Abstract
In a previous study, we reported the isolation and characterization of the two-component response regulator SSK1 gene of Candida albicans. This gene is a structural but not a functional homolog of the SSK1 and mcs4+ genes of Saccharomyces cerevisiae and Schizosaccharomyces pombe, respectively. In the present study, we have constructed and phenotypically characterized Δssk1 mutants of C. albicans. The results confirmed our previous observation that CaSSK1, unlike SSK1 or mcs4+, does not regulate cellular responses to either osmotic or oxidative stress. Instead, Δssk1 null strains showed severely reduced hyphal formation on serum agar and were totally defective in hyphal development on other solid media, such as medium 199 (pH 7.5) and Spider medium. In contrast, under conditions of low nitrogen availability on solid media, Δssk1 null strains dramatically hyperinvaded the agar. However, while forming germ tubes and hyphae in liquid media similar to those of the wild type, Δssk1 null strains flocculated in a manner similar to that of Δchk1 two-component histidine kinase mutants, which we have previously described. Finally, virulence studies indicated that SSK1 is essential for the pathogenesis of C. albicans, suggesting that the Ssk1p response regulator could be a good target for antifungal therapy.
Candida albicans is the most frequently isolated opportunistic fungal pathogen in humans. A number of factors have been associated with the virulence properties of C. albicans, such as adherence to host cells and the ability to undergo the transition from yeast to hyphal growth. This switch is induced in vitro by many environmental conditions, including temperature, pH, and the presence of serum. Diploid strains of Saccharomyces cerevisiae also switch their pattern of growth from unicellular yeast to chains of elongated cells that remain attached to each other (pseudohyphae) under conditions of nitrogen starvation (14). This switch requires STE20, STE11, STE7, KSS1, and STE12, genes of a conserved mitogen-activated protein (MAP) kinase pathway, but also depends on PHD1, a gene which functions in an STE12-independent pathway (3). Based upon functional complementation studies of the STE20, STE7, KSS1, and STE12 genes of S. cerevisiae, the homolog genes CST20 (15, 16), HST7 (15, 16), CEK1 (11), and CPH1 (18) of C. albicans, respectively, have been identified. Mutants of C. albicans containing mutations in each of these genes are unable to undergo the transition from yeast to hyphae on solid media, except when serum is included. In the same way, it was reported that the CPP1 gene, which encodes a phosphatase similar to the MAP kinase phosphatase Msg5p of S. cerevisiae, modulates the activity of the CPH1 pathway, likely by dephosphorylating Cek1p (10). Following a similar experimental approach, the C. albicans genes CaCLA4 (a CLA4 homolog) (17), EFG1 (a PHD1 homolog) (19, 28), CaTUP1 (a TUP1 homolog) (4), and CaRSR1 (an RSR1 homolog) (30) were also studied. The virulence of each of the single mutants described above (except for Δtup1 mutants, with which virulence studies were not performed) was reduced in a murine model of hematogenously disseminated candidiasis (10, 11, 16, 17, 19, 30), but a Δcph1 Δefg1 double mutant was shown to be avirulent (19). However, recent studies indicate that, in fact, the Δcph1 Δefg1 double mutant can still form hyphae when colonizing tissue, indicating that an additional EFG1- and CPH1-independent pathway that regulates morphogenesis in C. albicans may exist (23).
More recently, it has been demonstrated that the HOG1 gene of C. albicans, in addition to its role in cytokinesis and response to osmostress in C. albicans (2), also functions in morphogenesis (2). Interestingly, the HOG1 homolog of S. cerevisiae does not function in the filamentation-invasion pathway of S. cerevisiae but in the HOG pathway, which is regulated in part by a two-component cascade whose functional proteins have domains similar to the sensor histidine kinases (Sln1p and Ypd1) and response regulators (Sln1p and Ssk1p) of prokaryotes (3). In this regard, in C. albicans the two-component sensor histidine kinase gene CHK1 (7) seems to regulate the expression of hyphal surface components (5) and perhaps also virulence factors, since a Δchk1 null mutant is avirulent (8), while the NIK1/COS1 gene (21, 27) is required for hyphal development on solid media (1).
In previous work, we have identified the putative SSK1 response regulator gene of C. albicans (6). CaSSK1 encodes a protein (CaSsk1p) which is a structural homolog of Ssk1p and Mcs4 from S. cerevisiae and Schizosaccharomyces pombe, respectively. Ssk1p is a response regulator of the two-component cascade that regulates the HOG pathway of S. cerevisiae (20), and Mcs4 is an element of the Sty1 pathway of S. pombe (9, 26). Unlike Ssk1p, which functions only in osmosensing in S. cerevisiae, Mcs4 plays a role in regulating adaptive responses to different stresses, including osmotic and oxidative stresses, and coordinates the responses to these environmental stimuli with the cell cycle (9, 26). Interestingly, we have shown that CaSSK1 fails to complement the lack of SSK1 and mcs4+ in S. cerevisiae and S. pombe, respectively (6). This result indicates that the SSK1 gene of C. albicans may have functions other than modulating the response to osmotic or oxidative stress. In this paper, we present data indicating that SSK1 is involved in the morphogenesis and virulence of C. albicans.
MATERIALS AND METHODS
Strains and growth conditions.
