Abstract
Light initiates chloroplast biogenesis in Arabidopsis by eliminating PHYTOCHROME-INTERACTING transcription FACTORs (PIFs), which in turn de-represses nuclear photosynthesis genes, and synchronously, generates a nucleus-to-plastid (anterograde) signal that activates the plastid-encoded bacterial-type RNA polymerase (PEP) to transcribe plastid photosynthesis genes. However, the identity of the anterograde signal remains frustratingly elusive. The main challenge has been the difficulty to distinguish regulators from the plethora of necessary components for plastid transcription and other essential chloroplast functions, such as photosynthesis. Here, we show that the genome-wide induction of nuclear photosynthesis genes is insufficient to activate the PEP. PEP inhibition is imposed redundantly by multiple PIFs and requires PIF3’s activator activity. Among the nuclear-encoded components of the PEP holoenzyme, we identify four light-inducible, PIF-repressed sigma factors as anterograde signals. Together, our results elucidate that light-dependent inhibition of PIFs activates plastid photosynthesis genes via sigma factors as anterograde signals in parallel with the induction of nuclear photosynthesis genes.
Subject terms: Chloroplasts, Light responses, Plant signalling
Photoreceptors in the nucleus control plastid transcription by utilizing sigma factors as nucleus-to-plastid signals in parallel with the light regulation of nuclear photosynthesis genes.
Introduction
The control of organellar gene expression by the cell nucleus is critical to the cellular homeostasis of all eukaryotic organisms. In mammalian cells, the mitochondrial DNA encodes 13 proteins required for oxidative phosphorylation. The misregulation of mitochondrial genes results in or is associated with neurodegenerative diseases and cancer1. The plastids in plants encode substantially more genes; the plastome of the reference species Arabidopsis thaliana (Arabidopsis) harbors 85 protein-coding genes, including 46 genes essential for photosynthesis and therefore the plant’s life2,3. Because over 90% of mitochondrial and plastid proteins are encoded by the nuclear genome4, organellar gene expression relies on the expression of hundreds of nuclear-encoded gene products that participate in organellar transcription, post-transcriptional RNA processing, and translation. However, the mechanism of anterograde, i.e., nucleus-to-organelle, signaling by which the nucleus regulates organellar gene expression in response to developmental and environmental cues remains unclear. Anterograde signaling is often interpreted simply as the genetic dependency of organellar gene expression on the nuclear genome; but it is conceivable that not all nuclear-encoded organellar proteins are employed as signaling molecules. In fact, the main challenge to define anterograde signaling has been the difficulty in teasing apart regulators from the battery of nuclear-encoded components required for organellar biogenesis and/or functions5. Compared with mitochondria, the regulation of gene expression in plastids is expected to be more complex. This is not only attributable to the greater number of genes encoded by the plastid genome, but more importantly, due to the ability of plastids to differentiate into various cell-/tissue-specific types with distinct morphologies and functions6. Plastid differentiation is closely associated with plastid gene expression and is ultimately controlled by the host cell’s nucleus. As such, plastid differentiation presents a unique experimental paradigm to interrogate the mechanism of coordinating nuclear and organellar gene expression, particularly the nuclear control of organellar gene expression by anterograde signaling.
Chloroplasts are photosynthetically active plastids derived from undifferentiated proplastids in the meristematic cells. Chloroplast biogenesis is coupled with leaf development and the production of mesophyll cells – the cell type specialized for photosynthesis. Chloroplast biogenesis in angiosperms (flowering plants) also depends upon light6. In Arabidopsis, after seed germination under the ground or in darkness, seedlings adopt a dark-grown developmental program called skotomorphogenesis or etiolation, which promotes the elongation of the embryonic stem (hypocotyl) and inhibits the expansion of the embryonic leaves (cotyledons)7. Plastids in the cotyledon cells of dark-grown seedlings differentiate into nonphotosynthetic chloroplast precursors called etioplasts. Etioplasts are not green because chlorophyll biogenesis is blocked in the dark, as the conversion of protochlorophyllide to chlorophyllide a by protochlorophyllide oxidoreductase (POR) requires light8. During skotomorphogenesis, protochlorophyllide accumulates to high levels in etioplasts and, together with its associated POR, forms characteristic crystalline structures called prolamellar bodies. Upon exposure to light, seedlings switch to a light-grown developmental program called photomorphogenesis, which restricts hypocotyl elongation and instead promotes cotyledon expansion and leaf development7. The developmental switch from skotomorphogenesis to photomorphogenesis, called de-etiolation, is accompanied by the differentiation of nonphotosynthetic etioplasts into photosynthetic chloroplasts, thereby enabling seedlings to transition to autotrophic growth powered by photosynthesis. Making chloroplasts involves reorganizing the inner membrane system from prolamellar bodies to thylakoid membranes and building up the photosynthetic machinery, including the chlorophyll-containing light-harvesting complex for light perception, protein complexes for photosynthetic electron transport and ATP production – such as photosystems I and II, the cytochrome b6f complex, the NADH dehydrogenase-like complex, and ATP synthase – and Calvin cycle enzymes for carbon fixation. These photosynthetic components are encoded by both nuclear and plastid genes, referred to as photosynthesis-associated nuclear genes (PhANGs) and plastid genes (PhAPGs), respectively. The coordinated activation of PhANGs and PhAPGs is crucial for chloroplast biogenesis.
Chloroplast biogenesis is ultimately controlled by the host cell’s nucleus3. The transition from etioplasts to chloroplasts during de-etiolation is initiated by light through photoreceptors, including the red/far-red light-sensing phytochromes (PHYs) and the blue-light-absorbing cryptochromes (CRYs)7. Photoactivated PHYs and CRYs are localized in the nucleus but not in plastids9,10. The primary action of light signaling in initiating photomorphogenesis is to reprogram the nuclear genome, including activating the nuclear photosynthesis program, or PhANGs. PHYs and CRYs control nuclear gene expression by regulating the stability and activity of a family of basic helix-loop-helix transcription factors named PHYTOCHROME-INTERACTING FACTORs (PIFs)11–14. In Arabidopsis, PIFs include eight members (PIF1–8), of which PIF1, PIF3, PIF4, and PIF5 collectively and redundantly repress PhANGs in the nucleus15–17. A dark-grown pif1pif3pif4pif5 quadruple mutant (pifq) becomes de-etiolated, morphologically mimicking wild-type Col-0 seedlings grown in the light with early signs of chloroplast biogenesis such as the disappearance of the prolamellar bodies, the development of rudimentary prothylakoid membranes, and the activation of PhANGs15–17. Besides PIFs, the repression of PhANGs in darkness requires two additional transcriptional regulators ETHYLENE-INSENSITIVE 3 (EIN3) and EIN3-LIKE 1 (EIL1), which transduce the signal of mechanical pressure when seedlings are buried under the ground18. Dark-grown ein3/eil1 mutants, despite lacking obvious photomorphogenic phenotypes, show constitutive PhANG activation18. Corroborating the essential roles of PIFs and EIN3/EIL1 in repressing PhANGs, perturbing the stability of PIFs and/or EIN3/EIL1 in the classic constitutive photomorphogenic/de-etiolated/fusca (cop/det/fus) mutants also leads to activation of PhANGs in the absence of light19,20. CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) is a zinc finger protein that either acts as an E3 ubiquitin ligase by itself or works together with SUPPRESSOR OF PHYTOCHROME A-105 (SPA) proteins as the substrate recognition subunit of DAMAGE-SPECIFIC DNA BINDING PROTEIN 1 (DDB1) and CULLIN 4 (CUL4) based E3 ubiquitin ligases (i.e., COP1-SPA-DDB1-CUL4 complexes)21–23. COP1 stabilizes PIF3 and EIN3/EIL1 by promoting the degradation of their cognitive E3 ubiquitin ligases, EIN3-BINDING F BOX PROTEIN 1 or 224. In addition, the COP1-SPA complex interacts directly with PIF3 to block PIF3 phosphorylation by the protein kinase BRASSINOSTEROID INSENSITIVE 2 (BIN2) and the subsequent BIN2-dependent PIF3 degradation25. There are four SPA paralogs in Arabidopsis, named SPA1–4, of which SPA1 and SPA2 play prominent roles in repressing photomorphogenesis in the dark26. Moreover, DET1, together with COP10, constitutes the substrate recognition subunit of another DDB1-CUL4-based E3 ubiquitin ligase complex, COP10-DET1-DDB1-CUL427,28, which promotes PIF3 accumulation in the dark through direct DET1-PIF3 interaction29. PHYs and CRYs elicit photomorphogenesis by inhibiting the activity of the COP1-SPA complex in the light30–33. They also bind directly to PIFs to promote PIF phosphorylation, ubiquitylation, and proteasome-mediated degradation12,13,24,34–36. The light-dependent degradation of PIFs is considered a central mechanism to turn on the nuclear program for chloroplast biogenesis, particularly via the activation of PhANGs.
Light synchronizes PhAPG activation with PhANG expression via anterograde signaling3. Plastid genes are transcribed by two types of plastid RNA polymerases: a single-subunit, phage-type nuclear-encoded RNA polymerase (NEP) and a multi-subunit, bacterial-type plastid-encoded RNA polymerase (PEP)37–39. While the NEP preferentially transcribes housekeeping genes, including genes encoding the core PEP subunits, the PEP mainly transcribes PhAPGs38. Extensive biochemical and proteomics studies have demonstrated that a large fraction of the PEP is tightly associated with DNA and forms multisubunit complexes40,41. The PEP complex comprises the prokaryotic α, β, β’, β” core subunits surrounded by 12 PEP-associated proteins (PAPs) that are essential for the PEP activity40,41. Like the bacterial RNA polymerase, the PEP holoenzyme also requires a sigma factor (SIG) to specifically recognize promoter elements and initiate transcription42. There are 6 SIGs in Arabidopsis, named SIG1–6, which play unique and overlapping roles42. While the bacterial-like α, β, β’, β” core subunits are encoded in the plastid genome by the rpoA, rpoB, rpoC1, and rpoC2 genes, respectively, the 12 PAPs and 6 SIGs are encoded by the nuclear genome. We recently reported that PHY-mediated degradation of PIFs in the nucleus in monochromatic red light initiates an anterograde signaling pathway that triggers the assembly of the PEP into a 1000-kDa protein complex and transcription of PhAPGs43,44. These findings identify PIF degradation as a critical switch that synchronizes the activation of both PhANGs and PhAPGs.
The anterograde signaling mechanism downstream of PIFs to control the activity of the PEP remains elusive. The anterograde signal is likely encoded by a light-inducible gene repressed in darkness by PIFs directly or indirectly. However, the main challenge is still to discern the gene encoding the signal molecule from among a large set of light-induced, PIF-repressed genes that encode proteins required for chloroplast transcription or other chloroplast functions. For example, despite the essential roles of PAPs and SIGs in PEP activity, whether these proteins could serve as anterograde signals for light-dependent PEP activation remains ambiguous. Also, PhAPGs are thought to be coupled with PhANGs. Because PhANGs are also repressed by PIFs and induced by light15,16, the activation of PhANGs itself may constitute the anterograde signal. Supporting this idea, the ectopic expression of a light-harvesting complex protein is able to stimulate the formation of prothylakoid membranes in the dark18. Here, to dissect the anterograde signal, we first sought to determine whether light controls PhANGs and PhAPGs via hierarchical or parallel mechanisms. We characterized the relationship between the genome-wide induction of PhANGs and the activation of the PEP in Arabidopsis de-etiolated mutants that constitutively express PhANGs in darkness. These experiments surprisingly show that the genome-wide activation of PhANGs is insufficient to trigger PEP assembly in plastids, indicating that anterograde signaling controls plastid transcription via a separable pathway in parallel with the regulation of PhANGs. These experiments also allowed us to devise a strategy to distinguish anterograde signals from among essential components of the PEP holoenzyme. Together, our results further elucidate the framework of anterograde signaling that coordinates plastid transcription with the nuclear photosynthesis program during chloroplast biogenesis.
