | 286 | 0.029 |
| ¥3 000 000 to <¥5 000 000 | 27.0 | 102 | 27.6 | 417 | |
| ¥5 000 000 to <¥8 000 000 | 24.6 | 93 | 23.9 | 361 | |
| ¥8 000 000 or more | 14.8 | 56 | 15.4 | 233 | |
| Decline to answer | 14.0 | 53 | 14.2 | 215 | |
| Region | |||||
| Hokkaido | 3.2 | 12 | 2.9 | 44 | 0.063 |
| Tohoku | 6.6 | 25 | 7.1 | 107 | |
| Kanto | 39.4 | 149 | 40.7 | 615 | |
| Chubu | 14.6 | 55 | 14.6 | 221 | |
| Kansai/Kinki | 18.8 | 71 | 16.9 | 255 | |
| Chugoku | 4.2 | 16 | 4.4 | 67 | |
| Shikoku | 3.2 | 12 | 3.7 | 56 | |
| Kyushu/Okinawa | 10.1 | 38 | 9.7 | 147 | |
| Insurance type | |||||
| National health insurance | 52.1 | 197 | 50.8 | 768 | 0.034 |
| Social insurance | 44.7 | 169 | 46.1 | 697 | |
| Other (late-stage elderly insurance/other/none of the above) | 3.2 | 12 | 3.1 | 47 | |
| Employment status | |||||
| Currently not employed | 39.9 | 151 | 40.6 | 614 | 0.013 |
| Currently employed | 60.1 | 227 | 59.4 | 898 | |
| BMI | |||||
| Underweight (BMI <18.5 kg/m2) | 18.3 | 69 | 18.5 | 280 | 0.045 |
| Normal weight (18.5≤BMI<25 kg/m2) | 62.4 | 236 | 59.7 | 903 | |
| Preobese (25≤BMI<30 kg/m2) | 14.8 | 56 | 13.6 | 205 | |
| Obese (BMI ≥30 kg/m2) | 4.5 | 17 | 4.0 | 61 | |
| Decline to answer | 4.2 | 16 | 4.2 | 63 | |
| Smoking status | |||||
| Never smoker | 55.8 | 211 | 56.7 | 858 | 0.045 |
| Former smoker | 20.9 | 79 | 19.1 | 289 | |
| Current smoker | 23.3 | 88 | 24.1 | 365 | |
| Alcohol use | |||||
| Abstain | 40.5 | 153 | 40.8 | 617 | 0.007 |
| Currently consume alcohol | 59.5 | 225 | 59.2 | 895 | |
| Vigorous exercise in past 30 days | |||||
| No | 58.2 | 220 | 60.0 | 907 | 0.036 |
| Yes | 41.8 | 158 | 40.0 | 605 | |
| CCI* (mean (SD)) | 0.24 (0.86) | 0.08 (0.33) | 0.242 | ||
| HIT-6 impact grades* | |||||
| Little-to-no impact (HIT-6 score: 36–49) | 1.1 | 4 | – | – | – |
| Moderate impact (HIT-6 score: 50–55) | 6.6 | 25 | – | – | |
| Substantial impact (HIT-6 score: 56–59) | 8.7 | 33 | – | – | |
| Severe impact (HIT-6 score: 60–78) | 83.6 | 316 | – | – | |
*CCI and HIT-6 impact grades are not included in the propensity score matching.
BMI, body mass index; CCI, Charlson Comorbidity Index; HIT-6, Headache Impact Test-6; SMD, standardised mean difference.
Sociodemographic and general health characteristics of migraine patients and matched non-migraine respondents
Migraine patients (N=378) had an average age of 41.6 and were predominantly females (79.9%). The majority (83.6%) of migraine patients in this study had severe impact (HIT-6 score: 60–78). The CCI, which was not part of the PS matching, was higher in migraine patients than matched non-migraine respondents (mean: 0.24 vs 0.08) (table 1).
Psychiatric comorbidities in migraine patients versus matched non-migraine respondents
The psychiatric comorbidities with prevalence higher than 5.0% in migraine patients were depression (22.2%), post-traumatic stress disorder (PTSD) (7.7%), anxiety disorders (6.1% for phobias to 14.6% for anxiety) and obsessive–compulsive disorder (OCD) (5.0%).
