Abstract
Eucalyptol (1.8-cineole), an active component in traditional Chinese medicine Artemisia argyi for moxibustion. Previous studies have shown that eucalyptol has anti-tumor effects on leukemia and colon cancer. Nonetheless, the effect and mechanism of eucalyptol on neuroblastoma remains unclear. In the present study, we intended to reveal the effect and mechanism of eucalyptol treatment on the neuroblastoma cell line SH-SY5Y through transcriptome analysis. In the group treated with eucalyptol, 566 brain genes were up-regulated, while 757 genes were down-regulated. GO function analysis showed that positive regulation of cell cycle was down-regulated in biological processes. Meanwhile, cancer-related pathways were identified in KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis, including pathways in cancer, PI3K-Akt signaling pathway, cAMP signaling pathway, TGF-beta signaling pathway, Hippo signaling pathway, p53 signaling pathway, and additional pathways. Furthermore, we found a key gene, such as MYC, by constructing a network of cancer related pathways with differentially expressed genes and transcription factor analysis. In conclusion, our research indicates that MYC might play a central role in the anit-tumor mechanisms of eucalyptol.
Keywords: Eucalyptol, 1.8-cineole, Moxibustion, Anti-cancer, MYC, RNAseq
Introduction
Neuroblastoma is a developmental tumor of children from the neural crest. This disease is the primary cause of cancer-related death in children under 5 years of age [1]. Neuroblastoma is a heterogeneous pediatric tumor. Half of this disease is a high risk type and lacks effective cures [2]. SH-SY5Y cell line is a subclone of human neuroblastoma, originally derived from a child metastatic bone tumor biopsy [3]. This cell line is a common cell model to study neurotoxicity [4], neurodegenerative disease [5], neuron differentiation and neuroblastoma [6].
Eucalyptol is a cyclic ether and monoterpenoid, naturally produced by plants such as Artemisia argyi [7, 8]. It is an ingredient in some brands cough suppressants and is also used as a flavoring agent [9]. Eucalyptol has been reported to possess multiple pharmacological effects, including anti-inflammatory [10–12], antioxidant effects [13], pain reduction [14, 15], epilepsy inhibition [16], et al. In some in vitro studies, eucalyptol has anti-tumor effect on leukemia [17], ovarian cancer cells [18] and colon cancer cell line [19]. However, the anti-tumor effect of eucalyptol on neuroblastoma is still unknown.
In this research, we investigated the anti-tumor mechanism of eucalyptol by transcriptome sequencing and bioinformatic analysis. And we discovered that eucalyptol exerts anti-tumor activity on human neuroblastoma cell lines SH-SY5Y by regulating several cancer related pathways and genes. Our findings will be valuable for understanding the anti-tumor mechanism of eucalyptol in neuroblastoma cell proliferation and provide a new therapeutic candidate agent for neuroblastoma therapy.
Methods
Cell Culture of SH-SY5Y
The SH-SY5Y was kindly given by Professor Yun Wang of the Neuroscience Research Institute, Peking University. Cell cultures of SH-SY5Y were cultured in DMEM/F12 (Sigma, Darmstadt, Germany) with 10% (v/v) fetal bovine serum (FBS) (Gibco, Grand Island, USA) and 1% Penicillin-Streptomycin Solution (Gibco, Grand Island, USA). All cultures were incubated in a Thermo CO2 incubator at 37℃ with 95% air and 5% CO2 (v/v) and a humidity of 95%. The cell culture medium was changed twice a week. 70%~80% of confluent cultures used for passage to experiments.
Transcriptome Sequencing
Three pairs of cell samples were collected from untreated and 100 µM eucalyptol-treated SH-SY5Y cells for 6 days, and RNA was extracted for RNA-seq by Trizol (Invitrogen, Carlsbad, CA, USA). Two micrograms of RNA per sample were used as input material for the RNA sample preparations. Sequencing libraries were generated with the VAHTS mRNA-seq v2 Library Prep Kit for Illumina following the manufacturer’s recommendations. Index codes were added to attribute sequences to each sample. Then libraries were sequenced using an Illumina NovaSeq platform to generate 150 bp paired-end reads according to the manufacturer’s instructions.
