Abstract
Cytokines have been proposed to play an important role in Helicobacter pylori-associated gastroduodenal diseases, but the exact mechanism of the cytokine induction remains unclear. H. pylori urease, a major component of the soluble proteins extracted from bacterial cells, is considered to be one of the virulence factors for the inflammation in the gastric mucosa that is produced in H. pylori infection. However, the response of human gastric epithelial cells to the stimulation of urease has not been investigated. In the present study, we used human gastric epithelial cells in a primary culture system and examined whether H. pylori urease stimulates the gastric epithelial cells to induce proinflammatory cytokines by reverse transcription-PCR and enzyme-linked immunosorbent assay. First, by using peripheral blood mononuclear cells (PBMC) and a gastric cancer cell line (MKN-45 cells), we confirmed the ability of purified H. pylori urease to induce the production of proinflammatory cytokines. Furthermore, we demonstrated that the human gastric epithelial cells produced interleukin-6 (IL-6) and tumor necrosis factor alpha, but not IL-8, following stimulation with purified urease. The patterns of cytokine induction differed among human PBMC, MKN-45 cells, and human gastric epithelial cells. These results suggest that the human gastric epithelial cells contribute to the induction of proinflammatory cytokines by the stimulation of H. pylori urease, indicating that the epithelial cells were involved in the mucosal inflammation that accompanied H. pylori infection.
Helicobacter pylori, a microaerophilic gram-negative bacterium, has been identified as the cause of chronic gastritis and peptic ulcer disease in humans (18). H. pylori-associated gastroduodenal diseases are characterized by the severe infiltration of neutrophils, lymphocytes, monocytes, and plasma cells in the gastric mucosa. Indeed, cure of the infection results in a notable reduction in these cells in the gastric mucosa (3). Because the accumulation and activation of inflammatory cells have been induced by the local production of cytokines (16), cytokines are proposed to play an important role in the pathogenesis of H. pylori-associated gastroduodenal diseases. Many investigators have examined the relation between H. pylori infection and mucosal cytokines (7, 14, 17, 19). We have studied the cytokine expression patterns in gastric mucosal biopsy specimens by using reverse transcription-PCR (RT-PCR) and have found that the levels of interleukin-6 (IL-6), IL-7, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α) mRNA expression were significantly higher in H. pylori-positive than in H. pylori-negative patients (29).
Several potential virulence factors derived from H. pylori are considered to stimulate the cytokine induction in the gastric mucosa, thereby attracting and activating neutrophils and mononuclear cells (13, 21, 23). However, it remains unclear how these noninvasive bacteria, residing in the gastric mucous layer, produce the inflammation and cause the damage to the underlying epithelial tissue. Furthermore, the specific H. pylori protein responsible for stimulating the cytokine induction has not been identified, although the cag pathogenicity island was reported to be closely related to IL-8 production (5).
Urease plays a central role in the pathogenesis of H. pylori infection by protecting the bacteria from the acid environment of the stomach, promoting colonization, and inducing the production of ammonia. In addition, recent studies showed that urease is a potent chemoattractant factor for monocytes obtained from peripheral blood mononuclear cells (PBMC) and mucosal macrophages (11, 12). However, the response of the gastric epithelial cells to H. pylori urease has not been studied. The studies reported so far focused on the results of in vitro culture using gastric cancer cell lines (7, 13, 22).
In the present study, we established a primary culture system for human gastric epithelial cells derived from the human stomach tissue at surgery. To obtain insight into the mechanism of the cytokine induction, we examined the response of the human gastric epithelial cells to purified H. pylori urease.
MATERIALS AND METHODS
Preparation of purified H. pylori urease.
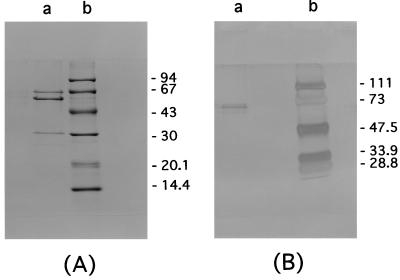
Purified H. pylori urease was provided by the Institute of Immunology, Co., Ltd. (Tokyo, Japan) and prepared as described previously (9). In brief, for the urease purification, H. pylori (ATCC 43504) was harvested from horse blood agar plates and washed with phosphate-buffered saline (PBS) three times by centrifugation at 5,000 × g for 10 min and resuspended in PBS. Intact bacterial cells were ruptured by French pressure (20,000 lb/in2) cell lysis, and urease was purified from the soluble protein by column chromatography on Sephacryl S-300HR (Pharmacia, Inc., Piscataway, N.J.). When this material was electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel (10% acrylamide) and stained with Coomassie blue, only three bands corresponding to the 62-, 60-, and 31-kDa proteins were detected, indicating a purified enzyme preparation (Fig. 1). The specific identification of the material was confirmed by Western blotting with antisera recognizing the 31- and 62-kDa H. pylori urease (UreA and UreB, respectively) polypeptide, and the 60-kDa H. pylori heat shock protein (hsp60) polypeptide. To prepare the specific material of H. pylori UreB, the 62-kDa protein was separated and concentrated after a sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The homogeneity of H. pylori UreB was verified by silver staining by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1). That there was no endotoxin in the purified urease preparation was confirmed by the manufacturer (detection limit, <0.03 ng/ml).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis profile of prepared protein. (A) Purified H. pylori urease stained with Coomassie blue. Lane a, purified H. pylori urease; lane b, molecular masses (in kilodaltons). (B) Highly purified urease, including only the UreB protein, after silver staining. Lane a, purified UreB; lane b, molecular masses (in kilodaltons).
