Abstract
The relationship of Helicobacter felis, a bacterium observed in the stomachs of cats, to gastric disease is unclear. The objective of this study was to determine if H. felis infection alters gastric histopathology, proinflammatory cytokine expression, and secretory function and evokes a humoral immune response in cats. Five specific-pathogen-free (SPF) Helicobacter-free cats were studied before and for 1 year after oral inoculation with H. felis (ATCC 49179). Four SPF H. felis-uninfected cats served as controls. The stomachs of all five H. felis-inoculated cats became colonized, as determined by urease activity, histopathology, PCR, culture, and transmission electron microscopy of serial gastric biopsies at 0, 3, 5, 8, and 12 months. Uninoculated cats remained Helicobacter free. Lymphoid follicular hyperplasia, atrophy, and fibrosis were observed primarily in the pylorus of infected cats. Mild mononuclear inflammation was detected in both infected and uninfected cats, but was more extensive in infected cats, with pangastric inflammation, eosinophilic infiltrates, and cardia gastritis observed only in infected cats. No upregulation of antral mucosal interleukin 1α (IL-1α), IL-1β, or tumor necrosis factor alpha was detected by reverse transcription-PCR in any cat. The gastric secretory axes, assessed by fasting plasma gastrin, antral mucosal gastrin and somatostatin immunoreactivity, and pentagastrin-stimulated gastric acid secretion, were similar in both infected and uninfected cats. Gradual seroconversion (immunoglobulin G) was observed in four of five infected cats, with enzyme-linked immunosorbent assay values reaching 4× to 12× baseline 12 months postinfection. These findings indicate that H. felis infection in cats induces lymphoid follicular hyperplasia, mild gastritis, and seroconversion, but is associated with normal gastric secretory function.
The discovery of the association of Helicobacter pylori with gastritis, peptic ulcers, and gastric neoplasia has led to fundamental changes in the understanding of gastric disease in humans (5, 38, 64, 67). Investigation of the relationship of gastric disease to Helicobacter spp. in other species has resulted in the discovery of Helicobacter mustelae in ferrets with gastritis and peptic ulcers, Helicobacter acinonyx in cheetahs with severe gastritis, and Helicobacter heilmannii in pigs with gastric ulcers (11, 19, 59).
Infection with Helicobacter spp. is also highly prevalent in cats, with spiral shaped bacteria 5 to 12 μm long, demonstrated in gastric biopsies from 86 to 100% of random-source cats (53, 62), 41 to 91% of clinically healthy pet cats (26, 30, 49, 73), 93 to 100% of laboratory cats (9, 54, 70), and 57 to 76% of cats with recurrent vomiting (26, 33, 73). H. pylori has been observed in a group of laboratory cats, but not pet cats, and is associated with gastritis in cats (22, 28). Excluding cats with H. pylori infection, the gastric Helicobacter-like organisms (HLOs) in cats are morphologically indistinguishable by light microscopy, but have been classified into several Helicobacter spp. on the basis of cultural characteristics, 16S rRNA sequencing, DNA hybridization, PCR with species-specific primers, electron microscopic appearance, and protein profiling (9, 15, 34, 36, 49, 51). To date, Helicobacter felis, H. heilmannii, Helicobacter pametensis, and H. felis- and H. heilmannii-like organisms have been identified (9, 15, 34, 36, 49, 51).
Despite their prevalence, the relationship of Helicobacter spp. to disease in cats is unresolved. Gastritis and glandular degeneration accompany infection in some, but not all, infected cats, and many are asymptomatic despite infection (30, 33, 53, 62, 73). Investigations of the pathogenicity of large gastric HLOs in cats have focused on describing the infecting bacteria and histopathology in cats with naturally acquired infections, and only a few studies have included Helicobacter-free cats (26, 30, 73). The host immune response to infection and gastric secretory function have not been examined. Consideration of those factors is important, because H. pylori infection in people is associated with gastritis, the induction of proinflammatory cytokines, seroconversion, and changes in gastric function. Increased acid secretion is associated with antral gastritis and duodenal ulceration (12, 13, 47), whereas achlorhydria is observed shortly after infection with H. pylori and when the gastric fundus and body is inflamed or atrophied (14, 44, 46, 65). Hypergastrinemia is a consistent finding in H. pylori-infected people and is present in asymptomatic individuals, those with achlorhydria, and those with duodenal ulcers (13, 27). Eradication of H. pylori has been associated with amelioration of gastritis and hypergastrinemia, decreased acid secretion in people with acid hypersecretion, and increased acid secretion in achlorhydric patients (13, 14, 47). It is presently unclear if Helicobacter spp. other than H. pylori can induce such changes and whether the alterations in gastric function which accompany infection with H. pylori are a consequence of bacterial products, such as urease, ammonia, or acid inhibitory factors, or the inflammatory response evoked by the bacterium.
There is a clear need to determine if H. felis is a gastric pathogen in cats and for animal models that will enable evaluation of the consequences of Helicobacter colonization for somatostatin and gastrin physiology, acid secretion, and mucosal inflammation. We report here the evaluation of gastric histopathology, antral interleukin 1α (IL-1α), IL-1β, and tumor necrosis factor alpha (TNF-α) mRNA expression, acid secretion, plasma gastrin, antral somatostatin and gastrin immunoreactivity, and circulating anti-H. felis immunoglobulin G (IgG) after experimental infection of cats with H. felis.
MATERIALS AND METHODS
Animals.
Five specific-pathogen-free (SPF), Helicobacter-free, male cats (7 months old) were studied before and for 1 year after oral inoculation with H. felis. Four SPF Helicobacter-free male cats (7 months old) which had not been inoculated with H. felis served as uninfected controls. The presence or absence of gastric Helicobacter spp. was ascertained in all cats prior to admission to the study by evaluating gastric biopsies for urease activity, impression smears and tissue sections for the presence of HLOs, and culture and gastric biopsies for H. felis DNA (see gastric biopsy). All cats were negative for Helicobacter spp. by all tests prior to inoculation. Cats were acclimatized to housing for 2 weeks prior to starting the study and for 4 weeks before infection with H. felis. Cats were fed a standard commercial diet and had constant access to water throughout the study. Infected cats were housed separately from uninfected cats. Cornell University operates under an approved Animal Welfare Assurance (A3347-01) and is fully accredited by the American Association for the Accreditation for Laboratory Animal Care. The project was approved by the Institutional Animal Care and Use Committee at Cornell University.
Infection with H. felis.
H. felis strain ATCC 49179 was used; it was originally isolated from the gastric mucosa of an adult cat (36) and was cultured as previously described (63). The bacteria were checked by Gram stain to ensure that there were few or no reversions from rod to coccoid forms. The bacterial suspension was standardized at a turbidity of a 0.5 McFarland standard, which would normally result in about 1.5 × 108 CFU/ml. However, since H. felis does not produce discrete colonies on agar, we could not do the standard colony counts to determine the actual number of H. felis cells in a 0.5 McFarland standard. An inoculum of 14.5 ml was administered to five cats via a stomach tube on days 1, 3, and 5 of the experiment.
Culture of gastric tissue samples.
Gastric tissue samples were transported to the laboratory in Trypticase soy broth tubes (BBL Microbiological Systems, Becton Dickinson, Cockeysville, Md.) on ice. Upon receipt in the laboratory, tissue samples were ground with a Ten Broeck tissue grinder and then cultured as previously described (63). Plates were checked daily for growth. Suspect colonies were subcultured to a brucella blood PRAS agar plate (Anaerobe Systems, Morgan Hill, Calif.) and reincubated; in addition, direct colony Gram staining was performed. Analysis of the 16S rRNA gene sequence was performed for suspect colonies to confirm that they were H. felis, as previously described (23).
Gastric tissue sampling.
