Abstract
Background
Cutibacterium acnes (C. acnes) is the most common pathogen responsible for post-operative shoulder infections. The purpose of this study was to evaluate the effectiveness of skin preparation methods against C. acnes in shoulder surgery.
Methods
A systematic review was conducted evaluating the effectiveness of skin preparation methods in the reduction of C. acnes in patients undergoing shoulder surgery. Outcomes were assessed based on the effectiveness of the method used; side effects and cost were also analysed.
Results
Of the 19 included studies, 9 evaluated pre-surgical home treatments: 8 assessed benzoyl peroxide (BPO) and 6 concluded it is effective in reducing C. acnes. Nine studies assessed surgical skin preparation and concluded that Chlorhexidine gluconate (CHG) was not effective; in contrast hydrogen peroxide reduced C. acnes. Finally, one study evaluated an aseptic protocol using CHG and concluded that it was not effective.
Conclusions
It was demonstrated that BPO as home treatment is effective in reducing C. acnes load on skin; it rarely causes side effects and is also cost-effective. This study highlights non-effectiveness of CHG. There was some evidence that the addition of hydrogen peroxide could have a positive effect in the reduction of C. acnes skin load; however, more studies are required.
Keywords: Cutibacterium acnes, Propionibacterium acnes, skin preparation methods, shoulder surgery
Introduction
Infections following shoulder surgery occur in 1.1% to 10% of operated patients, with some studies suggesting that the prevalence of shoulder surgery infections can reach 15%.1,2 Cutibacterium acnes (C. acnes) is the organism that most frequently causes shoulder infections, following all types of shoulder surgery.1–9
C. acnes (formerly Propionibacterium acnes) is a Gram positive anaerobic bacterium, which normally resides on the skin of healthy individuals and plays a significant role in its ecosystem, 10 even though occasionally it can act as an opportunistic pathogen. It is usually found on the sebaceous sites, including the upper chest and back.11,12
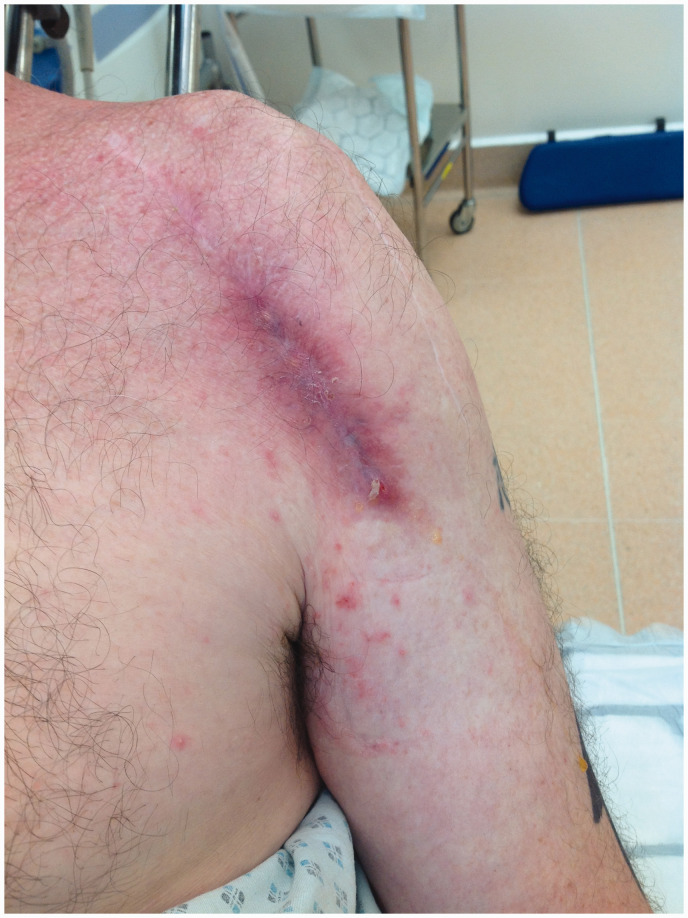
The main pathological characteristic of C. acnes, which is responsible for the pathogenesis of infection, is its ability to adhere to mechanical surfaces and form biofilms. Most orthopaedic C. acnes infections, which might take up to 2 years to develop, will present with symptoms such as fever, purulent discharge and joint effusion. Late infections may manifest as pain, joint stiffness or prosthesis dysfunction. 4 In order to deal with such complications, patients often require prolonged hospitalisation and antimicrobial therapy, and in the case of shoulder arthroplasty, revision shoulder surgery (Figure 1).
Figure 1.
Cutibacterium acnes shoulder infection.
