Key Points
A pilot randomized controlled trial of integrated palliative and nephrology care in patients with CKD stage 5 not on dialysis is feasible.
A pilot randomized controlled trial of integrated palliative and nephrology care in patients with nondialysis CKD 5 is acceptable.
Participants in the integrated care arm had lower symptom burden scores at the end of the trial, whereas the control group had higher scores.
Keywords: geriatric and palliative nephrology, palliative care, pilot projects, randomized controlled trials
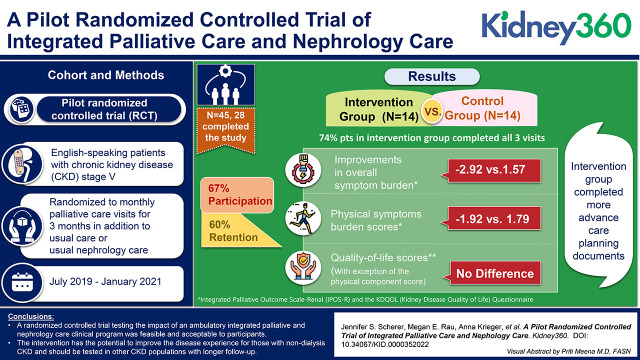
Visual Abstract
Abstract
Background
There has been a call by both patients and health professionals for the integration of palliative care with nephrology care, yet there is little evidence describing the effect of this approach. The objective of this paper is to report the feasibility and acceptability of a pilot randomized controlled trial testing the efficacy of integrated palliative and nephrology care.
Methods
English speaking patients with CKD stage 5 were randomized to monthly palliative care visits for 3 months in addition to their usual care, as compared with usual nephrology care. Feasibility of recruitment, retention, completion of intervention processes, and feedback on participation was measured. Other outcomes included differences in symptom burden change, measured by the Integrated Palliative Outcome Scale–Renal, and change in quality of life, measured by the Kidney Disease Quality of Life questionnaire and completion of advance care planning documents.
Results
Of the 67 patients approached, 45 (67%) provided informed consent. Of these, 27 patients completed the study (60%), and 14 (74%) of those in the intervention group completed all visits. We found small improvements in overall symptom burden (−2.92 versus 1.57) and physical symptom burden scores (−1.92 versus 1.79) in the intervention group. We did not see improvements in the quality-of-life scores, with the exception of the physical component score. The intervention group completed more advance care planning documents than controls (five health care proxy forms completed versus one, nine Medical Orders for Life Sustaining Treatment forms versus none).
Conclusions
We found that pilot testing through a randomized controlled trial of an ambulatory integrated palliative and nephrology care clinical program was feasible and acceptable to participants. This intervention has the potential to improve the disease experience for those with nondialysis CKD and should be tested in other CKD populations with longer follow-up.
Clinical Trials registry name and registration number
Pilot Randomized-controlled Trial of Integrated Palliative and Nephrology Care Versus Usual Nephrology Care, NCT04520984
Introduction
Patients with advanced CKD often have a high symptom burden that, when untreated, can negatively affect quality of life (1,2). In addition, mortality for older adults with kidney failure receiving hemodialysis can be as much as ten to 15 times higher than those not on dialysis (3). However, patients with kidney failure are less likely than those with other serious illnesses to engage in advance care planning, a process that includes discussions about future medical decisions and, often, end-of-life care (4,5). Palliative care is a specialty that assists with advance care planning and provides symptom relief for patients with serious illness (6). Importantly, it can be offered along with curative care at any stage of illness.
Integration of palliative care with CKD care has the potential to address the high symptom burden of the CKD population and their advance care planning needs (7). This possibility has been recognized by nephrology professional societies through recommendations for integrating palliative care principles into nephrology practice (8,9). Moreover, patient focus groups and other patient-centered research efforts have cited symptom management and psychosocial effects of kidney disease as research priorities (10–12). Despite these acknowledgments, palliative care is not typically integrated into nephrology practice, particularly in the United States. The reasons for this are multifactorial, including lack of palliative care training for nephrologists, a limited palliative care workforce, and an absence of consensus guidelines and established protocols for its delivery (13–17).
Clinical research testing the efficacy of palliative care in CKD care is needed to better understand the effect of palliative care in nephrology, and to determine the most effective way to deliver integrated care. Models of outpatient or home-based integrated nephrology and palliative care programs have been reported nationally and internationally (7,18–20). Oncology literature has described several models of integrated palliative and oncology care, citing outpatient palliative care as an opportunity to follow patients longitudinally throughout the disease trajectory (21). These models can be stand-alone clinics, embedded clinics, enhanced primary palliative care, or telehealth. We previously reported the feasibility of an ambulatory kidney palliative care program at New York University (NYU) Langone Health, named Kidney CARES (Comprehensive Advanced Renal disease and end stage kidney disease Support), to provide symptom management, advance care planning, and assistance with dialysis decision making for patients with advanced kidney disease, including those on dialysis (22). Qualitative analyses of interviews of patient and family attendees showed that the services offered in the program were valued and the clinical experience was positive (23).
To our knowledge, there are no randomized controlled trials testing the effectiveness of palliative care integrated with usual nephrology care (UC). In this paper, we report the feasibility and acceptability of a pilot randomized controlled trial designed to test the efficacy of integrated ambulatory palliative care with nephrology care versus UC on symptom burden, quality of life, and engagement in advance care planning, measured by completion of advance care planning documents, in patients with nondialysis CKD stage 5. As a pilot trial, we chose a focused patient population to conduct feasibility testing in a population with hypothetically similar needs. Patients with CKD stage 5, for the most part, are at a disease point where physical and emotional symptoms may already be present and shared decision making is imperative given the possible future need for kidney replacement therapy (KRT) (7). We hypothesized the study would be feasible and acceptable to participants and the intervention processes and data collection would be complete, allowing this trial to serve as a foundation for larger testing in a multisite randomized controlled trial with longer follow-up.