Endoscopic biopsies of the stomach were obtained at −2 weeks, 3.5 months, and 8 months with a pediatric endoscope and biopsy forceps. Endoscopic biopsies were procured from the pyloric antrum (incisura to pyloric sphincter), the body (greater curvature), and the cardia. Three biopsies were taken from each site for light microscopy, two were taken from each site for urease testing, and one was taken from each site for PCR. Endoscopic biopsy samples for PCR analysis were frozen at −80°C pending analysis. The endoscope was thoroughly cleaned and then sterilized with an activated aldehyde solution (Metrex; Parker, Co.). Biopsy forceps were sterilized in a similar fashion, and the biopsy cups were immersed in Chlorox (1:10 in water) for 10 min to destroy residual DNA. At necropsy (12 months), two full-thickness gastric tissue samples were obtained from 10 standardized sites as described by Lee et al. (37) with a sterile 6-mm skin biopsy punch. One sample from each site was evaluated for urease activity, and the other was evaluated by light microscopy. Additional samples for transmission electron microscopy were obtained adjacent to site 5 (body). Samples for cytokine analysis (1) and quantitation of gastrin and somatostatin immunoreactivity (1) were obtained next to sites 7 to 9 (pyloric antrum), snap-frozen in liquid nitrogen, and frozen at −80°C pending analysis.
Gastric urease.
Urease production by gastric tissue was evaluated as previously described (60). Gastric tissue samples were placed in sterile tubes containing 200 μl of a solution composed of urea, sodium azide, phenol red, and phosphate-buffered saline (pH 6.5). Samples were incubated for 48 h and observed at 4, 12, 24, and 48 h for a change in the color of the indicator medium. A change from orange-red to bright pink was considered a positive result, and the time of color change was recorded. Urease results were additionally scored as follows: 0, negative at 48 h; 4, positive at 4 h; 3, positive at 12 h; 2, positive at 24 h; and 1, positive at 48 h.
PCR.
Gastric biopsies collected endoscopically at 0, 3.5, and 8 months were frozen at −80°C. DNA was extracted from biopsies with a Qiamp tissue kit (Qiagen, Santa Clarita, Calif.). PCR was performed with primers which amplify the urease B gene of H. felis: F-5′-ATGAAACTAACGCCTAAAGAACTAG-3′ (forward) and R-5′-GGAGAGATAAAGTGAATATGCGT-3′ (reverse) (49). DNA samples (5 μl) were added to a reaction mixture containing 400 μM deoxynucleoside triphosphates (dNTPs; Pharmacia Biotech, San Francisco, Calif.), PCR buffer (Gibco BRL, Grand Island, N.Y.), 2 mM MgCl2 (Gibco BRL), 1.5 U of Taq DNA polymerase (Gibco BRL), 0.6 μM each primer, and distilled water in a total volume of 50 μl. PCR samples were heated to 94°C for 2.5 min once, followed by 40 cycles of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and extension at 72°C for 1 min, with a final extension at 72°C for 15 min in a Biometra (Tampa, Fla.) personal thermocycler. PCR products were subjected to electrophoresis on an agarose gel and visualized with ethidium bromide. When visualized over UV light, a single band at 1,150 bp was present when Helicobacter felis ATCC 49179 was used. This band was absent with DNA from H. pylori (human isolate 8826, Cornell cat strain 1), Helicobacter bizzozeronii (ATCC 700030), H. heilmannii (DNA from the stomach of an infected cat), Helicobacter salomonis (CCUG 37845), Helicobacter fenelliae (ATCC 35684), H. bilis (ATCC 51630), H. cinaedii (ATCC 35683), or Campylobacter jejuni (dog isolate).
Helicobacter-specific primers C97 and C05 were used to test for 16S rRNA amplicons (24) from the four uninfected cats at 3.5 months postinoculation. Two microliters of DNA was added to the PCR mixture described above in a total volume of 50 μl. The PCR cycle was the same as the H. felis cycle. A band with a size of 1,200 bp was apparent with these primers with DNA from H. pylori (human isolate 8826, Cornell cat strain 1), H. felis (ATCC 49179), H. bizzozeronii (ATCC 700030), H. salomonis (CCUG 37845), H. heilmannii (DNA from the stomach of an infected cat), H. fenelliae (ATCC 35684), H. bilis (ATCC 51630), H. cinaedii (ATCC 35683), H. hepaticus (ATCC 51450), and H. canis (ATCC 51401). This fragment did not amplify with DNA from Campylobacter jejuni (dog isolate) and Proteus mirabilis (cat isolate).
Southern blot analysis was performed as follows. The PCR amplification product was separated on a 1% agarose gel, stained with ethidium bromide, depurinated (0.25 M HCl, 15 min), and denatured (1.5 M NaCl, 0.5 M NaOH, 30 min), and transferred (10× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) to a Zeta-Probe membrane (Bio-Rad Laboratories, Hercules, Calif.) with a vacuum blotter (5 mm Hg, 90 min; Bio-Rad Laboratories) and UV cross-linked. The oligonucleotide (5′-GGAATAAGCGLATCT-3′), which was directed against the H. felis PCR product, was 3′-oligolabeled with a nonradioactive labeling kit (ECL [enhanced chemiluminescence] 3′-oligolabeling system; Amersham, Little Chalfont, England). Southern blot hybridization and detection were performed as described by the manufacturer.
Serology.
Serum samples collected at 0, 3, 6, 9, and 12 months after inoculation with H. felis and at 0, 3, 6, and 9 months in uninfected cats were evaluated by kinetic enzyme-linked immunosorbent assay (ELISA). High-molecular-weight cell-associated protein, purified from a detergent extraction of H. felis ATCC 49179 as described by Evans et al. (17), was used to coat ELISA plates at 1 μg/well (antigen-coated plates were a generous gift from Enteric Products Inc., Stony Brook, N.Y.). The ELISA was performed as previously described (63), except that bound IgG was detected with horseradish peroxidase-conjugated rabbit anti-cat IgG (Cappel/ICN, Costa Mesa, Calif.) diluted 1:3,000 in phosphate-buffered saline with 0.05% Tween 20 and 2% dried milk, and incubated for 30 min, followed by washing and incubation with tetramethylbenzidine dihydrochloride (TMB). The plates were read (650 nm; MRX plate reader; Dynatech, Chantilly, Va.) three times at 45-s intervals, with 30 s of shaking between readings. The results were expressed as optical density (OD) per minute.
Cytokine analysis.
Gastric tissue samples from the pyloric antrum were collected, snap-frozen in liquid nitrogen, and stored at −80°C pending analysis. RNA was extracted from the biopsies with an RNA extraction kit according to the manufacturer's instructions (Qiagen). To eliminate DNA contamination, samples were treated with 1 U of DNase according to the manufacturer's instruction (GIBCO BRL). mRNA expression for TNF-α, IL1-α, and IL-1β was determined by reverse transcription (RT)-PCR. RT reactions were carried out in a GeneAmp 9600 PCR system (Perkin-Elmer, Foster City, Calif.). One-tenth microgram of total RNA in 50 μl of RT buffer containing 1× PCR buffer II (Perkin-Elmer), 5.0 mM MgCl2 (Perkin-Elmer), 1.0 mM each dNTPs (Perkin-Elmer), 18 U of RNase inhibitor (Promega, Madison, Wis.), 200 U of Moloney murine leukemia virus reverse transcriptase (Amersham), and 2.5 mM oligo(dT)18 (New England Biolabs, Beverly, Mass.) was transcribed into cDNA at 24°C for 10 min, followed by 42°C for 30 min. The cDNA was then held at 98°C for 5 min. Five-microliter aliquots of each sample were transferred into new 96-well microtiter plates, sealed with adhesive sealer tape, and stored at −80°C until use. PCR primers for feline TNF-α, IL-1α, and IL-1β were used to amplify their respective cDNAs (Table 1). Primers originally designed to amplify a fragment from the published bovine actin sequence amplify a homologous segment of the feline actin and were used to monitor the amount of mRNA in the reaction. PCR was performed with a GeneAmp 9600 PCR system (Perkin-Elmer) in a 25-μl total reaction volume, which was prepared with 5 μl of the cDNA solution, 1× PCR buffer II (Perkin-Elmer), 1.0 μM each primer, and 0.6 U of Taq polymerase (Perkin-Elmer). The MgCl2 concentration was adjusted to 1.5 mM. The amplification protocol included DNA denaturation at 94°C for 2 min, followed by amplification cycles (actin, 30 cycles; TNF-α, 29 cycles; IL-1α, 40 cycles; IL-1β, 34 cycles) at 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min, and then the reaction was terminated with an extension step at 72°C for 6 min. PCR fragments were separated in 1.5% agarose gels and visualized with ethidium bromide.
TABLE 1.
