Abstract
Liver fibrosis is the most important prognostic factor in patients with nonalcoholic fatty liver disease (NAFLD). Several noninvasive markers for fibrosis, including blood-based markers and imaging based-markers have been developed. Indirect fibrosis markers (e.g., fibrosis-4 index and NAFLD fibrosis score) consist of standard laboratory data and clinical parameters. Given its availability and high negative predictive value for advanced fibrosis, these markers are suitable for screening at primary care. Blood-based fibrogenesis markers (enhanced liver fibrosis and N-terminal propeptide of type 3 collagen), ultrasound-based modalities (vibration-controlled transient elastography, point shear wave elastography [SWE], and two-dimensional SWE), and magnetic resonance elastography have high diagnostic accuracy for liver fibrosis and are suitable for diagnosing liver fibrosis at secondary care centers. Sequential use of these markers can increase diagnostic accuracy and reduce health care costs. Furthermore, combining noninvasive makers may assist in identifying candidates for pharmacological trials and reducing screening failure. Emerging data suggest that these noninvasive markers are associated with liver-related events (hepatocellular carcinoma and decompensation) and mortality. Furthermore, delta change in noninvasive markers over time is also associated with time-course change in fibrosis, liver-related event risk, and mortality risk. However, the association between liver fibrosis and cardiovascular disease (CVD) risk is still controversial. CVD risk may decrease in patients with decompensated liver disease and noninvasive markers may be useful for assessing CVD risk in these patients. Therefore, noninvasive markers may be utilized as measures of fibrosis as well as real-time prognostic tools, in place of liver biopsy.
Keywords: cardiovascular disease (CVD), fibrosis, hepatocellular carcinoma (HCC), nonalcoholic fatty liver disease (NAFLD), noninvasive
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease and affects about one-fourth of the population worldwide.1,2 A subset of patients with NAFLD progress to nonalcoholic steatohepatitis (NASH), hepatocellular carcinoma (HCC), liver failure, and death.3 NAFLD-related HCC has been increasing along with the rising prevalence of obesity and diabetes mellitus.4 Furthermore, NAFLD is associated with metabolic dysfunction and cardiovascular disease (CVD) is the main cause of death in NAFLD.5 Therefore, NAFLD has emerged as a major burden to national health care systems and the global economy.6,7
Liver fibrosis is the most important prognostic factor for liver-related morbidity and mortality in patients with NAFLD and accurate diagnosis of liver fibrosis is important in clinical practice.8,9 Liver biopsy is the gold standard for the assessment of liver fibrosis.10,11 However, liver biopsy has several limitations including sampling variability, and intra- and inter-observer reproducibility as well as its invasive nature and has potential risks including pain, infection, bleeding, perforation and rarely death.12 To circumvent the limitations of liver biopsy, several noninvasive modalities have been developed and used in clinical practice.13-15 High diagnostic accuracy of noninvasive modalities including serum-based markers and imaging-based modalities have been reported.13 Furthermore, recent studies have demonstrated noninvasive markers are associated with liver-related morbidity and mortality. In this review, we will discuss and compare the diagnostic accuracy among noninvasive markers of fibrosis. In addition, we review the clinical utility of noninvasive fibrosis measures as prognostic tools in patients with NAFLD.
NONINVASIVE FIBROSIS MARKERS IN NAFLD
Blood-based markers for liver fibrosis
Given the high prevalence of NAFLD, simple and widely available noninvasive modalities are needed. Since blood-based fibrosis markers may be performed without specialized equipment and are relatively inexpensive, these are good candidates for use in the general population. Several blood-based markers including indirect markers (e.g., fibrosis-4 index [FIB-4],16 NAFLD Fibrosis Score [NFS],17 and mac-2 binding protein glycosylation isomer [M2BPGi]) and direct markers for fibrogenesis (e.g., Enhanced Liver Fibrosis Score [ELF], type IV collagen 7S, N-terminal propeptide of type 3 collagen [Pro-C3]) have been developed and are used in clinical practice.
Indirect fibrosis markers
FIB-4 is composed of age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelets.16 NFS is composed of age, body mass index (BMI), impaired fasting glucose/diabetes, AST, ALT, platelets, and albumin.17 Since FIB-4 and NFS require only standard laboratory data and clinical parameters, these markers are suitable for use in the general population. In a meta-analysis including 13046 patients with biopsy-proven NAFLD, the area under the receiver operating characteristics (AUROC) of FIB-4 and NFS for advanced fibrosis (histological fibrosis stage 3 or 4) were 0.84 and 0.84, respectively.18 The diagnostic accuracy of FIB-4 and NFS were higher than other indirect blood-based markers but lower than imaging-based modalities. One utility of FIB-4 and NFS is that negative predictive values (NPV) of FIB-4 (<1.3) and NFS (<−1.45) for advanced fibrosis or cirrhosis are high (>90%). In Japanese biopsy-proven NAFLD, NPV of FIB-4 for advanced fibrosis was 98%,19 and therefore, these markers can be used to exclude patients with advanced fibrosis or cirrhosis, who are at high risk of liver-related morbidity or mortality.20 Based on these results, the American Association for the Study of Liver Diseases and the Japanese Society of Hepatology recommend FIB-4 and NFS as an initial screening method in primary care.21-23
Mac-2 binding protein glycosylation isomer (M2BPGi) has been developed in Japan as a serum fibrosis marker and has been used mainly in Asia. M2BP, a secreted glycoprotein present in the extracellular matrix, is associated with cell adhesion and correlates with liver fibrosis.24,25 Specific glycan structures of M2BP change as liver fibrosis progresses, and liver fibrosis is evaluated by measuring the proportion of M2BP with altered glycan structure.26 The change in the M2BP glycan structure is detected using the lectin Wisteria floribunda agglutinin and was found to be correlated with the progression of fibrosis. In a study that included 289 patients with biopsyproven NAFLD, the AUROC of M2BPGi for advanced fibrosis was0. 879 and it was higher than FIB-4 (AUROC: 0.857) and NFS (AUROC: 0.808).27 M2BPGi value of 1.0-1.2 COI is considered as an optimal threshold for advanced fibrosis in patients with NAFLD.28 Another study that compared M2BPGi and FIB-4 demonstrated that optimal cutoff values of M2BPGi for advanced fibrosis were similar in age-differ groups, while those of FIB-4 increased in parallel with age.29 Therefore, although thresholds of FIB-4 vary by age, M2BPGi can measure liver fibrosis independent of age and may be useful for assessing liver fibrosis in the general population.30 However, further validation studies are needed, especially in other regions outside Asia.
