Abstract
Objective
Cartilage defect treatment strategies are dependent on the lesion size and severity. Osteochondral explant models are a platform to test cartilage repair strategies ex vivo. Current models lack in mimicking the variety of clinically relevant defect scenarios. In this controlled laboratory study, an automated device (artificial tissue cutter, ARTcut®) was implemented to reproducibly create cartilage defects with controlled depth. In a pilot study, the effect of cartilage defect depth and oxygen tension on cartilage repair was investigated.
Design
Osteochondral explants were isolated from porcine condyles. 4 mm chondral and full thickness defects were treated with either porcine chondrocytes (CHON) or co-culture of 20% CHON and 80% MSCs (MIX) embedded in collagen hydrogel. Explants were cultured with tissue specific media (without TGF-β) under normoxia (20% O2) and physiological hypoxia (2% O2). After 28 days, immune-histological stainings (collagen II and X, aggrecan) were scored (modified Bern score, 3 independent scorer) to quantitatively compare treatment outcome.
Results
ARTcut® represents a software-controlled device for creation of uniform cartilage defects. Comparing the scoring results of the MIX and the CHON treatment, a positive relation between oxygen tension and defect depth was observed. Low oxygen tension stimulated cartilaginous matrix deposition in MIX group in chondral defects and CHON treatment in full thickness defects.
Conclusion
ARTcut® has proved a powerful tool to create cartilage defects and thus opens a wide range of novel applications of the osteochondral model, including the relation between oxygen tension and defect depth on cartilage repair.
Keywords: Cartilage defect, ex vivo model, Cartilage test system, Chondrocytes, MSC, Collagen type I hydrogel
1. Introduction
Defects of the articulating surface are frequently occurring diseases in the field of orthopedics, traumatology and sports medicine [1]. Around 14% of patients who suffered from a trauma induced knee injury during their middle ages, including fractures of the tibia, fibula, femur, or patella, develop osteoarthritis in the same knee joint at later age (>65 years) [2]. High risk of failure of more than 20% in cartilage defect repair require to rethink and improve current cartilage treatment concepts with the aim for long-term repair of the tissue [3,4].
Due to the avascular and aneural character of adult articular cartilage, large defects do not heal spontaneously [5]. Defects that are classified according to International Cartilage Repair Society (ICRS) grade III (defect depth >50% cartilage depth) and IV (defect down to the subchondral bone) need surgical intervention to repair [6]. Gold standard for defects larger than 2–3 cm2 is the autologous chondrocyte implantation (ACI), resulting in similar outcome as microfracture [7,8]. In a recent data analysis for cartilage defects, matrix-associated chondrocyte implantation (M-ACI) showed a significant lower reoperation rate than microfracture 2 years post-op [9]. M-ACI as well as ACI require two surgical interventions; the first to take a cartilage tissue sample for subsequent chondrocyte isolation and the second one for injection of isolated in vitro expanded autologous chondrocytes into defect site.
However, (M−)ACI has two main limitations: Due to the limited number of healthy and non-degenerated chondrocytes, cells have to be expanded in vitro to achieve sufficient number of cells needed for the implantation (ACI: 1 million cells per cm2, M-ACI: 20 million cells per cm3) [[10], [11], [12]]. Further, chondrocyte-based cartilage treatments, as well as microfracture techniques, result in formation of fibrocartilage, characterized by its inferior mechanical properties and thus lacking functional restoration compared to healthy hyaline cartilage [4,[13], [14], [15], [16], [17]]. One approach to overcome the limitation of cell number for (M−)ACI treatment are MSC-chondrocyte co-cultures, e.g., 80% MSCs and 20% chondrocytes, that reduces autologous chondrocyte cell number at same total cell density. Co-cultures of MSCs and chondrocytes have shown to increase cartilaginous matrix production and reduce hypertrophy, associated with MSCs during chondrogenic differentiation [18,19].
To bring cartilage treatments to the next level, cartilage repair needs to be studied and understood in more detail, starting with basic research questions in a physiologically relevant environment.
An ex vivo cartilage defect test system based on osteochondral explants represents a valuable ex vivo platform for biomaterial evaluation in critical size trauma-induced cartilage defects in terms of biocompatibility, biomaterial tissue integration, and cartilage repair with higher troughput compared to in vivo models [20]. Separated media compartments of the culture device allow for controlled, tissue- and cell-specific nutrient supply during ex vivo culture of osteochondral explants. This model also allows for direct comparison of different treatment approaches under controlled conditions and has been shown to stimulate cartilage-like tissue formation of chondrocytes or MSCs in osteochondral lesions [21,22].
However, this defect model was limited to full thickness defects created with a biopsy punch. To date, the creation of defects that do not fully penetrate the cartilage with the help of a biopsy punch or scalpel suffer from low reproducibility and are dependent on the operator. Therefore, an automated device with control over drilling depth to create defects with high reproducibility can overcome this limitation to study chondral wound healing. Of note, the drilling should not harm surrounding cartilage tissue and cell viability, neither by mechanical tissue disruption nor by friction induced heating. For the creation of defects in hard tissues like cartilage or even bone, there is no device available for automated defect creation that meets the above-mentioned requirements.
Following, in the present study the defect creation of the ex vivo defect model introduced by Schwab et al. was modified by implementation of a semi-automated drilling device, originally developed to mechanically induce standardized and reproducible wounds in full thickness skin equivalents [23]. Key features of this artificial tissue cutter (ARTcut®) are the sensor controlled optical barrier in combination with a moveable milling machine along x-, y- and z-axis. The light-barrier controlled drilling allows for creation of defects with defined depths in a reproducible set-up. Creation of tissue defects with ARTcut® can be performed under sterile conditions for subsequent in vitro or ex vivo culture with the possibility to adjust defect geometry and control of defect creation process.
Another fact that has been neglected in previous studies is the low oxygen tension present in the articular capsule [24]. It has been shown in literature that low oxygen tension is an important stimulus in chondrogenesis and increases the chondrogenic potential of articular cartilage progenitor cells and MSCs [[24], [25], [26]].
Taken together, the present study aimed to implement the ARTcut® as tool for automated wounding to the ex vivo model allowing to study the influence of defect depth (full thickness defects and chondral defects) on tissue repair. In a pilot study, the relation between oxygen tension (normoxia 20% vs. physiological hypoxia 2%), defect depth and treatment strategy was studied. For the treatment strategies either chondrocytes alone (CHON) or a mixture of 80% MSCs and 20% CHON (MIX) were embedded in a collagen type I hydrogel for implantation into cartilage defects in the ex vivo osteochondral model without further supplementation of growth factors to elusively study the effect of the culture conditions on cartilage repair.
