Abstract
Continuous renal replacement therapy (CRRT) is a form of renal replacement therapy that is used in modern intensive care units (ICUs) to help manage acute kidney injury (AKI), end stage kidney disease (ESKD), poisonings, and some electrolyte disorders. CRRT has transformed the care of patients in the ICU over the past several decades. In this setting, it is important to recognize CRRT-associated complications but also up-to-date management of these complications. Some of these complications are minor, but others may be more significant and even life-threatening. Some CRRT complications may be related to dialysis factors and others to specific patient factors. Our overarching goal in this article is to review and discuss the most significant CRRT-related complications at the different stage of management of CRRT. With the advent of newer solutions, there have been newer complications as well.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, bleeding, circuit, complications, CRRT, dialysis, drugs, electrolytes, ICU, pneumothorax
Introduction
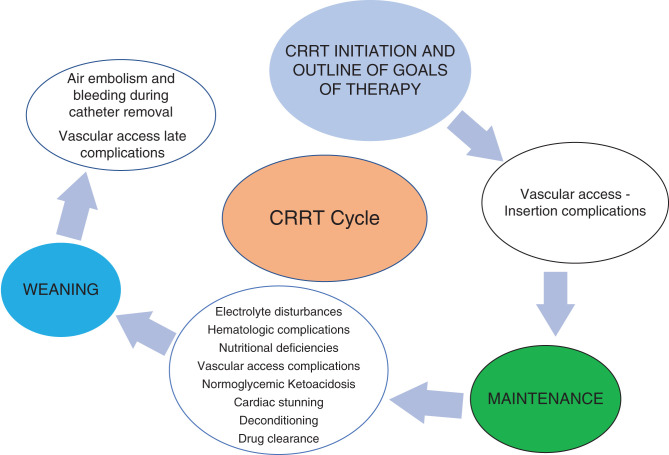
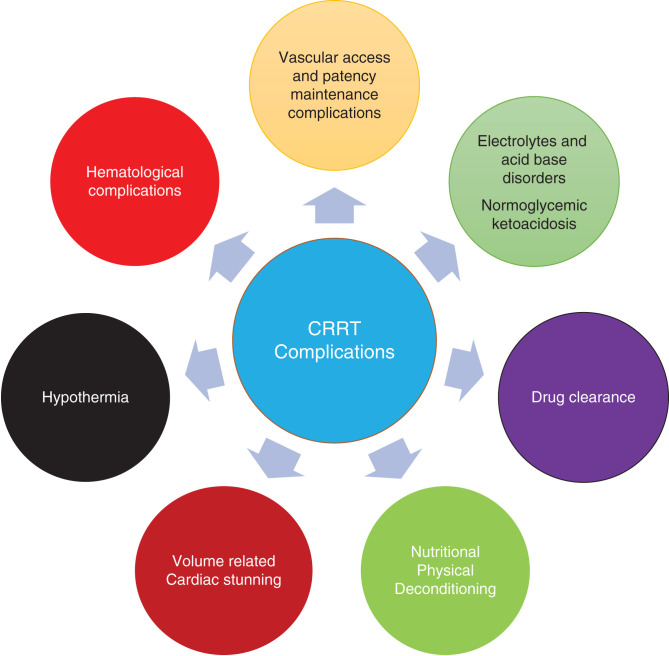
Continuous RRT (CRRT) is a form of RRT that is used in modern intensive care units (ICUs) to help manage AKI, ESKD, poisonings, and some electrolyte disorders. CRRT has transformed the care of patients in the ICU over the past several decades. As has been well documented in prior studies, AKI incidence has been increasing, in large part driven by an older population with more comorbidities (1,2). The delivery of care using CRRT has also evolved over the years, its indications has broadened, and it is being increasingly used. In this setting, it is important to recognize CRRT-associated complications but also up-to-date management of these complications. Some of these complications are minor, but others may be more significant and even life-threatening. Some CRRT-related complications may be related to dialysis factors and others to specific patient factors. Our overarching goal in this article is to review and discuss the most significant CRRT-related complications at different stages of management of CRRT (Figures 1 and 2).
Figure 1.
Continuous RRT (CRRT) cycle and complications.
Figure 2.
Complications associated with CRRT.
Herein, we describe and discuss the management of clinically relevant CRRT complications on the basis of those related to vascular access, the extracorporeal system, and biomembranes, metabolic support, and electrolytes, and those related to clearance and anticoagulation (Figure 3).
Figure 3.
Complications associated with CRRT.
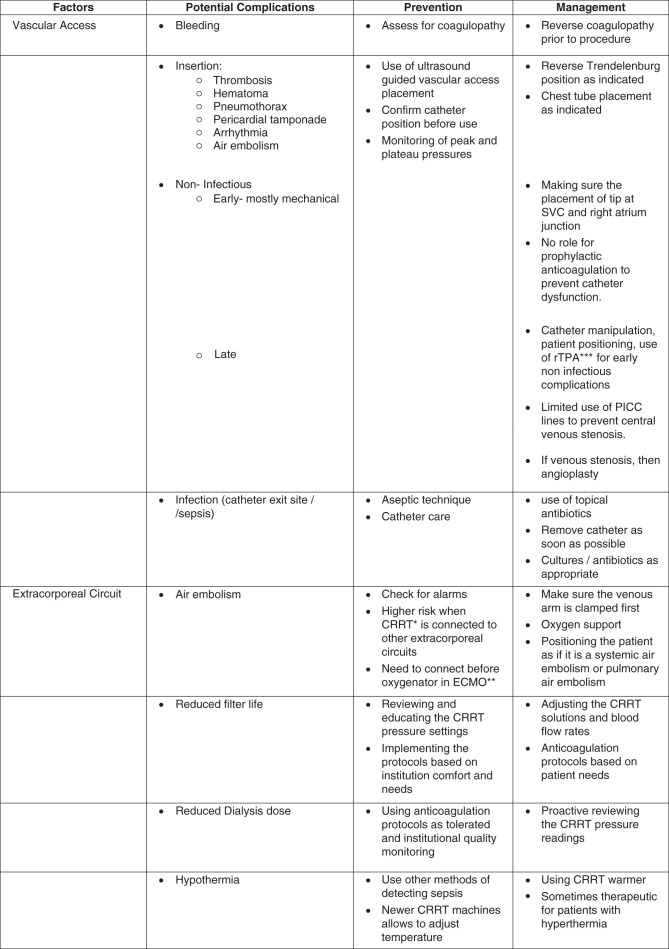
Vascular Access Complications
Vascular access is the lifeline of CRRT. Vascular access complications can arise during placement or during maintenance, whether it is in the acute setting or chronic settings, each of them having their own unique limitations and complications.