Prevention of surgical infection comprises various pre-operative, perioperative and post-operative interventions. Interventions related to skin preparation include treatment at home before admission (skin scrub and/or topical skin treatment) and surgical skin preparation methods. The National Institute for Health and Care Excellence (NICE), the World Health Organisation (WHO) and the Centers of Disease Control and Prevention (CDC) have produced guidelines regarding skin preparation methods for prevention of surgical site infections.13–15 Multiple studies have been conducted aiming to evaluate the effectiveness of skin preparation methods in reducing the C. acnes burden in the shoulder, leading to various recommendations by individual researchers regarding infection prevention.16,17
However, there is a paucity of high-quality systematic reviews regarding the evaluation of the effectiveness of skin preparation methods in the reduction of C. acnes in shoulder surgery and evidence-based recommendations. This has resulted in a variety of studies making contradictory recommendations regarding the effectiveness of surgical skin preparation methods, many of which question the effectiveness of Chlorhexidine gluconate (CHG) which is suggested by NICE and WHO for prevention of surgical site infections.18–21
The purpose of this systematic review was to evaluate the effectiveness of skin preparation methods in the reduction of C. acnes in shoulder surgery and to identify whether the current guidelines and standard approach in prevention of shoulder infections are effective. Additionally, it explored whether novel and non-standard skin preparation methods, trialled in the last 5 years, could potentially be more effective in reducing the C. acnes skin load prior to or during shoulder surgery. In order to fulfil the purpose of this study, three objectives were set. The primary objective was to determine the proportion of positive cultures following skin preparation intervention and/or estimation of the number of viable C. acnes – expressed as colony forming units per millilitre (CFU/mL) following intervention. Secondary objectives were the estimation of cost-effectiveness and identification of side effects of the assessed interventions.
Materials and methods
Search strategy
Using the PICOS (Population-Intervention-Comparison-Outcome-Study) framework 22 (supplementary Appendix 1) the research question of this study was formulated as: ‘Which pre-operative skin preparation method is most effective in reducing C. acnes in patients over 16 years old undergoing shoulder surgery?’
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines 23 using a PRISMA checklist and was not registered. The databases of PubMed, Cochrane library and EBSCOhost were searched by an independent reviewer (MS), focusing on publications published from 1 June 2015 until 31 May 2020 (supplementary Appendix 2). The references of the included studies and recent review articles on prevention of C. acnes infection, as well as titles included in ‘Similar articles’ section of PubMed were manually searched for any additional relevant studies, and these were included. For the purpose of this paper, a second independent reviewer (GA) validated the process. Search terms included ‘skin preparation’, ‘home treatment’, ‘shoulder surgery’, ‘shoulder arthroplasty’, ‘shoulder arthroscopy’, ‘Cutibacterium’, ‘Propionibacterium’, ‘antisepsis’ and ‘decolonisation’. Following that, bibliographic data of the search results were imported in reference management software (EndNote X 6.0.1., Thomson Reuters). A library was created and the reference application was used for removal of duplicates. Eight more papers, which were irrelevant and/or abstracts, and editorial comments were removed.
Study selection
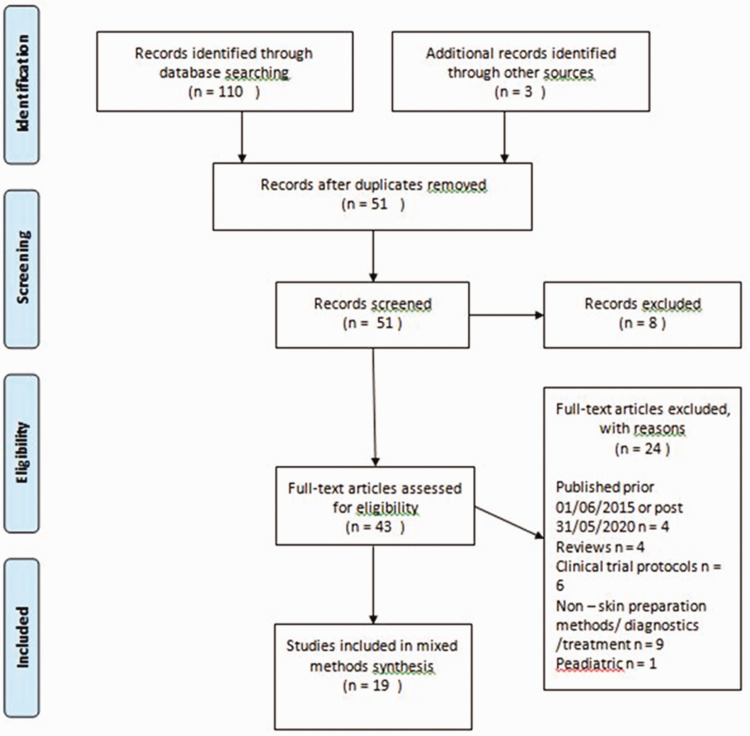
In total, 43 full-text articles remained which were screened for eligibility. Studies were eligible for inclusion if they were published between 1 June 2015 and 31 May 2020 in the English language. They were also eligible if their aim was the assessment of effectiveness of skin preparation methods in the reduction of C. acnes that had been carried out in patients undergoing any type of shoulder surgery. Clinical trials using healthy volunteers, as well as in vitro studies evaluating the effectiveness of skin preparation methods in the reduction of C. acnes skin load were also included. Papers were excluded if they were review studies or clinical trial protocols. They were also excluded if the study evaluated the effectiveness of preventive methods other than skin preparation (e.g. intravenous antibiotics) or diagnostic/treatment methods. Of the 43 full-text articles, 24 were excluded with reasons leaving 19 studies which were included in a mixed methods synthesis. Figure 2 shows the PRISMA diagram, depicting the literature search and selection process.
Figure 2.
PRISMA flowchart depicting the number of literature search, number of articles screened, full-texts retrieved and final select processed articles for the systematic review.