2. Methods
2.1. Osteochondral cylinders: isolation, defect creation and ex vivo culture
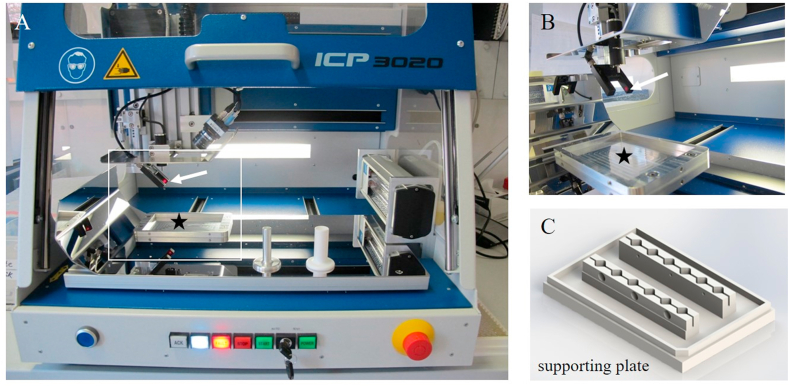
Osteochondral cylinders (diameter: 8 mm, height: 5 mm) were isolated from medial femoral condyles of 6-8-month-old domestic pigs as previously described [20]. Creation of defects in osteochondral cylinders was carried out either manually or automatically, depending on the intended defect depth: Full thickness defects were manually created with a biopsy punch (diameter 4 mm; Kai Medical, BPP-40F). Chondral defects of 1 mm depth were created using custom-built ARTcut® (Fig. 1A), a software-controlled device for automatic wound placement in tissues developed by Fraunhofer ISC and IGB, Wuerzburg, Germany [23]. Osteochondral cylinders were fixed in support plate (Fig. 1C) at specific x- and y-positions and onset of drilling 1 mm chondral defects (z-axis) was determined with light beam (Fig. 1B). For more details on the standardized defect creation process using ARTcut®, see chapter 1.1 in the supplementary information.
Fig. 1.
Artificial tissue cutter (ARTcut®): A) View of the computerized numerical controlled device equipped with B) optical barrier (white arrow indicates the laser light beam) and C) supporting plate to fix the samples and place on the bottom of the machine (marked with black star).
After creation of full thickness or 1 mm chondral defects, the cartilage defects were filled with cell embedded collagen type I hydrogels (Fig. 2). For subsequent ex vivo culture, osteochondral explants were transferred into custom-made culture platform [20] and cultured for 28 days with tissue specific media (Table 1) changed every 3–4 days. The ex vivo culture was carried out in a humidified atmosphere at 37 °C and 5% CO2 (BBD 6220 CO2 Incubator, Thermo Scientific™) either under normoxic (20%) or physiological hypoxic (2%) conditions.
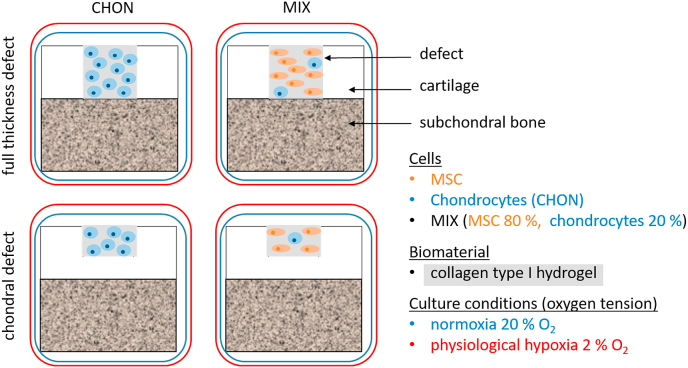
Fig. 2.
Schematic overview of all experimental groups: Full thickness and chondral defects of osteochondral explants were treated with collagen type I hydrogel (grey) either containing chondrocytes (CHON, blue) or MIX (co-culture of 80% MSCs [orange] and 20% chondrocytes), implanted in chondral or full thickness defects and cultured ex vivo under normoxic (20% O2, blue frame) or physiological hypoxic conditions (2% O2, red frame).
Table 1.
Composition of bone and cartilage media for ex vivo culture of osteochondral explants.
| Cartilage media | Bone media | Supplier | |
|---|---|---|---|
| DMEM GlutaMAX (high glucose) | 98% | 89% | Gibco 61965 |
| Antibiotics | 10 U/mL penicillin, 10 μg/mL streptomycin, 0.25 μg/mL amphotericin (1% (v/v)) | 10 U/mL penicillin, 10 μg/mL streptomycin, 0.25 μg/mL amphotericin (1% (v/v)) | Gibco, 15240 |
| Sodium-pyruvate | 1 mM | – | Gibco, 11360 |
| l-ascorbic acid-2-phosphate | 50 μg/mL | 50 μg/mL | Wako chemicals, 013-19641 |
| L-proline | 40 μg/mL | – | Sigma-Aldrich, P5607 |
| Dexamethasone | 100 nM | 100 nM | Sigma-Aldrich, D4902 |
| Fetal bovine serum (FBS) | 10% (v/v) | Gibco, 10270106 | |
| Insulin-transferrin-selenin | 1% (v/v) | – | ITS+-premix, Gibco, Life Technologies, 32430 |
| β-glycerophosphate | – | 10 mM | Sigma-Aldrich, G9422 |
2.2. Chondrocytes and mesenchymal stromal cells (MSCs): isolation and in vitro expansion
Porcine chondrocytes were isolated from lateral condyles of 6–8-month-old domestic pigs by enzymatic digestion as previously described [20]. Chondrocytes were used at passage 0 – directly after isolation - for embedding in collagen type I hydrogel.
Porcine MSCs were isolated from bone marrow aspirate (iliac crest) under the approval (reference number: 55.2 2532-2-256) of the District Government of Lower Franconia and the local animal welfare committee and performed according to the German Animal Welfare Act and the EU Directive 2010/63/EU according to the procedure described in chapter 1.2 in the supplementary.
2.3. Cell embedding in collagen type I hydrogel
In a pilot study, cartilage defects were treated with collagen type I hydrogel (see chapter 1.3 in the supplementary for details). For implantation, two different cell laden constructs, as illustrated in Fig. 2, were prepared and each cultured under normoxic and physiological hypoxic conditions: 1) CHON were prepared by embedding of chondrocytes in collagen type I hydrogel without expansion at a final density of 20 million cells/mL hydrogel volume; 2) MIX were prepared by mixing 80% MSCs with 20% chondrocytes at same cell density. CHON and MIX implants were filled into the cartilage defect for gelation at 37 °C.
