Abstract
Objective
To assess the effect of PRP on knee articular cartilage content (thickness/volume) and examine the correlation between cartilage changes and clinical outcomes in patients with knee OA.
Method
A systematic literature search was performed using the Cochrane methodology in four online databases. Studies were included if they reported on cartilage content with cross-sectional imaging pre- and post-injection. A random-effects model meta-analysis was performed. Correlation with clinical outcomes was evaluated.
Results
14 studies (n = 1099 patients) from 1452 records met the inclusion criteria: seven RCTs (n = 688), one prospective (n = 50), one retrospective (n = 68), and four case-series (n = 224). The PRP preparation process and treatment protocol varied widely (follow-up 6–12 months). In meta-analysis, PRP treatment was not associated with a significant increase in cartilage thickness (4 studies, n = 187, standardized mean difference: Hedges g: 0.079; 95%CI: 0.358 - 0.516; p = 0.723). Meta-analysis of 3 RCTs (n = 112) showed no significant difference in the change of overall knee cartilage content with PRP injections compared with no PRP (Hedges’ g: 0.217; 95%CI: 0.177 – 0.611; P = 0.281).
Conclusion
The current literature does not support the PRP as chondrogenic in treatment of knee OA. However, there is substantial heterogeneity in the evaluated studies which limits the robustness of any conclusion. An adequately powered RCT, with a standardized PRP regime and standardized high-resolution MRI is needed to definitely define any effect of PRP on knee cartilage content and its relation to clinical outcomes. Until such high-quality evidence becomes available, we recommend that PRP is not administered with the intention of promoting chondrogenesis.
Keywords: Platelet-rich plasma, PRP, Knee osteoarthritis, Knee OA, Articular cartilage
1. Introduction
Osteoarthritis (OA) is a leading cause of disability and reduced quality of life with the knee joint being the most common site of OA [1]. Treatments for knee OA are primarily aimed at improving patient symptoms, ranging from simple analgesia to surgery as part of the treatment management ladder [2]. Among the non-invasive treatment options, intra-articular (IA) therapies are considered the mainstay of management [3].
Different types of IA injectables exist e.g. corticosteroids, platelet-rich-plasma (PRP), bone marrow aspirate concentrate (BMAC), adipose-derived stem cells (ASCs), and hyaluronic acid (HA). Among these IA therapies, PRP has been increasingly used in recent years as it has been shown to improve knee OA symptoms and clinical outcomes [[4], [5], [6]]. Furthermore, considering the potential of activated platelets to release growth factors and cytokines stimulating cartilage growth, PRP has been increasingly used in clinical practice to promote tissue repair and regeneration [7,8], with a suggestion that it may change the cartilage content possibly slowing or reversing OA [9,10]. However, the level of evidence is low and controversial with no review or meta-analysis to assess the effect of PRP on knee articular cartilage [11].
The primary aim of the systematic review was to assess the effect of PRP on knee articular cartilage content and structure in patients with symptomatic knee OA. The secondary aim was to identify if there is any correlation of the changes in articular cartilage with clinical outcomes.
2. Methods
A systematic review was performed following the Cochrane methodology for systematic reviews [12]. The predefined protocol for the review was registered with the PROSPERO database (CRD42022325560). A systematic search of the literature was undertaken (ADP) in four electronic bibliographic databases in July 2022 without a limit on the publication year: MEDLINE (Interface: EBSCOhost); EMBASE (Interface: OvidSP); CINAHL (Interface: EBSCOhost); CENTRAL (Interface: Cochrane Library). Further searches of the reference lists of included studies and any identified systematic reviews were also carried out. Only studies available in English language were included. The search in all databases was performed with a combination of key-words, including wildcards (∗). The search was developed using the following set of key-words combined with the Boolean operator AND: [PRP OR platelet-rich-plasma OR platelet rich plasma OR platelet∗] AND [osteoarthriti∗ OR arthriti∗ OR OA] AND [cartilag∗ OR chondral OR MRI OR imag∗ OR map∗].
2.1. Inclusion/exclusion criteria
-
•
Study designs: Study designs included were RCTs, prospective and retrospective cohort studies, case-control studies and case series with minimum 3-month follow-up, as the highest clinical effect sizes of other injectables have been reported between 5 and 14 weeks [13]. Case reports, reviews, editorials, commentaries, personal opinions, surveys were excluded.
-
•
Population: Adults with knee OA.
-
•
Intervention/Comparators: Adults with knee OA having treatment with intraarticular injection with PRP. Studies which compared PRP with other injectables with regards to the effect on articular cartilage were included. Studies which looked at the effect of PRP on articular cartilage but did not compare it with other injectables were included in the systematic review and narrative presentation and synthesis of the results, but not in the meta-analysis.
-
•
Outcomes: Articular cartilage volume and structure measured and/or mapped using cross-sectional imaging.
Two reviewers (ADP, EM) independently screened the titles and abstracts of all retrieved studies for inclusion. Duplicates were removed. Full texts of studies considered eligible were retrieved and reviewed independently. Disagreements for inclusion were discussed between reviewers and if still unresolved with the senior author.
2.2. Data extraction
One reviewer (ADP) extracted relevant data from the included studies using a standardized data extraction form and input onto an Excel spreadsheet. Data extracted were study characteristics, patient demographics, OA severity and grade, PRP preparation and treatment protocol, cartilage measurements in cross-sectional imaging (thickness or volume or mapping values), clinical outcomes, and follow-up period.
2.3. Data analysis – statistical analysis
An initial descriptive analysis and synthesis of the characteristics and the study results was undertaken. The primary outcome was the change in cartilage thickness and/or the change in cartilage mapping values post-injection. For each study, cartilage thickness/volume or cartilage mapping values on MRI or US were reported in absolute numbers and rates and any significant difference post-injection was established (p < 0.05). For studies reporting on cartilage thickness, pre- and post-injection differences in means and 95% confidence intervals (CIs) were calculated and combined in a random-effects model meta-analysis [14]. When combining studies that reported on cartilage thickness or volume, the Hedges g and 95% CI were calculated and combined in a random-effects model meta-analysis [15]. Heterogeneity was assessed using tau [2], I2, Q and P values. Data were analyzed with Comprehensive Meta-analysis version 2 (Biostat).
2.4. Assessment of methodological quality of studies and quality of evidence
The methodological quality of the studies was assessed as per study design. The Cochrane Risk of Bias Tool was used for RCTs [16], the Newcastle-Ottawa scale for prospective cohort studies [17]; and the revised and validated version of Methodological Index for Non-Randomised Studies (MINORS) for retrospective studies [15]. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to assess the quality of evidence of the review [18].
3. Results
3.1. Findings of the database searches
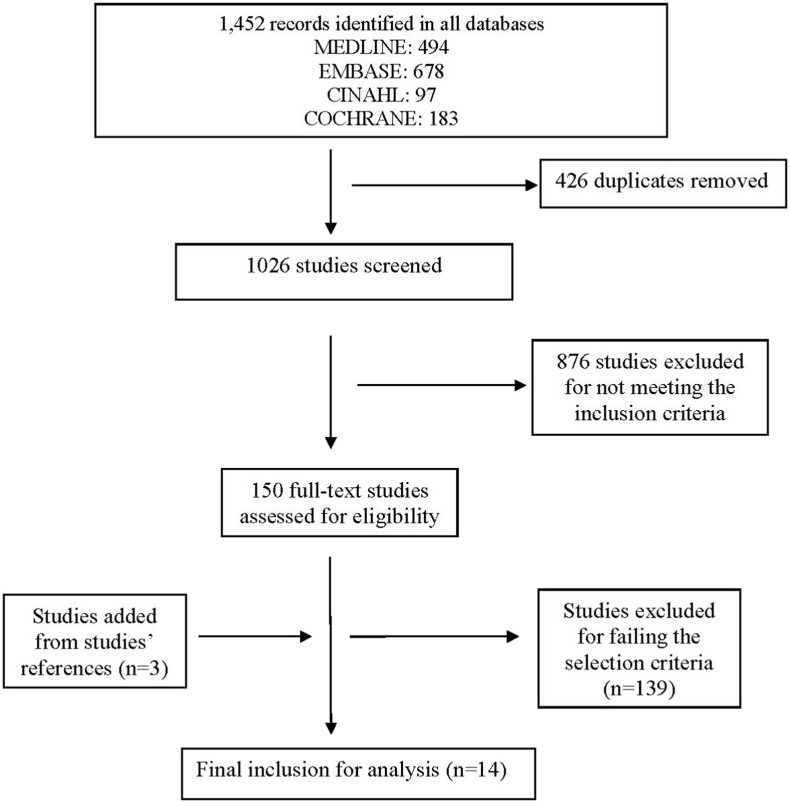
The search identified 1452 records by title, 14 of which met the inclusion criteria and were included for the analysis [10,11,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. Fig. 1 shows the Preferred Reporting Items for Systematic reviews and meta-analyses (PRISMA) flow diagram [29].
