The term “AKI” has evolved considerably since its development almost 60 years in 1964. AKI has gone through more than 30 definitions that have considered serum creatinine (sCr) and occasionally urine output. A rise in sCr value is an excellent biomarker to identify late changes in kidney function but not to detect an early injury or to identify structural damage (subclinical AKI). We know the strengths and weaknesses of sCr; it is the “Janus faces” for nephrologists when talking about AKI, hated and loved for decades. However, despite many weaknesses as a biomarker, it is still the most used test to identify kidney injury globally; it is inexpensive, available in most places, and a known marker of kidney injury not only by nephrologists but also by other health care professionals. It is unlikely that we will stop using sCr in the upcoming years (1).
For this reason, the use of novel biomarkers was proposed to improve the timely detection of early AKI, improve the differential diagnosis and prognostic assessment, provide early interventions, and improve its management. Different biomarkers with greater sensitivity and specificity have been discovered, including proteomics, which identifies changes in the metabolism of different zones of the nephron earlier in the event of a kidney insult. Such biomarkers have been a subject of intense ongoing interest (Table 1).
Table 1.
Serum creatinine, biomarkers, and its relationship between different AKI scenarios
| AKI Scenarios | Serum Creatinine | Biomarker | Example |
|---|---|---|---|
| Kidney stress | ✔ | ✔ | Cr: identifies patients with mild CKD who are most at risk for developing AKI |
| Biom: revealed when at risk of AKI | |||
| Subclinical AKI | × | ✔ | Cr: after the insult it takes up to 48 h to rise |
| Biom: some rises in the first hours | |||
| AKI diagnosis | ✔ | × | Cr: the diagnosis of AKI by KDIGO is made by an increase in serum creatinine and a decrease in urinary output |
| Biom: the ADQI group proposes to add biomarkers to the classification, not yet incorporated into KDIGO guideline | |||
| Prediction of severe AKI (2,3) | × | ✔ | Cr: does not identify which patient progressed to severe AKI |
| Biom: Nephrochek >0.3 and NGAL >450 ng/ml predicts AKI severity | |||
| Start KRT | × | × | Cr: does not identify which patient should start KRT |
| Biom: does not identify which patient should start KRT | |||
| Stop KRT | × | × | Cr: does not identify when to stop KRT |
| Biom: does not identify when to stop KRT | |||
| Acute tubular necrosis | × | × | Cr: does not differentiate ATN from other etiologies |
| Biom: does not differentiate ATN from other etiologies | |||
| Acute interstitial nephritis | × | ✔ | Cr: does not differentiate ATN from other etiologies |
| Biom: high values of TNF-α and IL-9 may identify AIN | |||
| Contrast-associated nephropathy | ✔ | ✔ | Cr: increases 12 h after contrast application |
| Biom: Cystatin C rises earlier and is more sensitive than creatinine | |||
| Sepsis-associated AKI | × | ✔ | Cr: reduced production of creatinine during sepsis |
| Biom: NGAL could have better performance identifying AKI than creatinine | |||
| Proximal tubular damage | × | ✔ | Cr: does not identify proximal tubular damage |
| Biom: Cystatin C, IL-18, NGAL, L-FABP, could identify proximal tubular damage | |||
| Kidney function improvement after AKI | × | ✔ | Cr: does not identify renal repair or improvement |
| Biom: KIM-1, NGAL, and NephroCheck have been shown to be associated with kidney improvement or repair | |||
| AKD or CKD progression from AKI | ✔ | ✔ | Cr: creatinine values have been associated with progression to AKD and CKD |
| Biom: KIM-1, Ang, NGAL, and NephroCheck have been associated with progression to CKD | |||
| Guide therapy | × | × | Cr: its values do not guide management or treatment |
| Biom: there is still not enough evidence to guide management or treatment due to its elevation | |||
| Availability | ✔ | × | Cr: universal availability and wide acceptance by health personnel |
| Biom: not available in many places, low acceptance by health personnel | |||
| Cost | ✔ | × | Cr: cheap and affordable |
| Biom: expensive |
ADQI, Acute Disease Quality Initiative; AIN, acute interstitial nephritis; AKI, acute kidney injury; AKD, acute kidney disease; Ang, angiotensin; ATN, acute tubular necrosis; Biom, biomarker; Cr, creatinine; KIM-1, kidney injury molecule 1; L-FABP, liver-type fatty acid–binding protein; NGAL, neutrophil gelatinase-associated lipocalin; KRT, kidney replacement therapy.
