Summary
Background
Sero-surveillance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can reveal trends and differences in subgroups and capture undetected or unreported infections that are not included in case-based surveillance systems.
Methods
Cross-sectional, convenience samples of remnant sera from clinical laboratories from 51 U.S. jurisdictions were assayed for infection-induced SARS-CoV-2 antibodies biweekly from October 25, 2020, to July 11, 2021, and monthly from September 6, 2021, to February 26, 2022. Test results were analyzed for trends in infection-induced, nucleocapsid-protein seroprevalence using mixed effects models that adjusted for demographic variables and assay type.
Findings
Analyses of 1,469,792 serum specimens revealed U.S. infection-induced SARS-CoV-2 seroprevalence increased from 8.0% (95% confidence interval (CI): 7.9%–8.1%) in November 2020 to 58.2% (CI: 57.4%–58.9%) in February 2022. The U.S. ratio of the change in estimated seroprevalence to the change in reported case prevalence was 2.8 (CI: 2.8–2.9) during winter 2020–2021, 2.3 (CI: 2.0–2.5) during summer 2021, and 3.1 (CI: 3.0–3.3) during winter 2021–2022. Change in seroprevalence to change in case prevalence ratios ranged from 2.6 (CI: 2.3–2.8) to 3.5 (CI: 3.3–3.7) by region in winter 2021–2022.
Interpretation
Ratios of the change in seroprevalence to the change in case prevalence suggest a high proportion of infections were not detected by case-based surveillance during periods of increased transmission. The largest increases in the seroprevalence to case prevalence ratios coincided with the spread of the B.1.1.529 (Omicron) variant and with increased accessibility of home testing. Ratios varied by region and season with the highest ratios in the midwestern and southern United States during winter 2021–2022. Our results demonstrate that reported case counts did not fully capture differing underlying infection rates and demonstrate the value of sero-surveillance in understanding the full burden of infection. Levels of infection-induced antibody seroprevalence, particularly spikes during periods of increased transmission, are important to contextualize vaccine effectiveness data as the susceptibility to infection of the U.S. population changes.
Funding
This work was supported by the Centers for Disease Control and Prevention, Atlanta, Georgia.
Keywords: SARS-CoV-2, Seroprevalence, United States, COVID-19
Research in context.
Evidence before this study
Analyses of trends in COVID-19 reported cases, deaths, emergency room visits, hospitalizations, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections have been important to understand the progression of the pandemic. Sero-surveillance of SARS-CoV-2 antibody prevalence can provide an additional and unique measure of disease burden by assess evidence of past undetected or unreported infections.
An initial SARS-CoV-2 seroprevalence study utilizing remnant serum from people seeking routine medical care was conducted in 10 US. geographic areas with known community transmission. The study estimated the cumulative number of infections to be 6 to 24 times higher than the number from reported, laboratory-confirmed COVID-19 case reports. Another nationwide study from blood donors found infection-induced seroprevalence reached approximately 20% by May 2021.
Added value of this study
The results of this study increase the knowledge base of SARS-CoV-2 infection and demonstrate the utility of SARS-CoV-2 sero-surveillance by describing trends in seropositivity and the ratios of changes in seroprevalence to changes in reported case prevalence, overall and among subpopulations, from October 25, 2020, through February 26, 2022, in the United States. Seroprevalence can be used to explore testing gaps, evaluate disease transmission, and identify population subgroups that are at higher risk of infection. Ratios of changes in serologically defined estimated infection rates to changes in reported case rates can add important context to the interpretation of case counts in the population and vaccine effectiveness data.
Implications of all the available evidence
Ratios of the change in seroprevalence to the change in reported case prevalence suggest that, during periods of increased transmission in the United States, a greater proportion of infections go unreported. The increased availability and use of self-administered viral antigen tests may lead to decreased reporting of cases. As self-testing becomes more commonplace, the U.S. CDC's updated community levels, which incorporate case counts as well as hospitalizations to measure COVID-19 impact, may become more variable; in that event, sero-surveillance will become more valuable. Sero-surveillance can more accurately characterize the infection burden, especially during periods of high transmission, and contextualize reported case counts. In addition, these sero-surveillance results highlight the utility of incorporating infection-induced immunity into vaccine effectiveness estimates, since infection-induced immunity also provides some protection against subsequent infection. Finally, these analyses demonstrate the higher infection burdens faced by certain subgroups, especially children and people in the Midwestern and southern United States.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in the United States in late January 20201 with initial waves of coronavirus disease 2019 (COVID-19) cases in the spring and summer 2020. A third wave started in the fall 2020 and case counts reached an apex in mid-January 2021, then started declining due to the population-level effectiveness of the vaccine rollout2 and continued a downward trajectory until reaching a nadir in early July 2021. Afterwards, reported case counts have risen sharply due to the B.1.617.2 (Delta)3 variant beginning in late June 2021 and B.1.1.529 (Omicron)4 variant beginning in December 2021. COVID-19 has imposed a tremendous burden of disease in the United States with approximately 97.2 million cases and 1.1 million deaths reported through October 28, 2022.5
Monitoring the COVID-19 burden over time in the United States has often utilized reported case and death counts,2,5, 6, 7 emergency department visits,2,7 or hospital admissions.2,7 Conversely, seroprevalence has often been measured at a single time point8, 9, 10 and before the popularity of at-home testing.11 Sero-surveillance studies with either cohort or repeated, cross-sectional designs have been limited in their ability to assess the national burden of COVID-19 infection because they were administered in subnational geographical areas12 or sampled specific patient populations.13, 14, 15, 16 Modeling or simulation-based approaches have attempted to fill these gaps.17, 18, 19
To better understand nationwide temporal trends of SARS-CoV-2 seroprevalence and contextualize case-based surveillance data, we analyzed data from an all-ages, national, repeated, cross-sectional SARS-CoV-2 seroprevalence study.20 Remnant sera from commercial laboratory specimens from patients who had blood drawn for routine screening or clinical care were collected regularly from the 50 United States and the District of Columbia (D.C.) and tested for SARS-CoV-2 antibodies using commercially available U.S. Food and Drug Administration Emergency Use Authorized test kits.
The objectives of the study were to a) examine antibody seroprevalence trends overall and in subgroups by age, sex, and urbanicity, and b) compare the change in serologically estimated infection prevalence to changes in prevalence derived from reported cases at different stages of the pandemic and by geographic region, including during case surges due to the omicron variant.