Fig. 1.
Methodology of identification and selection of studies (PRISMA flow chart) [29].
3.2. Characteristics of included studies
Table 1 summarises the characteristics of the 14 included studies [10,11,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. The methodology of the non-randomised studies was classified according to Mathes and Pieper (2017) [30]. Our analysis included seven RCTs (n = 688) [[10], [11], [19], [22], [26], [31], [32]]; two prospective (n = 119) [[24], [25]]; one retrospective cohort (n = 68) [21]; and 4 case-series (n = 244) [20,23,27,28]. The total number of participants included was 1119 (1,169TKAs).
Table 1.
Characteristics of all included studies in the systematic review.
| Lead author (Year) | Study design (Level of evidence, Country) | No. of patients (knees) | Diagnosis Stage of OA |
Age (years) | Gender (M:F) | BMI (kg/m2) | Treatment received (knees) |
|---|---|---|---|---|---|---|---|
| Bansal (2021) [19] | RCT (I, USA) | 132 (132) | Knee OA K-L grades I-III |
PRP: 64.4 (52–74) Control: 65.8 (54–73) |
PRP: 39:25 Control: 42:26 |
NR | PRP: 64 Control (HA): 68 |
| Bennell (2021) [11] | RCT (I, Australia) | 288 (288) | Knee OA K-L grades II, III |
PRP: 62.2 ± 6.3 Control: 30.1 ± 4 NSD |
PRP: 59:85 Control: 60:84 NSD |
PRP: 29 ± 3.7 Control: 29.6 ± 4.5 NSD |
PRP: 144 Control (placebo): 144 |
| Raeissadat (2020) [10] | RCT (I, Iran) | 42 (42) | Knee OA K-L grades I-III |
57.57 ± 5.9 | All females | 28.49 ± 3.24 | PRP: 21 Control (exercise): 21 |
| Elik (2020) [32] | RCT (I, Turkey) | 57 (57) | Knee OA K-L grades I-III |
60.77 ± 7.36 (50–75) | 4:53 PRP: 1:29 Control: 3:24 |
PRP: 30.37 ± 4.47 Control: 30.70 ± 3.97 |
PRP: 30 Control: 27 |
| Buendía-López (2019) [31] | RCT (I, Spain) | 99 (99) | Knee OA K-L grades I-II |
56.82 (50–63) | PRP: 16:17 HA: 15:17 NSAID: 17:16 |
25.1 (23.8–26.1) | PRP: 33 Control groups: HA: 32 NSAID: 33 |
| Elnemr (2019) [22] | RCT (I, Egypt) | 30 (30) | Knee OA (post-meniscal repair) No classification |
Range: 18-55 PRP: 27.7 ± 2.9 Control: 30.1 ± 4 P = 0.068 |
PRP: 14:1 Control: 13:2 NSD |
PRP:27.2 ± 4.3 Control: 25.5 ± 3.3 P = 0.23 |
PRP: 15 Control (exercise): 15 |
| Hart (2017) [26] | RCT (I, Czech Republic) | 40 (40) | Knee PFJ OA (chondromalacia) Outerbridge II, III |
Mean: 52.2 Range: 31-69 |
17:23 | Mean: 29.3 Range: 18.8–34.9 |
PRP: 20 Control (HA): 20 |
| Kenmochi (2020) [25] | Prospective, cross-sectional (II, Japan) | 44 (55) | Knee OA K-L grades I-IV |
Mean: 67.2 ± 9.6 Range: 36-84 |
6:38 | Mean: 25.3 Range: 19.6–33.8 |
PRP No control group |
| Hart (2013) [24] | Prospective cohort (II, Czech Republic) | 75 (75) | Knee PFJ OA (chondromalacia) Outerbridge II, III |
PRP: 58.1 (31–75) Control: 58.4 (36–74) |
PRP: 29:21 Control: 13:12 |
PRP: 28.1 (20.1–33.7) Control: 27.8 (19.6–34.7) |
PRP: 50 Control (1% mesocain): 25 |
| Cobianchi (2021) [21] | Retrospective cohort (III, Italy) |
68 (68) | Knee OA Outerbridge II, III, IV |
PRP: 41.8 ± 8.9 Range: 22-54 Control: Matched |
PRP: 22:12 Control: Matched |
NR | PRP: 34 Control: 34 |
| Sen (2020) [28] | Case series (IV, Turkey) | 71 (109) | Knee OA K-L grades II, III |
Mean: 47.4 ± 10.4 Range: 35-65 |
24:46 | Mean: 29.2 ± 4.9 | No control group |
| Guillibert (2019) [23] | Case series (IV, France) |
57 (57) | Knee OA K-L grades II, III |
Mean: 63.3 ± 9.6 | 24:33 | Mean: 25.4 ± 3.9 | No control group |
| Calis (2015) [20] | Case series (IV, Turkey) |
82 (103) | Knee OA K-L grades III, IV |
Mean: 63.5 ± 9.3 Range:40-88 |
13:69 | Mean: 33.5 ± 4.6 | No control group |
| Sampson (2010) [27] | Case series (IV, USA) | 14 (14) | Knee OA No grade reported |
Mean: 51.8 Range: 18-87 |
12:2 | Mean: 25 Range: 20.9–32.5 |
No control group |
RCT: Randomised Clinical Trial, USA: United States of America, OA: osteoarthritis, K-L: Kellgren-Lawrence, PFJ: Patellofemoral, PRP: Platelet-rich plasma, HA: hyaluronic acid, M: males, F: females, BMI: Body Mass Index, NR: not reported, NSD: no significant difference, p<0.05: significant
HA was used for the control group in three RCTs [19,24,31], a placebo (Normal saline) was used in one RCT [32], and conservative management with an exercise program was used in other two RCTs [10,22]. One RCT, being a cross-sectional randomized trial which injected PRP in all patients, did not have a control group [25]. In the prospective comparative study 5 ml of 1% mesocaine was used [24], whilst in the retrospective comparative study [21], conservative management with an exercise program was used for the control group. Four case-series did not have any control group [20,23,27,28].
Patient demographics (Table 1): Age range was 18–88 years. The mean BMI in all the studies was less than 30 kg/m2. Nine used the Kellgren-Lawrence (K-L) scale [10,11,19,20,23,25,28,31,32], while three used the Outerbridge scale to grade the OA severity [21,24,26].
