Abstract
Introduction
Delaying cancer treatment following diagnosis impacts health outcomes, including increasing patient distress and odds of mortality. Interventions to promote timely healthcare engagement may decrease patient-reported stress and improve quality of life. Community health workers (CHWs) represent an enabling resource for reducing delays in attending initial oncology treatment visits. As part of an ongoing programme evaluation coordinated by the Merck Foundation, we will implement a pilot navigation programme comprising CHW-conducted needs assessments for supporting patients and their caregivers. We aim to investigate (1) the programme’s influence on patients’ healthcare utilisation within the period between their first diagnosis and initial treatment visit and (2) the logistic feasibility and acceptability of programme implementation.
Methods and analysis
We will employ a hybrid implementation design to introduce the CHW navigation programme at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. CHW team members will use a consecutive sampling approach. Participants will complete the Problem-Checklist, Chronic Illness Distress Scale and the Satisfaction with Life Domains instruments. CHWs will provide tailored guidance by sharing information available on the Johns Hopkins Electronic Resource databases. The investigators will evaluate patients’ time to initial oncology treatment and healthcare utilisation by reviewing electronic medical records at 3 and 6 months postintervention. Bivariate analyses will be completed to evaluate the relationships between receiving the programme and all outcome measures.
Ethics and dissemination
This study’s protocol was approved by the Johns Hopkins School of Medicine’s institutional review board (IRB00160610). Informed consent will be obtained by phone by the CHW navigator. Dissemination planning is ongoing through regular meetings between members of the investigator team and public members of two community advisory groups. Study plans include collaborating with other experts from the Johns Hopkins Institute for Clinical and Translational Research and the Johns Hopkins Center for Health Equity for ideating dissemination strategies.
Keywords: ONCOLOGY, Adult oncology, PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The pilot intervention was translated from an established model of care (ie, community health worker) with robust evidence in the scientific literature showing efficacy in addressing a variety of health conditions among underserved populations.
Our targeted sampling strategy, based on assessing Area Deprivation Index scores, directed priority attention to populations residing in local zip codes with higher levels of poverty, substandard housing and unemployment.
The study design and implementation plan emerged through partnership and collaboration between academic investigators and members from a long-established community advisory group.
The target sample will be a heterogeneous population given the lack of an exclusion criterion based on cancer type.
The pilot intervention was developed within the unique infrastructure of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, and the intervention design may, therefore, be neither feasible nor acceptable within other healthcare systems and patient populations, respectively.
Introduction
Reliable healthcare utilisation during the period between a cancer diagnosis and receiving initial treatment is crucial for facilitating favourable outcomes. Prolonging the start of treatment leads to poorer outcomes such as patient distress, worsening the progression of disease, and increasing the odds of mortality.1–3 The average length of time for receiving initial cancer treatment has increased over time, highlighting a public health concern warranting attention. Khorana et al found a 38% increase in the time to initial cancer treatment following diagnosis across all cancer types in the USA between 2004 and 2013, using the data from the National Cancer Database.2 In addition, patients who identified as black had an increased time to initial treatment compared with white counterparts across cancer types.2 Although the exact cause of this increase in time to initial treatment following first cancer diagnosis is unclear, this delay is likely influenced by a combination of patient-level and system-level factors.1 4
Andersen’s behavioural model for healthcare utilisation can guide investigators’ understanding of how patient-level and system-level factors can affect timely access for initiating cancer treatments.5 This model posits that patients’ healthcare utilisation is a function of combined factors across the environment, including the healthcare system, along with population-level and individual-level patient characteristics that either enables or impedes certain health behaviours and use of health services.5 This in turn is theorised to impact health outcomes adversely. Therefore, the model may inform novel programme designs for reaching underserved demographics, such as patients with low income and/or from minoritised racial backgrounds, who may otherwise face higher risks for unfavourable outcomes due to structural and/or other social barriers associated with the environment and/or population characteristics.5
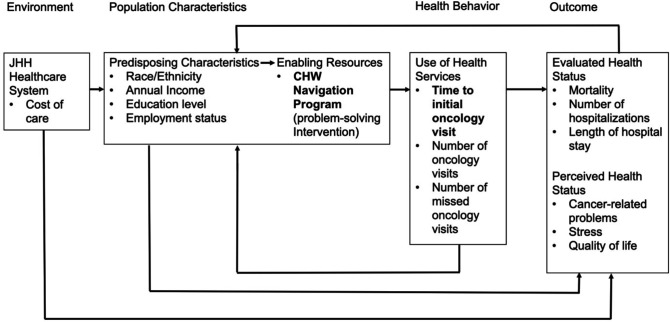