Direct fibrosis markers
Indirect fibrosis markers such as FIB-4 and NFS can estimate liver fibrosis, but they do not reflect directly fibrogenesis. To overcome this deficiency, direct fibrosis markers that reflect fibrogenesis have been developed. Enhanced liver fibrosis (ELF) consists of three components: type III procollagen peptide, hyaluronic acid, and tissue inhibitor of metalloproteinase-1. A meta-analysis that included 11 studies in patients with NAFLD demonstrated that the AUROC of ELF for advanced fibrosis was 0.83, with a sensitivity of 73% and specificity of 80%.31 ELF thresholds of 7.7 for excluding fibrosis and 10.18 for detecting advanced fibrosis were proposed in the study. Another study that investigated the diagnostic accuracy of ELF in 829 patients with NAFLD demonstrated that the AUROC for advanced fibrosis was 0.81. Furthermore, the performance of ELF was similar regardless of age or diabetes mellitus.32 Another study demonstrated that the diagnostic accuracy of ELF is comparable to an imaging-based modality.33
Type IV collagen 7S is a fragment of type IV collagen, which consists basement membrane. Basement membrane increase as liver fibrosis increases and type IV collagen 7S is associated with liver fibrosis. In a study that included 874 patients with biopsy-proven NAFLD, the diagnostic accuracy of type IV collagen 7S for advanced fibrosis was higher than other fibrosis markers including FIB-4 and NFS, regardless of diabetes mellitus status.34 Other studies also demonstrated high diagnostic accuracy of type IV collagen 7S for liver fibrosis in patients with NAFLD.35,36
The accumulation of excess extracellular matrix (ECM) leads to liver fibrosis progression, PRO-C3 is released during ECM formation (type III collagen) and PRO-C3 reflects the dynamic activity of the formation and degradation of ECM.37 In a study that included 431 patients with biopsy-proven NAFLD, the AUROC of PRO-C3 for detecting advanced fibrosis was 0.81–0.83, and the diagnostic accuracy was similar with available noninvasive markers (e.g., FIB-4 and NFS).38 To improve the diagnostic accuracy, a PRO-C3 based fibrosis algorithm that included age, presence of diabetes, PRO-C3, and platelet counts (ADAPT) was proposed.38 When ADAPT was used, the AUROC for advanced fibrosis improved to 0.86–0.87. Another study that included 517 patients with biopsy-proven NAFLD demonstrated that the diagnostic accuracy of ADAPT for advanced fibrosis was higher than other noninvasive markers such as FIB-4.39 Since ELF, type IV collagen 7S or PRO-C3 reflect fibrogenesis, it may be useful for determining treatment response or longitudinal changes in liver fibrosis. However, the validation studies are relatively small compared to FIB-4 or NFS, and further data are needed.
Imaging-based modalities for liver fibrosis
Recent advances in technology make it possible to measure liver stiffness, which is a reflection of liver fibrosis, using ultrasound-based modalities or magnetic resonance imaging (MRI)-based modality (magnetic resonance elastography [MRE]). Ultrasound-based modalities include vibration-controlled transient elastography (VCTE), point shear wave elastography (pSWE), and two-dimensional SWE (2D-SWE). Each modality is based on a different principle, but all modalities measure liver stiffness and can be used for assessing liver fibrosis.
Vibration-controlled transient elastography
VCTE (FibroScan®) was first developed as an ultrasound-based noninvasive modality to measure liver stiffness.40 In a meta-analysis comparing the diagnostic accuracy between VCTE and serum fibrosis markers, the AUROC of VCTE for advanced fibrosis was 0.85–0.88 and it was higher than serum fibrosis markers (0.76–0.84).18 Since the diagnostic accuracy of VCTE is well validated and VCTE has higher diagnostic accuracy than serum markers, it is widely used in clinical practice. However, one limitation of VCTE is the high failure rate (5%–27%).41 Obesity is associated with measurement failure of VCTE,42 and to mitigate measurement failure, an obesity specific (XL) probe has been developed.43 By combining the use of M and XL probes, measurement failure can be reduced to <10%44
Shear wave elastography
One disadvantage of VCTE is that the operator is blind to the exact location of the area being assessed. This may increase the failure rate of VCTE. On the other hand, SWE (both pSWE and 2D-SWE) is incorporated into standard B-mode ultrasound the area of interest being assessed may be visualized simultaneously, thereby reducing measurement failure.45 In a study investigating 291 patients with NAFLD using VCTE and pSWE contemporaneously, measurement failure occurred in 14.4% with VCTE and 0.7% with pSWE.46 In a meta-analysis that included 13 studies for patients with NAFLD, AUROC for advanced fibrosis was high as 0.94. However, the cutoff value had a relatively large range (1.33–2.20 m/s).47 2D-SWE can measure a larger area in the liver than VCTE or pSWE, which may allow for a more accurate assessment of liver stiffness. In a study that investigated 981 patients with chronic liver disease with 2D-SWE, measurement failure occurred in 2.1%.48 In studies that compared the diagnostic accuracy of 2D-SWE and VCTE in patients with NAFLD, the diagnostic accuracy of 2D-SWE for significant fibrosis (F2-4), advanced fibrosis, and cirrhosis was comparable to VCTE.49,50 pSWE and 2D-SWE can measure liver stiffness with comparable diagnostic accuracy to VCTE and less measurement failure. However, validation studies of 2D-SWE are relatively smaller than VCTE, and further investigation is needed. Several SWEs are made by different manufacturers and compatibility between SWEs has not been verified yet. Therefore, this point also should be validated in a future study.
