Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterised pathologically by the presence of extracellular amyloid plaques and the intracellular neurofibrillary tangles, along with inflammation, and a compromised antioxidant system. Significant insights into the neurobiology to better understand the pathophysiology of AD and to evaluate the possibility of cutting-edge therapy strategies, can be obtained through the selection of a well-designed experimental animal model. From the transgenic to chemical/drug-induced models, none of them represents the complete picture of Alzheimer pathology and incidence of cognitive dysfunction. Researchers did not explain why one model was preferred over another, did not consider how the pathological phenomena were formed (spontaneously, experimentally, or by genetic manipulation), and did not address the traits of the species that affect the results. There is a lack of concordance between preclinical models and clinical trials that could be due to variety of reasons such as incomplete models, choice of animal species, lack of variability, and the validity of the models. To provide greater translation of preclinical AD studies to clinical trials proper designing of the model is essential. This review provides a brief recap ranging from animal doses to their induction mechanism and common limitations of the chemical-induced AD models.
• Animal models may fail to replicate the exact pathology of the disease
• Validity of the model is essential for proper translation of pathology from animal models to human disease
• Appropriate induction doses need to be administered.
Keywords: Alzheimer's disease, Animal models, Induction dose, Validation
Graphical abstract
Specifications table
| Subject area | |
| More specific subject area | Alzheimer's disease |
| Name of the reviewed methodology | Induction of Alzheimer's disease in animal models |
| Keywords | Induction; Animal models; chemical-induced. |
| Resource availability | pubmed /Elsevier/ Nature/ Wiley / Springer /Medknow |
| Review question | Animal models of Alzheimer's disease |
| Name & Reference of original method | N.A. |
Method details
Introduction
Alzheimer's disease (AD) is the most common form of dementia. The prevalence increases logarithmically with age. According to the latest statistics, the prevalence of AD will raise to 13.8 million from 5.8 million in Americans by 2060. 121,499 deaths were reported in the year 2019 [1]. The clinical manifestations include memory impairment, language, visuospatial, behavioural perceptions, and disturbances [2]. AD pathogenesis can be evident earlier before the symptoms become deceptive [3].
Experimental animal models are essential for further understanding of AD pathology. Most animal models currently being used are chemical/drug-induced models. A well-designed experimental model is required not only to understand the underlying disease mechanisms but also to produce a novel therapeutic effect. It is exaggerating to know that over the past 19 years, nearly 400 clinical trials have failed (unsuccessful) in the synthesis of a new drug for AD. Data obtained from animal studies was not found to be properly translated into clinical studies [4]. Humans share extensive genetic and physiologic homology with mammals such as rodents, and where the likeness of a disease state can be demonstrated, knowledge gained by the study of the model may inform interpretation of human disease conditions. It is challenging to understand how this might be applied to successful translation of preclinical research in Alzheimer's disease, especially in cognitive and memory dysfunction, a condition whose phenomenology surpasses that which could be observed in animals. The animal models are categorised based on the disease characteristics, whether they are acquired by induction (chemical/drug/ physical), spontaneous (naturally occurring), genetic changes (transgenic models) [5]. In this study, we discussed the chemical/drug-induced models for Alzheimer's disease (Table 1).
Table 1.
Animal models for Alzheimer's disease.
| S.No | Model | Species, age, gender | Biomarkers | Dosage regimens | References |
|---|---|---|---|---|---|
| 1. | Streptozotocin induced AD. |
|
|
|
|
| 2. | Aβ-induced AD |
|
|
|
|
| 3. |
|
|
|
|
|
| 4. | AF64A induced memory deficits |
|
|
|
|
| 5. | Alcohol- induced memory deficits |
|
|
|
|
| 6. | Cholesterol and copper sulfate model |
|
|
|
|
| 7. | Colchicine model of AD. |
|
|
|
|
| 8. | Immunotoxin 192IgG-saporin model |
|
|
|
|
| 9. | Lysophosphatidic acid induced AD |
|
|
|
|
| 10. |
|
|
|
|
|
| 11. | Methionine |
|
|
|
|
| 12. | Okadaic (OKA) acid |
|
|
|
|
| 13. | (Excito)toxin |
|
|
|
|
| 14. | Clonidine-induced memory deficits |
|
|
|
|
| 15. | Clozapine-induced memory deficits |
|
|
|
|
| 16. | Lignocaine-induced memory deficits |
|
|
|
|
| 17. | Cycloheximide-induced memory deficits |
|
|
|
|
| 18. | Phenytoin induced memory deficits |
|
|
|
|
| 19. |
|
|
|
|
|
| 20 | Dizocilpine (MK-801) AD |
|
|
|
|
| 21. | Diazepam |
|
|
|
Animal models
The following models are a few commonly used models and have been proven to offer better efficacy in the development of new therapeutics for the development of AD-like models.
1. Streptozotocin-induced model of AD: Streptozotocin (STZ) is a glucosamine nitrosourea compound that belongs to the alkylating agents of anti-cancer drugs. This is the most widely employed model for induction of AD as it very closely resembles human sporadic AD. STZ at a sub-diabetogenic dose of 3mg/kg in two divided doses viz., i.c.v. route produces memory dysfunction like AD [7], [8], [9]. STZ infusion leads to enhanced oxidative and nitrosative stress, brain atrophy, neuronal loss, neuroinflammation, along with Aβ accumulation and tau hyperphosphorylation. Impaired synthesis of acetyl coenzyme A, ATP and creatine phosphate were observed. Increased activity of the acetylcholinesterase (AChE) enzyme along with apoptosis was reported with STZ administration. [6]
Advantages
It is a reliable model for the studies on Alzheimer's disease.
It replicates the major pathological hallmarks such as the expression of amyloid plaques, neurofibrillary tangles, elevated oxidative stress, neuroinflammation.
It resembles the pathology of human sporadic AD.
It is a valid model for studying the pathology of AD.
Limitations of the model
This is an invasive model that requires large number of animals because of its high mortality rate.
2. Amyloid-β model of Alzheimer's disease: Amyloid-β plaques are the major hallmarks of this disease. Administration of the Aβ peptide produces cognitive dysfunction, followed by neurodegeneration. Intracerebroventricular administration of Aβ into the 3rd ventricle for 14days leads to the accumulation of amyloid proteins and plaques in different regions of the brain [11], [12], [13], [14], particularly in regions of memory such as the hippocampus and cortex, followed by the amygdala and striatum [10]. This model produces behavioural changes apart from the alterations in biochemicals and neurochemicals, so it is proved to be more suitable for induction of AD [11], [12], [13], [14].