Methods
Study design
Remnants of serum specimens submitted to clinical laboratories for routine, clinical screening, or diagnostic testing from 51 U.S. jurisdictions (50 U.S. states and D.C.) between October 25, 2020, and February 26, 2022, were examined. Until July 11, 2021, specimens were tested for SARS-CoV-2 antibodies at biweekly time periods; after a break of 56 days, specimens were tested at monthly time periods beginning September 6, 2021. If SARS-CoV-2 antibody testing was requested by the ordering clinician on the same day as the specimen was identified in the convenience sample, the specimen was excluded to reduce selection bias. Three laboratories (A, B, and C) collected specimens from five, 23, and 23 jurisdictions, respectively. All laboratories serve or can serve patients in all 50 U.S. states, D.C., and Puerto Rico. Laboratory A serves approximately 259,000 of physicians in the U.S., laboratory B serves approximately 50% of physicians and hospitals in the U.S., and laboratory C serves 64% of hospitals and 400,000 physicians in the U.S. In each jurisdiction, participating laboratories selected a convenience sample of 1300 remnant sera specimens during each biweekly testing period, divided equally among four age groups (0–17, 18–49, 50–64, and ≥65 years); for the monthly samples, 1750 samples were included per jurisdiction per month divided among five age groups (0–11, 12–17, 18–49, 50–64, and ≥65 years). Laboratories were unable to provide specimens for the following time periods and states: September 6–October 3, 2021, from Indiana, Maryland, New Jersey, and Virginia; November 1–November 28, 2021, from North Dakota; and December 27, 2021–January 29, 2022, from Nevada. As a result, national seroprevalence estimates excluded these states in these time periods.
Data collected included information on patient age, sex, state, specimen collection date, ZIP code of residence, and ordering provider ZIP code, but not race, ethnicity, or vaccination status because these were not provided by the clinical laboratories.
Ethics
This activity was reviewed by the U.S. Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy.e Informed consent was waived as data were de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant.
Supporting data
Case count data from CDC's COVID Data Tracker5 were used to explore trends in case counts reported by jurisdictions over time, and to assess correlations with the trends in seroprevalence. Urbanicity is defined as metro or non-metro based on the U.S. Department of Agriculture's Rural-Urban Continuum Codes (metro 1–3, non-metro 4–9).21 The social vulnerability index (SVI)22 is a county-level measure of potential negative effects on communities caused by external stresses on human health that has been associated with higher SARS-CoV-2 seroprevalence.23 In modeling, we included SVI as a continuous variable but, for demographic information (Supplementary Table S2), was divided into terciles at the 33.3rd and 66.7th percentiles and defined as low, medium, and high.
Laboratory methods
Laboratories performed specimen processing and transportation according to their established procedures. Laboratory A tested all specimens at a central facility, laboratory B performed testing at 12 facilities, and laboratory C performed testing at 23 sites. Specimens were tested by either an assay detecting antibodies against the nucleocapsid (N) protein (the Abbott ARCHITECT SARS-CoV-2 IgG immunoassay or the Roche Elecsys Anti-SARS-CoV-2 pan-immunoglobulin immunoassay) or an assay detecting antibodies against the spike (S) protein (the Ortho-Clinical Diagnostics VITROS SARS-CoV-2 IgG immunoassay). All specimens tested with the VITROS platform after December 18, 2020 (n = 17,026 samples in 11 jurisdictions) were excluded from these analyses since the VITROS anti-S platform targets only the S-protein and could conflate antibodies from infection with antibodies from vaccination. All specimens from Puerto Rico were also excluded because they were tested with the VITROS platform and we were unable to establish an anti-N seroprevalence estimate. Beginning in September 2021, all specimens were tested using the Roche Elecsys assay which targets the N-protein; anti-N antibodies are produced by the body in response to infection, but not after vaccination with vaccines approved or authorized in the United States. All three assays were granted Emergency Use Authorization by the U.S. Food and Drug Administration and were used according to manufacturer instructions.
Statistical analysis
A generalized linear mixed effects model24 assuming a binomial distribution with a logit link function was used to associate the serologic test result (positive or negative) with multiple covariates. The testing round, age category, sex, metro or non-metro designation, SVI, biweekly period, census region, assay type, and an interaction between round and assay type were included as fixed effects. State and county were included as random effects to account for correlation among specimens collected in the same geographic area. After models were fit, seroprevalence estimates were generated from linear combinations of the regression coefficients. Seroprevalence estimates were standardized by adjusting to the parameters of the Roche Elecsys N-target assay, and weighting the age, sex, region, SVI, and metro or non-metro distributions to the U.S. population. The Roche Elecsys assay was chosen because, among the three assays used in this study, it has been shown to yield the most stable antibody responses over multiple months.25 Models were fit in R (The Comprehensive R Archive Network, version 4.0.3) with the lme4 package.26 Where appropriate, 95% confidence intervals are shown.
For a small number of specimens with missing data (Supplementary Table S1), imputation was performed for missing age, sex, U.S. County Federal Information Processing Standard (FIPS) code, and metro status data. A probabilistic method was used which imputes the missing data for each variable from the distribution of non-missing data within each jurisdiction. Records with missing SVI were excluded from the analysis.
The ratio of the change in seroprevalence to the change in reported case prevalence were calculated by finding the quotient of the difference between model-estimated seroprevalence at the beginning and end of a given period and the difference in population-based cumulative case counts prevalence during the same period. Seroprevalence was taken directly from the model-based point and confidence limit estimates, while the change in cumulative case prevalence was calculated by dividing the number of reported cases by the estimated 2019 population.27 To synchronize case prevalence data with the seroprevalence time periods, we used the median date within each time interval and then lagged the reported case count by 14 days to account for the time required to develop antibodies, such that, cumulatively, the reported case prevalence is estimated for a date that is 14 days earlier than the date of the seroprevalence. Further details on the statistical methods can be found in the Supplementary Material. This ratio is henceforth referred to as the change ratio.
Role of the funding source
The CDC was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Results
A total of 1,469,792 remnant serum specimens were collected between October 25, 2020, and February 26, 2022 (Table 1). Women accounted for 58.9% (n = 603,571) of specimens with a range of 58.1% to 59.8% across surveys. Specimens from children aged 0–17 years made up the smallest percentage among age groups (15.0%, n = 220,272, range: 11.5%–24.6%) while people aged 18–49 years made up the largest percentage (30.9%, n = 454,756, range 24.5%–33.1%). The oldest two age groups each contributed slightly over a quarter of specimens (50–64 years: 26.6%, n = 390,491, 21.1%−29.2%; 65+ years: 27.5%, n = 404,273, 26.4%−28.8%). Prior to December 18, 2020, 32.8% (n = 74,613) of specimens were tested with the Abbott ARCHITECT assay, 9.8% (n = 22,365) with the Ortho-Clinical Diagnostics VITROS assay, and 57.4% (n = 130,684) with the Roche Elecsys assay. After December 18, 2020, 29.4% (n = 365,005) of specimens were tested with the Abbott ARCHITECT assay and 70.6% (n = 877,125) with the Roche Elecsys assay. Specimens were collected from people divided evenly across SVI categories, and most came from metro areas (Supplementary Table S2).