Magnetic resonance elastography
MRE can measure liver stiffness throughout the liver.51 Although sampling error is a disadvantage of liver biopsy or ultrasound-based elastography, a more accurate assessment of liver fibrosis is possible with MRE. MRE has a low measurement failure rate (<5%).13 In a meta-analysis including 230 patients with biopsy-proven NAFLD, the diagnostic accuracy for significant fibrosis, advanced fibrosis or cirrhosis was higher in MRE than VCTE.52 The AUROC of MRE for significant fibrosis, advanced fibrosis, or cirrhosis was >0.90.53,54 In a study that compared observer reproducibility among MRE, VCTE, and 2D-SWE, infra- and inter-observer reproducibility were higher for MRE than VCTE and 2D-SWE.49 In a recent meta-analysis including 82 studies with 14609 patients with NAFLD, AUROCs for advanced fibrosis were 0.85 for VCTE, 0.91 for MRE, 0.86 for pSWE, and 0.75 for 2D-SWE. Similarly, AUROCs for cirrhosis were 0.89 for VCTE, 0.90 for MRE, 0.90 for pSWE, and 0.88 for 2D-SWE.55 MRE has the highest diagnostic accuracy for liver fibrosis among noninvasive modalities, and MRE is suitable for use as an inclusion criteria or primary endpoint in early phase NASH clinical trials instead of liver biopsy.56,57 Characteristics of each noninvasive modality are summarized in Table 1.
TABLE 1.
Characteristics of each noninvasive modality for the assessment of liver fibrosis
| Accuracy | Validation | Measurement failure |
Availability | Cost | Components | |
|---|---|---|---|---|---|---|
| Blood-based marker | ||||||
| FIB-4 | + | +++ | - | +++ | + | |
| NAFLD fibrosis score (NFS) | + | +++ | - | +++ | + | Age, AST, ALT, platelets |
| M2BPGi | ++ | + | - | +++ | ++ | Age, BMI, impaired fasting glucose/diabetes, AST, ALT, platelets, albumin |
| ELF | ++ | ++ | - | +++ | ++ | |
| PRO-C3 | ++ | + | - | +++ | ++ | Type III procollagen peptide, hyaluronic acid, tissue inhibitor of metalloproteinase-1 |
| Imaging-based marker | ||||||
| VCTE | +++ | +++ | ++ | ++ | +++ | |
| pSWE | +++ | ++ | + | +-++ | +++ | |
| 2D-SWE | +++ | + | + | +-++ | +++ | |
| MRE | ++++ | +++ | + | + | ++++ |
Notes: Plus signs indicate the score between each modality from low (+) to high (++++).
Abbreviations: 2D-SWE, two-dimensional shear wave elastography; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; ELF, enhanced liver fibrosis; M2BPGi, mac-3 binding protein glycosylatlon isomer; MRE, magnetic resonance elastography; PRO-C3, N-terminal propeptide of type 3 collagen; pSWE, point shear wave elastography; VCTE, vibration-controlled transient elastography.
Novel biomarkers
Emerging data suggest that the dysregulation of gut microbiome has been implicated in the progression of NAFLD to advanced fibrosis and cirrhosis, and liver fibrosis may be detected using a gut-microbiome-derived signature.58 In a study investigating patients with NAFLD and their first-degree relatives, gut-microbiome derived signatures can detect patients with NAFLD-cirrhosis and the utility was validated in their first-degree relatives.59 Furthermore, the utility of a gut-microbiome derived signature for detecting cirrhosis was examined in a geographically distinct cohort and high diagnostic accuracy (AUROC >0.90) was validated.60 Gut-microbiome signatures are associated with liver fibrosis in real-time, and may be useful as a non-invasive marker for treatment response, therapeutic targets, or future fibrosis progression. However, further studies are required.
Limitations and confounding factors of noninvasive markers
Limitations and confounding factors of each noninvasive marker are summarized in Table 2. FIB-4 and NFS include age in the formula. Therefore, these values change vary by age and the diagnostic accuracy decreases in elderly and younger patients.61 To mitigate these limitation, an age-specific threshold has been proposed.62,63 However, this limits the utility of FIB-4 and NFS when applied in a large population. On the other hand, M2BPGi is not affected by age and may be more appropriate for use in a large population.29 However, M2BPGi values increase in patients with viral hepatitis.28,64 Therefore, etiology-specific thresholds of M2BPGi are needed. Type III collagen is found in not only in liver but also other organs.65,66 Therefore, type III collagen level increases in other fibrotic disease such as lung disease and kidney disease and the diagnostic accuracy of ELF or PRO-C3 is influenced by these diseases.
TABLE 2.
Confounders and limitations of each noninvasive modality for liver fibrosis
| Confounders | Limitation | |
|---|---|---|
| Blood-based marker | ||
| FIB-4, NAFLD fibrosis score | Age, acute hepatitis, systemic inflammation | The diagnostic accuracy decreases in elderly or younger patients Age-specific thresholds are needed |
| M2BPGi | Viral hepatitis, systemic inflammation | Etiology-specific thresholds are needed |
| ELF, PRO-C3 | Other fibrotic diseases | Validation studies are limited |
| Imaging-based marker | ||
| VCTE | Obesity, food intake, acute hepatitis, systemic inflammation | The diagnostic accuracy decreases in patients with obesity Obesity specific probe can be used but the compatibility between probes is not fully evaluated |
| pSWE, 2D-SWE | Food intake, acute hepatitis, systemic inflammation | Validation studies are limited |
| MRE | Contraindications to MRI, iron overload | High cost and low availability |
Abbreviations: 2D-SWE, two-dimensional shear wave elastography; ELF, enhanced liver fibrosis; M2BPGi, mac-3 binding protein glycosylation isomer; MRE, magnetic resonance elastography; PRO-C3, N-terminal propeptide of type 3 collagen; pSWE, point shear wave elastography; VCTE, vibration-controlled transient elastography.