Advantages
It replicates the pathological hallmarks such as the amyloid plaques, neurofibrillary tangles, and neurodegeneration.
Behavioral alterations can be evaluated.
This is a specific model for screening of AD drugs.
Limitations of the model
Potential confounding effects of the invasive intracerebral injection.
3. Scopolamine induced AD model: The cholinergic system plays a vital role in the pathogenesis of AD. Reduced cholinergic activity leads to cognitive deficits [15]. Scopolamine, a known anticholinergic agent, blocks the cholinergic muscarinic receptor and leads to over release of acetylcholine which eventually damages the hippocampus, cortex, and nucleus basalis. Scopolamine can be administered via both i.p and i.c.v. routes. This drug disrupts the cholinergic tracts in areas responsible for cognition and memory [16], [17], [18].
Advantages
Provides ease of scopolamine administration.
Cholinergic deficits can be studied broadly using this model.
Limitations of the model
The model fails to replicate the pathological hallmarks of the AD.
4. (Ethyl choline mustard aziridinium ion) AF64A-induced AD model: AF64A is a neurotoxic product derived from choline. This chemical causes long-term damage to cholinergic neurons. Intracerebroventricular administration of AF64A produces cholinergic deficiency by altering acetylcholine levels and choline acetyltransferase activities in the cerebral cortex, hippocampus, and striatum [19,20], eventually leading to behavioural impairment along with neurochemical and biochemical changes.
Advantages
The model is advantageous to study the cognitive deficits involved in learning and memory.
Limitations of the model
Intracerebral injections could be difficult and may cause trauma in some animals.
The model fails to reproduce the hallmarks of the disease.
5. Alcohol-induced memory deficits: Increased and elevated doses of ethanol have been known to cause memory deficits by affecting the encoding, storage, and consolidation [25]. Ethanol is known to impair hippocampal dependent learning and memory, eventually leading to the cholinergic dysfunction [25]. Intraperitoneal administration of ethanol dose dependently (0.25, 0.5, 1g/kg) impaired memory retention in rodent models [21], [22], [23], [24],26].
Advantages
Does not require any invasive process for the induction of memory deficits.
Memory enhancing agents and the nootropic activity of the drugs can be evaluated.
It exhibits behavioural alterations similar to AD.
Cognitive deficits can be explored.
Limitations of the model
The slow and time-consuming process.
The pathological hallmarks of AD were not replicated in this model.
6. Cholesterol and copper sulfate model: Administration of cholesterol supplemented with copper sulfate in distilled water pathologically revealed the presence of senile plaques. The efflux of excess Aβ caused by cholesterol from the brain is attenuated by copper ions, which is the most likely mechanism for copper induced Aβ aggregation [27,28]. Advantages
Easy reliable and reproducible model.
The model is beneficial for exploring the fibrillar Aβ pathology.
Limitations
The pathological hallmarks such as the presence of neurofibrillary tangles, neurodegeneration, were not replicated.
7. Colchicine model of AD: Colchicine is being used in the treatment of inflammatory disorders such as gout for decades. Colchicine causes neuronal cell death by binding to tubulin, the main structural protein of the microtubule, and causing microtubular depolymerization and destabilization. This is followed by the blockage of axonal transit and mitosis [29].
Administration of colchicine (i.c.v), a causes significant cognitive dysfunction in rodents [29], by disrupting the cholinergic tracts. It also causes changes in the other monoaminergic neurotransmitters such as depletion in dopamine, serotonin and nor-epinephrine in the hippocampus, caudate nucleus, and cerebral cortex [30,31]. The neurotoxicity is mediated through oxidative stress, and excitotoxicity via NMDA activation [29], [30], [31].
Advantages
Replicates the behavioral, biochemical, and neurochemical alterations which are the main features of sporadic dementia of Alzheimer's type (SDAT).
Possible to screen nootropic agents and other cholinesterase inhibitors for memory.
NMDA activity and excitotoxicity can be explored.
Limitations of the model
The model may require large number of animals due to high mortality rate.
Adverse effects associated with the colchicine such as (myoclonic twitches, aggressive behaviour, acoustic startle behaviour and reduced threshold to pain) may affect/ alter the study and may lead to more suffering of the animals, so ethical considerations are the key issues in this model.
8. Immunotoxin Ig-saporin model: Neurons of the septum and diagonal band of Broca are the major source of cholinergic innervation in the hippocampus. Hippocampus is most effected region of the brain in AD, which is majorly associated with the learning and memory. Intracerebroventricular administration of 192IgG-saporin (Ig-saporin), induces degeneration of neurons and results in cholinergic deficits in various regions of the hippocampus [32,33]. This immunotoxin alters behavioral patterns in different behavioural tasks and impairs memory retention in animal models [34].
Advantages
Behavioural and electrophysiological consequences of degeneration of cholinergic neurons can be evaluated.
Insights into hippocampal alterations are possible with this model.
Neurotoxicity and underlying mechanisms can be explored.
Cholinergic deficits can be broadly evaluated.
Limitations
Invasive route of administration.
All pathological hallmarks were not expressed in this model.
9. Lysophosphatidic acid model: Growth cone collapse and neurite retraction are two effects of the bioactive phospholipid lysophosphatidic acid on neuronal cells [35,36]. Tau and other microtubule-associated proteins (MAPs) have higher levels of phosphorylation rate as a result of alterations caused by LPA in tubulin pools. Glycogen synthase kinase-3 is activated in response to LPA, which causes tau hyperphosphorylation. It happens in a variety of neuronal cells from many species in conjunction with the neurite retraction process [36]. It has been shown that LPA-induced neurite retraction in differentiated SY-SH5Y human neuroblastoma cells increases site-specific tau phosphorylation similar to that seen in Alzheimer's disease.
Advantages
Tau phosphorylation, tauopathies and the related mechanisms could be explored.
Limitations
Other pathological hallmarks were not expressed.
Limited to cell cultures.