Results
PEP is constitutively activated in all de-etiolated mutants except ein3/eil1
To determine whether the activation of PhANGs in the nucleus plays a role in anterograde signaling, we examined PEP assembly and PhAPG activation in de-etiolated mutants that constitutively express PhANGs even in darkness; these mutants included pifq15,17, cop1-445, det1-146, four spa triple mutants (spa234, spa134, spa124, and spa123)26, and ein3/eil147. We asked whether the constitutive activation of PhANGs was sufficient to trigger PEP assembly and PhAPG induction in plastids. PEP assembly has not been examined in any of these mutants except pifq43,44. Notably, the criteria for “constitutive activation of PhANGs” were loosely defined at this point because the genome-wide expression of PhANGs had not been precisely characterized in most of these mutants. Thus, “constitutive activation of PhANGs” here merely means that at least some PhANGs were reported to be induced in the mutant in darkness.
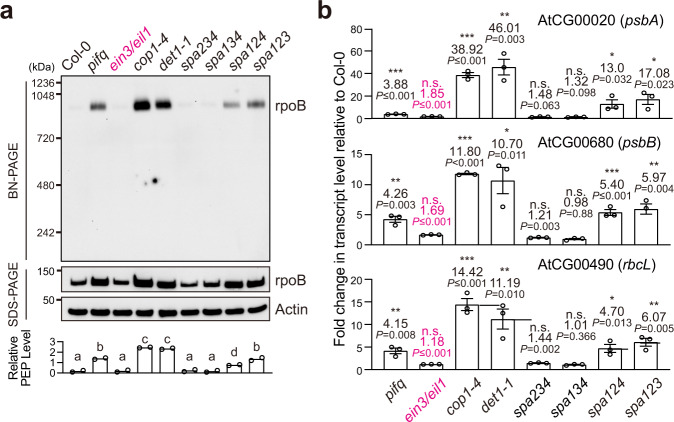
We have previously shown that the PEP assembles into a 1000-kDa protein complex in pifq seedlings regardless of the light signal, leading to the constitutive expression of PhAPGs in the dark (Fig. 1a, b)43,44. To compare the levels of PEP assembly between genotypes, we performed immunoblots to quantify the “relative PEP level”, which represents the relative level of rpoB from the PEP complex in total rpoB (Fig. 1). Consistent with the roles of COP1, DET1, and SPAs in stabilizing PIFs, the PEP was also constitutively assembled and activated in dark-grown cop1-4, det1-1, spa124, and spa123 (Fig. 1a, b). The PEP was not activated in spa234 or spa134 (Fig. 1a, b), corroborating the idea that SPA1 and SPA2 exert major roles in maintaining skotomorphogenesis26. Together, these results suggest that COP1-SPA1-DDB1-CUL4 and COP1-SPA2-DDB1-CUL4 redundantly repress the anterograde signal that activates the PEP in darkness, likely by stabilizing PIFs. The COP1-SPA and the COP10-DET1 E3 ubiquitin ligases appeared to play non-redundant roles, as knocking out either one led to PEP activation (Fig. 1a, b). Because both PhANGs and PhAPGs were constitutively activated, these de-etiolated mutants did not provide new insights into the role of PhANGs in anterograde signaling.
Fig. 1. The PEP is constitutively activated in all de-etiolated mutants except ein3/eil1.
a Immunoblots showing the levels of the PEP complex in 4-d-old dark-grown seedlings of Col-0, pifq, ein3/eil1, cop1-4, det1-1, spa234, spa134, spa124, and spa123. Total protein was isolated under either native or denaturing conditions and resolved via blue-native or sodium dodecyl sulfate polyacrylamide gel electrophoresis (BN-PAGE or SDS-PAGE) to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. The bar graph shows the qualification of the relative levels of the PEP complex, which were calculated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of the total rpoB (SDS-PAGE) in each sample. Different letters denote statistically significant differences in the relative PEP levels among the genotypes (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 2). b qRT-PCR results showing the fold changes in the steady-state transcript levels of psbA, psbB, and rbcL in 4-d-old dark-grown seedlings of pifq, ein3/eil1, cop1-4, det1-1, spa234, spa134, spa124, and spa123 relative to their respective levels in Col-0 seedlings. Asterisks indicate a statistically significant and at least two-fold change in the transcript level in the mutant compared with that in Col-0 based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001); if the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. The source data underlying the immunoblots in a and the qRT-PCR analysis in b are provided in the Source Data file.
A surprise came when we examined the ein3/eil1 mutant. Strikingly, neither PEP assembly nor PhAPG expression was induced in dark-grown ein3/eil1 seedlings (Fig. 1a, b), indicating that EIN3 and EIL1 do not participate in anterograde signaling. Because it was suggested that EIN3 and EIL1 work collaboratively with PIF3 to repress PhANGs18, the constitutive expression of PhANGs without the activation of PhAPGs in the ein3/eil1 mutant might imply that the induction of PhANGs is insufficient to activate PhAPGs in the plastids.
Only a subset of PhANGs are activated in ein3/eil1
Before we could reach a conclusion that the activation of PhANGs is insufficient to trigger PEP activation, we reasoned that we had to exclude the possibility that EIN3 and EIL1 could repress only a subset of PhANGs and PEP activation may require an activation of genome-wide PhANGs. To test this hypothesis, we decided to compare the global expression of PhANGs between dark-grown pifq and ein3/eil1 mutants. The full set of PhANGs have not been specifically characterized in de-etiolated mutants. Most previous studies used representative PhANGs to infer the expression pattern of PhANGs genome wide, while a detailed transcriptomic analysis of precisely defined PhANGs is still lacking. For our analysis, we defined PhANGs as the 149 nuclear-encoded genes whose products directly participate in the photosynthetic reactions, such as light-harvesting (including chlorophyll biosynthesis), photosynthetic electron transport, ATP synthesis, and the Calvin cycle for CO2 fixation (Supplementary Table 1)3. To identify possible distinct regulation between individual functional groups of PhANGs, we divided the PhANGs into three subcategories. Light harvesting for photosynthesis involves the light-harvesting complex that consists of chlorophyll molecules bound by LIGHT HARVESTING COMPLEX proteins (LHCs). All LHCs and chlorophyll biosynthetic enzymes are encoded by the nuclear genome; we named these two subcategories of PhANGs LHCs and CHLs (CHLorophyll biogenetic enzymes), respectively (Supplementary Table 1). We put the rest of the PhANGs, which encode components of photosystems I and II, electron transport, ATP synthase, and the Calvin cycle, into a third subcategory; because the components involved in these processes are encoded by both the nuclear and plastid genomes, we called this subcategory of PhANGs nuclear genes associated with Photosystems, Electron transport, ATP synthase, and the Calvin cycle (nPEACs) (Supplementary Table 1).
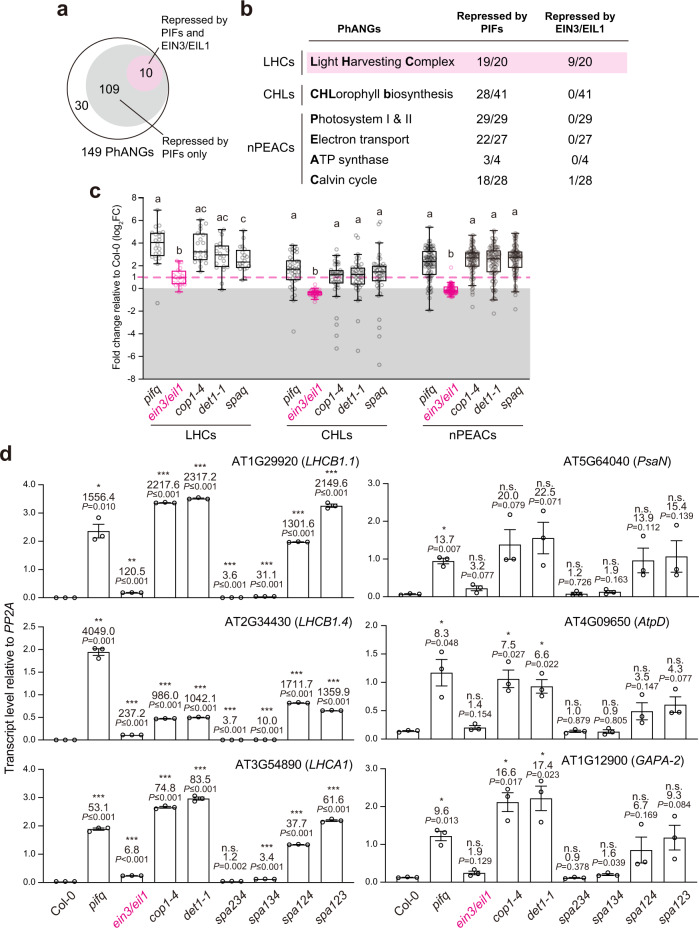
To assess the regulation of genome-wide PhANGs by PIFs and EIN3/EIL1, we reanalyzed published RNA-seq datasets on 4-d-old dark-grown pifq seedlings29 and 3-d-old ein3/eil1 seedlings48 together with their respective Col-0 controls (Supplementary Table 2). To allow comparisons across different experimental settings, we normalized the transcript level of each gene against that of the commonly used control gene PP2A (AT1G13320) and then quantified the fold changes of the transcript levels of the PhANGs in the mutants relative to their respective Col-0 controls. We used a statistically significant two-fold change as the cutoff to define the genes that were differentially expressed in the mutants (Supplementary Data 1). Intriguingly, PhANGs exhibited conspicuously different expression patterns between pifq and ein3/eil1. In pifq, 119 of 149 PhANGs were upregulated compared with Col-0, these PIF-repressed genes comprised the majority of the genes in all three subcategories, LHC, CHL, and nPEAC, confirming that PIFs are the main repressors of chloroplast biogenesis by controlling about 80% of PhANGs (Fig. 2a, b and Supplementary Data 1). In contrast, only 10 PhANGs were upregulated in ein3/eil1; these EIN3/EIL1-repressed PhANGs were also repressed by the PIFs (Fig. 2a and Supplementary Data 1). Interestingly, nine of the ten PIF- and EIN3/EIL1-corepressed PhANGs were LHCs, especially LHCBs associated with photosystem II (Fig. 2b and Supplementary Data 1). We then plotted the expression changes of LHCs, CHLs, and nPEACs separately in pifq and ein3/eil1 (Fig. 2c). The results demonstrated that whereas the majority of LHCs, CHLs, and nPEACs were upregulated in pifq, only LHCs were upregulated in ein3/eil1 (Fig. 2b, c). It is important to note that gene expression can vary significantly in RNA-seq data generated from seedlings grown in different conditions or at different developmental stages. A separate study showed that some nPEACs were also under the control of EIN3 and EIL118. Nonetheless, both studies corroborate the idea that EIN3 and EIL1 repress only a subset of PhANGs, especially LHCs. We then analyzed the expression of PhANGs using published RNA-seq datasets generated from dark-grown 3-d-old cop1-4 mutants49, 4-d-old det1-1 mutants29, and 3-d-old spa1234 quadruple mutants (spaq)49. Similar to pifq, all three subcategories of PhANGs were upregulated in cop1-4, det1-1, and spaq in the dark (Fig. 2c and Supplementary Data 1). To validate the RNA-seq results, we performed qRT-PCR experiments to measure the transcript levels of select LHCs and nPEACs in 4-d-old dark-grown Col-0, pifq, ein3/eil1, cop1-4, det1-1, spa234, spa134, spa124, and spa123 seedlings. These results confirmed that COP1, SPA1/2, DET1, and PIFs repress both LHCs and nPEACs, whereas EIN3 and EIL1 repress only LHCs (Fig. 2d). Additionally, the three tested LHCs were activated at much lower levels in ein3/eil1 than in the other de-etiolated mutants, suggesting that EIN3 and EIL1 play a relatively minor role compared with PIFs in repressing LHCs (Fig. 2d). Taken together, these results leave open the possibility that the lack of PEP activation in the ein3/eil1 mutant could be due to the incomplete activation of PhANGs globally.