PBMC preparation and the cell line.
Heparinized venous blood drawn from healthy adult volunteers was diluted in PBS. Mononuclear cells were separated by Ficoll-Hypaque (Pharmacia BiotechAB, Uppsala, Sweden) density gradient centrifugation as recommended by the manufacturer. PBMC were cultured at a final concentration of 106 cells/ml in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS) and 2 mM glutamine in 24-well plates at 37°C in 5% CO2.
In another series of experiments, the human gastric cancer cell line, MKN-45, established from a poorly differentiated adenocarcinoma metastasized to liver and obtained from the Japanese Cancer Research Resource Bank (Tsukuba, Japan), was used. MKN-45 cells were seeded in 24-well plates at 105 cells/ml in RPMI 1640 medium supplemented with 5% FBS, 2 mM glutamine, and 30 mg of streptomycin per ml at 37°C in 5% CO2.
Culture of human primary gastric epithelial cells.
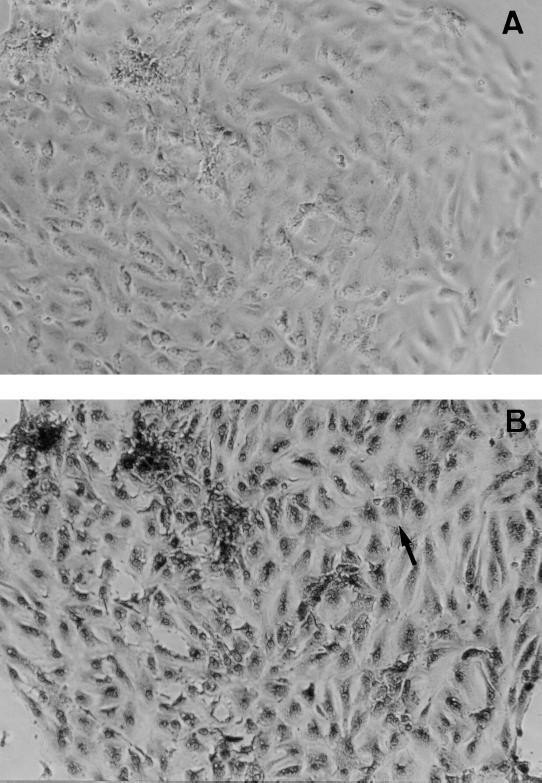
Human gastric epithelial cells were isolated enzymatically from the adult human stomach as described earlier (24, 25). A piece of healthy gastric mucosa (3 cm2) was obtained from the normal fundic gland area of the stomach at surgery. The patients underwent total gastrectomy because of early gastric cancer on the antrum. The patients gave informed consent, and this study was approved by the Human Research Committee of Kyoto Prefectural University of Medicine, Kyoto, Japan. The surface mucosal layer was carefully removed with a razor blade and minced immediately. The minced tissue was incubated in Ham's F-12 culture medium containing collagenase type I (0.2 mg/ml) (Gibco BRL, Gaithersburg, Md.) for 10 min. Cells from the final incubation were washed and cultured in Ham's F-12 medium supplemented with 10% FBS and 30 mg of streptomycin per ml at 37°C in a humidified 5% CO2 atmosphere. Human gastric epithelial cells in a 24-well collagen-coated dish were cultured at a final concentration of 106 cells/ml for 24 h before the experiment. When the stimulation studies were performed, F-12 medium was not supplemented with the FBS. Cultured cells had formed subconfluent monolayers within 24 h of the inoculation. For the histochemical identification of cultured cells, periodic acid-Schiff (PAS) reaction was employed after 24 h of incubation. The cultured cells in the monolayers had PAS-positive material in the cytoplasm, indicating that the population consisted of only mucus-producing epithelial cells without contamination by other cells (Fig. 2). Each experiment used gastric cells from a different patient. However, individual experiments were performed by using gastric cells from a single patient. The results were obtained from three different experiments.