10. AlCl3-induced AD model: Aluminium (Al) acts as a neurotoxin and cholino toxin, Al can gain easy access into the body and the chronic exposure induces oxidative stress and damages the CA fields of hippocampus, apart from the structural modifications of the nicotinic receptors leading to memory loss [37,42]. It induces oxidative stress, alters the blood-brain barrier, leading to the apoptotic death of hippocampal neurons. It also promotes the expression of the amyloid [41], tau [38] proteins and alters the acetylcholine and other monoamine levels [40]. In previous studies, it has also been shown to alter the behavioural aspects of rodents. Finally, leading to the expression of all the pathological hallmarks of Alzheimer's disease [37], [38], [39], [40], [41], [42].
Advantages
All the pathological hallmarks were replicated in this model.
Ease of aluminium administration.
This is an old, reliable, and reproducible model for the study of AD.
Less mortality rates.
Limitations
The amyloid plaques expressed in this model were pathologically different from the senile plaques of human AD.
11. Methionine model of AD: Treatment with L-methionine dramatically increases serum homocysteine levels and elevated homocysteine levels have been linked to oxidative stress and changes in the structure and function of cerebral blood vessels, both of which are important contributors to cerebral vascular dysfunction. It is known that oxidative stress and vascular dysfunction play a significant role in the pathology of AD and vascular dementias [43,44]. Memory acquisition and retrieval are significantly impaired in AD due to structural abnormalities in the cerebral capillaries, which also cause neuronal malfunction and death [44] Additionally, it has been demonstrated that hyperhomocysteinemia is neurotoxic [43]. This neurotoxicity is due to an excess of N-methyl-D-aspartate receptors, elevated oxidative stress, increased cholinesterase activity, tau phosphorylation and the toxicity caused by amyloid-β peptides [44].
Advantages
Beneficial for studying the vascular dementia.
Easy and safer route of administration of methionine.
Useful for screening of drugs with nootropic activity.
Limitations
Does not replicate the pathological hallmarks of AD.
12. Okadaic acid (OKA) model: OKA is a potent protein phosphate inhibitor (PPI/2A). Administration of OKA via the intracerebroventricular route for 14 days leads to cognitive dysfunction and significant pathological alterations and elevated oxidative stress. It causes the hyperphosphorylation of tau by expressing neurofibrillary tangles and also decreases the phospho-GSK3β [45,46].
Advantages
Beneficial for screening of dementia drugs.
All the pathological hallmarks were expressed.
Limitations
Administration of OKA could be difficult due to the invasive procedure, so there are chances of high mortality.
13. Toxin model of AD: Ibotenic acid is an excitotoxin and has been shown to cause damage to the nucleus basalis magnocellularis (NBM). Based on the finding that AD patients exhibit degenerative changes in the nucleus basalis of Mynert (nbM), the human counterpart of the NBM, lesions of the NBM have been proposed as an experimental model for the disease. Additionally, similar to what has been observed in AD patients, the NBM lesioned animal exhibits decrease in cholinergic markers, such as acetylcholine levels, release and turnover of acetylcholine, choline uptake, choline acetyl transferase and acetylcholinesterase activity, and number of muscarinic cholinergic receptors, in the frontal cortex [47,48].
Advantages
The cholinergic scenario in patients with AD matches with the pathology in rodent models hence, it is considered as a valid model for studying memory deficits in AD.
Limitations of the model
High mortality rate and invasive procedure.
14. Clonidine -induced memory deficits: Clonidine, a presynaptic adrenergic agonist, has become well established as a useful and relatively safe therapeutic agent. Clonidine has been described to inhibit the bioelectrical activity of noradrenergic neurons of the locus coeruleus, via activation of α2 adrenoceptors. clonidine also induces synchronization in cortical EEG pattern in rodents, cats, and rabbits [51]. Clonidine at doses of 0.1–1 mg/kg increases the firing of 5-HT cells in the dorsal raphe nucleus, facilitating the adrenergic influence on the 5-HT neurons, which suggest clonidine induced serotonergic transmission underlies the amnesic effect of the drug [50].
Advantages
The underlying mechanisms and the influence of monoamines can be evaluated.
Behavioural effects can be studied using this model.
Cognitive deficits can be evaluated.
Limitations
The model does not replicate the AD hallmarks.
The model is non-reliable.
The underlying mechanisms cause the depressive effects of clonidine such as inhibition of locomotor and exploratory activities, suppression of avoidance behaviour and suppression of self-stimulation in laboratory animals.
15. Lignocaine-induced memory deficits: Lignocaine, a local anaesthetic commonly employed for the arrythmias is known to block the voltage-gated sodium channels. Lidocaine injected bilaterally into the rodent brain, disrupted spatial information consolidation [54] and short-term and long-term memory [52] by disrupting the CA1 pyramidal cell function [52,53]. It is also known to impair memory processes by disrupting granule cell output functions [51].
Advantages
Long-term and short-term memory dysfunctions can be explored using this model.
Limitations
The model does not replicate the pathological hallmarks and not reliable AD model.
The route of administration is invasive, so there are chances of mortality.
The drug is known to have potential adverse effects such as drowsiness, mental clouding, dysphoria, muscle twitching, fall in blood pressure.
16. Clozapine-induced memory deficits: Behavioral changes are common in dementia, particularly in its later stages. Agitation, hostility, paranoid delusions, hallucinations, sleep disturbances, including nocturnal wandering, incontinence, and (stereotypical) vocalizations are the most prevalent disorders. These symptoms are often overlooked and treated with anti-psychotics, such as clozapine. Experimental evidence indicated working memory and attention impairment in rodents. Even though, the underlying mechanism appears to be unknown. However, the studies indicated that loss of nicotinic cholinergic β2 receptors are associated with the cognitive deficits [55,56].
Advantages
The underlying mechanisms and the influence of cholinergic system can be evaluated.
Behavioural effects can be studied using this model.
Only cognitive deficits can be evaluated.
Limitations
The model does not replicate the pathological hallmarks and not reliable AD model.
The underlying mechanisms cause the other adverse effects associated with the use of clozapine such as excessive drowsiness, vision problems, voluntary movement changes and the blood parameters have to be closely monitored even low doses are administered.
17. Cycloheximide-induced memory deficits: Cycloheximide, a potent protein synthesis inhibitor that interferes with translocation, and impairs development of long-term memory [57]. It is known to induce memory consolidation deficits majorly via disturbances in the cholinergic and GABAergic systems and increased serotonergic activity [57,58]. Administration of cycloheximide in rodents significantly impaired memory consolidation in the behavioural tasks [58].
Advantages
This model is beneficial to study the cognitive deficits associated, and the role of various neurotransmitters in memory deficits.
