Summary
Background:
Magnetic resonance elastography (MRE) has the highest diagnostic accuracy for liver fibrosis; however, the association between MRE-associated liver stiffness and the development of hepatic and extrahepatic complications as well as mortality remains unclear.
Aim:
In this study, we investigated the longitudinal association between MRE-associated liver stiffness and complications and mortality.
Methods:
This retrospective study included 2373 consecutive patients with chronic liver disease. All patients received standard of care and the development of complications was assessed every 1-6 months.
Results:
Newly diagnosed hepatocellular carcinoma (HCC), decompensation, major adverse cardiovascular events (MACE), extrahepatic cancer and death were observed in 99, 117, 73, 77 and 170 patients respectively. In multivariable analysis, the adjusted hazard ratios (aHR) (95% confidence interval [CI]) for HCC, decompensation, MACE, extrahepatic cancer and mortality were 1.28 (1.2-1.4), 1.34 (1.3-1.4), 0.96 (0.9-1.1), 1.00 (0.9-1.1) and 1.17 (1.1-1.2), respectively, with each 1-kPa increase in liver stiffness. Similarly, the aHR (95% CI) for HCC, decompensation, MACE, extrahepatic cancer and mortality were 4.20 (2.2-8.2), 67.5 (9.2-492), 0.83 (0.4-1.7), 0.90 (0.5-1.7) and 2.90 (1.6-5.4), respectively, in patients with cirrhosis (>4.7 kPa) compared to those with minimal fibrosis (<3 kPa).
Conclusions:
Increased MRE-associated liver stiffness was associated with increased risk for HCC, decompensation and mortality in a dose-dependent fashion but not with MACE or extrahepatic cancer, implicating a significant role for MRE in liver-related events and mortality; however, further studies are warranted to explore its role in MACE and extrahepatic cancer.
1 |. INTRODUCTION
Chronic liver disease can lead to decompensation as well as hepatocellular carcinoma (HCC), a leading cause of cancer-related deaths globally.1 Chronic liver disease is associated with not only liver-related complications but also extrahepatic complications, including major adverse cardiovascular events (MACE) and extrahepatic cancers.2,3 For example, non-alcoholic fatty liver disease (NAFLD) is associated with metabolic dysfunction and MACE is an important complication,2 whereas hepatitis C virus (HCV) infection is a systemic disease associated with metabolic alterations and can lead to MACE.4 Therefore, early detection of patients at high risk for liver-related and extrahepatic complications is an important clinical issue.
Liver fibrosis is an important factor associated with liver-related complications and mortality in patients with chronic liver disease.5–7 Liver biopsy, which is the gold standard for the assessment of liver fibrosis, has several limitations including invasiveness, sampling error, and intra- and inter-observer reproducibility.8 Non-invasive methods to assess liver fibrosis are currently used in clinical practice to resolve these issues.9–12
Magnetic resonance elastography (MRE) is a magnetic resonance imaging-based noninvasive modality to assess liver fibrosis, and liver stiffness determined with MRE has been demonstrated to be associated with histological stage of fibrosis.9,13,14 Among the currently utilized non-invasive modalities, MRE has the highest diagnostic accuracy for liver fibrosis and is used to determine inclusion criteria in clinical trials.15 However, the longitudinal association of liver stiffness determined with MRE and liver-related complications, that is HCC and decompensation, remains unclear. Furthermore, the association between liver fibrosis and extrahepatic complications is controversial, and the association between MRE-associated liver stiffness and extrahepatic complications also remains unclear. To fulfil the current gap in knowledge, we investigated the longitudinal association between MRE-associated liver stiffness and liver-related complications, including HCC and decompensation, extrahepatic complications including MACE and extrahepatic cancer, as well as mortality.
2 |. METHODS
2.1 |. Study design
This was a retrospective cohort study conducted at Musashino Red Cross Hospital in Tokyo, Japan, which is a tertiary center for liver disease. A total of 2827 consecutive patients with chronic liver disease who were evaluated with MRE between January 2015 and November 2020 were included. Patients who were followed up within six months (n = 452) and those aged <18 years (n = 2) were excluded from the study. Therefore, a total of 2373 patients with chronic liver disease were included in the final analyses. The causes of chronic liver disease were HCV infection (n = 1215, 51.2%), hepatitis B virus infection (n = 376, 15.8%), NAFLD (n = 487, 20.5%), alcohol use (n = 175, 7.4%)16 and others (primary biliary cholangitis, autoimmune hepatitis, idiopathic portal hypertension, primary sclerosing cholangitis, Wilson’s disease and cryptogenic, n = 120, 5.1%). NAFLD is defined as the presence of fatty liver based on imaging modalities or a hepatic steatosis index of >3017 after the exclusion of other causes. Alcohol use of <15 drinks/week for males and <10 drinks/week for females was used for the criteria of NAFLD16 and others (≥15 drinks/week for males and ≥10 drinks/week for females) were defined as alcohol use. All patients were evaluated for liver stiffness with MRE as part of routine clinical evaluation and received standard of care. All patients visited the outpatient clinic every 1-6 months. Since HCC screening every 6 months is recommended by guidelines published by the American Association for the Study of Liver Diseases and the Japan Society of Hepatology,18,19 the maximum interval between visits was set as 6 months. The study observation started at the time of assessment with MRE, and all patients were followed for the development of HCC, decompensation, MACE, extrahepatic cancers and death. Informed consent was obtained from all patients using the opt-out method. The study protocol was approved by the Clinical Research Ethics Committee of Musashino Red Cross Hospital and conformed to the ethical guidelines of the Declaration of Helsinki (Approval Number: 2007).