Table 1.
Demographic information and assay used for participants tested for SARS-CoV-2 antibodies for each cross-sectional survey period, 50 United States and District of Columbia, October 25, 2020–February 26, 2022.
| Date range | Total population or samples | Sex |
Age category |
Assay |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Male | 0–17 | 18–49 | 50–64 | ≥65 | Abbott Architect | Ortho VITROS | Roche Elecsys | ||
| ACS population total | 322,903,030 | 158,984,190 (49.2) | 73,553,240 (22.8) | 137,062,784 (42.4) | 6,3048,425 (19.5) | 49,238,581 (15.3) | – | – | – |
| Overall | 1,469,792 | 603,571 (41.1) | 220,272 (15.0) | 454,756 (30.9) | 390,491 (26.6) | 404,273 (27.5) | 439,618 (29.9) | 22,365 (1.5) | 1,007,809 (68.6) |
| Oct 25–Nov 15, 2020 | 63,111 | 25,595 (40.6) | 8360 (13.2) | 19,688 (31.2) | 18,064 (28.6) | 16,999 (26.9) | 21,117 (33.5) | 7364 (11.7) | 34,630 (54.9) |
| Nov 9–29, 2020 | 61,995 | 25,357 (40.9) | 7285 (11.8) | 20,547 (33.1) | 17,813 (28.7) | 16,350 (26.4) | 17,854 (28.8) | 4431 (7.1) | 39,710 (64.1) |
| Nov 23–Dec 12, 2020 | 57,859 | 24,226 (41.9) | 7444 (12.9) | 18,111 (31.3) | 16,286 (28.1) | 16,018 (27.7) | 20,315 (35.1) | 6008 (10.4) | 31,536 (54.5) |
| Dec 8–27, 2020 | 56,742 | 23,503 (41.4) | 6519 (11.5) | 18,452 (32.5) | 16,564 (29.2) | 15,207 (26.8) | 18,753 (33.0) | 4562 (8.0) | 33,427 (58.9) |
| Dec 22, 2020–Jan 10, 2021 | 50,969 | 21,119 (41.4) | 6227 (12.2) | 16,660 (32.7) | 14,361 (28.2) | 13,721 (26.9) | 19,902 (39.0) | 0 (0.0) | 31,067 (61.0) |
| Jan 4–24, 2021 | 55,529 | 22,930 (41.3) | 6729 (12.1) | 17,042 (30.7) | 15,979 (28.8) | 15,779 (28.4) | 21,315 (38.4) | 0 (0.0) | 34,214 (61.6) |
| Jan 19–Feb 7, 2021 | 57,819 | 24,215 (41.9) | 7736 (13.4) | 17,969 (31.1) | 16,361 (28.3) | 15,753 (27.2) | 23,802 (41.2) | 0 (0.0) | 34,017 (58.8) |
| Feb 1–21, 2021 | 59,009 | 24,623 (41.7) | 7631 (12.9) | 18,605 (31.5) | 16,517 (28.0) | 16,256 (27.5) | 27,293 (46.3) | 0 (0.0) | 31,716 (53.7) |
| Feb 15-Mar 7, 2021 | 59,008 | 24,281 (41.1) | 8620 (14.6) | 17,993 (30.5) | 16,654 (28.2) | 15,741 (26.7) | 27,471 (46.6) | 0 (0.0) | 31,537 (53.4) |
| Mar 1–21, 2021 | 61,779 | 25,210 (40.8) | 8605 (13.9) | 19,025 (30.8) | 17,110 (27.7) | 17,039 (27.6) | 27,211 (44.0) | 0 (0.0) | 34,568 (56.0) |
| Mar 15–Apr 4, 2021 | 60,280 | 24,515 (40.7) | 8295 (13.8) | 19,131 (31.7) | 16,541 (27.4) | 16,313 (27.1) | 26,998 (44.8) | 0 (0.0) | 33,282 (55.2) |
| Mar 29–Apr 18, 2021 | 60,359 | 24,570 (40.7) | 7726 (12.8) | 19,073 (31.6) | 17,176 (28.5) | 16,384 (27.1) | 26,841 (44.5) | 0 (0.0) | 33,518 (55.5) |
| Apr 12–May 2, 2021 | 58,767 | 23,816 (40.5) | 7867 (13.4) | 18,418 (31.3) | 16,409 (27.9) | 16,073 (27.4) | 26,896 (45.8) | 0 (0.0) | 31,871 (54.2) |
| Apr 26–May 16, 2021 | 60,640 | 24,813 (40.9) | 8009 (13.2) | 19,138 (31.6) | 16,907 (27.9) | 16,586 (27.4) | 26,936 (44.4) | 0 (0.0) | 33,704 (55.6) |
| May 10–30, 2021 | 58,501 | 23,805 (40.7) | 7566 (12.9) | 18,292 (31.3) | 16,618 (28.4) | 16,025 (27.4) | 26,677 (45.6) | 0 (0.0) | 31,824 (54.4) |
| May 24–Jun 13, 2021 | 59,841 | 24,276 (40.6) | 6940 (11.6) | 19,220 (32.1) | 17,202 (28.7) | 16,479 (27.5) | 26,727 (44.7) | 0 (0.0) | 33,114 (55.3) |
| Jun 7–27, 2021 | 62,584 | 25,392 (40.6) | 7540 (12.0) | 20,352 (32.5) | 17,295 (27.6) | 17,397 (27.8) | 27,196 (43.5) | 0 (0.0) | 35,388 (56.5) |
| Jun 21–Jul 11, 2021 | 58,591 | 23,970 (40.9) | 7370 (12.6) | 19,035 (32.5) | 16,155 (27.6) | 16,031 (27.4) | 26,314 (44.9) | 0 (0.0) | 32,277 (55.1) |
| Sep 6–Oct 3, 2021 | 63,199 | 26,464 (41.9) | 12,728 (20.1) | 19,200 (30.4) | 13,942 (22.1) | 17,329 (27.4) | 0 (0.0) | 0 (0.0) | 63,199 (100) |
| Oct 4–31, 2021 | 72,096 | 29,961 (41.6) | 16,414 (22.8) | 20,314 (28.2) | 15,241 (21.1) | 20,127 (27.9) | 0 (0.0) | 0 (0.0) | 72,096 (100) |