2.4. Live-dead viability staining
To visualize a possible effect of defect creation process on cell viability of tissue samples, live-dead viability staining was performed (live-dead staining kit for mammalian cells, L3224, Invitrogen). Osteochondral explants were incubated with 4 μM calcein AM and 2 μM ethidium homodimer-1 in DMEM HG and visualized with fluorescence microscope (494 nm/517 nm and 517 nm/617 nm wavelength) Keyence BZ-9000 (Biorevo). Living cells are stained by calcein AM in green, dead cells are stained by ethidium homodimer-1 in red.
2.5. Histological evaluation
Osteochondral explants, harvested on day 0 and day 28, were washed with phosphate buffered saline, fixed for 24 h with 4% formalin (Roti Histofix, Carl Roth, P087.3) and processed with plastic embedding (Technovit T9100, Heraeus Kulzer, 66006735) as detailed described in the supplementary.
For immune-histological stainings, antigens were enzymatically retrieved depending on antibody (Table 2). Before incubation with primary antibody over night at 4 °C, slides were blocked with 3% (v/v) H2O2 (Carl Roth, 8070) and incubated with 5% (w/v) bovine serum albumin. All stainings were visualized with horseradish-peroxidase kit (DCS Innovative, Dako, K3468) following manufacturer's instructions. Cell nuclei were counterstained with Mayer's hematoxylin (Morphisto, 11895), rehydrated and mounted with Entellan (Merck, 1079610500). All washing steps between blocking or chemical incubation were performed with PBS supplemented with 0.5% Tween-20 (VWR, 8.22184).
Table 2.
List of antibodies, including antibody concentrations and enzymes used for antigen retrieval in immuno histological stainings.
| Antibody | Clone | Dilution and final antibody concentration | Antigen retrieval | Antibody supplier |
|---|---|---|---|---|
| Collagen type I | EPR7785 | 1:1000; 0.875 μg/mL | pepsin & hyaluronidase | Abcam, ab138492 |
| Collagen type II | II-4C11 | 1:1000; 1 μg/mL | pepsin & hyaluronidase | MP Bio, 63171 |
| Collagen type X | COL-10 | 1:4000; 0.25 μg/mL | pronase | Sigma-Aldrich, C7974 |
| Aggrecan | EPR7785 | 1:2000; 0.5 μg/mL | pronase | Invitrogen, AHP0012 |
2.6. Histological scoring
Histological scoring for the evaluation of the immune histological stainings (collagen type I, II, X and aggrecan) was performed. For in vitro tissue engineered samples, the Bern score has been introduced, while cartilage tissue repair in clinical studies can be evaluated based on the International Cartilage Repair Society (ICRS) score II [27]. Further, Chang et al. introduced a modified ICRS score by adding two new criteria, namely the visual assessment of collagen type I and II staining [28]. The inclusion of extracellular markers is an important step to better evaluate the composition of the repair matrix. Therefore, in this pilot study, the scoring category A (Bern score), originally evaluating the uniformity and darkness of Safranin O-fast green stain, was translated to immune histological stainings of collagen type I, II, X and aggrecan [29]. Stained sections (n = 2 biological replicates) were evaluated blinded by three independent operators according to the criteria summarized in Table 3. Maximum possible score for the here reported study was 12 points.
Table 3.
Criteria for evaluating immune histological stainings with a maximum of 12 points (0–3 scores for each criteria assessing the uniformity and darkness of aggrecan, collagen type I, II and X staining). Table adapted from Grogan et al. and Chang et al. [28,29].
| Uniformity and darkness of aggrecan stain |
| 0: No stain, 1: Weak staining of poorly formed matrix, 2: Moderately even staining, 3: Even dark stain |
| Uniformity and darkness of collagen type II stain |
| 0: No stain, 1: Weak staining of poorly formed matrix, 2: Moderately even staining, 3: Even dark stain |
| Uniformity and darkness of collagen type I stain |
| 3: No stain, 2: Weak staining of poorly formed matrix, 1: Moderately even staining, 0: Even dark stain |
| Uniformity and darkness of collagen type X stain |
| 3: No stain, 2: Weak staining of poorly formed matrix, 1: Moderately even staining, 0: Even dark stain |
2.7. Statistics
Statistical analysis was performed with GraphPad Prism 6.07. Scoring values obtained from two independent experiments, each evaluated by three examinators were used for statistical analysis, resulting in n = 6 for each experimental group (Fig. 2). Two groups differing in only one culture parameter (oxygen tension, defect depth and applied cell type) were directly compared. Following tests for outlier and Gaussian distribution, the data was either compared using an unpaired t-test (normally distributed values) or a Mann-Whitney test (not normally distributed values). A p-value <0.05 was considered statistically significant.
3. Results
3.1. Chondral defect creation with ARTcut®
ARTcut® (Fig. 1) represents an automated device for reproducible creation of chondral defects (1 mm in depth; Fig. 3A). Main advantage of using the ARTcut® is the ability to create defined chondral defects with a flat bottom to allow a tide and uniform contact with any solid pre-formed cylindrical implant, for example bioprinted constructs with flat bottom.
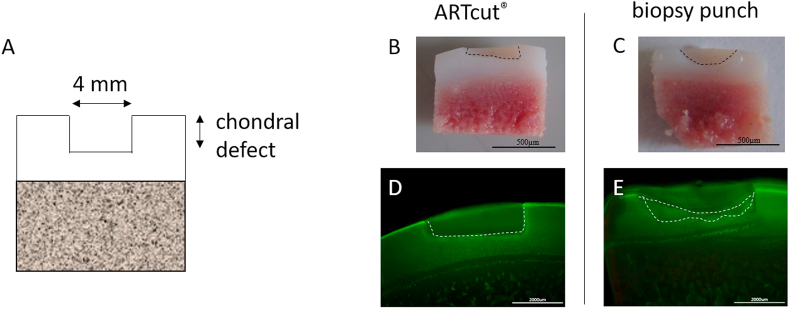
Fig. 3.
Osteochondral explant cross section with chondral defect. A) Schematic illustration of 4 mm chondral defect. B-E) Comparison of chondral defect created automated with ARTcut® (B) and manually with biopsy punch (C) (scale bar 500 μm). D-E) Live-dead staining of explant cross section highlighting accurate borders of defect induced with ARTcut® (D) and more irregular shaped geometry of manually induced defect (E). Scale bar 200 μm.