Raw data of FASTQ format was processed first through primary quality control. In this step, clean data were obtained by removing read pairs that contain N more than 3 or the proportion of base with quality value below 5 is more than 20%, in any end, or adapter sequence was founded. The clean data of each sample was more than 6 GB. All the downstream analyses were based on clean data with high quality.
Differential Expression Analysis and Venn Diagrams
Alignment of paired-end clean reads to the reference genome was with TopHat (v2.1.1). Differential expression analysis between two conditions was performed using Cufflinks (v2.2.1). Differently expressed genes (DEGs) were defined as those for which the P-value below 0.01 and the absolute value of log2(Fold change) more than 1. The Venn Diagrams were constructed by an interactive Venn diagram viewer [20].
Functional Enrichment Analysis
GO and KEGG enrichment analysis of DEGs sets were executed by the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8 [21]. GO terms and KEGG pathways with adjusted P-value below 0.05 were considered as significantly enriched by DEGs. The volcano map was drawn by R language with ggplot2. The bar and bubble graphs are plotted by the GOplot package in R. The network graph of cancer related pathways with DEGs was produced by Cytoscape 3.7.1 [22].
Transcription Factor Analysis
The target genes of MYC analyzed in this study were found by Gene Transcription Regulation Database (GTRD) [23] and Database of Human Transcription Factor Targets (hTFtarget) [24]. We found MYC target genes from GTRD in Homo sapiens with the promoter setting from − 1000 to + 100. And we got target genes of MYC from hTFtarget with the default mode. Then we took the intersection from the above two lists for further analysis.
Result
Differential Expression Analysis of Eucalyptol Treatment
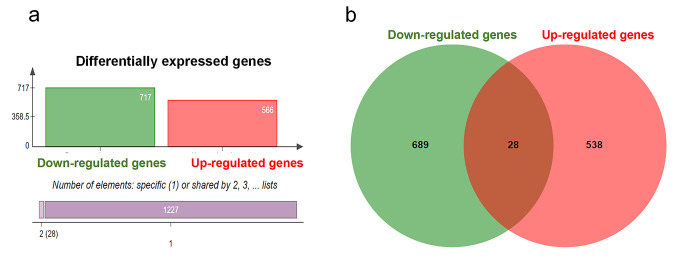
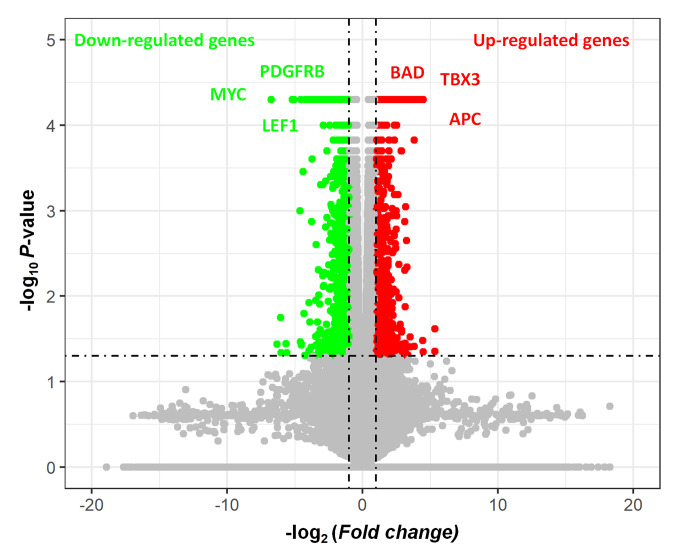
In order to identify the DEGs (up-regulated and down-regulated expression) in SH-SY5Y cells after eucalyptol treatment, we performed mRNA sequencing of normal SH-SY5Y and 100 µM treated SH-SY5Y on the 6th day. RNA-seq identification of DEGs was measured by TopHat and Cufflinks (See Methods). As the results shown in Fig. 1, a total of 1255 genes (1350 transcripts) were differentially expressed, including 566 up-regulated genes (593 transcripts) and 717 down-regulated genes (757 transcripts). There were 28 DEGs with both up-regulated transcripts and down-regulated transcripts. It can be shown from volcano map that anti-tumor genes BAD3, TBX3 and APC were up-regulated genes, at the same time, oncogenes LEF1, PDGFRB, and MYC were down-regulated genes (Fig. 2).
Fig. 1.