In Andersen’s behavioural model (figure 1), enabling resources are key components for supporting and promoting timely treatment initiation and healthcare utilisation by patients. One enabling resource for patients and their caregivers are community health worker (CHW) models of care, which commonly comprises trained lay individuals or paraprofessionals who serve as important linkages between patient populations and healthcare systems.6 7 Some examples of public health programmes adapting CHW models include community-based interventions for improving breast and cervical cancer screenings among Korean American women,8 health service outreach for residents with a history of stroke and hypertension in Harlem, New York (USA),9 and providing a socially accessible hearing care programme for older adults with hearing loss and low-to-moderate income in Baltimore, Maryland (USA).10 Evidence gathered through a systematic review of CHW-directed interventions reported treatment efficacy when compared with certain conventional models of healthcare and were cost-effective for managing certain health conditions.11 Trained CHWs often share varied degrees of either language, culture, lived experiences and/or geographical characteristics with their patient populations. The social concordance between CHWs and patient populations is a hallmark feature of CHW-directed programmes, which can be optimised for providing patient-centred services.12 Health systems, particularly ones with community-based partnerships like the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (SKCCC),13 can therefore also leverage CHW models as an enabling resource for addressing public health disparities.14
Figure 1.
Adapted Andersen’s behavioural model for healthcare utilisation. The CHW navigation programme serves as an enabling resource for promoting appointment-keeping and follow-up (ie, retention). In the model, the time to initial oncology treatment is defined as the time between the patient’s first cancer diagnosis and the start of cancer treatment. Furthermore, healthcare utilisation encompasses the provider’s recommended use of healthcare services, and the number of oncology visits, number of missed oncology visits, mortality, number of hospitalisations, and length of hospital stay are used as a proxy to measure this concept. CHW, community health worker.
The goal of the CHW navigation programme involves conducting a needs assessment and focusing on problem-solving for navigating the healthcare system. Prior studies of interventions focused on problem orientation and problem-solving with cancer patients and their caregivers have yielded evidence of promoting self-efficacy in managing care and in reducing stress.15–18 From adapting Andersen’s behavioural model,5 we theorise a relationship between the activation of respective health behaviours identified through a needs assessment and more timely treatment initiation. The pilot programme will be implemented at the systems level and all cancer patients ages 18 years and older are eligible as prospective service users. The Johns Hopkins Hospital system (JHH) includes the SKCCC, which has a centralised call centre for scheduling appointments regardless of cancer type and JHH provider team. Working through the SKCCC allows the intervention team to reach a broader range of new patients with various cancer diagnoses.
Our protocol manuscript describes a pilot programme featuring CHW-directed needs assessment and healthcare navigation services. We will assess the programme’s influence on patients’ treatment initiation and healthcare utilisation, as well as the feasibility of programme implementation. We hypothesised that implementing the programme would decrease the time leading up to the patient’s initial oncology treatment visit following their first cancer diagnosis. Secondary hypotheses include observing an increase in the total number of oncology provider visits (eg, patient retention), improvements in self-reported quality of life (QOL) and a decrease in the number of emergency department (ED) visits, hospitalisations, missed oncology appointments, reported stress and premature mortality attributed to delaying care.
Methods and analysis
Study design and hypotheses
This pilot study will be a pretest/post-test, hybrid effectiveness-implementation type-1 research design.19 20 This design will evaluate the effectiveness of the pilot CHW navigation programme in facilitating patients’ initiation of cancer treatment, healthcare utilisation for oncology care, and perceived health status (patient stress and QOL). Additional objectives include assessing the feasibility of technical and administrative processes in implementing such a programme (eg, recruitment using electronic medical record data, number of staff personnel needed), as well as the acceptability of programme for informing a larger-scale intervention study in the future. Feasibility and acceptability are key features to consider in addition to effectiveness of the programme to understand how the intervention may be incorporated into practice at the SKCCC.21
The following hypotheses will assess the impact of the pilot CHW navigation programme:
-
The CHW navigation programme will promote timely initiation of cancer treatment following the patient’s first cancer diagnosis.