Among the comorbidities with relatively high prevalence (5.0% or higher) in migraine patients, migraine patients had a significantly higher odds than matched non-migraine respondents for PTSD (PS-matched POR (95% CI): 11.3 (5.4 to 25.4)), various anxiety disorders (generalised anxiety disorder: 9.9 (95% CI 5.1 to 20.3) to social anxiety disorder (4.9 (95% CI 3.0 to 7.8)), OCD (5.3 (95% CI 2.5 to 11.3)) and depression (2.9 (95% CI 2.1 to 3.9)) (figure 2).
Somatic comorbidities in migraine patients versus matched non-migraine respondents
Figure 3 describes the somatic comorbidities of migraine patients compared to non-migraine respondents.
Gastrointestinal comorbidities
Most of the gastrointestinal comorbidities assessed in this study showed the prevalence higher than 5.0% in migraine patients: heartburn (42.6%), chronic constipation (28.8%), frequent diarrhoea (26.2%), gastro-oesophageal reflux disease (GERD) (20.6%), irritable bowel syndrome (19.8%) and ulcers (11.4%).
Among the gastrointestinal comorbidities with relatively high prevalence, compared with matched non-migraine respondents, migraine patients had a significantly higher odds for irritable bowel syndrome (PS-matched POR (95% CI): 3.8 (2.7 to 5.4)), heartburn (3.6 (95% CI 2.8 to 4.7)), GERD (3.5 (2.5, 4.8)), ulcers (3.1 (95% CI 2.0 to 4.8)), frequent diarrhoea (3.1 (95% CI 2.3 to 4.1)) and chronic constipation (2.5 (95% CI 1.9 to 3.3)).
Allergy-related comorbidities
All the allergy-related comorbidities assessed in this study have a prevalence higher than 5.0% in migraine patients: allergies (43.1%), hay fever (44.2%), atopic dermatitis (19.6%) and asthma (17.5%).
Compared with matched non-migraine respondents, migraine patients had a significantly higher odds for allergies (PS-matched POR (95% CI): 3.0 (2.3 to 3.8)), hay fever (2.9 (95% CI 2.2 to 3.6)), asthma (2.2 (95% CI 1.6 to 3.1)) and atopic dermatitis (2.0 (95% CI 1.5 to 2.7)).
Sleep comorbidities
The following sleep disorders presented in migraine patients with a prevalence higher than 5.0% included insomnia (35.4%) and sleep difficulties (12.7%). Migraine patients had a significantly higher odds for insomnia (PS-matched POR (95% CI): 4.3 (95% CI 3.3 to 5.7)) and sleep difficulties (4.1 (95% CI 2.7 to 6.3)), compared with matched non-migraine respondents.
Gynaecological comorbidities
The gynaecological disorders assessed in this study with a prevalence higher than 5.0% among migraine patients were premenstrual syndrome (32.1%), premenstrual dysphoric disorder (19.9%), fibroid (13.0%) and endometriosis (9.5%). Compared with matched non-migraine respondents, migraine patients had a significantly higher odds for premenstrual dysphoric disorder (PS-matched POR (95% CI): 5.5 (3.6 to 8.4)), premenstrual syndrome (5.3 (95% CI 3.8 to 7.4)), endometriosis (2.4 (95% CI 1.5 to 3.7)) and fibroids (1.8 (95% CI 1.2 to 2.6)).
Cardiovascular comorbidities
The cardiovascular disorders assessed in this study showed a lower than 5.0% prevalence among migraine patients except for high blood pressure (hypertension) (7.4%). Compared with matched non-migraine respondents, the odds of having hypertension, heart attack, stroke or ministroke/transient ischaemic attack were not significantly different in migraine patients.
Other comorbidities
Most other disorders assessed in this study showed the prevalence lower than 5.0% among migraine patients except for anaemia (23.3%), thyroid condition (5.8%) and restless leg syndrome (5.8%). Compared with matched non-migraine respondents, migraine patients had a significantly higher odds for restless leg syndrome (PS-matched POR (95% CI): 2.9 (1. 6 to 5.1)), thyroid condition (2.9 (95% CI 1. 6 to 5.1)) and anaemia (2.3 (95% CI 1.7 to 3.1)). Compared with matched non-migraine respondents, migraine patients had a significantly higher odds for fibromyalgia (prevalence among migraine patients: 1.6%; POR (95% CI): 12.2 (2.2 to 123.7)). For all other disorders assessed in this study (rheumatoid arthritis, lupus, Parkinson’s disease, epilepsy, genital herpes, genital warts and breast cancer), the odds among migraine patients were not different from matched non-migraine respondents.