Ease of route of administration.
Limitations
The model is better suited to study only cognitive deficits but not for studying the pathology of AD.
18. Phenytoin induced memory deficits: Phenytoin is a commonly used anti-epileptic drug, effective against all types of partial and tonic–clonic seizures. The treatment with this drug is known to be associated with cognitive decline [59] along with decrease in blood folic acid levels [60]. Administration of phenytoin to rodents caused alterations in the levels of neurotransmitters such as 5-HT along with changes in the cholinesterase activity [61].
Advantages
This model is beneficial to explore the cognitive deficits associated with dementia and epilepsy.
This model is beneficial for screening memory enhancing agents.
Limitations
This is a non-reliable model for exploring the pathology of AD.
19. D-Galactose induced cognitive impairment: D-galactose (D-gal) is a natural reducing sugar that is mainly derived from lactose in the milk. Studies have showed that an excess of D-gal leads to abnormal metabolism due to the reduction in Na+, K+, ATPase activity, excessive oxidative stress due to enhanced lipid peroxidation and downregulation of the superoxide dismutase activity [66], neuronal damage due to advanced glycation [62] and neuroinflammation [63], [64], [65] and increased senile plaques formation [66], tau phosphorylation [68] leading to cognitive impairment in rodents [67]. It is known to cause alteration in hippocampal gene expressions [64].
Advantages
Chronic D-gal administration has been the widely used animal model to investigate aging brain and AD pathology.
All the pathological hallmarks were observed in this model.
Safe and reliable model.
Limitations
Insulin resistance has been observed after D-galactose treatment, that could lead to diabetes.
20. (MK-801) Dizocilpine-induced model of AD: MK-801 is an NMDA antagonist with anaesthetic and anticonvulsant properties. This drug induces oxidative stress in the prefrontal cortex. MK-801 administration leads to neurodegeneration in areas responsible for cognition, hence memory deficits [69,70]. It inhibits caspase activation [71] and blockade of glutamate leads to elevated Ca2+. This elevation of calcium leads to neurotoxicity [72], due to activation of proteases, lipases, and nitric oxide synthases, thereby increasing free radical formation. It also increases glucose metabolism and induces heat shock protein and necrotic cell death in neurons [70,71]. Glutamate transmission has a significant role in behavioural systems such as motor activity, learning, and cognition [73,74]. MK-801 also alters dopamine levels in the prefrontal cortex, resulting in motor dysfunction [73].
Advantages
Cognitive deficits can be studied using this model.
Behavioural evaluation and screening of drugs for cognitive dysfunction can be studied.
Limitations
Animals exhibit schizophrenia-like behaviour.
This animal model failed to replicate the pathological hallmarks of AD.
21. Diazepam model of cognitive dysfunction: Diazepam belongs to the class of sedative and hypnotics. According to the research, benzodiazepines can cause amnesia by inhibiting long-term potentiation, a neuronal process that underlies memory and learning. Memory loss has been linked to the use of certain benzodiazepines. It has been demonstrated that benzodiazepine receptor agonists like diazepam (0.5 to 3 mg/kg i.p.) given 30 minutes before acquisition trials cause anterograde amnesia [15,75]. In addition, other benzodiazepines such as the lorazepam (0.06-0.5mg/kg), alprazolam (0.5-0.75 mg/kg), triazolam (0.05- 0.3 mg/kg) have been reported to produce anterograde and retrograde amnesia in rodent models [76,77].
Advantages
Cognitive deficits can be studied using this model and memory enhancing agents can be screened.
Limitations
This animal model failed to replicate the pathological hallmarks of AD.
Diazepam could cause CNS depression.
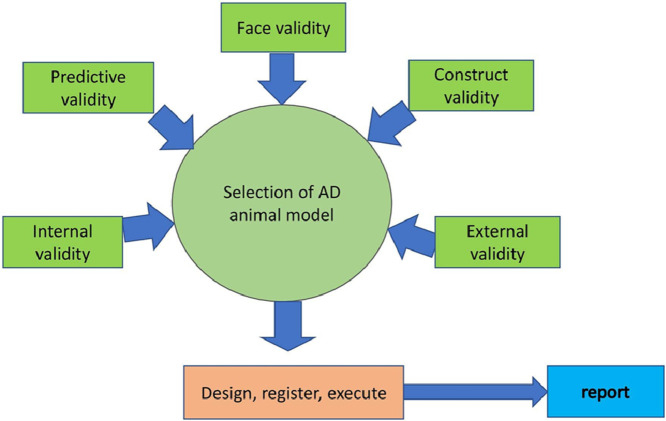
Many animal models are available for Alzheimer's disease. Each model has its own benefits and limitations. But it is important to note that no animal model completely replicates the whole pathology of human AD. However, the currently available animal models are useful in understanding and solving the key problems of AD neurobiology. Some key points must be considered while selecting an animal model, such as the expression of major hallmarks, i.e., amyloid plaques and neurofibrillary tangles.
Construct validity: It means how faithfully the disease pathogenesis is being presented in the selected animal species.
Face validity: Face validity is attained when there is a phenomenological resemblance between the model and the clinical condition. The model bears a resemblance to the condition or specific aspects.
Predictive validity: The predictive validity of an animal model is achieved if the expectations produced by the model can be authenticated in the clinical condition being modelled. They can also be used to find and study drugs that precipitate or aggravate a clinical condition. Predictive models are frequently used to screen new therapeutic targets.
Validation of animal models: Validate the model thoroughly and scientifically, such as assessment of housing and rearing conditions, age, and finally validate the model across different rodent strains.
Choice of animal species: The species differences among rodents (including a variant with more genetic similarity to humans) and the sex differences (females may have oestrogen protective action) are to be considered.
Inclusion of positive control: Many disease models include positive controls in the study. Exceptionally, in Alzheimer's disease, it is not possible. As the pathogenesis is multifactorial, the drugs currently being used for AD are cholinesterase inhibitors and memantine, which are useful only for treating the symptoms of Alzheimer's disease. AD pathogenesis includes tau hyperphosphorylation, amyloid deposition, increased oxidative stress and neuroinflammation, and alterations in various neurotransmitters along with memory impairment. So, it would not be possible for the currently available AD drugs to act as a standard drug/positive control.