The laser beam to detect the surface of every single osteochondral explant is the unique feature of the ARTcut® to define the onset of the drilling, thus all defects result with the same depth. Chondral cartilage defect induced with ARTcut® appeared as a well-defined rectangular defect boundary in cross section (diameter: 4 mm, height: 1 mm) with flat bottom (Fig. 3B). In contrast, the manually created defect (using biopsy punch) resulted in a half-round shape and required removing of residual cartilage tissue with a second tool (e.g., a sharp spoon) shown in Fig. 3C. Following, the accuracy of chondral defect geometry was much lower in manual procedure - using biopsy punch - compared to software – using automated ARTcut®.
The automated defect creation took less than 5 seconds and thus heating of tissue due to friction and rotation during drilling was very unlikely. Microscopic images of live-dead stainings of osteochondral explants with 1 mm cartilage defects (diameter: 4 mm) confirmed no indications of cell death after tissue defect creation, neither with ARTcut® nor with biopsy punch (Fig. 3D and E).
For the creation of full thickness cartilage defects, the ARTcut® was not required, since the bottom of the defect was given by the cartilage-bone interface. This two-tissue interface was easily separated using biopsy punch by pulling out the upper cartilage part.
3.2. Evaluation of cartilage treatments in the ex vivo model
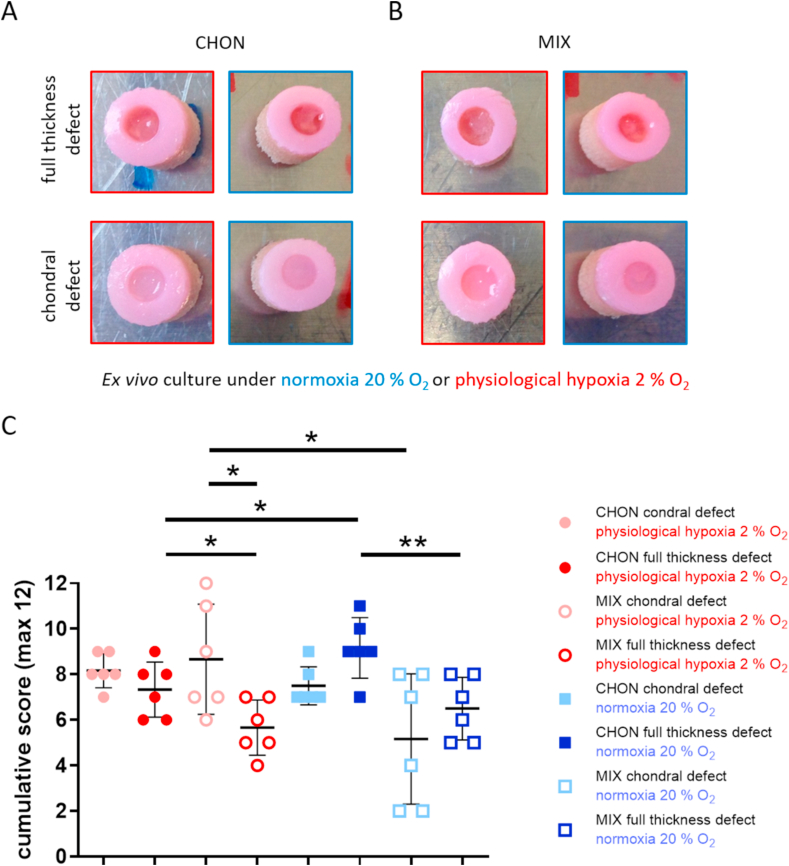
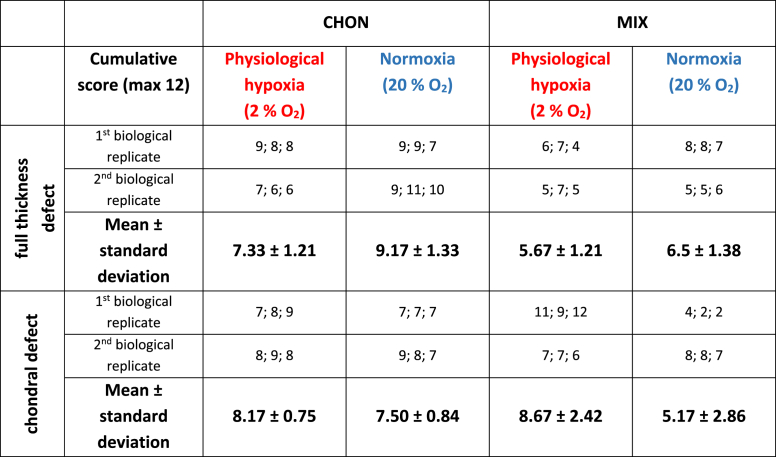
Macroscopic images of all treatment and culture groups of the osteochondral ex vivo model after 28 days of culture are illustrated in Fig. 4A and B. The results of the pilot study demonstrated the feasibility and reproducibility of the ex vivo cartilage test system as a platform to compare cartilage repair strategies. Results of the adapted Bern scoring are shown in Fig. 4 and Table 4 with scoring results of the co-culture (MIX) being close to the CHON treatment in chondral and full thickness defects. Representative sections of the scored samples are illustrated in Fig. 5, Fig. 6.
Fig. 4.
Evaluation of defect repair after 28 days ex vivo culture. Macroscopic images of full thickness and chondral defects (diameter 4 mm) treated with A) porcine chondrocytes (CHON) or B) co-culture of porcine MSCs and CHON (MIX). Red frame indicates culture under physiological hypoxic, blue frame under normoxic oxygen tension. Macroscopically, there are no obvious differences comparing full thickness and chondral defects. C) Scoring evaluation of cartilage repair strategies in the ex vivo model (mean ± standard deviation with single scoring results as dots resp. listed values for 1st and 2nd biological replicate). Unpaired t-test or Mann-Whitney test ∗ p < 0.05, ∗∗p < 0.01.
Table 4.
Scoring results to evaluate cartilage repair strategies in the ex vivo model. The table summarizes the cumulative score of the 3 independent scorer (mean ± standard deviation with single scoring results of both biological replicates) for the CHON (100% chondrocytes) and MIX (20% chondrocytes, 80% MSCs) treatment of the full thickness and chondral defects after 28 days culture. Values for samples cultured under physiological hypoxia (2% O2) and normoxia (20% O2) are displayed in separate columns.