The programme will decrease time leading up to the initial oncology treatment visit.
-
The CHW navigation programme will facilitate patient’s healthcare utilisation for recommended cancer care and treatment.
The programme will decrease the number of ED visits, hospitalisations and missed oncology appointments
The programme will increase the total number of provider-recommended oncology visits (mitigating healthcare overutilisation).
-
The CHW navigation programme will improve patients’ perceived health status.
The programme will decrease patient-reported stress.
The programme will increase patient-reported QOL.
This protocol previously underwent a scientific peer review by subject matter experts with support from the Merck Foundation (proposal #19-9714). The SKCCC-based programme is part of a broader network of initiatives across six cancer centres coordinated through the Alliance to Advance Patient-Centered Cancer Care, henceforth referred to as the ‘Alliance’.22
Study setting and sample
We will implement a CHW-directed needs assessment and navigation programme among patients newly diagnosed with cancer. Health disparities within Baltimore City are well documented with population life expectancies across local zip codes differing by as much as 20 years.23 Patients will be consecutively sampled from nine zip codes in East Baltimore (21202, 21205, 21206, 21212, 21213, 21218, 21224, 21231 and 21239). These areas within East Baltimore show high incidences of cancer mortality and Area Deprivation Index (ADI) scores. Rather than relying solely on race or ethnicity as proxy indicators of social factors associated with poorer health outcomes, we will use ADI to incorporate a more holistic approach (ie, incorporating social and structural factors) in reaching underserved demographics. ADI scores are calculated using sociodemographic characteristics of a neighbourhood. Individuals living in neighbourhoods with high ADI scores experience higher levels of poverty, mortality rates, substandard housing, unemployment and lower levels of educational attainment.22 Patients living in areas with higher ADIs are also more likely to have lower levels of health literacy, as literacy levels are associated with the social factors used to calculate ADI.24
Patients will be eligible to participate if they present with their first cancer diagnosis and are over 18 years old. Patients must be seeking cancer care in the JHH system and have previously contacted the SKCCC for an appointment. Recruitment will follow a consecutive sampling strategy,25 and all eligible patients will be contacted by phone for study recruitment by a two-member CHW team affiliated with the SKCCC. Through a review of the JHH Epic electronic medical health record data, there are approximately 2155 SKCCC patients over the age of 18 with a first-time cancer diagnosis who meet the inclusion criteria for recruitment.
Patient and public involvement
The SKCCC prioritises community engagement strategies for outreach and improving public trust in medical care.13 The investigator team maintains a partnership with two community advisory groups (CAGs) coordinated by the SKCCC based in Baltimore City and Prince George’s County (Maryland, USA), respectively, that were established over ten years ago.26 27 The CAGs meet monthly and comprise stakeholders from across the continuum of cancer care, including cancer survivors, caregivers and representatives from local health departments, community-based organisations and local faith-based communities. While CAG meetings are hosted by the SKCCC, their respective priorities and agendas are self-directed and self-managed by its membership. The CAGs contribute to research and practice by advising clinicians and researchers at SKCCC about patient and caregiver priorities such as barriers to accessing care and priority outcomes.13 Members are invited to weigh in on study designs, implementation plans, approaches in data analysis and interpretation, and the dissemination of study results.
Approximately 3 months prior to implementing the CHW navigator pilot programme, investigators from the National Cancer Institute (NCI)-supported Johns Hopkins Center to Reduce Cancer Disparities (JHCRCD) began engaging the CAGs about the current programme following members’ reports of unique challenges in accessing care following the first cancer diagnosis. The CAGs also identified issues related to maintaining contact with a care team and a lack of transportation as barriers to care and contributors to increased stress levels, which directly informed the current study’s priorities, outcomes of interest (stress and quality-of-life (QOL)) and intervention development. The investigator team continues to meet regularly with the CAGs, who in turn also support recruitment efforts by promoting the study within their respective social networks.