The C. albicans strains used in this work are listed in Table 1. All strains were routinely grown either in YPD complex medium (1% yeast extract, 2% peptone, 2% dextrose) or in SD minimal medium (0.67% yeast nitrogen base, 2% dextrose) at 28°C. For specific experiments, strains were also grown on solid Spider medium (1% nutrient broth, 1% mannitol, 0.2% K2HPO4), solid synthetic low-ammonium–dextrose (SLAD) medium (0.17% yeast nitrogen base without amino acids or ammonium sulfate, 2% dextrose, and 50 μM ammonium sulfate as the sole nitrogen source), serum (10% fetal bovine serum [Gibco BRL]), and medium 199 (M199) containing Earle's salts and glutamine but lacking sodium bicarbonate (Gibco BRL) and buffered with 155 mM Tris-HCl at pH 7.5 or 4.0. All liquid media were sterilized by filtration. Solid media were prepared by adding 1.5% agar (final concentration) (2% for SLAD plates) at 50°C after autoclaving. Prewarmed liquid media were inoculated to a density of 107 cells/ml and incubated at either 28 or 37°C with vigorous agitation. On solid media, the appropriate dilutions of cells were plated to obtain approximately 60 to 80 colonies per plate. In all cases, stationary-phase cells grown at 28°C in YPD complex medium were used as an inoculum.
TABLE 1.
C. albicans strains used in this study
| Strain | Relevant genotype | Generation time (h)a | Reference or source |
|---|---|---|---|
| CAF2 | Δura3::imm434/URA3 | 1.10 ± 0.01 | 12 |
| CAI4 | Δura3::imm434/Δura3::imm434 | ND | 12 |
| CSSK11-1 | Δura3::imm434/Δura3::imm434 Δssk1::hisG-URA3-hisG/SSK1 | 1.14 ± 0.04 | This work |
| CSSK11-2 | Δura3::imm434/Δura3::imm434 Δssk1::hisG-URA3-hisG/SSK1 | 1.16 ± 0.02 | This work |
| CSSK12-1 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/SSK1 | ND | This work |
| CSSK12-2 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/SSK1 | ND | This work |
| CSSK21-1 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/Δssk1::hisG-URA3-hisG | 1.36 ± 0.01 | This work |
| CSSK21-2 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/Δssk1::hisG-URA3-hisG | 1.33 ± 0.02 | This work |
| CSSK22-1 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/Δssk1::hisG | ND | This work |
| CSSK22-2 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/Δssk1::hisG | ND | This work |
| CSSK23-1 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/SSK1::URA3-hisG | 1.12 ± 0.02 | This work |
| CSSK23-2 | Δura3::imm434/Δura3::imm434 Δssk1::hisG/SSK1::URA3-hisG | 1.15 ± 0.03 | This work |
Calculated in YPD medium. Reported as the average of three independent experiments ± the standard deviation. ND, not determined.
DNA manipulation and analysis.
Standard molecular biology procedures for DNA manipulation were used (24). Genomic DNA from all C. albicans strains was obtained by the method described by Sherman et al. (25). For Southern blot analyses, 4 μg of DNA per lane was loaded on an 0.8% agarose gel and transferred by capillarity to positively charged nylon membranes (Hybond-N+; Amersham) by standard protocols (24). Probes were digoxigenin labelled by random priming (DIG DNA Labeling Kit; Boehringer Mannheim Biochemicals) and hybridized and detected by the chemiluminescence method as recommended by the manufacturer (DIG Nucleic Acid Detection Kit; Boehringer Mannheim Biochemicals).
Isolation of the SSK1 gene from C. albicans.
SSK1 was isolated as previously described (6). Briefly, a 2.77-kb EcoRI-XbaI DNA fragment which contains the entire open reading frame (ORF) of SSK1 was isolated from a λ-EMBL3 clone and subcloned into the EcoRI and NheI restriction sites of pBR322 (Gibco BRL) to generate plasmid pBR-CaSSK1 (6). The GenBank accession number for the SSK1 gene sequence is AF084608.
Construction of plasmids used for C. albicans transformation.