| Nov 1–28, 2021 | 79,649 | 32,860 (41.3) | 16,027 (20.1) | 22,538 (28.3) | 18,201 (22.9) | 22,883 (28.7) | 0 (0.0) | 0 (0.0) | 79,649 (100) |
| Nov 29–Dec 26, 2021 | 75,865 | 30,900 (40.7) | 14,070 (18.5) | 23,233 (30.6) | 17,444 (23.0) | 21,118 (27.8) | 0 (0.0) | 0 (0.0) | 75,865 (100) |
| Dec 27, 2021–Jan 29, 2022 | 70,091 | 28,887 (41.2) | 13,376 (19.1) | 21,580 (30.8) | 15,585 (22.2) | 19,550 (27.9) | 0 (0.0) | 0 (0.0) | 70,091 (100) |
| Jan 27–Feb 26, 2022 | 45,509 | 18,283 (40.2) | 11,188 (24.6) | 11,140 (24.5) | 10,066 (22.1) | 13,115 (28.8) | 0 (0.0) | 0 (0.0) | 45,509 (100) |
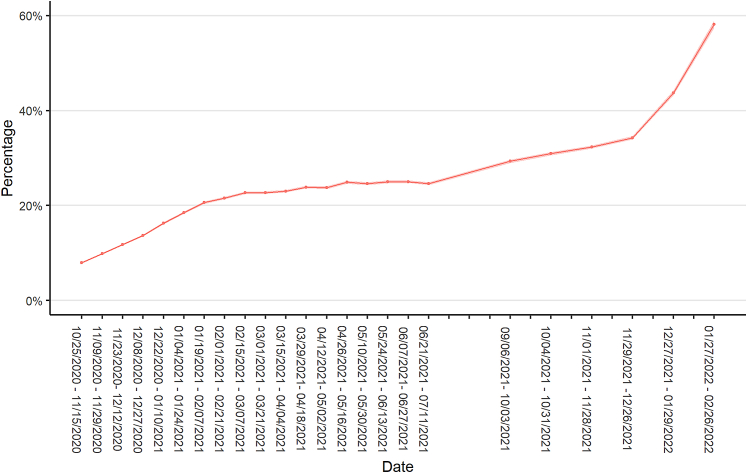
Estimated SARS-CoV-2 infection-induced seroprevalence in October 25–November 15, 2020, was 8.0% (95% confidence interval (CI): 7.9%–8.1%) (Fig. 1). Over the next three months, seroprevalence increased by at least 2 percentage points between each biweekly period until January 19-February 7, 2021, when seroprevalence was estimated at 20.6% (CI: 20.4%–20.9%). From then until June 28–July 11, 2021, seroprevalence increased very slowly. National seroprevalence was approximately 25% in June–July 2021. After a pause in data collection from July 12, 2021, to September 5, 2021, seroprevalence increased to 29.3% (CI: 29.0%–29.7%) on September 6–October 3, 2021. Infection-induced seroprevalence again increased slowly during the fall and early winter 2021 and then experienced two one-month increases of over 9 percentage points to 43.7% (CI: 43.3%–44.2%) in December 27, 2021–January 29, 2022, and an additional increase of 15 percentage points to 58.2% (CI: 57.4%–58.9%) in January 27–February 26, 2022.
Fig. 1.
Estimated SARS-CoV-2 antibody seroprevalence in the United States, October 25, 2020–February 26, 2022. Data collected as part of a national, repeated, cross-sectional study of convenience samples of specimens from patients who sought routine screening or clinical care. Footnotes: Data were collected from the 50 United States and the District of Columbia and tested for SARS-CoV-2 antibodies using the commercially available COVID-19 test kits specified in the methods section. Regression models were used to estimate associations between specimen positivity and covariates and then were used to create seroprevalence estimates as if all specimens were tested with Roche Elecsys Anti-SARS-CoV-2 pan-immunoglobulin immunoassay. Laboratories were unable to provide specimens for the following time periods and states and were excluded from analyses: September 6–October 3, 2021, from Indiana, Maryland, New Jersey, and Virginia; November 1–November 28, 2021, from North Dakota; and December 27, 2021–January 29, 2022, from Nevada.
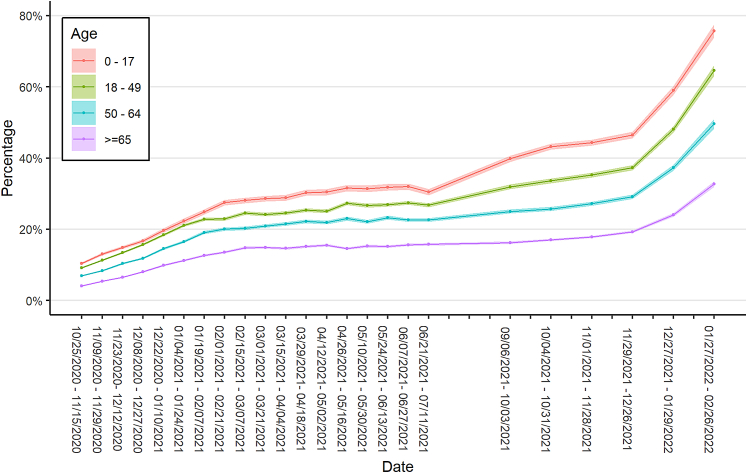
Infection-induced seroprevalence was associated with age; the youngest age group, aged 0–17 years, had the highest seroprevalence, which increased from 10.4% to 75.7% over the study period. The next highest seroprevalence was noted in those aged 18–49 years (increased from 9.2% to 64.5%) followed by people aged 50–64 years (increased from 7.0% to 49.6%) and people aged 65 years or older (increased from 4.1% to 32.7%) (Fig. 2). Infection-induced seroprevalence estimates had overlapping confidence intervals for males and females (Supplementary Fig. S1). Metro areas had consistently lower seroprevalence compared to non-metro areas, though the difference was 2.2 percentage points or less in every time interval (Supplementary Fig. S2). The Midwestern and southern U.S. regions had higher seroprevalence than the northeastern and western (Supplementary Fig. S3); the Midwest had the largest increase, from 9.8% to 62.6%, during the study period.