FIG. 2.
Identification of human gastric epithelial cells in primary culture. (A) Phase-contrast micrograph of human gastric epithelial cells in primary culture (magnification, ×40). (B) PAS staining of human gastric epithelial cells (magnification, ×40). The PAS-positive material in the cytoplasm can be seen; an example is indicated by the arrow.
Detection of cytokine mRNA expression by RT-PCR.
PBMC, MKN-45 cells, and human gastric epithelial cells were incubated for 3 h with purified H. pylori urease (0.1, 1, or 10 μg/ml), and total cellular RNA was extracted by the acid guanidinium isothiocyanate-phenol-chloroform method as described elsewhere (29). Aliquots (1 μg) of total RNA were incubated at 65°C for 5 min, chilled on ice, and reverse-transcribed in a final volume of 10 μl of a solution containing 50 mmol of Tris-HCl (pH 8.3), 75 mmol of KCl, 3 mmol of MgCl2, 10 mmol of dithiothreitol, and 200 mmol each of dATP, dCTP, dGTP, and dTTP (Pharmacia BiotechAB, Uppsala, Sweden) per liter, plus 1 mmol of oligo(dT) 16 primer per liter, 20 U of RNase inhibitor (Toyobo Co., Ltd., Tokyo, Japan), and 100 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL, Gaithersburg, Md.). The mixture was incubated at 43°C for 90 min, heated to 90°C for 10 min, and stored at −20°C until used.
The cDNA (5 μl) were added to 50-μl reaction mixtures containing 5 μl of 10× PCR reaction buffer, which consisted of 100 nmol of KCl, 20 nmol of Tris-HCl (pH 7.5), 15 nmol of MgCl2, 1 nmol of dithiothreitol, and 0.1 nmol of EDTA per liter, 200 ml of each deoxynucleotide (Pharmacia Biotech AB, Uppsala, Sweden), 200 nmol of each primer per liter, 1.0 U of the Taq DNA polymerase included in the Expand High Fidelity PCR system (Boehringer GmbH, Mannheim, Germany), and H2O. The PCR was performed with an automatic thermal cycler (Takara PCR Thermal Cycler TP-3000; Takara Biomedicals, Otsu, Japan). The amplification cycle consisted of an initial denaturation of target DNA at 95°C for 5 min and then denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and an extension step at 72°C for 1 min. The final cycle included an extension step for 7 min at 72°C to ensure full extension of the product. Aliquots (10 μl) of each PCR product were analyzed by electrophoresis on 1.5% Agarose S (Wako Chemical Co., Ltd., Osaka, Japan) gels containing ethidium bromide, and the bands were examined under UV light for the presence of the amplified DNA.
Oligonucleotide primers were designed based on the previous study and recent reports (10, 27, 29). The sense and antisense primers specific for each cytokine were designed to include at least one intron, permitting distinction between amplified cDNA and possible contaminating residual genomic DNA. Table 1 shows cytokine-specific oligonucleotide primers. The β-actin gene was assayed as a positive control for cytokine mRNA expression. Primer sequences were as follows: forward, 5′-GTGGGGCGCCCCAGGCACCA-3′; reverse, 5′-CTCCTTAATGTCACGCACGATTTC-3′.
TABLE 1.
Human cytokine-specific oligonucleotide primers for RT-PCR
| Cytokine | Direction | Primer sequence | Nucleotide position | Size of cDNA (bp) |
|---|---|---|---|---|
| IL-1β | Sense | ATAAGCCCACTCTACAGCT | 5449–5468 | 443 |
| Antisense | ATTGGCCCTGAAAGGAGAGA | 6593–6612 | ||
| IL-6 | Sense | GTACCCCCAGGAGAAGATTC | 242–261 | 819 |
| Antisense | CAAACTGCATAGCCACTTTC | 4535–4554 | ||
| IL-8 | Sense | GCTTTCTGATGGAAGAGAGC | 1396–1415 | 585 |
| Antisense | GGCACAGTGGAACAAGGACT | 2396–2415 | ||
| IL-10 | Sense | ATGCCCCAAGCTGAGAACCAAGAC | 312–339 | 353 |
| Antisense | TCTCAAGGGGCTGGGTCAGCTATCCCA | 638–664 | ||
| IL-12 | Sense | TCACAAAGGAGGCGAGGTTC | 283–302 | 378 |
| Antisense | TGAACGGCATCCACCATGAC | 641–660 | ||
| IL-18 | Sense | GCTTGAATCTAAATTATCAGTC | 297–318 | 342 |
| Antisense | GAAGATTCAAATTGCATCTTAT | 617–639 | ||
| IFN-γ | Sense | ATAATGCAGAGCCAAATTGTCTC | 2007–2029 | 300 |
| Antisense | CTGGGATGCTCTTCGACCTC | 4712–4731 | ||
| TNF-α | Sense | TCGGGCCAATGCCCTCCTGGCCAA | 1521–1540 | 468 |
| Antisense | GTAGACCTGCCCAGACTCGGCAAA | 2270–2289 |
Cytokine protein measurement.