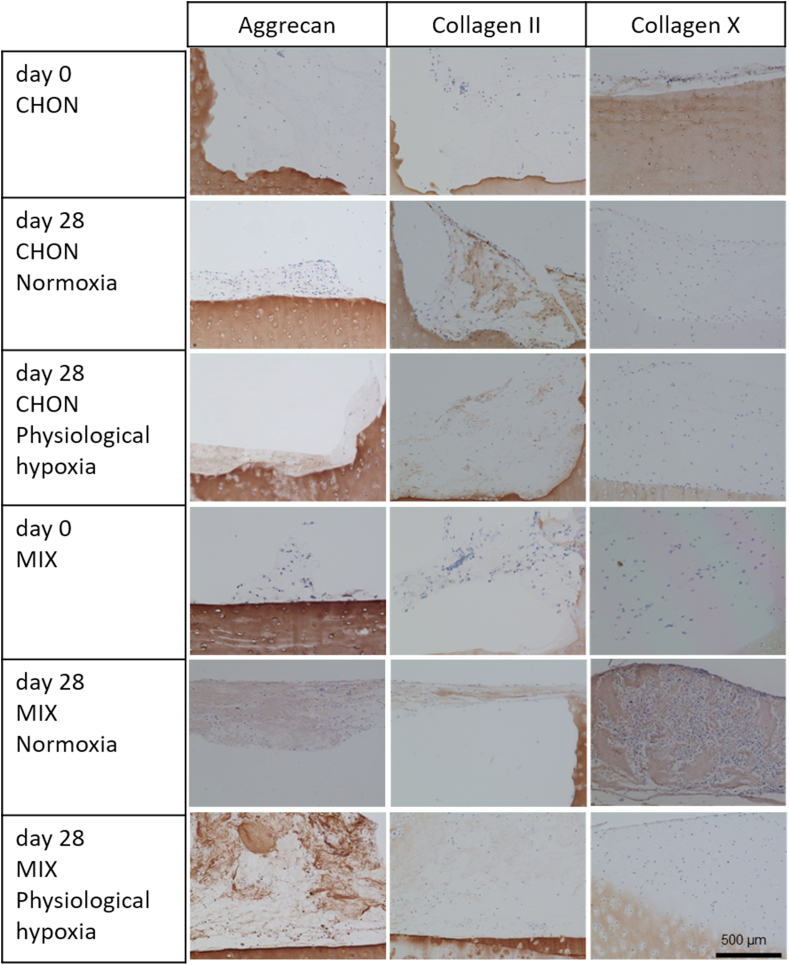
Fig. 5.
Chondral defects of the osteochondral defect model treated with either chondrocytes (CHON) or MSC-chondrocyte co-culture (MIX) embedded in a collagen type I hydrogel. Histological stainings (aggrecan, collagen II and X) of cross sections show the defect area at day 0 and after 28 days of ex vivo culture under normoxic (20% O2) or physiological hypoxic (2% O2) conditions. Scale bar 500 μm.
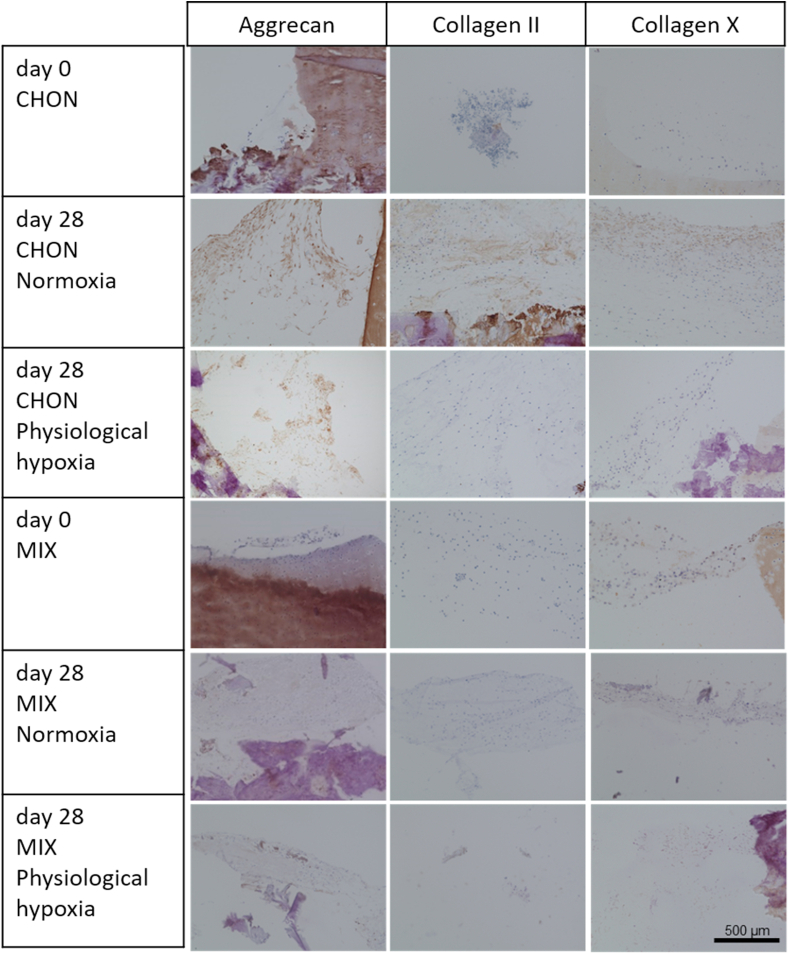
Fig. 6.
Full thickness cartilage defects of the osteochondral defect model treated with either chondrocytes (CHON) or MSC-chondrocyte co-culture (MIX) embedded in a collagen type I hydrogel. Histological stainings (aggrecan, collagen II and X) of cross sections show the defect area at day 0 and after 28 days of ex vivo culture under normoxic (20% O2) or physiological hypoxic (2% O2) conditions. Scale bar 500 μm.
Under physiological hypoxic conditions, scoring results of defect repair after co-culture treatment (MIX) was higher (p = 0.0218) in chondral defects than in full thickness defects. Scoring values of CHON treatment in chondral and full thickness experimental groups did not differ much (n.s.; p = 0.1828). At physiological hypoxic conditions, the scoring value was higher for CHON treatment compared to MIX treatment in full thickness defects (p = 0.0384).
Under normoxic conditions, full thickness defects reached higher scoring values compared to the chondral defects.