Fig. 2. Only a subset of PhANGs are constitutively activated in ein3/eil1.
a Venn diagram showing PhANGs repressed by PIFs and EIN3/EIL1 in dark-grown seedlings. b Table showing the number of PIF-repressed and EIN3/EIL1-repressed genes in the PhANG subcategories. c Box-and-whisker plots showing the fold changes in LHCs, CHLs, and nPEACs in pifq, ein3/eil1, cop1-4, det1-1, and spaq compared with Col-0 seedlings. Each data point represents a gene; data points above the dotted magenta line are statistically significantly upregulated in the mutants by at least two-fold. The lower and upper bounds of the box represent the 25th and 75th percentile respectively; the center bar represents the median. The lower and upper end of the whiskers represent minimum and maximum values respectively. Dots outside of the whiskers represent outliers based on Tukey’s fences. Different letters denote statistically significant differences in gene expression among the mutants within each subcategory (ANOVA, Tukey’s HSD, P ≤ 0.05; n = 3 biological replicates for pifq, cop1-4, det1-1, and spaq, and n = 2 biological replicates for ein3/eil1). d qRT-PCR analysis of the steady-state transcript levels of representative LHCs (LHCB1.1, LHCB1.4, and LHCA1) and nPEACs (PsaN, AtpD, and GAPA-2) in 4-d-old dark-grown seedlings of Col-0, pifq, ein3/eil1, cop1-4, det1-1, spa234, spa134, spa124, and spa123. The transcript levels were calculated relative to those of PP2A. The fold changes between the transcript levels in the mutants and Col-0 are shown. Asterisks indicate statistically significant differences between the transcript levels of the mutant and Col-0 based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). If the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. The source data underlying the statistical analysis in c and qRT-PCR analysis in d are provided in the Source Data file.
Genome-wide activation of PhAPGs is insufficient to fully activate the PEP in pifq
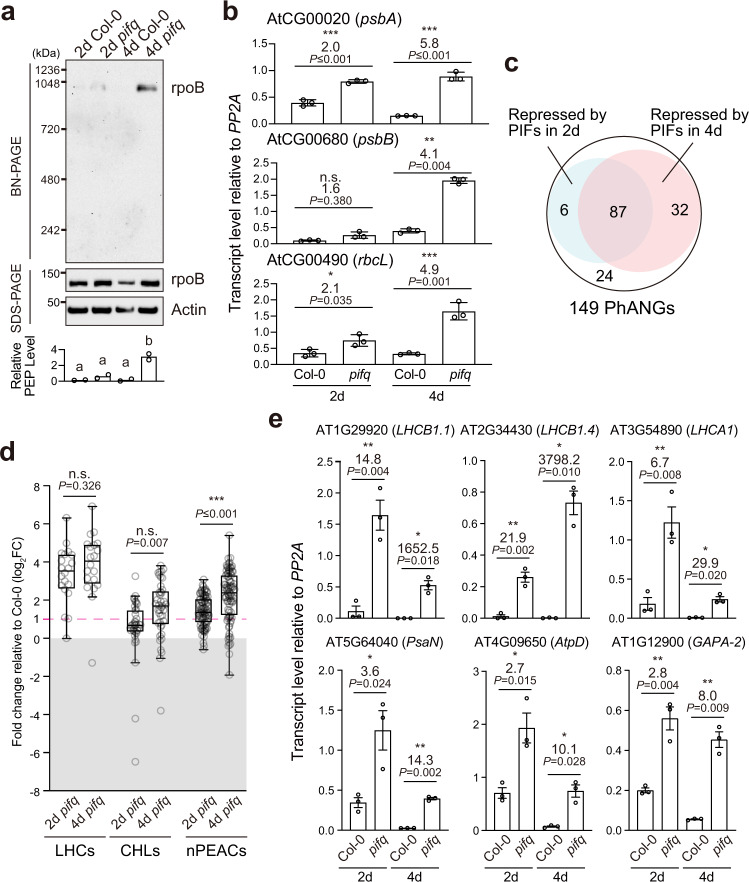
To further examine the relationship between PhANG induction and PEP activation, we turned to a previously observed scenario in which PhAPGs stay repressed in pifq in early seedling development43. We have shown that neither PEP assembly nor PhAPG expression can be fully activated during early seedling development in 2-d-old dark-grown pifq seedlings (Fig. 3a, b)43. We asked whether the lack of PEP activation in 2-d-old pifq seedlings was also accompanied by incomplete global activation of PhANGs. To that end, we used published RNA-seq datasets from studies using 2-d-old50 and 4-d-old29 dark-grown pifq seedlings to analyze the expression of the 149 PhANGs (Supplementary Table 2). Intriguingly, 87 of the 119 upregulated PhANGs in 4-d-old dark-grown pifq seedlings were also upregulated in 2-d-old dark-grown pifq seedlings (Fig. 3c and Supplementary Data 2). All three subcategories of PhANGs were de-repressed in 2-d-old dark-grown pifq (Fig. 3d). Our qRT-PCR analysis on select LHCs and nPEACs confirmed the conclusion that PhANGs were activated in both 2-d and 4-d old pifq seedlings (Fig. 3e). These results thus argue against the hypothesis that the lack of PEP activation in 2-d-old pifq was due to the incomplete activation of PhANGs globally; instead, they provide evidence supporting that genome-wide PhANG activation is insufficient to trigger full PEP activation and PhAPG expression in plastids. These results also confirm that a developmental signal exists during early seedling development to specifically repress the anterograde signal for PEP activation independently from the regulation of PhANGs.
Fig. 3. Genome-wide activation of PhANGs is insufficient to fully activate the PEP in pifq.
a Immunoblots showing the levels of the PEP complex and its core component rpoB in 2-d-old and 4-d-old Col-0 and pifq seedlings grown in the dark. Total protein was isolated under either native or denaturing conditions and resolved via BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. The bar graph shows the qualification of the relative levels of the PEP complex, which were calculated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of the total rpoB (SDS-PAGE) in each sample. Different letters denote statistically significant differences in the relative PEP levels among the genotypes (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 2). b qRT-PCR analysis of the steady-state transcript levels of psbA, psbB, and rbcL in 2-d-old and 4-d-old Col-0 and pifq seedlings grown in the dark. The transcript levels were calculated relative to those of PP2A. The fold changes between the transcript levels in the mutants and Col-0 are shown. Asterisks indicate statistically significant differences in the transcript levels between the mutant and Col-0 based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). If the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. c Venn diagram showing PIF-repressed PhANGs in 2-d-old and 4-d-old dark-grown seedlings. d Box-and-whisker plots showing the fold changes in LHCs, CHLs, and nPEACs in 2-d-old and 4-d-old dark-grown pifq seedlings compared with the Col-0 controls. Each data point represents a gene; data points above the dotted magenta line are statistically significantly upregulated in the mutants by at least two-fold. The lower and upper bounds of the box represent the 25th and 75th percentile respectively; the center bar represents the median. The lower and upper end of the whiskers represent minimum and maximum values respectively. Dots outside of the whiskers represent outliers based on Tukey’s fences. Asterisks indicate statistically significant differences between the 2-d and 4-d samples based on two-tailed Student’s t-test (***P ≤ 0.001, n = 6 biological replicates for 2-d-old dark-grown pifq, and n = 3 biological replicates for 4-d-old dark-grown pifq). n.s. indicates no significant change. e qRT-PCR analysis of the steady-state transcript levels of representative LHCs (LHCB1.1, LHCB1.4, and LHCA1) and nPEACs (PsaN, AtpD, and GAPA-2) in 2-d-old and 4-d-old dark-grown Col-0 and pifq seedlings. The transcript levels were calculated relative to those of PP2A. The fold changes between the mutants and Col-0 are shown. Asterisks indicate statistically significant differences between the mutant and Col-0 based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. The source data underlying the immunoblots in a and the qRT-PCR analysis in b and e are provided in the Source Data file.
Ectopic expression of LHCBs does not trigger PEP activation
It was previously shown that ectopically expressing LHCB1.1 or LHCB2.1 triggers the transition from etioplasts to chloroplasts in the dark18. Plastids in dark-grown seedlings overexpressing LHCB1.1 or LHCB2.1 (designated LHCB1.1ox and LHCB2.1ox, respectively) lacked prolamellar bodies, and instead, developed prothylakoid membranes, indicative of the initiation of chloroplast biogenesis. However, neither PEP assembly nor PhAPG expression was induced in these lines in the dark (Fig. 4a, b), supporting the idea that the induction of PhANGs alone is not sufficient to trigger PEP activation. These results also imply that the activation of PhAPGs does not necessarily correlate with the restructuring of the internal membrane system during the early etioplast-to-chloroplast transition.
Fig. 4. Ectopic expression of LHCBs does not activate the PEP.
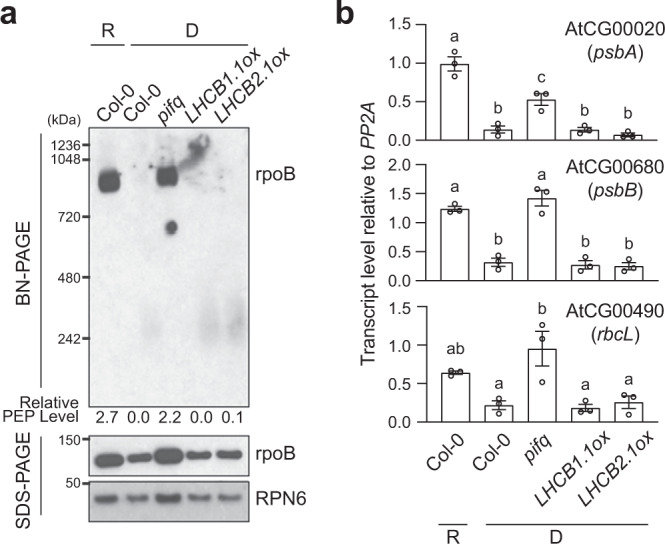
a Immunoblots showing the levels of the PEP complex, as well as its core component rpoB, in 4-d-old Col-0 seedlings grown in 10 μmol m−2sec−1 R light and 4-d-old Col-0, pifq, LHCB1.1ox, and LHCB2.1ox seedlings grown in the dark. Total protein was isolated under either native or denaturing conditions and resolved via BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. The numbers below the immunoblots represent the relative PEP levels, which were calculated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of denatured rpoB (SDS-PAGE) in each sample. b qRT-PCR analysis of the steady-state transcript levels of select PhAPGs, psbA, psbB, and rbcL, in 4-d-old Col-0 seedlings grown in 10 μmol m−2sec−1 R light and 4-d-old Col-0, pifq, LHCB1.1ox, and LHCB2.1ox seedlings grown in the dark. The transcript levels were calculated relative to those of PP2A. Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. Samples labeled with different letters exhibited statistically significant differences (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 3). The source data underlying the immunoblots in a and the qRT-PCR analysis in b are provided in the Source Data file.