PBMC and MKN-45 cells were incubated for 24 h with purified H. pylori urease (0.1, 1, or 10 μg/ml), and the supernatants were collected and stored at −80°C until assayed. In addition, PBMC and MKN-45 cells were incubated for 24 h with purified H. pylori UreB (0.1, 1, or 10 μg/ml), and the supernatants were collected. Human gastric epithelial cells were incubated for 24 h with purified H. pylori urease (10 μg/ml), and the supernatants were collected at 3, 6, 12, and 24 h after urease stimulation. In addition, the culture supernatants were collected at 12 h after the stimulation of purified H. pylori urease (0.1, 1, or 10 μg/ml).
Cytokine levels in the culture supernatants were determined by means of a specific enzyme-linked immunosorbent assay (ELISA). IL-6, IL-8, and TNF-α in the culture supernatants were measured by ELISA by using commercially available assay kits (BioSource International, Inc.). Assays were performed in duplicate according to the manufacturer's instructions. In these assays, the lower limits of detection were 2 pg/ml for IL-6, 10 pg/ml for IL-8, and 1 pg/ml for TNF-α. Regarding PBMC and MKN-45 cells, the same experiments were repeated three times, and the results from all experiments were used.
Statistical analysis.
All data in each experiment were expressed as the mean ± 1 standard error (SE). The statistical significance of the cytokine production response to urease protein was evaluated by using Student's t test as previously indicated (11). Differences between cytokine levels were considered significant when P was <0.05.
RESULTS
Cytokine mRNA expression by H. pylori urease in PBMC and MKN-45 cells.
To examine the ability of H. pylori urease to induce the production of cytokines, the cytokine-specific mRNA expressions in human PBMC and MKN-45 cells were analyzed by RT-PCR.
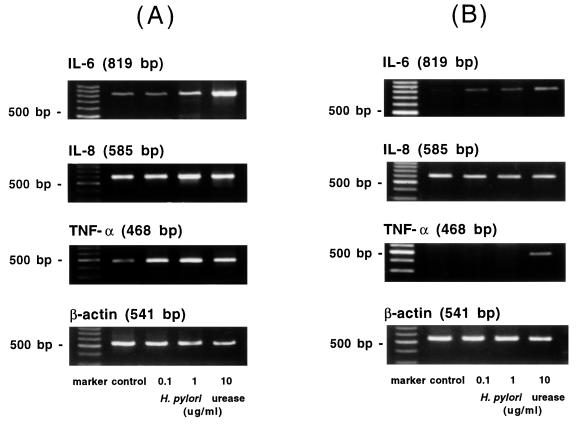
In PBMC, IL-1β, IL-6, IL-8, IL-10, IL-12, IL-18, and TNF-α mRNAs were constitutively detected before and after the stimulation of the purified urease. However, gamma interferon (IFN-γ) mRNA was detected neither before nor after the stimulation of the purified urease. The expressions of IL-6, IL-8, and TNF-α mRNAs are shown as representative results in Fig. 3.
FIG. 3.
Expressions of IL-6, IL-8, and TNF-α mRNA in PBMC and MKN-45 cells. The cells were exposed to purified H. pylori urease, and RT-PCR was carried out as described in Materials and Methods. These cells were exposed to medium alone (control) and increasing concentrations of H. pylori urease for 3 h. β-Actin gene was assayed as a positive control. The results from PBMC (A) and from MKN-45 cells (B) are shown.
The expression of IL-6 and TNF-α mRNA was induced in response to exposure to H. pylori urease in MKN-45 cells. IL-1β, IL-8, IL-12, and IL-18 mRNAs were constitutively detected both before and after the stimulation of the purified urease. IL-10 and IFN-γ mRNAs were detected neither before nor after the stimulation (Table 2 and Fig. 3).
TABLE 2.