Materials and Methods
Study Design and Recruitment
This pilot study was a randomized controlled trial. Eligible patients were recruited before or after routine nephrology clinic visits between July 2019 and January 2021 by an unblinded research assistant who received training from the principal investigator and online resources in recruitment for palliative care research (24). Nephrologists’ approval of patient participation was required before approaching participants for informed consent, with the ability to decline participation on behalf of their patients. Consented participants were randomized at the time of consent in blocks of two or four to monthly palliative care visits for 3 months along with their nephrology care (integrated care arm) or to UC. At the conclusion of the trial, all UC participants were offered appointments with the palliative care clinic. The study was originally designed to enroll 30 patients; however, we increased enrollment after early recruitment success. The study ended due to staffing turnover and an assessment that we had met feasibility criteria through percentage of participants enrolled and retained. Patients were given a $50 gift card for baseline and exit survey visits.
Patient Population
Inclusion criteria included individuals who were English speaking, aged ≥18 years, had an eGFR of ≤15 ml/min per 1.73 m2, and received care from an NYU nephrologist. Exclusion criteria included non-English speakers, receipt of KRT, cognitive impairment/dementia, pregnant women, prior visit with palliative care, or having received a kidney transplant. Cognitive impairment or dementia was determined from the nephrologist before approaching for consent, during patient interview by the research assistant in consultation with the principal investigator, or by electronic health record problem lists. Patients remained in the trial if they were started on dialysis after providing informed consent.
Changes to Trial Design
Starting in April 2020, due to coronavirus disease 2019 (COVID-19), all procedures, including consenting, were adapted to electronic or telephonic means; intervention visits were offered through telehealth and timing for baseline surveys was changed to up to 90 days from consenting, with similar flexibility regarding scheduling of intervention visits. We chose not to analyze patient satisfaction data because it became apparent, after review by the study team, that the data collected pertaining to this domain were not reflective of patients’ specific experience with nephrology or palliative care.
Study Setting
This study took place at NYU’s Kidney CARES Program. Kidney CARES is an ambulatory kidney palliative clinic embedded in NYU’s faculty nephrology group practice (22,25). The clinic has been open since May of 2016 with office hours of 0.5 d/wk and is staffed by a nephrologist board certified in palliative care (J.S.S.).
Intervention
Patients randomized to the integrated care arm were scheduled for three monthly visits with the ambulatory kidney palliative care clinic. All attempts were made to coordinate visits with nephrology visits. The Kidney CARES visits were solely for palliative care. Care was integrated via consistent communications with the primary nephrologist and the presentation of Kidney CARES as an extension of their UC at the NYU nephrology clinic. The intervention visits were not standardized to allow for an organic patient-doctor relationship to form; however, an electronic kidney palliative care navigator tab was developed for our electronic health record to standardize recorded data at each visit. Additionally, a templated note (Supplemental Appendix 1) was used for all clinic sessions to record clinical information and visit activities (22). Categories of visit activities were determined on the basis of previous work and were documented by the provider (J.S.S.) in the visit note (22,26). Predefined categories were advance care planning (defined broadly as completing or reviewing advance care planning documents or engaging in an advance care planning discussion), assistance with coping with disease, symptom management, disease education, assistance with dialysis decision-making, and building rapport (defined as reviewing the life narrative of the patient). Standard palliative care was delivered during clinic visits, including symptom management, emotional support, spiritual assessment, or shared decision making regarding decisions about RRT and advance care planning (22). Symptom management included pharmacologic and nonpharmacologic interventions, such as referral to integrative health services or psychology.
UC
Patients randomized to the UC arm received care at the discretion of their nephrologists.
Primary Study Objective
Our primary objective was feasibility and acceptability of the trial.
Feasibility
We defined feasibility as the ability to recruit and retain patients, completion of intended study processes and protocols, and completion of planned data collection. Categories of visit activities served as exploratory data for mechanisms of results.
Study Acceptability
Acceptability of the intervention was measured by patient responses in recorded exit interviews (Supplemental Appendix 2) that asked about patients’ experiences with the trial and with the clinic.
Clinical Outcomes
Symptom Burden
Our primary clinical outcome was the difference in change of symptom burden between the two groups from baseline to the end of participation. We measured symptom burden using the Integrated Palliative Outcome Scale–Renal (IPOS-Renal) survey, a survey tool with psychometric validation in a patients with kidney disease (Cronbach α for internal consistency of 0.84) (27). The IPOS-Renal consists of ten questions (including one with 15 subquestions asking about physical symptoms), with a score range of 0–90. We report scores as total score (range, 0–90), physical symptom score (15 subquestions asking how a symptom has affected a patient over the last week; range of scores 0–4, with 0=not all and 4= severe, total score of 0–60), physical symptom burden (total number of all symptoms reported; score range 0–15), psychologic symptom score (four questions; range 0–4, total score of 0–16), and a communication and practical subscale (four questions; range 0–4 or 0–2, total score of 0–14). A change of four points has been reported as clinically meaningful in a population of patients who are seriously ill (28).
Quality of Life
We measured quality of life using the Kidney Disease Quality of Life 36 (KDQOL-36) survey (29,30). The KDQOL is a commonly used tool in kidney disease that consists of five subscales, each scored between zero (worst) and 100 (best). It has been validated in a CKD population (31). These scales include the Short-Form 12 (12-items), Physical Component Summary (PCS), Mental Component Summary, four items on the burden of kidney disease, 12 items on symptoms/problems, and eight items on the effects of kidney disease.