Fig. 2.
Estimated SARS-CoV-2 antibody seroprevalence in the United States by age category, October 25, 2020–February 26, 2022. Data collected as part of a national, repeated, cross-sectional study of convenience samples from specimens of patients who sought routine screening or clinical care. Footnotes: Data were collected from the 50 United States and the District of Columbia and tested for SARS-CoV-2 antibodies using the commercially available COVID-19 test kits specified in the methods section. Regression models were used to estimate associations between specimen positivity and covariates and then were used to create seroprevalence estimates as if all specimens were tested with Roche Elecsys Anti-SARS-CoV-2 pan-immunoglobulin immunoassay. Laboratories were unable to provide specimens for the following time periods and states and were excluded from analyses: September 6–October 3, 2021, from Indiana, Maryland, New Jersey, and Virginia; November 1–November 28, 2021, from North Dakota; and December 27, 2021–January 29, 2022, from Nevada.
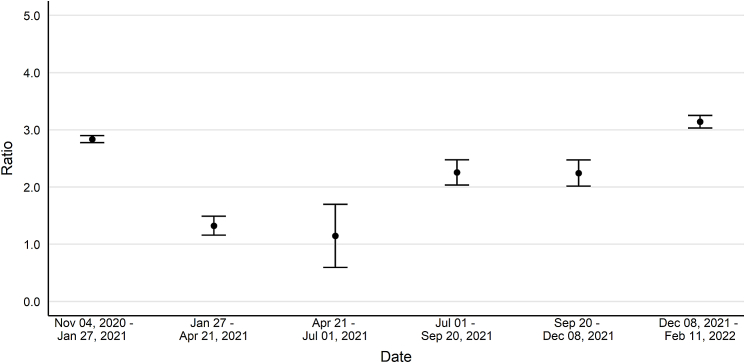
Change ratios, ratios of the change in seroprevalence to the change in reported case prevalence, followed a convex pattern over time (Fig. 3). From November 4, 2020, to January 27, 2021, the ratio was 2.8 (CI: 2.8–2.9) and then decreased, reaching a low point from April 21 to July 1, 2021 (1.1, CI: 0.6–1.7). These ratios increased to 2.3 (CI: 2.0–2.5) from July 1 to September 20, 2021, held steady from September 20 to December 8, 2021 (2.2, CI: 2.0–2.5), and increased again from December 8, 2021, to February 26, 2022 (3.1, CI: 3.0–3.3).
Fig. 3.
Estimated change in seroprevalence to change in reported case prevalence ratios for SARS-CoV-2 in the United States, October 25, 2020–February 11, 2022. Data collected as part of a national, repeated, cross-sectional study of convenience samples of specimens of patients who sought routine screening or clinical care. Footnotes: Bars represent 95% confidence intervals. Data were collected from the 50 United States and the District of Columbia and tested for SARS-CoV-2 antibodies using the commercially available COVID-19 test kits specified in the methods section. Regression models were used to estimate associations between specimen positivity and covariates. The associations were used to create seroprevalence estimates as if all specimens were tested with Roche Elecsys Anti-SARS-CoV-2 pan-immunoglobulin immunoassay and then compared to case counts from CDC's COVID Data Tracker. Laboratories were unable to provide specimens for the following time periods and states and were excluded from analyses: September 6–October 3, 2021, from Indiana, Maryland, New Jersey, and Virginia; November 1–November 28, 2021, from North Dakota; and December 27, 2021–January 29, 2022, from Nevada.
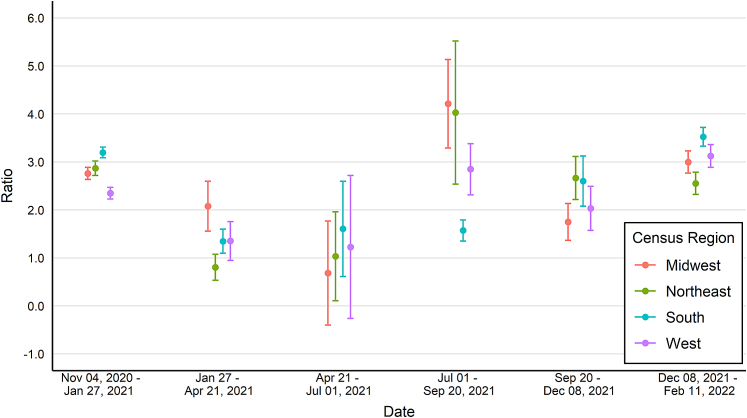
Trends of change ratios differed by U.S. Census region (Fig. 4). The South region had the highest ratios during the winter months (3.2, CI: 3.1–3.3; and 3.5, CI:3.3−3.7) but a ratio of approximately 1.5 during all other time periods. In contrast, the Northeast region had a ratio of approximately 1.0 from January 27 to July 1, 2021, and greater than 2.5 in all other time periods measured. Change ratios varied by U.S. Census division, e.g., some divisions did not have overlapping confidence intervals (Supplementary Fig. S4). Compared to non-metro locations, metro locations had a slightly higher ratio in two time periods but otherwise the two groups had overlapping confidence intervals (Supplementary Fig. S5).
Fig. 4.
Estimated change in seroprevalence to change in reported case prevalence ratios by census region for SARS-CoV-2 in the United States, October 25, 2020–February 11, 2022. Data collected as part of a national, repeated, cross-sectional study of convenience samples of specimens of patients who sought routine screening or clinical care. Footnotes: Bars represent 95% confidence intervals. Data were collected from the 50 United States and the District of Columbia and tested for SARS-CoV-2 antibodies using the commercially available COVID-19 test kits specified in the methods section. Regression models were used to estimate associations between specimen positivity and covariates. The associations were used to create seroprevalence estimates as if all specimens were tested with Roche Elecsys Anti-SARS-CoV-2 pan-immunoglobulin immunoassay and then compared to case counts from CDC's COVID Data Tracker. Laboratories were unable to provide specimens for the following time periods and states and were excluded from analyses: September 6–October 3, 2021, from Indiana, Maryland, New Jersey, and Virginia; November 1–November 28, 2021, from North Dakota; and December 27, 2021–January 29, 2022, from Nevada.