Multiple PIFs redundantly repress the anterograde signal
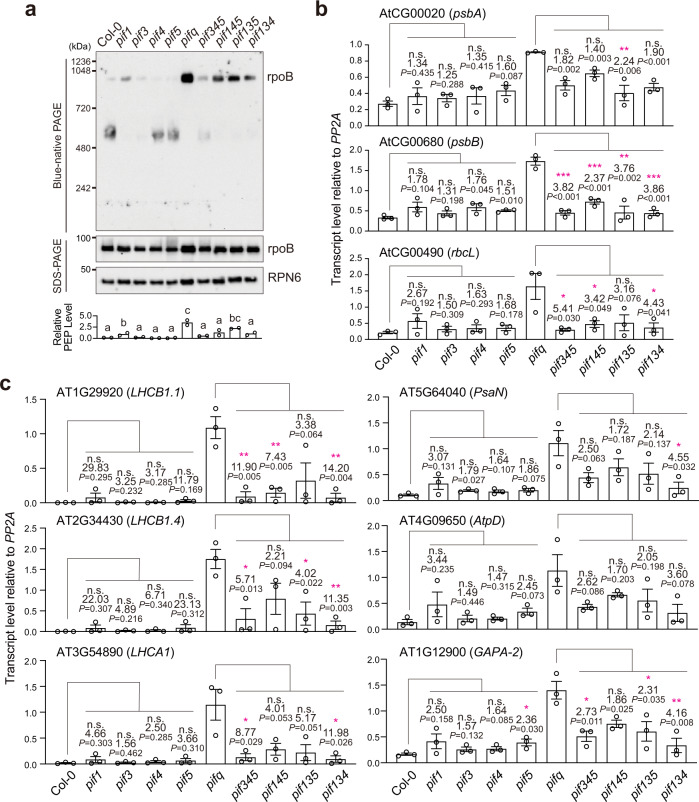
Photomorphogenesis is repressed in the dark primarily by four PIFs, PIF1, PIF3, PIF4, and PIF515,17. We asked whether the anterograde signal is repressed by individual PIFs or redundantly by multiple PIFs. To assess the role of individual PIFs in PEP inhibition, we examined PEP assembly and the expression of PhAPGs in pif1, pif3, pif4, and pif5 single mutants, as well as pif345, pif145, pif135, and pif134 triple mutants, which leave one of the four PIFs intact. Knocking out the four PIFs individually did not have a dramatic impact on PEP assembly, although the pif1 mutant showed a minor but significant increase in the level of the PEP complex compared with Col-0 (Fig. 5a). The individual pif mutants also did not show a significant increase in the transcript levels of select PhAPGs compared with Col-0 (Fig. 5b). These results indicate that PEP inhibition is imposed not by a particular PIF but rather redundantly by more than one PIF. Among the triple mutants, all exhibited a significant decrease in the level of the PEP complex compared with pifq (Fig. 5a), none of the four pif triple mutants could fully activate the three representative PhAPGs as strongly as could pifq (Fig. 5b), supporting the idea that the four PIFs repress the anterograde signal redundantly. The single pif mutants were also not effective in activating PhANGs, and none of the triple pif mutants could activate PhANGs to the same extent as pifq could (Fig. 5c). Therefore, the four PIFs act redundantly to repress both PhANGs and PhAPGs.
Fig. 5. PEP activation is repressed redundantly by multiple PIFs.
a Immunoblots showing the levels of the PEP complex and its core component rpoB in 4-d-old Col-0, pif1, pif3, pif4, pif5, pifq, pif345, pif145, pif135, and pif134 seedlings grown in the dark. Total protein was isolated under either native or denaturing conditions and resolved via BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. RPN6 was used as a loading control. The graph below the immunoblots shows the relative PEP levels, which were estimated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of denatured rpoB (SDS-PAGE) in each sample. Different letters denote statistically significant differences in relative PEP level either between the pif single mutants and Col-0 or between the pif triple mutants and pifq (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 2). b qRT-PCR analysis of the steady-state transcript levels of select PhAPGs, including psbA, psbB, and rbcL, in the 4-d-old dark-grown seedlings shown in a. c qRT-PCR analysis of the steady-state transcript levels of select PhANGs in the 4-d-old dark-grown seedlings described in a. For b and c, asterisks indicate statistically significant differences in the transcript levels either between the single pif mutants and Col-0 or between the pif triple mutants and pifq based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). If the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. The source data underlying the immunoblots in a and the qRT-PCR analysis in b and c are provided in the Source Data file.
Repression of the anterograde signal requires PIF3’s activator activity
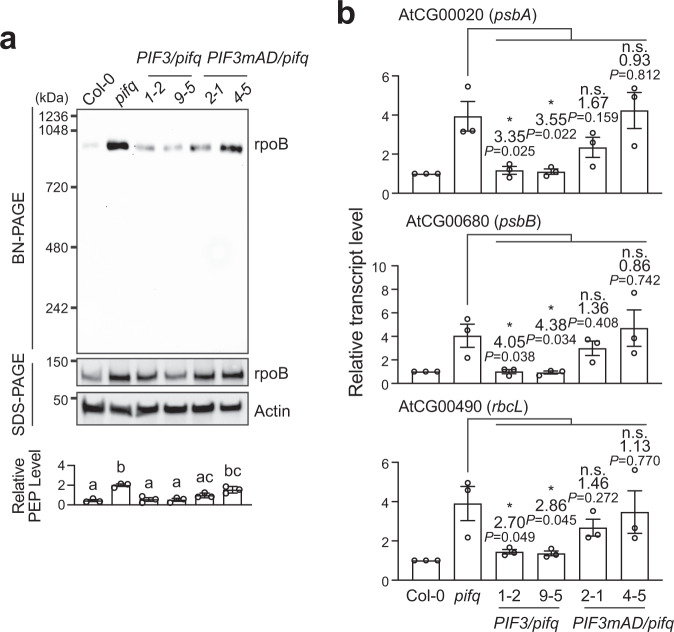
PIFs are transcriptional activators that promote skotomorphogenesis mainly by activating target genes, although PIFs can also repress gene expression14. Therefore, PIFs could repress the anterograde signal either directly or indirectly via PIF-induced genes. We recently identified a 24-amino-acid transcription activation domain (AD) in PIF3 that consists of a ΦxxΦΦ activator motif, where Φ indicates a bulky hydrophobic residue and x is any other amino acid, flanked by acidic residues11. A PIF3mAD mutant, which substitutes the ΦxxΦΦ activator motif with five alanines, abolishes the activator activity of PIF311. To determine whether the transactivation activity of PIF3 is required for repressing the anterograde signal, we examined PEP inhibition in dark-grown PIF3/pifq and PIF3mAD/pifq lines that express HA-YFP-PIF3 or HA-YFP-PIF3mAD, respectively, in the pifq background. As expected, the PIF3/pifq lines largely complemented the pifq mutant and could repress PEP assembly and PhAPG expression in darkness (Fig. 6a). In contrast, the PIF3mAD/pifq lines only partially repressed PEP assembly and PhAPGs remained fully activated as in pifq (Fig. 6a, b). These results thus suggest that PIF3 repress the anterograde signal indirectly via a PIF3-induced gene product. Given that PIFs play similar roles in repressing the anterograde signal (Fig. 5) and that all four PIFs have the same type of AD11, it is likely that other PIFs also repress the anterograde signal indirectly through their activator activity.
Fig. 6. PEP inhibition requires PIF3’s transactivation activity.
a Immunoblots showing the levels of the PEP complex and its core component rpoB in 4-d-old Col-0, pifq, PIF3/pifq (lines 1-2 and 9-5), and PIF3mAD/pifq (lines 2-1 and 4-5) seedlings grown in the dark. Total protein was isolated under either native or denaturing conditions and resolved via BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. Actin was used as a loading control. The graph below the immunoblots shows the relative PEP levels, which were estimated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of denatured rpoB (SDS-PAGE) in each sample. Different letters denote statistically significant differences in the relative PEP levels (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 3). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. b qRT-PCR analysis of the steady-state transcript levels of select PhAPGs, including psbA, psbB, and rbcL, in the 4-d-old dark-grown seedlings shown in a. The transcript levels were calculated relative to those of PP2A. Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. Asterisks indicate statistically significant differences between the indicated transgenic lines and pifq based on two-tailed Student’s t-test (*P ≤ 0.05). If the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). The source data underlying the immunoblots in a and the qRT-PCR analysis in b are provided in the Source Data file.
PIF-repressed sigma factors are anterograde signals
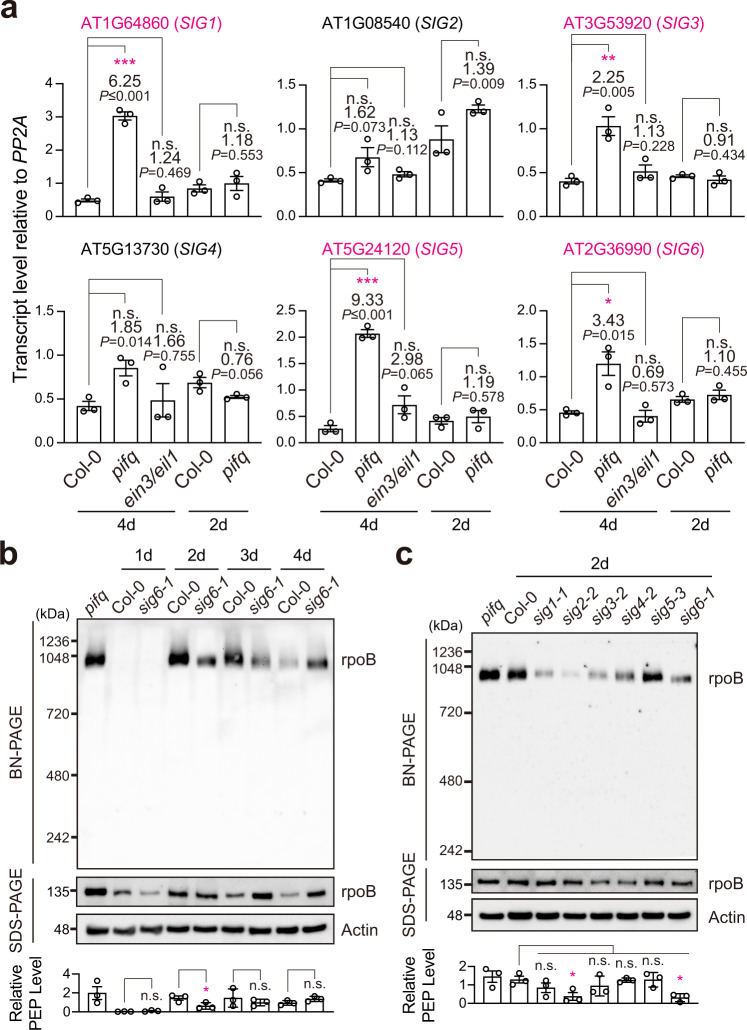
We next asked whether the nuclear-encoded components of the PEP – i.e., 12 PAPs and 6 SIGs – could serve as anterograde signals downstream of PIFs. Here we used the criteria that an anterograde signal must be encoded by a PIF-repressed gene that is also repressed by the developmental signal in early seedling development. To that end, we asked whether the expression patterns of any of these components match the pattern of PEP activation in pifq and ein3eil1; in other words, we examined whether any of the PEP components were induced in 4-d-old pifq but remained repressed in 4-d-old ein3eil1 and 2-d-old pifq. Intriguingly, none of the 12 PAPs were upregulated in pifq or the other de-etiolated mutants in the dark (Supplementary Fig. 1), suggesting that the PAPs are not regulated by PIFs at the transcript level. In contrast, four SIGs, including SIG1, SIG3, SIG5, and SIG6, were induced in 4-d-old pifq; we therefore refer to them as the PIF-repressed SIGs. More strikingly, these four PIF-repressed SIGs were not activated in 4-d-old ein3/eil1 and 2-d-old pifq (Fig. 7a), matching the pattern of PEP activation.