Cytokine mRNA expressions in MKN-45 cells and primary gastric epithelial cells in response to the purified urease protein examined by RT-PCRa
| Cytokine | MKN-45 cells
|
Primary gastric epithelial cells
|
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Urease protein
|
Control | Urease protein
|
|||||
| 0.1 μg/ml | 1.0 μg/ml | 10 μg/ml | 0.1 μg/ml | 1.0 μg/ml | 10 μg/ml | |||
| IL-1β | + | + | + | + | + | + | + | + |
| IL-6 | − | + | + | + | − | + | + | + |
| IL-8 | + | + | + | + | + | + | + | + |
| IL-10 | − | − | − | − | + | + | + | + |
| IL-12 | + | + | + | + | − | − | − | − |
| IL-18 | + | + | + | + | + | + | + | + |
| IFN-γ | − | − | − | − | − | − | − | − |
| TNF-α | − | − | − | + | − | + | + | + |
+, positive mRNA expression; −, negative mRNA expression.
Cytokine production by H. pylori urease and UreB in PBMC and MKN-45 cells.
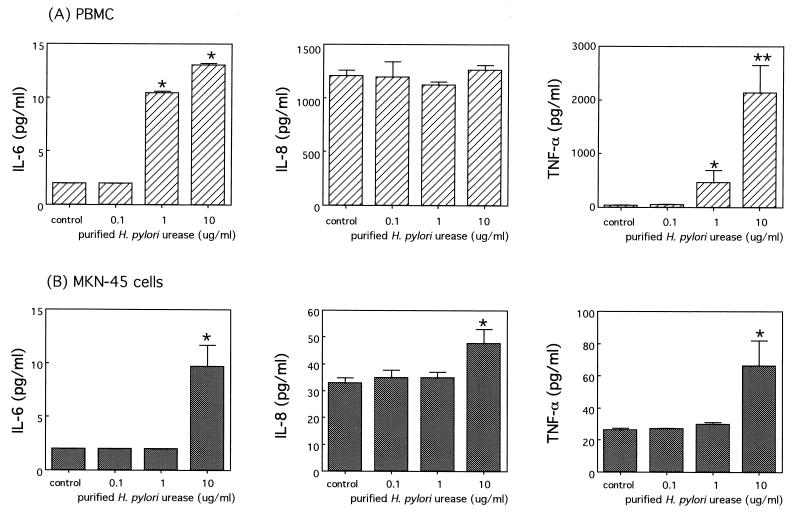
To examine the cytokine production after the stimulation of H. pylori urease, human PBMC and MKN-45 cells were incubated with purified urease for 24 h, and the culture supernatants were analyzed by ELISA. In addition, we assessed the response of human PBMC and MKN-45 cells to stimulation with highly purified H. pylori urease, including only the UreB protein.
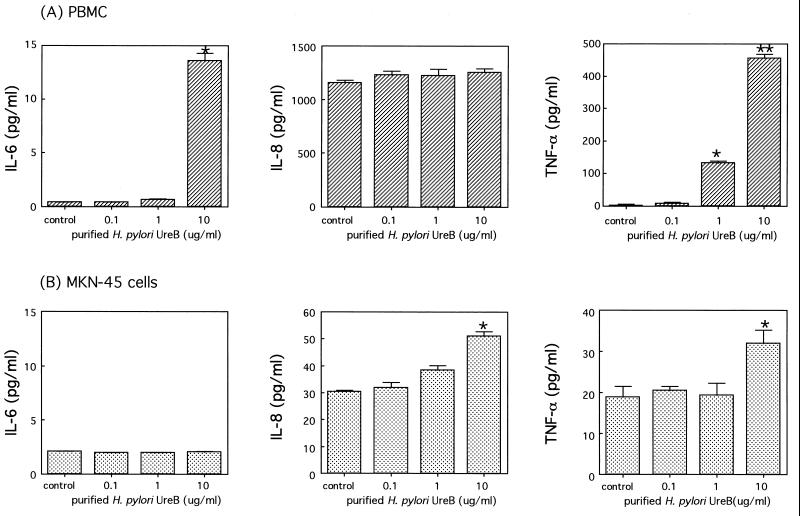
The production of IL-6 and TNF-α, but not IL-8, increased significantly in a dose-dependent manner after the addition of urease to PBMC. In particular, a large amount of TNF-α was detected in the supernatants (Fig. 4). In MKN-45 cells, the production of IL-6, IL-8, and TNF-α was significantly induced by the addition of 10 μg of urease per ml. However, TNF-α production was considerably lower than that in the PBMC (Fig. 4). Similarly, H. pylori UreB protein stimulated the production of IL-6 and TNF-α, but not IL-8, significantly in a dose-dependent manner in PBMC. The production of IL-8 and TNF-α, but not IL-6, was also significantly induced by the addition of UreB protein in MKN-45 cells (Fig. 5).
FIG. 4.
Productions of IL-6, IL-8, and TNF-α induced by purified H. pylori urease. Cells were incubated in triplicate, and the levels of each cytokine in the culture supernatants with purified H. pylori urease (0.1, 1, or 10 μg/ml) and medium alone (control) were assayed by ELISA as described in Materials and Methods. The results from PBMC (A) and from MKN-45 cells (B) are shown. The results are shown as the mean ± 1 SE. ∗ and ∗∗, statistically significant differences from the values for the corresponding medium alone (control) (P < 0.05 and P < 0.01, respectively).