Differentially expression genes of eucalyptol treated SH-SY5Y. a shows the classification of differentially expressed genes by bar graphs, b is the Venn Diagram of down-regulated genes and up-regulated genes
Fig. 2.
Volcano map of gene expression changing after eucalyptol treatment. Red dots represent up-regulated genes. Green dots represent down-regulated genes
GO Enrichment Analysis Identified the Biological Functions of DEGs in SH-SY5Y After Eucalyptol Treatment
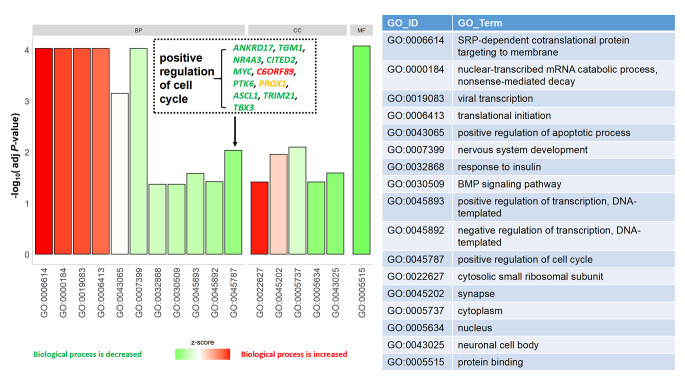
To further evaluate the biological functions of these DEGs, GO enrichment analysis was performed on the experimental group. The results revealed that there was significant enrichment of GO terms, which are grouped into three categories: molecular function (MF), cellular component (CC) and biological process (BP). In the biological process, GO: 0007399: nervous system development, GO: 0000184: nuclear-transcribed mRNA catabolic process, nonsense-mediated decay, GO: 0006614: SRP-dependent cotranslational protein targeting to membrane, GO: 0006413: translational initiation, GO: 0019083: viral transcription, GO: 0043065: positive regulation of apoptotic process, GO: 0045787: positive regulation of cell cycle, GO: 0045893: positive regulation of transcription, DNA-templated, GO: 0045892: negative regulation of transcription, DNA-templated, GO:0032868: response to insulin, GO: 0030509: BMP signaling pathway had the most abundant GO function items. In the molecular function, GO: 0005515: protein binding had the most abundant GO function items. In the cellular component, GO: 0005737: cytoplasm, GO: 0045202: synapse, GO: 0043025: neuronal cell body, GO: 0022627: cytosolic small ribosomal subunit, GO: 0005634: nucleus (Fig. 3).
Fig. 3.
Z-score coloured barplot of GO enrichment of DEGs. If the biological process (/molecular function/cellular components) is decreased, the colour of the bar is green. And If the biological process (/molecular function/cellular components) is increased, the colour of the bar is red
It should be mentioned that GO: 0045787 (positive regulation of cell cycle) was negatively regulated for the most involved DEGs in this term were down-regulated. Those decreasing DEGs were ANKRD17, TGM1, NR4A3, CITED2, MYC, PTK6, ASCL1, TRIM21, TBX3.
KEGG Enrichment Analysis Identified Cancer Related Pathways
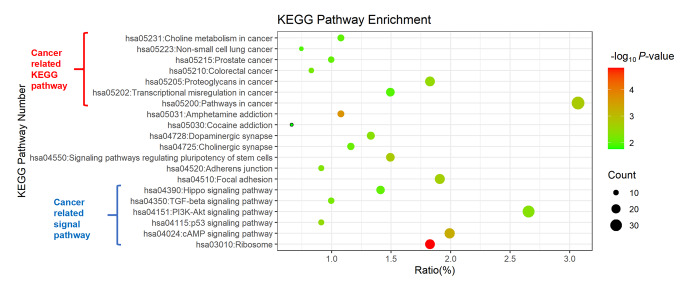
To analyse the DEGs, KEGG enrichment analysis was used for annotation. The top 20 enrichment KEGG pathways, including hsa03010: Ribosome, hsa05031: Amphetamine addiction, hsa04024: cAMP signaling pathway, hsa05200: Pathways in cancer, hsa04550: Signaling pathways regulating pluripotency of stem cells, hsa04510: Focal adhesion, hsa05205: Proteoglycans in cancer, hsa04115: p53 signaling pathway, hsa04728: Dopaminergic synapse, hsa04151: PI3K-Akt signaling pathway, hsa04520: Adherens junction, hsa04350: TGF-beta signaling pathway, hsa05210: Colorectal cancer, hsa04725: Cholinergic synapse, hsa05215: Prostate cancer, hsa04390: Hippo signaling pathway, hsa05231: Choline metabolism in cancer, hsa05202: Transcriptional misregulation in cancer, hsa05223: Non-small cell lung cancer, hsa05030: Cocaine addiction, were shown in Fig. 4.