Obesity is associated with an increasing risk of VCTE measurement failure. Measurement failure can be reduced by using XL probe. When the diagnostic accuracy between standard (M) probe and XL probes were compared, the diagnostic accuracy was similar, but probe-dependent cutoff values were needed.67,68 Therefore, further study is required to examine compatibility between probes. There are limited data on the association between obesity and SWE. Food intake increases liver stiffness due to an increase in portal blood flow and it decreases the diagnostic accuracy of VCTE, pSWE, and 2D-SWE.69 Patients who have contraindications to MRI, such as pregnancy or claustrophobia are not able to undergo MRE. Furthermore, iron overload leads to measurement failure and decrease the diagnostic accuracy of MRE.70 Another limitation of MRE is its high cost and low availability.
Combining noninvasive modalities
To detect liver fibrosis using noninvasive markers, two thresholds (rule-in and rule-out thresholds) have been proposed. Therefore, a subset of patients are classified as indeterminate and require further investigation. To mitigate this limitation, combination/sequential use of noninvasive markers has been proposed.71 A study including 3202 biopsy-proven NAFLD demonstrated that FIB-4 followed by ELF or VCTE can reduce the indeterminate subgroup of patients with acceptable performance.72 Recent studies including a meta-analysis also demonstrated a sequential combination, FIB-4 or NFS followed by VCTE increase the diagnostic accuracy than FIB-4 or VCTE alone with decreasing indeterminate patients.73,74 Indeterminate patients usually require a liver biopsy to discriminate advanced fibrosis, therefore combining noninvasive modalities is a useful strategy for reducing the need for liver biopsy. Furthermore, this strategy can be used in primary care centers. NPV of FIB-4 (< 1.3) for advanced fibrosis is comparable to MRE, and therefore, FIB-4 can be used as the initial screening modality at primary care.75 Patients with FIB-4 <1.3 have a low risk of advanced fibrosis and these patients do not need a referral to specialist care.76 Only patients with FIB-4 ≥1.3 require further investigation. Using this two-step strategy, patients with advanced fibrosis may be accurately detected and unnecessary referrals reduced, resulting in substantial cost savings.77,78
Candidates for pharmacological trials
There is currently no approved drug for the treatment of NASH. In phase 3 pharmacological trials in NASH, liver biopsy is necessary for the enrollment in trials, but a high screening failure rate (>70%) by liver biopsy is a major issue.79 To mitigate this, developing a noninvasive strategy is pivotal and a strategy for efficiently identifying patients for clinical trials has been proposed.80
Significant fibrosis (histological fibrosis stage ≥2) is used as inclusion criteria and MEFIB index (the combination of FIB-4 and MRE) for detecting patients with significant fibrosis has been proposed to identify suitable candidates.81 Patients with significant fibrosis can be detected using the MEFIB index (FIB-4 ≥1.6 and MRE ≥3.3 kPa) and the diagnostic accuracy of the MEFIB index was higher than FIB-4 alone or MRE alone.81 NASH (at least one grade of lobular inflammation, steatosis, and ballooning) with significant fibrosis (F ≥ 2) and NAFLD activity score (combining lobular inflammation, steatosis, and ballooning score) ≥ 4 is also used as inclusion criteria for pharmacological clinical trials. To detect these patients, FibroScan-AST score (combination of liver stiffness by VCTE [FibroScan], controlled attenuation parameter by FibroScan, and AST: FAST) has been proposed.82 The diagnostic accuracy of FAST was investigated in eight independent international cohorts and high diagnostic accuracy (AUROC: 0.74–0.95) was confirmed. Other studies also demonstrated the utility of FAST score.83,84 Therefore, MEFIB and FAST can be used as screening tools for detecting candidates for clinical trials and these contribute to reducing screening failure and excess liver biopsy. In a study comparing the diagnostic accuracy between MEFIB and FAST for significant fibrosis, MEFIB has higher diagnostic accuracy than FAST for significant fibrosis.85 Therefore, MRE and MRE-based indices are useful for detecting candidates for pharmacological trials in centers that are using MRI-based assessment, and VCTE (FibroScan)-based assessment may be used in centers where MRI is not readily available.
Liver steatosis ≥5% or ≥10% is the key inclusion criteria for pharmacological trials in NASH. Ultrasound-based modalities and multiparametric MRI could be estimated liver steatosis noninvasively and have high diagnostic accuracy for liver steatosis.14,51,86 Furthermore, these modalities could be estimated liver steatosis at the same time with the noninvasive assessment of liver fibrosis. Therefore, these modalities may be useful to decrease a screening failure rate and unnecessary liver biopsy. However, an effective screening strategy including liver fibrosis and steatosis as candidates for pharmacological trials has not been established yet and a further investigation is needed.