These novel biomarkers of structural AKI (subclinical AKI) were expected to provide critical diagnostic and prognostic stratification and complement sCr and urine output as proposed recently by the 10th Acute Disease Quality Initiative consensus meeting (2). Although several of these novel biomarkers have been assessed in diverse populations and various clinical scenarios, their implementation in routine clinical practice has not been embraced. Comparison to an imperfect gold standard (sCr), the unclear effect of CKD on biomarker performance and the use in different pathophysiologic disease processes (nephrotoxins, hemodynamic, sepsis-associated AKI, etc.) have delayed their use in clinical practice (3–5). Another important aspect that must be considered is that most novel AKI biomarkers studied so far have been measured in urine. Measuring biomarkers in the urine has some advantages, including being noninvasive, the reduced number of interfering proteins, and the increased specificity for kidney injury. However, disadvantages include the lack of samples from patients with severe oliguria and potential changes in urinary biomarker concentrations induced by the hydration status and diuretic therapy (6). A commonly used correction factor for urinary dilution is to express urinary biomarkers adjusted for urinary creatinine concentration in research studies. However, this correction may be inaccurate in the situation of AKI because creatinine production may be reduced in some forms of AKI, and both plasma and urine creatinine kinetics are significantly altered in the early phases of AKI (7). Although timed urine collection is a more accurate method to assess urinary biomarkers, in acute care settings, this is usually difficult. Indexing or adjusting spot urinary biomarker concentrations for urine creatinine concentration would not alter their prognostication of outcomes, but more studies are needed (8).
One of the great problems with biomarkers has been their indiscriminate use to study different types of AKI. We know that AKI is a heterogeneous syndrome with different presentations and does not usually follow a specific pattern. Its phenotype changes according to diverse etiologies and comorbidities; therefore, it is unlikely that a single biomarker will capture all clinical scenarios of AKI. It is more likely that a more complex, multicomponent predictive biomarker system will be required to implement biomarkers in routine clinical practice successfully. Ideally, these biomarkers would be able to detail the severity of AKI, the likelihood of AKI progressing to more severe stages or the need for kidney replacement therapy (KRT), the likelihood of renal recovery, and overall prognosis (9).
Biomarkers may pinpoint the site or mechanism of injury and in doing so may lead to targeted pharmacotherapy (10). The discovery of specific biomarkers of kidney tubular and glomerular function may help better understand the pathophysiologic process and determine the component and location of the injury. On the basis of this assumption, pairing biomarkers that identify different sites of injury or dysfunction can be useful in clinical practice. A great example is pairing of tissue inhibitor of metalloproteinases 2 and IGF binding protein 7, which together improved the area under the curve (AUC) for the detection of AKI over single biomarkers. Other examples exist such as the combined use of IL-18 and kidney injury molecule 1. A recent study in 32 patients with established acute interstitial nephritis by kidney biopsy reviewed by three pathologists independently, showed that urine TNF-α and IL-9 were consistently higher in patients with acute interstitial nephritis compared with those with other diagnoses, including acute tubular injury, glomerular diseases, and diabetic kidney disease, and those without any kidney disease (11). The combination of biomarkers, even after controlling for blood eosinophils, leukocyturia, and proteinuria, improved over clinicians’ prebiopsy diagnosis. In drug-induced interstitial nephritis, a combination of urinary biomarkers correlated and was predictive of the gradated severity of acute lesions (12).
As we have mentioned previously, the holy grail of biomarkers would be their use for improving our management, leading to change the clinical course of AKI and better outcomes. Unfortunately, there is still a lack of consistent evidence to show that they can be used in the therapeutic decision-making process. In different cohorts, the use of biomarkers to guide AKI treatment has been tested with controversial results. For example, significant effort has been made to incorporate neutrophil gelatinase-associated lipocalin (NGAL) in the KRT decision-making process. In the early versus late initiation of KRT in critically ill patients with AKI ELAIN study, patients with stage 2 AKI were randomized to early versus delayed start of KRT, excluding patients with plasmatic NGAL <150 ng/dl. The authors tried to select cases with a higher probability of having severe AKI, avoiding treating patients with KRT who may otherwise spontaneously recovered kidney function; it is worth mentioning that most patients had values >400 ng/dl (13). In a recent systematic review and meta-analysis including all trials evaluating biomarker performance for the prediction of KRT in AKI, the pooled AUCs for urine and blood NGAL were 0.72 (95% confidence interval [95% CI], 0.64 to 0.8) and 0.76 (95% CI, 0.71 to 0.8), respectively (14). Some biomarkers have reasonable potential to aid clinical decision making regarding when to start KRT in AKI. However, we consider that the current strength of evidence would essentially preclude their routine use pending further validation studies.