Fig. 7. PEP activation correlates with the expression of PIF-repressed sigma factors.
a qRT-PCR analysis of the steady-state transcript levels of SIG1-6 in dark-grown 4-d-old Col-0, pifq, and ein3/eil1, as well as 2-d-old Col-0 and pifq seedlings. The transcript levels were calculated relative to those of PP2A. The fold changes between the transcript levels in the mutants and their respective Col-0 controls are shown. Asterisks indicate statistically significant differences in the transcript level compared with that of Col-0 based on two-tailed Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). If the change was less than two-fold or not statistically significant, it is labeled as n.s. (not significant). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. b Immunoblots showing the levels of the PEP complex and the core PEP component rpoB in Col-0, and sig6-1 seedlings grown in 10 μmol m−2sec−1 R light for 1 to 4 days after imbibition. c Immunoblots showing the levels of the PEP complex and the core PEP component rpoB in 2-d-old Col-0, sig1-1, sig2-2, sig3-2, sig4-2, sig5-3, and sig6-1 seedlings grown in 10 μmol m−2sec−1 R light. For b and c, 4-d-old pifq seedlings grown in the dark were used as a control. Total protein was isolated under either native or denaturing conditions and resolved BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. Actin was used as a loading control. The graph below the immunoblots shows the relative PEP levels, which were estimated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of denatured rpoB (SDS-PAGE) in each sample. Asterisks indicate statistically significant differences with Col-0 in triplicates based on two-tailed Student’s t-test (*P ≤ 0.05). Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. The source data underlying the qRT-PCR analysis in a and the immunoblots in b and c are provided in the Source Data file.
SIGs are an essential component of the PEP holoenzyme for promoter recognition and transcription initiation. However, bacterial SIGs are not required for the assembly of the core subunits into the RNA polymerase complex51. We then tested whether the accumulation of the PEP complex was affected in sig mutants during chloroplast biogenesis in seedling development. To that end, we first examined PEP complex formation in Col-0 at different days after seed imbibition. Interestingly, the PEP complex and PhAPGs were repressed in 1-d-old seedlings, consistent with the idea that a developmental signal exists to inhibit the activation of plastid gene expression during early seedling establishment. The PEP complex became visible in 2-to-4 day old seedlings. Among the six SIGs, SIG2 and SIG6 are major general SIGs, as sig2-2 and sig6-1 mutants exhibit pale green phenotypes52. We then examined the PEP complex in the sig6-1 mutant because sig6-1 was the only mutant showing a visible phenotype among the mutants of the four PIF-repressed SIGs. The relative PEP level was only reduced significantly in 2-d-old sig6-1 but remained the same compared with Col-0 in 3-d and 4-d old sig6-1 (Fig. 7b). These results suggest that SIG6 is not required for the PEP complex, although the accumulation of the PEP could be influenced in the sig6-1 mutant likely as a secondary effect. We then examined the PEP complex in 2-d-old seedlings of the other sig mutants. Only sig2-2 – the other sig mutant with a visible phenotype – showed a reduction in the PEP complex (Fig. 7c). Therefore, we conclude that plastid SIGs are not required for the assembly of the PEP complex, despite their essential roles in promoter recognition and transcription initiation.
PIF-repressed sigma factors are rapidly induced by light
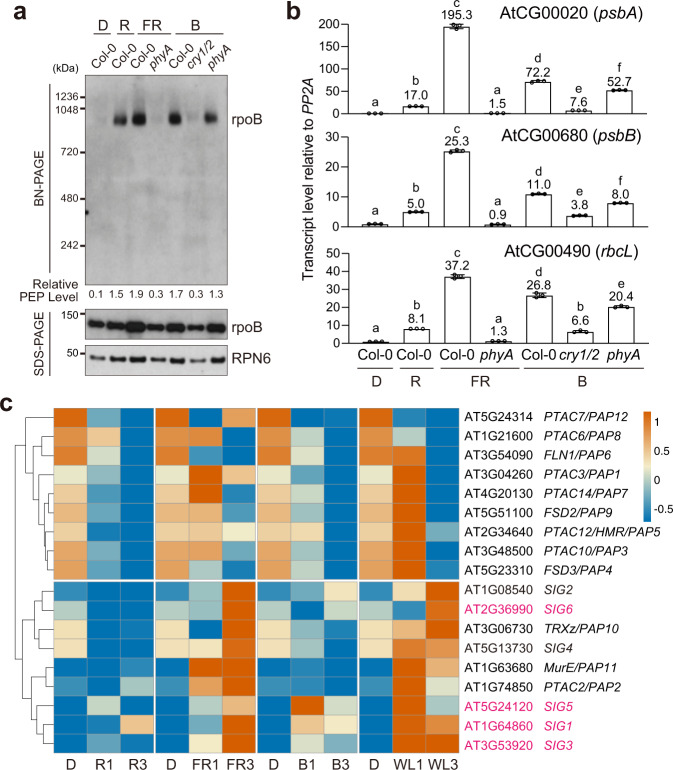
In addition to being PIF-repressed, anterograde signals triggering PEP activation should also be induced by light. The light-dependent inhibition of the stability and activity of PIFs is a master nuclear switch that activates both PhANGs and PhAPGs. PHYA and CRYs, which sense far-red and blue light, respectively, regulate PIFs in a similar manner as PHYB12,53. PhANGs can be activated by monochromatic light via PHYA and PHYB in red light15,16, by PHYA in far-red light54,55, and by CRYs in blue light56. To test whether PHYA and CRYs can also individually activate PhAPGs, we examined the assembly of the PEP and the expression of representative PhAPGs in monochromatic far-red and blue light. Growing Col-0 seedlings in monochromatic far-red light was sufficient to trigger both PEP assembly and PhAPG activation (Fig. 8a, b). The transcript levels of psbA, psbB, and rbcL were significantly higher in far-red than in red light (Fig. 8b), suggesting that either the plastid photosynthesis genes were more actively transcribed or their transcripts became more stable in far-red light. Both the far-red-mediated PEP assembly and PhAPG expression were blocked in phyA-211 (Fig. 8a, b), indicating that PHYA is responsible for PEP activation in far-red light. Monochromatic blue light was equally effective in eliciting PEP assembly and PhAPG activation (Fig. 8a, b). The blue light responses were abolished in the cry1/cry2 mutant, indicating that CRYs play an essential role in activating the PEP in blue light. PHYA can also detect blue light57, but PEP assembly and PhAPG expression could still be induced by blue light in phyA-211 (Fig. 8a, b), indicating that PHYA does not play a major role in regulating PhAPGs in blue light. Together, these results support the conclusion that PHYA and CRYs can individually initiate anterograde signaling to activate the PEP in monochromatic far-red and blue light, respectively. The PEP assembly was mostly unaffected in the sig mutants in far-red and blue light (Supplementary Fig. 2), supporting the conclusion that SIGs are not required for PEP assembly despite being an essential component for the activity of PEP holoenzyme.
Fig. 8. PIF-repressed sigma factors are rapidly induced by light.
a Immunoblots showing the levels of the PEP complex and the PEP component rpoB in 4-d-old Col-0, phyA-211 (phyA), and cry1/cry2 (cry1/2) seedlings grown in the dark (D) or in 10 μmol m−2s−1 monochromatic red (R), far-red (FR), or blue (B) light. Total protein was isolated under either native or denaturing conditions and resolved via BN-PAGE or SDS-PAGE to assess the fraction of rpoB in the PEP complex or the amount of total rpoB by immunoblots, respectively. RPN6 was used as a loading control. The numbers below the immunoblots represent the relative PEP levels, which were estimated using the relative level of rpoB in the PEP complex (BN-PAGE) divided by the relative level of denatured rpoB (SDS-PAGE) in each sample. b qRT-PCR analysis of the steady-state transcript levels of psbA, psbB, and rbcL in 4-d-old Col-0, phyA-211 (phyA), and cry1/cry2 (cry1/2) seedlings grown in the dark or the light conditions described in a. The transcript levels were calculated relative to those of PP2A. The numbers above the columns indicate the fold changes compared with Col-0 in the dark. Error bars represent the s.e. of three biological replicates, and the centers of the error bars represent the mean values. Samples labeled with different letters exhibited statistically significant differences (ANOVA, Tukey’s HSD, P ≤ 0.05, n = 3). c Heatmap showing the relative expression of 12 PAPs and 6 SIGs in 3-d-old Col-0 during the transition from dark to red (R), far-red (FR), blue (B), and while light (WL) for 1 or 3 h. The source data underlying the immunoblots in a, qRT-PCR analysis in b, and the heatmap in c are provided in the Source Data file.
We then analyzed the expression of 12 PAPs and 6 SIGs during the dark-to-light transition using a published RNA-seq dataset on 3-d-old dark-grown Col-0 seedlings treated with 1 or 3 h of red, far-red, blue, or white light58. Based on their expression patterns, the 18 nuclear-encoded PEP components were grouped into two clusters – a light-upregulated and a light-downregulated cluster (Fig. 8c). The majority of the PAPs were expressed at relatively high levels in the dark and their expression was downregulated during the dark-to-light transition. Intriguingly, all four PIF-repressed SIGs – SIG1, SIG3, SIG5, and SIG6 – belonged to the light-induced cluster, which included all six SIGs and three PAPs, PAP2, PAP10, and PAP11. The cluster of light-inducible PEP components were highly expressed in far-red light (also in white light), consistent with the elevated expression of PhAPGs in far-red light (Fig. 8b,c). These results further support the role of the four PIF-repressed SIGs as anterograde signals. The other light-inducible PEP components could also contribute to anterograde signaling; the fact that their expression was not significantly altered in pifq implies that anterograde signaling involves PIF-independent mechanisms.
Discussion
The nuclear control of plastid transcription is pivotal for the coordination of nuclear and plastid gene expression during chloroplast biogenesis3,4. We recently revealed that red-light-dependent photoinhibition of PIFs by PHYB in the nucleus triggers the assembly of the PEP and PhAPG transcription in plastids (Fig. 9)3,43,44. It is conceivable that, instead of all PIF-regulated plastid proteins being employed as signaling molecules, likely only some act as regulators or anterograde signals to confer the nuclear control of plastid gene expression in a spatial and temporal manner. For decades, the main challenge has been to identify the anterograde signals from among the battery of nuclear-encoded components required for plastid transcription or other essential chloroplast functions, such as photosynthesis5. In this study, we demonstrated that, although PIFs repress both PhANGs and PhAPGs, neither selective nor genome-wide induction of PhANGs is sufficient to activate PhAPGs. Therefore, PhANGs themselves do not constitute the anterograde signal; instead, anterograde signaling activates the PEP via a separate mechanism in parallel with the regulation of PhANGs. Based on the pattern of PEP activation in de-etiolated mutants, we devised a strategy – using transcriptomic analysis of the pifq mutant at two seedling developmental stages in combination with the analysis of early light-responsive genes in Col-0 – to distinguish anterograde signals from essential components of the PEP. Among the 18 nuclear-encoded components of the PEP holoenyzme, this approach identified four light-inducible, PIF-repressed SIGs, including SIG1, SIG3, SIG5, and SIG6, as anterograde signals downstream of PIFs to control PEP activation (Fig. 9). Our results also predict the existence of a developmental mechanism that represses the anterograde signal during early seedling establishment (Fig. 9). Moreover, we show that far-red and blue light could individually turn on anterograde signaling via PHYA and CRYs, respectively (Fig. 9). Together, these results further elucidate the framework of light signaling that coordinates nuclear and plastid photosynthesis genes, in which PHYs and CRYs synchronize plastid transcription via PIF-repressed SIGs as anterograde signals in parallel with the regulation of nuclear photosynthesis genes (Fig. 9).
Fig. 9. Framework of anterograde signaling for the nuclear control of plastid transcription.