Post hoc subgroup analysis by MHDs
Majority of the baseline characteristics remained balanced at the subgroup level (results not shown). Post hoc analysis demonstrated consistent results in both subgroups of the migraine patients MHDs <15 and MHDs ≥15 with the overall analysis. The most prevalent psychiatric and somatic comorbidities were similar in both migraine patients with MHDs <15 and migraine patients with MHDs ≥15 (online supplemental tables 1 and 2).
bmjopen-2022-065787supp001.pdf (58.3KB, pdf)
Migraine patients with MHDs ≥15 tended to have higher point estimates for POR although formal statistical testing was not performed to assess the interaction for the association between comorbidities and migraine by MHD.
Discussion
This study showed that patients with migraine have a significantly higher prevalence for various psychiatric and somatic comorbidities, compared with matched non-migraine respondents. Our study is the first to report the broad range of comorbidities associated with migraine as a population-based study in Japan.
Potential pathological mechanisms for comorbidities were proposed—for example, unidirectional or bidirectional causality or shared environmental or genetic risk factors.23 Although various explanations for the association between migraines and psychiatric conditions have been proposed, exact causal relationships and mechanisms for the associations are yet to be elucidated in this study.
Psychiatric comorbidities
Psychiatric disorders that our study found associated with migraine such as PTSD, anxiety disorders, OCD and depression have been described in previous literature.8–11 Various neurotransmitter systems and brain regions implicated in psychiatric disorders have been postulated to overlap with that in migraine.7 11 23 Neurotransmitters such as serotonin and other monoamines, and ovarian hormonal influences, might lead to serotonergic processing dysfunction and hypothalamic–pituitary–adrenal axis dysregulation which underlie most psychiatric disorders.23 For instance, migraine patients exhibit ictal or interictal alterations in neurotransmitters blood levels, which share common pathophysiology to depression, stress and bipolar disorder.7 23 Genetic factors could also potentially influence the association between migraine and psychiatric disorders. A genome-wide association study with over 1 million individuals found significantly overlapped genetic risks of migraine with psychiatric conditions such as attention-deficit/hyperactivity disorder or depression.30 Emerging functional neuroimaging hint at that possibility of long-term chronic migraine could alter brain activity and increase disease burden,7 11 implying a need to understand the shared pathophysiology of psychiatric comorbidities and migraine to facilitate better management of both conditions among patients.
Somatic comorbidities
Gastrointestinal comorbidities
We found that bowel movement dysregulations, such as constipation, diarrhoea and irritable bowel syndrome, are more prevalent in migraine patients than matched non-migraine respondents, which was consistent with previous literature.14–16 Autonomic nervous system dysfunction and other mechanisms such as dysregulation of neuroendocrine, immunological factors, the brain–gut axis or intestinal microbiota15 were thought as potential mechanisms to alter visceral sensitivity and common pathology with migraine.
Both GERD16 and gastric ulcer6 16 were shown to have higher prevalence in migraine patients than matched non-migraine respondents in this study. Association of migraine with Helicobacter pylori infection has been shown in a meta-analysis of observational studies,31 and this may explain the high prevalence of GERD and gastric ulcer in migraine patients15 Other possible explanations for the association between migraine and gastrointestinal disorders includes analgesics use in migraine patients.15 18
Allergy-related comorbidities
In this study, migraine patients were more likely to have allergy-related comorbidities, which are consistent with previous research.6 17 18 The association between migraine and allergy-related comorbidities could in part be due to common pathophysiology of inflammatory (including neuroinflammation), immune and genetic factors.17 23
Sleep comorbidities
Migraine patients in this study had a significantly increased prevalence for sleep disorders including insomnia and sleep difficulties, compared with non-migraine respondents, consistent with previous studies.6 19 20 A large population-based study reported that those with comorbid migraine and poor sleep showed significantly poorer anxiety and depression scores.20 The association between migraine and sleep disorders is thought to be bidirectional: headache was shown to be a risk factor for insomnia, while insomnia (and oversleeping) had been reportedly to be a contributing factor of migraine attacks.11 32 It was suggested that aminergic neurotransmitter systems such as those involved in the sleep-wake cycle could underlie the association between migraine and sleep disorders.11 23 32
Gynaecological comorbidities
Headache has been known to be a common symptom for perimenstrual syndrome.33 Furthermore, the association between endometriosis and migraine was previously reported,21 23 in which the occurrence of endometriosis was more frequent among women with migraines than women without.23 The findings from this study were consistent with the previous research. The association between migraines and gynaecological comorbidities could be due to hormonal changes in women throughout the menstrual cycle or during menopause which may cause migraine through estrogen-mediated pathways including the association of oestrogen receptor markers from genetic studies.34
Cardiovascular comorbidities
Previous studies pointed out the association between migraine and cardiovascular conditions such as hypertension,6 myocardial infarction6 13 and stroke,6 12 13 and the association specifically on migraine with aura12 13 or other risk factors such as age, smoking habit and use of oral contraceptives.12 However, no association was observed between migraine and cardiovascular conditions such as hypertension, probably due to the younger age of migraine patients in this study or small numbers of high-risk migraine patients for cardiovascular conditions.