3.3. Characteristics of PRP used (supplementary material: Table 1)
The PRP preparation process varied widely. Six studies used a commercial kit/method [10,11,20,23,27,32], with the rest using independent methods [19,21,22,[24], [25], [26],28,31]. Nine studies used double-spin [10,19,20,22,23,25,28,31,32], and four used single spin centrifugation [11,24,26,27]. There was no consistency in the PRP volume injected in the studies, with three injecting < 4 ml [20,25,28], seven injecting 4–6 ml [10,11,22,24,26,27,31,32], and three injecting ≥8 ml [19,21,23]. Platelet concentration ranged from 1.4 to 10 times the blood concentration. Interestingly one RCT injected one dose of 10 billion platelets in 8 ml volume of PRP showing sustained therapeutic benefit in 1 year [19]. The number of PRP injections and the time interval between injections varied significantly [22,[24], [25], [26]]. Most studies used an anticoagulant, with six using a Citrate Dextrose solution [10,20,23,24,26,27], and 4 using Calcium Chloride [21,22,31,32]. Classifying the PRPs according to the Dohan Ehrenfest classification for platelet concentrates into Leukocyte Rich-PRP (LR-PRP), Leukocyte Poor PRP (LP-PRP) or Pure-PRP (P-PRP) [33], five studies used LR-PRP [10,18,22,25,28,32], four used LP-PRP [11,21,26,31], and three using P-PRP. Two studies did not clarify if their PRP concentrate contained WBC [20,27].
3.4. Outcomes: cartilage thickness/volume (Table 2)
Table 2.
Cartilage thickness reported in all studies before and after treatment.
| Lead author (Year) | Control group | Imaging modality | Compartments evaluated | Follow-up (months) | Cartilage evaluation pre-treatment (PRP) | Cartilage evaluation post-treatment (PRP) | Cartilage evaluation pre-treatment (Control) | Cartilage evaluation post-treatment (Control) | Statistical analysis |
|---|---|---|---|---|---|---|---|---|---|
| Bansal (2021) [19] | HA 4 ml (Monovisc®) | MRI 1.5T |
Cartilage thickness (mm) MFC |
12 | 4.48–4.98 | 53 (82.8%) unchanged 11 (17.1%) reduced |
4.34–5.00 | 42 (61.7%) unchanged 16 (23.5%) reduced |
PRP vs Control: Unchanged p < 0.05 Reduced p > 0.05 |
| Bennell (2021) [11] | Placebo (5 ml Normal Saline) | MRI T1 FS 3T |
Medial tibia cartilage volume (mm3) | 12 | 1337 ± 488 | −1.4 ± 7.2 | 1309 ± 479 | −1.2 ± 6.8 |
Difference in change: −0.2 (95%CI: 1.9 to 1.5) P = 0.81 |
| Raeissadat (2020) [10] | Exercise and 500 mg Paracetamol | MRI FS PD 1.5T |
Patellofemoral cartilage volume (mm3) | 8 | 1041.47 ± 323.01 K-L I: 26.3% K-L II: 52.6% K-L III: 21.1% |
1336.88 ± 295.83 | 1012.68 ± 259.24 K-L I: 26.3% K-L II: 52.6% K-L III: 21.1% |
1105.1 ± 262.62 |
PRP: P = 0.001 Control: P = 0.05 PRP vs Control: p = 0.001 |
| Elik (2020) [32] | Placebo (4 ml Normal Saline) | US (high-resolution) | Cartilage thickness (mm) MFC, LFC, intercondylar femur |
6 | No numbers reported | NSD | No numbers reported | NSD | NR |
| Buendía-López (2019) [31] | HA 2 ml (60mg/2 ml Durolane®) NDAID for 52 weeks (60 mg etoricoxib Acoxxel®) |
MRI T2 FS PD 1.5T slice 3 mm 1 mm intersection gap |
MOAKS BML (distal femur, proximal tibia) | 12 | Femur: Central: 1.73 ± 0.4 Tibia: Central: 1.82 ± 0.4 Anterior: 1.42 ± 0.26 Posterior: 1.28 ± 0.2 |
Reduced No increase |
Femur: Central: 1.73 ± 0.4 Tibia: Central: 1.82 ± 0.4 Anterior: 1.42 ± 0.26 Posterior: 1.28 ± 0.2 |
Reduced No increase |
PRP vs HA vs NSAID: NSD |
| Elnemr (2019) [22] | No PRP | US (high-resolution) | Cartilage thickness (mm) MFC, LFC, MTC, LTC |
12 | MFC: 2.5 ± 0.5 LFC: 2.1 ± 0.4 MTC: 2.6 ± 0.5 LTC: 3 ± 0.5 Total: 10.12 ± 1.76 |
MFC: 2.2 ± 0.5 LFC: 2 ± 0.4 MTC: 2.4 ± 0.5 LTC: 2.9 ± 0.5 Total: 9.51 ± 1.80 %degeneration: ↓6.16 ± 3.33 |
MFC: 2.4 ± 0.5 LFC: 2.2 ± 0.4 MTC: 2.6 ± 0.4 LTC: 2.7 ± 0.5 Total: 9.87 ± 1.62 |
MFC: 2.1 ± 0.4 LFC: 2.1 ± 0.4 MTC: 2.2 ± 0.5 LTC: 2.6 ± 0.5 Total: 8.99 ± 1.56 %degeneration: ↓ 9.07 ± 3.66 |
PRP vs Control: MFC: P = 0.366 LFC: P = 0.562 MTC: P = 0.338 LTC: P = 0.122 %degeneration: P = 0.031 |
| Hart (2017) [26] | HA 2 ml (Erectus® Medicom International, Czech Republic) | MRI T1+T2 1.5T slice 3 mm 0.5 mm intersection gap |
Cartilage thickness (mm) MFC, LFC, MTC, LTC |
12 | 1.51 ± 0.463 Grade II: 12 Grade III: 8 |
1.35 ± 0.668 Increased in 1 patient Grade II: 13 Grade III: 7 Grade improved (III-II): 1/20 |
1.52 ± 0.472 Grade II: 11 Grade III: 9 |
1.37 ± 0.715 Grade II: 12 Grade III: 8 Grade improved (III-II): 1/20 |
PRP: P = 0.941 Control: P = 0.929 |
| Kenmochi (2020) [25] | No control | MRI 3T | MOAKS BML (15 regions: 2 patellar, 6 femoral, 7 tibial) | 6 | MOAKS BML 7.44 |
MOAKS BML 6.6 |
NA | NA | P = 0.007 |
| Hart (2013) [24] | 5 ml 1% Mesocain | MRI T1+T2 1.5T slice 3 mm 0.5 mm intersection gap |
Cartilage thickness (mm) MFC, LFC, MTC, LTC |
12 | 2.15 ± 0.75 (1.00–4.30) Grade II: 21 (42%) Grade III: 29 (58%) |
2.22 ± 0.93 (0.50–4.30) | Not measured Grade II: 9 (36%) Grade III: 16 (64%) |
Not measured |
PRP: P = 0.23 Control: N/A |
| Cobianchi (2021) [21] | No PRP | MRI T1+T2 3T slice 4 mm 0.4 mm intersection gap |
T2 cartilage mapping (ms) Patellofemoral (medial + lateral patellar, MFC, LFC) |
6.4 ± 1.9 (4–12) |
T2 relaxation times Medial patellar: 40.4 ± 3.8 Latellar patellar: 40.1 ± 5.1 MFC: 48.5 ± 3.1 LFC: 47.6 ± 3.7 Global: 44.2 ± 2.5 Modified WORMS: 14 ± 3.4 (10.5–18) Grades: Grade II: 4 Grade III: 18 Grade IV: 4 |
T2 relaxation times Medial patellar: 37.6 ± 4.3 Latellar patellar: 40.1 ± 5.1 MFC: 44.7 ± 3.7 LFC: 45.7 ± 2.9 Global: 41.5 ± 2.5 Modified WORMS: 12.5 ± 2.4 (10–15) Improvement: 10.5% (mean) Grades: Grade II: 4 Grade III: 22 Grade IV: 0 Grade improvement: 4 (11%) |
T2 relaxation times Medial patellar: 42.5 ± 1.5 Lateral patellar: 41.2 ± 5.5 MFC: 46.1 ± 3.5 LFC: 46.6 ± 3.9 Global: 43.2 ± 1.8 Modified WORMS: 15 ± 2.9 (11.6–19) Grades: Grade II: 5 Grade III: 15 Grade IV: 5 |
T2 relaxation times Medial patellar: 42.2 ± 3.9 Lateral patellar: 41.1 ± 4.5 MFC: 45.9 ± 3.9 LFC: 36.1 ± 3.1 Global: 43.1 ± 2.1 Modified WORMS: 15.5 ± 2.6 (11–17) Improvement: 3.3% (mean) Grade II: 5 Grade III: 14 Grade IV: 6 Grade improvement: 1 (3%) worsening |
PRP: Medial Patellar: P<0.001 Lateral Patellar: P<0.001 MFC: P<0.001 LFC: P<0.001 Global: P<0.001 Modified WORMS: P<0.001 Control: Medial patellar: P > 0.05 Lateral Patellar: P > 0.05 MFC: P > 0.05 LFC: P > 0.05 Global: P = 0.121 Modified WORMS: P = 0.132 |
| Sen (2020) [28] | No control | US (high-resolution) | Cartilage thickness (mm) MFC, LFC, ICA |