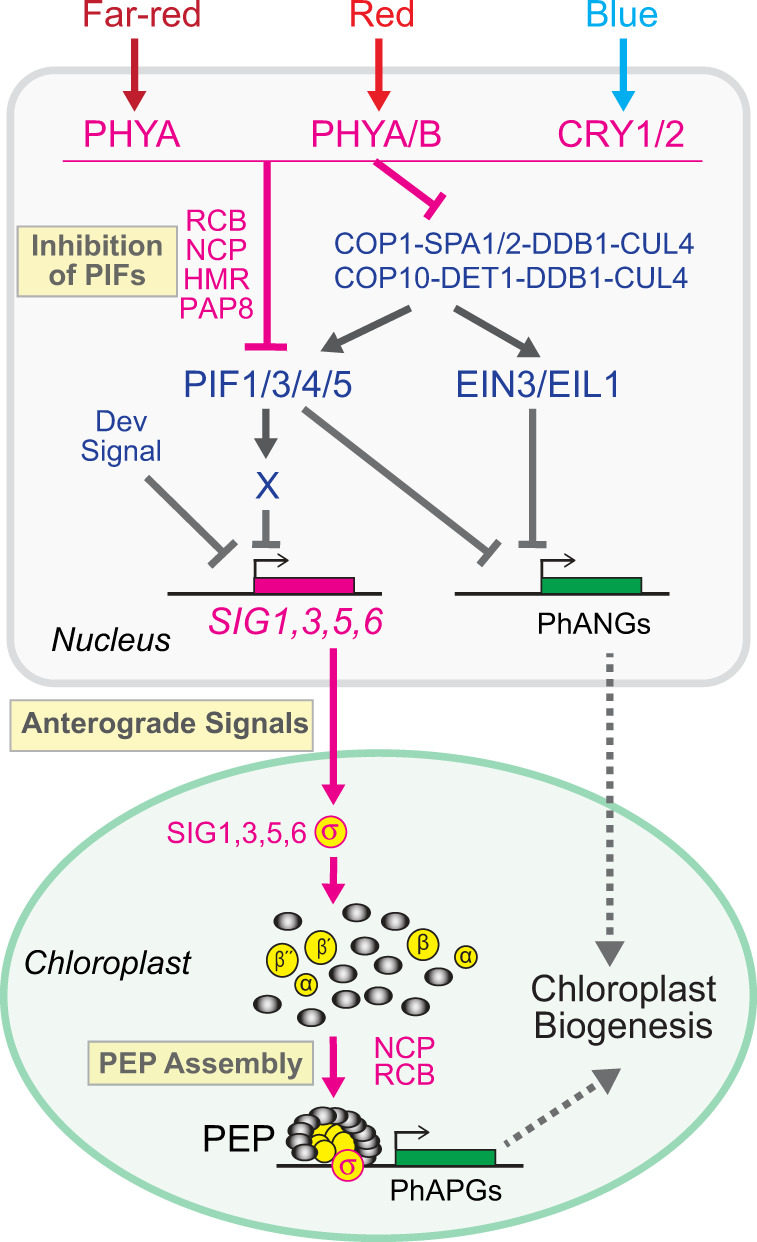
Monochromatic far-red, red, and blue light – which are perceived by PHYA, PHYA/PHYB (PHYA/B), and CRY1/CRY2 (CRY1/2), respectively – can individually initiate anterograde signaling by blocking the accumulation and activity of PIF1, PIF3, PIF4, and PIF5. PHYs and CRYs inhibit PIFs either directly via physical interaction or indirectly by attenuating the activity of COP1-SPA- and COP10-DET1-containing CUL4-based E3 ubiquitin ligases, which stabilize PIFs and also EIN3/EIL1. PIFs simultaneously repress both PhANGs and PhAPGs via parallel mechanisms. While PIFs work with EIN3/EIL1 to repress PhANGs in the nucleus, PIFs alone separately control the activation of the PEP in plastids by repressing the expression of four light-inducible SIGs, SIG1, SIG3, SIG5, and SIG6. PIFs likely repress SIGs indirectly via a yet unknown PIF-induced factor X. During early seedling establishment, anterograde signaling is suppressed by a developmental signal independently of PIFs. Several dual-targeted proteins, including RCB, NCP, HMR/PAP5/pTAC12 and PAP8/pTAC6, participate in the regulation of anterograde signaling in both the nucleus and plastids.
The provocative conclusion of a genetically uncoupled regulation of PhANGs and PhAPGs extends the conventional view emphasizing mostly on the coupled expression of PhANGs and PhAPGs by providing a novel mechanism that allows the flexibility to uncouple PhANGs and PhAPGs in response to developmental and environmental cues. The expression of PhANGs and PhAPGs has been widely accepted to be “coupled” primarily because of their synchronous expression along with the repression and activation of chloroplast biogenesis in the dark and light, respectively. We now know that the synchronization of PhANGs and PhAPGs by light is achieved by the regulation of the master nuclear regulators PIFs (Fig. 9). However, how PIFs coordinate the expression PhANGs and PhAPGs remained unclear. There are two possible mechanisms. PIFs could synchronize PhANGs and PhAPGs via a hierarchical mechanism, in which the activation of PhANGs itself serves as an anterograde signal to induce PhAPGs. Alternatively, PIFs could regulate PhANGs and PhAPGs via parallel mechanisms. Here, by examining precisely defined PhANGs in de-etiolated mutants, we showed that the genome-wide activation of PhANGs is insufficient to elicit PEP assembly and PhAPG activation, suggesting that PhANGs and PhAPGs are regulated by genetically separable, parallel mechanisms (Fig. 9). This conclusion is supported by the distinct regulation of PhANGs and PhAPGs by EIN3/EIL1 (Fig. 2) and a developmental signal (Fig. 3). Corroborating this idea, the ectopic expression of LHCBs, which has been shown to promote the formation of prothylakoid membranes in plastids in darkness, was unable to trigger PEP assembly or PhAPG activation (Fig. 4).
Our model of a paralleled regulation of PhANGs and PhAPGs by PIFs provides the mechanistic basis to explain the observations of uncoupled expression of PhANGs and PhAPGs. The expression of PhANGs and PhAPGs is also coupled by the retrograde GUN (Genome UNcoupled) signaling4. The current model of GUN signaling posits that the PEP activity generates a signal that promotes the expression PhANGs52. As such, disrupting plastid transcription downregulates PhANGs. However, in the GUN signaling mutant gun1, the expression of PhANGs remains high even when plastid transcription is blocked4. Intriguingly, we showed that the same type of uncoupled expression of PhANGs and PhAPGs also occurred during early seedling development (Figs. 3 and 7). Our results not only provide a mechanistic explanation for the genome uncoupled phenomenon in gun mutants but also suggest that an uncoupled expression of PhANGs and PhAPGs represents a temporal state in plant development. Recently, the uncoupled expression of PhANGs and PhAPGs was shown to be required for generating reactive oxygen species to mediate salicylic acid signaling59. PhANGs and PhAPGs can be uncoupled by SIGMA FACTOR BINDING PROTEIN 1, which is dual-targeted to the nucleus and chloroplasts to induce PhANGs and repress PhAPGs59. The differential expression of PhANGs and PhAPGs results in an imbalance between photosystems II and I, thereby generating singlet oxygen species to trigger programmed cell death59. These observations further support the idea that the genetic architecture of the paralleled control of PhANGs and PhAPGs may play a profound functional role in cell signaling and adaptation to abiotic and biotic stress.
We devised a strategy that identified SIG1, SIG3, SIG5, and SIG6 as anterograde signals downstream of PIFs (Fig. 9). The light-dependent regulation of SIG1, SIG3, SIG5, and SIG6 as well as their critical functions in plastid transcription have been extensively reported16,60–62. The contribution of this work is to provide evidence distinguishing them as anterograde signals from the other PEP components. The first clue supporting this conclusion came from the correlation between the induction of these SIGs and PEP assembly and activation in de-etiolated mutants. The expression of SIG1, SIG3, SIG5, and SIG6 correlates with the pattern of PEP activation – i.e., they were activated in 4-d-old pifq but remained inactive in 4-d-old ein3/eil1 and 2-d-old pifq (Fig. 7a). These PIF-repressed SIGs were rapidly induced by light during the dark-to-light transition (Fig. 8c), further supporting their role as anterograde signals. Our conclusion is consistent with the recent findings that the circadian and light-intensity dependent expression of plastid photosynthesis genes is controlled by the nucleus via SIG563,64. Our results showed that PIF3 represses PhAPGs through its activator activity (Fig. 6). Therefore, PIF3 and possibly also other PIFs likely repress SIGs indirectly by promoting the expression of a repressor protein X (Fig. 9).
Our results indicate that PIFs do not regulate PAPs at the transcript level (Supplementary Fig. 1)43. These results contradict the studies using cultured Arabidopsis cell lines, which showed that the expression of some PAPs was under the control of PIFs and the PEP complex became fully assembled in etioplasts65,66. These discrepancies are likely due to the different experimental model and/or assay conditions. For example, sucrose had to be used to sustain the growth of Arabidopsis cell culture, whereas, in our experiments, no supplemental sucrose was used because sucrose is known to influence light signaling and photosynthesis gene expression significantly67. It is important to note that we have focused on identifying PIF-dependent anterograde signals. Light also induces the expression of PIF-independent PEP components, including SIG2, SIG4, PAP2, PAP10, and PAP11 (Fig. 8c). The regulation of SIG2 by PHYs has been extensively reported68. SIG2 is involved in the regulation of PHY signaling, including PHY-dependent hypocotyl elongation and the expression of PhANGs68,69. Therefore, it is highly likely that the PIF-independent PEP components, such as SIG2, also contribute to the light-dependent activation of the PEP via PIF-independent mechanisms.
The current data indicate that SIG1, SIG3, SIG5, and SIG6 are not the only anterograde signals downstream of PIFs. Similar to bacterial SIGs, plastid SIGs are most likely a dispensable subunit not required for the assembly of the PEP complex (Fig. 7b, c and Supplementary Fig. 2). Therefore, the light-dependent assembly of the PEP must be regulated by a yet unidentified anterograde signal. Two good candidates are REGULATOR OF CHLOROPLAST BIOGENESIS (RCB) and NUCLEAR CONTROL OF PEP ACTIVITY (NCP), which are dual-targeted proteins that participate in PHY-mediated PIF regulation in the nucleus and PEP assembly in plastids (Fig. 9)43,44,70. Also, we cannot exclude the possibility that some PAPs are involved in the regulation of PEP assembly by light. Although PAPs are not regulated by PIFs at the transcript level, they could still be regulated by light at the post-translational level. In fact, PHYB promotes the accumulation of HMR/PAP5/pTAC12 via direct interaction71. Moreover, because HMR/PAP5/pTAC12 and PAP8/pTAC6 are also dual-targeted to the nucleus and plastid and participate in both nuclear PHY signaling and PEP assembly (Fig. 9)72–74, their subcellular partitioning could be another mechanism to modulate PEP assembly. Future investigations will focus on dissecting the anterograde signal for PEP assembly.
Our new findings substantially advanced the understanding of anterograde signaling in the control of plastid transcription by light. We demonstrated that, in addition to monochromatic red light, far-red and blue light – perceived by PHYA and CRYs, respectively – can individually initiate anterograde signaling to turn on the PEP and the expression of PhAPGs (Figs. 8 and 9). These results are consistent with the fact that CRYs regulate the stability and activity of PIFs in a similar manner as PHYs12,53. Arabidopsis seedlings grown in far-red light lack chlorophyll because POR, the enzyme catalyzing the conversion of protochlorophyllide to chlorophyll a, requires light with a shorter wavelength than far-red for its activity8. Therefore, the control of plastid transcription by anterograde signaling operates independently of chlorophyll biosynthesis and photosynthesis. This conclusion is further supported by the PEP activation in de-etiolated mutants in darkness (Fig. 1). It is also important to note that, although the general principle of anterograde signaling is expected to be the same under various light conditions, the extent of activation of the PIF-repressed SIGs was clearly different under distinct monochromatic light conditions (Fig. 8c), which may provide an explanation for the light quality-dependent regulation of plastid gene expression, such as the activation of a unique blue-light responsive promoter of psbD by SIG575.