Methodological quality
The methodological quality of the included papers was assessed using the Critical Appraisal Skills Programme (CASP) criteria. 24 Of the 19 included studies, 7 were Randomised Control Trials (RCT), 7 were cohort studies, 3 were case series, 1 study was prospective case control study and 1 was proof of concept pilot study (in vitro). The only study that was not assessed with CASP was that of Hernandez et al., 25 because CASP does not include quality assessment tool for this type of study (in vitro). Overall, the basic study designs of the RCTs were valid and methodologically sound. Additionally, all the cohort studies, the case series and the case control study were conducted in an acceptable way; therefore, all 19 eligible studies were included in this review (supplementary Appendix 3).
Data extraction
Following assessment of methodological quality, data were extracted using spreadsheet software (Microsoft excel, 2010). Studies were divided into three groups according to the skin preparation method that was evaluated. The first group involved studies assessing the effectiveness of home treatment. The second group included studies evaluating surgical skin preparation method. The third group comprised studies evaluating both home treatment and surgical skin preparation. We extracted the authors, publication year, aim of the paper, type of study, sample size, demographics, type of procedure, skin preparation method, sampling method, patient compliance, follow-up, cost of treatment, complications and results reported as ‘most effective’.
Data synthesis and statistical analysis
In the first part of the review, a Bayesian approach, which allows final meta-aggregation of individual syntheses by transforming data into a mutually compatible format, was used to translate the finding of the quantitative and semi-quantitative studies to qualitative values. 26 These were pooled together with the qualitative studies into a combined mixed methods synthesis.
Descriptive statistics were used to report the general demographics of the review including data related to age, sex and type of procedure. Findings regarding the outcome of this study, which was the evaluation of effectiveness of skin preparation methods in the reduction of skin C. acnes load in shoulder surgery, were reported from ‘most effective’ to ‘least effective’ in reducing C. acnes. Since the data for the standard skin preparation, as well as for the evaluated skin preparation, for all groups, were significantly skewed towards either most effective or least effective, it was not feasible to proceed to in-depth meta-analysis.
Results
Nineteen studies have been included in this review.25,27–44 (supplementary Appendix 4). The total number of participants was 957, with a mean age of 59 years (range: 17–89) and 64% were male (Table 1). Four studies used participants undergoing shoulder arthroplasty,27–30 four studies used patients undergoing shoulder arthroscopy,31–34 one study was conducted using patients undergoing surgery for proximal humerus fracture, 35 one study used patients undergoing arthroplasty and arthroscopy, 36 six studies used healthy volunteers,37–42 two studies recruited patients undergoing open shoulder surgery43,44 and one study was in vitro. 25
Table 1.
Demographic data.
| Total all of the group |
Home treatment group |
Surgical skin prep group |
Aseptic protocol group |
|||||
|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |
| Participants | ||||||||
| Total | 957 | 426 | 501** | 30 | ||||
| Male | 622 | 64.05 | 264 | 61.97 | 351 | 70.05 | 7 | 23.33 |
| Female | 335 | 35.95 | 162 | 38.03 | 150 | 29.95 | 23 | 76.67 |
| Patients | 807 | 84.32 | 310 | 72.76 | 467 | 93.21 | 30 | 100 |
| Healthy volunteers | 150 | 15.68 | 116 | 27.24 | 34 | 6.79 | ||
| Age (Mean) | 58.99 years | 50.16 years* | 52.83 years | 74 years | ||||
| Procedure | ||||||||
| Arthroplasty | 5 | 3 | 1 | 1 | ||||
| Arthroscopy | 4 | 3 | 1 | |||||
| Proximal humeral fracture surgery | 1 | 1 | ||||||
| Open shoulder surgery | 2 | 2 | ||||||
| No procedure | 7 | 4 | 3 | |||||
*one study didn’t report mean age of participants (Sheer et al., 2018); **one study didn’t use participants (in vitro) (Hernandez et al., 2019).
Home treatment group
Nine studies evaluated skin preparation methods used as home treatment prior to shoulder surgery. Eight assessed the effectiveness of benzoyl peroxide (BPO)28,31,32,36,37,40–42 and one CHG alone. 30
Of the eight BPO studies, two assessed the effectiveness of BPO alone,32,37 three compared it against CHG,28,36,41 one study compared it against BPO/clindamycin and clindamycin alone, 40 one study compared it with placebo 42 and one reported the combination of BPO and clindamycin without control. 31 Six studies reported findings regarding side effects31,32,36,37,40,42 and two provided information regarding skin preparation’s cost.32,36
Six studies concluded that 5% w/v BPO gel was the most effective treatment against C. acnes.32,36,37,40–42 One study assessed the efficacy of BPO combined with clindamycin and concluded that the treatment effectively reduced C. acnes on the skin of patients undergoing arthroscopic shoulder surgery, 31 and only one study reported that neither BPO nor CHG reduced C. acnes successfully. 28 Finally, one study assessed the efficacy of CHG alone and found it is not effective in reducing C. acnes burden. 30
Side effects were reported in six studies,31,32,36,37,40,42 all of which assessed the efficacy of BPO in a combined total of 271 participants. Of these 271 participants, 9 (3.32%) developed side effects due to the treatment: 1 (0.37%) reported mild dermatitis, 2 (0.73%) reported itching, 2 (0.73%) developed redness of the skin, 1 (0.37%) reported dry skin, 1 (0.37%) reported flaking and 2 (0.73%) had a mild rash. With regards to cost–effectiveness, only Kolakowski et al. 36 and Sabetta et al. 32 reported on cost of BPO, which was estimated at $10 and $8.6 per patient, respectively (Table 2).