PCR primers for bovine actin and feline TNF-α, IL1-α, and IL-1β used to amplify their respective cDNAs
| Primer | Sequence (5′→3′) | Expected fragment length (bp) |
|---|---|---|
| BAC-1 | ATG TTC AGG GAC TTT GGA CG | 380 |
| BAC-2 | ACC AGC CAT CCA GAC AAA AC | |
| IL-1α-F | TTT GAA GAC CTG AAG AAC TGT TAC | 545 |
| IL-1α-R2 | GTT TTT GAG ATT CTT AGA GTC AC | |
| IL-1β-F-Cat | CAC AGT TTT CTG GGA GAT GAG G | 272 |
| IL-1β-R2-Cat | TGG CTT ATG TCT CGG AAC GTG T | |
| TNF-α-F | CTC TTC TGC CTG CTG CAC | 288 |
| TNF-α-R | GCC CTT GAA GAG GAC CTG |
Histopathology.
Samples for histopathology were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 4 to 6 μm. Serial sections of each block were stained with hematoxylin and eosin (H&E) and modified Steiner's stain (25). Samples were examined in a blinded fashion by our pathologist (E.S.) and evaluated for the presence of HLOs and degree of colonization, degree of inflammation, and presence of mucosal lymphoid nodules. The degree of colonization by HLOs was graded as follows: 0, no organisms seen; +1, presence of Helicobacter in <5% of gastric glands; +2, presence of Helicobacter in 5 to 50% of gastric glands; and +3, presence of Helicobacter in >50% of gastric glands. The degrees of inflammation, fibrosis, and atrophy were graded as follows: +1, mild; +2, moderate; and +3, severe. The number of lymphoid follicles was evaluated in samples obtained at necropsy and graded as follows (per specimen): +1, 1 lymphoid follicle; +2, 2 to 3 lymphoid follicles; and +3, >3 lymphoid follicles. Samples obtained for histopathology at necropsy were grouped according to site (cardia or fundus, biopsies 1 to 3; body, biopsies 4 to 6; and pyloric antrum, biopsies 7 to 10) (37).
Electron microscopy.
Gastric tissue was fixed by immersion in a solution containing 2.5% gluteraldehyde cacodylate (0.1 M) buffered to pH 7.2. Samples were postfixed in 1% osmium tetroxide, dehydrated, infiltrated, and embedded in Epon araldite. Semithin sections cut at 0.5 μm were stained with azure blue. Thin sections (approximately 80 nm thick) were stained with uranyl acetate and lead citrate and examined at 80 kV with a Philips 201 transmission electron microscope (FEI/Philips, Hillsboro, Oreg.).
Measurement of acid secretion.
Gastric acid secretion was evaluated for anesthetized cats prior to and 3.5, 8, and 12 months after the oral administration of H. felis. Following initial sedation with ketamine (10 to 15 mg/kg of body weight intramuscular), cats were induced, intubated, and maintained with halothane and oxygen. A gastric tube (12 Fr Levin tube; Davol, Inc., Cranston, R.I.) was positioned endoscopically in the dependent part of the stomach. The tube position was checked by injecting and recovering 6 ml of sterile water. Gastric juice was then continuously aspirated by gentle manual suction for 75 min. Basal (30 min) and pentagastrin-stimulated fractions (30 to 45, 45 to 60, and 60 to 75 min) were collected on ice. The collection, stimulation, and quantitation of gastric acid secretion were performed as described by Happe and DeBruijne (29), except that pentagastrin (Bachem Bioscience, Inc., King of Prussia, Pa.) was administered as a continuous intravenous infusion (0.9% NaCl, 0.1% albumin) at 8 μg/kg of body weight/h. Acid secretion was determined by pH measurement and titration to pH 7.0 with 0.1 M NaOH at room temperature. Maximal acid output was calculated by using values from the 15-min period with the highest acidity, and acid output was expressed as peak pH, millimoles of HCl per milliliter, and millimoles of HCl per kg0.75 (metabolic body mass) per hour.
Gastrin and somatostatin analysis. (i) Plasma gastrin.
Two weeks prior to the oral administration of H. felis and at 2, 4, 6, 9, 13, 15, 20, 25, 33, 45, and 50 weeks after inoculation, blood was collected into EDTA. All sampling periods were preceded by an overnight fast. Blood samples were placed in ice and centrifuged at 4°C, and plasma was stored at −80°C until analysis. Concentrations of gastrin in plasma were determined by radioimmunoassay (Gastrin 125I; Becton Dickinson, Cockeysville, Md.) at the Department of Endocrinology, The Ohio State University, Columbus.
(ii) Extraction of somatostatin and gastrin.
Antral gastrin and somatostatin content was determined in gastric tissue samples obtained between sample sites 7 and 9. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C pending analysis.
Gastric mucosal samples were weighed and immediately placed in 1 ml of distilled boiling water. For gastrin extraction, the samples were boiled for 10 min; this was followed by another 10 min in 3% acetic acid for somatostatin extraction. The supernatant was collected for radioimmunoassay.
(iii) Radioimmunoassay.
Somatostatin- and gastrin-specific radioimmunoassays were performed by previously described established methods (6, 52). K2 antibody (raised to somatostatin-14 synthetic human peptide in rabbits) at a dilution of 1:10,000 was used for the somatostatin assay and Gastrin 04281 (raised against human synthetic gastrin 1 in rabbits) at a final dilution of 1:100,000 was used for the gastrin assay: both were kindly provided by S. R. Bloom of the Department of Metabolic Medicine, Imperial College, London, United Kingdom. Addition of dextran-coated charcoal followed by centrifugation at 800 × g for 20 min at 4°C precipitated the free from the antibody-bound label. The supernatant (bound hormone) was separated from the charcoal pellet (free hormone) with a Pasteur pipette, and both fractions were counted for radiation content. The assays were performed on duplicate samples, and the mean hormone concentration was expressed per milligram of wet weight.
(iv) Immunohistochemistry for gastrin and somatostatin cells.
Immunohistochemistry was performed with deparaffinized tissue from the pyloric antrum with polyclonal rabbit anti-human gastrin 2-17 (1:2,000) (Peninsula Europe; lot no. 801374) and anti-synthetic somatostatin {−14[som-28(15–28)]; 1:2,000; Genosys; batch no. C1002} antibodies and by a streptavidin-biotin immunoperoxidase technique with aminoethyl carbazole as the chromagen. Nonimmune rabbit serum at 1:80 was used as a negative control. The numbers of immunoreactive cells in the pyloric antrum were quantitated by counting all immunoreactive cells observed in tissue from the pyloric antrum (biopsy sites 7 to 10). The results were expressed as the total number of each cell type and as the ratio of immunoreactive gastrin to somatostatin cells.
Statistical analysis.
Differences in gastric bacterial colonization density, inflammation, lymphoid follicles, atrophy, and fibrosis between infected and uninfected cats were evaluated with a Mann-Whitney test. Differences in these variables between gastric region (pylorus, body, and cardia) and over time (0, 3.5, 8, and 12 mo) were evaluated by Friedman analysis of variance. Two-way analysis of variance was conducted to determine the effects of group (infected, noninfected) and time on acid output (pentagastrin-stimulated peak, 15-min-period peak pH, millimoles of HCl per milliliter, and millimoles of HCl per kg0.75 per hour) and serum gastrin, before and after administration of H. felis. Differences in the gastrin and somatostatin contents of pyloric tissue and the number of immunoreactive somatostatin and gastrin cells in infected and uninfected cats were evaluated with a students' t test, or Mann-Whitney test when an F test was significant. The correlation between inflammation, lymphoid follicles, the number of organisms in gastric biopsies, and degree of seroconversion was assessed by using the Kendall rank correlation coefficient (tau). All statistical analyses were performed with Statview software (Abacus Concepts, Inc., Berkeley, Calif.). Significance was set at P < 0.05.
RESULTS
Clinical signs.
No abnormal clinical signs were evident in the infected and uninfected cats throughout the study.
Infection with H. felis.