During placement, the common complications, similar to all central vascular access line placements, are arterial puncture and venous rupture, leading to bleeding, and, in addition, for chest line procedures, pneumothorax, myocardial rupture, and arrhythmias.
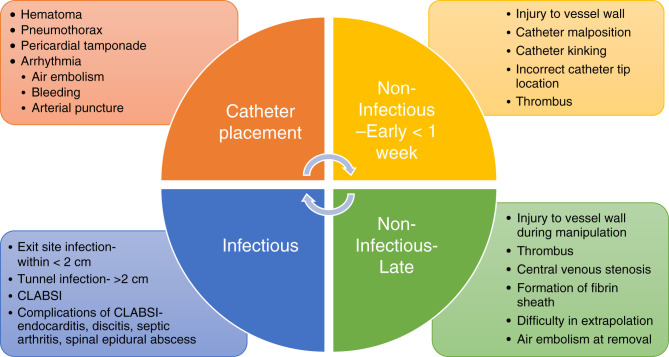
Although these are complications at the time of catheter placement, the catheters can have further complications on the basis of timing of the catheter placement, which can be infectious or noninfectious (Figure 4). Noninfectious complications can be within the duration of CRRT or can persist over a number of years such as central venous stenosis or even difficulty with catheter extraction. The nontunneled catheters have limitations with possible increased risk of infections and also mechanical complications, with comparisons discussed in the section below.
Figure 4.
Complications of catheter placement.
However, with clinical practice guidelines recommending the use of ultrasound-guided techniques to place vascular access, the initial complications have been minimized (3,4). Catheter-related thrombosis is another dreaded complication, and patients on CRRT are at increased risk, given concurrent illness and hypercoagulable state. If found, systemic anticoagulation is indicated as long as the catheter remains in place (5).
Nosocomial Complications
Most common vascular access complications are similar to catheter access issues seen during intermittent hemodialysis. Infectious complications of vascular accesses are one of the impediments of caring for patients in ICUs. The infectious process typically starts from the spectrum of skin colonization to biofilm development to local exit site infection to tunnel infection to bacteremia; however, intraluminal bacteremia may also be acquired. Organisms are generally Staphylococcus aureus and coagulase-negative Staphylococcus, but Gram-negative bacteria and candida are other possible causes of sepsis (6). Multiple observational studies and guidelines recommend avoiding femoral catheter sites because of an increased risk of nosocomial infections; however, in a concealed, randomized, multicenter, evaluator-blinded, parallel-group trial of 750 patients of nine tertiary care centers, no clinically relevant benefit of jugular site catheterization was found compared with femoral site catheterization in terms of nosocomial complication in critically ill adults requiring RRT (4). Jugular catheters had a statistically significantly higher rate of hematoma formation compared with the femoral group (4% versus 1%; P=0.03) but no difference in arterial puncture. In regard to catheter-related infections and catheter colonization at the time of catheter removal, there was no statistically significant difference between infection in the jugular and femoral catheter groups. Further, in subgroups analysis, the researchers found a statistically significant higher rate of catheter colonization at the time of catheter removal with femoral catheters in patients in the highest tercile of body mass index (>28.4 kg/m2). Jugular catheters had statistically significantly more Gram-positive bacteria (P=0.04), and femoral catheters had a higher colonization of Gram-negative bacteria (P=0.03). Duration of catheter did not seem to have a statistically significant change in catheter colonization at the time of removal when the two groups compared were ≤5 days and >5 days (4).
The 2019 Kidney Disease Outcomes Quality Initiative guidelines recommend limiting the use of a temporary, noncuffed, nontunneled dialysis catheter to 2 weeks due to increased risk of infections in patients who need emergent vascular access, which certainly has to be reviewed on a patient-to-patient basis as described below (7). There are variations to this practice too on the basis of the patient’s needs and the ability to maintain CRRT patency and minimize treatment or dialysis interruption. This is a change from previous guideline recommendations that a noncuffed, nontunneled dialysis catheter should not exceed 3 weeks for jugular and 5 days for femoral access (8,9). In a retrospective study of 595 patients receiving CRRT, looking at rates of adverse events, other catheter-related complications included bleeding (23%), arterial puncture (1%), hematoma (2.85%), line-related infection (5%), and other (12%; pneumothorax, catheter misplacement, and air embolism) (10). Although nontunneled dialysis catheter placement has been standard and common, a 16-month observational prospective cohort study involving 154 patients showed that compared with nontunneled dialysis catheters, tunneled dialysis catheters were associated with better dialysis delivery and fewer mechanical complications. Interestingly, there was no difference in the rate of positive blood cultures per catheter (11). Further randomized studies are needed to confirm these findings.
Extracorporeal Circuit Complications
AN69 and Angiotensin-Converting Enzyme Inhibitors
AN69 is polyacrylonitrile synthetic dialysis membrane that was developed to improve biocompatibility. AN69 membranes have been associated with anaphylactoid reactions when used in combination with angiotensin-converting enzyme inhibitors due to activation of bradykinin. These reactions have been partially mitigated by surface treatment of the AN69 membrane (AN-69ST) (12).