6 | MFC: 1.8 ± 0.2 LFC: 1.9 ± 0.2 ICA: 2.1 ± 0.2 |
MFC: 1.9 ± 0.2 LFC: 2.0 ± 0.2 ICA: 2.2 ± 0.2 |
NA | NA | MFC: P = 0.108 LFC: P = 0.063 ICA: P = 0.684 |
| Guillibert (2019) [23] | No control | MRI 1.5T |
Cartilage thickness (mm) MFC MTP LFC LTP MPF LPF |
6 | MFC: 1.16 ± 0.72, MTP: 1.67 ± 0.85, LFC: 1.6 ± 0.6, LTP: 2.08 ± 0.91, MPF: 2.27 ± 0.75, LPF: 2.61 ± 1.03 |
MFC: 1.14 ± 0.77, MTP: 1.64 ± 0.89, LFC: 1.62 ± 0.6, LTP: 2.14 ± 1.01, MPF: 2.33 ± 0.77, LPF: 2.68 ± 1.06 |
NA | NA | MFC: P = 0.72 MTP: P = 0.82 LFC: P = 0.75 LTP: P = 0.26 MPF: P = 0.22 LPF: P = 0.22 |
| Calis (2015) [20] | No control | US (high-resolution) | Cartilage thickness (mm) MFC |
6 | 0.6 ± 0.2 | 0.8 ± 0.2 | NA | NA | P<0.05 |
| Sampson (2010) [27] | No control | US (high-resolution) | Cartilage thickness (mm) MFC, LFC, ICA |
6 | MFC: 2.53 ± 0.64 LFC: 2.50 ± 0.97 ICA: 3.32 ± 1.00 |
MFC: 2.53 ± 0.95 LFC: 2.73 ± 0.81 ICA: 3.38 ± 1.06 |
NA | NA | MFC: P = 0.22 LFC: P = 0.46 ICA: P > 0.05 |
PRP: Platelet-rich plasma, MRI: Magnetic Resonance Imaging, T: Tesla, US: Ultrasound scan, K-L: Kellgren-Lawrence, MFC: medial femoral condyle, LFC: lateral femoral condyle, ICA: intercondylar area, MTC: medial tibial condyle, LTC: lateral tibial condyle, MPF: medial patellofemoral, LPF: lateral patellofemoral, WORMS: Whole-organ MRI score, MOAKS BML: MRI Osteoarthritis Knee Score Bone Marrow Lesion, p<0.05: significant.
The cartilage thickness was evaluated with either high-resolution US or MRI before treatment and at follow-up. Nine used MRI [10,11,19,21,[23], [24], [25], [26],31], and five high-resolution US [20,22,27,28,32]. Among the studies that used MRI, three used a 3.0 T scanner [11,21,25], and six used a 1.5 T scanner [10,19,23,24,26,31]. Usually, the MRI slice thickness was 3 mm (0.5 mm intersection gap). Most studies did measurements in the medial (MFC) and lateral femoral condyle (LFC), medial (MTP) and lateral tibial plateau (LTP) [[19], [20], [21], [22], [23], [24],[26], [27], [28],31,32]. The follow-up ranged from 6 to 12 months.
Among the nine studies that used MRI to evaluate cartilage thickness [10,11,19,21,[23], [24], [25], [26],31], three reported significant improvement post-PRP injections [10,21,25]. One study evaluated the patellofemoral cartilage volume (as the sum of cartilage area in all images multiplied by thickness of the slide) 8 months post-treatment showing significant increase in cartilage volume (p = 0.001), with the improvement in cartilage volume for the PRP group being significantly better as compared to the control group (exercise and analgesia) (p = 0.001) [10]. The second study used the MRI Osteoarthritis Knee Score Bone Marrow Lesion (MOAKS BML) [34] to assess articular cartilage before and after treatment [25]. It showed significant improvement 6 months post-PRP injection (p = 0.007). The third study used T2 mapping evaluation to assess cartilage thickness [21]. It used the modified whole-organ MRI score (WORMS) for quantitative analysis [35]. For quantitative analysis it recorded the T2 relaxation times in 3–5 regions in medial and lateral patella and femoral condyle (normal range was considered: 28.3–41.2 ms). It showed a significant improvement in T2 relaxation times in all regions (p < 0.001) and better modified WORMS score post-PRP injection (p < 0.001). A recent RCT (RESTORE) of 288 patients compared treatment with PRP injections with a placebo, and measured the medial tibia cartilage volume before and after treatment [11]. It showed a decrease in cartilage volume following both PRP treatment and placebo treatment (−1.4 ± 7.2 and −1.2 ± 6.8 respectively), with the difference between two groups being not significant (p = 0.81). A recent RCT comparing a single injection of inactivated PRP high-concentration in platelets (10 billion) with high-molecular-weight HA showed that there was no increase in cartilage thickness on MRI in either group [19]. Another RCT, comparing PRP with HA and NSAIDs and using the MOAKS BML to assess cartilage, showed that the MOAKS BML was reduced and not improved post-treatment in all groups with no significant difference between groups without reporting on the actual post-treatment values [31].
Among the five studies that used US to evaluate cartilage thickness [20,22,27,28,32], one case series (n = 103) reported significant improvement in cartilage thickness on the MFC six months following three PRP injections [20]. In two other case-series (n = 123) [27,28], measurement of cartilage thickness 6 months post-PRP injections was not significantly different. However, 6 of the 14 patients in one study had increased femoral articular cartilage [27]. One RCT (n = 30) showed no significant difference in change of cartilage thickness between two groups (follow-up 12 months), one group having six PRP injections and the other having none [22]. Interestingly, cartilage thickness was decreased in all areas measured with the percentage of degeneration being worse 12 months post-treatment. Similarly, another RCT (n = 57) showed no significant difference in cartilage thickness post-treatment between two groups (follow-up 6 months), one group having three PRP injections and the other having placebo injections, but did not report the actual values in mm [32].
4. Meta-analysis
4.1. Differences in mean articular cartilage thickness/volume following PRP treatment (Table 3)
Table 3.
Estimated differences in mean articular cartilage thickness in different intraarticular areas post-PRP treatment (as compared with cartilage thickness pre-treatment).
| Areas of measurement | No. of studies (knees) | Estimated difference in means (95%CI) | Heterogeneity |
|||
|---|---|---|---|---|---|---|
| τ2 | I2 | Q value | P value | |||
| MFC | 5 (313) [20,22,23,27,28] | 0.068 (−0.05 - 0.185), p = 0.259 | 0.01 | 86.284 | 29.163 | <0.001 |
| LFC | 4 (210) [22,23,27,28] | 0.064 (−0.02 - 0.148), p = 0.136 | 0.002 | 24.568 | 3.977 | 0.264 |
| MTP | 2 (87) [22,23] | −0.105 (−0.290 - 0.079), p = 0.263 | <0.001 | <0.001 | 0.804 | 0.370 |
| LTP | 2 (87) [22,23] | −0.019 (−0.214 – 0.176), p = 0.848 | <0.001 | <0.001 | 0.648 | 0.421 |
| Overall (MFC, LFC, MTP, LTP) | 3 (145) [22,24,26] | −0.075 (−0.300 – 0.150), p = 0.513 | 0.017 | 44.705 | 3.617 | 0.164 |
PRP: Platelet-Rich Plasma, CI: Confidence Interval, MFC: medial femoral condyle, LFC: lateral femoral condyle.