Fig. 4.
Bubble map of KEGG pathway enrichment of DEGs. The size of bubbles represents the count of DEGs. The color of bubbles represents P-value
The results demonstrated that the seven enriched pathways were directly cancer-related KEGG pathways, as shown in the top 7 pathways in Fig. 4. We also found that five cancer-related cellular signaling pathways, including Hippo signaling pathways, TGF-beta signaling pathways, PI3K-Akt signaling pathway, p53 signaling pathway, cAMP signaling pathway. These results suggest that eucalyptol may exert antitumor effects through the above signaling pathways.
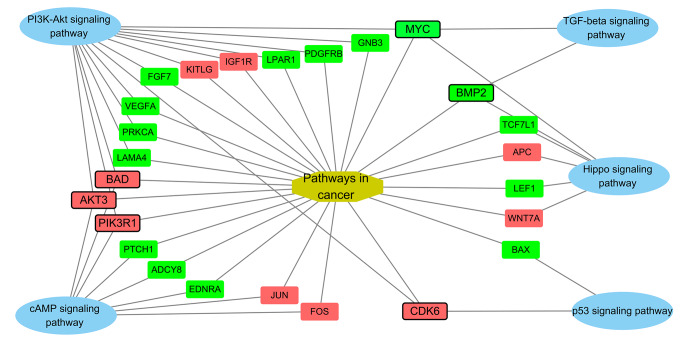
System Biological Analysis Identified the Key Genes in the Network of Cancer Related Pathways with DEGs
For clarify the mechanism of anti-proliferation, we constructed a network of the KEGG enriched cellular signaling pathways (Hippo signaling pathways, TGF-beta signaling pathways, PI3K-Akt signaling pathway, p53 signaling pathway, cAMP signaling pathway) and Pathways in cancer with DEGs (Fig. 5). We found that MYC, BMP2, CDK6, PIK3R1, AKT3 and BAD are linked with more than 3 pathways, demonstrating those genes are important roles in anti-proliferation effect of eucalyptol on SH-SY5Y.
Fig. 5.
The network of cancer-related KEGG pathways with DEGs. The up-regulated genes are in red boxes, while down-regulated genes are in green boxes
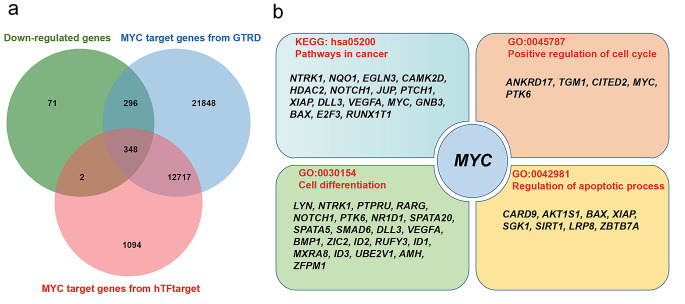
Transcription Factor Analysis Showed Multiple Biology Functions of MYC in the Antitumor Mechanism of Eucalyptol
MYC is an important cancer-related transcription factor gene and sits on the most important gene node in the network (Fig. 5), suggesting that it plays an important role in the antitumor mechanism of eucalyptol. In this study, it was found that eucalyptol caused a pronounced down-regulation of MYC expression and then might result in the down-regulation of MYC target genes (MTGs). Therefore, we conducted a transcription factor analysis on MYC. First, we found 35,769 MTGs from GTRD and 14,741 MTGs from hTFtarget. Then we intersected the list of down-regulated genes with the list of these two MTG lists to determine the down-regulated genes regulated by MYC (Fig. 6a). After analysis, we found that about half of the down-regulated genes were MYC target genes (48.5%, 348/717). By KEGG and GO analysis of these MTGs, we found that these genes were enriched in KEGG: HSA05200 Pathways in cancer, GO:0045787 Positive regulation of cell cycle, GO:0030154 Cell differentiation, GO:0042981 regulates the passage of apoptotic process (Fig. 6b).