Hypothermia
One of the most noted significant adverse events of CRRT is hypothermia, defined as a temperature <35°C, and was found in up to 44% of patients in one study (10). Below 34°C, hypothermia can cause depressed brain and cardiovascular function and arrhythmia, and mask fevers, delaying recognition of infections and the initiation of antibiotics (13). Critically ill patients on CRRT are predisposed to hypothermia from many factors including, but not limited to, sedation, paralytics, shock, endocrine disorders, intoxications, and central nervous system lesion/injury. Of note, arterial and venous line temperatures differences during CRRT have been studied. The largest temperature difference between blood in arterial and venous lines was 5.5°C±0.2°C when blood flow (Qb) was 100 ml/min and dialysate flow (Qd) was 1500 ml/h. The lowest temperature difference was 1.9°C±0.1°C when Qb was 200 ml/min and Qd was 500 ml/h, showing that slower Qb and higher Qd caused greater energy loss during CRRT (14). In another study, heat loss was calculated to average 750 kcal/d, worsening caloric deficit in these already critically ill patients. However, in some situation, cooling may be beneficial such as in patients with significant hyperthermia and status post cardiac arrest (13). Milder degrees of hypothermia may contribute to more hemodynamic stability by causing an increase in pulse, cardiac output, and systemic vascular resistance. In a prospective crossover randomized study, 30 patients on continuous venovenous hemofiltration had a heating device set to 38°C and 36°C for 6 hours each. The authors found that patients core temperature did not change significantly; however, patients with continuous venovenous hemofiltration with a heating device set at 36°C had higher mean arterial pressure and required lower catecholamine infusion doses (15).
Suggestions for the treatment of hypothermia include passive external rewarming (blankets allow for natural thermogenesis to raise core temperature by 0.5°C/h if shivering mechanism is intact), but also active external rewarming (warming devices are reported to raise temperature by 1–2.5°C/h) and active internal/core rewarming (intravenous fluids warmed up to 42°C, peritoneal dialysate, isotonic crystalloids into the stomach or bladder). Of note, modern dialysis machines are equipped with warming devices to help counter heat loss as well (13).
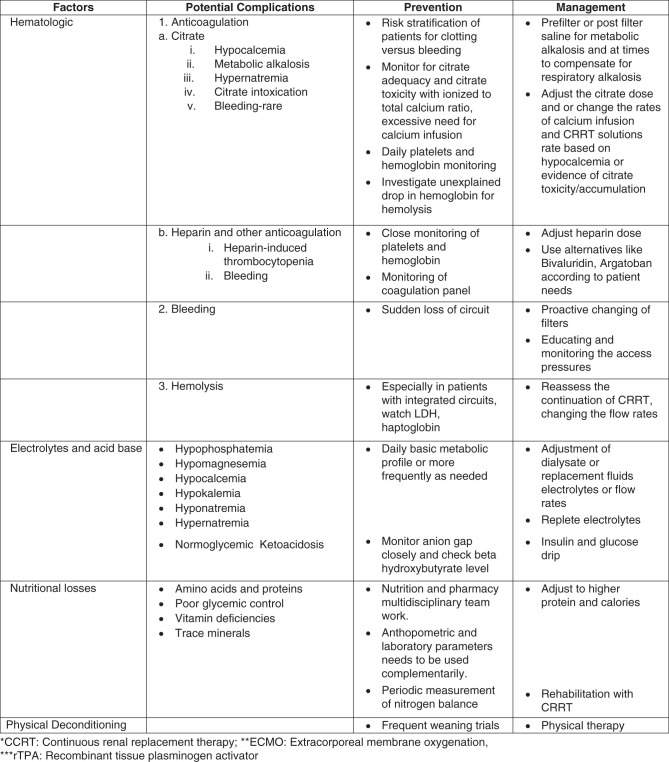
Citrate Toxicity
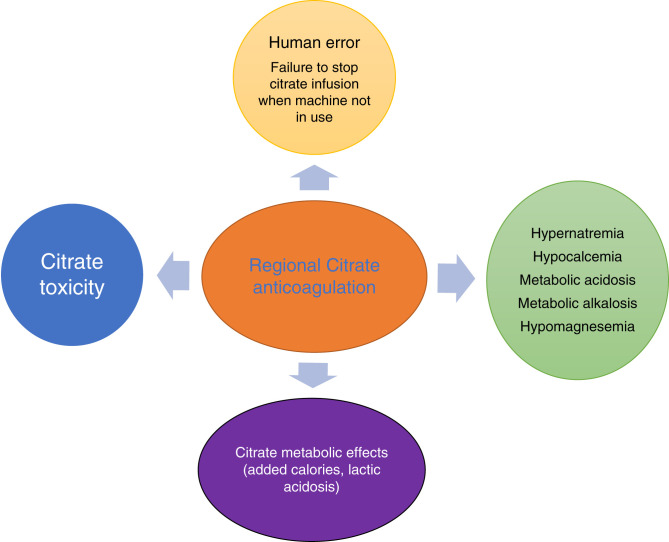
Because of the nature of the extracorporeal circuit, contact of blood with the biomembranes, and procoagulant state, critically ill patients on CRRT frequently require anticoagulation to prolong the life of the filter and to minimize interruptions to dialysis therapy (16). The most common anticoagulants used during CRRT are intravenous heparin or regional citrate anticoagulation (RCA) (17). The RICH study showed that the patients with intravenous heparin had a higher rate of bleeding compared with RCA, but RCA had a higher rate of culture-proven infection compared with intravenous heparin (18). In addition, it is also important to recognize associated metabolic complications (hypocalcemia, hypercalcemia, hypernatremia, metabolic alkalosis) and citrate toxicity (Figure 5) (19). However, it is important to note that a recent post hoc analysis of the RICH trial revealed that a longer mean filter lifespan (>48 hours) was associated with an increased rate of new infections, independent of the type of anticoagulation used (20).
Figure 5.
Complications of regional citrate anticoagulation.
Citrate toxicity can be identified by a low ionized calcium, a disproportional rise in total calcium with a total calcium/ionized calcium ratio of >2.5, and high anion gap metabolic acidosis or with escalating rates of calcium infusion (19). Citrate excess has also been associated with metabolic alkalosis, which occurs when citrate is metabolized to bicarbonate in the liver (21). These complications can be managed by decreasing the citrate rate or increasing the dialysis or effluent rate, all of which would be geared toward decreasing citrate delivery (22). Importantly, RCA is best avoided in patients who have acute liver failure or cardiogenic shock with high lactate levels (>8 mmol/L) because they have a high risk of impaired citrate metabolism with a high risk of citrate accumulation and citrate toxicity. For other rare instances of citrate dynamics, it is important to recognize there can be three potential scenarios that can cause different acid base and electrolyte disorders. Citrate accumulation can cause metabolic acidosis due to delayed metabolism of citrate, leading to lactic acidosis. This can be fatal, but given intensive monitoring, it is rare. Increased citrate infusion with can lead to metabolic alkalosis and hypernatremia (23,24).