Comparing the outcome of same defect depth and treatment group (cell type), but cultured under physiological hypoxic or normoxic conditions, the oxygen tension plays only a significant role in chondral defects using MIX (p = 0.0451, higher scoring values under physiological hypoxic conditions) and in full thickness defects treated with CHON (p = 0.0316, lower scoring values under physiological hypoxic conditions).
Overall, treatment of the two defect depths with MIX showed slightly reduced scoring values compared to the CHON treatment group. Only exception is MIX chondral (physiological hypoxia) with a slightly higher value as the corresponding one for CHON. The results of the MIX treatment groups also tend to spread more than the CHON treatment group independently of defect depth and oxygen tension.
4. Discussion
The decision for the treatment approach for cartilage repair is dependent on the size and depth of the cartilage defect and thus the severity of pathological condition.
To mimic these different pathological defect scenarios, the implementation of the ARTcut® allowed to overcome the low reproducibility of manual induced chondral defects. So far, there is no other automated defect creation device published that has comparable features, namely possibility to select different drilling parameters within one run and the application of one device for a wide range of soft and hard tissues or tissue equivalents, as it is demonstrated with the ARTcut®. The induction of defects in osteochondral explants allows to compare different cell-based treatment approaches by implantation of cell loaded materials into the defects or to study the effect of defect depth on repair in an ex vivo model. The software-controlled drilling parameters allow for standardized and reproducible defect creation in other soft and hard tissues including bone tissue as well. In vitro as well as ex vivo studies require sample procession at high throughput rates under a negative microbial environment. ARTcut® device addresses these demands, resulting in higher methodological accuracy and reproducibility, compared to manual procedure using biopsy punch.
Current treatments of small or large cartilage defects mainly aim on pain and symptom relief rather than on functional tissue repair [30]. From a scientific point of view, there is way for improvement of cartilage defects, especially on long term results. Material-assisted as well as cell-based therapies, including (M−) ACI, AMIC and microfracture, tend to result in formation of mechanical inferior fibrocartilage in long term follow up [[30], [31], [32]]. Moreover, the treatment of large defects with autologous cells are limited due to the high number of chondrocytes needed for the current techniques. Therefore, in this laboratory-controlled study, an ex vivo osteochondral defect model was modified to screen cell-based approaches to treat chondral and full thickness cartilage defects. Different cell types, namely chondrocytes and MSCs, were embedded in collagen type I hydrogel (CHON or MIX) and implanted in trauma induced chondral and full thickness defects. A tendency of a stimulative and beneficial effect of MIX treatment (20% CHON and 80% MSCs) was observed regarding cartilaginous matrix formation with the main advantage to reduce the overall number of CHON used for traditional ACI treatment. It has been demonstrated in previous studies that the cells in cartilage and subchondral bone remained metabolic active up to 56 days of ex vivo culture [20].
Promising results regarding cartilage matrix production were obtained in this pilot study comparing CHON and MIX in chondral and full thickness defects. The most pronounced increase in proteoglycan content normalized to DNA amount resulted for the MIX treatment in both conditions, chondral and full thickness defect, cultured under physiological hypoxia (Supplementary Figure S1). The MIX treatment reduces the amount of autologous cartilage tissue needed for chondrocyte isolation, since chondrocytes represented only 20% of the total cell number in our model. Several in vitro studies and in silico models have shown that chondrocytes in co-culture with MSCs increase the chondrogenic differentiation potential [18,19,33,34]. Scalzone et al. reported of an enhanced chondrocyte activity measured by proteoglycan and collagen type II production after co-culture with MSCs compared to chondrocyte monoculture both embedded in chitosan based hydrogels [19].
One advantage of the co-culture approach – when clinically applied – would be the one-step procedure, addressing the surgical intervention of the knee joint, for the treatment of the cartilage defect: The reduced number of chondrocytes in the MIX treatment can be isolated intra-operatively without expansion and thus reduced the risk of chondrocyte dedifferentiation [35]. Further, the required MSCs, e.g., can be harvested independently form the knee surgery from bone marrow aspirate in a minimal invasive procedure with subsequent expansion prior to the cartilage repair procedure to ensure a sufficient number of MSCs.
Considering the absence of TGF-β in the culture media, which is known to drive MSC chondrogenesis and matrix production [36], the here reported cartilage repair solely derives from the stimulating effect of the surrounding osteochondral tissue, cell-cell signaling of the cell laden implants and the culture conditions. Therefore, the overall matrix deposition is rather low due to missing stimuli of exogenous growth factors in the here described model.
Results of a similar ex vivo model, based on horse and bovine osteochondral explants, suggested that the presence of the osteochondral tissue in the explants increases cartilage-like matrix deposition compared to free swelling conditions of cell seeded material only [21,22].
Beside the cell source, the influence of oxygen tension (normoxia 20% O2, physiological hypoxia 2% O2) was studied during the ex vivo culture on chondrogenic matrix deposition, resulting in different outcomes between the treatment groups in chondral and full thickness defects. Based on the histological scoring, the results of the pilot study showed that the oxygen tension seems to only play a role in chondral defects - marked by elevated matrix production. In contrast, full thickness defects showed different response in cartilage-like matrix deposition related to the treatment approach: While higher scoring values were reached with CHON treatment under normoxic conditions, the MIX treatment did not show differences comparing physiological hypoxic and normoxic conditions. Physiological hypoxic conditions resulted in an increase in cartilaginous matrix deposition in chondral defects compared to full thickness defects independently of the used cell type (CHON, MIX). Once the defect reaches the cartilage-bone interface, the cells in the implant receive stimuli from the subchondral bone that seem to counteract with the hypoxic induced stimuli in the here presented model. One explanation of the differential cell response to oxygen tension in chondral and full thickness defects may originate from the variation in oxygen tension present in the human body. Chondrocytes are exposed to lower oxygen tension in avascular cartilage (2–5%) [37], synovial fluid and the synovial capsule (6.5–9.0%) [25,38], with an increasing oxygen level in the bone marrow of subchondral bone (>7%) [26,39] and a maximum of 12% in arterial blood [40]. Once the cartilage defect progresses to the exposure of the subchondral bone in patients, chondrocytes within the defect are exposed to higher levels of oxygen present in the bone supplied by vascular invasion [41]. It has been shown that low oxygen inhibits the degradation of hypoxia induced factors (HIF) [42]. HIFs are described to be essential for maintaining CHON homeostasis and extracellular matrix synthesis and activate the transcription of genes [43].