Other comorbidities
As found in our study, anaemia35 and thyroid dysfunction36 have been described as comorbidities of migraine. Similarly, restless leg syndrome25 has been shown for the association, although the association may be controversial.37
The observation on the relationship between migraine and fibromyalgia in this study is consistent with previous studies.38 Fibromyalgia may share a pathological pathway with migraine through chronic hypothalamic neuroendocrine dysfunction, resulting in abnormal central nervous system sensory processing.39
Clinical implications
Although some of the associations are not solidly established with potential pathological explanation, the findings from this study appear to have clinical importance. Various comorbidities are at high prevalence among migraine patients. Migraine patients may experience impairment for daily life and loss of productivity not only because of the migraine but also its comorbidities. In the treatment of migraine, comorbidities may influence therapeutic choices in two ways. First, by taking comorbidities into consideration, medication regimens could be selected to treat for both migraine and comorbidities simultaneously. Second, therapeutic options for migraine may be limited if there are comorbidities as contraindications to migraine medications.40 Therefore, accounting for comorbidities which could complicate care in clinical practice is warranted when considering appropriate therapeutic options for migraine management.24 40 In addition, with the growing migraine prevalence,2 elucidating the associated comorbidities may provide insights to the shared physiopathology with migraine which could facilitate effective management and treatment of migraine.7
Limitations
There are a few limitations to be mentioned for this study. NHWS is cross-sectional and causal relationship between migraine and comorbidities cannot be concluded, that is, if the comorbidities were induced by migraine or treatment for migraine or vice versa. In addition, respondents self-reported their migraine symptoms as well as comorbidities, which may lead to potential recall bias. Although ICHD-3-like criteria were created for this study using available self-reported data as objective classification criteria and to minimise misclassification, discrepancies between the criteria used in this study and the formal ICHD-3-like criteria still exist. As such, the interpretation of the findings in this study would only be valid in comparison with the groups defined within this study. In addition, NHWS is an online survey and thus respondents without access to internet or internet-related technology (such as those with more severe comorbidities or older respondents) were not included in the study and may not be well represented in this study. Lastly, patient demographics such as gender and lifestyle could potentially influence the association of migraine with psychiatric and/or somatic comorbidities that was not explored and warrants further investigation.
Conclusion
Our study found that migraine patients are more likely to have psychiatric and somatic comorbidities compared with matched non-migraine respondents, some of which are novel ones previously unreported (eg, OCD, gastrointestinal comorbidities, sleep comorbidities, gynaecological comorbidities) in Japan. This study showed that migraine patients in Japan face an additional comorbid burden and provided insights to types of comorbidities that patients with migraine may suffer. As such, accounting for comorbidities, which could usually complicate care, when treating migraine would help in good clinical practice and improve outcomes among migraine patients.
Supplementary Material
Acknowledgments
The authors would like to thank Amanda Woo from Cerner Enviza for support with development, writing and editing of the manuscript.
Footnotes
Contributors: TT led and all authors (SK, YC, KI, MH, KA, and TT) conceptualised and designed the study. YC analyzed the data. SK, YC, KI, MH, KA, and TT interpreted the results and contributed to the original draft of the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by Amgen K.K. grant number (funding/grant number: not available).