Table 2.
Results of home treatment group.
| Study | Sample size | Type of procedure | Skin preparation | Sampling method | Results reported as ‘most effective’ |
|---|---|---|---|---|---|
| Dizay et al. 31 | 65 patients (43 male/ 22 female) | Arthroscopy | C/BPO 1.2%/5% gel Application once per day (randomisation: 1–10 applications) | Skin swabs Tissue swabs | C/BPO effective Positive cultures in pre-surgical skin swab: > 1 application of C/BPO = 25.8% = 1 application of C/BPO = 33.3% Positive cultures in post-surgical tissue swab: C/BPO = 3.1% |
| Duvall et al. 37 | 34 healthy volunteers (23 male/11 female) | No procedure | 5% BPO gel Total three applications | Deep sebaceous glands samples | BPO effective in decreasing Cutibacterium acnes but not permanently Differences pre-treatment vs. rebound CFU counts: Anterior: (p = 0.29), Lateral: (p = 0.33), Posterior: (p = 0.66) Axilla: (p = 0.69) |
| Heckman et al. 40 | 12 healthy volunteers (10 male/ 2 female) | No procedure | 5% BPO gel vs. 1% clindamycin vs. 5% BPO + 1% clindamycin Total six applications | Dermal punch biopsy specimen | None effective in eradicating C. acnes BPO in decreasing C. acnes Positive biopsy cultures results: NC vs. BPO (p = 0.0833), NC vs. C/BPO (p = 0.1573), NC vs. CL (p = 0.1573) |
| Hsu et al. 28 | 49 male patients | Arthroplasty | 4% CHG solution vs. 10% BPO soap Total two applications | Skin surface swabs Dermal edge swabs | None Skin swabs: SpCuV similar in both groups (CHG 1.6 ± 1.1 vs. BPO 1.5 ± 1.4, p = 0.681) with 100% positivity Dermal edge swabs: similar in both groups (CHG 0.8 ± 1.0 vs. BPO 0.8 ± 1.4, p = 0.991) and positive cultures CHG 61% vs. BPO 46%, p = 0.369] |
| Kolakowski et al. 36 | 80 patients (37 male/ 43 female) | Arthroplasty (n = 27) Arthroscopy (n = 53) | 5% BPO gel vs. 4% CHG solution Total three applications | Deep sebaceous glands samples | BPO Decrease of positive cultures BPO treated site > BPO non-treated site (p = 0.0003), CHG treated site = CHG non-treated site (p = 0.80), BPO (anterior) > CHG (anterior) (p = 0.027), BPO (posterior) > CHG (posterior) (p = 0.005), BPO (lateral) > CHG (lateral) (p = 0.081), BPO (axilla) = CHG (axilla) (p = 0.99) |
| Matsen et al. 30 | 66 patients (44 male/ 22 female) | Arthroplasty | 4% CHG solution vs. no comparison Total two applications | Skin surface swabs Dermal edge swabs | None Average SpCuV 1.0 ± 0.9 prior CHG application = 1.0 ± 1.1 post CHG application and prior surgery (p = 0.585) |
| Sabetta et al. 32 | 50 patients (23 male/ 27 female) | Arthroscopy | 5% BPO vs. no comparison Total five applications | Skin swabs Joint fluid aspirate Deep tissue samples | BPO Anterior deltoid: BPO = 16% vs. no treatment = 32% (p = 0.001) Axilla BPO = 8% vs. no treatment = 28% (p = 0.013) |
| Scheer et al. 41 | 40 healthy volunteers (24 male/16 female) | No operation | 5% BPO gel vs. 4% CHG solution BPO: total five applications CHG: total three applications | Skin swabs | BPO BPO = 5% vs. CHG = 35% (p = 0.044) |
| van Diek et al. 42 | 30 healthy volunteers (11 male/19 female) | No operation | 5% BPO gel vs. placebo Total five applications | Skin swabs | BPO BPO = 20% vs. Placebo = 71.4% (p = 0.003) |
BPO: benzoyl peroxide; C/BPO: clindamycin/benzoyl peroxide; CFU: colony forming units; CHG: chlorhexidine gluconate; CL: clindamycin; NC: negative control; SpCuV: specimen Cutibacterium value.
Surgical skin preparation group
Nine studies assessed the effectiveness of surgical skin preparation methods against C. acne.25,27,33–35,38,39,43,44 Six were clinical trials and evaluated various concentrations of CHG either alone 43 or in combination with BPO, 38 in combination with iodine, 35 and in combination with isopropyl alcohol.34,39,44 Two studies evaluated hydrogen peroxide (H2O2) in combination with ChloraPrep (2% CHG and 70% isopropyl alcohol).27,33 The ninth was an in vitro study assessing H2O2 alone 25 (Table 3).
Table 3.