The manifestations of citrate overload depend on the metabolic state, rate of citrate infusion, or type of citrate used. Citrate chelates with calcium, and it has to be used as proximally as possible to the access to reduce the initiation of coagulation cascade. The citrate binds with calcium to form the creatinine calcium citrate complex (CCC), most of which would be cleared with CRRT (21,24). But as it escapes to the systemic circulation, citrate is metabolized to bicarbonate and also releases sodium. Thus, metabolic alkalosis and hypernatremia can occur. The severity of hypernatremia depends on the type of citrate used such as trichloroacetic acid, which has 420 mmol/L of sodium, compared with acid citrate dextrose, which has 224 mmol/L of sodium (24).
Hematologic Complications
Hematologic complications are one of the most underrecognized complications observed in patients during CRRT. These complications could be related to anticoagulation (heparin, citrate) or the result of extracorporeal circuit–related issues. The most common complication is thrombocytopenia, but anemia has also been reported.
Thrombocytopenia
CRRT may be associated with thrombocytopenia and can confound the diagnosis and management of other causes of thrombocytopenia seen in critically ill patients such as sepsis, heparin-induced thrombocytopenia (HIT), and drug-related thrombocytopenia (25). The temporal relationship of CRRT and decrease in platelet count with CRRT initiation and follow-up was evaluated at a quaternary regional referral center where 80 patients received CRRT for >48 hours and were followed for thrombocytopenia (defined by a platelet count of <100,000/μL) with a stable platelet count for at least 4 days before CRRT initiation. During a 5-day course, there was significant worsening thrombocytopenia in 59% of patients at day 5, including 30% of patients who developed even more severe thrombocytopenia of <50,000/μl. In this study, only the Sequential Organ Failure Assessment score at time of CRRT initiation on multivariate analysis predicted the development of thrombocytopenia. In regard to HIT, of the 20% of patients suspected and evaluated, 81% had a low to intermediate pretest probability of HIT, but only one patient had laboratory-confirmed HIT (26).
The mechanism of thrombocytopenia in CRRT is unclear and likely multifactorial because critically ill patients on CRRT have many comorbidities that can be associated with thrombocytopenia. Platelet destruction, adsorption, and activation are likely to play a role. In one study, indium-labeled platelets in an in vitro system showed considerable platelet deposition on a variety of dialysis membranes (27,28). Seen in other extracorporeal membranes, platelet activation was postulated to have a role in thrombocytopenia in CRRT through peripheral consumption. However, evidence for platelet activation has been mixed (27,29). Thrombocytopenia may also have prognosis values at the time of CRRT initiation. In a recent study by Griffin et al., the authors reported that a >40% decrease in platelet count was associated with increased risk of secondary infections. Interestingly, the same research group reported that thrombocytopenia was associated with lower rates of renal recovery and higher mortality (28,30,31).
Management is variable and could consist of higher blood flows, which is postulated to decrease transit time, improved rheology, and decreased hemoconcentration (28). Transition to intermittent hemodialysis is postulated to decrease contact time with the dialysis filter. However, not many studies have evaluated thrombocytopenia across different RRT modalities. In one study, CRRT was associated with a platelet decrease compared with intermittent hemodialysis but only in univariate analysis. Nonetheless, the results were attenuated when accounting for severity of illness, liver disease, and filter losses. In the same study, the intermittent hemodialysis group had twice as much filter exposure and more thrombocytopenia compared with the conventional group, but this was not statistically significant (28,32).
Anemia
Anemia may occur in patients on CRRT for a variety of reasons. In a retrospective study assessing adverse events in adults on CRRT, one study found 31% of the 595 patients to have new-onset anemia, defined as a hemoglobin <10 g/dl. One leading cause of anemia is blood lost through the extracorporeal circuit. A subgroup analysis comparing blood loss in CRRT with that in intermittent hemodialysis showed that patients with CRRT had increased RRT-related blood loss, but the transfusion events were similar (33). The clotting cascade is activated with shearing and turbulence induced by the nonendothelialized surface of the filter, circuit tubing, and catheter. RCA has been evaluated extensively. A meta-analysis of 11 randomized controlled trials of approximately 2000 filters and 1000 patients demonstrated RCA for CRRT was able to reduce the risk of extracorporeal circuit blood loss compared with regional and systemic heparin administration and is the recommended anticoagulant in the most recent Kidney Disease Improving Global Outcomes (KDIGO) guidelines if there are no contraindications (4,34). Compared with systemic heparin administration, circuit loss (circuit termination for any reason) was significantly reduced by 24% with RCA. On the other hand, compared with regional heparin anticoagulation, circuit loss had a 48% significant reduction with RCA. Information regarding filter failure (filter clotting or high transmembrane filter pressure) was available in six randomized controlled trials and was found in the pool data to favor the citrate group, with the important caveat of high intertrial heterogeneity. Catheter dysfunction was similar between both groups. In the nine trials that compared systemic heparin to RCA, the bleeding risk was significantly reduced with RCA (35).
Another reason for anemia observed during CRRT is mechanical hemolysis from the extracorporeal circuit itself. More commonly seen in extracorporeal membrane oxygenator circuits (ECMO), small studies looking into plasma-free hemoglobin (PFHb) from CRRT circuits showed a statistically significant rise in PFHb but not to the levels of clinically significant hemolysis reported in the ECMO literature, whereas filter clotting and peak circuit pressures did not have any statistically significant change in PFHb in another small study (32).