In conclusion, our study further elucidates the light signaling framework that coordinates nuclear and plastid transcription during chloroplast biogenesis. We identified four SIGs as anterograde signals and surprisingly unveiled that anterograde signaling operates in parallel with the regulation of PhANGs. The latter provides a conceptual basis for the uncoupled regulation of PhANGs and PhAPGs in response to developmental and environmental stimuli. Our study also reveals gaps in anterograde signaling for future investigations, including a developmental mechanism that gates the anterograde signal during early seedling development and a yet-unidentified mechanism for controlling PEP assembly by light.
Methods
Plant materials, growth conditions, and hypocotyl measurements
Arabidopsis Colombia (Col-0) was used as the wild-type control for PEP complex assembly and gene expression under various light conditions. The mutant lines, including pifq17, cop1-445, det1-146, spa23426, spa13426, spa12426, spa12326, ein3/eil147, LHCB1.1ox18, LHCB2.1ox18, pif117, pif317, pif417, pif517, pif34517, pif14517, pif13517, pif13427, PIF3/pifq (1-2 and 9-5)11, PIF3mAD/pifq (2-1 and 4-5)11, sig1-152, sig2-252, sig3-252, sig4-252, sig5-352, sig6-152, phyA-21155, and cry1/cry276, have been previously reported. Seeds were surface sterilized in 50% bleach with 0.01% Triton X-100 for 10 min and then washed four times with ddH2O. Seeds were then plated on half-strength Murashige and Skoog (½ MS) medium containing Gamborg’s vitamins (MSP0506, Caisson Laboratories), 0.5 mM MES (pH 5.7), and 0.8% (w/v) agar (A038, Caisson Laboratories). Seeds were stratified in the dark at 4 °C for five days before being placed in an LED chamber (Percival Scientific) under the indicated conditions for four days. For the dark-grown samples, seeds were exposed to far-red light for 3 h to trigger germination before placing them back into the dark for an additional 93 h. Light intensity was measured using an Apogee PS200 spectroradiometer (Apogee Instruments).
RNA extraction and quantitative real-time PCR
Total RNA from seedlings of the indicated genotypes and growth conditions was isolated using a Quick-RNA MiniPrep kit with on-column DNase I treatment (Zymo Research). cDNA was synthesized from total RNA using a Superscript II First-Strand cDNA Synthesis kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Oligo(dT) primers were used for the analysis of nuclear gene expression, and a mixture of plastidial-gene-specific primers was used for the analysis of plastidial genes. qRT-PCR was performed with Bio-Rad iQ SYBR Green Supermix on a Roche LightCycler 96 system. The mRNA level of each gene was normalized to that of PP2A. Primers for qRT-PCR are listed in Supplementary Tables 3 and 4.
Blue-native gel electrophoresis for analyzing PEP assembly
PEP assembly was analyzed via blue-native polyacrylamide gel electrophoresis (BN-PAGE) using a NativePAGE Sample Prep kit and a NativePAGE Novex Bis-Tris Gel system (Thermo Fisher Scientific) with immunoblot77. One hundred milligrams of seedlings grown under the indicated conditions was ground in liquid nitrogen and resuspended in 3 volumes of BN lysis buffer (100 mM Tris-Cl, pH 7.2; 10 mM MgCl2; 25% glycerol; 1% Triton X-100; 10 mM NaF; 5 mM β-mercaptoethanol; 1× EDTA-free protease inhibitor cocktail). Protein extracts were divided into two tubes, one for BN-PAGE and the other for SDS-PAGE. For BN-PAGE, protein extracts were mixed with BN sample buffer (1× NativePAGE sample buffer, 50 mM 6-aminocaproic acid, 1% n-dodecyl β-D-maltoside (DDM), and Benzonase nuclease) and incubated for 60 min at room temperature to degrade DNA/RNA and solubilize the PEP complex. Samples were mixed with 0.25% NativePAGE Coomassie blue G-250 sample additive and centrifuged at 17,500 × g for 10 min at 4 °C. Proteins from the supernatant were separated on a 4–16% NativePAGE Bis-Tris protein gel using a NativePAGE Running Buffer kit (Thermo Fisher Scientific) according to the manufacturer’s protocol and with the following modifications. NativeMark Unstained Protein Standard (Thermo Fisher Scientific) was used to determine protein size in BN-PAGE. Briefly, electrophoresis was performed at 30–40 V for 3 h at 4 °C until the blue dye migrated through one third of the gel. The Dark Blue Cathode Buffer was replaced with Light Blue Cathode Buffer, and electrophoresis was continued at 20–25 V overnight at 4 °C. After electrophoresis was complete, the separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane using 1× NuPAGE Transfer Buffer (Thermo Fisher Scientific, Waltham, MA) at a constant 70 V for 7 h at 4 °C. After transfer, the membrane was fixed with fixation buffer (25% methanol, 10% acetic acid) for 15 min and washed with water. The membrane was incubated with methanol for 5 min to destain the dye, and then it was washed with water and immunoblotting continued. The membrane was blocked with 2% non-fat milk in 1× TBS (20 mM Tris-Cl pH 7.6, 150 mM NaCl), probed with the indicated monoclonal mouse anti-rpoB antibodies (PHY1700, PhytoAB Inc.), washed with 1× TBS containing 0.05% Tween-20, and then incubated with anti-mouse secondary antibodies conjugated with horseradish peroxidase (1706516, Bio-Rad). Primary and secondary antibodies were used at 1:1000 and 1:5000 dilutions, respectively. The signals were detected using SuperSignal West Dura Extended Duration Chemiluminescent Substrate (Thermo Fisher Scientific).
Protein extraction and immunoblot analysis
Total protein was extracted from Arabidopsis seedlings grown under the indicated conditions. Plant tissues were ground in liquid nitrogen and resuspended in extraction buffer (100 mM Tris-HCl pH 7.5, 100 mM NaCl, 1% SDS, 5 mM EDTA pH 8.0, 20 mM DTT, 40 μM MG132, 40 μM MG115, and 1× EDTA-free protease inhibitor cocktail), boiled for 10 min and then centrifuged at 20,000 × g for 10 min at room temperature. Protein extracts were separated via SDS-PAGE, transferred to nitrocellulose membranes, probed with the indicated primary antibodies, and then incubated with HRP-conjugated secondary antibodies. Mouse monoclonal anti-actin (A0480, Sigma-Aldrich) was used at a 1:4000 dilution, and anti-RPN6 (BML-PW8370-0100, Enzo Life Sciences) was used at a 1:1000 dilution. Goat anti-mouse (1706516, Bio-Rad) and anti-rabbit (1706515, Bio-Rad) secondary antibodies were used at a 1:5000 dilution. Signals were detected via SuperSignal West Dura Extended Duration Chemiluminescent Substrate (Thermo Fisher Scientific).
RNA-seq data analysis
RNA-seq data analysis was performed with the pRNASeqTools pipeline (https://github.com/grubbybio/pRNASeqTools). Briefly, the raw reads were mapped to the Araport1178 genome using STAR version 2.7.7a79, and the number of reads mapped uniquely to each annotated gene was counted using FeatureCounts version 2.0.180. Transcript levels were measured in fragments per kilobase per million total mapped fragments (FPKM). Differentially expressed genes were identified using DEseq2 version 3.1481 with a fold change of two and P < 0.01 as the parameters. The relative transcript levels of PhANGs were normalized to that of PP2A.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are grateful to Joanne Chory (Salk Institute) for providing the det1-1 mutant, Xing Wang Deng (Peking University) for providing the cop1-4 mutant, Peter Quail for sharing the pifq mutant, Hong Qiao (University of Texas) for providing the ein3/eil1 mutant, Jesse Woodson (University of Arizona) for providing the sig2-2 and sig6-1 mutants, Beronda Montgomery (Michigan State University) for providing the sig1-1, sig3-2, sig4-2, and sig5-3 mutants, and Chentao Lin (University of California) for providing the cry1/cry2 mutant. We thank Thomas Girke, Jordan Hayes, and the UCR High-Performance Computing Center for providing assistance with the bioinformatics tools used in the study. We thank Elise Pasoreck for valuable suggestions and comments on the manuscript. This work was supported by National Institute of General Medical Sciences grant R01GM132765 to M.C. and National Science Foundation grant IOS-2034015 to X.C. and M.C.
Source data
Author contributions
Y.H., S.H., C.Y.Y., X.C. and M.C. conceived of the original research plan; M.C. supervised the experiments; Y.H., S.H., C.Y.Y., L.H. and M.C. performed the experiments; H.S. and S.Z. provided the LHCB1.1ox and LHCB2.1ox lines; U.H. provided the spa mutants; Y.H., S.H., C.Y.Y., L.H., C.Y., B.H.L., H.S., S.Z., U.H., X.C., and M.C. analyzed the data; Y.H. and M.C. wrote the article with contributions from all authors.
Peer review
Peer review information
Nature Communications thanks Xin Hou, Elena Monte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Arabidopsis mutants used in the current study are available from the corresponding author. The published RNA-seq data used in this study are listed in Supplementary Table 2. The source data underlying Figs. 1a–b, 2c–d, 3a–b, 3e, 4a–b, 5a–c, 6a–b, 7a–c, 8a–c, Supplementary Fig. 1, and Supplementary Fig. 2a–b are provided as a Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Youra Hwang, Soeun Han, Chan Yul Yoo.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35080-0.
References
- 1.Gustafsson CM, Falkenberg M, Larsson N-G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016;85:133–160. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- 2.Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S. Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 1999;6:283–290. doi: 10.1093/dnares/6.5.283. [DOI] [PubMed] [Google Scholar]
- 3.Yoo, C. Y., Han, S. & Chen, M. Nucleus‐to‐Plastid Phytochrome Signalling in Controlling Chloroplast Biogenesis. Annu. Plant Rev.3, 251–280 (2020).
- 4.Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008;9:383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor WC. Regulatory Interactions between Nuclear and Plastid Genomes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:211–233. doi: 10.1146/annurev.pp.40.060189.001235. [DOI] [Google Scholar]
- 6.Kirk, J. T. O. & Tilney-Bassett, R. A. E. The plastids: their chemistry, structure, growth and inheritance. (Elsevier/North-Holland, 1978).