The expression of various core markers/factors such as oxidative stress markers, neuroinflammatory markers, and changes in neurotransmitter levels should be considered. It is hard to decipher the other downstream pathological targets in detail. But the expression of Amyloid Precursor Protein (APP), Presenilin 1, and other cleaved by-products of an APP like AICD may also exert toxicity.
The most important point to be considered is cognitive dysfunction. Most models show memory impairment to some extent, but the timing and the type (stress-related/ age-related) must be cautiously considered in animal studies. For example, memory impairment arises at a different stage of pathogenesis in transgenic rodent models in contrast to humans. Memory impairment occurring at or before the onset of plaque development in rodents should be noted.
Behavioural assessment: Cognitive testing in humans includes learning, speech, social cognition, perception, short-term and long-term memory, and these are determined according to a series of tests like the Cambridge Neuropsychological Test Automated Battery (CANTAB) [78]. However, the distinct cognitive damage is mainly dependent on the disease stage and existing comorbidities. In animal models, this differentiation in disease severity is not reflected and mostly single outcome measures are used. Moreover, the cognitive validation of maze tests is still a topic of dispute.
Poor internal validity: The results of the study might also be influenced by variations in how the test is carried out. For example, in the behavioural assessment by Morris water maze test, differences in the size of the pool, water temperature and days of training, which are being used most frequently.
Animal models have their apparent advantages, such as testing for in vivo toxicity studies for new therapeutics, cognitive and behavioural testing, and many more. Selection and validation of the model is the core factor to be considered for any pre-clinical study to get better output.
Poor study design: Animal studies are to be designed, executed, and reported properly using PREPARE, ARRIVE, guidelines. The compliance must be verified by the head/chief.
3.Conclusion
AD remains as a disease with a complex pathogenesis having multiple aetiologies, so designing therapeutic strategies for a disease having potential cross-talks remains difficult. Animal models of AD continue to play an important role in pre-clinical research, mainly for the identification of new therapeutics. Drug development for Alzheimer's disease has generally failed, with a failure rate of more than 99%. These failures were caused, in part, by the fact that data acquired from animal models were not being translated to the clinical level. Given this circumstance, it has been questioned whether using animal models to study AD is even worthwhile, particularly when evaluating the effectiveness of novel therapeutics. Low internal validity has primarily been the main reason for poor translation of animal model. Animal studies are frequently poorly designed, underpowered, and poorly standardized (e.g., non-randomized, non-blinded) and it is not possible to replicate all pathological events in a single animal model.
This article presents selection an animal model for AD that is appropriate, such as one that has been used previously or is often utilized, may not be the best choice. We have tried to cover the regimen for induction of AD and major guidelines to be noted for the selection of the animal model. We presumed complete face validity of the animal models, i.e. are the same symptoms present in the model and the human disease, since we only included studies where memory deficits was observed and evaluated. Based on this, the most commonly employed chemically induced models were STZ and β-amyloid models, while d-galactose, scopolamine, aluminium-induced models are being used due to their non-invasive nature, reproducibility, and compatibility. Of the many accessible animal models, transgenic models have proven useful in studying key disease mechanisms implicated in AD. It is important to adjust and revise the models to represent clinical conditions. Yet, regardless of many constraints, animal models have provided valuable knowledge concerning the pathogenesis of AD.
Ethics statements
Not Applicable.
CRediT authorship contribution statement
Deepthi Rapaka: Conceptualization, Methodology, Writing – original draft. Paul C. Adiukwu: Visualization, Supervision. Veera Raghavulu Bitra: Writing – review & editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Authors declare no conflict of interest.
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Twitter A/C: Not available.
Data availability
No data was used for the research described in the article.
References
- 1.Alzheimer's Association. Alzheimer's disease facts and figures, Alzheimer's Dement. 2021. doi: 10.1002/alz.12328. [DOI] [PubMed]
- 2.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurol. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Rapaka D., Bitra V.R., Challa S.R., Adiukwu P.C. Potentiation of microglial endocannabinoid signaling alleviates neuroinflammation in Alzheimer's disease. Neuropeptides. 2021;90 doi: 10.1016/j.npep.2021.102196. [DOI] [PubMed] [Google Scholar]
- 4.Anand A., Patience A.A., Sharma N., Khurana N. The present and future of pharmacotherapy of Alzheimer's disease: a comprehensive review. Euro. J. Pharmacol. 2017;815:364–375. doi: 10.1016/j.ejphar.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 5.VeeningGriffioen D.H., Ferreira G.S., VanMeer P.J.K., Boon W.P.C., Wied C.C.Gispen de, Moors E.H.M., Schellekens H. Are some animal models more equal than others? A case study on the translational value of animal models of efficacy for Alzheimer's disease. Eur. J. Pharmacol. 2019;859 doi: 10.1016/j.ejphar.2019.172524. [DOI] [PubMed] [Google Scholar]
- 6.Lannert H., Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 7.Noor N.A., Hosny E.N., Khadrawy Y.A., Mourad I.M, Othman A.I, Aboul Ezz H.S., Mohammed H.S. Effect of curcumin nanoparticles on streptozotocin-induced male Wistar rat model of Alzheimer's disease. Metab. Brain Dis. 2022;37:343–357. doi: 10.1007/s11011-021-00897-z. [DOI] [PubMed] [Google Scholar]