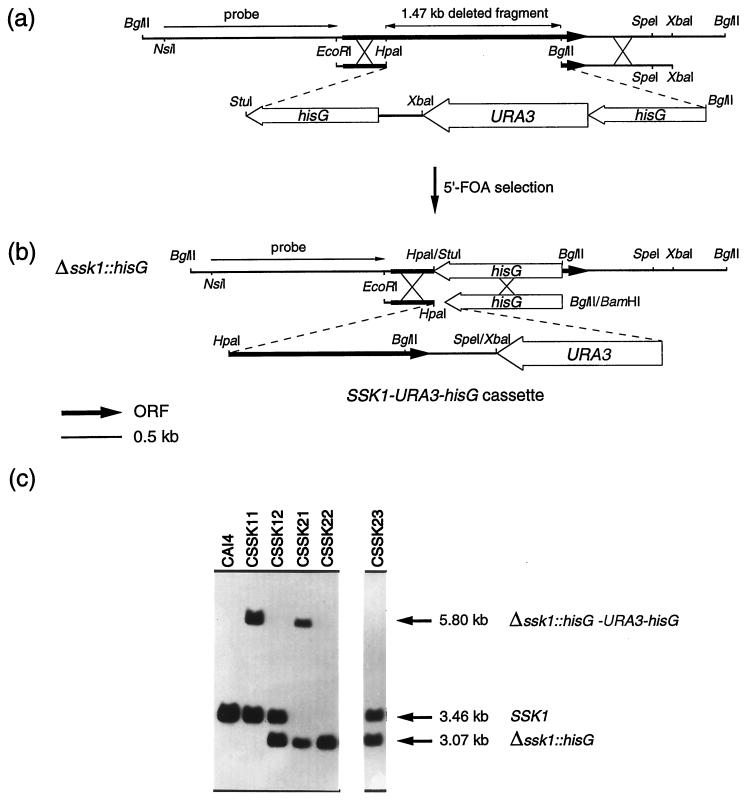
To obtain Δssk1 mutants, we constructed plasmid pBR1, which carries a cassette designed to delete most of the ORF of SSK1. To construct this plasmid, a 3.80-kb StuI-BglII DNA fragment from pMB7 (12), which contains the hisG-URA3-hisG cassette, was used to replace most of the ORF of SSK1 (Fig. 1a). Plasmid pBR1 was linearized by digestion with AatII, which cuts once in the plasmid outside of the cassette, and approximately 2 μg of DNA was used to transform Ura− C. albicans CAI4 by electroporation (29). Electroporation was chosen for transformation experiments, since the most commonly used lithium acetate procedure (13) consistently failed to yield transformants, even when a large amount of DNA (up to 30 μg) was used. Transformed cells were selected as Ura+ on SD minimal medium, and spontaneous Ura− derivatives from a Ura+ independent clone were selected on SD minimal medium containing 5′-fluoro-orotic acid (1 mg/ml) and uridine (25 μg/ml). The transformants were then used to delete the second allele of SSK1. In order to obtain a reconstituted strain with one SSK1 allele, we designed vector pBR2. To construct this plasmid, a 2.37-kb XbaI-BglII DNA fragment from pMB7, which contains the URA3-hisG cassette, was introduced into the only SpeI and BamHI sites of pBR-CaSSK1, which are located downstream of SSK1, generating the SSK1-URA3-hisG cassette (Fig. 1b). Plasmid pBR2 was linearized and used to transform a Ura− Δssk1 null strain as described above.
FIG. 1.
Schematic representations of the construction of the cassette used to disrupt ssk1 (a) and the cassette SSK1-URA3-hisG, used to reintroduce one wild-type SSK1 allele (b). (c) Corresponding Southern blot analyses of strains CSSK11-1, CSSK12-1, CSSK21-1, and CSSK22-1, obtained during the disruption process, and a revertant strain (CSSK23-1). Genomic DNAs from these strains were BglII digested and hybridized with a 1.4-kb NsiI-EcoRI fragment located at the 5′ end of the SSK1 gene as a probe. The exact size and genotype of the expected hybridizing DNA fragments are indicated on the right. 5′-FOA, 5′-fluoro-orotic acid.
The construction of expression plasmids pLJ19 and pCCa1 (kindly provided by D. Harcus), which carry the CPH1 and CPP1 genes of C. albicans, respectively, under the control of the ADH1 promoter, was previously described (10, 11). To construct plasmid pYPB1-ADHpt-HOG1, the coding region of the HOG1 gene of C. albicans flanked by BglII sites was amplified by PCR with the 5′ oligonucleotide 5′-GCAGATCTGAAAATGTCTGCAGATGGAG-3′ and the 3′ oligonucleotide 5′-TTAGATCTTTGAAGATTAAGCTCCGTTGGC-3′ and with plasmid pBSK8 containing the HOG1 gene as a template. The flanking regions of the PCR product were confirmed by sequencing, digested with BglII, and inserted into the BglII site of plasmid pYPB1-ADHpt (kindly provided by D. Harcus), containing the ADH1 promoter, C. albicans URA3 as a selectable marker, and an autonomously replicating sequence (10, 11, 16).
Determination of generation time.
Preinoculum cultures of each strain were always prepared in YPD medium for 24 h at 28°C. The optical density at 600 nm (OD600) of precultures was determined, and 250-ml flasks containing 50 ml of prewarmed fresh medium (YPD or M199 [pH 4.0]) were inoculated to a final OD600 of 0.1 with the appropriate preinoculum and incubated at 28°C and 200 rpm. The OD600 was measured every 1.5 h until the stationary phase of the growth curve was reached. The generation time (μ) during the log phase (exponential growth) was determined by the formula μ = (tf − t0)/n, where n is the number of generations calculated from the formula n = (logNf − logN0)/log2, in which Nf is the number of cells at the end of the time period (tf) and N0 is the number of cells at the beginning of the time period (t0). The generation times calculated for each strain are the averages of three independent experiments.
Animal model of hematogenously disseminated candidiasis.
The C. albicans strains used in these experiments included a parental control with only one functional URA3 allele (strain CAF2) and five strains in which either one (CSSK11-1, CSSK23-1, and CSSK23-2) or both (CSSK21-1 and CSSK21-2) alleles have been deleted. CSSK23 strains were included in all experiments to ensure that all phenotypic traits observed with the CSSK21 strains were due solely to the SSK1 mutation rather than to unrelated mutations that may have occurred during construction of the Δssk1 null strains. All strains were grown, harvested, and resuspended to a density of 2 × 106 cells per ml in phosphate-buffered saline (pH 7.5), and 0.5 ml (106 cells) of this cell suspension was injected intravenously per mouse as previously described (8). For the survival experiments, each C. albicans strain was used to inoculate seven mice. Concomitantly, 15 mice were also inoculated with each strain. Five members from each group were sacrificed after 24, 48, and 72 h postinfection to quantitate the CFU per gram of tissue and for histological examination as described previously (8).