Discussion
Sero-surveillance data are an important source of data on the burden of infection, particularly during periods of increased transmission and changing testing practices. First, the change ratios, ratios estimating the change in seroprevalence compared to the change in reported case prevalence, can be used as a multiplier to enhance the understanding of the infection burden represented by officially reported case rates. Second, sudden increases in infection rates are essential to consider in vaccine efficacy studies since infection-induced immunity can also provide some protection from infection. The increasing infection-induced seroprevalence rates shown in this study demonstrate that accurate estimates of vaccine effectiveness will need to incorporate previous infection, including in pediatric populations.28 Sudden increases in infection rates impact the disease susceptibility of the unvaccinated population and are vital in the interpretation of vaccine effectiveness data.
The change ratios were highest during periods of high transmission, specifically during winter case surges. While increased seroprevalence compared to case prevalence during surges are concerning, they demonstrate the improvement in access to testing compared to spring 2020, when early infection to reported case ratios ranged from 6 to 24 infections per case in metropolitan areas29,30 due to a shortage of diagnostic tests. The increased availability and uptake of home testing11 may be an important factor driving winter 2021–2022 increases in seroprevalence compared to case prevalence. Changes in seroprevalence may continue to increase relative to changes in reported case prevalence as home testing becomes more common and fewer jurisdictions engage in active case finding through case interviews and contact tracing. This illustrates the importance of continued sero-surveillance for understanding the true infection burden represented by officially reported case counts and other metrics; interpretation of the significance of reported case counts may require revision in the event of continued increases in this ratio.
During times of lower transmission, smaller changes in seroprevalence between time periods suggest a greater proportion of infections were included in reported case counts, though with greater uncertainty. At the nadir of the ratio of the change in seroprevalence compared to the change in case prevalence in April–July 2021 (1.1; CI: 0.6–1.7), the confidence interval was widest because small changes in both seroprevalence and reported case rates resulted in a high coefficient of variation. These data from the current analysis are reinforced by findings from 2020, when jurisdictional infection to reported case ratios declined (range: 1.0–12.5) as transmission decreased during summer 2020 and testing became more widely accessible,20 though the variability in jurisdiction-level change ratios also underscore the importance of sub-national surveys to more accurately depict seroprevalence.
Serosurveys can highlight population subgroups that are at higher risk of infection and help target interventions to those subgroups. For example, children had higher seroprevalence31 and have had higher infection to case ratios32 despite suggestions that seroprevalence in children might be underestimated compared with adults.33 Additionally, fewer infections per case were reported during periods of high transmission, especially in certain regions.
These seroprevalence results possess some differences from prior seroprevalence estimates, which may be because convenience sampling limits the generalizability of the sample pool. Approximately 14.3% (range, 11.6%–18.5%) of the U.S. population were estimated to have been infected by mid-November 2020,34 which is slightly higher than our estimate of 10.7% from November 9–29, 2020. Our lower estimate may be due to the possibility of underestimation of seroprevalence from the available specimens obtained from clinical laboratories. People engaged in routine medical care and clinical screening likely have greater access to and utilization of healthcare resources, while people with less access are more likely to belong to racially- and ethnically-minoritised groups, disproportionately affected by chronic conditions and COVID-19.35 The lack of race and ethnicity data is especially limiting since disparities have been noted in other seroprevalence surveys.30,36, 37, 38 The combined bias is likely to underestimate seroprevalence and the ratio of change in seroprevalence to change in reported case prevalence, though a comparison in a diverse location revealed a higher seroprevalence estimate.39 Persons with specimens collected under routine screening for high-risk conditions may be more likely to be vaccinated than those with high-risk conditions unable to utilize healthcare resources.40 This could be a result of health insurance status and workplace sick leave policies.41 Compared to estimates from a longitudinal study of blood donors from November 2020 through May 2021,14 seroprevalence estimates in this study were consistently 1.0–4.5% higher. This could be explained by the exclusion of the pediatric age group from the blood donor estimate, higher vaccination rates in blood donors,14 or the underrepresentation in blood donor pools42 of people from racially- and ethnically-minoritised groups or other groups disproportionately affected by the COVID-19 pandemic.43
The lack of probabilistic sampling, which has been highlighted as a potential source of bias in serosurveys,44 was one of multiple limitations in this investigation. Several other limitations also may have led to an underestimation of seroprevalence, including the exclusion of specimens from people specifically seeking SARS-CoV-2 antibody testing, the inability of these sero-surveillance methods to detect reinfection (particularly during the Omicron phase45,46), and the potential that some fully vaccinated people who are subsequently infected may develop levels of N-antibody that fall below the assay's limit of detection.47 In addition, the likelihood of a given assay to detect antibody post-infection varies by the time since infection and the type of antibody binding.48,49 Although the Roche Elecsys N-target assay is less affected by antibody waning compared to other assays, antibody concentrations wane and specimens could fall below the limit of detection.25 While we were unable to control or adjust for antibody waning directly, we mitigated these effects by adjusting results to a single assay, and by performing all testing with a single assay type (Roche Elecsys) since September 2021. Nevertheless, we did not directly adjust our estimates for errors in SARS-CoV-2 antibody measurement from the assays used in this study. Qualitative testing cannot quantify the level of SARS-CoV-2 antibody in the blood,50 and current FDA emergency use authorized SARS-CoV-2 antibody assays are not validated to measure a specific level of immunity or protection from SARS-CoV-2 infection.51 Thus, a SARS-CoV-2 N-target-based seroprevalence estimate does not necessarily indicate the percentage of the population susceptible or immune to SARS-CoV-2 infection or reinfection. Also, while we hoped to be able to calculate an infection-to-reported-case ratio in this manuscript, our ratios of the change in seroprevalence to the change in reported case prevalence are only an approximation. Finally, our power of 70% for detecting a 2% increase in seroprevalence may mean our study was underpowered.
Nevertheless, these results provide information to more fully understand the burden of SARS-CoV-2 infection in the United States from late 2020 to early 2022, and to more accurately interpret case reporting to aid public health decision-making.52 The U.S. CDC utilizes case surveillance, hospital admissions, and staffed inpatient beds to evaluate the community-level impact of COVID-19 illness.53 These analyses highlight the importance of a multi-faceted approach to surveillance since reported case rates must be interpreted in the context of variable ratios of the change in seroprevalence to the change in reported case prevalence by geographic area and across time.
Conclusions
Sero-surveillance data suggest that reported case counts did not completely capture the SARS-CoV-2 infection burden in the U.S. between late 2020 and early 2022, especially during periods of high transmission. Some subgroups, such as children and people living in the South and Midwest regions, experienced a higher infection burden compared to that suggested by case-based surveillance. Sero-surveillance data can aid in the appropriate interpretation of vaccine effectiveness data, demonstrate the increasing importance of accounting for previous infection, provide a more complete picture of COVID-19 impact for community-level decision-making, and identify subgroups at higher risk for infection. With the potential for increased use of at home, viral-based testing, national sero-surveillance will become pivotal in efforts to estimate disease burden and appropriately interpret case rates over time and by geographic region.