MTP: medial tibial plateau, LTP: lateral tibial plateau, p<0.05: significant.
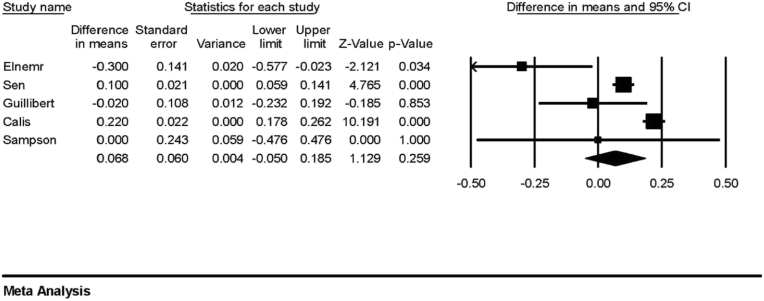
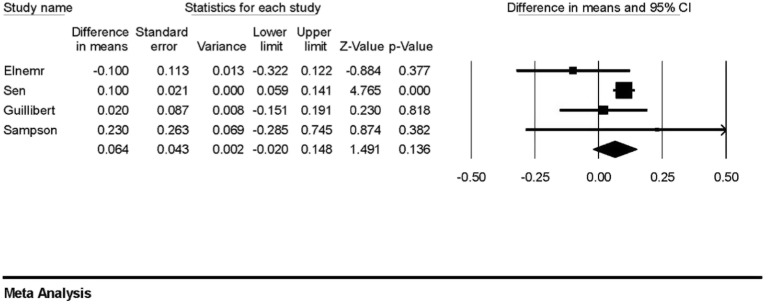
Five studies (n = 313) measured the cartilage thickness in MFC pre- and post-PRP treatment [20,22,23,27,28], and meta-analysis did not show a significant increase in cartilage thickness post-PRP treatment (Fig. 2: estimated difference in means: 0.068; 95%CI: 0.050 - 0.185; p = 0.259). Four studies (n = 210) measured the cartilage thickness in LFC [22,23,27,28], and meta-analysis did not show a significant increase in cartilage thickness post-PRP treatment (Fig. 3: estimated difference in means: 0.064; 95%CI: 0.02 – 0.148; P = 0.136).
Fig. 2.
Forest plot for the estimated differences in mean cartilage thickness of medial femoral condyle post-PRP treatment showing no significant increase in cartilage thickness.
Fig. 3.
Forest plot for the estimated differences in mean cartilage thickness of lateral femoral condyle post-PRP treatment showing no significant increase in cartilage thickness.
Meta-analysis of two studies (n = 87) [22,23], measuring the cartilage thickness in MTP and LTP, showed a decrease in cartilage thickness post-PRP treatment which was not significant (estimated difference in means respectively: 0.105; 95%CI: 0290 – 0.079; P = 0.263. −0.019; 95%CI: 0.214 – 0.176; P = 0.848). Meta-analysis of three studies (n = 145) which reported difference in overall cartilage thickness in four areas (MFC, LFC, MTP, LTP) [22,24,26], showed a non-significant decrease (estimated difference in means: 0.075; 95%CI: 0.300 – 0.150; P = 0.513). Meta-analysis of four studies (n = 187) confirmed difference of cartilage thickness overall [10,22,24,26], including one study showing non-significant increase in cartilage content (thickness/volume) (Hedges’ g: 0.079; 95%CI: 0.358 – 0.516; P = 0.723) [10].
4.2. Comparison with control group
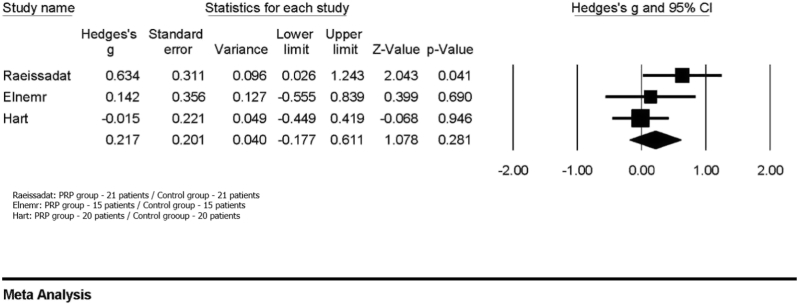
Three RCTs (n = 112) compared PRP treatment with a control [10,22,26]. One (n = 40) compared PRP with HA [26], whilst the other two compared PRP with exercise program [10,22]. Meta-analysis showed no significant difference in cartilage thickness and/or volume with PRP (Fig. 4: Hedges’ g: 0.217; 95%CI: 0.177 – 0.611; P = 0.281; heterogeneity: τ2 = 0.039; Ι2 = 31.548; Q = 2.922; P = 0.232).
Fig. 4.
Forest plot for the differences in mean cartilage thickness/volume comparing PRP with a control group showing no significant difference in cartilage thickness and/or volume with PRP.
4.3. Effect of PRP on cartilage and clinical outcomes (Table 4)
Table 4.
Clinical outcomes (pre- and post-PRP treatment) in the studies of the systematic review reporting on clinical outcomes.