While the here presented results of the pilot study are consistent, traceable, and in line with literature, there are still limitations in the current experimental setup: Due to limitations in availability of human healthy osteochondral tissue – absence of any osteoarthritic phenotype – the authors decided to use porcine tissue instead, as introduced by Schwab et al. [20]. The performance of an extensive comparative ex vivo study comprising three parameters – namely 1) defect depth (chondral vs. full thickness), 2) different cell types for defect filling and treatment (100% chondrocytes vs. 20% chondrocytes and 80% MSCs), and 3) oxygen tension (physiological hypoxia vs. normoxia) requires tissue material in the required quantity and of similar quality. Due to the well-known donor issues associated with donor variability of human tissue and the already mentioned limited availability of healthy human osteochondral tissue, this could not be achieved for human tissue [44,45]. In contrast, the use of porcine tissue isolated from one pig population (similar age; grown up under equal conditions) minimizes the donor variation and represents a tissue source for healthy and non-degenerated tissues with higher reproducibility. To proof the findings and the trend of the here presented study and to show clear differences between treatment groups, this experimental set-up needs to be repeated with more biological replicates.
The results obtained in this pilot study indicate that the surrounding tissue of the osteochondral explants plays a crucial role in the defect repair, addressing chondral or full thickness defects, and that the oxygen tension additionally stimulates the outcome in a defect depth dependent way. However, the exact mechanisms controlling success or failure of the treatment approach are not fully understood and require further research. Despite the advantage of the here reported model, the mimicry of the complex 3D environment present in the knee joint with the additional ability to reproducibly create (trauma induced) defects, this model has its potential in pre-screening several biomaterials or treatment approaches, but best candidates still require further pre-clinical and clinical testing to confirm obtained results.
To conclude, in this ex vivo study two cell-based approaches (CHON vs. MIX embedded in collagen type I hydrogel) for the treatment of chondral and full thickness defects, cultured under normoxic and physiological hypoxic conditions, were compared on their cartilage repair potential. Independently of defect depth, the scoring results of the co-culture of chondrocytes and MSCs (MIX) are close to those of CHON treatment. Hence, usage of MIX could be a promising approach to reduce the number of chondrocytes to one fifth and thus the amount of tissue for harvest and accompanied side morbidities for the patient. Further, in this experimental set up the oxygen tension showed an influence in a defect depth dependent way that has not been reported in literature before. The here presented advanced osteochondral cartilage defect model provides a promising platform to deeper understand the underlying mechanism.
Author contribution
Conception and design of the study: Franziska Ehlicke and Andrea Schwab; Acquisition of data: Alexa Buss and Andrea Schwab; Analysis and interpretation of data: Andrea Schwab, Alexa Buss, Franziska Ehlicke and Oliver Pullig; Manuscript drafting: Andrea Schwab and Alexa Buss; Revision of the manuscript: Franziska Ehlicke, Oliver Pullig and Andrea Schwab; Final approval: Franziska Ehlicke, Andrea Schwab, Alexa Buss and Oliver Pullig; Funding: Franziska Ehlicke and Alexa Buss.
Role of funding sources
This work was financially supported by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no 309962 (HydroZONES). Alexa Buss was supported by a medical scholarship of the German Excellence Initiative to the Graduate School of Life Sciences, University of Wuerzburg. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Ethics approval and consent to participate
Animal experiment was approved (reference number: 55.2 2532-2-256) by the District Government of Lower Franconia and the local animal welfare committee and performed according to the German Animal Welfare Act and the EU Directive 2010/63/EU. Following heparinization of the pig, porcine MSCs were obtained by bone marrow aspiration.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank Andreas Diegeler (Fraunhofer ISC, Bronnbach, Germany) for technical support of the ARTcut®. Thanks also to Sebastian Naczenski for establishing the protocol for wounding of osteochondral explants using ARTcut® device. Finally, we would like to thank Heike Walles for her support. We acknowledge the authors Andrea Schwab and Alexa Buss for the permission to use some data produced in the light of their doctoral thesis (Fig. 1, Fig. 3 from Schwab 2017 [46] and Fig. 5, Fig. 6 from Buss 2021) [47].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100173.
Contributor Information
Andrea Schwab, Email: andrea.schwab@aofoundation.org.
Alexa Buss, Email: alexa.buss@kwm-klinikum.de.
Oliver Pullig, Email: oliver.pullig@isc.fraunhofer.de.
Franziska Ehlicke, Email: franziska.ehlicke@uni-wuerzburg.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Gelber A.C., Hochberg M.C., Mead L.A., Wang N.Y., Wigley F.M., Klag M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 2000;133(5):321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 3.Martín A.R., Patel J.M., Zlotnick H.M., Carey J.L., Mauck R.L. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. npj Regenerative Medicine. 2019;4(1):12. doi: 10.1038/s41536-019-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wylie J.D., Hartley M.K., Kapron A.L., Aoki S.K., Maak T.G. Failures and reoperations after matrix-assisted cartilage repair of the knee: a systematic review. Arthroscopy. 2016;32(2):386–392. doi: 10.1016/j.arthro.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole B.J., Pascual-Garrido C., Grumet R.C. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91(7):1778–1790. [PubMed] [Google Scholar]
- 8.Dewan A.K., Gibson M.A., Elisseeff J.H., Trice M.E. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. BioMed Res. Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemeyer P., Schubert T., Grebe M., Hoburg A. Matrix-associated chondrocyte implantation is associated with fewer reoperations than microfracture: results of a population-representative, matched-pair claims data analysis for cartilage defects of the knee. Orthop J Sports Med. 2019;7(10) doi: 10.1177/2325967119877847. 2325967119877847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 11.Mumme M., Barbero A., Miot S., Wixmerten A., Feliciano S., Wolf F., Asnaghi A.M., Baumhoer D., Bieri O., Kretzschmar M., Pagenstert G., Haug M., Schaefer D.J., Martin I., Jakob M. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet (London, England) 2016;388:1985–1994. doi: 10.1016/S0140-6736(16)31658-0. 10055. [DOI] [PubMed] [Google Scholar]