Electrolyte Disturbances
Hypophosphatemia
Like many of the adverse events seen in critically ill patients receiving acute dialysis, the etiology of hypophosphatemia is multifactorial as well (34). It is important to note that hypophosphatemia is typically an avoidable complication. Malnourishment, refeeding syndrome, sepsis, insulin, and phosphorous removal during CRRT all contribute to hypophosphatemia in this critically ill population. Some studies that looked at the effectiveness of established phosphate repletion protocols still resulted in patients developing hypophosphatemia (23,36). Even though there is no evidence that intensive CRRT doses improve the survival rate of critically ill patients with AKI, there are instances where intensive CRRT is needed such as acute hyperammonemia or severe labile hyperkalemia (37,38). However, this intensive treatment may increase the risk of developing hypophosphatemia (39). Further, there is no consensus agreement about the phosphate goal during CRRT, but there is evidence that hypophosphatemia at all ranges is associated with worse outcomes (23). Hypophosphatemia <0.6 mmol/L (1.86 mg/dl) has been reported to increase the incidence and duration of mechanical ventilation (38). Similarly, hypophosphatemia <0.67 mmol/L (2 mg/dl) has been associated with an increased need for tracheostomy (40).
Some have advocated for phosphate-containing dialysate as a different approach to prevent CRRT-induced hypophosphatemia. Commercially available dialysate replacement fluid containing phosphate at 1.2 mmol/L has been studied and indeed helped to maintain normophosphatemia in the majority of patients (23,41). However, rare cases of hyperphosphatemia, metabolic acidosis, and hypocalcemia have been reported. Currently, there is no consensus about the optimal phosphorous target in CRRT patients, and importantly, there are concerns about serum levels not being reflective of intracellular phosphorous concentrations and subsequent ATP synthesis (38). So, further studies are needed to evaluate adequate phosphorus targets to avoid complications associated with CRRT-induced hypophosphatemia (38,40). Of note, phosphate-containing solutions contain no glucose, and recently, there have been reports of patients developing normoglycemic ketoacidosis. This phenomenon is increasingly recognized in patients who are using glucose-free CRRT solutions and sometimes even with glucose-containing CRRT solutions. Normoglycemic ketoacidosis is identified with anion gap metabolic acidosis, serum ketones, and low/normal glucose. The treatment of this phenomenon involves an infusion of glucose and insulin (42,43).
In addition to calcium, we also need to monitor magnesium levels while the patient is using RCA because the citrate also chelates magnesium and the patient’s magnesium levels become systematically depleted (44).
In addition to phosphorus, importantly, there is a need to monitor other electrolyte imbalances such as potassium, calcium, and sodium. Although potassium and calcium disorders can be mitigated by changing the dialysate or replacement fluid electrolyte mixture, sodium disorders require additional management. Severe AKI and hyponatremia with risk of overcorrection can be managed by adding hypotonic fluids through the circuit or adjusting the CRRT solutions (45). Customizing CRRT solutions by adjusting the sodium concentrations in the solution is possible with a multidisciplinary effort by pharmacists according to the sodium levels (46). Sodium follows urea kinetics, and using this model, it is possible to predict the change in sodium levels by making changes to the CRRT solution (47). Similarly, circumstances that require hypernatremia, such as acute neurologic injuries including intracranial hemorrhage and stroke, will also require custom CRRT solutions or hypertonic fluids.
Treatment Delivery and Volume Management
Although there is no proven lower threshold of CRRT dose in AKI, the KDIGO guidelines recommend aiming to deliver effluent of 20–25 ml/kg per hour for CRRT in this setting; however, because of downtime for different reasons such as imaging studies, CRRT breakdown, and need for surgery, patients don’t always get the desired dose (48,49). Venkatraman et al. showed that patients who were prescribed 24 ml/kg per hour in fact received 16 ml/kg per hour, with RRT running for 16 hours (67%) on average (50). Therefore, to ensure a minimum delivered dose of 20–25 ml/kg per hour, it may be necessary to prescribe approximately 25–30 ml/kg per hour, and it is also necessary to minimize CRRT downtime to ≤4 h/d (48–50).
Cardiac Stunning
In addition to clearance, more recently we have had a better understanding of the effect of ultrafiltration rates on patient survival. Too aggressive ultrafiltration can cause hypotension and myocardial stunning. In hemodialysis patients, this has shown to increase the risk of sudden cardiac arrest. In critically ill patients, initiation of CRRT has been associated with cardiac stunning (51). Cardiac stunning is not only related in patients with aggressive ultrafiltration but also in patients without aggressive ultrafiltration, and these patients have extremely high mortality (52).
Dialysis Disequilibrium Syndrome
Dialysis disequilibrium syndrome (DDS) is one of the complications that can occur after initiating patients on RRT due to rapid shifts of solutes, although CRRT has been postulated to have slower clearance of solutes, thereby decreasing the risks of DDS; however, there have been a few case reports of DDS occurring in patients receiving CRRT (53). Education on overriding the alarms is needed, with careful adjustment of electrolyte mixtures to prevent further electrolyte derangements, especially with commercially available solutions.
Metabolic Support/Nutritional Losses
Patients in the ICU are typically in a catabolic state and require a high intake of amino acids and micronutrients. In addition, most of these patients are hypoalbuminemic due to their critical illness but also as a consequence of their CRRT treatment. In addition to providing clearance for solute and ultrafiltration, the CRRT membranes also clear micronutrients and macronutrients. Consequently, patients on CRRT lose water-soluble amino acids. Nutritional losses represent a significant concern for patients on RRT. Careful administration of calories and nutrients in close coordination with the nutritionist would be desirable, and while switching modalities, the changing clearance of amino acids and micronutrients needs to be considered. KDIGO guidelines recommend a protein intake of up to 1.7 g/kg per day in patients on CRRT (49,54,55). We also need to adjust the addition of calories with citrate or lactate because they also provide extra calories (56).
Deconditioning
One of the barriers to patients receiving CRRT is delayed mobility due to being connected to machines in addition to their critical illness and often endotracheal intubation with mechanical ventilation. However, physical therapy in the ICU has been reported to improve outcomes and even physical functioning. Specifically in the setting of ongoing CRRT treatment, more recently there have been reports showing good safety profile and feasibility of physical therapy. In addition, when possible, the use of hybrid RRT may allow for early mobilization. As a reminder, any attempts to wean off CRRT early should be on the checklist of ICU rounding (57,58).