RESULTS
Chromosomal deletion of the SSK1 gene of C. albicans.
It was previously shown that the SSK1 gene of C. albicans was unable to rescue the lack of SSK1 or mcs4+ in S. cerevisiae or S. pombe, respectively, suggesting that despite their structural homology, these genes may not be functional homologs (6). Thus, to further investigate the function of SSK1 in the growth, morphogenesis, and virulence of C. albicans, we used the Urablaster technique (12) to obtain Δssk1 mutants. The hisG-URA3-hisG cassette was used to replace a 1.47-kb fragment of SSK1 that includes the coding region for the conserved putative aspartate residue which is phosphorylated (Fig. 1a). After the first round of transformation, Ura+ transformants were selected on SD minimal medium, and several isolates were tested by Southern blotting to confirm the replacement. DNA from a representative CSSK11 isolate exhibited two hybridizing bands, a 3.46-kb BglII-BglII fragment characteristic of the parental strain and an additional fragment of 5.80 kb, consistent with the replacement of one allele of SSK1 with the hisG-URA3-hisG cassette (Fig. 1c). Two independent Ura+ transformants (CSSK11-1 and CSSK11-2) were used as parental strains to obtain two independent Ura− segregants (CSSK12-1 and CSSK12-2). A representative CSSK12 intrachromosomal recombinant strain was tested by Southern blotting (Fig. 1c). The 5.80-kb BglII-BglII fragment seen in the CSSK11 strains which contained the Δssk1::hisG-URA3-hisG disruption was absent, and a new, 3.07-kb hybridizing fragment was present in the CSSK12 strain. The size of this fragment is consistent with the desired event, the loss of URA3 and one copy of hisG.
Following the same protocol, a second round of transformation of CSSK12-1 and CSSK12-2 was performed, and the remaining allele was disrupted to generate strains CSSK21-1 and CSSK21-2. In Fig. 1c, a representative CSSK21 Ura+ isolate and a CSSK22 Ura− segregant are shown. Two independent strains with one allele reconstituted (CSSK23-1 and CSSK23-2) were also constructed to ensure that the resulting phenotype was not due to extraneous mutations that could occur during transformation. To do that, both strains CSSK22-1 and CSSK22-2 were transformed with the SSK1-URA3-hisG cassette. This cassette allowed the integration event to occur between the remaining 5′-end ORF of SSK1 and the hisG that had replaced 1.47 kb of the SSK1 sequence in the CSSK22 strains (Fig. 1b). Ura+ transformants were selected on SD minimal medium, and the integration of the transforming DNA into the Δssk1::hisG locus was verified by Southern blot analysis of several isolates. Southern blot analysis of a representative CSSK23 isolate is shown in Fig. 1c. The 3.46-kb BglII-BglII fragment that was detected is consistent with the replacement of one disrupted copy of SSK1 (Δssk1::hisG) in the CSSK22 strains with the SSK1-URA3-hisG cassette, restoring one SSK1 allele.
SSK1 is not involved in the response to osmotic or oxidative stress.
In order to corroborate our previous findings obtained by complementation analyses, which indicated that SSK1 does not function in regulating the response to either osmotic or oxidative stress (6), the Δssk1 null strains and strain CAF2, used as a control, were grown in M199 (pH 7.5) at 37°C and in M199 (pH 4.0) at 28°C (both solid and liquid) to induce filamentous or yeast growth under conditions of osmotic or oxidative stress, for which media were supplemented with 1 M sorbitol or 2 mM H2O2, respectively. The results indicated that under these conditions of stress, the ability of the Δssk1 null strains either to grow as a yeast or to form germ tubes was similar to that of wild-type cells, although both wild-type and null strains were growth retarded compared to untreated cultures. Thus, the increase observed in either the generation time or the initiation of germ tube formation for the Δssk1 null strains in the presence of stress, compared to the results for untreated cultures, did not differ significantly from the increases observed for the wild-type strain (P, >0.05). However, interestingly, the Δssk1 null mutants flocculated extensively in liquid M199 (pH 7.5), forming clumps of cells through interactions of their germ tubes which sedimented rapidly (data not shown), similar to the previously described behavior of CHK1 mutants (5). Also, we observed that the Δssk1 null strains were unable to undergo the morphological transition from yeast to hyphae on solid M199 (pH 7.5) in either the presence or the absence of stress factors. Taken together, these results indicate that SSK1 does not function in regulating the response to osmotic and oxidative stress in C. albicans, consistent with our previous results obtained by complementation analyses (6). Moreover, these results indicate that SSK1 could play a role in morphogenesis and might be associated with changes in the expression of hyphal cell surface components, since its absence results in flocculation.
SSK1 is required for hyphal formation on solid media.