Contributors
Dr. Wiegand and Dr. Clarke had full access to the data presented in the study and take responsibility for data integrity and accuracy of analysis.
Study concept and design: Wiegand, Y. Deng, X. Deng, Lee, Jones, Iachan, Clarke.
Acquisition, analysis, or interpretation of data: Wiegand, Y. Deng, X. Deng, Lee, Meyer, Letovsky, Jones, Iachan, Clarke.
Drafting of the manuscript: Wiegand, Y. Deng, X. Deng, Lee, Jones, Iachan, Clarke.
Critical revision of the manuscript for important intellectual content: all authors.
Obtained funding: Gundlapalli, Charles, MacNeil, Hall, Clarke.
Administrative, technical, or material support: Meyer, Letovsky, Charles, Gundlapalli, MacNeil, Hall, Jones, Clarke.
Study supervision: Meyer, Letovsky, Charles, Jones, Iachan, Clarke
Disclaimer: The findings and conclusions in the article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention or the corporate employers of the authors.
Data sharing statement
A public use dataset containing a data dictionary and seroprevalence estimates for all rounds of data collection is available at https://data.cdc.gov/Laboratory-Surveillance/Nationwide-Commercial-Laboratory-Seroprevalence-Su/d2tw-32xv. Individual-level data will not be made available.
Declaration of interests
BioReference Laboratories, Inc., ICF Inc., Laboratory Corporation of America Holdings, and Quest Diagnostics, Inc. were awarded federal contracts from the U.S. Centers for Disease Control and Prevention (CDC) for the execution of this project. No other disclosures were reported.
Acknowledgments
We thank the following members of CDC for administrative and technical support: Anna Bratcher, PhD, Elizabeth Cole-Greenblatt, JD, Elise Nycz, MHS, Lauren Peel, JD. We thank Tonja Kyle, MS, from ICF Inc. for administrative and technical support. We thank Quest Diagnostics, BioReference Laboratories, and Laboratory Corporation of America for testing specimens. From Quest Diagnostics: Sara Ansari, PhD, Scott Deschenes, Brooke Ethington, MS, MBA, Sara Peters, Caterina Powell, BS, Dianna Tate, Brian Young, AA. From BioReference Laboratories: James Weisberger, MD. From Laboratory Corporation of America: Dorothy Adcock, MD, Kelly Chun PhD, Marla Williams. These individuals were not compensated directly by CDC for their participation in this specific study.
Funding: This work was supported by the CDC, Atlanta, Georgia.
Footnotes
See e.g., 45 C.F.R. part 46; 21 C.F.R. part 56; 42 U.S.C. §241(d), 5 U.S.C. §552a, 44 U.S.C. §3501 et seq.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100403.
Appendix A. Supplementary data
References
- 1.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara L.A., Wiegand R.E., Burke R.M., et al. Estimating the early impact of the US COVID-19 vaccination programme on COVID-19 cases, emergency department visits, hospital admissions, and deaths among adults aged 65 years and older: an ecological analysis of national surveillance data. Lancet. 2021;399(10320):152–160. doi: 10.1016/S0140-6736(21)02226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Rio C., Malani P.N., Omer S.B. Confronting the delta variant of SARS-CoV-2, summer 2021. JAMA. 2021;326(11):1001–1002. doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team SARS-CoV-2 B.1.1.529 (omicron) variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731–1734. doi: 10.15585/mmwr.mm7050e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention CDC COVID data tracker. 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 6.Neelon B., Mutiso F., Mueller N.T., Pearce J.L., Benjamin-Neelon S.E. Spatial and temporal trends in social vulnerability and COVID-19 incidence and death rates in the United States. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel D.A., Reses H.E., Cool A.J., et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 Years - United States, August 2020-August 2021. MMWR Mortal Wkly Rep. 2021;70(36):1249–1254. doi: 10.15585/mmwr.mm7036e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez L., Nguyen T., Weber G., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in the staff of a public school system in the midwestern United States. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0243676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogawski McQuade E.T., Guertin K.A., Becker L., et al. Assessment of seroprevalence of SARS-CoV-2 and risk factors associated with COVID-19 infection among outpatients in Virginia. JAMA Netw Open. 2021;4(2):e2035234. doi: 10.1001/jamanetworkopen.2020.35234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S., Montez-Rath M., Han J., et al. Estimated SARS-CoV-2 seroprevalence in US patients receiving dialysis 1 year after the beginning of the COVID-19 pandemic. JAMA Netw Open. 2021;4(7):e2116572. doi: 10.1001/jamanetworkopen.2021.16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rader B., Gertz A., Iuliano A.D., et al. Use of at-home COVID-19 tests — United States, August 23, 2021–March 12, 2022. MMWR Mortal Wkly Rep. 2022;71(13) doi: 10.15585/mmwr.mm7113e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malecki K., Nikodemova M., Schultz A., et al. Population changes in seroprevalence among a statewide sample in the United States. medRxiv. 2021 2020.12.18.20248479. [Google Scholar]
- 13.Fisher B.T., Sharova A., Boge C.L.K., et al. Evolution of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) seroprevalence among employees of a US academic children's hospital during coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021:1–9. doi: 10.1017/ice.2021.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones J.M., Stone M., Sulaeman H., et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326(14):1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker A.G., Sibbel S., Wade C., et al. SARS-CoV-2 antibody seroprevalence among maintenance dialysis patients in the United States. Kidney Med. 2021;3(2):216–222.e1. doi: 10.1016/j.xkme.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zyskind I., Rosenberg A.Z., Zimmerman J., et al. SARS-CoV-2 seroprevalence and symptom onset in culturally linked orthodox jewish communities across multiple regions in the United States. JAMA Netw Open. 2021;4(3):e212816. doi: 10.1001/jamanetworkopen.2021.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu W.A., Ndeffo-Mbah M.L. Using test positivity and reported case rates to estimate state-level COVID-19 prevalence and seroprevalence in the United States. PLoS Comput Biol. 2021;17(9) doi: 10.1371/journal.pcbi.1009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noh J., Danuser G. Estimation of the fraction of COVID-19 infected people in U.S. states and countries worldwide. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reif J., Heun-Johnson H., Tysinger B., Lakdawalla D. Measuring the COVID-19 mortality burden in the United States. Ann Intern Med. 2021;174(12):1700–1709. doi: 10.7326/M21-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajema K.L., Wiegand R.E., Cuffe K., et al. Estimated SARS-CoV-2 seroprevalence in the US as of september 2020. JAMA Intern Med. 2021;181(4):450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Agriculture Economic Research Service Rural-urban Continuum codes. 2013. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/