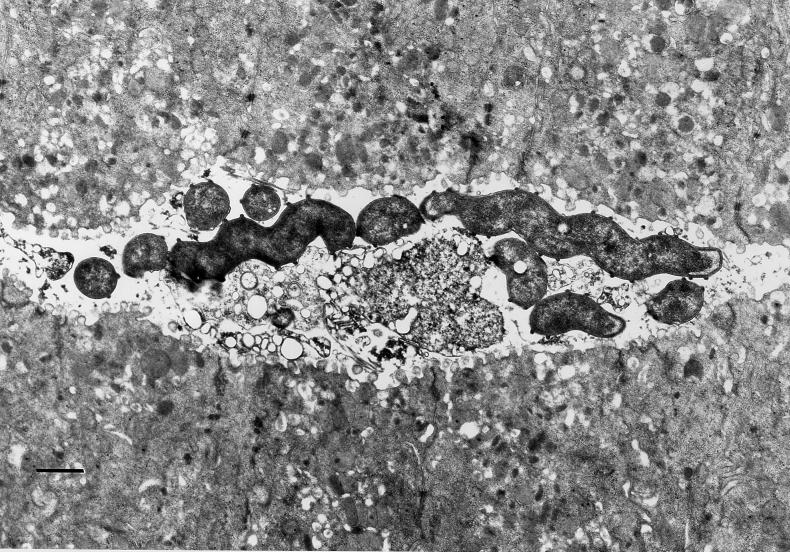
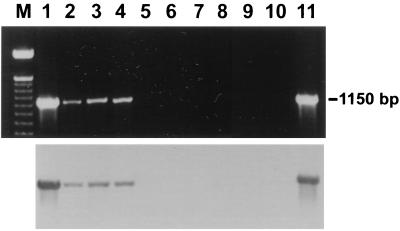
Gastric spiral organisms consistent with H. felis were visualized in modified-Steiner-stained sections in five of five infected cats at 3.5, 8, and 12 months (Table 2). Transmission electron microscopy confirmed the presence of spiral bacteria with periplasmic fibrils consistent with H. felis (Fig. 1). Culture recovered H. felis from one infected cat (cat 3) at 8 months and two infected cats (cats 1 and 5) at necropsy. Analysis of the 16S rRNA sequence from one of those isolates confirmed that the sequence was only 1 base different from the published sequence of H. felis ATCC 49179, which was used for infection. PCR of gastric biopsies with primers for H. felis urease was positive in biopsies from four of five cats at 3.5 months and five of five cats at 8 months (Table 2 and Fig. 2). Southern blot analysis with a labeled oligonucleotide probe specific for the H. felis urease B gene confirmed that PCR products were specific (Fig. 2). No positive PCR results were obtained in uninfected cats with the H. felis primers at 0, 3.5, and 8.0 months. Evaluation of gastric biopsies from uninfected cats at 3.5 months with Helicobacter genus-specific primers confirmed that those cats were also free from infection with other Helicobacter spp.
TABLE 2.
H. felis colonization determined by the presence or absence of gastric spiral organisms (modified Steiner stain), urease production, and H. felis DNA (PCR) in gastric biopsies
| Cat | Result by method ata:
|
|||||
|---|---|---|---|---|---|---|
| 3.5 mo
|
8 mo
|
|||||
| MS | Ure | PCR | MS | Ure | PCR | |
| H. felis infected | ||||||
| 1 | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + |
| 3 | + | + | − | + | − | + |
| 4 | + | + | + | + | − | + |
| 5 | + | + | + | + | + | + |
| Control | ||||||
| 6 | − | − | − | − | − | − |
| 7 | − | − | − | − | − | − |
| 8 | − | − | − | − | − | − |
| 9 | − | − | − | − | − | − |
The results of biopsies taken from the pyloric antrum, body, and cardia or fundus were pooled and are presented as positive (+) or negative (−). Biopsies were obtained by endoscopy 3.5 and 8.0 months after infection with H. felis. All cats were negative by all tests prior to inclusion in the study. MS, modified Steiner stain; Ure, urease production.
FIG. 1.
Electron micrograph of a gastric biopsy from an H. felis-infected cat (cat 2). Note the spiral shape and distinctive periplasmic fibrils. Bar, 833 nm.
FIG. 2.
Detection of H. felis DNA in gastric biopsy specimens (3.5 months) by PCR (upper bands) and confirmation by Southern blotting (lower bands). Lanes: M, DNA ladder; 1, cat 1; 2, cat 4; 3, cat 5; 4, cat 2; 5, cat 3; 6, cat 6; 7, cat 7; 8, cat 8; 9, cat 9; 10, negative control; 11, DNA from H. felis ATCC 49179.
Urease tests were positive in five of five cats at 3.5 months, three of five cats at 8 months, and five of five cats at 12 months (Tables 2 and 3). Urease tests of endoscopic biopsies were more frequently positive in the cardia (6 of 10 evaluations) than the body (4 of 10 evaluations) and pyloric antrum (4 of 10 evaluations). Urease mapping at 26 weeks confirmed that the cardia, fundus, and body were generally more heavily colonized than the pyloric antrum (Table 3), although one cat (cat 1) was strongly urease positive at all sites.
TABLE 3.
Urease activity in H. felis-infected and control cats
| Cat | Time to color change at sitea
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (cardia) | Fundus
|
Body
|
Pylorus
|
10 (pyloric canal) | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| H. felis infected | ||||||||||
| 1 | 4 | 2 | 4 | 2 | 4 | 0 | 4 | 4 | 4 | 3 |
| 2 | 4 | 3 | 2 | 2 | 3 | 1 | 0 | 0 | 2 | 0 |
| 3 | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 3 | 3 | 0 | 3 | 3 | 2 | 0 | 0 | 0 | 0 |
| 5 | 4 | 4 | 2 | 3 | 3 | 2 | 2 | 1 | 0 | 0 |
| Control | ||||||||||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Gastric tissue samples were obtained from 10 standardized sites at 12 months (37). The time taken for a color change in the indicator solution from red to pink was recorded as follows: 4, <4 h; 3, <12 h; 2, <24 h; 1, <48 h; and 0, negative after 48 h.
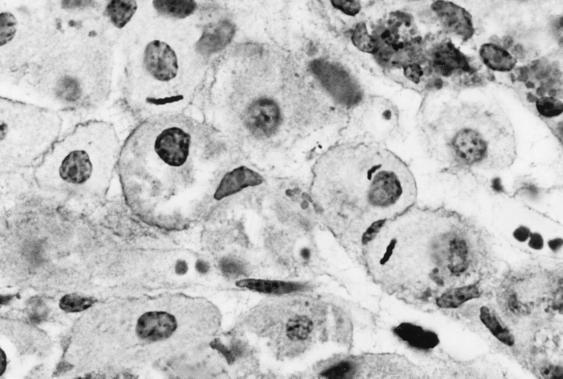
When the density and site of colonization by H. felis were assessed by light microscopy, it was apparent that the body and cardia were more densely colonized than the pyloric antrum in three cats, with similar degrees of colonization at all sites in the other two cats (Table 4). Helicobacter-like organisms were most frequently observed in the superficial gastric mucus layer, but were also observed within gastric glands and parietal cells of five of five infected cats (Fig. 3). Intracellular organisms accounted for approximately 5% of the organisms observed in a section. The degrees of colonization visualized in endoscopic biopsies were similar at 3.5 and 8 months after infection. Helicobacter-like organisms were not visualized in tissue samples from the four uninfected cats at any time point.
TABLE 4.
Histopathological findings 12 months after inoculation with H. felis
| Cat | Histopathology scorea:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonization density
|
Mononuclear infiltrates
|
Lymphoid follicles
|
Atrophy
|
Fibrosis
|
|||||||||||
| P | B | C | P | B | C | P | B | C | P | B | C | P | B | C | |
| H. felis infected | |||||||||||||||
| 1 | 3 | 2 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| 2 | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 1 |
| 3 | 0 | 3 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| 4 | 1 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| 5 | 1 | 3 | 3 | 1 | 1 | 1 | 3 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Control | |||||||||||||||
| 6 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| 8 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Colonization density, mononuclear infiltrates, lymphoid follicles, atrophy and fibrosis were graded on a scale of 0 to 3. P, pyloric antrum; B, body; C, cardia or fundus.
FIG. 3.
Intracellular localization of H. felis in the fundus of cat 3 (modified Steiner stain).
Gastric histopathology.
No gross mucosal abnormalities were observed in any cat during upper gastrointestinal endoscopy at 0, 3.5, 8, and 12 months or at necropsy at 12 months.