Completion of Advance Care Planning Documents
We defined this outcome as documentation of a health care proxy or surrogate form or an electronic Medical Order for Life Sustaining Treatment. This information was obtained through chart review.
Study Procedures
Baseline demographics (age, sex, race, ethnicity, eGFR, hemoglobin, albumin, Charlson Comorbidity Index, Karnofsky Performance Scale, etiology of kidney disease, and education level) were recorded upon study enrollment through patient interview and chart review. Survey data (IPOS-Renal and KDQOL) were collected at baseline and at three months, either in person or over the phone. All patients in the intervention arm were asked to complete a recorded interview about their experience with integrated care at the conclusion of their participation.
Statistical Analysis
Continuous variables are reported as mean± standard deviation (SD). Categoric variables are reported as n (%). Given this was a pilot study, we did not predetermine a sample size on the basis of power to detect differences and we did not test differences for significance. All analyses were performed using R (version 3.5.2).
This study adheres to the Declaration of Helsinki and was approved by the NYU Grossman School of Medicine’s Institutional Review Board and registered in clinical trials.gov (NCT04520984) after study onset; no changes in the protocol were made between study onset and registration.
Results
Feasibility
Patient Recruitment
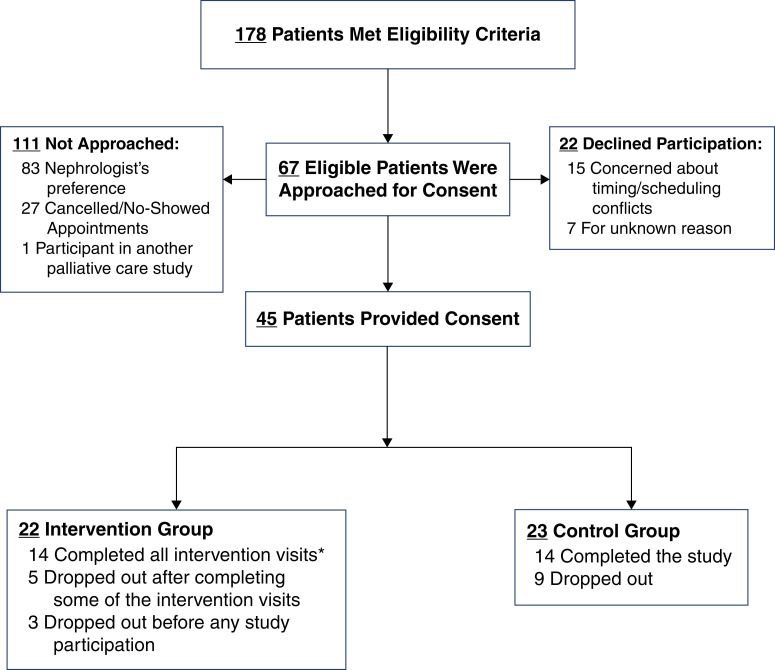
We identified 178 patients who met eligibility criteria (Supplemental Table 1). Of those, 111 (62%) were not approached for the following reasons: nephrologist request (n=83, 75%), not showing for their appointment or logistics (n=27, 24%), or participation in another palliative care study (n=1, 0.01%) (Figure 1). A total of 67 individuals (38%) were approached for consent. Of these, 45 patients (67%) provided informed consent. Twenty-two patients (33%) declined participation with reasons including patient/family concerns, scheduling concerns, or lack of follow-up after expressing interest. A total of 22 patients were randomized to the intervention group and 23 patients to the control group.
Figure 1.
Flow chart demonstating patient recruitment for the pilot trial. *One participant did not complete the exit interview.
Retention
Seventeen patients (38%) dropped out of the study; ten patients (22%) dropped out before any participation and seven (16%) after completing some portion of the study procedures, five in the intervention group and two in the control. Reasons for dropout included patients wanting to make an appointment with the palliative care clinic rather than participating in a trial (n=2), loss to follow-up (n=3), changed mind (n=7), “guinea-pig” fear (n=1), scheduling conflicts (n=1), death (n=1), deemed ineligible after rechecking eGFR (n=1), and for unknown reasons (n=1). Therefore, 27 patients (60%) completed the study.
Completion of Intervention and Data Collection
Fourteen patients (74%) in the intervention group completed all three visits. One person in the intervention group who attended all three visits did not complete the exit data collection visit.
Visit Activities
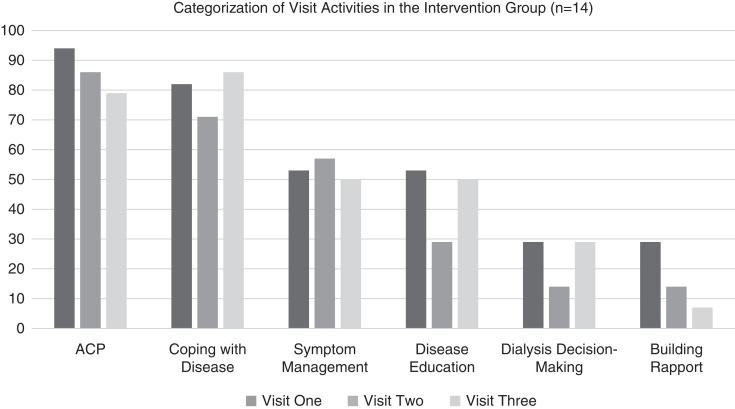
In most visits (79%–94%), advance care planning and coping with disease (71%–86%) was done. Symptom management was done in nearly half of all visits, whereas disease education was done in 50% of visits one and three, with dialysis decision-making recording in 29% of visits one and three and 14% of visits two (Figure 2).