Screening strategy for liver fibrosis in patients with NAFLD
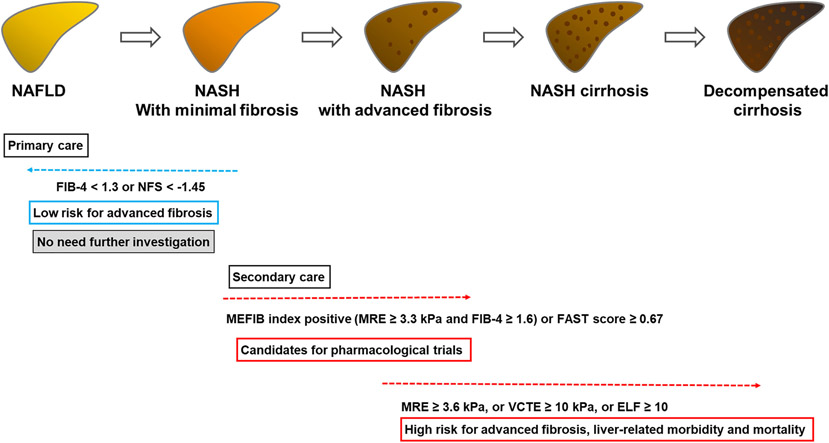
A screening strategy for liver fibrosis in patients with NAFLD is summarized in Figure 1. Since FIB-4 and NFS can be calculated using only standard laboratory data and has a high NPV for advanced fibrosis, FIB-4 (< 1.3) or NFS (<−1.45) is recommended to exclude patients with advanced fibrosis.21-23 Patients with FIB-4 <1.3 or NFS < −1.45 do not need further screening, while patients with FIB-4 ≥1.3 or NFS ≥ −1.45 should be referred to specialist centers. At the specialist center, further investigation using direct either direct fibrosis markers, VCTE, or MRE is advised. Thresholds of ELF ≥ 10,87 VCTE ≥10 kPa,88 or MRE ≥3.6 kPa52 for advanced fibrosis can be used and patients with exceeding these thresholds are a high probability of advanced fibrosis. Since patients with advanced fibrosis are at high risk for liver-related morbidity and mortality (detailed in next section), aggressive intervention and screening for complications are needed in these patients. Furthermore, rapid promotion of clinical trials in NASH is unmet needs but a high screening failure rate (> 70%) by liver biopsy is a major issue. Patients within the MEFIB index (FIB-4 ≥ 1.6 and MRE ≥ 3.3 kPa) or FAST ≥ 0.67 are more likely to be a candidate for clinical trials, and enrollment in clinical trials can be considered in these patients. Using this strategy, unnecessary and excessive liver biopsies can be avoided, contributing to reduced health care cost and burden. Patients who do not fulfill these criteria (e.g., patients with FIB-4: 1.3-1.6) have a low risk of fibrosis and could be follow-up. However, an adequate follow-up interval is not evaluated well, and further investigation is needed on this issue.
FIGURE 1.
Screening strategy for liver fibrosis in patients with NAFLD
PREDICTION OF PROGNOSIS USING NONINVASIVE MARKERS
Noninvasive markers and liver-related events
Liver fibrosis is a significant prognostic factor for comorbidity and mortality in patients with NAFLD and patients with advanced fibrosis are at high risk for the development of liver-related events including HCC and decompensation and mortality.89 Emerging data suggest that noninvasive markers for liver fibrosis are significantly associated with the development of liver-related events and mortality in patients with NAFLD as well as other chronic liver disease.90-92 Patients with NAFLD and a high level of blood-based markers (e.g., FIB-4 > 2.67 or NFS >0.675) are at high risk for liver-related events.93,94 Furthermore, since FIB-4 or NFS can be measured easily using standard laboratory data, the association between liver-related events and these markers can be evaluated in the general population. In studies investigating the general population, liver-related events risk increased in patients with a higher FIB-4 or NFS.95 Similarly, the significant association between liver stiffness measured by VCTE or MRE and liver-related events has been reported and increased liver stiffness by VCTE or MRE is associated with increased risk for liver-related events.96,97 Therefore, these noninvasive markers may be used as a prognostic marker for liver-related events in place of a liver biopsy.
One advantage of these noninvasive markers is that it can be measured repeatedly and the delta change over time can be evaluated. In a study including patients who received a paired liver biopsy, change in FIB-4 or MRE was associated with change in histological fibrosis stage, suggesting that fibrosis progression/regression can be estimated using repeat measurement of noninvasive markers.98-100 In studies investigating the change in noninvasive markers (e.g., FIB-4 or VCTE), the delta change in noninvasive markers was associated with time-course deterioration/improvement risk of liver-related events.101-103 Therefore, change in fibrosis and risk for liver-related events can be evaluated by repeat measurement of noninvasive markers, avoiding the risks of repeated liver biopsies. Recent studies demonstrated that MRI-based assessment is better than liver biopsy in assessing quantitative changes in liver features.104-106 Therefore, noninvasive markers may be more useful to assess disease progression in clinical practice and noninvasive markers can be used as a real-time fibrosis and prognostic marker in NAFLD.
Noninvasive markers and cardiovascular events
When comparing CVD events occurrence between patients with NAFLD and control (non-NAFLD), patients with NAFLD have a higher risk for CVD than control.107 However, among patients with NAFLD, the association between liver fibrosis and CVD risk is still controversial. In a study that investigated 10422 patients with biopsy-proven NAFLD, CVD risk increased as liver fibrosis increased.108 In a study including 101 patients with biopsy-proven NAFLD, coronary artery lesions are more common in patients with NASH than in those with simple steatosis.109 However, in a study that investigated 1773 patients with biopsy-proven NAFLD, there was no significant association between CVD incidence and liver fibrosis.110 In another study including 458 patients with biopsy-proven NAFLD and fibrosis stage 3 or 4, CVD incidence was higher in patients with F3 than those with F4.111 Among studies investigating the general population, an increased FIB-4 score was associated with increased risk of CVD.112,113 In a study that used MRE as a noninvasive marker, CVD incidence was higher in patients with moderate-advanced fibrosis (MRE: 3.0–4.7 kPa) than those with cirrhosis including decompensation cirrhosis (MRE >4.7 kPa).97 One possible reason for this discrepancy is the distribution of liver fibrosis among studies. In patients with decompensated cirrhosis, arterial pressure, systemic vascular resistance and serum cholesterol levels decrease.114,115 Due to these phenomena, CVD risk may decrease in patients with severe liver stiffness (decompensation). However, liver biopsy is usually avoided among decompensated patients because of the high risk for complications and these patients were rarely included in biopsy-based studies. Therefore, further investigation is needed regarding the association between liver fibrosis and CVD risk. Among patients with decompensated liver disease, the use of noninvasive markers for fibrosis are safer and may be more appropriate than liver biopsy.
Noninvasive makers and mortality
Several studies demonstrated the significant association between noninvasive markers and mortality, and the association is similar to the association between noninvasive markers and liver-related events. Therefore, mortality risk increases as noninvasive markers increase. In a study investigating 5033 patients with NAFLD, high NFS (>0.675) was associated with increased mortality with the relative risk of 4.54.116 Another study also revealed the significant association between NFS and mortality in patients with NAFLD as well as in the general population.117,118 Liver stiffness by imaging modalities is also associated with mortality.90,119 In a study investigating 2373 patients with chronic liver disease, the hazard ratio of MRE for mortality was 1.17 with each 1-kPa increase in MRE-based stiffness.120 Similarly, the hazard ratio of cirrhosis defined by MRE (>4.7 kPa) was 2.90 compared to those with minimal fibrosis (<3 kPa). Noninvasive markers are associated with liver-related events and mortality and can be used as a real-time monitor. Given a high prevalence of NAFLD in the general population, the need for non-invasive markers is expected to increase further.