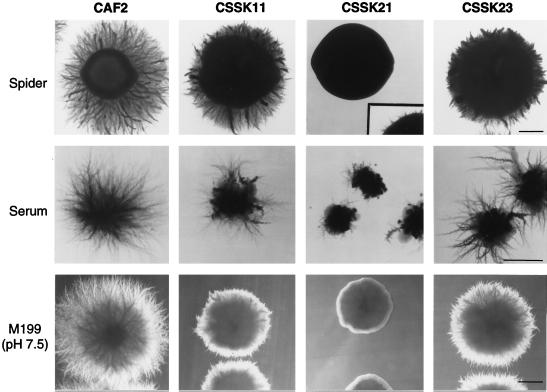
To study the role of SSK1 in the morphological transition from yeast to hyphae, the Δssk1 null and heterozygote Ura3+ strains were grown in several media (solid and liquid) that induce this morphological switch, including Spider, serum, and M199 (pH 7.5) (Fig. 2). Colonies from the wild-type (CAF2) and the heterozygote (CSSK11) strains developed radial filaments emerging from the edge of the colonies after 2 days in M199 (pH 7.5) and after 3 days in Spider medium. In both media, radial filaments had grown extensively after 5 days, even though the heterozygote strain showed a severely reduced extension of these filaments compared with the wild-type strain (Fig. 2). In contrast, the Δssk1 null strains showed suppressed hyphal formation on M199 (pH 7.5) and Spider medium after 5 days of culturing, as revealed by the observation of smooth colonies (Fig. 2). However, after 8 days of incubation, short filaments emerged from the edge of the colonies on Spider medium (Fig. 2, inset). The CSSK23 strains, in which one wild-type copy of SSK1 was reintroduced, regained the ability to form hyphae, similar to the ability of the CSSK11 heterozygote strains grown under the same conditions. Additionally, the Δssk1 null mutants, when grown on agar with 10% serum, showed a severe reduction in hyphal development in comparison with CAF2, forming irregular smooth colonies with yeast growth in the center of the colonies from which some filaments emerged. The heterozygote strains formed colonies intermediate between those of the Δssk1 null strains and the wild-type strain (Fig. 2).
FIG. 2.
Phenotypes of the Δssk1 mutants (strains CSSK11-1, CSSK21-1, and CSSK23-1) grown on solid media which induce hyphal development. Most of the plates were incubated for 5 days at 37°C. The Spider plates were incubated for up to 8 days at 37°C, and the same CSSK21 colony was photographed again (insert) to show the formation of short filaments emerging from the edge of the colony. Bars, 1 mm.
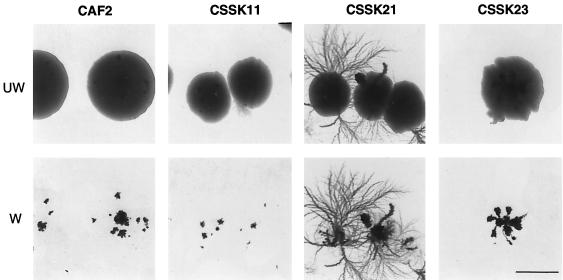
On the other hand, the growth of the Δssk1 null strains was dramatically influenced by low nitrogen availability in SLAD medium (Fig. 3). Contrary to the growth of the wild-type strain (CAF2) on solid Spider medium or M199 (pH 7.5), where hyphal formation creates fuzzy colonies, on SLAD agar, hyphae did not radiate from the edge but formed beneath the colonies, growing into the agar in a way typically known as agar invasion. Thus, when these colonies were washed off the agar, only the agar-invasive section of the colonies remained. Interestingly, we observed that the Δssk1 null strains invaded the agar much more extensively than the heterozygote strains which, in fact, invaded the agar in a manner similar to the wild-type strain. The abnormal hyperinvasion of the agar by the Δssk1 null strains was completely suppressed by the reintroduction of one wild-type copy of SSK1 (Fig. 3), indicating that the hyperinvasive growth was a recessive phenotype of the Δssk1 null strains. In addition, when ammonium sulfate as a nitrogen source was used at a final concentration of 40 mM instead of 50 μM, the Δssk1 null strains did not show hyperinvasive growth, suggesting that the low concentration of the nitrogen source was the critical factor that affected the invasion of the agar by the Δssk1 null mutants.
FIG. 3.
Phenotypes of CAF2 and the Δssk1 mutants (strains CSSK11-1, CSSK21-1, and CSSK23-1) grown under conditions of low nitrogen availability on solid SLAD medium. Plates were incubated for 8 days at 30°C. Invasion of the agar initially was observed after 5 days of incubation and increased with time. The colonies in the top row (UW, unwashed) show darker spots more clearly in the thinner areas of the colonies; these correspond to cells of the colonies that have invaded the agar. In the bottom row (W, washed), the same colonies are shown after the noninvading cells were washed off the surface of the agar. Bar, 1 mm.
Since the yeast-to-hypha switch phenotype depends not only on media and growth conditions but also on the physical state of the media (solid or liquid) (10, 15, 16, 18), we analyzed the ability of the Δssk1 null mutants to form hyphae in liquid media. Thus, in all media tested, the Δssk1 null strains developed hyphae identical to those of the wild type, and no differences in the pattern and timing of germ tube formation or elongation of hyphae could be observed between the CSSK21 strains and CAF2. Again, the most remarkable finding was that in M199 broth (pH 7.5), cells flocculated at all cell densities tested (105, 106, or 107) (data not shown) in the same way as the Δchk1 mutants, a result which we recently reported (5).
Avirulence of Δssk1 mutants.