- 22.Flanagan B.E., Gregory E.W., Hallisey E.J., Heitgerd J.L., Lewis B. A social vulnerability index for disaster management. J Homel Secur Emerg Manag. 2011;8(1) [Google Scholar]
- 23.Li Z., Hallisey E., Lewis B., et al. Social vulnerability and rurality associated with higher severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection–induced seroprevalence: a nationwide blood donor study—United States, July 2020–June 2021. Clin Infect Dis. 2022;75(1):e133–e143. doi: 10.1093/cid/ciac105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow N.E., Clayton D.G. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88(421):9–25. [Google Scholar]
- 25.Takahashi S., Peluso M.J., Hakim J., et al. SARS-CoV-2 serology across scales: a framework for unbiased seroprevalence estimation incorporating antibody kinetics and epidemic recency. medRxiv. 2021 doi: 10.1093/aje/kwad106. 2021.09.09.21263139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 27.United States Census Bureau County population totals: 2010-2019. 2021. https://www.census.gov/data/datasets/time-series/demo/popest/2010s-counties-total.html
- 28.Hall V., Foulkes S., Insalata F., et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havers F.P., Reed C., Lim T., et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180(12):1576–1586. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Routledge I., Epstein A., Takahashi S., et al. Citywide serosurveillance of the initial SARS-CoV-2 outbreak in San Francisco using electronic health records. Nat Commun. 2021;12(1):3566. doi: 10.1038/s41467-021-23651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke K.E.N., Jones J.M., Deng Y., et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies — United States, september 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(17):606–608. doi: 10.15585/mmwr.mm7117e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couture A., Lyons B.C., Mehrotra M.L., et al. SARS-CoV-2 seroprevalence and reported COVID-19 cases in U.S. Children, August 2020—May 2021. Open Forum Infect Dis. 2022;9(3):ofac044. doi: 10.1093/ofid/ofac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toh Z.Q., Anderson J., Mazarakis N., et al. Comparison of seroconversion in children and adults with mild COVID-19. JAMA Netw Open. 2022;5(3):e221313. doi: 10.1001/jamanetworkopen.2022.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angulo F.J., Finelli L., Swerdlow D.L. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open. 2021;4(1):e2033706. doi: 10.1001/jamanetworkopen.2020.33706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai D.B.G., Shah A., Doubeni C.A., Sia I.G., Wieland M.L. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand S., Montez-Rath M., Han J., et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feehan A., Velasco C., Fort D., et al. Racial and workplace disparities in seroprevalence of SARS-CoV-2, Baton Rouge, Louisiana, USA. Emerg Infect Dis. 2021;27(1):314. doi: 10.3201/eid2701.203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrott J.C., Maleki A.N., Vassor V.E., et al. Prevalence of SARS-CoV-2 antibodies in New York City adults, June–October 2020: a population-based survey. J Infect Dis. 2021;224(2):188–195. doi: 10.1093/infdis/jiab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajema K.L., Dahlgren F.S., Lim T.W., et al. Comparison of estimated SARS-CoV-2 seroprevalence through commercial laboratory residual sera testing and a community survey. Clin Infect Dis. 2020;73(9):e3120–e3123. doi: 10.1093/cid/ciaa1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galanis P., Vraka I., Siskou O., Konstantakopoulou O., Katsiroumpa A., Kaitelidou D. Predictors of COVID-19 vaccination uptake and reasons for decline of vaccination: a systematic review. medRxiv. 2021 2021.07.28.21261261. [Google Scholar]
- 41.Peipins L.A., Soman A., Berkowitz Z., White M.C. The lack of paid sick leave as a barrier to cancer screening and medical care-seeking: results from the National Health Interview Survey. BMC Publ Health. 2012;12(1):520. doi: 10.1186/1471-2458-12-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaz B.H., James A.B., Hillyer K.L., Schreiber G.B., Hillyer C.D. Demographic patterns of blood donors and donations in a large metropolitan area. J Natl Med Assoc. 2011;103(4):351–357. doi: 10.1016/s0027-9684(15)30316-3. [DOI] [PubMed] [Google Scholar]
- 43.Van Dyke M.E., Mendoza M.C.B., Li W., et al. Racial and ethnic disparities in COVID-19 incidence by age, sex, and period among Persons aged <25 Years - 16 U.S. Jurisdictions, January 1-december 31, 2020. MMWR Mortal Wkly Rep. 2021;70(11):382–388. doi: 10.15585/mmwr.mm7011e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shook-Sa B.E., Boyce R.M., Aiello A.E. Estimation without representation: early severe acute respiratory syndrome coronavirus 2 seroprevalence studies and the path forward. J Infect Dis. 2020;222(7):1086–1089. doi: 10.1093/infdis/jiaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulliam J.R.C., van Schalkwyk C., Govender N., et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron in South Africa. Science. 2022;376(6593):eabn4947. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altarawneh H.N., Chemaitelly H., Hasan M.R., et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitaker H.J., Gower C., Otter A.D., et al. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off. medRxiv. 2021 2021.10.25.21264964. [Google Scholar]
- 48.Alfego D., Sullivan A., Poirier B., Williams J., Adcock D., Letovsky S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Germanio C., Simmons G., Kelly K., et al. SARS-CoV-2 antibody persistence in COVID-19 convalescent plasma donors: dependency on assay format and applicability to serosurveillance. Transfusion. 2021 doi: 10.1111/trf.16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gundlapalli A.V., Salerno R.M., Brooks J.T., et al. SARS-CoV-2 serologic assay needs for the next phase of the US COVID-19 pandemic response. Open Forum Infect Dis. 2021;8(1):ofaa555. doi: 10.1093/ofid/ofaa555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Food & Drug Administration Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination: FDA safety communication. 5/19/2021 2021. https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety
- 52.Murhekar M.V., Clapham H. COVID-19 serosurveys for public health decision making. Lancet Global Health. 2021;9(5):e559–e560. doi: 10.1016/S2214-109X(21)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention COVID-19 community levels. March. 2022;17:2022. https://www.cdc.gov/coronavirus/2019-ncov/science/community-levels.html [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.