Results of surgical skin preparation group.
| Study | Sample size | Type of procedure | Skin preparation | Sampling method | Results reported as ‘most effective’ |
|---|---|---|---|---|---|
| Blonna et al. 35 | 40 patients (8 male/ 32 female) | Proximal humeral fracture | Single surgical skin preparation (1% iodine povidone/50% isopropyl alcohol) vs. double skin preparation (4% CHG followed by 1% iodine povidone/50% isopropyl alcohol) | Skin swabs | Single surgical skin preparation = double skin preparation PC: Single surgical skin prep = 17.5% vs. double skin prep = 17.5% (p = 1) Bacterial load: Single surgical skin prep CFU = 9.61*102 vs. double skin prep = 1.61*102 (p = 0.07) |
| Chalmers et al. 27 | 61 patients (29 male/ 32 female) | Arthroplasty | 3% H2O2 + standard surgical skin preparation vs. standard surgical skin prep (Standard surgical skin prep: 70% ethyl alcohol + 2 ChloraPrep) | Skin swabs Dermis swabs Joint swabs | 3% H2O2 + standard surgical skin prep Standard prep = 27% (25/93) vs. H2O2 = 16% (14/90) |
| Hancock et al. 38 | 22 male healthy volunteers | No procedure | 5% BPO + standard surgical skin preparation vs. standard surgical skin preparation (Standard surgical skin preparation: ChloraPrep) | Skin swabs | None 5%BPO + standard prep: n = 9 (20%) vs. standard prep: n = 6 (14%) p = 0.57 |
| Heckman et al. 39 | 12 male healthy volunteers | No procedure | 70% isopropyl alcohol vs. ChloraPrep (2% CHG and 70% isopropyl alcohol) vs. 2% CHG and 70% isopropyl alcohol with a 2 min mechanical scrub vs. 4% CHG and 70% isopropyl alcohol with a 2 min mechanical scrub | Dermal biopsies samples | None 70% isopropyl alcohol n = 7 (58%) vs. ChloraPrep n = 5 (42%) vs. 2% CHG and 70% isopropyl alcohol with a 2 min mechanical scrub n = 6 (50%) vs. 4% CHG and 70% isopropyl alcohol with a 2 min mechanical scrub n = 6 (50%) |
| Hernandez et al. 25 | N/a | N/a | 0%, 1%, 3%, 4%, 6%, 8% , 10% H2O2 in saline or water vs. 3% topical H2O2 solution | N/a | 3% H2O2 solution applied for 5 min 3% H2O2 for 5 min vs. water-only control: p < 0.0001 3% H2O2 vs. 3% H2O2 in water or saline: p < 0.0001 |
| MacLean et al. 43 | 50 patients (22 male/ 28 female) | Open shoulder surgery | 0.1% aqueous chlorhexidine | Dermal swabs | None Total PC: 38/150 (25%) Pre-prep vs. 60 min post: p = 0.043 5 min post vs. 60 min post: p = 0.123 Pre-prep vs. 5 min post: p = 0.617 |
| Phadnis et al. 44 | 50 patients (30 male/ 20 female) | Open shoulder surgery | ChloraPrep | Skin swabs Dermal swabs Dermal biopsy | None Positive samples pre-prep vs. post-prep vs. dermal swabs vs. dermal biopsy: pre-prep n = 21 (42%) vs. post-prep n = 7 (33%) vs. dermal swabs n = 26 (52%) vs. dermal biopsy n = 20 (40%) |
| Stull et al. 33 | 140 male patients | Arthroscopy | 3% H2O2 + standard surgical skin preparation vs. standard surgical skin preparation (Standard surgical skin preparation: 2% CHG + 7.5% povidone-iodine solution + 2 ChloraPrep | Punch biopsy samples | 3% H2O2 + standard surgical skin preparation H2O2 = 17.1% vs. control = 34.2% (p = 0.033) |
| Yamakado 34 | 126 patients (88 men and 38 women) | Arthroscopy | 1% CHG and 70% alcohol with drape vs. 1% CHG and 70% alcohol without drape vs. povidone iodine with drape vs. povidone iodine without drape | Skin swabs | 1% CHG and 70% alcohol with drape 1% CHG + 70% alcohol with drape n = 3/32 (9.3%) vs. 1% CHG + 70% alcohol without drape n = 10/30 (33%) vs. povidone iodine with drape n = 11/33 (33%) vs. povidone iodine without drape n = 14/31 (47%) |
BPO: benzoyl peroxide; CFU: colony forming units; ChloraPrep: 2% CHG + 70% isopropyl alcohol; CHG: Chlorhexidine gluconate; H2O2: hydrogen peroxide.
Chlorhexidine gluconate
Of the six studies assessing CHG, five concluded that CHG in various concentrations alone and/or combined with other solutions (BPO, iodine and isopropyl alcohol) was not effective in reducing C. acnes on the skin.35,38,39,43,44 Yamakado’s 34 study suggested that 1% CHG and 70% alcohol with drape was the most effective surgical skin preparation method in reducing C. acnes burden on the shoulder compared with the same solution used without drape, and iodine povidone with and without drape.
Three studies reported findings regarding side effects: these were the two studies evaluating H2O2 which reported no side effects.27,33 The third study was that of Heckmann et al. 39 which reported that use of CHG and isopropyl alcohol did not cause side effects. Cost of treatment was estimated by Chalmers et al. 27 for the H2O2 ($2 per 250 cc container) and by Hernandez et al. 25 for the same solution ($1.3 for a 473.18 ml).