Implementation studies using biomarkers to identify high-risk patients have been published recently. In the PrevAKI trial, the authors examined the feasibility of implementing the Kidney Disease Improving Global Outcomes (KDIGO) bundle of care in high-risk patients undergoing cardiac surgery identified by urinary biomarkers (NephroCheck). The occurrence of moderate and severe AKI was significantly lower in the intervention group compared with the control group (14% versus 24%; absolute risk reduction of 10% [95% CI, 0.9 to 19.1]; P=0.03) (15). In the pediatric population, the Nephrotoxic Injury Negated by Just-in time Action (NINJA) program is a good example of the use of biomarkers to identify high-risk populations coupled with a systematic approach that could improve AKI care. In this study, investigators observed a significant and sustained 24% decrease in nephrotoxic medication-associated AKI (16). Another clinical trial attempted to identify patients with a high risk of kidney-related complications in the emergency room using NephroCheck values >0.3 ([tissue inhibitor of metalloproteinases 2]×[IGF binding protein 7]) to evaluate if early treatment prevented the progression and severity of AKI. Patients were randomized either to nephrology-guided early intervention that consisted of corrections of glucose values, withdrawal of nephrotoxic drugs, and hemodynamic and fluid optimization, or to standard care (without nephrology intervention). Unfortunately, the frequency of patients developing AKI was similar in both groups, despite early nephrology intervention (17).
The combination of novel biomarkers with instruments designed for predicting persistent or for detection of severe AKI such as the renal angina index could improve the performance of this instrument as shown by Matsuura et al. A combination of the renal angina index and urinary liver-type fatty acid–binding protein also contributed greatly to stratifying higher-risk patients with severe AKI (18). Finally, novel biomarkers have been evaluated to enable prediction of persistence of renal dysfunction and renal nonrecovery. In the RUBY study, the urinary C-C motif chemokine ligand 14 (CCL14) was identified with the most predictive capacity of persistent stage 3 AKI, with an AUC of 0.83 (95% CI, 0.78 to 0.87) (19).
Currently, the only FDA-approved biomarker is NephroCheck, which is not available in several places, and its cost is not affordable in low-middle-income countries. In high-income countries such as the United States, coverage policy differences across insurance health plans have limited accessibility to their use, and patients may incur burdensome out-of-pocket costs, depending on their insurance plan benefits. This inequality may also create barriers to testing and contribute to health disparities. The variable cutoff values in published articles, risk of confounding by comorbidities, lack of standardization, availability, and high expenses are important barriers to access and sustainability of AKI biomarker implementation. These issues could explain why in an international survey of AKI management, 40% of nephrologists and intensivists have used novel biomarkers in routine clinical practice but only 23% used them for research purposes (20).
The synergistic role of biomarkers should be coupled with standard clinical parameters to improve the outcome for AKI patients (21). The main goal of novel biomarkers is not to replace clinical judgment or older markers but instead to add prediction value when applied together. Recently, urine NGAL (uNGAL) and sCr samples from intensive care unit admission were studied in 178 children. Patients with uNGAL+/sCr– had almost four-fold increased risk for all-stage day 3 AKI (≥KDIGO stage 1) compared with those with uNGAL–/sCr–. Compared with uNGAL–/sCr+, patients uNGAL+/sCr+ had 12-fold increased risk for severe day 3 AKI (≥KDIGO stage 2). The only patients to suffer all-stage day 3 AKI and mortality were uNGAL+ (3% uNGAL+/sCr–; 7% uNGAL+/sCr+) (22).
Establishing this multicomponent biomarker for a given clinical scenario will still require prospective validation in large cohorts, including patients with diverse AKI causes. In order to have a clinically useful predictive power through a multicomponent marker, it is likely that we will need first to decrease cost and expand the use of the available markers.