Fig. 6.
Transcription factor analysis of MYC target gene. a shows the Venn Diagram of the list of down-regulated genes with the list of these two MYC target gene lists from GTRD and hTFtarget. b shows MYC targets genes of cancer related pathways by the KEGG and GO enrichment
Discussion
Moxibustion is an effective supportive cancer care in inhibiting tumor growth [25, 26] and alleviating side effects of chemotherapy and radiotherapy [27]. Such as, moxibustion can inhibit nausea and vomiting after chemotherapy [28, 29]. In addition, moxibustion can also be used to treat cancer-related fatigue [30]. The mechanism of moxibustion therapy for cancer is still unclear. Eucalyptol is the main component of Artemisia argyi [11, 12]. Eucalyptol has been reported to inhibit the proliferation of many cancer cells [22, 23, 31–33]. In this study, we revealed the anti-tumor effect and mechanism of eucalyptol against human neuroblastoma SH-SY5Y cells by transcriptome sequencing.
The mechanism of the anti-tumor effect of eucalyptol is complex and has not been fully clarified. Suppression of growth by eucalyptol in leukemia, ovarian cancer cells and colorectal cell lines was reported to the induction of apoptosis [21–23, 32]. It was reported that eucalyptol also inhibited cell proliferation by promoting G0/G1 arrest in HepG2 cells [32]. In our study, we found that eucalyptol has a negative effect on “positive regulation of cell cycle (GO: 0045787)” by reducing the expression of most genes in this GO terms (Fig. 3), indicating that eucalyptol intervene cancer cell growth not only by inducing apoptosis but also with anti-proliferation.
Cancer is a complex disease characterized by excessive proliferation of cancer cells with selective growth advantage [34]. Many cell signaling pathways related to cancer development, such as PI3K-Akt signaling pathway [35, 36], Ras signaling pathway [37–40], STAT signaling [40–43], MAPK signaling pathway [35, 37], TGF-beta signaling pathway [44, 45], NOTCH signaling pathway [46–49], p53 signaling pathway [50–52], cAMP signaling pathway [53, 54], Hippo signaling pathway [55, 56], Wnt signaling pathway [57], and so on. In our study, we found that some cancer-related signaling pathways were enriched based on DEGs, including Hippo signaling pathways, TGF-beta signaling pathways, PI3K-Akt signaling pathway, p53 signaling pathway, cAMP signaling pathway. This result suggests that eucalyptol can regulate multiple cancer-related signaling pathways to achieve its anti-cancer effect.
With system biological analysis of the network constructed by cancer related pathways and DEGs, we found that MYC is a key gene in the network of eucalyptol’s anti-tumor mechanism. MYC is an important and well-known oncogene, which regulates cell growth and proliferation [58, 59]. A previous study reported that eucalyptol inhibited protein expression of MYC in AGE-treated podocytes and diabetic kidneys [60]. In our study, we found eucalyptol can down-regulated MYC transcription in neuroblastoma SH-SY5Y cell. And half of the down-regulated genes were MYC target genes. Some of those genes are related to positive regulation of cell cycle, cell differentiation, and apoptotic process, indicating that eucalyptol may be implicated in its anti-tumor effects by down-regulating MYC and its target genes involved in cell division, differentiation, and apoptosis pathways.
In conclusion, our findings demonstrate that eucalyptol can exert its anti-tumor activity by regulating multiple cancer-related cellular signal pathways in human SH-SY5Y cells in vitro. Eucalyptol shows promise as an effective and safe therapeutic agent for neuroblastoma.
Acknowledgements
We thank Professor Yun Wang from Neuroscience Research Institute, Peking University for kindly providing the SH-SY5Y cell. This work was supported by the National Natural Science Foundation of China (grant number: 82171435, 81971211, 81601131), by the National Key Research and Development Program of China (grant number: 2020YFA0804000), by Beijing Natural Science Foundation (grant number: 7212109), by Key Project of Clinical Medicine Research of National Clinical Research Center for Child Health and Disorders, Children’s Hospital of Chongqing Medical University (grant number: NCRCCHD-2021-KP-02), by the capital health research and development of special (2020-1-4071), by the Beijing Key Laboratory of Molecular Diagnosis and Study on Pediatric Genetic Diseases (grant number: BZ0317), and by the Fundamental Research Funds for the Central Universities (grant number: BMU2017JI002, BMU2018XY006, PKU2017LCX06). The authors declare no competing financial interests.