2.2 |. Definitions of HCC, decompensation, MACE and extrahepatic cancer
HCC is defined as a tumour displaying vascular enhancement in the early phase and washout in the later phase according to the guidelines.18,19 Tumour biopsy is used to diagnose tumours with non-typical imaging findings. Decompensation is defined as the development of ascites or hepatic encephalopathy or the need for preventive treatment for gastro-oesophageal variceal or gastro-oesophageal variceal bleeding. MACE is defined as the development of coronary heart disease, cerebrovascular disease, peripheral vascular disease or heart failure. Extrahepatic cancer was diagnosed by oncologists in each field. All patients were followed up by hepatologists and hepatologists diagnosed HCC and decompensation. When patients had any symptoms of suspicious MACE or extrahepatic cancer, patients were referred to cardiologists or oncologists and received screening and diagnosed as MACE or extrahepatic cancer. Extrahepatic cancer recurrence was not included in the analyses. HCC or extrahepatic cancer diagnosed within 3 months after assessment with MRE is excluded from the study analyses given that their presence at the time of assessment with MRE could not be ruled out. History of HCC, decompensation, MACE and extrahepatic cancers were present in 582 (24.5%), 79 (3.3%), 67 (2.8%) and 93 (3.9%) patients, respectively, who were excluded from analyses related to these outcomes. All patients were included in the analysis of mortality.
2.3 |. MRE assessment
MRE was performed using Signa HDxt 1.5T (GE Medical Systems, Waukesha, WI, US) and MR Touch (GE Healthcare), as previously described.20 In summary, shear waves were generated by external vibration at 60 Hz using a passive driver as the vibration device slightly placed to the right, lateral to the xiphoid process. Cross-sectional elastography images were created by the stiffness generated from the wave propagation information obtained using the gradient echo sequence. The region of interest was placed at the right hepatic lobe on each slice of the stiffness map, carefully avoiding the liver surface, liver edge, gallbladder, blood vessels, bile ducts, tumours and artefacts. The mean stiffness value of three circular regions of interest placed at different slices was used for analysis. Based on MRE-associated liver stiffness, patients were stratified into three groups as follows: minimal fibrosis (<3 kPa), moderate/advanced fibrosis (3-4.7 kPa) and cirrhosis (>4.7 kPa). The thresholds were based on those determined in previous studies.21
2.4 |. Clinical and laboratory data
Patient characteristics and laboratory data were collected within 6 months of MRE assessment. Information on age, sex, body mass index, alcohol intake and comorbidities (diabetes mellitus [DM], dyslipidaemia and hypertension) were recorded, and standard blood count and biochemistry tests were conducted.
2.5 |. Statistical analysis
Patient characteristics were compared among those with minimal fibrosis, moderate-advanced fibrosis and cirrhosis using the Kruskal-Wallis or Fisher’s exact test. The cumulative incidence rates of HCC, decompensation, MACE, extrahepatic cancer and mortality were evaluated using the Kaplan-Meier method, and differences among the groups were analysed using the log-rank test. The post hoc analysis was conducted using Bonferroni correction to compare the complication incidence or mortality between two groups (minimal vs moderate/advanced and moderate/advanced vs cirrhosis). The Cox proportional hazards model was used for multivariable analysis. Age, sex, aetiology of liver disease, DM, dyslipidaemia and hypertension were chosen as a priori factors and used for the multivariable analysis of complications. For analysis of mortality, these factors as well as history of HCC, decompensation, MACE and extrahepatic cancer were chosen as a priori factors and used for the multivariable analysis of mortality. A value of P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan), a graphical user interface for R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
3 |. RESULTS
3.1 |. Patient characteristics
The characteristics of 2373 patients included in the study are shown in Table 1. Briefly, the median (interquartile range [IQR]) age was 68 (58-75) years, and DM, dyslipidaemia and hypertension were present in 483 (20.4%), 838 (35.3%) and 843 (35.5%) patients respectively. There were 783, 729 and 861 patients with minimal fibrosis (<3.0 kPa), moderate/advanced fibrosis (3.0-4.7 kPa) and cirrhosis (>4.7 kPa), respectively, based on liver stiffness measurement with MRE. The patients with cirrhosis were older and had higher prevalence rates of DM, hypertension, and history of HCC and decompensation compared to those with minimal or moderate/advanced fibrosis. Additionally, 61.5% (n = 747) of the patients with HCV infection received antiviral therapy and achieved sustained virological response. Furthermore, 56.5% (n = 213) of the patients with hepatitis B virus infection had started on nucleotide/nucleoside analogue therapy before study enrolment and continued treatment during follow-up.
TABLE 1.