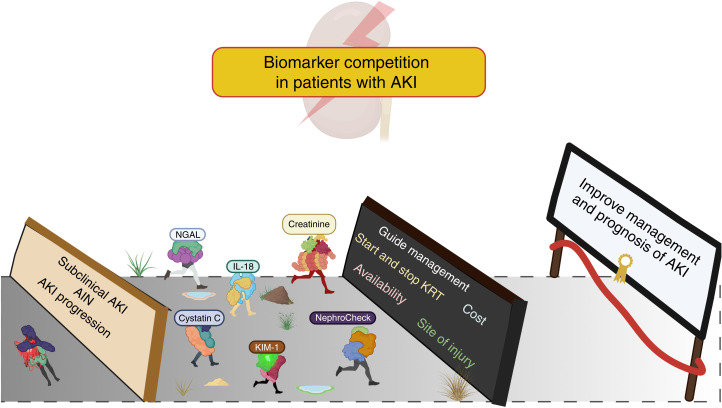
We believe that biomarkers could improve care in AKI, and despite their limitations and barriers to their clinical use, biomarkers will permit assessment of renal stress or injury before permanent damage occurs, allowing the implementation of a combination of therapies that may reverse the course or blunt the severity of kidney injury (Figure 1) (23,24). The association of early elevation of biomarkers with clinically meaningful outcomes such as early AKI, progression of AKI, and need for KRT is clear, and their application can potentially help to stratify patients with different pathophysiology and guide us on more appropriate and individualized therapies (25).
Figure 1.
Biomarker competition in AKI. There may be successful biomarkers among the current candidates for becoming an ideal biomarker and help improve early detection, management and outcomes of AKI. However, there are many barriers for their implementation like: availability, costs, unique set of AKI case-mix presentation and pathophysiology, the need of individualized panel of biomarkers for each setting, etc. Further research is needed to advance biomarkers to bedside.
Disclosures
R. Claure-Del Granado reports consultancy for Medtronic; honoraria for Nova Biomedical; and an advisory or leadership role for Acute Kidney Injury Committee of the Latin American Society of Nephrology and Hypertension (member); International Society of Nephrology (member at large of the executive committee). E. Macedo reports an advisory or leadership role for the International Society of Nephrology. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Author Contributions
R. Claure-Del Granado was responsible for the conceptualization; R. Claure-Del Granado, E. Macedo, and J.S. Chávez-Íñiguez reviewed and edited the manuscript; and all authors wrote the original draft of the manuscript.
References
- 1.Ostermann M, Joannidis M: Acute kidney injury 2016: Diagnosis and diagnostic workup. Crit Care 20: 299, 2016. 10.1186/s13054-016-1478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, Bell M, Forni L, Guzzi L, Joannidis M, Kane-Gill SL, Legrand M, Mehta R, Murray PT, Pickkers P, Plebani M, Prowle J, Ricci Z, Rimmelé T, Rosner M, Shaw AD, Kellum JA, Ronco C: Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative Consensus Conference: A consensus statement. JAMA Netw Open 3: e2019209, 2020. 10.1001/jamanetworkopen.2020.19209 [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Betensky RA, Emerson SC, Bonventre JV: Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 23: 13–21, 2012. 10.1681/ASN.2010111124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyner JL, Coca SG, Thiessen-Philbrook H, Patel UD, Shlipak MG, Garg AX, Parikh CR; Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium; Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury TRIBE-AKI Consortium : Urine biomarkers and perioperative acute kidney injury: The impact of preoperative estimated GFR. Am J Kidney Dis 66: 1006–1014, 2015. 10.1053/j.ajkd.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM: Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79: 1119–1130, 2011. 10.1038/ki.2010.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devarajan P, Murray P: Biomarkers in acute kidney injury: Are we ready for prime time? Nephron Clin Pract 127: 176–179, 2014. 10.1159/000363206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009. 10.1681/ASN.2008070669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wettersten N, Katz R, Shlipak MG, Scherzer R, Waikar SS, Ix JH, Estrella MM: Urinary biomarkers and kidney outcomes: Impact of indexing versus adjusting for urinary creatinine. Kidney Med 3: 546–554.e1, 2021. 10.1016/j.xkme.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Dodkins J, Doyle JF, Forni LG: Acute kidney injury biomarkers: What do they tell us? Contrib Nephrol 193: 21–34, 2018. 10.1159/000484960 [DOI] [PubMed] [Google Scholar]