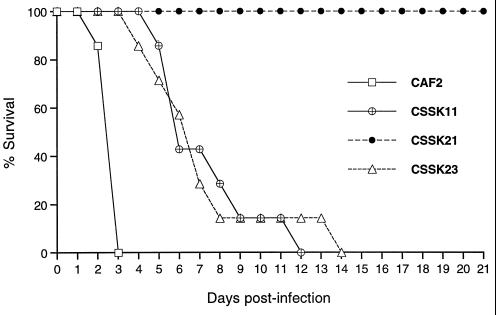
In order to determine whether SSK1 was required for virulence, the ability of Δssk1 C. albicans null strains to establish infection in a murine model of hematogenously disseminated candidiasis was investigated. Prior to animal studies, we evaluated two factors that may also affect the virulence of the mutants, such as their generation time (22) and orotidine 5′-monophosphate (OMP) decarboxylase activity. The generation time of the Δssk1 null strains was slightly higher than that calculated for the wild-type, heterozygote, and revertant strains, whose generation times did not differ significantly from one another (P, >0.09). The OMP decarboxylase activity of each Δssk1 mutant did not differ significantly from the OMP decarboxylase activity of CAF2 (P, >0.1). The data in Fig. 4 show that mice infected with the CSSK21 strains survived throughout the experiment. In contrast, all mice infected with the parental control strain (CAF2) succumbed to infection within 3 days, and survival times observed for mice injected with either the heterozygote (CSSK11) or the revertant (CSSK23) strain were longer than that observed for mice inoculated with the parental strain (CAF2).
FIG. 4.
Survival of mice following infection with C. albicans Δssk1 mutants (CSSK11-1, CSSK21-1, and CSSK23-1) and parental strain CAF2. Product-limit survival estimates were calculated by the Kaplan-Meier method, and the log rank test was used to examine the homogeneity of survival curves among the four strains. The overall differences in survival among strains were highly statistically significant (P, 0.0001). Individual comparisons did not vary from the overall pattern: CSSK21 > CSSK23 ≈ CSSK11 (P, 0.0001), CSSK21 > CAF2 (P, 0.0003), CSSK23 > CAF2 (P, 0.0003), CSSK11 > CAF2 (P, 0.0005), and CSSK23 ≈ CHK11 (P, 0.50).
Quantitative determinations of the level of each C. albicans strain associated with host tissues suggest that the CSSK21 strains were slowly cleared from the kidneys but quickly cleared from the liver (Table 2). The levels of both CSSK11 and CSSK23 strains are similar to or lower than that observed for the parental control strain. Both strains persisted in tissues at 72 h. In order to determine the significance of differences observed among strains for each target organ at each of three times (24, 48, and 72 h), a general linear-model procedure was used. Tukey's multiple-comparison test was used to hold the type I error (α) constant at 0.01. In terms of virulence in the kidneys and liver, several statistically significant differences were seen at a P value of ≤0.01 in mean log10 CFU per gram at each of the times (Table 2). Histological examinations of kidney tissue support these observations (data not shown). Thus, all strains had formed mycelia in infected tissue, but smaller amounts of fungal burden were observed in tissue infected with the CSSK21 strains than in those infected with the CSSK11 or CSSK23 strains or CAF2, probably because a more effective clearing of the CSSK21 strains was performed by murine phagocytes.
TABLE 2.
Recovery of C. albicans from infected tissues
| Time postinfection (h) | Strain | Log10 CFU/g (mean ± SD) in:
|
|
|---|---|---|---|
| Kidneysa | Liverb | ||
| 24 | CAF2 | 6.20 ± 0.27 | 3.88 ± 0.10 |
| CSSK11-1 | 5.11 ± 0.11 | 3.43 ± 0.10 | |
| CSSK21-1 | 4.13 ± 0.12 | 3.28 ± 0.17 | |
| CSSK23-1 | 5.57 ± 0.50 | 3.53 ± 0.19 | |
| 48 | CAF2 | 6.66 ± 0.76 | 3.98 ± 0.34 |
| CSSK11-1 | 4.74 ± 0.44 | 2.42 ± 0.24 | |
| CSSK21-1 | 3.69 ± 0.24 | 2.39 ± 0.25 | |
| CSSK23-1 | 5.80 ± 0.94 | 3.47 ± 0.52 | |
| 72 | CAF2 | —c | — |
| CSSK11-1 | 4.68 ± 0.21 | 1.93 ± 0.16 | |
| CSSK21-1 | 3.52 ± 0.24 | 0.68 ± 0.85 | |
| CSSK23-1 | 5.56 ± 0.50 | 2.87 ± 0.25 | |
At 24 h, CAF2 > CSSK23 ≈ CSSK11 > CSSK21. At 48 h, CAF2 > CSSK23 > CSSK11 > CSSK21. At 72 h, CSSK23 and CSSK11 > CSSK21, and CSSK23 ≈ CSSK11.
At 24 h, CAF2 > CSSK11 > CSSK21, CSSK11 ≈ CSSK23, and CSSK23 > CSSK21. At 48 h, CAF2 > CSSK23 > CSSK11 and CSSK21, and CSSK11 ≈ CSSK21. At 72 h, CSSK23 > CSSK11 > CSSK21.
—, all mice succumbed to CAF2 infection by 72 h.
The avirulence of the Δssk1 null strains indicates that SSK1 is required for the pathogenesis of C. albicans. Furthermore, several experiments indicated that the virulence of the CSSK11 and CSSK23 strains was due to the presence of one functional allele, while the avirulence of the CSSK21 strains was due to the absence of any SSK1 allele. (i) The virulence of strain CSSK21-1 was restored by the reintroduction of a parental copy of SSK1 (strain CSSK23-1). (ii) Similar results in survival and tissue counts were obtained with a second independent Δssk1 null mutant (CSSK21-2), the virulence of which was also restored after one wild-type copy of SSK1 was reintroduced (strain CSSK23-2) (data not shown). (iii) The generation time of the Δssk1 mutants was similar to the generation time of the wild type. However, the longer generation time of the null strain likely also contributes to its avirulence. In this context, it has been reported recently that a linear relationship between the generation time of mutants and the survival time of mice infected with these mutants exists (22). (iv) No differences were observed in the OMP decarboxylase activities of the Δssk1 mutants and the wild type (CAF2).