Patient characteristics
| All patients (n = 2373) | Minimal fibrosis (<3 kPa) (n = 783) | Moderate/advanced fibrosis (3-4.7 kPa) (n = 729) | Cirrhosis (>4.7 kPa) (n =861) | P value | |
|---|---|---|---|---|---|
| Age (years) | 68 (58-75) | 63 (52-72) | 69 (60-76) | 70 (63-78) | |
|
| |||||
| Male (n, %) | 1196 (50.4%) | 351 (44.8%) | 369 (50.6%) | 476 (55.3%) | <.001 |
|
| |||||
| Aetiology | <.001 | ||||
| HCV | 1215 (51.2%) | 324 (41.4%) | 418 (57.3%) | 473 (54.9%) | |
| HBV | 376 (15.8%) | 224 (28.6%) | 102 (14.0%) | 50 (5.8%) | |
| NAFLD | 487 (20.5%) | 166 (21.2%) | 142 (19.%) | 179 (20.8%) | |
| Alcohol use | 175 (7.4%) | 16 (2.0%) | 33 (4.5%) | 126 (14.6%) | |
| Other | 120 (5.1%) | 53 (6.8%) | 34 (4.7%) | 33 (3.8%) | |
|
| |||||
| Comorbidities (n, %) | |||||
| DM | 483 (20.4%) | 51 (6.5%) | 142 (20.0%) | 290 (33.7%) | <.001 |
| Dyslipidaemia | 838 (35.3%) | 266 (34.0%) | 303 (41.6%) | 269 (31.2%) | <.001 |
| Hypertension | 843 (35.5%) | 149 (19.0%) | 266 (36.5%) | 428 (49.7%) | <.001 |
| History of HCC | 582 (24.5%) | 80 (10.2%) | 168 (23.0%) | 334 (38.8%) | <.001 |
| History of decompensation | 79 (3.3%) | 0 (0%) | 7 (1.0%) | 72 (8.4%) | <.001 |
| History of MACE | 67 (2.8%) | 14 (1.8%) | 25 (3.4%) | 28 (3.3%) | .09 |
| History of extrahepatic cancer | 93 (3.9%) | 30 (3.8%) | 22 (3.0%) | 41 (4.8%) | .2 |
|
| |||||
| Laboratory data | |||||
| Albumin (g/dl) | 4.2 (3.8-4.4) | 4.3 (4.1-4.5) | 4.3 (4.0-4.5) | 3.8 (3.5-4.2) | <.001 |
| AST (IU/L) | 33 (24-51) | 26 (21-35) | 32 (34-47) | 45 (32-68) | <.001 |
| ALT (IU/L) | 28 (18-49) | 22 (15-35) | 28 (18-50) | 36 (23-60) | <.001 |
| Bilirubin (mg/dl) | □0.7 (0.5-0.9) | 0.6 (0.5-0.8) | 0.6 (0.5-0.8) | 0.8 (0.6-1.1) | <.001 |
| Total cholesterol (mg/dl) | 185 (159-209) | 199 (177-221) | 187 (164-209) | 166 (142-190) | <.001 |
| HDL (mg/dl) | 58 (47-71) | 63 (51-77) | 53 (43-70) | 54 (44-55) | <.001 |
| LDL (mg/dl) | 103 (84-125) | 114 (96-133) | 108 (88-124) | 90 (73-112) | <.001 |
| TG (mg/dl) | 107 (77-153) | 101 (72-148) | 116 (79-160) | 105 (79-151) | .003 |
| HbA1c (%) | 5.8 (5.5-6.5) | 5.7 (5.5-6.1) | 5.9 (5.5-6.6) | 6.0 (5.4-6.9) | <.001 |
| Platelet counts (109/L) | 159 (117-205) | 191 (155-233) | 170 (134-206) | 117 (80-154) | <.001 |
|
| |||||
| MRE finding | |||||
| Liver stiffness (kPa) | 3.78 (2.7-5.8) | 2.41 (2.1-2.7) | 3.70 (3.3-4.2) | 6.65 (5.6-8.5) | <.001 |
| Follow-up period (months) | 34 (17-52) | 29 (14-48) | 37 (19-55) | 34 (18-55) | <.001 |
Note: Data are shown in median (interquartile range).
Patients were stratified into three groups based on MRE-associated liver stiffness.
P value indicates differences among three groups (minimal fibrosis, moderate/advanced fibrosis and cirrhosis).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; HbA1c, haemoglobin A1c; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL. low-density lipoprotein; MACE, major adverse cardiovascular event; MRE, magnetic resonance elastography; NALFD, non-alcoholic fatty liver disease; TG, triglyceride.
3.2 |. Rates of HCC, decompensation, MACE, extrahepatic cancer and mortality
The median (IQR) follow-up duration was 2.80 (1.5-4.3) years, and the newly developed HCC, decompensation, MACE and extrahepatic cancer were observed in 99, 117, 73 and 77 patients respectively. Of the 77 patients with extrahepatic cancer, 27.3% (21/77) were colon, 19.5% (15/77) were gastric, 13.0% (10/77) were haematologic malignancy, 9.1% (7/77) were lung, pancreas and bile duct, 2.6% (2/77) were urinary tract, oesophageal and uterine, and 1.3% (1/77) were breast, thyroid, head and neck, and endocrine tumour respectively. Patients with a history of HCC, decompensation, MACE and extrahepatic cancer were excluded from analyses related to these outcomes. The 1-, 3- and 5-year incidence rates of HCC were 1.6%, 5.5% and 9.7%, respectively (Figure 1), and the 1-, 3- and 5-year incidence rates of decompensation, MACE and extrahepatic cancer were 1.8%, 5.5% and 8.3%; 1.0%, 3.1% and 5.2%; and 1.1%, 3.3% and 5.6% respectively.
FIGURE 1.