Drug Delivery and Clearance
Generally, for patients on intermittent RRT, the drug dosing is for a GFR of <10 ml/min per 1.73 m2; however, there is risk of inappropriate drug clearance with CRRT or prolonged intermittent RRT, especially antibiotics in septic patients, resulting in underdosing of antibiotics or any other medications (59). This is important especially if you are treating a patient with septic shock or status epilepticus. There is a paucity of data on individual clearance of medications by CRRT, and there is a different degree of clearance for individual drugs on the basis of the modality and dose of CRRT, volume of distribution, sieving coefficient, and protein binding of the drugs. In addition, the medication dosing can be estimated by multiplying the effluent with (1-protein binding) and adjusting for prefilter dilution (60,61).
Conclusions
CRRT plays a very important role in the modern ICU, and we need to be mindful about the common complications observed with this renal replacement modality and how to mitigate some more difficult to avoid complications associated with CRRT. Often, the patients have a septic profile with increasing comorbidities. There needs to be more vigilance in nutritional support and volume management. Although CRRT has been around for a few decades, there is a need to utilize safety and quality mechanisms to standardize the care, undergo root cause analysis, and collaborate with different types of ICUs (55). Ultimately, excellent coordination with multidisciplinary teams, including nurses, pharmacists, nutritionists, and intensivists, is key to the success of CRRT in the modern ICU setting.
Disclosures
S.C. Gautam reports ownership interest in BNGO, Criper, Invitae (stockholder), Sensonics, and Pacific Biosciences. B.G. Jaar reports honoraria from the American Board of Internal Medicine—Nephrology; patents or royalties from UpToDate; and an advisory or leadership role for the American Board of Internal Medicine, BMC Medicine, BMC Nephrology, the Clinical Journal of the American Society of Nephrology, and the National Kidney Foundation. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
S.C. Gautam and B.G. Jaar were responsible for the conceptualization; S.C. Gautam and J. Lim wrote the original draft of the manuscript; S.C. Gautam and B.G. Jaar reviewed and edited the manuscript; and B.G. Jaar was responsible for supervision.
References
- 1.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. 10.1681/ASN.2007080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding JL, Li Y, Burrows NR, Bullard KM, Pavkov ME: US trends in hospitalizations for dialysis-requiring acute kidney injury in people with versus without diabetes. Am J Kidney Dis 75: 897–907, 2020. 10.1053/j.ajkd.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saugel B, Scheeren TWL, Teboul JL: Ultrasound-guided central venous catheter placement: A structured review and recommendations for clinical practice. Crit Care 21: 225, 2017. 10.1186/s13054-017-1814-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parienti JJ, Thirion M, Mégarbane B, Souweine B, Ouchikhe A, Polito A, Forel JM, Marqué S, Misset B, Airapetian N, Daurel C, Mira JP, Ramakers M, du Cheyron D, Le Coutour X, Daubin C, Charbonneau P; Members of the Cathedia Study Group : Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: A randomized controlled trial. JAMA 299: 2413–2422, 2008. 10.1001/jama.299.20.2413 [DOI] [PubMed] [Google Scholar]
- 5.Geerts W: Central venous catheter-related thrombosis. Hematology (Am Soc Hematol Educ Program) 2014: 306–311, 2014. 10.1182/asheducation-2014.1.306 [DOI] [PubMed] [Google Scholar]
- 6.Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, Kiaii M, Lok C, Luscombe R, Moist L, Oliver M, MacRae J; Canadian Society of Nephrology Vascular Access Work Group : Hemodialysis tunneled catheter-related infections. Can J Kidney Health Dis 3: 2054358116669129, 2016. 10.1177/2054358116669129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP; National Kidney Foundation : KDOQI clinical practice guideline for vascular access: 2019 update [published correction appears in Am J Kidney Dis 77: 551, 2021 10.1053/j.ajkd.2021.02.002]. Am J Kidney Dis 75: S1–S164, 2020. 10.1053/j.ajkd.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 8.Ng YH, Ganta K, Davis H, Pankratz VS, Unruh M: Vascular access site for renal replacement therapy in acute kidney injury: A post hoc analysis of the ATN study. Front Med (Lausanne) 4: 40, 2017. 10.3389/fmed.2017.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benichou N, Lebbah S, Hajage D, Martin-Lefèvre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Schortgen F, Tubach F, Ricard JD, Dreyfuss D, Gaudry S: Vascular access for renal replacement therapy among 459 critically ill patients: A pragmatic analysis of the randomized AKIKI trial. Ann Intensive Care 11: 56, 2021. 10.1186/s13613-021-00843-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhoundi A, Singh B, Vela M, Chaudhary S, Monaghan M, Wilson GA, Dillon JJ, Cartin-Ceba R, Lieske JC, Gajic O, Kashani K: Incidence of adverse events during continuous renal replacement therapy. Blood Purif 39: 333–339, 2015. 10.1159/000380903 [DOI] [PubMed] [Google Scholar]
- 11.Mendu ML, May MF, Kaze AD, Graham DA, Cui S, Chen ME, Shin N, Aizer AA, Waikar SS: Non-tunneled versus tunneled dialysis catheters for acute kidney injury requiring renal replacement therapy: A prospective cohort study. BMC Nephrol 18: 351, 2017. 10.1186/s12882-017-0760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokubo K, Kurihara Y, Kobayashi K, Tsukao H, Kobayashi H: Evaluation of the biocompatibility of dialysis membranes. Blood Purif 40: 293–297, 2015. 10.1159/000441576 [DOI] [PubMed] [Google Scholar]
- 13.Finkel KW, Podoll AS: Complications of continuous renal replacement therapy. Semin Dial 22: 155–159, 2009. 10.1111/j.1525-139X.2008.00550.x [DOI] [PubMed] [Google Scholar]