Hydrogen peroxide
Two clinical trials assessed the efficacy of H2O2 combined with ChloraPrep and reported a significant reduction of C. acnes burden.27,33 Specifically, Chalmers et al. 27 found a statistically significant difference in joint positive cultures between the standard skin preparation group and the H2O2 group (p = 0.024). Similar findings were reported by Stull et al. 33 who found a statistically significant difference in dermal biopsy cultures between the two groups (p = 0.033). Furthermore, the in vitro study assessing the effectiveness of H2O2 alone also concluded that it was effective in reducing C. acnes burden significantly. 25
Aseptic protocol study
One study assessed the efficacy of a perioperative aseptic protocol in reducing C. acnes in patients undergoing shoulder arthroplasty. 29 The aseptic protocol comprised home treatment with 4% chlorhexidine – impregnated scrub, which patients used to shower 24 h prior to surgery – and surgical skin preparation which comprised 4% chlorhexidine – impregnated scrub followed by two applications of ChloraPrep (2% CHG and 70% isopropyl alcohol). No statistically significant reduction in C. acnes burden was observed after applying the aseptic protocol. Cost and side effects were not reported 29 (Table 4).
Table 4.
Results of aseptic protocol study.
| Study | Sample size | Type of procedure | Skin preparation | Sampling method | Results reported as ‘most effective’ |
|---|---|---|---|---|---|
| Koh et al. 29 | 30 patients (7 male/ 23 female) | Arthroplasty | Home treatment + Surgical skin preparation. Home treatment: 4% chlorhexidine – impregnated scrub – shower 24 h prior surgery. Surgical skin preparation: 4% chlorhexidine – impregnated scrub followed by two applications of ChloraPrep (2% chlorhexidine gluconate and 70% isopropyl alcohol) | Skin swabs Dermal swabs | None Total number of patients with positive C. acnes cultures: n = 22 (73%) S1 = 47% vs. S2 = 40% (p = 0.13) vs. S3 = 27% (p = 0.76) vs. D4 = 43% (p = 0.19) vs. D5 = 37% (p = 0.53) vs. S6 = 43% (p = 0.53) |
D: dermal swab; S: skin swab.
Discussion
This study reviewed studies that have been published from 1 June 2015 until 31 May 2020 aiming to identify the most effective home and surgical skin preparation method to reduce C. acnes skin load in shoulder surgery, with the anticipation that this might reduce the risk of subsequent C. acnes shoulder infections following surgery. To the authors’ knowledge, this is the first systematic review that evaluates the efficacy of home treatments as well as surgical skin preparation solutions in eliminating C. acnes, an anaerobic Gram positive bacterium that has been identified as a significant cause of the majority of post-operative shoulder infections.1–9
Nineteen studies were included in this review,25,27–44 which were divided into three groups: those assessing pre-surgical home treatments,28,30–32,36,37,40–42 those evaluating surgical skin preparation methods25,27,33–35,38,39,43,44 and those assessing an aseptic protocol. 29
Benzoyl peroxide gel
This systematic review demonstrated the effectiveness of 5% (w/v) BPO gel as pre-surgical home treatment in reducing the skin load of C. acnes in patients undergoing shoulder surgery. 5% (w/v) BPO gel can be effective in reduction of C. acnes skin load, if it is applied at least three times (once per morning) pre-operatively, with the last application being on the morning of surgery. From the studies reviewed, there was no evidence that 5% (w/v) and 10% (w/v) BPO can fully eradicate C. acnes, however.
BPO is relatively safe, with the main side effect being mild skin irritation. Application of 5% (w/v) BPO gel for five times (in 2.5 days) might result in development of mild side effects, even though the study of Heckmann et al. 40 used 5% (w/v) BPO gel six times (twice per day for 3 days and with last application being on the morning of specimen collection) without reporting side effects.
The efficacy of BPO could be explained by its ability to penetrate the follicles of the sebaceous glands, in which C. acnes normally resides. 45 BPO has a direct bactericidal effect destroying both surface and ductal bacterial organisms and yeast, without altering bacteria that normally reside on the skin.46,47 BPO has not been linked with development of C. acnes resistance, compared with other antimicrobial agents such as clindamycin and erythromycin.48,49 Therefore, its use as monotherapy may be preferable, considering there is no evidence of an additional benefit in combining BPO and clindamycin in reducing C. acnes load.
Effectiveness of home pre-operative treatment relies on patient compliance. Of the nine included studies in the home treatment group, five studies failed to monitor compliance and this poses a limitation.28,30,31,37,40 However, the fact that BPO was found to be the most effective treatment in the vast majority of studies reduces the possibility of results being affected by this omission. This review signifies that BPO is a simple treatment and apparently easy for patients to adhere to.
Chlorhexidine gluconate
This review confirmed findings of previous studies,18–21 which suggested that CHG is not effective in reducing C. acnes burden on shoulder either as home treatment or as surgical skin preparation method, despite the fact that CHG has been proven effective in vitro in eradicating up to 100% of Gram-positive and Gram-negative bacteria and fungi. 50
It appears that CHG cannot effectively penetrate the dermal layer of the skin and therefore cannot eliminate C. acnes which resides in the sebaceous glands. This finding is similar to studies previously published.7,16,17,20 However, it is important to note that CHG is very effective in reducing coagulase negative Staphylococci (CoNs) which are also responsible for post-operative shoulder infections. 51 This finding was also demonstrated by studies included in this review, which have reported effectiveness of CHG against CoNs.34,35,44
Hydrogen peroxide
No strong recommendation regarding the effectiveness of surgical skin preparation in reducing the skin load of C. acnes could be made by this review. The reason being that most included studies evaluated CHG solutions, which have been proven to be ineffective. Only two clinical trials27,33 and one in vitro study 25 have been performed in the last 5 years testing the efficacy of an alternative surgical skin preparation method such as H2O2.