FIG. 5.
Productions of IL-6, IL-8, and TNF-α induced by purified H. pylori UreB. Cells were incubated in triplicate, and the levels of each cytokine in the culture supernatants with purified UreB (0.1, 1, 10 μg/ml) and medium alone (control) were assayed by ELISA as described in Materials and Methods. The results from PBMC (A) and from MKN-45 cells (B) are shown. The results are shown as the mean ± 1 SE. ∗ and ∗∗, statistically significant differences from the values for the corresponding medium alone (control) (P < 0.05 and P < 0.01, respectively).
Although there was little difference in the pattern of cytokine production between PBMC and MKN-45 cells, these results indicated that purified H. pylori urease, especially the component of UreB, could stimulate PBMC and MKN-45 cells to produce proinflammatory cytokines.
Cytokine mRNA expression and production by purified H. pylori urease in human gastric epithelial cells.
To obtain further insight into the mechanism of the cytokine induction, we examined the effect of purified H. pylori urease in human gastric epithelial cells in the primary culture system.
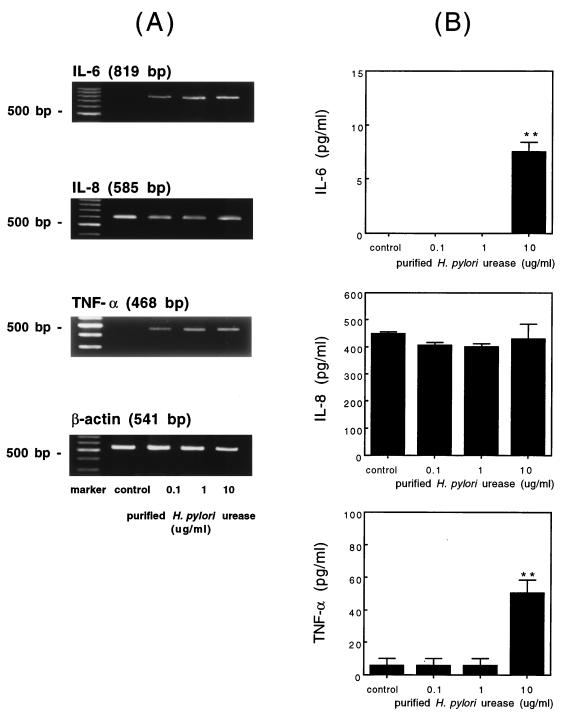
Human gastric epithelial cells were incubated with purified H. pylori urease for 3 h, and the expressions of mRNAs were analyzed by RT-PCR. The expressions of IL-6 and TNF-α mRNAs were induced after the stimulation with the purified urease, and there was no expression of mRNAs in the unstimulated cells. The expressions of IL-1β, IL-8, IL-10, and IL-18 mRNAs were constitutively observed both before and after the stimulation with the purified urease. IL-12 and IFN-γ mRNA expressions, on the other hand, were detected neither before nor after the stimulation. There was a difference in the pattern of the cytokine expression between the cell line, the MKN-45 cells, and the primary gastric epithelial cells (Table 2 and Fig. 6).
FIG. 6.
Expressions of cytokine-specific mRNA and productions of IL-6, IL-8, and TNF-α induced by purified H. pylori urease in human gastric epithelial cells. (A) Human gastric epithelial cells were exposed to medium alone (control) and increasing concentrations of H. pylori urease for 3 h. The expressions of cytokine mRNAs were analyzed by RT-PCR as described in Materials and Methods. (B) Cells were incubated in triplicate for 12 h, and the levels of each cytokine in the culture supernatants with medium alone (control) and purified H. pylori urease (0.1, 1, 10 μg/ml) were measured by ELISA as described in Materials and Methods. The results are shown as the mean ± 1 SE. ∗ and ∗∗, statistically significant differences from the value of the corresponding medium alone (control) (P < 0.05 and P < 0.01, respectively).