| Lead author (Year) | Effect of PRP on cartilage (MRI/US) | WOMAC | KOOS | IKDC | VAS | SF-36 | Lysholm | Tegner |
|---|---|---|---|---|---|---|---|---|
| Bansal (2021) [19] | Thickness (MRI) 82.8% unchanged 17.1% reduced |
WOMAC total Pre: 55 (48–66) Post: 52 (47–60) NSD |
NR | Pre: 53.6 Post: 62.8 P<0.01 |
NR | NR | NR | NR |
| Bennell (2021) [11] | Volume (MRI) Decreased NSD |
NR |
KOOS pain Pre: 52.9 ± 115.2 Post: 68 ± 18.2 P<0.05 KOOS other: Pre: 53.9 ± 15.9 Post: 67.2 ± 18.9 P<0.05 KOOS Knee-QoL: Pre: 33.8 ± 15.8 Post: 51.1 ± 20.1 P<0.05 |
NR | NR | NR | NR | NR |
| Raeissadat (2020) [10] | Volume (MRI) Decreased (MRI) NSD |
WOMAC pain Pre: 8.14 ± 4.56 Post: 3.85 ± 1 3.4 P = 0.001 WOMAC stiffness Pre: 1.5 ± 2.2 Post: 0.76 ± 0.88 P = 0.001 WOMAC functional Pre: 24.28 ± 10.95 Post: 10.15 ± 8.3 P = 0.001 |
NR | NR | Pre: 6 ± 2.07 Post: 2.76 ± 2.07 P = 0.001 |
NR | NR | NR |
| Elik (2020) [32] | Thickness (US) NSD |
WOMAC pain Pre: 11.13 ± 4.27 Post: 4.73 ± 3.58 P < 0.001 WOMAC total Pre: 56.40 ± 18.71 Post: 24.87 ± 18.79 |
NR | NR |
Rest: Pre: 3.87 ± 2.14 Post: 1.20 ± 1.56 P < 0.001 Movement: Pre: 7.10 ± 2.52 Post: 2.80 ± 2.32 P < 0.001 |
Components: Physical: p<0.001 Mental: p = 0.003 |
NR | NR |
| Buendía-López (2019) [31] | MOAKS BML (MRI) Decreased |
WOMAC pain: Pre: 6.09 ± 1.4 Post: 4.84 ± 0.7 WOMAC total Pre: 42.57 ± 7.3 Post: 34.51 ± 1.2 |
NR | NR | Pre: 6.15 ± 1.1 Post: 5.03 ± 1.7 |
NR | NR | NR |
| Elnemr (2019) [22] | Thickness (US) Decreased NSD |
NR | Pre: 62 ± 9.8 Post: 86.2 ± 4 P = 0.014 |
NR | Pre: 9 (7–10) Post: 1 (1–3) P = 0.001 |
NR | NR | NR |
| Hart (2017) [26] | Thickness (MRI) Decreased NSD |
WOMAC total Pre: 37.1 ± 12.9 Post: 13.5 ± 13.7 P = 0.0005 |
NR | Pre: 48.6 ± 15.5 Post: 73.7 ± 13.5 P = 0.0005 |
NR | NR | Pre: 58.5 ± 17.4 Post: 82.2 ± 9.6 P = 0.0002 |
Pre: 3.6 ± 1.2 Post: 6.1 ± 1.1 P = 0.00001 |
| Kenmochi (2020) [25] | MOAKS BML (MRI) Improved P = 0.007 |
NR | Pre: 56.2 Post: 69.1 P<0.01 |
NR | Pre: 5.8 Post: 3.1 P<0.05 |
NR | NR | NR |
| Cobianchi (2021) [21] | T2 relaxation times (MRI) Improved P<0.001 |
WOMAC pain Pre: 18.3 ± 4.5 Post: 7.3 ± 3.2 P<0.05 |
NR | NR | Pre: 7 Post: 2 P<0.05 |
NR | NR | NR |
| Sen (2020) [28] | Thickness (US) Increased NSD |
WOMAC pain P<0.001 WOMAC stiffness P<0.001 WOMAC functional P<0.001 |
NR | NR |
VAS resting pain Pre: 2.0 ± 2.3 Post: 0.7 ± 1.2 P<0.001 VAS activity pain Pre: 4.8 ± 2.1 Post: 2.3 ± 1.9 P<0.001 |
Components: Physical: P<0.05 Mental: NSD |
NR | NR |
| Guillibert (2019) [23] | Thickness (MRI) Decreased NSD |
NR | Pre: 43.5 ± 14.3 Post: 66.4 ± 21.7 P<0.001 |
NR | NR | Components: Physical: P<0.001 Mental: NSD |
NR | NR |
| Calis (2015) [20] | Thickness (US) Increased P<0.05 |
WOMAC total Pre: 81.5 ± 14.5 Post: 62.2 ± 18.5 P = 0.001 WOMAC stiffness Pre: 5.8 ± 2.4 Post: 4.6 ± 2 P = 0.001 WOMAC functional Pre: 58.9 ± 11 Post: 45.1 ± 13.5 P = 0.001 |
NR | NR | Pre: 8.1 ± 2.1 Post: 4.4 ± 2.9 P<0.001 |
NR | NR | NR |
| Sampson (2010) [27] | Thickness (US) Increased NSD |
NR |
KOOS pain Pre: 35.3 ± 4.96 Post: 48.1 ± 4.96 P = 0.0295 KOOS other: Pre: 31.6 ± 4.84 Post: 43.9 ± 4.84 P = 0.0437 KOOS Knee-QoL: Pre: 1.0 ± 6.68 Post: 13.4 ± 6.18 P = 0.1048 |
NR |
VAS resting pain Pre: 2.5 (0–6) Post: 0.8 (0–3) P = 0.0011 VAS activity pain Pre: 4.6 (1–9) Post: 2.5 (0–7) P = 0.0003 |
NR | NR | NR |
PRP: Platelet-rich plasma, MRI: Magnetic Resonance Imaging, US: Ultrasound scan, WOMAC: Western Ontario and McMaster Universities Arthritis Index, KOOS: Knee Injury and Osteoarthritis Outcome Score, IKDC: International Knee Documentation Committee, VAS: Visual Analogue Scale, SF-36: Short Form Health Survey, NSD: no significant difference, p<0.05: significant.
Thirteen studies reported on various clinical outcomes such as WOMAC, KOOS, IKDC, VAS, SF-36, Lysholm and Tegner score. Clinical outcomes significantly improved in all studies irrespective of the effect on cartilage. Interestingly, even in studies where cartilage thickness or volume decreased (non-significant) [10,11,22,23,26], clinical outcomes were significantly improved at follow-up as compared with their baseline.
5. Assessment of methodological quality of studies and quality of evidence
RCTs – Cochrane Risk of Bias Tool [16] (Supplementary material: Table 2): Five RCTs were assessed as low risk of bias [11,19,26,31,32], two as unclear risk of bias having insufficient information for at least one domain [10,22], and one as high risk [25].
Prospective cohort studies – Newcastle Ottawa Scale [17] (Supplementary material: Table 3): One prospective cohort study was rated as “good quality” scoring high in the NOS scale.
Retrospective cohort studies – MINORS criteria [15] (Supplementary material: Table 4): Two studies scored 18 out of 24 points [20,21], while the other three scored 15 out of 24 points [23,27,28].
Quality of evidence: The GRADE approach was used to assess the overall quality of evidence which was “low” [18]. The review included five RCTs, and six non-randomised studies. There was some inconsistency with methodological and clinical heterogeneity, but there was no significant variability in the reported results.
6. Discussion
This systematic review and meta-analysis concluded that treatment of knee OA with PRP is not associated with a significant increase in articular cartilage content and any change in cartilage content was not correlated to clinical outcomes. These findings held both by examining studies that reported pre and post treatment cartilage content as well as studies that compared PRP to a control group.
Hong et al. [4], in a recent systematic review and meta-analysis assessed the safety and efficacy of PRP injections versus placebo (or other conservative management) and showed that PRP is more effective in relieving symptoms (follow-up 6 months). There was no difference between triple versus single PRP injection with regards to their curative effect in short-term. However, they only looked at the clinical outcomes and did not assess the effect of injections on articular cartilage. Another systematic review compared the safety and efficacy of PRP versus HA injections for knee OA [36]. It showed that PRP injections improved clinical outcomes as compared with HA. Moreover, they reported that LP-PRP may be a superior treatment for knee OA as compared with LR-PRP, although admitting that further studies are needed to compare the effect of PRP's leukocyte content on outcomes. However, The American Academy of Orthopaedic Surgeons (AAOS) in its most recent guidelines from August 2021 concluded that PRP may reduce pain and improve function in patients with symptomatic OA of the knee, but downgraded two levels the strength of recommendation to limited due to inconsistent evidence [37]. In line with this, The National Institute for Health and Care Excellence in United Kingdom (UK) in its 2019 guidelines regarding PRP injections for knee OA in adults, suggested that current evidence shows no major safety concerns for PRP injections for knee OA, but with regards to efficacy, evidence is limited and low quality [38].
In addition to any clinical effect, there has been widespread interest as to whether PRP may influence cartilage content, to slow or reverse the process of OA. Such an effect could revolutionize the management of arthritic knees. This was based on encouraging in-vitro and in-vivo studies showing the positive biological effects of platelet-rich products on osteoarthritic chondrocytes and cartilage [[39], [40], [41], [42]], and it was also supported by clinical studies which reported that PRP can improve grade of knee OA on MRI [43,44]. One RCT (n = 58) showed that nearly 50% of patients who had LP-PRP injections had more than one grade OA improvement 6 months post-injections, as compared to only 8% with HA injections (p < 0.003) [44]. Another series of 15 patients with knee OA having PRP injection and MRI follow-up one year post-treatment, reported no significant worsening of the OA (Outerbridge grading) in the patellofemoral joint in 80% of patients, and no change in the medial and lateral compartments in 73% [43]. A recent RCT showed that an absolute count of near 10 billion platelets in the injected PRP is needed to have long-term chondroprotective effect up to one year in patients with moderate knee OA [19]. Another important factor seems to be the proteomic analysis of the injected PRP and identification of proteins which contribute more to tissue healing. PRPs containing high concentration of platelets contain high quantity of bioactive proteins (such as growth factors and cytokines) which can promote tissue healing and regeneration and this has been and still is extensively researched [45,46].