Hydrogen peroxide is a reactive oxygen species which can act directly as a molecular oxidant and indirectly through free radical generation causing oxidative stress.52,53 Hydrogen peroxide is not an antibiotic, therefore cannot contribute to antimicrobial resistance; it is cheap and widely available. 25 It has multiple clinical uses in dermatology, as well as an antiseptic surgical solution. In concentration up to 6%, it has shown antimicrobial properties useful for wound care with limited side effects. 53 The 3% (w/v) H2O2 solution added to standard surgical skin preparation with CHG and alcohol showed effectiveness in reducing C. acnes;27,33 similar results were also reported by the in vitro study of Hernandez et al. 25
NICE, WHO and CDC suggest the use of CHG as the surgical skin preparation method of choice, which is not effective in reducing C. acnes skin load. The addition of H2O2 to standard skin preparation could be an effective and safe improvement of surgical skin preparation in shoulder surgery and could also result in a reduction of CoNs skin burden as well as C. acnes. We believe that more studies will be needed to form a stronger recommendation regarding the effectiveness of adding H2O2 to the surgical skin preparation.
Importance of C. acnes infection
The relationship between pre-operative C. acnes shoulder skin load and the risk of subsequent post-operative shoulder joint infection with C. acnes is much debated. Of the 18 clinical trials of this review, only one study reported rates of post-operative infections. 44 One could argue that finding C. acnes positive cultures in skin or dermis in patients undergoing shoulder surgery might not be clinically significant. Studies have suggested that the role of C. acnes in shoulder infection is overestimated and that positive cultures might be contaminants and therefore not posing real risk for development of infection. 7 Additionally, studies have suggested that even ‘true positive’ C. acnes culture may not indicate a real infection as patients with positive cultures do not show symptoms suggestive of infection.54,55 However, it is important to notice that C. acnes is a microorganism with very slow growth, causing low-grade infections which can take up to 2 years to develop. Most of the studies regarding infection prevention in shoulder surgery fail to follow-up their patients for so long. 56 This was also confirmed by this study. Of the 18 clinical trials included in this review, 12 recruited patients.27–36,43,44 Of those 12 studies, only 7 followed up their patients for 3–12 months post-surgery.27,31,32,34–36,43 Furthermore, the number of studies reporting C. acnes positive cultures in patients with septic arthritis, osteomyelitis, and particularly, prosthetic joint infection following shoulder surgery are multiple.57–59 It is also possible that C. acnes colonisation is generally underreported, since its culture in the laboratory requires incubation for up to 14 days. Therefore, patients attending post-operatively with symptoms of stiffness and pain, which are not typical for infection but often found in confirmed C. acnes infections, might not get screened; but even if they do, they might receive a negative result, because the majority of labs proceed their culture samples for up to 5 days. Improving lab techniques for identification of C. acnes could lead to earlier diagnosis of post-operative shoulder infection and consequently to instigation of treatment. Bokshan et al. 60 suggest that the use of an automated regulated anaerobic incubation system resulted in decrease of C. acnes culture growth from 6.5 days to 4.9 days.
The importance of decreasing C. acnes colonisation in patients undergoing shoulder surgery can be fully appreciated by understanding the way that C. acnes causes shoulder infection. C. acnes likely inoculates the surgical wound once incision is made through the sebaceous glands. For both primary and revision arthroplasties, the load of bacteria on the epidermis is predictive of the load of bacteria on the dermal wound edge and predictive of deep cultures, respectively.61,62 Following leakage into the surgical wound, C. acnes can participate in the formation of a biofilm on the implant surfaces, which could lead to failure of the joint arthroplasty many years later. 63 Therefore, decolonisation of the skin prior to shoulder surgery is thought to be important.
Additionally, infection prevention methods, such as administration of preoperative antibiotics, have been proven ineffective in decreasing C. acnes load. Matsen et al. 63 administered 2 g ceftriaxone and 1 g vancomycin intravenously to patients undergoing shoulder arthroplasty 30 min and 1 h prior to skin incision, respectively. They reported that deep tissue cultures which were taken after administration of antibiotics could still yield C. acnes. 63 Furthermore, in an RCT performed by Namdari et al., 64 patients undergoing shoulder arthroscopy were randomised to receive either 100 mg oral doxycycline twice per day for 7 days or no drug. Authors reported that doxycycline did not reduce C. acnes skin load significantly. 64 These findings highlight the importance of identifying effective skin preparation methods, like BPO and H2O2 which are not antibiotics, considering also the emergence of C. acnes antibiotic resistance.65,66
Strengths and limitations
The strength of this study is that it was a comprehensive systematic review with a large number of included studies. A limitation is that only 7 out of 19 studies were RCTs and 8 out of 19 lacked a control group. However, the included studies were qualitative. Another limitation of this study was the small number of participants that some studies recruited. However, the total number of participants in this review was 957, with most of them being patients undergoing surgery.
A major limitation is that it was impossible to reach a conclusion regarding the effectiveness of skin preparation methods in reducing post-operative C. acnes shoulder infection rates. However, considering that infections caused by C. acnes can take up to 2 years to manifest, and that few studies have followed up patients long term, it was decided that the aim of this study should be the evaluation of effectiveness in reducing C. acnes skin load rather than the rates of post-surgical joint infection.