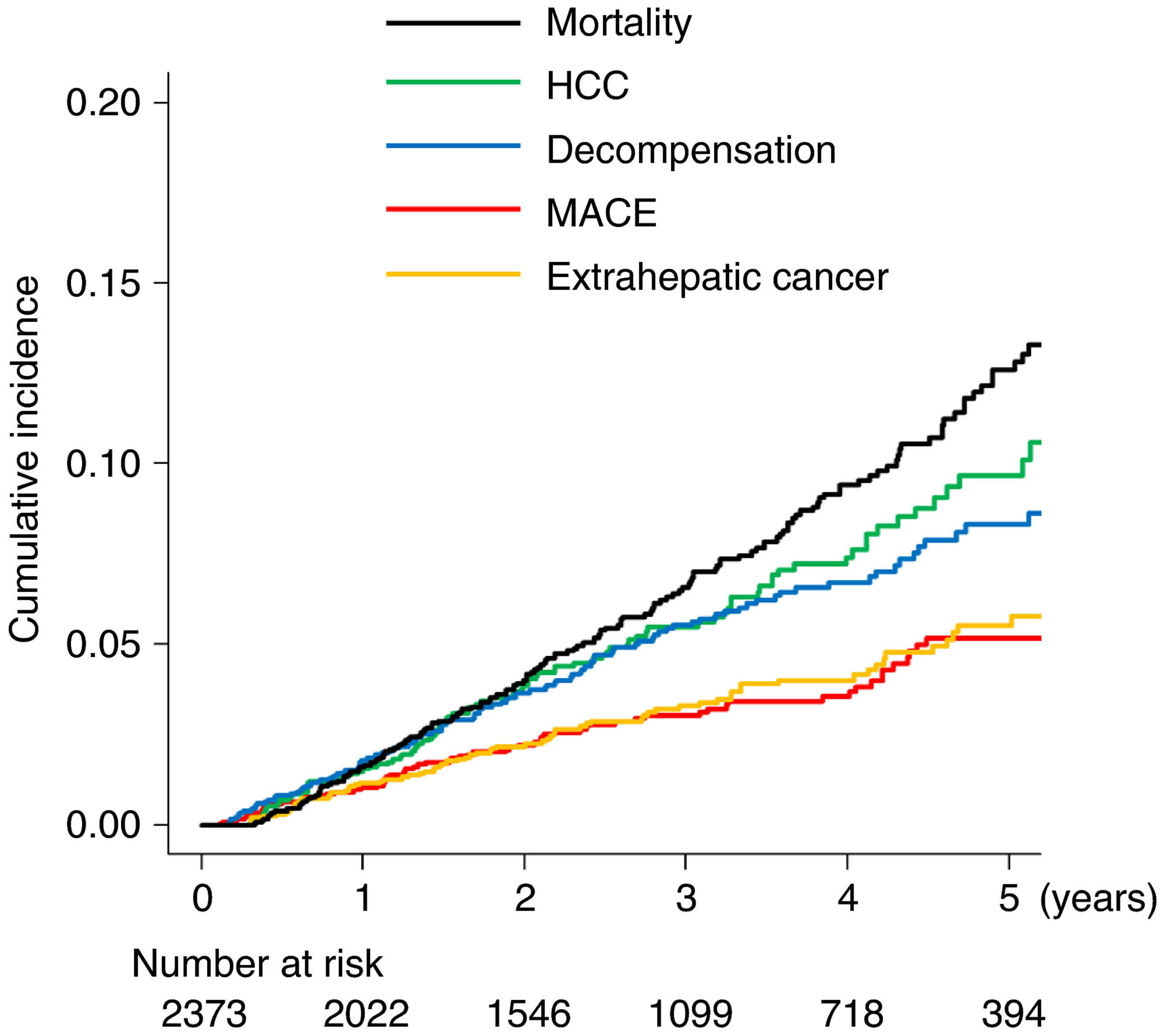
Cumulative incidence of newly diagnosed complications and mortality. HCC, hepatocellular carcinoma; MACE, major adverse cardiovascular events
A total of 170 patients died during the follow-up period. The causes of death were HCC, decompensation, MACE, extrahepatic cancer and other aetiologies in 97, 40, 8, 16 and 9 patients respectively. The 1-, 3- and 5-year mortality rates were 1.6%, 6.6% and 12.6% respectively.
3.3 |. Risk stratification for complications of MRE-associated liver stiffness
The cumulative incidence of specific complications and mortality rates were investigated in patient groups stratified based on MRE-associated liver stiffness. The 1-, 3- and 5-year HCC incidence rates were 0.3%, 1.8% and 3.5%; 0.5%, 2.9% and 6.8%; and 4.0%, 12.7% and 19.2%, respectively, in patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis, respectively (P < 0.001, Figure 2A). The incidence of HCC was significantly higher in patients with cirrhosis than in those with moderate/advanced fibrosis (P < 0.001), and there was no difference between patients with minimal fibrosis and those with moderate/advanced fibrosis (P = 0.1). Similarly, the 1-, 3- and 5-year incidence rates of decompensation were 0.1%, 0.1% and 0.1%; 0.4%, 1.6% and 1.6%; and 4.6%, 13.9% and 21.0%, respectively, in patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis respectively (P < 0.001, Figure 2B). The incidence of decompensation was significantly higher in patients with cirrhosis than in those with moderate/advanced fibrosis (P < 0.001) and it was also higher in patients with moderate/advanced fibrosis than in those with minimal fibrosis (P = 0.008). The 1-, 3- and 5-year incidence rates of MACE were 0.7%, 1.7% and 3.2%; 1.5%, 3.6% and 7.0%; and 1.0%, 3.7% and 5.0%, respectively, in patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis respectively (P = 0.06, Figure 2C). The incidence of MACE was a high tendency in patients with moderate/advanced fibrosis than in those with minimal fibrosis (P = 0.07); however, there was no difference in MACE incidence between patients with moderate/advanced fibrosis and those with cirrhosis (P = 1). In contrast, no significant differences in extrahepatic cancer incidence were found among the patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis (P = 0.8, Figure 2D). Finally, the 1-, 3- and 5-year mortality rates were 0.1%, 1.5% and 3.4%; 1.6%, 4.8% and 6.6%; and 3.0%, 12.0% and 23.3%, respectively, in patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis, respectively (P < 0.001, Figure 2E); the rate of mortality increased with increasing MRE-associated liver stiffness severity (minimal vs moderate/advanced, P = 0.02 and moderate/advanced, P < 0.001).
FIGURE 2.