CONCLUSION
NAFLD is extremely common in the general population and is a significant burden to the global economy and health care system. Noninvasive assessments of liver fibrosis have become part of routine clinical care in patients with NAFLD. They may be used for screening purposes in the general population, as well as for accurate diagnosis in specialist centers, in place of liver biopsy. Noninvasive markers are significant predictors of liver-related events and mortality, and a change in these markers can predict change in prognosis. Therefore, the use of noninvasive markers can potentially reduce the economic and health burden of NAFLD.
ACKNOWLEDGMENTS
Masayuki Kurosaki received funding support from the Japan Agency for Medical Research and Development (JP20fk0210067h0001, 22fk0210072s0203). Rohit Loomba received funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019).
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: P30DK120515, R01DK106419, R01DK121378, R01DK124318, U01DK061734, U01DK130190; National Center for Advancing Translational Sciences, Grant/Award Number: 5UL1TR001442; Japan Agency for Medical Research and Development, Grant/Award Numbers: 22fk0210072s0203, JP20fk0210067h0001; National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: U01AA029019; National Heart, Lung, and Blood Institute, Grant/Award Number: P01HL147835
Abbreviations:
- 2D-SWE
two-dimensional shear wave elastography
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristics
- BMI
body mass index
- CVD
cardiovascular disease
- ECM
extracellular matrix
- ELF
Enhanced Liver Fibrosis Score
- FIB-4
Fibrosis-4
- HCC
hepatocellular carcinoma
- M2BPGi
mac-2 binding protein glycosylation isomer
- MRE
magnetic resonance elastography
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD Fibrosis Score
- NPV
negative predictive value
- Pro-C3
N-terminal propeptide of type 3 collagen
- pSWE
point shear wave elastography
- VCTE
vibration-controlled transient elastography
Footnotes
CONFLICT OF INTEREST
Rohit Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-lngelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, lonis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc. Masayuki Kurosaki received lecture fees from Gilead Sciences Inc., Abbvie, Eisai Co., Ltd., Bayer AG, Otsuka Holdings Co., Ltd. The other authors disclose no conflicts.
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- 3.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–25. [DOI] [PubMed] [Google Scholar]
- 4.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691–705. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70:1119–33. [DOI] [PubMed] [Google Scholar]
- 7.Tampi RP, Wong VW, Wong GL, Shu SST, Chan HLY, Fung J, et al. Modelling the economic and clinical burden of non-alcoholic steatohepatitis in East Asia: data from Hong Kong. Hepatol Res. 2020;50:1024–31. [DOI] [PubMed] [Google Scholar]
- 8.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasui Y, Abe T, Kurosaki M, Higuchi M, Komiyama Y, Yoshida T, et al. Elastin fiber accumulation in liver correlates with the development of hepatocellular carcinoma. PLoS One. 2016;11:e0154558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 12.Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–32. [DOI] [PubMed] [Google Scholar]
- 13.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamaki N, Kurosaki M, Yasui Y, Tsuchiya K, Izumi N. Attenuation coefficient (ATT) measurement for liver fat quantification in chronic liver disease. J Med Ultrason. 2021;48:481–7. [DOI] [PubMed] [Google Scholar]
- 15.Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metabol. 2021;50:101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 18.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–501. [DOI] [PubMed] [Google Scholar]
- 19.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellana M, Donghia R, Guerra V, Procino F, Castellana F, Zupo R, et al. Fibrosis-4 index vs nonalcoholic fatty liver disease fibrosis score in identifying advanced fibrosis in subjects with nonalcoholic fatty liver disease: a meta-analysis. Am J Gastroenterol. 2021;116:1833–41. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 22.Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021;51:1013–25. [DOI] [PubMed] [Google Scholar]
- 24.Gantumur D, Harimoto N, Muranushi R, Hoshino K, Batbayar C, Hagiwara K, et al. Hepatic stellate cell as a Mac-2-binding protein-producing cell in patients with liver fibrosis. Hepatol Res. 2021;51:1058–63. [DOI] [PubMed] [Google Scholar]
- 25.Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Mori K, et al. A novel noninvasive diagnostic method for nonalcoholic steatohepatitis using two glycobiomarkers. Hepatology. 2015;62:1433–43. [DOI] [PubMed] [Google Scholar]
- 26.Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776–84. [DOI] [PubMed] [Google Scholar]
- 28.Tamaki N, Kurosaki M, Loomba R, Izumi N. Clinical utility of mac-2 binding protein glycosylation isomer in chronic liver diseases. Ann Lab Med. 2021;41:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamaki N, Higuchi M, Kurosaki M, Kirino S, Osawa L, Watakabe K, et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci Rep. 2019;9:10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamaki N, Kurosaki M, Takahashi Y, Itakura Y, Kirino S, Inada K, et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as a screening tool for significant liver fibrosis in health checkup. Int J Mol Sci. 2020:22:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vali Y, Lee J, Boursier J, Spijker R, Löffler J, Verheij J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2020;73:252–62. [DOI] [PubMed] [Google Scholar]
- 32.Younossi ZM, Felix S, Jeffers T, Younossi E, Nader F, Pham H, et al. Performance of the enhanced liver fibrosis test to estimate advanced fibrosis among patients with nonalcoholic fatty liver disease. JAMA Netw Open. 2021;4:e2123923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inadomi C, Takahashi H, Ogawa Y, Oeda S, Imajo K, Kubotsu Y, et al. Accuracy of the Enhanced Liver Fibrosis test, and combination of the Enhanced Liver Fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol Res. 2020;50:682–92. [DOI] [PubMed] [Google Scholar]
- 34.Ishiba H, Sumida Y, Seko Y, Tanaka S, Yoneda M, Hyogo H, et al. Type IV collagen 7S is the most accurate test for identifying advanced fibrosis in NAFLD with type 2 diabetes. Hepatol Commun. 2021;5:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa Y, Honda Y, Kessoku T, Tomeno W, Imajo K, Yoneda M, et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1795–803. [DOI] [PubMed] [Google Scholar]