- 14.Yagi N, Leblanc M, Sakai K, Wright EJ, Paganini EP: Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis 32: 1023–1030, 1998. 10.1016/S0272-6386(98)70078-2 [DOI] [PubMed] [Google Scholar]
- 15.Robert R, Méhaud JE, Timricht N, Goudet V, Mimoz O, Debaene B: Benefits of an early cooling phase in continuous renal replacement therapy for ICU patients. Ann Intensive Care 2: 40, 2012. 10.1186/2110-5820-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macedo E, Mehta RL: Continuous dialysis therapies: Core curriculum 2016. Am J Kidney Dis 68: 645–657, 2016. 10.1053/j.ajkd.2016.03.427 [DOI] [PubMed] [Google Scholar]
- 17.Oudemans-van Straaten HM: Citrate anticoagulation for continuous renal replacement therapy in the critically ill. Blood Purif 29: 191–196, 2010. 10.1159/000245646 [DOI] [PubMed] [Google Scholar]
- 18.Zarbock A, Küllmar M, Kindgen-Milles D, Wempe C, Gerss J, Brandenburger T, Dimski T, Tyczynski B, Jahn M, Mülling N, Mehrländer M, Rosenberger P, Marx G, Simon TP, Jaschinski U, Deetjen P, Putensen C, Schewe JC, Kluge S, Jarczak D, Slowinski T, Bodenstein M, Meybohm P, Wirtz S, Moerer O, Kortgen A, Simon P, Bagshaw SM, Kellum JA, Meersch M; RICH Investigators and the Sepnet Trial Group : Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: A randomized clinical trial. JAMA 324: 1629–1639, 2020. 10.1001/jama.2020.18618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW: Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: A meta-analysis of randomized controlled trials. Am J Kidney Dis 59: 810–818, 2012. 10.1053/j.ajkd.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 20.Gerss J, Meersch M, Kindgen-Milles D, Brandenburger T, Willam C, Kellum JA, Zarbock A: The effect of filter lifespan during continuous renal replacement therapy in critically ill patients with acute kidney injury on the rate of new-onset infection: Analysis from the RICH randomized controlled trial. Am J Respir Crit Care Med 206: 511–514, 2022. 10.1164/rccm.202201-0063LE [DOI] [PubMed] [Google Scholar]
- 21.Schneider AG, Journois D, Rimmelé T: Complications of regional citrate anticoagulation: Accumulation or overload? Crit Care 21: 281, 2017. 10.1186/s13054-017-1880-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindgen-Milles D, Amman J, Kleinekofort W, Morgera S: Treatment of metabolic alkalosis during continuous renal replacement therapy with regional citrate anticoagulation. Int J Artif Organs 31: 363–366, 2008. 10.1177/039139880803100414 [DOI] [PubMed] [Google Scholar]
- 23.Chua HR, Schneider AG, Baldwin I, Collins A, Ho L, Bellomo R: Phoxilium vs Hemosol-B0 for continuous renal replacement therapy in acute kidney injury. J Crit Care 28: 884, 2013. 10.1016/j.jcrc.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 24.Davenport A, Tolwani A: Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus 2: 439–447, 2009. 10.1093/ndtplus/sfp136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Droege CA, Ernst NE, Messinger NJ, Burns AM, Mueller EW: Evaluation of thrombocytopenia in critically ill patients receiving continuous renal replacement therapy. Ann Pharmacother 52: 1204–1210, 2018. 10.1177/1060028018779200 [DOI] [PubMed] [Google Scholar]
- 26.Remuzzi A, Boccardo P, Benigni A: In vitro platelet adhesion to dialysis membranes. Nephrol Dial Transplant 6: 36–39, 1991 [PubMed] [Google Scholar]
- 27.Mulder J, Tan HK, Bellomo R, Silvester W: Platelet loss across the hemofilter during continuous hemofiltration. Int J Artif Organs 26: 906–912, 2003. 10.1177/039139880302601006 [DOI] [PubMed] [Google Scholar]
- 28.Griffin BR, Jovanovich A, You Z, Palevsky P, Faubel S, Jalal D: Effects of baseline thrombocytopenia and platelet decrease following renal replacement therapy initiation in patients with severe acute kidney injury. Crit Care Med 47: e325–e331, 2019. 10.1097/CCM.0000000000003598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Pont AC, Bouman CS, Bakhtiari K, Schaap MC, Nieuwland R, Sturk A, Hutten BA, de Jonge E, Vroom MB, Meijers JC, Büller HR: Predilution versus postdilution during continuous venovenous hemofiltration: A comparison of circuit thrombogenesis. ASAIO J 52: 416–422, 2006. 10.1097/01.mat.0000227733.03278.5f [DOI] [PubMed] [Google Scholar]
- 30.Griffin BR, Ten Eyck P, Faubel S, Jalal D, Gallagher M, Bellomo R: Platelet decreases following continuous renal replacement therapy initiation as a novel risk factor for renal nonrecovery. Blood Purif 51: 559–566, 2021. 10.1159/000517232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin BR, Wu C, O’Horo JC, Faubel S, Jalal D, Kashani K: The association of platelet decrease following continuous renal replacement therapy initiation and increased rates of secondary infections. Crit Care Med 49: e130–e139, 2021. 10.1097/CCM.0000000000004763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bierer P, Holt AW, Bersten AD, Plummer JL, Chalmers AH: Haemolysis associated with continuous venovenous renal replacement circuits. Anaesth Intensive Care 26: 272–275, 1998. 10.1177/0310057X9802600307 [DOI] [PubMed] [Google Scholar]
- 33.Pschowski R, Briegel S, Von Haehling S, Doehner W, Bender TO, Pape UF, Hasper D, Jörress A, Schefold JC: Effects of dialysis modality on blood loss, bleeding complications and transfusion requirements in critically ill patients with dialysis-dependent acute renal failure. Anaesth Intensive Care 43: 764–770, 2015. 10.1177/0310057X1504300615 [DOI] [PubMed] [Google Scholar]
- 34.Pistolesi V, Zeppilli L, Fiaccadori E, Regolisti G, Tritapepe L, Morabito S: Hypophosphatemia in critically ill patients with acute kidney injury on renal replacement therapies. J Nephrol 32: 895–908, 2019. 10.1007/s40620-019-00648-5 [DOI] [PubMed] [Google Scholar]
- 35.Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, Sun S: Citrate versus heparin anticoagulation for continuous renal replacement therapy: An updated meta-analysis of RCTs. Intensive Care Med 41: 2098–2110, 2015. 10.1007/s00134-015-4099-0 [DOI] [PubMed] [Google Scholar]