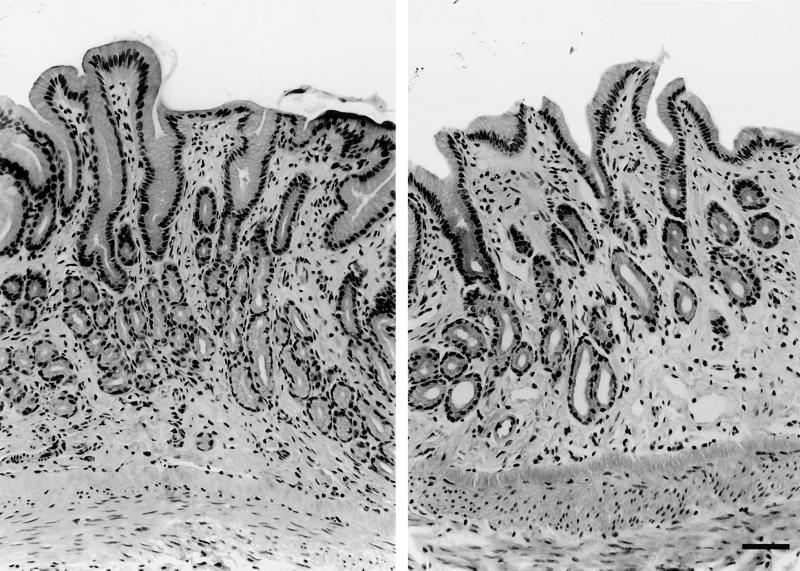
No lymphoid follicles and only one area of mild mononuclear infiltrates (grade 1) in the pylorus of three cats were detected in biopsies taken before entry into the study. Blinded evaluation of tissue specimens taken at necropsy (12 months [Table 4]) revealed mild mononuclear infiltrates in one or more gastric sites from three of five infected and three of four uninfected cats (P > 0.05). Inflammation was more extensive in infected cats, with pangastric mononuclear infiltrates (cats 2 and 5) and cardia gastritis (cats 2, 3, and 5) observed only in infected cats (Table 4). Furthermore, eosinophilic infiltrates were observed in three infected cats (cats 2, 3, and 5). At 12 months, lymphoid follicles were present in the pyloric mucosa of all infected cats and in one uninfected cat (P < 0.05) (Fig. 4). Atrophy and fibrosis were observed predominantly in infected cats (P < 0.05), with the pylorus more severely affected in infected cats than the fundus or cardia (P < 0.05) (Fig. 5). There was no relationship between total gastric colonization density and mononuclear infiltrates, although the degree of bacterial colonization in the cardia was related to the degree of mononuclear infiltrates (tau = 0.722, P = 0.007). Associations between colonization density and the presence of lymphoid follicles (tau = 0.643, P < 0.02), atrophy (tau = 0.750, P = 0.005), and fibrosis (tau = 0.803, P = 0.003) and associations between atrophy and fibrosis (tau = 0.813, P < 0.002) were also observed. Analysis of endoscopic biopsies taken at 0, 3.5, and 8 months showed an effect of time (P < 0.05) for mononuclear infiltrates, lymphoid follicles, atrophy, and fibrosis in infected cats but not uninfected cats.
FIG. 4.
Pyloric antrum from cat 4. A lymphoid follicle is shown (H&E stain).
FIG. 5.
Pylorus of an uninfected cat (left; cat 9, fibrosis grade 1) and an infected cat (right; cat 5, atrophy grade 2, fibrosis grade 2). H&E stain was used.
Gastric cytokines.
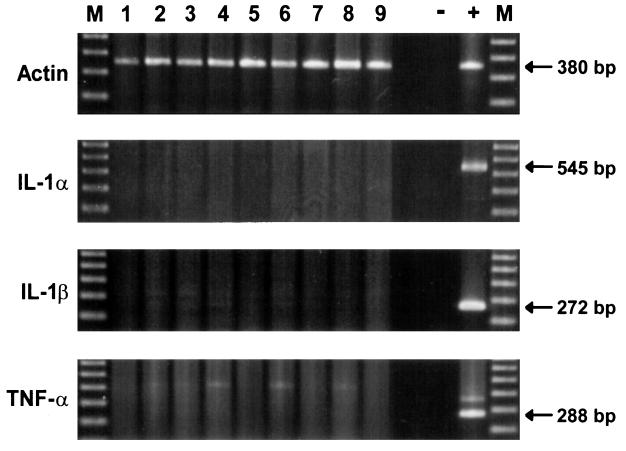
RT-PCR analysis of gastric biopsies showed actin amplification in all samples (Fig. 6) and appropriate reactions for positive (cat bronchial macrophages) and negative control samples. There was no evidence of upregulation of IL-1α, IL-1β, or TNF-α mRNA in either uninfected or infected cats.
FIG. 6.
Detection of mRNA for actin, IL-1α, IL-1β, and TNF-α in gastric tissue specimens by RT-PCR. Agarose gel electrophoresis of DNA products. Lanes: 1 to 4, uninfected cats; 5 to 9, infected cats; −, negative control; +, positive control (cat bronchial macrophages); M, DNA ladder.
Serology.
Four of the infected cats (cats 2 to 5) showed evidence of seroconversion, with progressive increases in OD per minute to 4× to 12× preinoculation values at 12 months (Fig. 7). The uninfected cats showed little change in OD per minute throughout, although one uninfected cat (cat 9) showed a gradual increase to 2× baseline at 9 months. There was no correlation between the relative increase in ELISA values and the degree of bacterial colonization, inflammation, or lymphoid follicles in infected cats.
FIG. 7.
Serological responses (IgG) of SPF cats to infection with H. felis (solid symbols) and uninfected cats (open symbols).
Acid secretion.
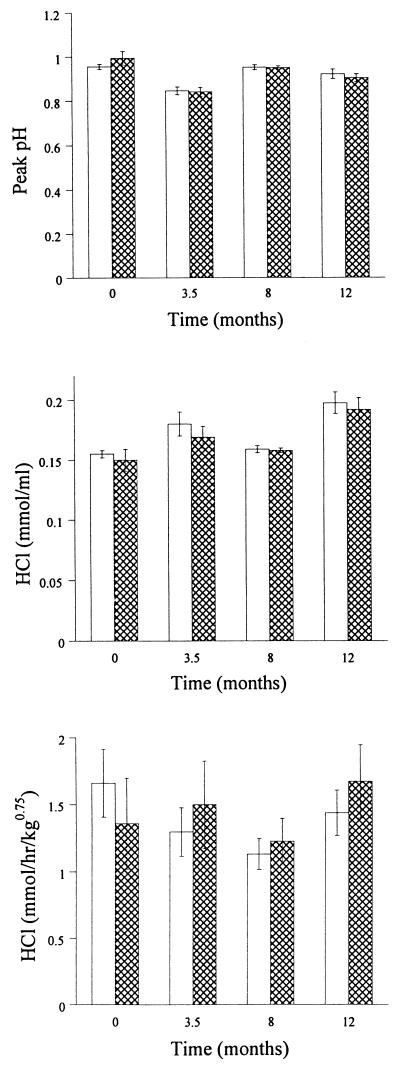
Gastric secretion during the basal period (0 to 30 min) was low in volume, and titratable acidity could not be reliably determined. Pentagastrin-stimulated acid output was usually maximal during the 60- to 75-min period. The acidities of gastric juice during maximal output (pH and millimoles of HCl per milliliter) and total acid output (millimoles per kg0.75 per hour) were similar in infected and uninfected cats throughout the study (Fig. 8).
FIG. 8.
Pentagastrin-stimulated gastric acid secretion during maximal acid output (mean ± SE) before and after infection with H. felis. Open bars, uninfected cats; crosshatched bars, infected cats.
Plasma gastrin.
There was no significant difference in fasting gastrin concentrations between groups over the 50-week period (Fig. 9).
FIG. 9.
Fasting plasma gastrin concentrations (picograms per milliliter [mean ± SE]) before and after infection with H. felis. Open bars, uninfected cats; crosshatched bars, infected cats.
Antral somatostatin and gastrin. (i) Immunohistochemistry.