- 7.Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 8.Santel HJ, Apel K. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). The effect of light on the NADPH:protochlorophyllide oxidoreductase. Eur. J. Biochem. 1981;120:95–103. doi: 10.1111/j.1432-1033.1981.tb05674.x. [DOI] [PubMed] [Google Scholar]
- 9.Fankhauser C, Chen M. Transposing phytochrome into the nucleus. Trends Plant Sci. 2008;13:596–601. doi: 10.1016/j.tplants.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, et al. Beyond the photocycle-how cryptochromes regulate photoresponses in plants? Curr. Opin. Plant Biol. 2018;45:120–126. doi: 10.1016/j.pbi.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo, C. Y. et al. Direct photoresponsive inhibition of a p53-like transcription activation domain in PIF3 by Arabidopsis phytochrome B. Nat. Commun.12, 5614 (2021). [DOI] [PMC free article] [PubMed]
- 12.Pedmale UV, et al. Cryptochromes Interact Directly with PIFs to Control Plant Growth in Limiting Blue Light. Cell. 2016;164:233–245. doi: 10.1016/j.cell.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin J, et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl Acad. Sci. USA. 2009;06:7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leivar P, et al. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell. 2009;21:3535–3553. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leivar P, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, X. et al. EIN3 and PIF3 Form an Interdependent Module That Represses Chloroplast Development in Buried Seedlings. Plant Cell29, 3051–3067 (2017). [DOI] [PMC free article] [PubMed]
- 19.Wei N, Deng XW. The role of the COP/DET/FUS genes in light control of arabidopsis seedling development. Plant Physiol. 1996;112:871–878. doi: 10.1104/pp.112.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed JW, Chory J. Mutational analyses of light-controlled seedling development in Arabidopsis. Semin. Cell Biol. 1994;5:327–334. doi: 10.1006/scel.1994.1039. [DOI] [PubMed] [Google Scholar]
- 21.Saijo Y, et al. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17:2642–2647. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo HS, et al. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, et al. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell. 2010;22:108–123. doi: 10.1105/tpc.109.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J, et al. Light-Dependent Degradation of PIF3 by SCFEBF1/2 Promotes a Photomorphogenic Response in Arabidopsis. Curr. Biol. 2017;27:2420–2430.e6. doi: 10.1016/j.cub.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling J-J, Li J, Zhu D, Deng XW. Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness. Proc. Natl Acad. Sci. USA. 2017;114:3539–3544. doi: 10.1073/pnas.1700850114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laubinger S, Fittinghoff K, Hoecker U. The SPA quartet: A family of WD-repeat proteins with a central role in suppression of photomorphogenesis in Arabidopsis. Plant Cell. 2004;16:2293–2306. doi: 10.1105/tpc.104.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H, et al. Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl Acad. Sci. 2015;112:3817–3822. doi: 10.1073/pnas.1502405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagawa Y, et al. Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev. 2004;18:2172–2181. doi: 10.1101/gad.1229504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong J, et al. Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell. 2014;26:3630–3645. doi: 10.1105/tpc.114.130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheerin DJ, et al. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell. 2015;27:189–201. doi: 10.1105/tpc.114.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X-D, et al. Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol. Plant. 2015;8:467–478. doi: 10.1016/j.molp.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Lian H-L, et al. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 2011;25:1023–1028. doi: 10.1101/gad.2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Zuo Z, Liu H, Liu X, Lin C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 2011;25:1029–1034. doi: 10.1101/gad.2025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma D, et al. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl Acad. Sci. USA. 2016;113:224–229. doi: 10.1073/pnas.1511437113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni W, et al. PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3. Nat. Commun. 2017;8:15236. doi: 10.1038/ncomms15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni W, et al. A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science. 2014;344:1160–1164. doi: 10.1126/science.1250778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weihe A, Hedtke B, Börner T. Cloning and characterization of a cDNA encoding a bacteriophage-type RNA polymerase from the higher plant Chenopodium album. Nucl. Acids Res. 1997;25:2319–2325. doi: 10.1093/nar/25.12.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajdukiewicz PT, Allison LA, Maliga P. The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J. 1997;16:4041–4048. doi: 10.1093/emboj/16.13.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hricová A, Quesada V, Micol JL. The SCABRA3 nuclear gene encodes the plastid RpoTp RNA polymerase, which is required for chloroplast biogenesis and mesophyll cell proliferation in Arabidopsis. Plant Physiol. 2006;141:942–956. doi: 10.1104/pp.106.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner S, Schröter Y, Pfalz J, Pfannschmidt T. Identification of essential subunits in the plastid-encoded RNA polymerase complex reveals building blocks for proper plastid development. Plant Physiol. 2011;157:1043–1055. doi: 10.1104/pp.111.184515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfalz J, Pfannschmidt T. Essential nucleoid proteins in early chloroplast development. Trends Plant Sci. 2013;18:186–194. doi: 10.1016/j.tplants.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Schweer J, Türkeri H, Kolpack A, Link G. Role and regulation of plastid sigma factors and their functional interactors during chloroplast transcription - recent lessons from Arabidopsis thaliana. Eur. J. Cell Biol. 2010;89:940–946. doi: 10.1016/j.ejcb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Yoo CY, et al. Phytochrome activates the plastid-encoded RNA polymerase for chloroplast biogenesis via nucleus-to-plastid signaling. Nat. Commun. 2019;10:2629. doi: 10.1038/s41467-019-10518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang EJ, et al. NCP activates chloroplast transcription by controlling phytochrome-dependent dual nuclear and plastidial switches. Nat. Commun. 2019;10:2630. doi: 10.1038/s41467-019-10517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng XW, et al. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992;71:791–801. doi: 10.1016/0092-8674(92)90555-Q. [DOI] [PubMed] [Google Scholar]
- 46.Chory J, Peto C, Feinbaum R, Pratt L, Ausubel F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell. 1989;58:991–999. doi: 10.1016/0092-8674(89)90950-1. [DOI] [PubMed] [Google Scholar]
- 47.Alonso, J. M. et al. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc. Natl Acad. Sci. USA100, 2992–2997 (2003). [DOI] [PMC free article] [PubMed]
- 48.Zhang, X. et al. Integrated regulation of apical hook development by transcriptional coupling of EIN3/EIL1 and PIFs in Arabidopsis. Plant Cell30, 1971–1988 (2018). [DOI] [PMC free article] [PubMed]
- 49.Pham, V. N., Xu, X. & Huq, E. Molecular bases for the constitutive photomorphogenic phenotypes in Arabidopsis. Development145, dev169870 (2018). [DOI] [PMC free article] [PubMed]
- 50.Pfeiffer A, Shi H, Tepperman JM, Zhang Y, Quail PH. Combinatorial complexity in a transcriptionally centered signaling hub in Arabidopsis. Mol. Plant. 2014;7:1598–1618. doi: 10.1093/mp/ssu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feklístov A, Sharon BD, Darst SA, Gross CA. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu. Rev. Microbiol. 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 52.Woodson JD, Perez-Ruiz JM, Schmitz RJ, Ecker JR, Chory J. Sigma factor-mediated plastid retrograde signals control nuclear gene expression. Plant J. 2013;73:1–13. doi: 10.1111/tpj.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pham VN, Kathare PK, Huq E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018;176:1025–1038. doi: 10.1104/pp.17.01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH. Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl Acad. Sci. USA. 2001;98:9437–9442. doi: 10.1073/pnas.161300998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and Phytochrome B Have Overlapping but Distinct Functions in Arabidopsis Development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin JHC, Prado K, Sutton P, Toledo-Ortiz G. Coordinating light responses between the nucleus and the chloroplast, a role for plant cryptochromes and phytochromes. Physiol. Plant. 2020;169:515–528. doi: 10.1111/ppl.13148. [DOI] [PubMed] [Google Scholar]
- 57.Lariguet P, Fankhauser C. Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J. 2004;40:826–834. doi: 10.1111/j.1365-313X.2004.02256.x. [DOI] [PubMed] [Google Scholar]
- 58.Kurihara, Y., Makita, Y., Shimohira, H. & Matsui, M. Time-Course Transcriptome Study Reveals Mode of bZIP Transcription Factors on Light Exposure in Arabidopsis. Int. J. Mol. Sci. 21, 1993 (2020). [DOI] [PMC free article] [PubMed]
- 59.Lv R, et al. Uncoupled Expression of Nuclear and Plastid Photosynthesis-Associated Genes Contributes to Cell Death in a Lesion Mimic Mutant. Plant Cell. 2019;31:210–230. doi: 10.1105/tpc.18.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macadlo LA, Ibrahim IM, Puthiyaveetil S. Sigma factor 1 in chloroplast gene transcription and photosynthetic light acclimation. J. Exp. Bot. 2020;71:1029–1038. doi: 10.1093/jxb/erz464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zghidi W, Merendino L, Cottet A, Mache R, Lerbs-Mache S. Nucleus-encoded plastid sigma factor SIG3 transcribes specifically the psbN gene in plastids. Nucl. Acids Res. 2007;35:455–464. doi: 10.1093/nar/gkl1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishizaki Y, et al. A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J. 2005;42:133–144. doi: 10.1111/j.1365-313X.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 63.Noordally ZB, et al. Circadian control of chloroplast transcription by a nuclear-encoded timing signal. Science. 2013;339:1316–1319. doi: 10.1126/science.1230397. [DOI] [PubMed] [Google Scholar]
- 64.Belbin FE, et al. Integration of light and circadian signals that regulate chloroplast transcription by a nuclear-encoded sigma factor. New Phytol. 2017;213:727–738. doi: 10.1111/nph.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubreuil C, Ji Y, Strand Å, Grönlund A. A quantitative model of the phytochrome-PIF light signalling initiating chloroplast development. Sci. Rep. 2017;7:13884. doi: 10.1038/s41598-017-13473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji, Y. et al. A fully assembled plastid‐encoded RNA polymerase complex detected in etioplasts and proplastids in Arabidopsis. Physiol. Plant.171, 435–446 (2021). [DOI] [PubMed]
- 67.Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SC. Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell. 1997;9:583–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh S, Montgomery BL. Phytochrome-induced SIG2 expression contributes to photoregulation of phytochrome signalling and photomorphogenesis in Arabidopsis thaliana. J. Exp. Bot. 2013;64:5457–5472. doi: 10.1093/jxb/ert308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh S, Strand DD, Kramer DM, Chen J, Montgomery BL. Transcriptome and phenotyping analyses support a role for chloroplast sigma factor 2 in red-light-dependent regulation of growth, stress, and photosynthesis. Plant Direct. 2018;2:e00043. doi: 10.1002/pld3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu Y, et al. RCB initiates Arabidopsis thermomorphogenesis by stabilizing the thermoregulator PIF4 in the daytime. Nat. Commun. 2021;12:2042. doi: 10.1038/s41467-021-22313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galvão RM, et al. Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev. 2012;26:1851–1863. doi: 10.1101/gad.193219.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liebers M, et al. Nucleo-plastidic PAP8/pTAC6 couples chloroplast formation with photomorphogenesis. EMBO J. 2020;39:e104941. doi: 10.15252/embj.2020104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen, M. et al. Arabidopsis HEMERA/pTAC12 Initiates Photomorphogenesis by Phytochromes. Cell141, 1230–1240 (2010). [DOI] [PMC free article] [PubMed]
- 74.Nevarez PA, et al. Mechanism of Dual Targeting of the Phytochrome Signaling Component HEMERA/pTAC12 to Plastids and the Nucleus. Plant Physiol. 2017;173:1953–1966. doi: 10.1104/pp.16.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsunoyama Y, et al. Blue light-induced transcription of plastid-encoded psbD gene is mediated by a nuclear-encoded transcription initiation factor, AtSig5. Proc. Natl Acad. Sci. USA. 2004;101:3304–3309. doi: 10.1073/pnas.0308362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mockler TC, Guo H, Yang H, Duong H, Lin C. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development. 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- 77.Pfalz J. Investigating the Assembly Status of the Plastid Encoded Polymerase Using BN-PAGE and Sucrose Gradient Centrifugation. BIO-PROTOCOL. 2016;6:1873. doi: 10.21769/BioProtoc.1873. [DOI] [Google Scholar]
- 78.Cheng C-Y, et al. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017;89:789–804. doi: 10.1111/tpj.13415. [DOI] [PubMed] [Google Scholar]
- 79.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 81.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Arabidopsis mutants used in the current study are available from the corresponding author. The published RNA-seq data used in this study are listed in Supplementary Table 2. The source data underlying Figs. 1a–b, 2c–d, 3a–b, 3e, 4a–b, 5a–c, 6a–b, 7a–c, 8a–c, Supplementary Fig. 1, and Supplementary Fig. 2a–b are provided as a Source Data file. Source data are provided with this paper.