Intervention: CHW navigation programme
The CHW navigation programme will be provided to patients and caregivers to address cancer care-related problems during the period between first diagnosis and the initial treatment visit. The CHW navigation programme adopts the C.O.P.E model to provide patients and caregivers with guidance and expert information. The C.O.P.E. model includes four principles: Creativity, Optimism, Planning and Expert information. It focuses on social problem-solving for both the patient and family caregiver(s) to optimise problem orientation and problem-solving.15 17 18 The C.O.P.E. model has been widely documented in the scientific literature with oncology patients and their caregivers to assist with problem-solving. The CHW navigation programme incorporates these principles of C.O.P.E. to empower and engage patients and caregivers in their care. The implementation of the CHW navigation programme is intended to influence patients’ healthcare utilisation, as well as stress and QOL.
The CHW team from SKCCC will contact eligible patients or their caregivers to assess interests in participating before obtaining informed consent. On enrolling, the CHWs will ask patients a series of scripted questions as a needs assessment. The questions asked will be tailored to whether the patient already received a confirmed diagnosis via biopsy report. This allows a more flexible approach for the delivery of the programme and helps tailor the intervention accordingly for the respective needs of different patients and/or caregivers. This flexibility was prioritised by the Alliance to accommodate the unique infrastructures and capacities of the six NCI-designated cancer centres within the project’s network,22 and represents an important feature of many complex interventions.21 28 29 For patients who do not have a biopsy-confirmed diagnosis on record, the CHW will facilitate scheduling a biopsy visit, as needed, and direct patients to the appropriate JHH department for follow-up care (eg, Surgery, Medical Oncology or Radiation Therapy). The patient can leave the navigation programme if they decide to seek cancer care elsewhere, or if the CHW is unable to meet the patient’s specific needs at that time. All points of contact with patients and/or caregivers throughout the programme will be documented as encounter notes by the CHW navigators.
Alternatively, CHWs will focus on scheduling the initial appointment with oncology for patients who have a biopsy-confirmed diagnosis on record. During a call, the CHW will identify any barriers inhibiting the patient from scheduling their first oncology visit. This may include logistical barriers, such as a lack of transportation, childcare or understanding how to use MyChart, an online personal health record system used to schedule appointments, view medications and test results, and connect with providers. The CHW may also identify psycho-emotional barriers, such as fear, mistrust or a lack of support. After the CHW identifies these barriers, they will connect the patient with the appropriate online community resources from Johns Hopkins Electronic Resource databases. The CHW may initiate discussions for scheduling either an in-home or office visit for the patient’s initial oncology treatment, if appropriate. CHWs will anticipate and prepare for multiple phone calls with patients who have more complex needs to provide sufficient information and navigation support.
Outcome of interests
The primary outcome is time for the initial treatment visit following a cancer diagnosis. Secondary outcome variables include healthcare utilisation at 3 and 6 months after the completion of the programme, cost of care, number of problems resolved, number of community resources used, stress and QOL. The acceptability of the programme by patient and caregiver populations will be assessed by the established CAGs through semi-structured discussions hosted by the investigator team. Members from the CAGs vetted all proposed questionnaires and procedures before receiving formal approval from a research ethics review board and the project sponsor. See table 1 for specific short-term and long-term outcome variables.
Table 1.
Short-term and long-term outcome variables
| Variable | Measurement | |
| Short-term outcomes* | Cancer-related problem | Problem-Checklist (25-item) |
| Stress | Chronic Illness Distress Scale (7-item) | |
| Quality of Life | Satisfaction with Life Domains (16-item) | |
| Long-term outcomes† | Time to first oncology treatment visit‡ | Time to first oncology treatment visit following patient’s cancer-confirmed biopsy |
| Healthcare utilisation‡ | No of ED visits No of hospitalisations Length of hospital stay Mortality No of oncology visits (telehealth/in-person) No of missed oncology visits |
|
| Cost of care‡ | Pharmacy charges Radiology charges |
*During CHW Navigation Programme Contact.
†A 3-month and 6-month postprogramme.
‡Data provided from Epic (electronic health record).
CHW, community health worker; ED, emergency department.