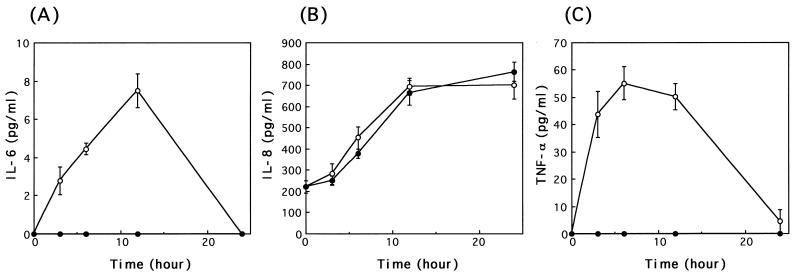
We further studied the time kinetics of cytokine production in primary gastric epithelial cells at various time intervals (Fig. 7). When stimulated with purified urease (10 μg/ml), epithelial cells secreted IL-6 and TNF-α, but not IL-8, in a time-dependent manner. IL-6 production could be detected in the culture supernatant within 3 h after the addition of urease, and the most active secretion occurred at 12 h. IL-8 was constitutively produced in agreement with a previous finding (25), and there was no difference in the production level between control and stimulated cells. TNF-α production was detectable at 3 h of exposure to urease and continued for up to 24 h after exposure, reaching a peak of 60 pg/ml.
FIG. 7.
Time course of IL-6 (A), IL-8 (B), and TNF-α (C) production induced by purified H. pylori urease in human gastric epithelial cells. Cells were incubated in triplicate, and the levels of each cytokine in the culture supernatants with purified H. pylori urease (10 μg/ml; ●) and medium alone (control; ○) were measured by ELISA as described in Materials and Methods. The error bars indicate the SE values from three determinations.
In a subsequent experiment, cytokine production was examined at 12 h after stimulation with various concentrations of purified H. pylori urease (0.1, 1, or 10 μg/ml) (Fig. 6). The production of IL-6 and TNF-α, but not IL-8, was significantly induced by the addition of 10 μg of urease per ml compared to the level of the unstimulated cells. With this assay, IL-6 production was not detected upon the addition of 0.1 and 1 μg of urease per ml, although IL-6 mRNA expression was observed after 3 h of stimulation.
DISCUSSION
Several studies have indicated that infection with H. pylori induced the expression and production of various cytokines in the gastric mucosa, and cytokines contribute to the pathogenesis of H. pylori-associated gastroduodenal diseases (3, 7, 17, 19, 29). However, the contribution of the human gastric epithelial cells to the cytokine induction has not been studied. To examine the mechanism of the cytokine induction in the epithelial cells, gastric cancer cell lines have been used in other investigations as an in vitro system (13, 23). However, this approach has limitations for examining the natural properties of cytokine induction because the cell lines were obtained from human cancer cells. Therefore, we have successfully established a primary culture system and examined the property of cytokine induction in human gastric epithelial cells.
It was proposed that the ability to stimulate cytokine induction is associated with several gene products of H. pylori (13, 23). In spite of searches for the components of H. pylori related to the cytokine stimulation, the mechanism still remains unclear, though the cag pathogenicity island was found to be closely related to IL-8 induction (5, 26). Since H. pylori exists in the gastric mucus layer overlying the epithelium and does not invade the epithelial tissue, it is still unclear how a noninvasive bacterium stimulates the induction of proinflammatory cytokines in the gastric mucosa. H. pylori urease, a component of soluble proteins released outside the bacterial cells (20), plays a central role in the pathogenesis of infection by promoting colonization and inducing the production of ammonia. In addition, the present study indicates that urease may act as a virulence factor by inducing the production of IL-6 and TNF-α in gastric epithelial cells. These results also extend other observations that H. pylori urease induced the production of certain cytokines in human peripheral blood monocytes and mucosal macrophages (11, 12). Compared to the response of mucosal macrophages, it is of interest to note that the level of TNF-α production was nearly the same but that the level of IL-6 production was decreased in the epithelial cells. Despite differences in the amounts of cytokine produced, the dose responses were similar regardless of the cell origin. However, the time kinetic responses could not compared because there were no data except ours.
IL-6 has broad biological effects on mononuclear cells, including B-cell and T-cell differentiation and activation of macrophages (2). These biological properties may be relevant to the pathogenesis of gastroduodenal inflammation. We have previously shown that there were some factors other than the cag pathogenicity island which induced IL-6 production in H. pylori infection (30). The present study showed that IL-6 production was induced in response to urease in human gastric epithelial cells. Meanwhile, immunohistochemistry revealed positive staining for IL-6 in the epithelial cells localized to the superficial and the neck regions of the stomach glands in H. pylori-infected patients (16). These results indicated that H. pylori urease contributes to IL-6 production, at least in part, in human gastric epithelial cells, whereas ELISA did not detect IL-6 production in low-dose urease stimulation that had been judged positive by RT-PCR. The apparent contradiction of these data may be explained by methodological problems. The RT-PCR system is usually chosen to catch the minimal expression of mRNA because of its high level of sensitivity and its reproducibility.