The numbers of immunoreactive somatostatin cells in antral biopsies varied widely between cats but were not significantly different (P > 0.05) in the groups of uninfected (median, 75; range, 0 to 256) and H. felis-infected (median, 0; range, 0 to 39) cats. The numbers of immunoreactive gastrin cells were more constant and were similar (P > 0.05) in uninfected (median, 551; range, 256 to 964) and infected (median, 527; range, 145 to 1,510) cats. The ratios of immunoreactive gastrin to somatostatin cells were not significantly different (P > 0.05) in uninfected (median, 6.65; range, 3 to 64) and infected (median, 39; range, 4 to 74) cats.
(ii) Somatostatin and gastrin products.
The amounts of somatostatin (femtomoles per milligram) and gastrin (picomoles per milligram) in antral biopsies were similar (P > 0.05) (median, range, or mean ± standard error [SE]) in uninfected (somatostatin, median, 50; range, 19 to 98; gastrin, mean ± SE, 18.9 ± 10.3; gastrin/somatostatin ratio, median, 156; range, 36 to 2,000) and H. felis-infected (somatostatin, median, 75; range, 3 to 330; gastrin, mean ± SE, 17.8 ± 12.4; gastrin/somatostatin ratio, 158; range, 15 to 508) cats.
DISCUSSION
The oral administration of H. felis resulted in the infection of five of five cats. Infection was achieved solely by administration of a suspension of H. felis. It was not necessary to suppress gastric acid secretion or use gnotobiotic animals, as previously reported by others, to infect cats with H. pylori and dogs with H. felis (22, 37). The pattern of colonization, with a tendency for the strongest urease activity to occur in the cardia and fundus, was similar to that observed in cats and dogs with naturally acquired gastric helicobacteriasis and gnotobiotic and SPF dogs infected with H. felis (9, 30, 37, 53, 63, 73). While H. felis was cultured from gastric tissue samples of infected cats on only three occasions, positive PCR with H. felis-specific primers, the presence of HLOs with periplasmic fibrils on electron microscopy, and 16S rRNA sequence analysis indicate that the gastric HLOs observed in biopsies were H. felis (37, 49). Uninfected cats were negative for Helicobacter spp. throughout the study.
The marked lymphoid follicular hyperplasia in the H. felis-infected cats in the present study is similar to that reported in cats with naturally acquired infection with HLOs (30, 33, 53, 62) and cats infected with H. pylori (22, 28). An antral predominance of lymphoid follicular hyperplasia has also been previously observed in cats with naturally acquired infection and those infected with H. pylori (22, 28, 30, 53). Our observations lend support to those of Happonen et al. (30) and Hermanns et al. (33), who described an absence of lymphoid follicles in uninfected cats and a relationship between colonization density and lymphoid follicles. However, we did observe lymphoid follicles in the pylorus of one of four uninfected cats (cat 8). It is of note that previous studies that have evaluated full-thickness gastric tissue samples (30, 33, 53, 62), rather than endoscopic biopsies (49, 51, 54, 73), have also found a relationship between infection and lymphoid follicle hyperplasia in cats. Our observations are of potential importance with respect to the development of mucosa-associated lymphoid tissue (MALT) lymphoma. H. pylori infection in people is strongly associated with the development of gastric MALT and MALT lymphoma, and the eradication of Helicobacter has caused remission of MALT lymphoma in people (55, 66, 71). H. felis infection has also been associated with MALToma-like lesions in BALB/c mice (16). Because lymphoma accounts for 26 to 33% of malignant tumors in cats, and alimentary lymphoma is the most common anatomic form (42, 68), the potential relationship of Helicobacter spp. to gastric lymphoma in cats merits further investigation.
The mononuclear inflammation observed in the present study is consistent with previous studies with cats with naturally acquired infection with large HLOs (30, 49, 51, 70, 73). The lack of uninfected cats in the majority of previous studies has made it difficult to determine if the histopathological abnormalities were related to infection with Helicobacter spp. In the present study, pangastric inflammation, eosinophilic infiltrates, and cardia gastritis were observed only in infected cats, and a relationship between colonization and inflammation of the cardia was detected. In some, but not all, previous studies that have examined infected and uninfected cats, a correlation between colonization density and inflammation of the fundus, or glandular degeneration, has been shown (30, 33, 73). Conversely, mononuclear infiltration and lymphoid follicular hyperplasia are often more severe in the pyloric antrum, which is generally less heavily colonized (9, 30, 53, 73). Neutrophilic infiltrates were not detected in the present study and contrast with the active gastritis noted in people and cats infected with H. pylori (22, 28, 38). Eosinophilic infiltrates were detected in three infected cats in the present study and have also been observed in gnotobiotic dogs infected with H. pylori (60) and dogs and rats infected with H. felis (20, 37). In the present study, fibrosis and atrophy were more common in infected cats and appeared to be related to each other. A relationship between colonization and fibrosis has been reported by some (33) but not by others (9, 73).
The development of RT-PCR assays to measure IL-1α, IL-1β, and TNF-α mRNA in cats enabled us to evaluate the relationship between infection and cytokine induction in the gastric mucosa. The mild inflammatory response and absence of cytokine induction in the cats in the present study, despite substantial colonization with H. felis, contrasts markedly with the chronic active gastritis, cytokine expression, and peptic ulceration observed in H. pylori-infected people. Those pathological abnormalities in infected people are linked to changes in gastric function; antral gastritis and peptic ulceration are associated with increased acid secretion (13, 47), whereas inflammation or atrophy of the fundus and body is associated with achlorhydria (14, 44, 46, 65). Eradication of H. pylori results in decreased acid secretion in patients with acid hypersecretion and increased acid secretion in achlorhydric patients (13, 14, 47). Decreased inhibition of gastrin release by somatostatin, with resultant hypergastrinemia and increased parietal cell mass, has been postulated as the cause of hyperacidity and duodenal ulceration (48, 58). Decreased mucosal somatostatin and increased gastrin may be a consequence of antral inflammation or perhaps chronic ammonia exposure (10, 40). The proinflammatory cytokines IL-1β and TNF-α, which are expressed in H. pylori-infected people (41, 50, 72), inhibit somatostatin release (3) and stimulate gastrin release (1, 2, 39) by antral G cells in vitro. The precise mechanisms of cytokine induction are unclear, but bacterium-associated factors such as urease have been shown to release cytokines from macrophages (32).
Many of the factors that are thought to promote gastrin release in the pyloric antrum are associated with inhibition of acid secretion in the fundus. For example TNF-α and IL-1β decrease acid secretion by affecting parietal (4) and possibly enterochromaffin-like cells (57). In addition, inhibitory substances produced by H. felis and H. pylori have been shown to decrease parietal cell acid secretion in vitro (7, 69). Because the degrees of hypergastrinemia and the antral cytokine milieus appear similar in humans with and without duodenal ulcers (41, 50, 72), it is likely that it is the balance between parietal cell stimulation and hyperplasia on one hand and inhibition and atrophy on the other that determines the outcome. Because eradication is associated with a decrease in inflammation (14), it has been difficult to separate the effects of bacterial colonization per se from those induced by inflammation. The absence of abnormalities in gastric acid secretion, plasma gastrin, and antral somatostatin and gastrin in the present study in the face of substantial colonization with H. felis strongly argues against a direct effect of H. felis, urease, and ammonia on the gastric secretory axis in the cat. The lack of severe gastric inflammation in the cats in the present study may explain our findings. This hypothesis is supported by a recent study of rats infected with either H. felis or H. heilmannii, in which fasting or stimulated gastrin and acid secretion were similarly unchanged in the face of dense colonization (8).
It is possible that the evaluation of acid secretion in response to pentagastrin, rather than bombesin or gastrin-releasing peptide and measurement of fasting, rather than meal-stimulated, gastrin, may have failed to expose hyperacidity or hypergastrinemia (43). However, because pentagastrin stimulation is the most sensitive method for detecting hypochlorhydria, it is unlikely that decreased acid secretion was present (43).
While the lack of severe inflammation and abnormalities in gastric secretory function observed in the present study may be due to differences in the pathogenic attributes of the various species or strains of Helicobacter, it has been previously illustrated that the species and strain of the host can also determine the density of bacterial colonization and the degree and type of inflammation observed in response to infection with H. felis (20, 45, 61). Our observation that the pylorus was more affected than the cardia and the fundus, despite being least densely colonized with HLOs, is different from observations of gnotobiotic dogs infected with H. felis, where lymphoid follicle hyperplasia and bacterial colonization were most evident in the fundus and body and subglandular infiltrates of lymphocytes, plasma cells, and eosinophils were widespread (37). Conversely, H. felis-infected mice have more inflammation in the fundus than the pyloric antrum, although the pyloric antrum is generally more densely colonized (45). Hence the variable outcome of H. felis infection likely reflects inter- and intraspecies differences, such as the genetic makeup, age, and origins of the animals.