- 8.Yeo H.G., Lee Y., Jeon C.Y., Jeong K.J., Jin Y.B., Kang P., Kim S.U., Kim J.S., Huh J.W., Kim Y., Sim B.W., Song B.S., Park Y.H., Hong Y., Lee S.R., Chang K.T. Characterization of cerebral damage in a Monkey Model of Alzheimer's disease induced by intracerebroventricular injection of streptozotocin. J. Alzheimers Dis. 2015;46:989–1005. doi: 10.3233/JAD-143222. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.M., Qu Z.Q., Wu J.L., Chung P., Zeng Y.S. Mitochondrial protective and anti-apoptotic effects of Rhodiola crenulata extract on hippocampal neurons in a rat model of Alzheimer's disease. Neural. Regen. Res. 2017;12:2025–2034. doi: 10.4103/1673-5374.221160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Q.H., Lou F.L., Hou W.X., Liu M., Guo H., Zhang X.M. Acetyl puerarin reduces inflammation and improves memory function in a rat model of Alzheimer's disease induced by Abeta1-42. Pharmazie. 2013;68:904–908. [PubMed] [Google Scholar]
- 11.Nakamura S., Murayama N., Noshita T., Annoura H., Ohno T. Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide. Brain Res. 2001;912 doi: 10.1016/s00068993(01)02704-4. [DOI] [PubMed] [Google Scholar]
- 12.Geula C., Wu C.K., Saroff D., Lorenzo A., Yuan M., Yankner B.A. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat. Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 13.X. Wang, X. Zhou, B. Uberseder, J. Lee, C.S. Latimer, C.M. Furdui, C.D. Keene, T.J. Montine, T.C. Register, S. Craft, C.A. Shively, T. Ma, Isoform-specific dysregulation of AMP-activated protein kinase signaling in a non-human primate model of Alzheimer's disease. Neurobiol. Dis., 158 (2021) 105463. doi: 10.1016/j.nbd.2021.105463. [DOI] [PMC free article] [PubMed]
- 14.Hruska Z., Dohanich G.P. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1-42) and ibotenic acid. Horm. Behav. 2007;52 doi: 10.1016/j.yhbeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Mani V., Ramasamy K., Ahmad A., Parle M., Shah S.A., Majeed A.B. Protective effects of total alkaloidal extract from Murraya koenigii leaves on experimentally induced dementia. Food Chem. Toxicol. 2012;50:1036–1044. doi: 10.1016/j.fct.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 16.A.H, Abu Almaaty, R.M. Mosaad, M.K, Hassan, E. Ali, G.A. Mahmoud, H. Ahmed, N. Anber, S. Alkahtani, M.M. Abdel-Daim, L. Aleya, S. Hammad, S. Urtica dioica extracts abolish scopolamine-induced neuropathies in rats. Environ. Sci. Pollut. Res. Int., 28 (2021) 18134–18145. doi: 10.1007/s11356-020-12025-y. [DOI] [PubMed]
- 17.Khurana K., Kumar M., Bansal N. Lacidipine prevents scopolamine-induced memory impairment by reducing brain oxido-nitrosative stress in mice. Neurotox. Res. 2021;39:1087–1102. doi: 10.1007/s12640-021-00346-w. [DOI] [PubMed] [Google Scholar]
- 18.Riedel G., Kang S.H., Choi D.Y., Platt B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav. Brain Res. 2009;2014:217–225. doi: 10.1016/j.bbr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Fisher A., Hanin I. Potential animal models for senile dementia of Alzheimer's type, with emphasis on AF64A-induced cholinotoxicity. Annu. Rev. Pharmacol. Toxicol. 1986;26:161–181. doi: 10.1146/annurev.pa.26.040186.001113. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K., Furukawa S., Iwasaki T., Ichitani Y. Nicotine improves AF64A-induced spatial memory deficits in Morris water maze in rats. Neurosci. Lett. 2010;469(1):88–92. doi: 10.1016/j.neulet.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi Nosrat Abadi T., Vaghef L., S.Babri M.Mahmood-Alilo, Beirami M. Effects of different exercise protocols on ethanol-induced spatial memory impairment in adult male rats. Alcohol. 2013;47(4):309–316. doi: 10.1016/j.alcohol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Bai S., Wang W., Zhang Z., Li M., Chen Z., Wang J., Zhao Y., An L., Wang Y., Xing S., Fu X., Ma J. Ethanol alleviates amyloid-β-induced toxicity in an alzheimer's disease model of Caenorhabiditis elegans. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.762659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaghef L., Farajdokht F., Erfani M., Majdi A., Sadigh-Eteghad S., Karimi P., Sandoghchian Shotorbani S., Seyedi Vafaee M., Mahmoudi J. Cerebrolysin attenuates ethanol-induced spatial memory impairments through inhibition of hippocampal oxidative stress and apoptotic cell death in rats. Alcohol. 2019;79:127–135. doi: 10.1016/j.alcohol.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Baird T.J., Vanecek S.A., Briscoe R.J., M.Vallett K.L.Carl, Gauvin D.V. Moderate, long-term alcohol consumption potentiates normal, age-related spatial memory deficits in rats. Alcohol Clin. Exp. Res. 1998;22:628–636. doi: 10.1111/j.1530-0277.1998.tb04304.x. [DOI] [PubMed] [Google Scholar]
- 25.M.J. Spinetta, M. T. Woodlee, L.M. Feinberg, C. Stroud, K. Schallert, L.K. Cormack, T. Schallert, Alcohol-induced retrograde memory impairment in rats: prevention by caffeine. Psychopharmacology 201(3), 361–371. doi: 10.1007/s00213-008-1294-5. [DOI] [PubMed]
- 26.Rezayof A., Alijanpour S., Zarrindast M.R., Rassouli Y. Ethanol state-dependent memory: involvement of dorsal hippocampal muscarinic and nicotinic receptors. Neurobiol. Learn. Mem. 2008;89(4):441–447. doi: 10.1016/j.nlm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.D.L.Sparks Cholesterol. Copper, and accumulation of thioflavine S-Reactive Alzheimer's like amyloid-β in rabbit brain. J. Mol. Neurosci. 2004;24:97–104. doi: 10.1385/jmn:24:1:097. [DOI] [PubMed] [Google Scholar]
- 28.Sparks D.L., Schreurs B.G. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Seghal N., Naidu P., Padi S.S., Goyal R. Colchicines-induced neurotoxicity as an animal model of sporadic dementia of Alzheimer's type. Pharmacol Rep. 2007;59(3):274–283. [PubMed] [Google Scholar]
- 30.Ganguly R., Guha D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer's disease & protection by Moringa oleifera. Indian J. Med. Res. 2008;128:744–751. [PubMed] [Google Scholar]