Competing interests: SK and TT received consultation fee for this study. YC is an employee at Cerner Enviza, Singapore. Cerner Enviza received funding from Amgen K.K. for conduction of the analysis and manuscript development. MH and KI are employees at Amgen K.K., and KA is a former employee at Amgen K.K. and is currently an employee of IQVIA Solutions Japan, K.K. MH holds and KA held stocks of Amgen Inc.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as online supplemental information. Study data to support our findings are available from Cerner Enviza, but availability of the data is restricted and was used under license for this study and are not publicly available. Data are, however, available from the authors on reasonable request and with the permission of Cerner Enviza.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The NHWS was granted exemption status uponon review by the Pearl Pathways Institutional Review Board (Indiana, USA, IRB Study Number: 17-KANT-150). All NHWS respondents provided informed consent prior to participation. This analysis was granted exemption by the Public Health Research Foundation Ethical Review Committee (Tokyo, Japan).
References
- 1.Steiner TJ, Stovner LJ, Jensen R, et al. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain 2020;21:137. 10.1186/s10194-020-01208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stovner LJ, Hagen K, Linde M, et al. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain 2022;23:34. 10.1186/s10194-022-01402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshima T, Ishizaki K, Fukuhara Y, et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache 2004;44:8–19. 10.1111/j.1526-4610.2004.04004.x [DOI] [PubMed] [Google Scholar]
- 4.Suzuki N, Ishikawa Y, Gomi S, et al. Prevalence and characteristics of headaches in a socially active population working in the Tokyo metropolitan area -surveillance by an industrial health Consortium. Intern Med 2014;53:683–9. 10.2169/internalmedicine.53.1700 [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Fanning KM, Buse DC, et al. Identifying natural subgroups of migraine based on comorbidity and concomitant condition profiles: results of the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2018;58:933–47. 10.1111/head.13342 [DOI] [PubMed] [Google Scholar]
- 6.Buse DC, Reed ML, Fanning KM, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (mast) study. J Headache Pain 2020;21:23. 10.1186/s10194-020-1084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dresler T, Caratozzolo S, Guldolf K, et al. Understanding the nature of psychiatric comorbidity in migraine: a systematic review focused on interactions and treatment implications. J Headache Pain 2019;20:51. 10.1186/s10194-019-0988-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry 2016;87:741–9. 10.1136/jnnp-2015-312233 [DOI] [PubMed] [Google Scholar]
- 9.Ziplow J. The psychiatric comorbidities of migraine in children and adolescents. Curr Pain Headache Rep 2021;25:69. 10.1007/s11916-021-00983-y [DOI] [PubMed] [Google Scholar]
- 10.Pompili M, Serafini G, Di Cosimo D, et al. Psychiatric comorbidity and suicide risk in patients with chronic migraine. Neuropsychiatr Dis Treat 2010;6:81–91. 10.2147/NDT.S8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsan N, Goadsby PJ. Migraine is more than just headache: is the link to chronic fatigue and mood disorders simply due to shared biological systems? Front Hum Neurosci 2021;15:646692. 10.3389/fnhum.2021.646692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hassany L, Linstra KA, Terwindt GM. Cardiovascular Risk of Migraine in Men and Women. In: Maassen van den Brink A, MacGregor EA, eds. Gender and migraine. Cham: Springer International Publishing, 2019: 17–29. [Google Scholar]
- 13.Adelborg K, Szépligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 2018;360:k96. 10.1136/bmj.k96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau C-I, Lin C-C, Chen W-H, et al. Association between migraine and irritable bowel syndrome: a population-based retrospective cohort study. Eur J Neurol 2014;21:1198–204. 10.1111/ene.12468 [DOI] [PubMed] [Google Scholar]
- 15.Cámara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, et al. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol 2016;22:8149–60. 10.3748/wjg.v22.i36.8149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aamodt AH, Stovner LJ, Hagen K, et al. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT study. Cephalalgia 2008;28:144–51. 10.1111/j.1468-2982.2007.01486.x [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Deng Z-R, Zu M-D, et al. The comorbid relationship between migraine and asthma: a systematic review and meta-analysis of population-based studies. Front Med 2020;7:609528. 10.3389/fmed.2020.609528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aamodt AH, Stovner LJ, Langhammer A, et al. Is headache related to asthma, hay fever, and chronic bronchitis? the Head-HUNT study. Headache 2007;47:204–12. 10.1111/j.1526-4610.2006.00597.x [DOI] [PubMed] [Google Scholar]
- 19.Uhlig BL, Engstrøm M, Ødegård SS, et al. Headache and insomnia in population-based epidemiological studies. Cephalalgia 2014;34:745–51. 10.1177/0333102414540058 [DOI] [PubMed] [Google Scholar]
- 20.Song T-J, Cho S-J, Kim W-J, et al. Poor sleep quality in migraine and probable migraine: a population study. J Headache Pain 2018;19:58. 10.1186/s10194-018-0887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tietjen GE, Conway A, Utley C, et al. Migraine is associated with menorrhagia and endometriosis. Headache 2006;46:422–8. 10.1111/j.1526-4610.2006.00290.x [DOI] [PubMed] [Google Scholar]
- 22.Caponnetto V, Deodato M, Robotti M, et al. Comorbidities of primary headache disorders: a literature review with meta-analysis. J Headache Pain 2021;22:71. 10.1186/s10194-021-01281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altamura C, Corbelli I, de Tommaso M, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci 2021;15:640574. 10.3389/fnhum.2021.640574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki N, Takeshima T, Ando N, et al. Clinical practice guideline for chronic headache 2013, 2019. Available: https://onlinelibrary.wiley.com/doi/abs/10.1111/ncn3.12322 [Accessed 17 Jul 2020].