- 36.Heung M, Mueller BA: Prevention of hypophosphatemia during continuous renal replacement therapy—An overlooked problem. Semin Dial 31: 213–218, 2018. 10.1111/sdi.12677 [DOI] [PubMed] [Google Scholar]
- 37.Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ; US Acute Liver Failure Study Group : Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 67: 711–720, 2018. 10.1002/hep.29488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki S, Egi M, Schneider AG, Bellomo R, Hart GK, Hegarty C: Hypophosphatemia in critically ill patients. J Crit Care 28: 536, 2013. 10.1016/j.jcrc.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008. 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demirjian S, Teo BW, Guzman JA, Heyka RJ, Paganini EP, Fissell WH, Schold JD, Schreiber MJ: Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant 26: 3508–3514, 2011. 10.1093/ndt/gfr075 [DOI] [PubMed] [Google Scholar]
- 41.Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G: Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand 55: 39–45, 2011. 10.1111/j.1399-6576.2010.02338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ting S, Chua HR, Cove ME: Euglycemic ketosis during continuous kidney replacement therapy with glucose-free solution: A report of 8 cases. Am J Kidney Dis 78: 305–308, 2021. 10.1053/j.ajkd.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 43.Sriperumbuduri S, Clark E, Biyani M, Ruzicka M: High anion gap metabolic acidosis on continuous renal replacement therapy. Kidney Int Rep 5: 1833–1835, 2020. 10.1016/j.ekir.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zakharchenko M, Leden P, Rulíšek J, Los F, Brodska H, Balik M: Ionized magnesium and regional citrate anticoagulation for continuous renal replacement therapy. Blood Purif 41: 41–47, 2016. 10.1159/000440972 [DOI] [PubMed] [Google Scholar]
- 45.Rosner MH, Connor Jr MJ: Management of severe hyponatremia with continuous renal replacement therapies. Clin J Am Soc Nephrol 13: 787–789, 2018. 10.2215/CJN.13281117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neyra JA, Ortiz-Soriano VM, Ali D, Morris PE, Johnston CM: A multidisciplinary approach for the management of severe hyponatremia in patients requiring continuous renal replacement therapy. Kidney Int Rep 4: 59–66, 2018. 10.1016/j.ekir.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yessayan LT, Szamosfalvi B, Rosner MH: Management of dysnatremias with continuous renal replacement therapy. Semin Dial 34: 472–479, 2021. 10.1111/sdi.12983 [DOI] [PubMed] [Google Scholar]
- 48.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179–c184, 2012. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group : Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013. 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venkataraman R, Kellum JA, Palevsky P: Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care 17: 246–250, 2002. 10.1053/jcrc.2002.36757 [DOI] [PubMed] [Google Scholar]
- 51.Slessarev M, Salerno F, Ball IM, McIntyre CW: Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial Int 23: 325–332, 2019. 10.1111/hdi.12760 [DOI] [PubMed] [Google Scholar]
- 52.Dorairajan S, Chockalingam A, Misra M: Myocardial stunning in hemodialysis: What is the overall message? Hemodial Int 14: 447–450, 2010. 10.1111/j.1542-4758.2010.00495.x [DOI] [PubMed] [Google Scholar]
- 53.Tuchman S, Khademian ZP, Mistry K: Dialysis disequilibrium syndrome occurring during continuous renal replacement therapy. Clin Kidney J 6: 526–529, 2013. 10.1093/ckj/sft087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashani K, Rosner MH, Haase M, Lewington AJP, O’Donoghue DJ, Wilson FP, Nadim MK, Silver SA, Zarbock A, Ostermann M, Mehta RL, Kane-Gill SL, Ding X, Pickkers P, Bihorac A, Siew ED, Barreto EF, Macedo E, Kellum JA, Palevsky PM, Tolwani AJ, Ronco C, Juncos LA, Rewa OG, Bagshaw SM, Mottes TA, Koyner JL, Liu KD, Forni LG, Heung M, Wu VC: Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 14: 941–953, 2019. 10.2215/CJN.01250119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rewa OG, Tolwani A, Mottes T, Juncos LA, Ronco C, Kashani K, Rosner M, Haase M, Kellum J, Bagshaw SM; ADQI Consensus Meeting Members on behalf of ADQI XXII : Quality of care and safety measures of acute renal replacement therapy: Workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care 54: 52–57, 2019. 10.1016/j.jcrc.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 56.Rogers AR, Jenkins B: Calorie provision from citrate anticoagulation in continuous renal replacement therapy in critical care. J Intensive Care Soc 22: 183–186, 2021. 10.1177/1751143720937451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer KP, Joseph-Isang E, Robinson LE, Parry SM, Morris PE, Neyra JA: Safety and feasibility of physical rehabilitation and active mobilization in patients requiring continuous renal replacement therapy: A systematic review. Crit Care Med 48: e1112–e1120, 2020. 10.1097/CCM.0000000000004526 [DOI] [PubMed] [Google Scholar]
- 58.Mayer KP, Hornsby AR, Soriano VO, Lin TC, Cunningham JT, Yuan H, Hauschild CE, Morris PE, Neyra JA: Safety, feasibility, and efficacy of early rehabilitation in patients requiring continuous renal replacement: A quality improvement study. Kidney Int Rep 5: 39–47, 2019. 10.1016/j.ekir.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Awdishu L, Bouchard J: How to optimize drug delivery in renal replacement therapy. Semin Dial 24: 176–182, 2011. 10.1111/j.1525-139X.2011.00826.x [DOI] [PubMed] [Google Scholar]
- 60.Jang SM, Infante S, Abdi Pour A: Drug dosing considerations in critically ill patients receiving continuous renal replacement therapy. Pharmacy (Basel) 8: 18, 2020. 10.3390/pharmacy8010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang SM, Awdishu L: Drug dosing considerations in continuous renal replacement therapy. Semin Dial 34: 480–488, 2021. 10.1111/sdi.12972 [DOI] [PubMed] [Google Scholar]