Instruments
The time to initial oncology treatment visit and healthcare utilisation data will be obtained from Epic, an electronic medical record database. Time to initial oncology treatment visit is defined as the time from the patient’s biopsy-confirmed diagnosis to their first oncology treatment. Healthcare utilisation encompasses the number of oncology visits (via telehealth or in-person), missed oncology visits, ED visits, hospitalisations and length of hospital stay and patient mortality. The cost of care (eg, pharmacy and radiology charges) will also be tracked through reviewing data from Epic.
The current study will use the following instruments as directed by the Alliance for harmonising outcomes across the six cancer centres.22 The Problem-Checklist is a 25-item inventory of problems cancer patients have reported during and after diagnosis and treatment. The Problem-Checklist will assist in identifying problems to focus on during the intervention. To measure QOL, the patient will complete the Satisfaction with Life Domains Scale, a 16-item scale. The scale uses a seven-point Likert rating system, ranging from 1 (most satisfied) to 7 (most dissatisfied). This scale was previously used with breast cancer survivors and patients who received bone marrow transplants and were positively received by both patient populations.30 31 The Chronic Illness Distress scale will measure stress. This scale is a 16-item, 4-point Likert scale ranging from not upset to very upset. These brief questionnaires will be completed on entry into the programme with interim follow-up data collected at 3 and 6 months.
Patient sociodemographic information will be collected using a Patient Demographics Questionnaire. This questionnaire includes items about age, marital status, race/ethnicity, education, employment status, annual household income, cancer diagnosis and time of diagnosis.
Data collection
Short-term outcome variables will be collected by CHW team members during the first series of phone calls with the participant. CHWs will review each questionnaire with the participant and record their responses. Each CHW will also maintain a journal of interactions (encounter notes) with participants, detailing the participant’s problem, resources used to address the problem, who they spoke with (patient or informal/formal caregiver), and the number of phone calls made. All data will be secured in accordance with university policies for upholding study confidentiality. Long-term outcome variables will be collected through Epic and stored in Research Electronic Data Capture (REDCap). REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources.32 33 For assessing the low-cost feature of the CHW navigation programme, our team will document the resources expended during implementation such as staffing hours and costs of communication resources. We will also document the number of patients served by the programme to demonstrate our reach.
Analytical plan
We will collect all measures (table 1) before and after implementation of the CHW Navigation Programme. CHWs will collect information by phone through the Problem Checklist, Chronic Illness Distress scale and Satisfaction with Life Domains scale before providing guidance on cancer-related problem(s). In addition, we will assess the time to the initial oncology treatment visit and healthcare utilisation through evaluating data from Epic at 3 and 6 months following the programme. Data analyses will occur at each timepoint of data collection (baseline and postprogramme; immediate, 3 months and 6 months). Univariate analyses will be completed to describe the sample. Bivariate analyses will be completed to understand relationships between receipt of the programme and all outcome variables. We will use independent t-tests to assess the programme’s impact on participant stress, QOL, number of problems reported and healthcare utilisation. We will use repeated paired t-tests (under a Bonferroni correction) on the following: average pretest to each post-test data collection point for distress, severity of symptoms and problem-solving behaviours. Additional paired t-tests will be conducted to determine if there is a difference in the same variables over time.
Ethics and dissemination
This study completed an ethical review through the Johns Hopkins School of Medicine’s institutional review board (IRB00160610) and received initial approval on 1 August 2019. This study includes an IRB-approved informed consent process where we will obtain oral consent by phone from each participant prior to enrolment in the programme. At the time of this writing (2022), the current protocol is approved through 6 July 2023, coinciding with the projected study conclusion after confronting implementation delays due to local public health guidelines implemented during the COVID-19/SARS-CoV-2 pandemic which restricted research and clinical activities at SKCCC. Discussions surrounding the study’s dissemination plans are ongoing through regular meetings between members of the investigator team (JHCRCD) and members of the public (CAGs). The JHCRCD and CAGs together will also be collaborating with other entities such as the Johns Hopkins Institute for Clinical and Translational Research and the Johns Hopkins Center for Health Equity (formerly the Johns Hopkins Center to Eliminate Cardiovascular Health Disparities),34 35 who offer additional expertise drawn from experience in forging academic-community partnerships for study conduct and disseminating findings.36
Discussion
We will implement a pilot CHW navigation programme to support patient populations in Baltimore who face a higher risk of delaying initial cancer treatment visits or becoming lost to follow-up throughout the care continuum. Our sampling strategy targets residents of select urban zip codes with high ADI scores where patients are more likely to confront barriers to timely healthcare engagement37 and are less likely to participate in research studies,38 attributed to respective social determinants of health. CHWs are shown to be effective in improving access and engagement with healthcare in underserved populations6 39 and are well positioned to support cancer patients in reducing the time to initial cancer treatment.