Authors’ Contributions
Jian Lu and Yuwu Jiang supervised, designed, carried out and wrote the manuscript. Kai Gao performed cell culture of SH-SY5Y, drug treatment and RNAseq. Congying Wu analyzed RNAseq data, GO and KEGG enrichment, and wrote the manuscript. Yanlong Li help to perform the data analysis and manuscript writing. All authors read and approved the final manuscript.
Funding
The funding agencies had no role in the study design, the experiments, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Data Availability
The original data presented in the study are included in the article materials, further inquiries can be directed to the author/corresponding authors.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Kai Gao and Congying Wu contributed equally.
The original online version of this article was revised due to addition of article note.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/21/2022
A Correction to this paper has been published: 10.1007/s11064-022-03823-6
Contributor Information
Jian Lu, Email: lvjian2999@126.com.
Yuwu Jiang, Email: jiangyw@263.net.
References
- 1.Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. 2017;17:369–386. doi: 10.1080/14737140.2017.1285230. [DOI] [PubMed] [Google Scholar]
- 3.Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21. doi: 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam M, Huh YJ, Lee YI. The Impairments of alpha-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front Neurosci. 2019;13:1028. doi: 10.3389/fnins.2019.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teppola H, Sarkanen JR, Jalonen TO, Linne ML. Morphological Differentiation Towards Neuronal Phenotype of SH-SY5Y Neuroblastoma Cells by Estradiol, Retinoic Acid and Cholesterol. Neurochem Res. 2016;41:731–747. doi: 10.1007/s11064-015-1743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang WJ, You CX, Yang K, Chen R, Wang Y, Wu Y, Geng ZF, Chen HP, Jiang HY, Su Y, Lei N, Ma P, Du SS, Deng ZW. Bioactivity of essential oil of Artemisia argyi Levl. et Van. and its main compounds against Lasioderma serricorne. J Oleo Sci. 2014;63:829–837. doi: 10.5650/jos.ess14057. [DOI] [PubMed] [Google Scholar]
- 8.Ge YB, Wang ZG, Xiong Y, Huang XJ, Mei ZN, Hong ZG. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J Nat Med. 2016;70:531–538. doi: 10.1007/s11418-016-0972-6. [DOI] [PubMed] [Google Scholar]
- 9.Seol GH, Kim KY. Eucalyptol and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;929:389–398. doi: 10.1007/978-3-319-41342-6_18. [DOI] [PubMed] [Google Scholar]
- 10.Juergens LJ, Worth H, Juergens UR. New Perspectives for Mucolytic, Anti-inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv Ther. 2020;37:1737–1753. doi: 10.1007/s12325-020-01279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res (Stuttg) 2014;64:638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- 12.Yin C, Liu B, Wang P, Li X, Li Y, Zheng X, Tai Y, Wang C, Liu B. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. Br J Pharmacol. 2020;177:2042–2057. doi: 10.1111/bph.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciftci O, Ozdemir I, Tanyildizi S, Yildiz S, Oguzturk H. Antioxidative effects of curcumin, beta-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol Ind Health. 2011;27:447–453. doi: 10.1177/0748233710388452. [DOI] [PubMed] [Google Scholar]
- 14.Zheng XB, Zhang YL, Li Q, Liu YG, Wang XD, Yang BL, Zhu GC, Zhou CF, Gao Y, Liu ZX. Effects of 1,8-cineole on neuropathic pain mediated by P2 × 2 receptor in the spinal cord dorsal horn. Sci Rep. 2019;9:7909. doi: 10.1038/s41598-019-44282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YL, Liu YG, Li Q, Wang XD, Zheng XB, Yang BL, Wan B, Ma JM, Liu ZX. 1,8-cineole decreases neuropathic pain probably via a mechanism mediating P2 × 3 receptor in the dorsal root ganglion. Neurochem Int. 2018;121:69–74. doi: 10.1016/j.neuint.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Bahr TA, Rodriguez D, Beaumont C, Allred K (2019) The Effects of Various Essential Oils on Epilepsy and Acute Seizure: A Systematic Review. Evid Based Complement Alternat Med 2019:6216745 [DOI] [PMC free article] [PubMed]