The above findings led to MRI studies which aimed to more accurately evaluate the cartilage content of arthritic knees in relation to PRP injection. MRI is generally considered a reliable and sensitive tool to assess cartilage status and chondral lesion progression especially in osteoarthritic knees [47]. One technique is to measure the cartilage thickness or volume in a lot of areas inside the knee (MFC, LFC, MTP, LTP) which most studies did. Another more detailed technique, which only one study did [21], is to measure T2 relaxation times (T2 mapping) across the knee joint. It has been shown that T2 relaxation time measurements in the knee are sensitive to early cartilage degeneration and reflect the histological changes inside the cartilage matrix [48,49]. Moreover, some recent studies based on large cohorts showed the predictive and prognostic role of T2 mapping in detection of progression of radiological degenerative changes and morphological lesions in osteoarthritic knees, even when radiographic changes are not apparent [50,51]. The most reliable and reproducible methods to assess articular cartilage morphology and repair is with the use of objective assessment tools, such as the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) [52], or the WORMS score [35], or the MOAKS BML [34]. Unfortunately, only two studies in our review used such tools.
Our study has shown that the available evidence does not support a chondrogenic role for PRP. There is inconsistency in the included studies with variable effect on cartilage content, with some reporting an increase and some reporting a decrease following PRP treatment, and the meta-analysis confirmed that. If OA has a natural history of deterioration, even if PRP is chondrogenic, it may not match the rate of cartilage degeneration, and that may explain the variability seen between studies, and this may also depend on which stage of OA the patient is at the time of treatment.
However, our findings must be considered in the light of the limitations of the available literature. All available and eligible studies were small and differed in their protocols in multiple ways which makes it difficult to draw meaningful conclusions. There is lack of standardization in PRP treatment protocol for knee OA in terms of preparation, administration and dosing. The substantial variability in treatment protocols included multiple different preparation techniques, different centrifugation protocols, various administration protocols with different number of injections (1–6), different time intervals between injections (1–4 weeks), different volumes of PRP injections (2–10 ml), different platelet concentrations (1.4–10 times blood concentration), different WBC concentration (from none to LR-PRP), and use of a different activator. A standardized PRP preparation needs to be defined by further review of the scientific evidence as to the efficacy of different PRP preparations in improving clinical outcomes and a consensus approach amongst clinical experts. The quality of evidence is limited by the inclusion of non-randomised studies. We have included seven RCTs with a control group, but a meta-analysis with only Level I studies was not possible due to the significant heterogeneity and the small number of studies. The meta-analysis entailed an overlap of prospective and retrospective studies, but in all included studies data were reliably and prospectively collected. The method of imaging to assess articular cartilage was also not unified in all studies. This is acknowledged and a relevant recommendation is made, but overall the majority of the studies described in detail how they assessed cartilage with these methods and data were reliably reported by experienced radiologists (sometimes more than one). Lastly, there was not enough studies using objective assessment tools to do a sub-group analysis of their results which would strengthen our results and conclusions.
In conclusion, the current literature does not support the PRP as chondrogenic in treatment of knee OA. There is substantial heterogeneity in the evaluated studies which limits the robustness of any conclusion. Given the limitations of the available literature, further research is needed to draw a definite conclusion. A multi-centre adequately powered RCT with a standardized PRP preparation and treatment protocol and standardized high-resolution MRI along with a quality assessment to ensure a reproducible composition of the injectate is needed to definitely define any effect of PRP in knee cartilage content and its relation to clinical outcomes. Until such high-quality evidence becomes available, we recommend that PRP is not administered with the intention of promoting chondrogenesis.
Author contributions
Conception and design: All authors. Analysis and interpretation of data: ADP, CPC, RV, HP. Drafting of the article: All authors. Critical revision of the article: ADP, CPC, RV, HP. Final approval of the article: All authors. Statistical analysis: ADP, CPC. Collection and assembly of data: ADP, EM.
Responsibility for the integrity of the work as a whole is taken by Apostolos D. Prodromidis, MD, MSc (first author: apostolos.prodromidis@nhs.net).
Competing interests
No conflicts of interest to declare.
Role of funding source
No funding was received for this study.
Authorship
All authors should have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. By signing below each author also verifies that he (she) confirms that neither this manuscript, nor one with substantially similar content, has been submitted, accepted or published elsewhere (except as an abstract). Each manuscript must be accompanied by a declaration of contributions relating to sections (1), (2) and (3) above. This declaration should also name one or more authors who take responsibility for the integrity of the work as a whole, from inception to finished article. These declarations will be included in the published manuscript.
Declaration of funding
All sources of funding should be declared as an acknowledgement at the end of the text.
Role of the funding source
Authors should declare the role of study sponsors, if any, in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. If the study sponsors had no such involvement, the authors should state this.
Studies involving humans or animals
Clinical trials or other experimentation on humans must be in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Randomized controlled trials should follow the Consolidated Standards of Reporting Trials (CONSORT) guidelines and be registered in a public trials registry.
Studies involving experiments with animals were in accordance with institution guidelines.
Please sign below to certify your manuscript complies with the above requirements and then upload this form at
Declaration of competing interest
At the end of the text, under a subheading “Conflict of interest statement” all authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and research grants or other funding.
Acknowledgement of other contributors
All contributors who do not meet the criteria for authorship as defined above should be listed in an acknowledgements section. Examples of those who might be acknowledged include a person who provided purely technical help, writing assistance, or a department chair who provided only general support. Such contributors must give their consent to being named. Authors should disclose whether they had any writing assistance and identify the entity that paid for this assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2022.100318.
Contributor Information
Apostolos D. Prodromidis, Email: apostolos.prodromidis@nhs.net.
Charalambos P. Charalambous, Email: mr.charalambous@nhs.net.
Emma Moran, Email: emma_moran@outlook.com.
Ram Venkatesh, Email: ram.venkatesh@nhs.net.