Figure 2.
Categorization of visit activities in the intervention group. ACP, advance care planning.
Study Acceptability
The exit survey and interview were offered to the 14 patients randomized to the intervention arm. Of these, thirteen patients (93%) completed the survey and interview. Eleven respondents (85%) had never taken part in a research study before. Eight respondents (62%) said they did not find the process cumbersome, whereas 12 (92%) said they would participate again and would encourage others to participate. Eleven patients (92%) said the program was helpful for physical symptom management. Six participants (46%) said advance care planning was a novel process. Although six patients (46%) responded that discussions regarding of advance care planning were not desired, eight (62%) said asking about advance care planning did not make them uncomfortable. Eight participants (62%) found the clinic helpful with dialysis decision making, nine (69%) said it was helpful to explain conservative kidney management (CKM), whereas nine (69%) reported they had not heard of CKM before. Eleven patients (92%) said that the clinic was helpful to their spiritual well-being. Table 2 shows sample quotes from open-ended questions during their interview. Areas for improvement identified by patients included the presence of a translator and education on disease prevention. Some participants thought a patient support group may have been more effective than one-on-one sessions.
Table 2.
Baseline demographics of study patients
| Characteristic | Control (n=16) | Intervention (n=19) |
|---|---|---|
| Age (yr), mean (SD) | 68 (14) | 69 (11) |
| Sex, n (%) | ||
| Female | 8 (50) | 11 (58) |
| Male | 8 (50) | 8 (42) |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0.0) | 1 (5) |
| Asian | 0 (0.0) | 1 (5) |
| Black or African American | 6 (38) | 4 (21) |
| White | 10 (63) | 13 (68) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 0 (0) | 3 (16) |
| Non-Hispanic or Latino | 16 (100) | 16 (84) |
| Education, n (%) | ||
| Less than high school | 1 (7) | 0 (0) |
| High school/GED | 3 (21) | 9 (47) |
| College | 5 (36) | 4 (21) |
| Graduate/professional degree | 5 (36) | 6 (32) |
| Comorbidities, n (%) | ||
| Cancer (history of or in remission) | 3 (19) | 1 (5) |
| Congestive heart failure | 1 (6) | 0 (0) |
| Coronary artery disease | 3 (19) | 2 (11) |
| Diabetes | 5 (31) | 10 (53) |
| Liver disease | 1 (6) | 0 (0) |
| None of the above | 0 (0) | 3 (16) |
| Other | 3 (19) | 3 (16) |
| Charlson Comorbidity Index, mean (SD) | 6 (2) | 5 (2) |
| Karnofsky Performance Score, mean (SD) | 66 (26) | 78 (19) |
| GFR (ml/min per 1.73 m2), mean (SD) | 11 (2) | 11 (5) |
| Cause of kidney failure, n (%) | ||
| Diabetes | 4 (25) | 4 (21) |
| Glomerular disease | 2 (13) | 0 (0) |
| Hypertension | 5 (31) | 10 (53) |
| Other | 5 (31) | 5 (26) |
| Albumin (g/dl), mean (SD) | 3.8 (0.40) | 3.9 (0.4) |
| Hemoglobin (g/dl), mean (SD) | 9.5 (1.90) | 10.0 (1.6) |
| Lives alone, n (%) | 7 (50) | 8 (42) |
| Presence of a health care proxy, n (%) | 11 (79) | 11 (58) |
| Presence of advance care planning documents, n (%) | 5 (36) | 4 (21) |
GED, General Education Development Test.
Baseline Characteristics
Table 1 shows the baseline characteristics of all consented participants we collected baseline information from. Supplemental Table 1 shows the baseline data of all participants who completed the study. There were no significant differences in any baseline measures between UC and intervention participants. The mean age of both groups was >65, with the intervention group being approximately 1 year older than control. Most participants were white and non-Hispanic. There were no significant between-group differences in distributions of sex, self-reported race or ethnicity, or comorbidities. Patients had a Charlson Comorbidity Index of approximately five, with diabetes being the most common comorbidity. All patients had albumin levels >3 g/dl, but were anemic. The most common etiology of kidney disease recorded was hypertension. In both groups, most patients reported having a health care proxy at baseline, with a minority of patients having other advance care planning documents (Tables 1 and 2). Supplemental Table 1 shows the demographics of all patients who completed the study.
Table 1.
Representative quotes from exit interviews of participants in the intervention group
| Domain/Question | Quotes |
|---|---|
| Program value/usefulness | |
| What about the palliative care clinic was useful? Please rank the most important elements. Why were these things useful? | “It was helpful to clear things up about the illness. It opened up questions that had not been asked and allowed me to feel and question things myself.” |
| “The talks and conversations regarding kidney disease. I learned a lot in depth, how to apply the changes in my everyday life going forward.” | |
| “Thinking about and understanding DNR form and requirements.” | |
| Spiritual well-being | |
| Why was the palliative care clinic helpful to your spiritual well-being? | “I liked to have these surveys and these questions that doctors don’t usually ask.” |
| “Gave me reassurance to my expectations as to my direction with my health plan and kidney care/dialysis decision.” | |
| “I think it makes the end of life less scary.” | |
| What was it like to have someone ask you about your spirituality? | “It was a little different. I don’t want to say weird, but I think it was good. It made me think about how you have to stay grounded in your faith. Your spirituality never leaves you.” |
| “It was comforting and with concern in an expressive and caring way.” | |
| “It was good. I had no problem with that.” | |
| Feelings to advance care planning | |
| What did it mean to you to discuss these difficult topics (advance care planning) with the kidney palliative care team? | “It was comfortable. It was refreshing. It was mind opening.” |
| “I had always thought of it as something in the distance that doesn’t relate to me. It provided what I needed at the right time.” | |
| “It was good information and support. I was comfortable and they seemed interested.” | |
| Areas for improvement | |
| What about the palliative care clinic was not useful to you and why? | “I believe palliative care has to do with helping people understand and cope with their illness. I had already done much of the processing” |
| Is there anything that the palliative care clinic could have done to allow you to participate more fully? | “The sessions, which were one-on-one, were less effective than a possible group of patients offering ideas or support.” |
| What services would you have wanted that are not provided already? | “I believe that a group or collective exchange support would have been more effective, or perhaps educational presentations.” |
DNR, do not resuscitate.