- 25.Suzuki S, Suzuki K, Miyamoto M, et al. Evaluation of contributing factors to restless legs syndrome in migraine patients. J Neurol 2011;258:2026–35. 10.1007/s00415-011-6064-3 [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Moriwaki K, Oiso H, et al. High prevalence of comorbidity of migraine in outpatients with panic disorder and effectiveness of psychopharmacotherapy for both disorders: a retrospective open label study. Psychiatry Res 2011;185:145–8. 10.1016/j.psychres.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 27.Kikui S, Chen Y, Todaka H, et al. Burden of migraine among Japanese patients: a cross-sectional National health and wellness survey. J Headache Pain 2020;21:110. 10.1186/s10194-020-01180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge Abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 29.Fumihiko S, Yasuo F, Makoto I. Evaluation of reliability of the Japanese version “Headache Impact Test (HIT-6)". J-GLOBAL 臨床医薬 2004;20:1045–54. [Google Scholar]
- 30.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science 2018;360. doi: 10.1126/science.aap8757. [Epub ahead of print: 22 06 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su J, Zhou X-Y, Zhang G-X. Association between Helicobacter pylori infection and migraine: a meta-analysis. World J Gastroenterol 2014;20:14965–72. 10.3748/wjg.v20.i40.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waliszewska-Prosół M, Nowakowska-Kotas M, Chojdak-Łukasiewicz J, et al. Migraine and sleep-an unexplained association? Int J Mol Sci 2021;22:5539. 10.3390/ijms22115539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician 2016;94:236–40. [PubMed] [Google Scholar]
- 34.Cupini LM, Corbelli I, Sarchelli P. Menstrual migraine: what it is and does it matter? J Neurol 2021;268:2355–63. 10.1007/s00415-020-09726-2 [DOI] [PubMed] [Google Scholar]
- 35.Tayyebi A, Poursadeghfard M, Nazeri M, et al. Is there any correlation between migraine attacks and iron deficiency anemia? A case-control study. Int J Hematol Oncol Stem Cell Res 2019;13:164. [PMC free article] [PubMed] [Google Scholar]
- 36.Spanou I, Bougea A, Liakakis G, et al. Relationship of migraine and tension-type headache with hypothyroidism: a literature review. Headache 2019;59:1174–86. 10.1111/head.13600 [DOI] [PubMed] [Google Scholar]
- 37.Trenkwalder C, Allen R, Högl B, et al. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology 2016;86:1336–43. 10.1212/WNL.0000000000002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penn I-W, Chuang E, Chuang T-Y, et al. Bidirectional association between migraine and fibromyalgia: retrospective cohort analyses of two populations. BMJ Open 2019;9:e026581. 10.1136/bmjopen-2018-026581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valença MM, Medeiros FL, Martins HA, et al. Neuroendocrine dysfunction in fibromyalgia and migraine. Curr Pain Headache Rep 2009;13:358–64. 10.1007/s11916-009-0058-1 [DOI] [PubMed] [Google Scholar]
- 40.Lipton RB, Silberstein SD. Why study the comorbidity of migraine? Neurology 1994;44:S4–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065787supp001.pdf (58.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as online supplemental information. Study data to support our findings are available from Cerner Enviza, but availability of the data is restricted and was used under license for this study and are not publicly available. Data are, however, available from the authors on reasonable request and with the permission of Cerner Enviza.