The CHW will serve as a linkage between patients newly diagnosed with cancer and their oncology provider team at the JHH. As an enabling resource for supporting cancer care utilisation by patient populations facing higher risks for adverse outcomes, a CHW navigation programme also supports pathways for promoting health equity. Like other CHW models of care, the health worker does not directly provide medical consultation or primary care. Rather, they are trained to identify patients’ and caregivers’ needs and support problem-solving skills for navigating their healthcare. The CHWs also provide support by connecting patients to relevant resources and help coordinate care to reduce time to initiating treatment. Since healthcare utilisation during the time between first diagnosis and initial treatment represents a critical period that can significantly modify health outcomes, reducing this time improves the prospects of survivorship and experiencing a better QOL.
There will be several limitations worth noting in implementing this programme. First, there will be no exclusion criterion based on cancer type. This results in a heterogeneous sample of patients with varying needs; some may require several contacts with a CHW to sense adequate support while others may only require one point of contact. Second, only patients who have previously contacted the SKCCC will be reached, thus introducing population selection bias to consider. Inherent structural factors that impede access to health systems such as the SKCCC should also be considered when designing programmes aiming to address barriers to cancer care. Furthermore, our programme is connecting patients who are still early in their cancer care trajectory, and therefore, may not yet be knowledgeable enough to articulate their complete needs to the CHW. However, engagements with a CHW navigator still offer an initial point of linkage to resources that patients and caregivers may access during relevant periods later in their care trajectory. Finally, the programme will be operating within the context of the JHH system and therefore other institutions with different infrastructures may benefit from considering another implementation strategy.
Our CHW navigation programme represents an innovative approach to address disparities early in cancer care and supports the odds of survivorship by leveraging a model commonly used for reaching underserved patient populations.40 The role of the CHW has proliferated due to its demonstrated effectiveness in community outreach, social support, informal counselling and health education.41 The paraprofessional workforce is associated with improved care access and reduced healthcare costs,6 42 particularly among socially minoritised populations.43 44 While the CHW navigation programme will be based within JHH, conducting outreach to patients who are not yet fully integrated within the healthcare system presents another unique advantage. Patients usually need to be completely onboarded before being eligible to receive navigation services by either a trained CHW or staff nurse. Our hospital-based CHW, who may also be perceived as more approachable than healthcare providers by patients, serves as a linkage with patients in the Baltimore community before they spend the additional time usually needed for integrating with a healthcare team. Waiting for patients to be fully onboarded before receiving navigation services may otherwise risk further delaying initial treatment. The CHW navigator, therefore, operates within a ‘transitional space’ for patients in their care trajectory when they may face higher risk of becoming lost to follow-up due to barriers in healthcare systems, including those attributable to legacies of structural racism. A CHW navigator may also provide further assurance for a medical provider team who would know that their patients can remain better connected to vital healthcare through the CHW-directed service. Finally, a CHW navigation programme may also represent a lower-cost approach6 42 in reaching underserved demographics with cancer diagnoses. Resources for operating such a programme include properly allocating staff hours (including time for CHW training, programme delivery and continuing education as needed), adequate workspaces and communications equipment. Taken together, we propose our CHW navigation programme for cancer care as a cost-effective approach to facilitate better outcomes for both patients, their caregivers and the healthcare system.
We will pilot our novel CHW navigation programme and evaluate its logistic feasibility and acceptability among populations with recent cancer diagnoses who are traditionally underserved by healthcare systems in Baltimore City. Our programme represents an enabling resource5 to support healthcare utilisation for patients and their caregivers during a critical period when initiating treatment by oncology on time is crucial for favourable outcomes. Our programme protocol describes key guiding frameworks, programme features and an analytical plan that we aim to follow for implementation.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support of the Merck Foundation in this program of research. The authors would also like to acknowledge Dr. Debra Roter and Ms. Marielle (Mari) Bugayong for their guidance and expertise.