Change in Symptom Burden
The intervention group had lower baseline IPOS-Renal scores than the UC group. The overall IPOS-Renal scores decreased for participants in the intervention group (−2.92±10.2), whereas scores increased for the control participants (1.57±10.5). Intervention participants also had lower physical symptom scores at the end of participation (−1.92±9.4), whereas the control participants had higher scores (1.79±6.7) (Table 3). The number of physical symptoms decreased in the intervention group and increased in the control participants (−1.36±4.0 versus 0.57±3.2; Tables 2 and 3).
Table 3.
Change in symptom burden in each treatment group between exit and baseline measurements
| IPOS Domain | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | Exit | Change | ||||
| Control (n=14) | Intervention (n=14) | Control (n=14) | Intervention (n=13)a | Control (Exit−Baseline) (n=14) | Intervention (Exit–Baseline) (n=13) | |
| Total score | 25.29 (16.05) | 21.21 (11.33) | 26.86 (15.52) | 18.31 (12.76) | 1.57 (10.49) | −2.92 (10.2) |
| IPOS total physical symptom burden score | 13.50 (10.17) | 12.50 (9.12) | 15.29 (10.39) | 10.38 (9.04) | 1.79 (6.70) | −1.92 (9.36) |
| IPOS physical symptom number score | 7.29 (4.46) | 7.14 (3.08) | 7.86 (4.38) | 5.79 (3.60) | 0.57 (3.18) | −1.36 (3.99) |
| Number of severe physical symptoms | 1.64 (2.47) | 1.57 (2.95) | 1.86 (2.14) | 0.93 (1.73) | 0.21 (1.48) | −0.64 (2.41) |
| Psychologic symptom burden, total score | 6.93 (3.75) | 5.36 (3.10) | 6.71 (3.91) | 4.92 (3.07) | −0.21 (3.31) | −0.38 (3.18) |
| Communication and practical questions total burden score | 4.86 (3.53) | 3.36 (2.87) | 4.86 (3.94) | 3.00 (3.11) | 0.00 (2.88) | −0.62 (2.43) |
IPOS, Integrated Palliative Outcome Scale.
One participant did complete all three visits but did not complete the exit data visit.
Change in Quality-of-Life Scores
With the exception of the PCS of the KDQOL, the intervention group had better quality-of-life scores at the start of the study. The PCS was the only component score that was higher in the intervention group at the conclusion of the study (5.81±8.80 versus −3.98±7.30 for control; P=0.004). All other domains worsened for all participants, except for the Mental Component Summary (Table 4).
Table 4.
Change in quality-of-life scores between exit and baseline measurements in each treatment group
| KDQOL Domain | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | Exit | Change | ||||
| Control (n=14) | Intervention (n=14) | Control (n=14) | Intervention (n=13)a | Control (Exit A−Baseline A) (n=14) | Intervention (Exit B–Baseline B) (n=13) | |
| Burden of kidney disease | 49.55 (36.15) | 57.59 (26.54) | 47.32 (35.16) | 52.88 (34.20) | −2.23 (16.74) | −5.77 (30.13) |
| Symptoms and problems of kidney disease | 75.27 (22.31) | 83.28 (12.63) | 70.06 (21.32) | 78.76 (17.77) | −5.21 (15.39) | −5.16 (17.90) |
| Effect of kidney disease on daily life | 65.85 (25.70) | 77.01 (18.77) | 59.38 (29.37) | 78.12 (21.35) | −6.47 (8.62) | −0.96 (23.26) |
| Physical Component Score | 37.41 (13.03) | 34.77 (11.34) | 33.43 (9.76) | 42.02 (9.82) | −3.98 (7.30) | 5.81 (8.80) |
| Mental Component Score | 43.09 (12.58) | 50.47 (9.89) | 43.74 (10.84) | 47.03 (11.30) | 0.66 (7.32) | −3.02 (9.03) |
The KDQOL consists of five parts, (Short-Form 12 consisting of a Mental Component Score, a Physical Component Score, burden of kidney disease, symptoms/problems, and effects of kidney disease), all with scores between zero and 100, with higher scores correlating with better well-being. KDQOL, Kidney Disease Quality of Life.
One participant did complete all three visits but did not complete the exit data visit.
Completion of Advance Care Planning Documents
Five patients (36%) in the intervention group completed health care proxies during their visits, with four (29%) completed on the first visit and one (7%) on the second visit. Nine patients (64%) in the intervention arm had electronic Medical Orders for Life Sustaining Treatment completed: four were done on visit three (29%), four on visit two (29%), and one on visit one (7%). In comparison, the only advance care planning in the control group was one patient who completed a health care proxy form while hospitalized during their engagement time with the study.
Discussion
We conducted the first pilot and feasibility testing of a randomized controlled trial of integrated ambulatory palliative and nephrology care for patients with patients with CKD stage 5 not on dialysis. We successfully enrolled 45 participants (67% participation), of which 27 completed the study (60% retention). We found that patients with CKD stage 5 not on dialysis were willing to participate in a palliative care–focused trial, a majority of those randomized to the intervention group completed the study, and that study processes were not viewed as burdensome by participants. Documentation of advance care planning increased in the intervention group and symptom burden scores were lowered in this group by the conclusion of participation.