- 36.Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol. 2007;42:375–81. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen MJ, Nedergaard AF, Sun S, Veidal SS, Larsen L, Zheng Q, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–15. [PMC free article] [PubMed] [Google Scholar]
- 38.Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, et al. ADAPT: an algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075–86. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen MJ, Leeming DJ, Goodman Z, Friedman S, Frederiksen P, Rasmussen DGK, et al. Comparison of ADAPT, FIB-4 and APRI as non-invasive predictors of liver fibrosis and NASH within the CENTAUR screening population. J Hepatol. 2021;75:1292–300. [DOI] [PubMed] [Google Scholar]
- 40.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mai F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. [DOI] [PubMed] [Google Scholar]
- 41.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong GL, Chan HL, Choi PC, Chan AWH, Lo AOS, Chim AML, et al. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11:295–302. e1-3. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich-Rust M, Hadji-Hosseini H, Kriener S, Herrmann E, Sircar I, Kau A, et al. Transient elastography with a new probe for obese patients for non-invasive staging of non-alcoholic steatohepatitis. Eur Radiol. 2010;20:2390–6. [DOI] [PubMed] [Google Scholar]
- 44.Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneda M, Honda Y, Nogami A, Imajo K, Nakajima A. Advances in ultrasound elastography for nonalcoholic fatty liver disease. J Med Ultrason. 2020;47:521–33. [DOI] [PubMed] [Google Scholar]
- 46.Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–27. [DOI] [PubMed] [Google Scholar]
- 47.Lin Y, Li H, Jin C, Wang H, Jiang B. The diagnostic accuracy of liver fibrosis in non-viral liver diseases using acoustic radiation force impulse elastography: a systematic review and meta-analysis. PLoS One. 2020;15:e0227358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakegawa T, Sugimoto K, Kuroda H, Suzuki Y, Imajo K, Toyoda H. Diagnostic accuracy of two-dimensional shear wave elastography for liver fibrosis: a multicenter prospective study. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.08.021 [DOI] [PubMed] [Google Scholar]
- 49.Imajo K, Honda Y, Kobayashi T, Nagai K, Ozaki A, Iwaki M, et al. Direct comparison of US and MR elastography for staging liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020. 10.1016/j.cgh.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 50.Sharpton SR, Tamaki N, Bettencourt R, Madamba E, Jung J, Liu A, et al. Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2021;14:175628482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imajo K, Honda Y, Yoneda M, Saito S, Nakajima A. Magnetic resonance imaging for the assessment of pathological hepatic findings in nonalcoholic fatty liver disease. J Med Ultrason. 2020;47:535–48. [DOI] [PubMed] [Google Scholar]
- 52.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–37. e7. [DOI] [PubMed] [Google Scholar]
- 54.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2021;75:770–85. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor a modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1263–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54:1150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharpton SR, Schnabl B, Knight R, Loomba R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metabol. 2021;33:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, et al. A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metabol. 2020;32:878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurosaki M, Izumi N. External validation of FIB-4: diagnostic accuracy is limited in elderly populations. Hepatology. 2008;47:352. [DOI] [PubMed] [Google Scholar]
- 62.Ishiba H, Sumida Y, Tanaka S, Tanaka S, Yoneda M, Hyogo H, et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216–24. [DOI] [PubMed] [Google Scholar]
- 63.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayashi T, Tamaki N, Kurosaki M, Wang W, Okada M, Higuchi M, et al. Use of the serum Wisteria floribunda agglutinin-positive Mac2 binding protein as a marker of gastroesophageal varices and liver-related events in chronic hepatitis C patients. Diagnostics. 2020;10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madahar P, Duprez DA, Podolanczuk AJ, Bernstein EJ, Kawut SM, Raghu G, et al. Collagen biomarkers and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Respir Med. 2018;140:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heickendorff L, Frost L, Madsen JK, Pedersen EB. Serum propeptides of type I and III procollagens in renal transplant recipients. A comparison of cyclosporine and azathioprine treatment. Nephron. 1994;67:203–8. [DOI] [PubMed] [Google Scholar]
- 67.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 68.Oeda S, Takahashi H, Imajo K, Seko Y, Ogawa Y, Moriguchi M, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan(®) M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55:428–40. [DOI] [PubMed] [Google Scholar]
- 69.Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500–6. [DOI] [PubMed] [Google Scholar]
- 70.Ghoz HM, Kröner PT, Stancampiano FF, Bowman AW, Vishnu P, Heckman MG, et al. Hepatic iron overload identified by magnetic resonance imaging-based T2* is a predictor of non-diagnostic elastography. Quant Imag Med Surg. 2019;9:921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoneda M, Imajo K, Nakajima A. Non-invasive diagnosis of nonalcoholic fatty liver disease. Am J Gastroenterol. 2018;113:1409–11. [DOI] [PubMed] [Google Scholar]
- 72.Anstee QM, Lawitz EJ, Alkhouri N, Wong VWS, Romero-Gomez M, Okanoue T, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70:1521–30. [DOI] [PubMed] [Google Scholar]
- 73.Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan WK, Treeprasertsuk S, Goh GB, Fan J-G, Song MJ, Charatcharoenwitthaya P, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17:2570–80. e37. [DOI] [PubMed] [Google Scholar]
- 75.Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Yoneda M, et al. FIB-4 first in the diagnostic algorithm of metabolic-dysfunction-associated fatty liver disease in the era of the global metabodemic. Life. 2021;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamaki N, Imajo K, Sharpton SR, Jung J, Sutter N, Kawamura N, et al. Two-step strategy, FIB-4 followed by magnetic resonance elastography, for detecting advanced fibrosis in NAFLD. Clin Gastroenterol Hepatol. 2022. 10.1016/j.cgh.2022.01.023 [DOI] [PubMed] [Google Scholar]