- 17.Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol Rep. 2002;9:757–760. [PubMed] [Google Scholar]
- 18.Abdalla AN, Shaheen U, Abdallah QMA, Flamini G, Bkhaitan MM, Abdelhady MIS, Ascrizzi R, Bader A (2020) Proapoptotic Activity of Achillea membranacea Essential Oil and Its Major Constituent 1,8-Cineole against A2780 Ovarian Cancer Cells. Molecules 25 [DOI] [PMC free article] [PubMed]
- 19.Murata S, Shiragami R, Kosugi C, Tezuka T, Yamazaki M, Hirano A, Yoshimura Y, Suzuki M, Shuto K, Ohkohchi N, Koda K. Antitumor effect of 1, 8-cineole against colon cancer. Oncol Rep. 2013;30:2647–2652. doi: 10.3892/or.2013.2763. [DOI] [PubMed] [Google Scholar]
- 20.Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao X, Sherman BT, Huang da W, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics. 2012;28:1805–1806. doi: 10.1093/bioinformatics/bts251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolmykov S, Yevshin I, Kulyashov M, Sharipov R, Kondrakhin Y, Makeev VJ, Kulakovskiy IV, Kel A, Kolpakov F. GTRD: an integrated view of transcription regulation. Nucleic Acids Res. 2021;49:D104–D111. doi: 10.1093/nar/gkaa1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Liu W, Zhang HM, Xie GY, Miao YR, Xia M, Guo AY. hTFtarget: A Comprehensive Database for Regulations of Human Transcription Factors and Their Targets. Genomics Proteom Bioinf. 2020;18:120–128. doi: 10.1016/j.gpb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Yang M, Fan Y, Pei X. Moxibustion as a Therapy for Breast Cancer-Related Lymphedema in Female Adults: A Preliminary Randomized Controlled Trial. Integr Cancer Ther. 2019;18:1534735419866919. doi: 10.1177/1534735419866919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Huang J, Li S, Pan Z, Guo Y, Yang Y, Li L, Wang C, Gong Y, Wang J, Lu S, Xu Z, Guo Y (2020) The Combinatorial Effect of Cisplatin and Moxibustion on Tumor Growth Inhibition with Special Reference to Modulation of the Immune Microenvironment in Lewis Lung Cancer Mice. Evid Based Complement Alternat Med 2020:3170803 [DOI] [PMC free article] [PubMed]
- 27.Zhang HW, Lin ZX, Cheung F, Cho WC, Tang JL. Moxibustion for alleviating side effects of chemotherapy or radiotherapy in people with cancer. Cochrane Database Syst Rev. 2018;11:CD010559. doi: 10.1002/14651858.CD010559.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, Choi TY, Park JE, Lee SS, Ernst E. Moxibustion for cancer care: a systematic review and meta-analysis. BMC Cancer. 2010;10:130. doi: 10.1186/1471-2407-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Wang YL, Fu RY, Li JX, Guo XQ, Xu B, Feng SH, Guan XJ. [Ginger-partitioned moxibustion in the prevention of nausea and vomiting induced by chemotherapy in lung cancera randomized controlled trial] Zhen Ci Yan Jiu. 2020;45:574–577. doi: 10.13702/j.1000-0607.190568. [DOI] [PubMed] [Google Scholar]
- 30.Han K, Kim M, Kim EJ, Park YC, Kwon O, Kim AR, Park HJ, Park YC, Cho JH, Kim JH, Lee JH. Moxibustion for treating cancer-related fatigue: A multicenter, assessor-blinded, randomized controlled clinical trial. Cancer Med. 2021;10:4721–4733. doi: 10.1002/cam4.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodenak Kladniew B, Polo M, Montero Villegas S, Galle M, Crespo R, Garcia de Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chem Biol Interact. 2014;214:57–68. doi: 10.1016/j.cbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Rodenak-Kladniew B, Castro A, Stärkel P, Galle M, Crespo R. 1,8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020;243:117271. doi: 10.1016/j.lfs.2020.117271. [DOI] [PubMed] [Google Scholar]