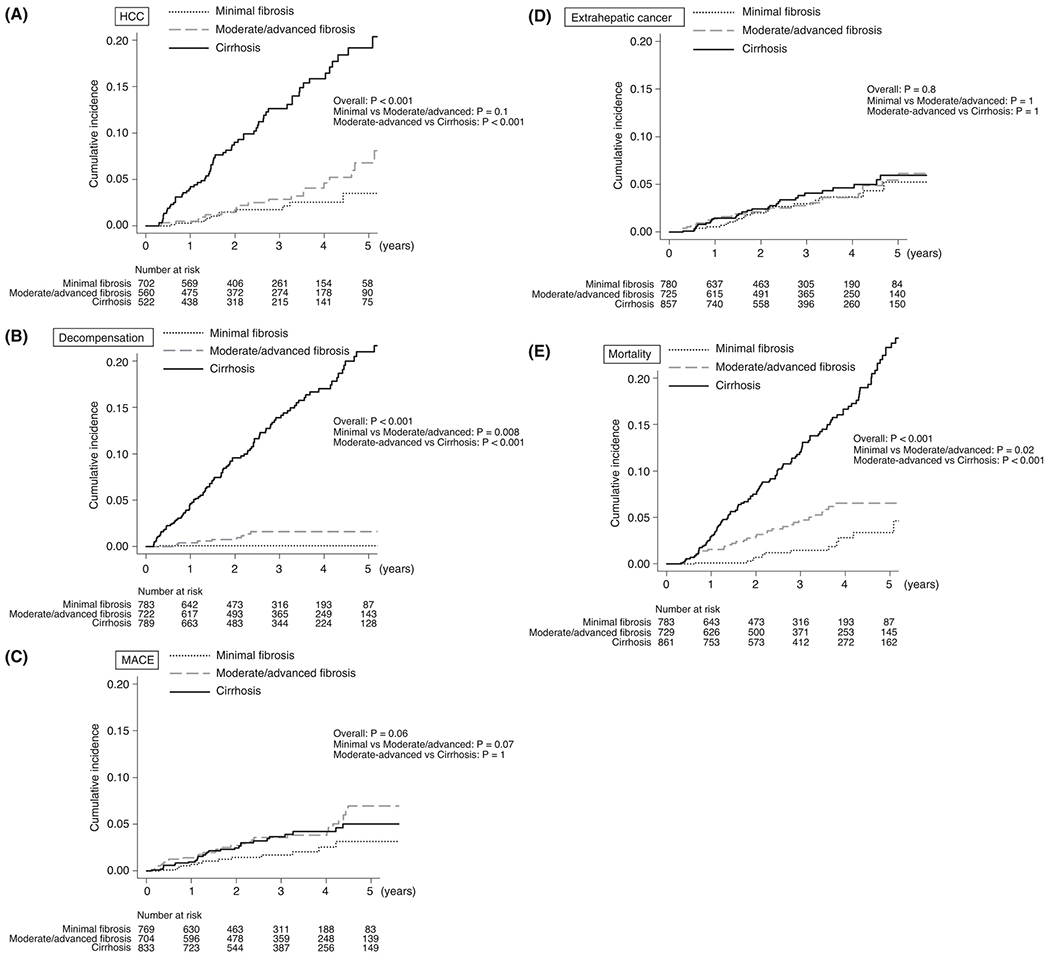
Cumulative incidence of complications and mortality in patients stratified based on MRE-associated liver stiffness. (A) HCC, (B) Decompensation, (C) MACE, (D) extrahepatic cancer, (E) mortality. Patients were stratified into three groups based on MRE-associated liver stiffness as follows: minimal fibrosis (<3 kPa), moderate/advanced fibrosis (3-4.7 kPa) and cirrhosis {>4.7 kPa). HCC, hepatocellular carcinoma; MACE, major adverse cardiovascular events; MRE, magnetic resonance elastography
In the subgroup of patients with NAFLD, there was a significant difference in the incidence of HCC (P < 0.001) and decompensation (P < 0.001) among patients with minimal fibrosis, moderate/advanced fibrosis and cirrhosis, but no significant difference was observed in the incidence of MACE (P = 0.1) and extrahepatic cancer (P = 0.5) among the three groups.
3.4 |. Hazard ratio of MRE-associated liver stiffness for complications and mortality
Hazard ratios (HRs) of MRE-associated liver stiffness (as continuous value or categorized value) for complications and mortality were investigated. For the analysis for complications, age, sex, aetiology of liver fibrosis, DM, dyslipidaemia, hypertension and MRE-associated liver stiffness were used for the multivariable analyses. For the analysis of mortality, age, sex, aetiology, DM, dyslipidaemia, hypertension, history of HCC, decompensation, MACE, extrahepatic cancer and MRE-associated liver stiffness were used for the multivariable analysis. The analyses using MRE-associated liver stiffness as a continuous parameter revealed that the adjusted HRs for HCC, decompensation, MACE, extrahepatic cancer and mortality were 1.28 (95% confidence interval [CI] 1.2-1.4, P < 0.001), 1.34 (95% CI 1.3-1.4, P < 0.001), 0.96 (95% CI 0.9-1.1, P = 0.5), 1.00 (95% CI 0.9-1.1, = 0.9) and 1.17 (1.1-1.2, P < 0.001), respectively, for each 1-kPa increase in liver stiffness. MRE-associated liver stiffness was significantly associated with HCC, decompensation and mortality but not with MACE or extrahepatic cancer (Figure 3A).
FIGURE 3.