- 77.Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–8. [DOI] [PubMed] [Google Scholar]
- 78.Vilar-Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N. Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Clin Gastroenterol Hepatol. 2020;18:2305–14. e12. [DOI] [PubMed] [Google Scholar]
- 79.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. [DOI] [PubMed] [Google Scholar]
- 80.Loomba R, Ratziu V, Harrison SA. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology. 2021. 10.1053/j.gastro.2021.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021;70:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan W-K, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oeda S, Takahashi H, Imajo K, Seko Y, Kobayashi T, Ogawa Y, et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: comparison between M and XL probes. Hepatol Res. 2020;50:831–9. [DOI] [PubMed] [Google Scholar]
- 84.Puri P, Jain S, Fuchs M. Use of FibroScan-AST score to stratify high-risk nonalcoholic steatohepatitis in US veterans. Clin Gastroenterol Hepatol. 2020;18:3060–1. [DOI] [PubMed] [Google Scholar]
- 85.Tamaki N, Imajo K, Sharpton S, Jung J, Kawamura N, Yoneda M, et al. MRE plus FIB-4 (MEFIB) versus FAST in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology. 2021. 10.1002/hep.32145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imajo K, Toyoda H, Yasuda S, Suzuki Y, Sugimoto K, Kuroda H, et al. Utility of ultrasound-guided attenuation parameter for grading steatosis with reference to MRI-PDFF in a large cohort. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.11.003 [DOI] [PubMed] [Google Scholar]
- 87.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–42. [DOI] [PubMed] [Google Scholar]
- 88.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83. [DOI] [PubMed] [Google Scholar]
- 90.Gidener T, Yin M, Dierkhising RA, Allen AM, Ehman RL, Venkatesh SK. MRE for prediction of long-term progression and outcome in chronic liver disease: a retrospective study. Hepatology. 2021. 10.1002/hep.32151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsui N, Imajo K, Yoneda M, Kessoku T, Honda Y, Ogawa Y, et al. Magnetic resonance elastography increases usefulness and safety of non-invasive screening for esophageal varices. J Gastroenterol Hepatol. 2018;33:2022–8. [DOI] [PubMed] [Google Scholar]
- 92.Tamaki N, Kurosaki M, Higuchi M, Kirino S, Inada K, Yamashita K, et al. Validation of albumin, bilirubin, and platelet criteria for avoiding screening endoscopy in patients with advanced fibrosis. Hepatol Res. 2020;50:996–9. [DOI] [PubMed] [Google Scholar]
- 93.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagström H, Nasr P, Ekstedt M, Stål P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring systems in assessing risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:1148–56.e4. [DOI] [PubMed] [Google Scholar]
- 95.Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology. 2020;158:200–14. [DOI] [PubMed] [Google Scholar]
- 96.Shili-Masmoudi S, Wong GL, Hiriart JB, Liu K, Chermak F, Shu SS, et al. Liver stiffness measurement predicts long-term survival and complications in non-alcoholic fatty liver disease. Liver Int. 2020;40:581–9. [DOI] [PubMed] [Google Scholar]
- 97.Tamaki N, Higuchi M, Kurosaki M, Loomba R, Izumi N. Risk difference of liver-related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17:1877–85.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ajmera VH, Liu A, Singh S, Yachoa G, Ramey M, Bhargava M, et al. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology. 2020;71:849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tamaki N, Kurosaki M, Tanaka K, Suzuki Y, Hoshioka Y, Kato T, et al. Noninvasive estimation of fibrosis progression overtime using the FIB-4 index in chronic hepatitis C. J Viral Hepat. 2013;20:72–6. [DOI] [PubMed] [Google Scholar]
- 101.Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73:1023–9. [DOI] [PubMed] [Google Scholar]
- 102.Petta S, Sebastiani G, Viganò M, Ampuero J, Wai-Sun Wong V, Boursier J, et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol. 2021;19:806–15. e5. [DOI] [PubMed] [Google Scholar]
- 103.Tamaki N, Kurosaki M, Yasui Y, Mori N, Tsuji K, Hasebe C, et al. Change in fibrosis 4 index as predictor of high risk of incident hepatocellular carcinoma after eradication of hepatitis C virus. Clin Infect Dis. 2021;73:e3349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le T-A, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tamaki N, Munaganuru N, Jung J, Yonan AQ, Loomba RR, Bettencourt R, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2021. 10.1136/gutjnl-2021-324264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 108.Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2021. 10.1136/gutjnl-2021-325724 [DOI] [PubMed] [Google Scholar]
- 109.Niikura T, Imajo K, Ozaki A, Kobayashi T, Iwaki M, Honda Y, et al. Coronary artery disease is more severe in patients with non-alcoholic steatohepatitis than fatty liver. Diagnostics. 2020;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–57. el7. [DOI] [PubMed] [Google Scholar]
- 112.Tamaki N, Kurosaki M.Takahashi Y, Itakura Y, Inada K, Kirino S.et al. Liver fibrosis and fatty liver as independent risk factors for cardiovascular disease. J Gastroenterol Hepatol. 2021;36:2960–6. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi Y, Kurosaki M, Tamaki N, Yasui Y, Hosokawa T, Tsuchiya K, et al. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res. 2015;45:667–75. [DOI] [PubMed] [Google Scholar]
- 114.Iwakiri Y Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017;16:s27–42. [DOI] [PubMed] [Google Scholar]
- 116.Jaruvongvanich V, Wijarnpreecha K, Ungprasert P. The utility of NAFLD fibrosis score for prediction of mortality among patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of cohort study. Clin Res Hepatol Gastroenterol. 2017;41:629–34. [DOI] [PubMed] [Google Scholar]
- 117.Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, Akita T, et al. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boursier J, Vergniol J, Guillet A, Hiriart J-B, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–8. [DOI] [PubMed] [Google Scholar]
- 120.Higuchi M, Tamaki N, Kurosaki M, Inada K, Kirino S, Yamashita K, et al. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Aliment Pharmacol Ther. 2022;55:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]