- 12.Davies R.L., Kuiper N.J. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel) 2019;6(1) doi: 10.3390/bioengineering6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamplot J.D., Schafer K.A., Matava M.J. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sports Med. 2018;6(3) doi: 10.1177/2325967118761871. 2325967118761871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkely G., Ogura T., Ackermann J., Barbieri Mestriner A., Gomoll A.H. Cartilage; 2019. Clinical Outcomes after Revision of Autologous Chondrocyte Implantation to Osteochondral Allograft Transplantation for Large Chondral Defects: A Comparative Matched-Group Analysis. 1947603519833136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., Mandelbaum B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am. J. Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 16.Kreuz P.C., Steinwachs M.R., Erggelet C., Krause S.J., Konrad G., Uhl M., Sudkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Carey J.L. Fibrocartilage Following Microfracture Is Not as Robust as Native Articular Cartilage: commentary on an article by Aaron J. Krych, MD, et al. Activity Levels Are Higher After Osteochondral Autograft Transfer Mosaicplasty Than After Microfracture for Articular Cartilage Defects of the Knee. A Retrospective Comparative Study”, JBJS. 2012;94(11) doi: 10.2106/JBJS.L.00319. [DOI] [PubMed] [Google Scholar]
- 18.Bian L., Zhai D.Y., Mauck R.L., Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage, Tissue engineering. Part A. 2011;17(7–8):1137–1145. doi: 10.1089/ten.TEA.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scalzone A., Ferreira A.M., Tonda-Turo C., Ciardelli G., Dalgarno K., Gentile P. The interplay between chondrocyte spheroids and mesenchymal stem cells boosts cartilage regeneration within a 3D natural-based hydrogel. Sci. Rep. 2019;9(1):14630. doi: 10.1038/s41598-019-51070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab A., Meeuwsen A., Ehlicke F., Hansmann J., Mulder L., Smits A., Walles H., Kock L. Ex vivo culture platform for assessment of cartilage repair treatment strategies. ALTEX. 2017;34(2):267–277. doi: 10.14573/altex.1607111. [DOI] [PubMed] [Google Scholar]
- 21.Mouser V.H.M., Dautzenberg N.M.M., Levato R., van Rijen M.H.P., Dhert W.J.A., Malda J., Gawlitta D. Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair. ALTEX. 2018;35(1):65–76. doi: 10.14573/altex.1704171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries-van Melle M.L., Narcisi R., Kops N., Koevoet W.J., Bos P.K., Murphy J.M., Verhaar J.A., van der Kraan P.M., van Osch G.J. Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone. Tissue Eng. 2014;20(1–2):23–33. doi: 10.1089/ten.TEA.2013.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi A., Appelt-Menzel A., Kurdyn S., Walles H., Groeber F. Generation of a three-dimensional full thickness skin equivalent and automated wounding. JoVE. 2015;96 doi: 10.3791/52576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattappa G., Schewior R., Hofmeister I., Seja J., Zellner J., Johnstone B., Docheva D., Angele P. Physioxia has a beneficial effect on cartilage matrix production in interleukin-1 beta-inhibited mesenchymal stem cell chondrogenesis. Cells. 2019;8(8) doi: 10.3390/cells8080936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattappa G., Johnstone B., Zellner J., Docheva D., Angele P. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson D.E., Markway B.D., Bond D., McCarthy H.E., Johnstone B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res. Ther. 2016;7(1):154. doi: 10.1186/s13287-016-0419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutgers M., van Pelt M.J.P., Dhert W.J.A., Creemers L.B., Saris D.B.F. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18(1):12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Chang F., Ishii T., Yanai T., Mishima H., Akaogi H., Ogawa T., Ochiai N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J. Orthop. Res. 2008;26(1):18–26. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 29.Grogan S.P., Barbero A., Winkelmann V., Rieser F., Fitzsimmons J.S., O'Driscoll S., Martin I., Mainil-Varlet P. Visual histological grading system for the evaluation of in vitro–generated neocartilage. Tissue Eng. 2006;12(8):2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- 30.Horas U., Pelinkovic D., Herr G., Aigner T., Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 32.Carey J.L. Fibrocartilage following microfracture is not as robust as native articular cartilage: commentary on an article by Aaron J. Krych, MD, et al. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. A retrospective comparative study", J Bone Joint Surg Am. 2012;94(11):e80. doi: 10.2106/JBJS.L.00319. [DOI] [PubMed] [Google Scholar]
- 33.Chen M.J., Whiteley J.P., Please C.P., Schwab A., Ehlicke F., Waters S.L., Byrne H.M. Inducing chondrogenesis in MSC/chondrocyte co-cultures using exogenous TGF-β: a mathematical model. J. Theor. Biol. 2018;439:1–13. doi: 10.1016/j.jtbi.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Kim T.W., Lee M.C., Bae H.C., Han H.-S. Direct coculture of human chondrocytes and synovium-derived stem cells enhances in vitro chondrogenesis. Cell J. 2018;20(1):53–60. doi: 10.22074/cellj.2018.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von der Mark K., Gauss V., von der Mark H., Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S., Cui Z., Urban J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage–bone interface: a modeling study. Arthritis Rheum. 2004;50(12):3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 38.Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970;13(6):769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- 39.Carreau A., El Hafny-Rahbi B., Matejuk A., Grillon C., Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011;15(6):1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marenzana M., Arnett T.R. The key role of the blood supply to bone. Bone Research. 2013;1(1):203–215. doi: 10.4248/BR201303001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipowska J., Tomaszewski K.A., Niedzwiedzki L., Walocha J.A., Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291–302. doi: 10.1007/s10456-017-9541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremer A., Wussmann M., Herrmann M., Raghunath M., Walles H. Ciclopirox olamine promotes the angiogenic response of endothelial cells and mesenchymal stem cells. Clin. Hemorheol. Microcirc. 2019;73(2):317–328. doi: 10.3233/CH-190559. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Torres J., Zamudio-Cuevas Y., Martinez-Nava G.A., Lopez-Reyes A.G. Hypoxia-Inducible Factors (HIFs) in the articular cartilage: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 2017;21(12):2800–2810. [PubMed] [Google Scholar]
- 44.Chang H.X., Yang L., Li Z., Chen G., Dai G. Age-related biological characterization of mesenchymal progenitor cells in human articular cartilage. Orthopedics. 2011;34(8):e382–e388. doi: 10.3928/01477447-20110627-06. [DOI] [PubMed] [Google Scholar]
- 45.Kim M., Erickson I.E., Huang A.H., Garrity S.T., Mauck R.L., Steinberg D.R. Donor variation and optimization of human mesenchymal stem cell chondrogenesis in hyaluronic acid. Tissue Eng. 2018;24(21–22):1693–1703. doi: 10.1089/ten.TEA.2017.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwab A. Julius-Maximilians University; Wuerzburg: 2017. Development of an Osteochondral Cartilage Defect Model. [Google Scholar]
- 47.Buss A. Julius-Maximilians University; Wuerzburg: 2021. Evaluation of Cartilage Regeneration Strategies in an Osteochondral Ex Vivo Cartilage Defect Model. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.