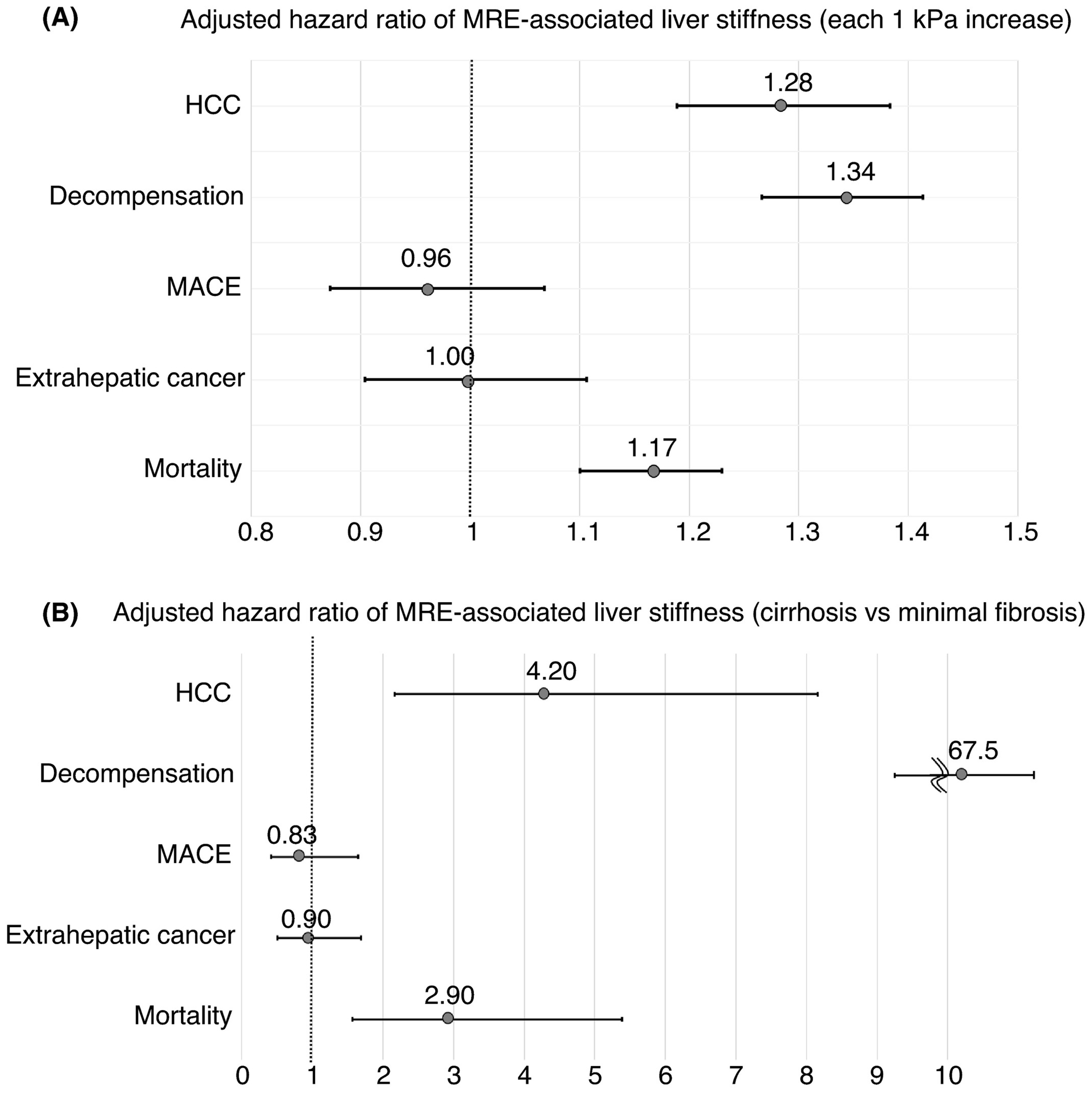
Hazard ratio of MRE-associated liver stiffness for complication development and mortality. Adjusted hazard ratios for complications and mortality with MRE-associated liver stiffness are shown; (A) each 1 kPa increase in MRE-associated liver stiffness, (B) cirrhosis vs minimal fibrosis based on MRE assessment. MRE-associated liver stiffness, age, sex, aetiology, DM, dyslipidaemia and hypertension were used for the multivariable analysis of the development of complications. For the analysis of mortality, history of HCC, decompensation, MACE and extrahepatic cancer were used, in addition to these factors. HCC, hepatocellular carcinoma; MACE, major adverse cardiovascular events; MRE, magnetic resonance elastography
The analyses using MRE-associated liver stiffness as a categorical parameter (cirrhosis vs minimal fibrosis) revealed that the adjusted HRs (95% CI) for HCC, decompensation, MACE, extrahepatic cancer and mortality were 4.20 (2.2-8.2, P < 0.001), 67.5 (9.2-492, P < 0.001), 0.83 (0.4-1.7, P = 0.6), 0.90 (0.5-1.7, P = 0.7) and 2.90 (1.6-5.4, P < 0.001), respectively, in patients with cirrhosis (>4.7 kPa) compared to minimal fibrosis (<3.0 kPa) (Figure 3B). Cirrhosis based on MRE assessment was a significant risk factor for HCC, decompensation and mortality but not for MACE or extrahepatic cancer, compared to minimal fibrosis.
4 |. DISCUSSION
4.1 |. Main findings
In this study, we found that the risk of HCC, decompensation and mortality increased with increasing MRE-associated liver stiffness in a dose-dependent manner, although we did not find an association between MRE-associated liver stiffness and MACE or extrahepatic cancer. The rates of newly developed HCC and decompensation were high, with 5-year incidence rates of 9.7% and 8.3%, respectively, and the risk for HCC, decompensation and mortality increased with increasing liver stiffness measured with MRE. These results provide evidence that MRE-associated liver stiffness could be used as a surrogate marker for HCC, decompensation and mortality. On the other hand, MRE-associated liver stiffness was not associated with the development of MACE or extrahepatic cancer. However, the 5-year incidence rates of MACE and extrahepatic development were 5.8% and 5.6%, respectively, indicating their role as important complications of chronic liver disease. Therefore, further studies are warranted to assess the role of liver stiffness measured with MRE as a risk marker for MACE and extrahepatic cancer.
4.2 |. In context with published literature
HCC and decompensation are major causes of mortality in patients with chronic liver disease. Histological stage of liver fibrosis is significantly associated with liver-related complications and mortality in patients with viral hepatitis, NAFLD and alcoholic liver disease as well as in those with other chronic liver diseases.5–7 Several studies demonstrated that MRE-associated liver stiffness was significantly associated with HCC, decompensation and mortality in patients with chronic liver disease related to various aetiologies.22–25 We also found that the risk of HCC, decompensation and mortality increased with increasing MRE-associated liver stiffness in a dose-dependent fashion. The current study, which comprised over 2000 patients with chronic liver disease, utilized a larger cohort to strengthen the evidence on the previously reported association of liver stiffness measured with MRE with liver-related complications and mortality.