- 10.Okusa MD, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Workgroup : Therapeutic targets of human AKI: Harmonizing human and animal AKI. J Am Soc Nephrol 27: 44–48, 2016. 10.1681/ASN.2015030233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moledina DG, Wilson FP, Pober JS, Perazella MA, Singh N, Luciano RL, Obeid W, Lin H, Kuperman M, Moeckel GW, Kashgarian M, Cantley LG, Parikh CR: Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4: e127456, 2019. 10.1172/jci.insight.127456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM: Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol 5: 1954–1959, 2010. 10.2215/CJN.02370310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315: 2190–2199, 2016. 10.1001/jama.2016.5828 [DOI] [PubMed] [Google Scholar]
- 14.Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, Joannidis M: Biomarkers for prediction of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Intensive Care Med 44: 323–336, 2018. 10.1007/s00134-018-5126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarbock A, Küllmar M, Ostermann M, Lucchese G, Baig K, Cennamo A, Rajani R, McCorkell S, Arndt C, Wulf H, Irqsusi M, Monaco F, Di Prima AL, García Alvarez M, Italiano S, Miralles Bagan J, Kunst G, Nair S, L’Acqua C, Hoste E, Vandenberghe W, Honore PM, Kellum JA, Forni LG, Grieshaber P, Massoth C, Weiss R, Gerss J, Wempe C, Meersch M: Prevention of cardiac surgery–associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: The PrevAKI-multicenter randomized controlled trial. Anesth Analg 133: 292–302, 2021. 10.1213/ANE.0000000000005458 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, Askenazi DJ, Henderson T, Dill L, Somers MJG, Kerr J, Gilarde J, Zaritsky J, Bica V, Brophy PD, Misurac J, Hackbarth R, Steinke J, Mooney J, Ogrin S, Chadha V, Warady B, Ogden R, Hoebing W, Symons J, Yonekawa K, Menon S, Abrams L, Sutherland S, Weng P, Zhang F, Walsh K: A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97: 580–588, 2020. 10.1016/j.kint.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Schanz M, Wasser C, Allgaeuer S, Schricker S, Dippon J, Alscher MD, Kimmel M: Urinary [TIMP-2]·[IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant 34: 1902–1909, 2019. 10.1093/ndt/gfy186 [DOI] [PubMed] [Google Scholar]
- 18.Matsuura R, Srisawat N, Claure-Del Granado R, Doi K, Yoshida T, Nangaku M, Noiri E: Use of the renal angina index in determining acute kidney injury. Kidney Int Rep 3: 677–683, 2018. 10.1016/j.ekir.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Haase M, Zacharowski K, Wunderink R, Heung M, Lissauer M, Self WH, Koyner JL, Honore PM, Prowle JR, Joannidis M, Forni LG, Kampf JP, McPherson P, Kellum JA, Chawla LS; RUBY Investigators : Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensive Care Med 46: 943–953, 2020. 10.1007/s00134-019-05919-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Digvijay K, Neri M, Fan W, Ricci Z, Ronco C: International survey on the management of acute kidney injury and continuous renal replacement therapies: Year 2018. Blood Purif 47: 113–119, 2019. 10.1159/000493724 [DOI] [PubMed] [Google Scholar]
- 21.Molitoris BA: Urinary biomarkers: Alone are they enough? J Am Soc Nephrol 26: 1485–1488, 2015. 10.1681/ASN.2014111145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanski N, Menon S, Goldstein SL, Basu RK: Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care 53: 1–7, 2019. 10.1016/j.jcrc.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 23.Malhotra R, Siew ED: Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 12: 149–173, 2017. 10.2215/CJN.01300216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzger J, Kirsch T, Schiffer E, Ulger P, Mentes E, Brand K, Weissinger EM, Haubitz M, Mischak H, Herget-Rosenthal S: Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int 78: 1252–1262, 2010. 10.1038/ki.2010.322 [DOI] [PubMed] [Google Scholar]
- 25.Guzzi LM, Bergler T, Binnall B, Engelman DT, Forni L, Germain MJ, Gluck E, Göcze I, Joannidis M, Koyner JL, Reddy VS, Rimmelé T, Ronco C, Textoris J, Zarbock A, Kellum JA: Clinical use of [TIMP-2]•[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: Guidance from an expert panel. Crit Care 23: 225, 2019. 10.1186/s13054-019-2504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]