- 33.Santos FA, Rao VS. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 2000;14:240–244. doi: 10.1002/1099-1573(200006)14:4<240::AID-PTR573>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laha D, Nilubol N, Boufraqech M. New Therapies for Advanced Thyroid Cancer. Front Endocrinol (Lausanne) 2020;11:82. doi: 10.3389/fendo.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masliah-Planchon J, Garinet S, Pasmant E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget. 2016;7:38892–38907. doi: 10.18632/oncotarget.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore AR, Rosenberg SC, McCormick F, Malek S. Author Correction: RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19:902. doi: 10.1038/s41573-020-0089-1. [DOI] [PubMed] [Google Scholar]
- 39.Vaseva AV, Yohe ME. Targeting RAS in pediatric cancer: is it becoming a reality? Curr Opin Pediatr. 2020;32:48–56. doi: 10.1097/MOP.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prior IA, Hood FE, Hartley JL. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rani A, Murphy JJ. STAT5 in Cancer and Immunity. J Interferon Cytokine Res. 2016;36:226–237. doi: 10.1089/jir.2015.0054. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Liu Z. STAT1 in cancer: friend or foe? Discov Med. 2017;24:19–29. [PubMed] [Google Scholar]
- 44.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Targeting the TGFbeta pathway for cancer therapy. Pharmacol Ther. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Vinson KE, George DC, Fender AW, Bertrand FE, Sigounas G. The Notch pathway in colorectal cancer. Int J Cancer. 2016;138:1835–1842. doi: 10.1002/ijc.29800. [DOI] [PubMed] [Google Scholar]
- 47.Kontomanolis EN, Kalagasidou S, Pouliliou S, Anthoulaki X, Georgiou N, Papamanolis V, Fasoulakis ZN (2018) The Notch Pathway in Breast Cancer Progression. ScientificWorldJournal 2018:2415489 [DOI] [PMC free article] [PubMed]
- 48.Li L, Tang P, Li S, Qin X, Yang H, Wu C, Liu Y. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy. Med Oncol. 2017;34:180. doi: 10.1007/s12032-017-1039-6. [DOI] [PubMed] [Google Scholar]
- 49.Aster JC, Pear WS, Blacklow SC. The Varied Roles of Notch in Cancer. Annu Rev Pathol. 2017;12:245–275. doi: 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Liu J, Xu D, Zhang T, Hu W, Feng Z. Gain-of-function mutant p53 in cancer progression and therapy. J Mol Cell Biol. 2020;12:674–687. doi: 10.1093/jmcb/mjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacroix M, Riscal R, Arena G, Linares LK, Le Cam L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol Metab. 2020;33:2–22. doi: 10.1016/j.molmet.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017;83:258–265. doi: 10.1016/j.ejca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Bergantin LB. Diabetes and cancer: Debating the link through Ca(2+)/cAMP signalling. Cancer Lett. 2019;448:128–131. doi: 10.1016/j.canlet.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Bergantin LB. A Hypothesis for the Relationship between Depression and Cancer: Role of Ca2+/cAMP Signalling. Anticancer Agents Med Chem. 2020;20:777–782. doi: 10.2174/1871520620666200220113817. [DOI] [PubMed] [Google Scholar]
- 55.Zeng X, Ju D (2018) Hedgehog Signaling Pathway and Autophagy in Cancer.Int J Mol Sci19 [DOI] [PMC free article] [PubMed]
- 56.Luo J, Yu FX (2019) GPCR-Hippo Signaling in Cancer. Cells 8 [DOI] [PMC free article] [PubMed]
- 57.Gartel AL. FOXM1 in Cancer: Interactions and Vulnerabilities. Cancer Res. 2017;77:3135–3139. doi: 10.1158/0008-5472.CAN-16-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and Cancer. Cancer Discov. 2015;5:1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim DY, Kang MK, Lee EJ, Kim YH, Oh H, Kang YH. Eucalyptol Inhibits Advanced Glycation End Products-Induced Disruption of Podocyte Slit Junctions by Suppressing Rage-Erk-C-Myc Signaling Pathway. Mol Nutr Food Res. 2018;62:e1800302. doi: 10.1002/mnfr.201800302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data presented in the study are included in the article materials, further inquiries can be directed to the author/corresponding authors.