- 31.Sil S., Ghosh T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer's Disease. J. Neuroimmunol. 2016;15:115–124. doi: 10.1016/j.jneuroim.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T.J., Herzog C.D., Gandhi C., Stackman R.W., Wiley R.G. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res. 1996;726:69–79. [PubMed] [Google Scholar]
- 33.Dobryakova Y.V., Kasianov A., Zaichenko M.I., Stepanichev M.Y., Chesnokova E.A., Kolosov P.M., Markevich V.A., Bolshakov A.P. Intracerebroventricular administration of 192IgG-saporin alters expression of microglia-associated genes in the dorsal but not ventral hippocampus. Front. Mol. Neurosci. 2018;10:429. doi: 10.3389/fnmol.2017.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T.J.Walsh R.M.Kelly, Dougherty K.D., Stackman R.W., Wiley R.G., Kutscher C.L. Behavioural and neurobiological alterations induced by the immunotoxin 192-IgG-saporin: cholinergic and non-cholinergic effects following i.c.v. injection. Brain Res. 1995;702(1-2):233–245. doi: 10.1016/0006-8993(95)01050-x. [DOI] [PubMed] [Google Scholar]
- 35.Shi Jing, Dong Yunzhou, Cui Mei-Zhen, Xu Xuemin. Lysophosphatidic acid induces increased BACE1 expression and Aβ formation. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832(1):29–38. doi: 10.1016/j.bbadis.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayas C.L., Avila J., Wandosell F. Regulation of neuronal cytoskeleton by lysophosphatidic acid: role of GSK-3. Biochim. Biophys. Acta. 2002;1582:144–153. doi: 10.1016/s1388-1981(02)00149-x. [DOI] [PubMed] [Google Scholar]
- 37.Bitra V.R., Rapaka D., Mathala N., Akula A. Effect of wheatgrass powder on aluminium-induced Alzheimer's disease in Wistar rats. Asian Pac. J. Trop. Med. 2014;S1:S278–S281. doi: 10.1016/s1995-7645(14)60246-7. [DOI] [PubMed] [Google Scholar]
- 38.Rapaka D., Bitra V.R., Vishala T.C., Akula A. Vitis vinifera acts as anti-Alzheimer's agent by modulating biochemical parameters implicated in cognition and memory. J. Ayurveda Integr. Med. 2019;10:241–247. doi: 10.1016/j.jaim.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan K.A., Kumar N., Nayak P.G., Nampoothiri M., Shenoy R.R., Krishnadas N., Rao C.M., Mudgal J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol. 2013;65:1745–1752. doi: 10.1111/jphp.12126. [DOI] [PubMed] [Google Scholar]
- 40.Shuchang H., Qiao N., Piye N., Mingwei H., Xiaoshu S., Feng S., Sheng W., Opler M. Protective effects of gastrodia elata on aluminium-chloride-induced learning impairments and alterations of amino acid neurotransmitter release in adult rats. Restor. Neurol. Neurosci. 2008;26:467–473. [PMC free article] [PubMed] [Google Scholar]
- 41.Rapaka D., Bitra V.R., Ummidi R., Akula A. Benincasa hispida alleviates amyloid pathology by inhibition of Keap1/Nrf2-axis: emphasis on oxidative and inflammatory stress involved in Alzheimer's disease model. Neuropeptides. 2021;88 doi: 10.1016/j.npep.2021.102151. [DOI] [PubMed] [Google Scholar]
- 42.Gulya K., Rakonczay Z., Kása P. P. Cholinotoxic effects of aluminium in rat brain. J. Neurochem. 1990;54(3):1020–1026. doi: 10.1111/j.1471-4159.1990.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 43.Sain H., Sharma B., Jaggi A.S., Singh N. Pharmacological investigations on potential of peroxisome proliferator-activated receptor-gamma agonists in hyperhomocysteinemia-induced vascular dementia in rats. Neuroscience. 2011;192:322–333. doi: 10.1016/j.neuroscience.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Khodir S.A., Faried M.A., Abd-Elhafiz H.I., Sweed E.M. Sitagliptin attenuates the cognitive deficits in L-methionine-induced vascular dementia in rats. Biomed. Res. Int. 2022 doi: 10.1155/2022/7222590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X.Y., Hu J.F., Chu S.F., Zhang Z., Xu S., Yuan Y.H., Han N., Liu Y., Niu F., He X., Chen N.H. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3β/tau signaling pathway and the Aβ formation prevention in rats. Eur. J. Pharmacol. 2013;710(1-3):29–38. doi: 10.1016/j.ejphar.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z., Simpkins J.W. An okadaic acid-induced model of tauopathy and cognitive deficiency. Brain Res. 2010;1359:233–246. doi: 10.1016/j.brainres.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Li P., Wang Y., Liu J., Zhang Z., Cheng W., Wang Y. Ameliorative effects of a combination of baicalin, jasminoidin and cholic acid on ibotenic acid-induced dementia model in rats. PLoS One. 2013;8(2):e56658. doi: 10.1371/journal.pone.0056658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He L., Deng Y., Gao J., Zeng L., Gong Q. Icariside II ameliorates ibotenic acid-induced cognitive impairment and apoptotic response via modulation of MAPK pathway in rats. Phytomedicine. 2018;41:74–81. doi: 10.1016/j.phymed.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Genkova-Papazova M., Petkova B.P., Lazarova-Bakarova M., Boyanova E., Staneva-Stoytcheva D. Effects of flunarizine and nitrendipine on electroconvulsive shock and clonidineinduced amnesia. Pharmacol. Biochem. Behav. 1997;56:583–587. doi: 10.1016/s0091-3057(96)00406-6. [DOI] [PubMed] [Google Scholar]
- 50.Fein, G., Merrin, E. L., Davenport, L., & Buffum, J. C. (1987). Memory deficits associated with clonidine. Gen. Hosp. Psychiatry, 9 (1987) 154–155. doi: 10.1016/0163-8343(87)90029-6. [DOI] [PubMed]
- 51.Dyr W., Kostowski W., Zacharski B., Bidzinski A. Differential clonidine effects on EEG following lesions of the dorsal and median raphe nuclei in rats. Pharmacol. Biochem. Behav. 1983;19:177–185. doi: 10.1016/0091-3057(83)90036-9. [DOI] [PubMed] [Google Scholar]
- 52.Holahan M.R., Routtenberg A. Lidocaine injections targeting CA3 hippocampus impair long-term spatial memory and prevent learning-induced mossy fiber remodeling. Hippocampus. 2011;21:532–540. doi: 10.1002/hipo.20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pérez-Ruiz C., Prado-Alcalá R.A. Retrograde amnesia induced by lidocaine injection into the striatum: protective effect of the negative reinforcer. Brain Res. Bull. 1989;22(4):599–603. doi: 10.1016/0361-9230(89)90076-2. [DOI] [PubMed] [Google Scholar]
- 54.Cimadevilla J.M., López F., Nieto L., Aguirre M.J., Fernández R. Lidocaine, tetrodotoxin and their effect on consolidation of spatial memory. Psicothema. 2009;21(3):471–474. [PubMed] [Google Scholar]