The stimulation of TNF-α production was found in human PBMC, MKN-45 cells, and human gastric epithelial cells. TNF-α was correlated with the severity of infiltration of mononuclear and polymorphonuclear cells in H. pylori-positive specimens (4, 30). In addition, TNF-α has been shown to stimulate the gastrin secretion in vitro (28), suggesting a role for this cytokine in H. pylori-induced hypergastrinemia (15), which is linked to increased gastric acid secretion. Based on these findings, we speculate that certain bacterial components, including urease, are related to the gastric acid secretion through the cytokine production. However, at this point, further investigations using other techniques are needed.
By H. pylori urease, IL-8 was induced only in MKN-45 cells, not in human PBMC nor the human gastric epithelial cells, although the site of IL-8 induction was considered to be the epithelial cells (6). This discrepancy might be explained by the difference of cell characteristics between the cancer cell line and the primary culture cells. Furthermore, it was proposed that the receptors for H. pylori in host cells or other components contributed to the differences between the cell lines and PBMC (1, 8). These results showed that H. pylori urease was unrelated to the IL-8 production, providing indirect evidence that some other H. pylori protein induces the IL-8 production in the epithelial cells.
In conclusion, the present study is the first to indicate that the human gastric epithelial cells were sensitive to H. pylori urease and participated in the production of proinflammatory cytokines, such as IL-6 and TNF-α. These results suggest that the gastric epithelial cells play a role in the mucosal inflammation that accompanied H. pylori infection.
ACKNOWLEDGMENTS
We thank Hisakazu Yamagishi and Chohei Sakakura (First Department of Surgery, Kyoto Prefectural University of Medicine, Kyoto, Japan) for generously donating the surgical specimen of human stomach. We are also grateful to Yukito Kawakami (Institute of Immunology, Co., Ltd., Tokyo, Japan) for providing the purified H. pylori urease.
REFERENCES
- 1.Aihara M, Imagawa K, Funakoshi Y, Ohmoto Y, Kikuchi M. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig Dis Sci. 1998;43:160S–166S. [PubMed] [Google Scholar]
- 2.Akira S, Hirano T, Taga T. Biology of multifocal cytokines: IL6 and related molecules (IL1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 3.Ando T, Kusugami K, Ohsuga M, Ina K, Shinoda M, Konagaya T, Sakai T, Imada A, Kasuga N, Nada T, Ichiyama S, Blaser M J. Differential normalization of mucosal interleukin-8 and interleukin-6 activity after Helicobacter pylori eradication. Infect Immun. 1998;66:4742–4747. doi: 10.1128/iai.66.10.4742-4747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Investig. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree J E, Xiang Z, Lindley I J, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di T A, Xiang Z, Bugnoli M, Pileri P, Figura N, Bayeli P F, Rappuoli R, Abrignani S, De M M. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun. 1995;63:1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D J, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 10.Goodman R E, Nestle F, Naidu Y M, Green J M, Thompson C B, Nickoloff B J, Turka L A. Keratinocyte-derived T cell costimulation induces preferential production of IL-2 and IL-4 but not IFN-gamma. J Immunol. 1994;152:5189–5198. [PubMed] [Google Scholar]
- 11.Harris P R, Ernst P B, Kawabata S, Kiyono H, Graham M F, Smith P D. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J Infect Dis. 1998;178:1516–1520. doi: 10.1086/314426. [DOI] [PubMed] [Google Scholar]
- 12.Harris P R, Mobley H L, Perez P G, Blaser M J, Smith P D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, O'Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karttunen R A, Karttunen T J, Yousfi M M, el-Zimaity H M, Graham D Y, el-Zaatari F A. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–27. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 15.Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989;i:1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm A-M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss S F, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 19.Noach L A, Bosma N B, Jansen J, Hoek F J, van Deventer S J, Tytgat G N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 20.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieder G, Hatz R A, Moran A P, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappaB in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 23.Sharma S A, Tummuru M K, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smoot D T, Resau J H, Naab T, Desbordes B C, Gilliam T, Bull H K, Curry S B, Nidiry J, Sewchand J, Mills R K. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect Immun. 1993;61:350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi M, Ogura K, Maeda S, Mori K, Mafune K, Mikami Y, Terano A, Omata M. Promoters of epithelialization induce expression of vascular endothelial growth factor in human gastric epithelial cells in primary culture. FEBS Lett. 1997;418:115–118. doi: 10.1016/s0014-5793(97)01354-9. [DOI] [PubMed] [Google Scholar]
- 26.Tummuru M K, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 27.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 28.Weigert N, Schaffer K, Schusdziarra V, Classen M, Schepp W. Gastrin secretion from primary cultures of rabbit antral G cells: stimulation by inflammatory cytokines. Gastroenterology. 1996;110:147–154. doi: 10.1053/gast.1996.v110.pm8536851. [DOI] [PubMed] [Google Scholar]
- 29.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]