DISCUSSION
The morphological switch from yeast to hyphae is one of the most important biological features that enables C. albicans to colonize, invade, and survive in the host tissues during infection. Although S. cerevisiae is rarely an opportunistic human pathogen and apparently does not require this dimorphic transition as a mechanism to survive in tissue, by use of functional complementation studies of mutations in genes related to the filamentation-invasion of S. cerevisiae as a model, several C. albicans homologs have been identified; these include CST20, HST7, CEK1, and CPH1 (11, 15, 16, 18), which function in a C. albicans STE12-like MAP kinase cascade, as well as EFG1 (19, 28) which, like its S. cerevisiae homolog PHD1, is probably part of an independently regulated, unknown pathway (3). Initially, studies with a Δcph/Δefg1 strain of C. albicans indicated that the signalling pathways that regulate the activity of the Cph1p and Efg1p transcription factors constitute the main regulatory mechanisms of the yeast-to-hypha transition in C. albicans (19). However, the CaHOG1 gene, unlike its S. cerevisiae homolog, exhibits functional properties related to morphogenesis in C. albicans (2). Furthermore, in C. albicans there are genes without an S. cerevisiae counterpart that also play a role in morphogenesis, such as the sensor histidine kinase genes CHK1 (5, 7) and NIK1/COS1 (1). In this work, we have reported and discussed the function of SSK1, the only response regulator kinase gene described so far in C. albicans (6).
As we have previously shown by complementation analyses, CaSSK1 does not restore the normal growth of either Δssk1 or Δmcs4 strains of S. cerevisiae or S. pombe, respectively, suggesting that CaSSK1 may have a role in C. albicans other than the adaptation of cells to stress (6). The phenotypic characterization of Δssk1 mutants supports our previous observation and confirms that CaSSK1 is not functionally related to either SSK1 or mcs4+. Moreover, CaSSK1 is essential for hyphal formation on solid inducing media and virulence, even though it is not required for the formation of hyphae in liquid media.
It has been proposed that, like Cph1p, Efg1p must be the final element of a morphogenesis pathway (19, 28). Thus, like Δssk1 null strains, Δefg1 mutants are unable to form true hyphae either on solid or in liquid media (19). However, in contrast to Δssk1 mutants that form hyphae in liquid media similar to those of the wild type, Δefg1 mutants grow as a yeast in liquid media, even in the presence of serum (19). This phenotypic difference between Δssk1 and Δefg1 strains makes it unlikely that SSK1 can influence the activity of the EFG1 pathway. Also, it has previously been reported that mutations in the genes CST20, HST7, CEK1, and CPH1 make C. albicans unable to undergo the yeast-to-hypha transition on solid media (11, 15, 16, 18), while CPP1 mutants undergo hyperfilamentation under conditions that normally do not induce filamentation (10). The phenotype of the Δssk1 null strains resembles the defect in hyphal formation on solid media observed for mutants with mutations in genes of the CPH1 filamentation pathway but, in contrast, strains with mutations in genes of the CPH1 pathway are able to form hyphae on solid serum, are not totally avirulent, and fail to hyperinvade agar under conditions of low nitrogen availability (11, 15, 16, 18). In addition, although the hyperinvasive phenotype of the Δssk1 null strains resembles that of the Δcpp1 mutants, the Δssk1 null strains show this phenotype only under conditions of low nitrogen, while the Δcpp1 mutants show derepressed hyperinvasion of agar under both normally noninducing and inducing conditions with a wide variety of rich and defined solid media (10). In any case, if SSK1 affects the activity of the CPH1 pathway, then the overproduction of downstream components of the CPH1 pathway in a Δssk1 background should rescue the lack of SSK1. However, in preliminary experiments, we were unable to rescue the normal filamentous growth of a Δssk1 null mutant by overexpressing either CPH1 or CPP1. Similar results were previously described by others for a Δnik1/cos1 background (1). Overexpression of the HST7 gene did not rescue the normal filamentous growth of a Δcos1 null strain (1), suggesting that NIK1/COS1 may lie in a different pathway or interact with components downstream of HST7 (1). Interestingly, like the Δssk1 null strains, the Δcos1 mutants cannot undergo the yeast-to-hypha transition on Spider agar and show reduced hyphal formation on serum agar (1). On the other hand, the flocculation displayed by Δssk1 null strains under conditions of germ tube formation occurs in a manner similar to that for Δchk1 mutants (5). Both Δssk1 and Δchk1 strains show similar growth rates and are totally avirulent in a murine model of hematogenously disseminated candidiasis (8). These observations, together with the nonrestoration of filamentous growth by overexpression of the Δssk1 background of either CPP1 or CPH1 (which, unlike HST7, has been proposed to be the last element of the pathway), indicate that SSK1 may also lie in a CPH1-independent, two-component filamentation pathway.