As a feasibility trial, our findings identify barriers and facilitators for implementing a larger nephrology palliative care trial and provide useful experiences in recruitment, retention, data collection, and patient acceptability of clinical care and trial processes. The significance of feasibility studies in clinical trial research is their ability to prospectively recognize difficulties in protocols before larger trials and to determine if larger testing of research questions is valuable and worthwhile (32–35). In palliative care, feasibility studies are of particular importance given that serious illnesses often have unpredictable disease trajectories and present significant challenges to recruitment and retention (32). Hagen et al. (33) argue that a feasibility study of a proposed palliative care trial should be done before larger trial execution to identify barriers to implementation to be addressed before subsequent trials. Our findings support further study of the effect of palliative care in nephrology through larger trials with longer follow-up and expanded patient inclusion criteria, while also identifying elements to modify in future work.
Although we were able to demonstrate the ability to recruit and retain trial participants with nondialysis CKD stage 5 in an ambulatory care setting, we were also able to demonstrate flexibility in our protocol and intervention by accommodating the shift to telehealth due to COVID-19. We do recognize that our population was one free of the time burden inherent to dialysis therapies; however, it is likely that they were at a disease point where more frequent medical visits were competing factors for time. Nonetheless, intervention completion rates were high. Importantly, our pilot estimates of intervention efficacy were consistent with improvement in both in the IPOS-Renal measurement and the PCS component of the KDQOL survey. Our analysis of visit activities showed that the clinic delivered symptom management, disease education, and assistance with coping with disease and advance care planning in nearly half or more of visits, allowing us to identify possible mechanisms of our results for further exploration. Additionally, participation was not viewed as burdensome, suggesting there can be adequate data collection in future work. One note of caution, however, is that baseline IPOS-Renal physical symptom burden scores were lower than what has previously been reported (22,36), possibly reflecting a healthier population in our study—although the scores were still consistent with a high symptom burden in absolute terms. Our patient acceptability data shows that our ambulatory care model that integrates palliative care and nephrology is viewed positively by patients in addition to acceptability of the trial itself. Patients welcomed clinic procedures, viewed them as valuable, and would recommend others to participate in similar trials. Comments favoring a focus on spirituality and education about CKM were positive notations we did not anticipate, and we will measure this aspect in future trials.
This trial had several limitations and identified barriers. It was conducted in a single center with a specialized kidney palliative care program, with an intervention delivered by a single palliative care and nephrology trained provider (J.S.S.), limiting its generalizability and not allowing us to test fidelity to the intervention across multiple providers. The majority of participants were White and non-Hispanic, which is not representative of the typical American patient with kidney disease. Furthermore, our participants were healthier than what we observed in our nonstudy patients (22) and at a particular stage of their disease, limiting generalizability. We also experienced some significant delays in follow-up visits, primarily due to COVID-19 and staff turnover. Our trial design was limited by the requirement of the nephrologist’s approval for participation, which likely biased our sample and lowered our recruitment total. However, with education efforts and success of the trial there has been a practice change and willingness by these same providers to refer to palliative care. Thus, our overall impression is that a necessary ingredient for future trial success is adequate education and engagement with local providers. Our decision to limit exit interviews to intervention participants missed an opportunity to explore feelings about palliative care from those without exposure or from dropouts. Additionally, the survey used lacked several questions that could have provided value, such as exploration of the burden of appointments. We will include these steps in future studies. Postenrollment dropout raises the need for better outreach postconsent. Family engagement may address some of this concern. Given the small sample size, it is possible the small improvements in measured outcomes we observed were due to random effects rather than to our intervention. Finally, in future trials, we will be deliberate in choosing patient-satisfaction tools that are specific to the intervention because we did not analyze our planned data tool in this domain due to finding the answers to be nonspecific.
Despite these limitations, our study suggests that a randomized clinical trial testing the efficacy of palliative care in an advanced CKD population is feasible and acceptable to participants. Our outcomes support larger and more rigorous studies that test the effectiveness of palliative care in advanced CKD.
Disclosures
A. Brody reports having ownership interest in Accomplish Health, and having consultancy agreements with David Horowitz (PC), Ryan Ryan Deluca, and Victoria Crown Law (PC). D.M. Charytan reports having consultancy agreements with Allena Pharmaceuticals (data and safety monitoring board), Amgen, AstraZeneca, CSL Behring, Eli Lilly/Boehringer Ingelheim, GlaxoSmithKline, Janssen (steering committee), Fresenius, Gilead, Medtronic, Merck, Novo Nordisk, PLC medical (clinical events committee), Renalytix, and Zogenix; receiving grants and personal fees from Amgen, Gilead, Medtronic, and Novo Nordisk; receiving personal fees from AstraZeneca, Boehringer/Eli Lily, CSL Behring, Fresenius, GlaxoSmithKline, Merck, and Zoll; serving in an advisory or leadership role for CJASN; receiving personal fees and other from Janssen; and receiving expert witness fees related to proton pump inhibitors. J. Chodosh reports serving in an advisory or leadership role for the Journal of American Geriatrics Society. M.E. Rau reports having ownership interest in Doximity. J.S. Scherer reports serving in an advisory or leadership role for Cara Pharmaceuticals and Monogram Health (clinical advisory board), and receiving honoraria from Vifor Pharmaceuticals. All remaining authors have nothing to disclose. H. Zhong receives funding from National Institute of Aging grants 1R011AG065330-01, 5R01AG 054467-04, and 3R01AG54467-04S1All remaining authors have nothing to disclose.