Seroconversion was observed in four of five infected cats and the OD per minute gradually increased to values 4× to 12× higher than the baseline after 12 months. Previous studies of H. felis infection in gnotobiotic dogs and mice have demonstrated fairly uniform seroconversion 2 weeks after infection (21, 37). The variable serological responses we observed are more similar to those in SPF dogs infected with H. felis and cats infected with H. pylori, in studies in which some animals did not seroconvert until 6 months after infection and had titers only twofold greater than baseline (22, 63). In all of those studies, the degree of seroconversion was not related to colonization density, inflammation, or lymphoid follicles, and the reasons for the differences in the time and degree of seroconversion are unclear.
A number of methods were used to detect H. felis in gastric biopsies. When the results of culture and biopsies taken from infected cats at 3.5, 8, and 12 months are combined, it is apparent that culture was positive for H. felis at 3 of 15 sampling points, PCR was positive for H. felis at 9 of 10 sampling points, modified Steiner staining was positive for HLOs at 15 of 15 sampling points, and urease tests were positive at 13 of 15 sampling points. These observations concur with studies with H. felis-infected mice and dogs with naturally acquired helicobacteriosis, in which microscopy was more sensitive than culture and urease (31, 45). Our observation that PCR was sensitive and specific is in agreement with studies of mice and dogs infected with H. felis and humans and cats infected with H. pylori (18, 35, 56, 63). However, the patchy distribution of Helicobacter within the stomach, particularly in areas of low colonization density, suggests that the confirmation of Helicobacter infection is probably best undertaken by evaluating multiple biopsies from different sites by a combination of methods.
In summary, H. felis infection in cats was associated with lymphoid follicular hyperplasia and seroconversion. Gastritis was more extensive in infected cats, but was mild and was not associated with alterations in mRNA of antral IL-1α, IL-1β, and TNF-α or changes in the gastric-secretory axis. The development of substantial lymphoid follicular hyperplasia is relevant to the pathogenesis of gastric lymphoma in cats.
ACKNOWLEDGMENTS
This study was supported by grants from the Winn Feline Foundation, Enteric Products, Inc. (Stony Brook, N.Y.), New York State Science and Technology Foundation, and Cornell Unrestricted Alumni.
We thank Bruce Paster for 16S rRNA sequencing, Brent Howe at the Ohio State University for gastrin analysis, Anita Alisio and Shannon Caldwell for electron microscopy, and Hollis Erb for statistical advice.
REFERENCES
- 1.Beales I, Blaser M J, Srinivasan S, Calam J, Perez Perez G I, Yamada T, Scheiman J, Post L, DelValle J. Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology. 1997;113:465–471. doi: 10.1053/gast.1997.v113.pm9247465. [DOI] [PubMed] [Google Scholar]
- 2.Beales I L P, Post L, Calam J, Yamada T, Delvalle J. Tumour necrosis factor alpha stimulates gastrin release from canine and human antral G cell: possible mechanism of the Helicobacter pylori-gastrin link. Eur J Clin Investig. 1996;26:609–611. doi: 10.1046/j.1365-2362.1996.2040517.x. [DOI] [PubMed] [Google Scholar]
- 3.Beales I L P, Calam J. The H3 histamine receptor agonist Nα-methylhistamine produced by Helicobacter pylori does not alter somatostatin release from cultured rabbit fundic D-cells. Gut. 1998;43:176–181. doi: 10.1136/gut.43.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beales I L P, Calam J. Interleukin-1β and tumour necrosis factor-α inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser M J. The bacteria behind ulcers. Sci Am. 1996;February:104–107. doi: 10.1038/scientificamerican0296-104. [DOI] [PubMed] [Google Scholar]
- 6.Bryant M G, Adrian T E. Gastrin. In: Bloom S, Long R, editors. Radioimmunoassay of gut regulatory peptides. W. B. London, United Kingdom: Saunders; 1982. pp. 138–145. [Google Scholar]
- 7.Cave D R. Helicobacter pylori and its interaction with chief and parietal cells. Yale J Biol Med. 1996;69:91–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Danon S J, Moss N D, Larsson H, Arvidsson S, Ottosson S, Dixon M F, Lee A. Gastrin release and gastric acid secretion in the rat infected with either Helicobacter felis or Helicobacter heilmannii. J Gastroenterol. 1998;13:95–103. doi: 10.1111/j.1440-1746.1998.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 9.De Majo M, Pennisis M G, Carbone M, Fera M T, Masucci M, Meli F, Cavallari V. Occurrence of Helicobacter spp. in gastric biopsies of cats living in different kinds of colonies. Eur J Comp Gastroenterol. 1998;3:13–18. [Google Scholar]
- 10.Dial E J, Hall L R, Romero J J, Lichtenberger L M. Rats with gastritis have increased sensitivity to the gastrin stimulatory effects of luminal ammonia. Gastroenterology. 1996;110:801–808. doi: 10.1053/gast.1996.v110.pm8608890. [DOI] [PubMed] [Google Scholar]
- 11.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Fox J G, Morgan D R. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Vet Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- 12.El-Omar E, Penman I, Ardill J E S, McColl K E L. A substantial proportion of non-ulcer dyspepsia patients have the same abnormality of acid secretion as duodenal ulcer patients. Gut. 1995;36:534–538. doi: 10.1136/gut.36.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Omar E, Penman I D, Ardill J E S, Chittajallu R S, Howie C, McColl K E L. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 14.El-Omar E M, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill J E S, McColl K E L. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 15.El-Zaatari F A K, Woo J S, Badr A, Osato M S, Serna H, Lichtenberger L M, Genta R M, Graham D Y. Failure to isolate Helicobacter pylori from stray cats indicates that H. pylori in cats may be an anthroponosis—an animal infection with a human pathogen. J Med Microbiol. 1997;46:372–376. doi: 10.1099/00222615-46-5-372. [DOI] [PubMed] [Google Scholar]
- 16.Enno A, O'Rourke J L, Howlett C R, Jack A, Dixon M F, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 17.Evans D J, Evans D G, Graham D Y, Klein P D. A sensitive and specific serological test for the detection of Campylobacter pylori infection. Gastroenterology. 1989;96:1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- 18.Fabre R, Sobhani I, Laurent-Puig P, Hedef N, Yazigi N, Vissuzaine C, Rodde I, Potet F, Mignon M, Etienne J P, Braquet M. Polymerase chain reaction assay for the detection of Helicobacter pylori in gastric biopsy specimens: comparison with culture, rapid urease test, and histopathological tests. Gut. 1994;35:905–908. doi: 10.1136/gut.35.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J G, Correa P, Taylor N S, Lee A, Otto G, Murphy J C, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 20.Fox J G, Lee A, Otto G, Taylor N S, Murphy J C. Helicobacter felis gastritis in gnotobiotic rats: an animal model of Helicobacter pylori gastritis. Infect Immun. 1991;59:785–791. doi: 10.1128/iai.59.3.785-791.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox J G, Blanco M, Murphy J C, Taylor N S, Lee A, Kabok Z, Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993;61:2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox J G, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox J G, Yan L L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox J G, Dewhirst F E, Shen Z, Feng Y, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 25.Garvey W, Fathi A, Bigelow F. Modified Steiner for the demonstration of spirochetes. J Histotechnol. 1985;8:15–17. [Google Scholar]
- 26.Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993;133:18–19. doi: 10.1136/vr.133.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Gillen D, El-Omar E M, Wirz A A, Ardill J E S, McColl K E L. The acid response to gastrin distinguishes duodenal ulcer patients from Helicobacter pylori-infected healthy subjects. Gastroenterology. 1998;114:50–57. doi: 10.1016/s0016-5085(98)70632-8. [DOI] [PubMed] [Google Scholar]