The association of liver fibrosis with extrahepatic complications, especially MACE, remains controversial.26–28 Furthermore, no study to date has investigated the association between MRE-associated liver stiffness and extrahepatic complications in patients with chronic liver disease. In a study investigating 101 patients with biopsy-proven NAFLD, the risk of coronary artery lesions increased as histological liver fibrosis increased.29 Recent studies demonstrated that advanced fibrosis, defined based on assessments using liver biopsy, serum markers, or ultrasound-based elastography, was associated with an increased risk of MACE.27,30,31 In contrast, in the present study, we found that increased liver stiffness measured with MRE was not associated with an increased risk of MACE. One potential explanation for this discrepancy is the difference in the prevalence of liver fibrosis among the studies. Previous studies were conducted in biopsy-proven patients or population-based cohorts. Therefore, few patients with severe liver stiffness (decompensation), who usually avoided liver biopsy, were included. In contrast, the present study included a high number of patients with severe liver stiffness (36.3% of the patients had a liver stiffness >4.7 kPa), because assessment with MRE can be easily conducted with no restrictions even in patients with decompensation. Although the incidence of MACE was high tendency in patients with moderate/advanced fibrosis than in those with minimal fibrosis, no further increase in MACE risk was observed between the patients with moderate/advanced fibrosis and those with cirrhosis. We previously demonstrated that the risk of MACE was higher in patients with moderate/advanced fibrosis based on MRE but the risk was lower in those with higher liver stiffness based on MRE among patients with NAFLD.32 Therefore, although the risk of HCC and decompensation increased with increasing liver stiffness in a dose-dependent manner based on the assessment of patients with MRE, there is no significant association between the incidence of MACE and liver fibrosis. The previous studies indicated that the incidence of MACE and liver fibrosis may not be linear and the risk of MACE may decrease with increasing liver stiffness determined with MRE in patients with cirrhosis or decompensation.28,32 This study results also indicated a similar tendency with the previous studies and further investigation is needed to evaluate the association between MACE and liver fibrosis.
In patients with cirrhosis and more severe liver stiffness, intrahepatic resistance leads to portal hypertension and results in decreased arterial pressure and systemic vascular resistance.33 Cholesterol is synthesized primarily in the liver, and cirrhosis results in impaired cholesterol synthesis and decreased serum cholesterol levels.34 These underlying mechanisms may contribute to a decrease or a lack of increase in MACE risk in patients with cirrhosis compared to those with moderate/advanced fibrosis. The present study provides new evidence between MACE risk and liver stiffness and highlights the need for further investigation to determine whether MRE-associated liver stiffness might be used as a risk marker for MACE.
The risk of extrahepatic cancer increases in patients with chronic liver disease. Viral hepatitis is associated with increased rates of haematologic malignancies and non-HCC cancers involving pancreas, colon, stomach and lungs compared to healthy individuals.35,36 Similarly, patients with NAFLD are at a higher risk for non-HCC cancers, including those of the colon, pancreas and kidney/bladder, compared to healthy individuals.2,37 However, evidence regarding the association between liver fibrosis and the risk of extrahepatic cancer is limited. In the present study, we found that MRE-associated liver stiffness was not associated with extrahepatic cancer risk. Therefore, although the presence of chronic liver disease is associated with increased risk of extrahepatic cancer, extrahepatic cancer risk does not appear to increase with increased liver fibrosis or disease severity. The 5-year rate of newly diagnosed extrahepatic cancer was 5.6% in the present study and not only HCC but also extrahepatic cancer is an important complication in patients with chronic liver disease. Since patients from a variety of aetiologies were included in the study, future studies should elucidate the utility of liver stiffness assessed with MRE as a surrogate marker for extrahepatic cancer for each aetiology of liver disease.
4.3 |. Strengths and limitations
This study included over 2000 patients with chronic liver disease, and all patients received standard of care and routine surveillance for complications. However, the study was conducted at a single tertiary centre for liver disease. The study included many patients with advanced liver disease, including those with a history of HCC or decompensation (27.8%) and those with liver stiffness >4.7 kPa (36.3%), because our centre has been designated as a treatment centre for HCC. These patients with advanced liver disease were followed up more frequently than patients without advanced liver disease, and the occurrence of complications may underestimate in patients without advanced liver disease than those with advanced liver disease. Furthermore, the occurrence rate of extrahepatic complications may be underestimated in patients with advanced liver disease because of competitive risks. Therefore, multicentre prospective studies are needed to generalize the current study findings.
4.4 |. Future implications
Increased liver stiffness based on evaluation with MRE was associated with HCC, decompensation and mortality risk in a dose-dependent fashion. Furthermore, recent studies demonstrated that changes in liver stiffness detected using MRE were associated with changes in histologically determined fibrosis.38 Therefore, MRE may be used as an approach to assess not only fibrosis but also the risk of complications and mortality, as an alternative to liver biopsy. Furthermore, magnetic resonance imaging (MRI)-based assessment of liver features including proton density fat fraction (PDFF) is better than histological evaluation in assessing quantitative changes in liver features.39–41 Combining these MRI-based assessments (MRE and PDFF) might be able to assess a more accurate prognosis and might provide significant benefit in patients with chronic liver disease.
We found that increased liver stiffness based on MRE was not associated with MACE risk and there was no risk increase in patients with cirrhosis compared to those with moderate/advanced fibrosis. The range of liver stiffness is wide in patients with cirrhosis, who are however considered in the same broad category of cirrhosis based on liver biopsy results. However, MACE risk may differ based on liver stiffness even among patients with histologically defined cirrhosis. Furthermore, liver biopsy is challenging in patients with severe liver stiffness, ie, in those with decompensation, which may lead to selection bias and inaccurate assessment of MACE risk. Therefore, MRE may have more utility than liver biopsy in determining the association between MACE risk and liver fibrosis. The incidence of MACE is an important complication of chronic liver disease. Therefore, screening and prevention of MACE are also necessary and future studies should investigate whether MRE might be used as a method for MACE risk assessment.