Funding
This trial was funded by the National Kidney Foundation Young Investigator Award and the NYU Doris Duke Fund to Retain Clinical Scientists to J.S. Scherer. J.S. Scherer also receives funding from National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK-125840.
Author Contributions
A. Brody, D.M. Charytan, J. Chodosh, A. Kreiger, J.S. Scherer, Y. Xia, M.E. Rau, and H. Zhong were responsible for formal analysis; A. Brody, D.M. Charytan, J. Chodosh, M.E. Rau, J.S. Scherer, and H. Zhong were responsible for investigation; A. Brody, D.M. Charytan, J. Chodosh, J.S. Scherer, Y. Xia, and H. Zhong were responsible for methodology; A. Brody, D.M. Charytan, J. Chodosh, and J.S. Scherer provided supervision and were responsible for project administration; A. Brody, D.M. Charytan, J. Chodosh, J.S. Scherer, and H. Zhong conceptualized the study; J. Chodosh and J.S. Scherer were responsible for funding acquisition; J.S. Scherer wrote the original draft and was responsible for validation; Y. Xia and H. Zhong were responsible for resources and software; and all authors reviewed and edited the manuscript and were responsible for data curation.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000352022/-/DCSupplemental.
Template of the kidney palliative care clinic note. Download Supplemental Appendix 1, PDF file, 177 KB (176.4KB, pdf) .
Exit interview questions asked to the intervention group. Download Supplemental Appendix 2, PDF file, 177 KB (176.4KB, pdf) .
Demographic table of all patients who completed the trial. Download Supplemental Table 1, PDF file, 177 KB (176.4KB, pdf) .
References
- 1.Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, Arnold RM: Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant 18: 1345–1352, 2003. 10.1093/ndt/gfg105 [DOI] [PubMed] [Google Scholar]
- 2.Davison SN, Jhangri GS: Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage 39: 477–485, 2010. 10.1016/j.jpainsymman.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System : 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Chapter 6: Mortality. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. Available at: https://adr.usrds.org/2021/end-stage-renal-disease/6-mortality. Accessed August 29, 2021 [Google Scholar]
- 4.Kurella Tamura M, Liu S, Montez-Rath ME, O’Hare AM, Hall YN, Lorenz KA: Persistent gaps in use of advance directives among nursing home residents receiving maintenance dialysis. JAMA Intern Med 177: 1204–1205, 2017. 10.1001/jamainternmed.2017.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachterman MW, Lipsitz SR, Lorenz KA, Marcantonio ER, Li Z, Keating NL: End-of-life experience of older adults dying of end-stage renal disease: A comparison with cancer. J Pain Symptom Manage 54: 789–797, 2017. 10.1016/j.jpainsymman.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center to Advance Palliative Care : About palliative care. Available at: https://www.capc.org/about/palliative-care/. Accessed February 1, 2018
- 7.Lam DY, Scherer JS, Brown M, Grubbs V, Schell JO: A conceptual framework of palliative care across the continuum of advanced kidney disease. Clin J Am Soc Nephrol 14: 635–641, 2019. 10.2215/CJN.09330818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renal Physician’s Association : Shared Decision-Making in the Appropriate Initiation of and Withdrawal From Dialysis, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 9.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR; American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012. 10.2215/CJN.04970512 [DOI] [PubMed] [Google Scholar]
- 10.Urquhart-Secord R, Craig JC, Hemmelgarn B, Tam-Tham H, Manns B, Howell M, Polkinghorne KR, Kerr PG, Harris DC, Thompson S, Schick-Makaroff K, Wheeler DC, van Biesen W, Winkelmayer WC, Johnson DW, Howard K, Evangelidis N, Tong A: Patient and caregiver priorities for outcomes in hemodialysis: An international nominal group technique study. Am J Kidney Dis 68: 444–454, 2016. 10.1053/j.ajkd.2016.02.037 [DOI] [PubMed] [Google Scholar]
- 11.Tong A, Sainsbury P, Carter SM, Hall B, Harris DC, Walker RG, Hawley CM, Chadban S, Craig JC: Patients’ priorities for health research: Focus group study of patients with chronic kidney disease. Nephrol Dial Transplant 23: 3206–3214, 2008. 10.1093/ndt/gfn207 [DOI] [PubMed] [Google Scholar]
- 12.Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Crowe S, Harris T, Van Biesen W, Winkelmayer WC, Sautenet B, O’Donoghue D, Tam-Tham H, Youssouf S, Mandayam S, Ju A, Hawley C, Pollock C, Harris DC, Johnson DW, Rifkin DE, Tentori F, Agar J, Polkinghorne KR, Gallagher M, Kerr PG, McDonald SP, Howard K, Howell M, Craig JC; Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) Initiative : Developing a set of core outcomes for trials in hemodialysis: An international Delphi survey. Am J Kidney Dis 70: 464–475, 2017. 10.1053/j.ajkd.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 13.Combs SA, Culp S, Matlock DD, Kutner JS, Holley JL, Moss AH: Update on end-of-life care training during nephrology fellowship: A cross-sectional national survey of fellows. Am J Kidney Dis 65: 233–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamal AH, Bull JH, Swetz KM, Wolf SP, Shanafelt TD, Myers ER: Future of the palliative care workforce: Preview to an impending crisis. Am J Med 130: 113–114, 2017. 10.1016/j.amjmed.2016.08.046 [DOI] [PubMed] [Google Scholar]