- 28.Handt L K, Fox J G, Dewhirst F E, Fraser G J, Paster B J, Yan L L, Rozmiarek H, Rufo R, Stalis I H. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Happe R P, DeBruijne J J. Pentagastrin stimulated gastric secretion in the dog (orogastric aspiration technique) Res Vet Sci. 1982;33:232–239. [PubMed] [Google Scholar]
- 30.Happonen I, Saari S, Castren L, Tyni O, Hanninen M L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. J Vet Med Assoc. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 31.Happonen I, Saari S, Castren L, Tyni O, Hanninen M L, Westermarck E. Comparison of diagnostic methods for detecting gastric Helicobacter-like organisms in dogs and cats. J Comp Pathol. 1996;115:117–127. doi: 10.1016/s0021-9975(96)80034-x. [DOI] [PubMed] [Google Scholar]
- 32.Harris P R, Ernst P B, Kawabata S, Kiyono H, Graham M F, Smith P D. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J Infect Dis. 1998;178:1516–1520. doi: 10.1086/314426. [DOI] [PubMed] [Google Scholar]
- 33.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 34.Jalava K, On S L W, Vandamme P A R, Happonen I, Sukura A, Hänninen M-L. Isolation and identification of Helicobacter spp. from canine and feline gastric mucosa. Appl Environ Microbiol. 1998;64:3998–4006. doi: 10.1128/aem.64.10.3998-4006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong L, Smith J G, Bramhill D, Abruzzo G K, Bonfiglio C, Cioffe C, Flattery A M, Gill C J, Lynch L, Scott P M, Silver L, Thompson C, Kropp H, Bartizal K. A sensitive and specific PCR method to detect Helicobacter felis in a conventional mouse model. Clin Diagn Lab Immunol. 1996;3:73–78. doi: 10.1128/cdli.3.1.73-78.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee A, Hazell S L, O'Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee A, Krakowka S, Fox J G, Otto G, Eaton K A, Murphy J C. Role of Helicobacter felis in chronic canine gastritis. Vet Pathol. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- 38.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann F S, Golodner E H, Wang J, Chen M C Y, Avedian D, Calam J, Walsh J H, Dubinett S, Soll A H. Mononuclear cells and cytokines stimulate gastrin release from canine antral cells in primary culture. Am J Physiol. 1996;270:G783–G788. doi: 10.1152/ajpgi.1996.270.5.G783. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenberger L M, Dial E J, Romero J J, Lechago J, Jarboe L A, Wolfe M M. Role of luminal ammonia in the development of gastropathy and hypergastrinemia in the rat. Gastroenterology. 1995;108:320–329. doi: 10.1016/0016-5085(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 41.Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm A-M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahony O M, Moore A S, Cotter S M, Engler S J, Brown D, Penninck D G. Alimentary lymphoma in cats: 28 cases (1988–1993) J Am Vet Med Assoc. 1995;207:1593–1598. [PubMed] [Google Scholar]
- 43.McColl K E L, El-Omar E. Review article: gastrin releasing peptide and its value in assessing gastric secretory function. Aliment Pharmacol Ther. 1995;9:341–347. doi: 10.1111/j.1365-2036.1995.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 44.McGowan C G, Cover T L, Blaser M J. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996;110:926–938. doi: 10.1053/gast.1996.v110.pm8608904. [DOI] [PubMed] [Google Scholar]
- 45.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 47.Moss S F, Calam J. Acid secretion and sensitivity to gastrin in patients with duodenal ulcer: effect of eradication of Helicobacter pylori. Gut. 1993;34:888–892. doi: 10.1136/gut.34.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss S F, Legon S, Bishop A E, Polak J M, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- 49.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noach L A, Bosma N B, Jansen J, Hoek F J, van Deventer S J H, Tytgat G N J. Mucosal tumor necrosis factor-α, interleukin-1β, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 51.Norris C R, Marks S L, Eaton K A, Torabian S Z, Munn R J, Solnick J V. Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J Clin Microbiol. 1999;37:189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Shaughnessy D. Somatostatin. In: Bloom S, Long R, editors. Radioimmunoassay of gut regulatory peptides. W. B. London, United Kingdom: Saunders; 1982. pp. 138–145. [Google Scholar]
- 53.Otto G, Hazell S H, Fox J G, Howlett C R, Murphy J C, O'Rourke J L, Lee A. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol. 1994;32:1043–1049. doi: 10.1128/jcm.32.4.1043-1049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papasouliotis K, Gruffydd-Jones T J, Werrett G, Brown P J, Pearson G R. Occurrence of “gastric Helicobacter-like organisms” in cats. Vet Rec. 1997;140:369–370. doi: 10.1136/vr.140.14.369. [DOI] [PubMed] [Google Scholar]
- 55.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman J D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 56.Perkins S E, Yan L L, Shen Z, Hayward A, Murphy J C, Fox J G. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy. Antimicrob Agents Chemother. 1996;40:1486–1490. doi: 10.1128/aac.40.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prinz C, Neumayer N, Mayr S, Classen M, Scepp W. Functional impairment of rat enterochromaffin-like cells by interleukin-1β. Gastroenterology. 1997;112:364–365. doi: 10.1053/gast.1997.v112.pm9024290. [DOI] [PubMed] [Google Scholar]
- 58.Queiroz D M M, Mendes E N, Rocha G A, Moura S B, Resende L M H, Barbosa A J A, Coelho L G V, Passos M C E, Castro L P, Oliveira C A, Lima G F. Effect of Helicobacter pylori eradication on antral gastrin- and somatostatin-immunoreactive cell density and gastrin and somatostatin concentrations. Scand J Gastroenterol. 1993;28:858–864. doi: 10.3109/00365529309103125. [DOI] [PubMed] [Google Scholar]
- 59.Queiroz D M M, Rocha G A, Mendes E N, Moura S B, Oliveira A M R, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- 60.Radin M J, Eaton K A, Krakowka S, Morgan D R, Lee A, Otto G, Fox J. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606–2612. doi: 10.1128/iai.58.8.2606-2612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakagami T, Dixon M E, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serna J H, Genta R M, Lichtenberger L M, Graham D Y, El-Zaatari F A K. Invasive Helicobacter-like organisms in feline gastric mucosa. Helicobacter. 1997;2:40–43. doi: 10.1111/j.1523-5378.1997.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 63.Simpson K W, McDonough P L, Strauss-Ayali D, Chang Y F, Harpending P, Valentine B A. Helicobacter felis infection in dogs: effect on gastric structure and function. Vet Pathol. 1999;36:237–248. doi: 10.1354/vp.36-3-237. [DOI] [PubMed] [Google Scholar]
- 64.Smoot D T, Hamilton F A. Summary of the National Institutes of Health consensus development conference on Helicobacter pylori. Gastrointest Dis Today. 1995;4:1–10. [Google Scholar]
- 65.Sobala G M, Crabtree J, Dixon M F, Schorah C J, Taylor J D, Rathbone B J, Heatley R V, Axon A T R. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiede C, Morgner A, Alpen B, Wundisch T, Herrmann J, Ritter M, Ehninger G, Stolte M, Bayerdorffer E, Neubauer A. What role does Helicobacter pylori eradication play in gastric MALT and gastric MALT-lymphoma. Gastroenterology. 1997;113:S61–S64. doi: 10.1016/s0016-5085(97)80014-5. [DOI] [PubMed] [Google Scholar]
- 67.Tompkins L S, Falkow S. The new path to preventing ulcers. Science. 1995;267:1621–1622. doi: 10.1126/science.7886448. [DOI] [PubMed] [Google Scholar]
- 68.Vail D M, Moore A S, Ogilvie G K, Volk L M. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med. 1998;12:349–354. doi: 10.1111/j.1939-1676.1998.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 69.Vargas M, Lee A, Fox J G, Cave D R. Inhibition of acid secretion from parietal cells by non-human-infecting Helicobacter species: a factor in colonization of gastric mucosa? Infect Immun. 1991;59:3694–3699. doi: 10.1128/iai.59.10.3694-3699.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla in the fundic glands of dogs and cats. Am J Vet Res. 1958;19:677–680. [PubMed] [Google Scholar]
- 71.Wotherspoon A C, Doglioni C, Diss T C, Pan L X, Moschini A, Debomi M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 72.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K. Induction of various cytokines and development of severe mucosal inflammation by cag A gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998;212:529–533. [PubMed] [Google Scholar]