Hemant Pandit, Email: H.Pandit@leeds.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2019;72(2):149–162. doi: 10.1002/acr.24131. 2020 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannuru R.R., Schmid C.H., Kent D.M., Vaysbrot E.E., Wong J.B., McAlindon T.E. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann. Intern. Med. 2015 Jan 6;162(1):46–54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 4.Hong M., Cheng C., Sun X., Yan Y., Zhang Q., Wang W., et al. Efficacy and safety of intra-articular platelet-rich plasma in osteoarthritis knee: a systematic review and meta-analysis. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/2191926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLarnon M., Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC Muscoskel. Disord. 2021 Jun 16;22(1):550. doi: 10.1186/s12891-021-04308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H., Knapik D.M., Polce E.M., Eikani C.K., Bjornstad A.H., Gursoy S., et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am. J. Sports Med. 2021 Aug 17 doi: 10.1177/03635465211029659. [DOI] [PubMed] [Google Scholar]
- 7.Andia I., Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013 Dec;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 8.Garbin L.C., Olver C.S. Platelet-rich products and their application to osteoarthritis. J. Equine Vet. Sci. 2020 Mar;86 doi: 10.1016/j.jevs.2019.102820. [DOI] [PubMed] [Google Scholar]
- 9.Kabiri A., Esfandiari E., Esmaeili A., Hashemibeni B., Pourazar A., Mardani M. Platelet-rich plasma application in chondrogenesis. Adv. Biomed. Res. 2014;3:138. doi: 10.4103/2277-9175.135156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raeissadat S.A., Ghorbani E., Sanei Taheri M., Soleimani R., Rayegani S.M., Babaee M., et al. MRI changes after platelet rich plasma injection in knee osteoarthritis (randomized clinical trial) J. Pain Res. 2020;13:65–73. doi: 10.2147/JPR.S204788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennell K.L., Paterson K.L., Metcalf B.R., Duong V., Eyles J., Kasza J., et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021 Nov 23;326(20):2021–2030. doi: 10.1001/jama.2021.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P.T.T.J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021) Cochrane; Cochrane: 2021. www.training.cochrane.org/handbook Available from: [Google Scholar]
- 13.Bellamy N., Campbell J., Robinson V., Gee T., Bourne R., Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2005 Apr 18;(2) doi: 10.1002/14651858.CD005321. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 2015 Nov;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003 Sep;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G.A.S.B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. 2008. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2021 January]; Available from: [Google Scholar]
- 18.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal H., Leon J., Pont J.L., Wilson D.A., Bansal A., Agarwal D., et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci. Rep. 2021 Feb 17;11(1):3971. doi: 10.1038/s41598-021-83025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calis H.T., Sütbeyaz S.T., Güler E., Halici C., Sayan H., Koc A., et al. Efficacy of intra-articular autologous platelet rich plasma application in knee osteoarthritis. Turkish J. Rheumatology. 2015;30(3):198. [Google Scholar]
- 21.Cobianchi Bellisari F., De Marino L., Arrigoni F., Mariani S., Bruno F., Palumbo P., et al. T2-mapping MRI evaluation of patellofemoral cartilage in patients submitted to intra-articular platelet-rich plasma (PRP) injections. La radiologia medica. 2021 2021/08/01;126(8):1085–1094. doi: 10.1007/s11547-021-01372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elnemr R.A., Abdelnaby H.M., Elshafei M.M. Does intra-articular platelet rich plasma injection improve meniscal repair outcomes? Asian J. Sports Med. 2019;10(3) [Google Scholar]
- 23.Guillibert C., Charpin C., Raffray M., Benmenni A., Dehaut F.X., El Ghobeira G., et al. Single injection of high volume of autologous pure PRP provides a significant improvement in knee osteoarthritis: a prospective routine Care study. Int. J. Mol. Sci. 2019 Mar 15;20(6) doi: 10.3390/ijms20061327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart R., Safi A., Komzák M., Jajtner P., Puskeiler M., Hartová P. Platelet-rich plasma in patients with tibiofemoral cartilage degeneration. Arch. Orthop. Trauma. Surg. 2013 Sep;133(9):1295–1301. doi: 10.1007/s00402-013-1782-x. [DOI] [PubMed] [Google Scholar]
- 25.Kenmochi M. Clinical outcomes following injections of leukocyte-rich platelet-rich plasma in osteoarthritis patients. J. Orthop. 2020 Mar-Apr;18:143–149. doi: 10.1016/j.jor.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart R., Safi A., Jajtner P., Puskeiler M., Hartova P. Tibiofemoral chondromalacia treated with platelet-rich plasma and hyaluronic acid. Curr. Orthopaedic Pract. 2017;28(1):58–65. [Google Scholar]
- 27.Sampson S., Reed M., Silvers H., Meng M., Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am. J. Phys. Med. Rehabil. 2010 Dec;89(12):961–969. doi: 10.1097/PHM.0b013e3181fc7edf. [DOI] [PubMed] [Google Scholar]
- 28.Şen E., Yıldırım M.A., Yeşilyurt T., Kesiktaş F.N., Dıraçoğlu D. Effects of platelet-rich plasma on the clinical outcomes and cartilage thickness in patients with knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2020;33(4):597–605. doi: 10.3233/BMR-181209. [DOI] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathes T., Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med. Res. Methodol. 2017 Jul 17;17(1):107. doi: 10.1186/s12874-017-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buendía-López D., Medina-Quirós M., Fernández-Villacañas Marín M. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J. Orthop. Traumatol. 2018 Aug 20;19(1):3. doi: 10.1186/s10195-018-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elik H., Doğu B., Yılmaz F., Begoğlu F.A., Kuran B. The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2020;33(1):127–138. doi: 10.3233/BMR-181374. [DOI] [PubMed] [Google Scholar]
- 33.Dohan Ehrenfest D.M., Andia I., Zumstein M.A., Zhang C.Q., Pinto N.R., Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014 Jan;4(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter D.J., Guermazi A., Lo G.H., Grainger A.J., Conaghan P.G., Boudreau R.M., et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011 Aug;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterfy C.G., Guermazi A., Zaim S., Tirman P.F., Miaux Y., White D., et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004 Mar;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Belk J.W., Kraeutler M.J., Houck D.A., Goodrich J.A., Dragoo J.L., McCarty E.C. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am. J. Sports Med. 2021 Jan;49(1):249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Orthopaedic Surgeons (AAOS) 2021. Management of Osteoarthritis of the Knee (Non-arthroplasty) Evidence-Based Clinical Practice Guideline.https://www.aaos.org/oak3cpg [updated Published 08/31/2021; cited 2022 06/06/2022]; Available from: [Google Scholar]
- 38.National Institute for Health and Care Excellence (NICE) 2019. Platelet-rich Plasma Injections for Knee Osteoarthritis.https://www.nice.org.uk/guidance/ipg637 [cited 2022 25/01/2022]; Available from: [Google Scholar]
- 39.Akeda K., An H.S., Okuma M., Attawia M., Miyamoto K., Thonar E.J., et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006 Dec;14(12):1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Saito M., Takahashi K.A., Arai Y., Inoue A., Sakao K., Tonomura H., et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin. Exp. Rheumatol. 2009 Mar-Apr;27(2):201–207. [PubMed] [Google Scholar]
- 41.van Buul G.M., Koevoet W.L., Kops N., Bos P.K., Verhaar J.A., Weinans H., et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am. J. Sports Med. 2011 Nov;39(11):2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 42.Wanstrath A.W., Hettlich B.F., Su L., Smith A., Zekas L.J., Allen M.J., et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in a canine population. Vet. Surg. 2016 Aug;45(6):764–774. doi: 10.1111/vsu.12512. [DOI] [PubMed] [Google Scholar]
- 43.Halpern B., Chaudhury S., Rodeo S.A., Hayter C., Bogner E., Potter H.G., et al. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin. J. Sport Med. 2013 May;23(3):238–239. doi: 10.1097/JSM.0b013e31827c3846. [DOI] [PubMed] [Google Scholar]
- 44.Lisi C., Perotti C., Scudeller L., Sammarchi L., Dametti F., Musella V., et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin. Rehabil. 2018 Mar;32(3):330–339. doi: 10.1177/0269215517724193. [DOI] [PubMed] [Google Scholar]
- 45.Fréchette J.P., Martineau I., Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J. Dent. Res. 2005 May;84(5):434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 46.Lee H.W., Choi K.H., Kim J.Y., Kim K.O., Haotian B., Yuxuan L., et al. Proteomic classification and identification of proteins related to tissue healing of platelet-rich plasma. Clin. Orthop. Surg. 2020 Mar;12(1):120–129. doi: 10.4055/cios.2020.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckstein F., Cicuttini F., Raynauld J.P., Waterton J.C., Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(Suppl A):A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Li X., Cheng J., Lin K., Saadat E., Bolbos R.I., Jobke B., et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn. Reson. Imaging. 2011 Apr;29(3):324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishioka H., Hirose J., Nakamura E., Oniki Y., Takada K., Yamashita Y., et al. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J. Magn. Reson. Imag. 2012 Jan;35(1):147–155. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 50.Liebl H., Joseph G., Nevitt M.C., Singh N., Heilmeier U., Subburaj K., et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann. Rheum. Dis. 2015 Jul;74(7):1353–1359. doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad A.P., Nardo L., Schooler J., Joseph G.B., Link T.M. T₁ρ and T₂ relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013 Jan;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marlovits S., Singer P., Zeller P., Mandl I., Haller J., Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006 Jan;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.