Footnotes
Twitter: @suenjonathan
Contributors: All authors contributed to the conceptualisation of this manuscript (JJS, EP, AH, KP, OS, JW, JRZ and ASD). JJS and EP drafted the manuscript as co-first authors and all other authors contributed substantial feedback and writing for revisions, including a member of the CHW team at SKCCC (OS). ASD and JRZ advised the manuscript’s development as co-senior authors and principal investigators of the grant awarded by the Merck Foundation.
Funding: This work was coordinated through the Alliance to Advance Patient-Centered Cancer Care, with support from the Merck Foundation (proposal #19-9714), after a competitive call for Alliance-affiliated projects with program oversight from a designated National Program Office within the University of Michigan School of Nursing. JJS is supported in part by the National Institute on Aging/National Institutes of Health (F31AG071353) and the Johns Hopkins Cochlear Center for Hearing and Public Health, which is supported in part by a philanthropic gift from Cochlear.
Disclaimer: The funders listed above have no role in both the conduct and outcomes of the study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open 2020;3:e2030072. 10.1001/jamanetworkopen.2020.30072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One 2019;14:e0213209. 10.1371/journal.pone.0213209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin 2018;68:199–216. 10.3322/caac.21452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1–10. 10.2307/2137284 [DOI] [PubMed] [Google Scholar]
- 6.Witmer A, Seifer SD, Finocchio L, et al. Community health workers: integral members of the health care work force. Am J Public Health 1995;85:1055–8. 10.2105/AJPH.85.8_Pt_1.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olaniran A, Smith H, Unkels R, et al. Who is a community health worker? – a systematic review of definitions. Glob Health Action 2017;10:1272223. 10.1080/16549716.2017.1272223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han H-R, Song Y, Kim M, et al. Breast and cervical cancer screening literacy among Korean American women: a community health worker–Led intervention. Am J Public Health 2017;107:159–65. 10.2105/AJPH.2016.303522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter RW, Bengen B, Alsup PA, et al. The community health worker. A resource for improved health care delivery. Am J Public Health 1974;64:1056–61. 10.2105/AJPH.64.11.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suen JJ, Han H-R, Peoples CY, et al. A community health worker training program to deliver accessible and affordable hearing care to older adults. J Health Care Poor Underserved 2021;32:37–49. 10.1353/hpu.2021.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Choi JS, Choi E, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health 2016;106:e3–28. 10.2105/AJPH.2015.302987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katigbak C, Van Devanter N, Islam N, et al. Partners in health: a conceptual framework for the role of community health workers in facilitating patients’ Adoption of healthy behaviors. Am J Public Health 2015;105:872–80. 10.2105/AJPH.2014.302411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Sidney Kimmel Comprehensive Cancer Center Community Outreach and Engagement . Available: https://www.hopkinsmedicine.org/kimmel_cancer_center/community_outreach_engagement/ [Accessed 05 Aug 2022].