- 55.Levin E.D., Perkins A., Brotherton T., M.Qazi C.Berez, Montalvo-Ortiz J., Davis K., Williams P., Christopher N.C. Chronic underactivity of medial frontal cortical beta2-containing nicotinic receptors increases clozapine-induced working memory impairment in female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(2):296–302. doi: 10.1016/j.pnpbp.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pocivavsek A., Icenogle L., Levin E.D. Ventral hippocampal alpha7 and alpha4beta2 nicotinic receptor blockade and clozapine effects on memory in female rats. Psychopharmacology. 2006;188:597–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinton E.E., Kramarcy N.R. Memory impairment correlates closely with cycloheximide dose and degree of inhibition of protein synthesis. Brain Res. 1977;131(1):184–190. doi: 10.1016/0006-8993(77)90041-5. [DOI] [PubMed] [Google Scholar]
- 58.Lu M.C., Hsieh M.T, Wu C.R., Cheng H.Y., Hsieh C.C., Lin Y.T., Peng W.H. ameliorating effect of emodin, a constitute of Polygonatum multiflorum, on cycloheximide-induced impairment of memory consolidation in rats. J. Ethnopharmacol. 2007;112(3):552–556. doi: 10.1016/j.jep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Reeta K.H., Mehla J., Gupta Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009;8(1301):52–60. doi: 10.1016/j.brainres.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Hernández R., Fernández M., Miranda G., Suástegui R. Disminución de ácido fólico y alteraciones cognitivas en pacientes con epilepsia tratados con fenitoína o carbamazepina, estudio piloto [Decrease of folic acid and cognitive alterations in patients with epilepsy treated with phenytoin or carbamazepine, pilot study] Rev. Invest. Clin. 2005;57:522–531. [PubMed] [Google Scholar]
- 61.Sudha S., Lakshmana M.K., Pradhan N. Chronic phenytoin induced impairment of learning and memory with associated changes in brain acetylcholine esterase activity and monoamine levels. Pharmacol. Biochem. Behav. 1995;52:119–124. doi: 10.1016/0091-3057(95)00059-6. [DOI] [PubMed] [Google Scholar]
- 62.Chowdhury A.A., Gawali N.B., Bulani V.D., Kothavade P.S., Mestry S.N., Deshpande P.S., Juvekar A.R. In vitro antiglycating effect and in vivo neuroprotective activity of Trigonelline in d-galactose induced cognitive impairment. Pharmacol. Rep. 2018;70(2):372–377. doi: 10.1016/j.pharep.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Zhong J., Wang Z., Xie Q., Li T., Chen K., Zhu T., Tang Q., Shen C., Zhu J. Shikonin ameliorates D-galactose-induced oxidative stress and cognitive impairment in mice via the MAPK and nuclear factor-κB signaling pathway. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106491. [DOI] [PubMed] [Google Scholar]
- 64.Wei H., Cai Y., Chu J., Li C., Li L. Temporal gene expression profile in hippocampus of mice treated with D-galactose. Cell. Mol. Neurobiol. 2008;28:781–794. doi: 10.1007/s10571-007-9177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong J., Wang F., Wang Z., Shen C., Zheng Y., Ma F., Zhu T., Chen L., Tang Q., Zhu J. Aloin attenuates cognitive impairment and inflammation induced by dgalactose via downregulating ERK, p38 and NFκB signaling pathway. Intern. Immunopharmacol. 2019;72:48–54. doi: 10.1016/j.intimp.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 66.Tsai S.J., Chiu C.P., Yang H.T., Yin M.C. s-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by D-galactose. J. Agric. Food Chem. 2011;59:6319–6326. doi: 10.1021/jf201160a. [DOI] [PubMed] [Google Scholar]
- 67.Kenawy S., Hegazy R., Hassan A., El-Shenawy S., Gomaa N., Zaki H., Attia A. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: protective role of metformin and saxagliptin. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong X.P., Chen T., Yin N.N., Han Y.M., Yuan F., Duan Y.J., Shen F., Zhang Y.H., Chen Z.B. Puerarin ameliorates D-galactose induced enhanced hippocampal neurogenesis and tau hyperphosphorylation in rat brain. J. Alzheimers Dis. 2016;51:605–617. doi: 10.3233/JAD-150566. [DOI] [PubMed] [Google Scholar]
- 69.Sadek B., Khan N., Darras F.H., Pockes S., Decker M. The dual-acting AChE inhibitor and H3 receptor antagonist UW-MD-72 reverses amnesia induced by scopolamine or dizocilpine in passive avoidance paradigm in rats. Physiol. Behav. 2016;15(165):383–391. doi: 10.1016/j.physbeh.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 70.Kovacic P., Somanathan R. Clinical physiology and mechanism of dizocilpine (MK-801): electron transfer, radicals. Oxid. Med. Cell Longev. 2010;3(1):13–22. doi: 10.4161/oxim.3.1.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alavez S., Blancas S., Moran J. Effect of N-methyl-D-aspartate receptor blockade on caspase activation and neuronal death in the developing rat cerebellum. Neurosci. Lett. 2006;404:176–181. doi: 10.1016/j.neulet.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 72.Rapaka D., Bitra V.R., Medapati J.R., Akula A. Calcium regulation and Alzheimer's disease. Asian Pac. J. Trop. Dis. 2014;4:S513–S518. [Google Scholar]
- 73.Tsukada H., Nishiyama S., Fukumoto D., Sato K., Kakiuchi T. E.F. Domino Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in prefrontal cortex of conscious monkeys. Neuropsychopharmacol. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
- 74.Sawahata M., Asano H., Nagai T., Ito N., Kohno T., Nabeshima T., Hattori M., Yamada K. Microinjection of Reelin into the mPFC prevents MK-801-induced recognition memory impairment in mice. Pharmacol. Res. 2021;173 doi: 10.1016/j.phrs.2021.105832. [DOI] [PubMed] [Google Scholar]
- 75.Dhingra D., Parle M., Kulkarni S.K. Memory enhancing activity of Glycyrrhiza glabra in mice. J. Ethnopharmacol. 2004;91 doi: 10.1016/j.jep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 76.Singh N., Sharma A., Singh M. Possible mechanism of alprazolam-induced amnesia in mice. Pharmacology. 1998;56:46–50. doi: 10.1159/000028181. [DOI] [PubMed] [Google Scholar]
- 77.Neha R.K.Sodhi, Jaggi A.S., Singh N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014;109(2):73–86. doi: 10.1016/j.lfs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Millan M., Agid Y., Brüne M., et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.