Finally, the fact that CaSSK1 is not functionally related to SSK1 does not preclude the possibility that a structurally homologous two-component Sln1p-Ypd1-Ssk1p cascade (3) that affects the activity of a putatively homologous HOG MAP kinase pathway exists in C. albicans. In this context, some phenotypic properties of the Δssk1 mutants resemble those observed for the Δhog1 mutants of C. albicans (2): both are unable to form hyphae on Spider agar, are more invasive in SLAD medium than the wild type, and are avirulent. However, we were unable to rescue the normal phenotype of a Δssk1 null strain by overexpressing CaHOG1 in the Δssk1 background. In spite of this result, it still remains possible that Ssk1p functions in a two-component phosphorelay cascade which may or may not influence the activity of Hog1p. In addition, since Ssk1p should function as a response regulator in this putative cascade, like other response regulators (3), it must lie downstream of a sensor histidine kinase component, such as Chk1p or Nik1p/Cos1p. However, since the Δssk1 mutants displayed two unrelated phenotypes that resemble those of the Δchk1 and Δnik1/Δcos1 mutants, our hypothesis is that two Chk1p- and Nik1p-independent branches of the same two-component cascade could modulate the phosphorylation state of Ssk1p through one or more intermediate Ypd1-like histidine kinases. In this regard, we have recently isolated a C. albicans gene which encodes a Ypd1p-like protein (unpublished data).
In summary, our data indicate that SSK1 links two of the most important aspects of the biology of C. albicans, i.e., the morphological transition from yeast to hyphae and changes in the expression of a hyphal surface compound(s) that occur during hyphal growth. Also, in addition to the STE12-like MAP kinase cascade that is known to regulate morphogenesis in C. albicans, these results suggest that a two-component cascade may have been adopted by C. albicans to modulate its morphological switching and that other undiscovered elements or pathways that regulate the transition from yeast to hyphae in liquid media in C. albicans must exist. Furthermore, two-component signal transduction cascades have not been found in mammalian cells, emphasizing the interest of both Ssk1p and Chk1p as targets for the development of antifungal agents.
ACKNOWLEDGMENTS
This work was supported by a Public Health Service grant to R.C. (NIH-AI-43465). J.A.C. is a recipient of a postdoctoral fellowship from the Ministerio de Educación y Cultura of the Spanish government.
We thank William Fonzi for performing the OMP decarboxylase assays. We also thank Doreen Harcus (CNRC-NRC, Montreal, Quebec, Canada) for plasmids pLJ19 and pCCa1.
REFERENCES
- 1.Alex L A, Korch C, Selitrennikoff C P, Simon M I. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci USA. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Monge R, Navarro-García F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sánchez M, Nombela C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banuett F. Signaling in the yeast: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 5.Calera J A, Calderone R. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology. 1999;145:1431–1442. doi: 10.1099/13500872-145-6-1431. [DOI] [PubMed] [Google Scholar]
- 6.Calera J A, Calderone R. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast. 1999;15:1243–1254. doi: 10.1002/(SICI)1097-0061(19990915)15:12<1243::AID-YEA449>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Calera J A, Choi G H, Calderone R. Identification of a putative histidine kinase two-component phosphorelay gene (CaHK1) in Candida albicans. Yeast. 1998;14:665–674. doi: 10.1002/(SICI)1097-0061(199805)14:7<665::AID-YEA246>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Calera J A, Zhao X J, De Bernardis F, Sheridan M, Calderone R. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect Immun. 1999;67:4280–4284. doi: 10.1128/iai.67.8.4280-4284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottarel G. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics. 1997;147:1043–1051. doi: 10.1093/genetics/147.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csank C, Makris C, Meloche S, Schroppel K, Rollinghoff M, Dignard D, Thomas D Y, Whiteway M. Derepressed hyphal growth and reduced virulence in a VH1 family-related protein phosphatase mutant of the human pathogen Candida albicans. Mol Biol Cell. 1997;8:2539–2551. doi: 10.1091/mbc.8.12.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gietz D, Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1991;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 15.Kohler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A, Brown A J, Thomas D Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol. 1997;7:539–546. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Kohler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 19.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 20.Maeda T, Wurgler-Murphy S M, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 21.Nagahashi S, Mio T, Ono N, Yamada-Okabe T, Arisawa M, Bussey H, Yamada-Okabe H. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology. 1998;144:425–432. doi: 10.1099/00221287-144-2-425. [DOI] [PubMed] [Google Scholar]
- 22.Reig G, Fu Y, Ibrahim A S, Zhou X, Filler S G, Edwards J E., Jr Unanticipated heterogeneity in growth rate and virulence among Candida albicans AAF1 null mutants. Infect Immun. 1999;67:3193–3198. doi: 10.1128/iai.67.7.3193-3198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggle P J, Andrutis K A, Chen X, Tzipori S R, Kumamoto C A. Invasive lesions containing filamentous forms produced by a Candida albicans mutant that is defective in filamentous growth in culture. Infect Immun. 1999;67:3649–3652. doi: 10.1128/iai.67.7.3649-3652.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 26.Shieh J C, Wilkinson M G, Buck V, Morgan B A, Makino K, Millar J B. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- 27.Srikantha T, Tsai L, Daniels K, Enger L, Highley K, Soll D R. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology. 1998;144:2715–2729. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 28.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J R, Register E, Curotto J, Kurtz M, Kelly R. An improved protocol for the preparation of yeast cells for transformation by electroporation. Yeast. 1998;14:565–571. doi: 10.1002/(SICI)1097-0061(19980430)14:6<565::AID-YEA251>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Yaar L, Mevarech M, Koltin Y. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology. 1997;143:3033–3044. doi: 10.1099/00221287-143-9-3033. [DOI] [PubMed] [Google Scholar]