- 15.Eneanya ND, Labbe AK, Stallings TL, Percy S, Temel JS, Klaiman TA, Park ER: Caring for older patients with advanced chronic kidney disease and considering their needs: A qualitative study. BMC Nephrol 21: 213, 2020. 10.1186/s12882-020-01870-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladin K, Pandya R, Kannam A, Loke R, Oskoui T, Perrone RD, Meyer KB, Weiner DE, Wong JB: Discussing conservative management with older patients with CKD: An interview study of nephrologists. Am J Kidney Dis 71: 627–635, 2018. 10.1053/j.ajkd.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM: Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: A national qualitative study. Am J Kidney Dis 75: 167–176, 2020. 10.1053/j.ajkd.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamar FB, Tam-Tham H, Thomas C: A description of advanced chronic kidney disease patients in a major urban center receiving conservative care. Can J Kidney Health Dis 4: 2054358117718538, 2017. 10.1177/2054358117718538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josland E, Brennan F, Anastasiou A, Brown MA: Developing and sustaining a renal supportive care service for people with end-stage kidney disease. Renal Soc Australas 8: 12–18, 2012 [Google Scholar]
- 20.Chao CT, Tsai HB, Shih CY, Hsu SH, Hung YC, Lai CF, Ueng RH, Chan DC, Hwang JJ, Huang SJ: Establishment of a renal supportive care program: Experience from a rural community hospital in Taiwan. J Formos Med Assoc 115: 490–500, 2016. 10.1016/j.jfma.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 21.Hui D, Bruera E: Models of palliative care delivery for patients with cancer. J Clin Oncol 38: 852–865, 2020. 10.1200/JCO.18.02123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer JS, Harwood K, Frydman JL, Moriyama D, Brody AA, Modersitzki F, Blaum CS, Chodosh J: A descriptive analysis of an ambulatory kidney palliative care program. J Palliat Med 38: 259–263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bristol AA, Chaudhry S, Assis D, Wright R, Moriyama D, Harwood K, Brody AA, Charytan DM, Chodosh J, Scherer JS: An exploratory qualitative study of patient and caregiver perspectives of ambulatory kidney palliative care. Am J Hosp Palliat Care 38: 1242–1249, 2021. 10.1177/1049909120986121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palliative Care Research Cooperative Group : Training videos. Available at: https://palliativecareresearch.org/resources/training-videos. Accessed February 28, 2022
- 25.Scherer JS, Wright R, Blaum CS, Wall SP: Building an outpatient kidney palliative care clinical program. J Pain Symptom Manage 55: 108–116.e2, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Yoong J, Park ER, Greer JA, Jackson VA, Gallagher ER, Pirl WF, Back AL, Temel JS: Early palliative care in advanced lung cancer: A qualitative study. JAMA Intern Med 173: 283–290, 2013. 10.1001/jamainternmed.2013.1874 [DOI] [PubMed] [Google Scholar]
- 27.Raj R, Ahuja K, Frandsen M, Murtagh FEM, Jose M: Validation of the IPOS-Renal Symptom Survey in advanced kidney disease: A cross-sectional study. J Pain Symptom Manage 56: 281–287, 2018. 10.1016/j.jpainsymman.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 28.Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, Denzel J, Guo P, Bernhardt F, Schildmann E, van Oorschot B, Hodiamont F, Streitwieser S, Higginson IJ, Bausewein C: A brief, patient- and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med 33: 1045–1057, 2019. 10.1177/0269216319854264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rand Health Care : Kidney Disease Quality of Life Instrument. Available at: https://www.rand.org/health-care/surveys_tools/kdqol.html. Accessed October 10, 2019
- 30.Peipert JD, Bentler PM, Klicko K, Hays RD: Psychometric properties of the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36) in the United States. Am J Kidney Dis 71: 461–468, 2018. 10.1053/j.ajkd.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 31.Ricardo AC, Hacker E, Lora CM, Ackerson L, DeSalvo KB, Go A, Kusek JW, Nessel L, Ojo A, Townsend RR, Xie D, Ferrans CE, Lash JP; CRIC Investigators : Validation of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36) US Spanish and English versions in a cohort of Hispanics with chronic kidney disease. Ethn Dis 23: 202–209, 2013 [PMC free article] [PubMed] [Google Scholar]
- 32.Jones TA, Olds TS, Currow DC, Williams MT: Feasibility and pilot studies in palliative care research: A systematic review. J Pain Symptom Manage 54: 139–151.e4, 2017. 10.1016/j.jpainsymman.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 33.Hagen NA, Biondo PD, Brasher PM, Stiles CR: Formal feasibility studies in palliative care: Why they are important and how to conduct them. J Pain Symptom Manage 42: 278–289, 2011. 10.1016/j.jpainsymman.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 34.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, Bond CM: Defining feasibility and pilot studies in preparation for randomised controlled trials: Development of a conceptual framework. PLoS One 11: e0150205, 2016. 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flythe JE, Hilliard T, Castillo G, Ikeler K, Orazi J, Abdel-Rahman E, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie C, Mehrotra R: Symptom prioritization among adults receiving in-center hemodialysis: A mixed methods study. Clin J Am Soc Nephrol 13: 735–745, 2018. 10.2215/CJN.10850917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SA, Tyrer FC, Clarke AL, Lloyd-Davies LH, Stein AG, Tarrant C, Burton JO, Smith AC: Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J 10: 788–796, 2017. 10.1093/ckj/sfx057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Template of the kidney palliative care clinic note. Download Supplemental Appendix 1, PDF file, 177 KB (176.4KB, pdf) .
Exit interview questions asked to the intervention group. Download Supplemental Appendix 2, PDF file, 177 KB (176.4KB, pdf) .
Demographic table of all patients who completed the trial. Download Supplemental Table 1, PDF file, 177 KB (176.4KB, pdf) .