- 14.Anderson LM, Adeney KL, Shinn C, et al. Community coalition-driven interventions to reduce health disparities among racial and ethnic minority populations. Cochrane Database Syst Rev 2015;6:CD009905. 10.1002/14651858.CD009905.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bevans M, Castro K, Prince P, et al. An individualized Dyadic problem-solving education intervention for patients and family caregivers during allogeneic hematopoietic stem cell transplantation. Cancer Nurs 2010;33:E24–32. 10.1097/NCC.0b013e3181be5e6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevans M, Wehrlen L, Castro K, et al. A problem-solving education intervention in caregivers and patients during allogeneic hematopoietic stem cell transplantation. J Health Psychol 2014;19:602–17. 10.1177/1359105313475902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MJL W, Bucher JA. The cope model. J Psychosoc Oncol 1999;16:93–117. 10.1300/j077v16n03_07 [DOI] [Google Scholar]
- 18.Houts PS, Nezu AM, Nezu CM, et al. The prepared family caregiver: a problem-solving approach to family caregiver education. Patient Educ Couns 1996;27:63–73. 10.1016/0738-3991(95)00790-3 [DOI] [PubMed] [Google Scholar]
- 19.Hwang S, Birken SA, Melvin CL, et al. Designs and methods for implementation research: advancing the mission of the CTSA program. J Clin Transl Sci 2020;4:159–67. 10.1017/cts.2020.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical Research Council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ver Hoeve ES, Simon MA, Danner SM, et al. Implementing patient navigation programs: considerations and lessons learned from the alliance to advance Patient‐Centered cancer care. Cancer 2022;128:2806–16. 10.1002/cncr.34251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baltimore City Health Department Community Health Assessment , 2017. Available: https://health.baltimorecity.gov/sites/default/files/health/attachments/Baltimore%20City%20CHA%20-%20Final%209.20.17.pdf
- 24.Literacy I of M (US) C on H . Health Literacy: A Prescription to End Confusion. Washington (DC): National Academies Press (US), 2004. [PubMed] [Google Scholar]
- 25.Polit DF, Beck CT. Essentials of nursing research. 7th ed. Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 26.Brown QL, Elmi A, Bone L, et al. Community engagement to address cancer health disparities: a process evaluation using the partnership self-assessment tool. Prog Community Health Partnersh 2019;13:97–104. 10.1353/cpr.2019.0012 [DOI] [PubMed] [Google Scholar]
- 27.Mbah O, Ford JG, Qiu M, et al. Mobilizing social support networks to improve cancer screening: the coach randomized controlled trial study design. BMC Cancer 2015;15:907. 10.1186/s12885-015-1920-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new medical Research Council guidance. J Epidemiol Community Health 2012;66:1182–6. 10.1136/jech-2011-200375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnola S, Zabora J, BrintzenhofeSzoc K, et al. The satisfaction with life domains scale for breast cancer (SLDS-BC). Breast J 2003;9:463–71. 10.1046/j.1524-4741.2003.09603.x [DOI] [PubMed] [Google Scholar]
- 31.Baker F, Curbow B, Wingard JR. Development of the satisfaction with life domains scale for cancer. J Psychosoc Oncol 1992;10:75–90. 10.1300/J077V10N03_05 [DOI] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper LA. Training and mentoring the next generation: insights from the field. Ethn Dis 2018;28:579–85. 10.18865/ed.28.4.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper LA, Purnell TS, Showell NN, et al. Progress on major public health challenges: the importance of equity. Public Health Rep 2018;133:15S–19. 10.1177/0033354918795164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper LA, Purnell TS, Ibe CA, et al. Reaching for health equity and social justice in Baltimore: the evolution of an academic-community partnership and conceptual framework to address hypertension disparities. Ethn Dis 2016;26:369–78. 10.18865/ed.26.3.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martires KJ, Kurlander DE, Minwell GJ, et al. Patterns of cancer screening in primary care from 2005 to 2010. Cancer 2014;120:253–61. 10.1002/cncr.28403 [DOI] [PubMed] [Google Scholar]
- 38.Halbert CH, Jefferson M, Allen CG, et al. Racial differences in patient portal activation and research enrollment among patients with prostate cancer. JCO Clinical Cancer Informatics 2021;6:768–74. 10.1200/CCI.20.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: state of the art or is it science? Cancer 2008;113:1999–2010. 10.1002/cncr.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman HP. The history, principles, and future of patient navigation: commentary. Semin Oncol Nurs 2013;29:72–5. 10.1016/j.soncn.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Pérez LM, Martinez J. Community health workers: social justice and policy advocates for community health and well-being. Am J Public Health 2008;98:11–14. 10.2105/AJPH.2006.100842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocque GB, Pisu M, Jackson BE, et al. Resource use and Medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol 2017;3:817. 10.1001/jamaoncol.2016.6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earp JA, Eng E, O'Malley MS, et al. Increasing use of mammography among older, rural African American women: results from a community trial. Am J Public Health 2002;92:646–54. 10.2105/AJPH.92.4.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eng E, Smith J. Natural helping functions of lay health advisors in breast cancer education. Breast Cancer Res Treat 1995;35:23–9. 10.1007/BF00694741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.