In conclusion, increased liver stiffness measured with MRE was associated with increased risk for HCC, decompensation and mortality in a dose-dependent fashion but not with MACE and extrahepatic cancer in patients with chronic liver disease. These findings indicate that liver stiffness measured with MRE might be important for the assessment of liver-related events and mortality; however, additional studies are needed to determine the utility of MRE for the assessment of MACE and extrahepatic cancer.
Funding information
Masayuki Kurosaki received funding support from the Japan Agency for Medical Research and Development (JP20fk0210067h0001). Rohit Loomba received funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019).
Declaration of personal interests:
Namiki Izumi received lecture fees from Gilead Sciences Inc, and Abbvie. Masayuki Kurosaki received lecture fees from Gilead Sciences Inc, Abbvie, Eisai Co., Ltd., Bayer AG, Otsuka Holdings Co., Ltd. Rohit Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc, Madrigal, Metacrine, Inc, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc The other authors have no conflicts of interest to declare.
Footnotes
ETHICAL APPROVAL
The study was approved by the Clinical Research Ethics Committee of Musashino Red Cross Hospital and conformed to the ethical guidelines of the Declaration of Helsinki. (Approval Number: 2007).
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article. Data details can be provided upon request to credible investigators on verification for patient confidentiality.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. [DOI] [PubMed] [Google Scholar]
- 3.Pol S, Vallet-Pichard A, Hermine O. Extrahepatic cancers and chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2018;15:283–290. [DOI] [PubMed] [Google Scholar]
- 4.Petta S, Maida M, Macaluso FS, et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2016;150(1):145–155.e4; quiz e15-6. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–25.e12. [DOI] [PubMed] [Google Scholar]
- 6.Yasui Y, Abe T, Kurosaki M, et al. Elastin fiber accumulation in liver correlates with the development of hepatocellular carcinoma. PLoS One. 2016;11:e0154558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahina Y, Tsuchiya K, Nishimura T, et al. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. [DOI] [PubMed] [Google Scholar]
- 8.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. [DOI] [PubMed] [Google Scholar]
- 9.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamaki N, Kurosaki M, Loomba R, et al. Clinical utility of Mac-2 binding protein glycosylation isomer in chronic liver diseases. Ann Lab Med. 2021;41:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki N, Kurosaki M, Higuchi M, et al. Validation of albumin, bilirubin, and platelet criteria for avoiding screening endoscopy in patients with advanced fibrosis. Hepatol Res. 2020;50:996–999. [DOI] [PubMed] [Google Scholar]
- 12.Kakegawa T, Sugimoto K, Kuroda H, et al. Diagnostic accuracy of two-dimensional shear wave elastography for liver fibrosis: a multicenter prospective study. Clin Gastroenterol Hepatol. 2021;S1542-3565(21)00901-0. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki N, imajo K, Sharpton S, et al. MRE plus FIB-4 (MEFIB) versus FAST in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology. 2021. 10.1002/hep.32145 [DOI] [Google Scholar]
- 14.Tamaki N, Higuchi M, Kurosaki M, et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci Rep. 2019;9:10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima A, Eguchi Y, Yoneda M, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(10):1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokushige K, Ikejima K, Ono M, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021;51:1013–1025. [DOI] [PubMed] [Google Scholar]
- 17.Lee J-H, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. [DOI] [PubMed] [Google Scholar]
- 18.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. [DOI] [PubMed] [Google Scholar]
- 19.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 20.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–13.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17:630–7.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidener T, Ahmed OT, Larson JJ, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD. Clin Gastroenterol Hepatol. 2021;19:1915–24.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver Int. 2020;40:2242–2251. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi M, Tamaki N, Kurosaki M, et al. Prediction of hepatocellular carcinoma after sustained virological responses using magnetic resonance elastography. Clin Gastroenterol Hepatol. 2019;17:2616–2618. [DOI] [PubMed] [Google Scholar]
- 25.Gidener T, Yin M, Dierkhising RA, et al. MRE for prediction of long-term progression and outcome in chronic liver disease: a retrospective study. Hepatology. 2021. 10.1002/hep.32151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagström H, Nasr P, Ekstedt M, et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. [DOI] [PubMed] [Google Scholar]
- 27.Henson JB, Simon TG, Kaplan A, et al. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–57.e17. [DOI] [PubMed] [Google Scholar]
- 29.Niikura T, Imajo K, Ozaki A, et al. Coronary artery disease is more severe in patients with non-alcoholic steatohepatitis than fatty liver. Diagnostics (Basel). 2020;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonmann Y, Yeshua H, Bentov I, et al. Liver fibrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig Liver Dis. 2021;53:79–85. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki N, Kurosaki M, Takahashi Y, et al. Liver fibrosis and fatty liver as independent risk factors for cardiovascular disease. J Gastroenterol Hepatol. 2021;36(10):2960–2966. [DOI] [PubMed] [Google Scholar]
- 32.Tamaki N, Higuchi M, Kurosaki M, et al. Risk difference of liver-related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwakiri Y Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HH, Garruti G, Liu M, et al. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017;16:s27–s42. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub P, Saadoun D. Extrahepatic manifestations of chronic HCV infection. N Engl J Med. 2021;384:1038–1052. [DOI] [PubMed] [Google Scholar]
- 36.Hong CY, Sinn DH, Kang D, et al. Incidence of extrahepatic cancers among individuals with chronic hepatitis B or C virus infection: a nationwide cohort study. J Viral Hepat. 2020;27:896–903. [DOI] [PubMed] [Google Scholar]
- 37.Simon TG, Roelstraete B, Sharma R, et al. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology. 2021;74(5):2410–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajmera VH, Liu A, Singh S, et al. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology. 2020;71:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamaki N, Munaganuru N, Jung J, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2021;gutjnl-2021-324264. 10.1136/gutjnl-2021-324264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article. Data details can be provided upon request to credible investigators on verification for patient confidentiality.