This study met its objectives, which were to determine the proportion of positive cultures following skin preparation intervention and/or estimation of the number of viable C. acnes ,expressed as CFU/mL following intervention, and to examine side effects and assess cost-effectiveness of skin preparation methods.
Conclusion
C. acnes infection following shoulder surgery is an uncommon but devastating complication leading to prolonged antibiotic treatment and revision surgery. This review demonstrated that standard skin preparation methods are not effective in reducing C. acnes skin burden. CHG has been shown not to reduce C. acnes skin burden effectively, even though it is efficient in killing other pathogenic microorganisms. Therefore, it is recommended that 5% (w/v) BPO gel should be applied at least three times prior to shoulder surgery, with the last application being on the morning of surgery. Additionally, this review suggests that the use of 3% (w/v) H2O2 combined with ChloraPrep as surgical skin preparation could decrease C. acnes colonisation on shoulder without significantly increasing the cost of treatment and without causing significant side effects. However, more studies are required to form a more reliable evidence-based recommendation. Future studies could examine the effectiveness of an antiseptic protocol, which could combine a pre-operative home treatment preparation with 5% (w/v) BPO gel and a surgical skin preparation with 3% (w/v) H2O2 combined with CHG and alcohol (ChloraPrep). Considering paucity of evidence regarding C. acnes infection rates, future studies should follow up patients for at least 2 years in order to monitor the development of C. acnes joint infections. This could also provide more reliable evidence regarding the link between post-operative C. acnes positive skin and wound cultures and the development of C. acnes joint infections.
Supplemental Material
Supplemental material, sj-pdf-1-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-2-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-3-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-4-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplementary material: Supplementary material is available at: http://journals.sagepub.com
ORCID iD: Maria Sagkrioti https://orcid.org/0000-0002-4108-9836
Ethical Review and Patient Consent
Ethics approval was not required for the study. The image used was taken following written consent from the patient.
References
- 1.Fink B, Sevelda F. Periprosthetic joint infection of shoulder arthroplasties: diagnostic and treatment options. Biomed Res Int 2017; 2017: 4582756–4582756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saper D, Capiro N, Ma R, et al. Management of Propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med 2015; 8: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang MJ, Jancosko JJ, Mendoza V, et al. The incidence of Propionibacterium acnes in shoulder arthroscopy. Arthroscopy 2015; 31: 1702–1707. [DOI] [PubMed] [Google Scholar]
- 4.Kanafani ZA, Sexton DJ and Baron EL. Invasive Cutibacterium (formerly Propionibacterium) infections. UpToDate, https://www.uptodate.com/contents/invasive-cutibacterium-formerly-propionibacterium-infections?search=Cutibacterium%20(formerly%20Propionibacterium)%20acnes%20infections%20associated%20with%20implantable%20devices&usage_type=default&source=search_result&selectedTitle=1∼150&display_rank=1#H4 (2020, accessed 29 August 2020).
- 5.Levy PY, Fenollar F, Stein A, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis 2008; 46: 1884–1886. [DOI] [PubMed] [Google Scholar]
- 6.Mercurio M, Castioni D, Iannò B, et al. Outcomes of revision surgery after periprosthetic shoulder infection: a systematic review. J Shoulder Elbow Surg 2019; 28: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 7.Mook WR, Klement MR, Green CL, et al. The incidence of Propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. J Bone Joint Surg Am 2015; 97: 957–963. [DOI] [PubMed] [Google Scholar]
- 8.Nelson GN, Davis DE, Namdari S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2016; 25: 1337–1345. [DOI] [PubMed] [Google Scholar]
- 9.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am 2012; 94: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JE, Bumgarner RE, Matsen FA, 3rd. Propionibacterium in shoulder arthroplasty: what we think we know today. J Bone Joint Surg Am 2016; 98: 597–606. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood D, Barer M, Slack R, et al. Medical Microbiology. 18th ed. London: Churchill Livingstone Elsevier, 2012.
- 12.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011; 9: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017; 152: 784–791. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment, https://www.nice.org.uk/guidance/ng125/chapter/Recommendations#preoperative-phase (2019, accessed 10 March 2020).
- 15.World Health Organisation. Global guidelines for the prevention of surgical site infection, https://apps.who.int/iris/bitstream/handle/10665/250680/9789241549882-eng.pdf?sequence=8 (2016, accessed 10 March 2020).
- 16.Lee MJ, Pottinger PS, Butler-Wu S, et al. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am 2014; 96: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 17.Saltzman MD, Nuber GW, Gryzlo SM, et al. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am 2009; 91: 1949–1953. [DOI] [PubMed] [Google Scholar]
- 18.Clark JJC, Abildgaard JT, Backes J, et al. Preventing infection in shoulder surgery. J Shoulder Elbow Surg 2018; 27: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 19.Garrigues GE, Zmistowski B, Cooper AM, et al. Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: prevention of periprosthetic shoulder infection. J Shoulder Elbow Surg 2019; 28: S13–S31. [DOI] [PubMed] [Google Scholar]
- 20.Hackett DJ, Jr, Crosby LA. Infection prevention in shoulder surgery. Bull Hosp Jt Dis (2013) 2015; 73(Suppl 1): S140–S144. [PubMed] [Google Scholar]
- 21.Singh AM, Sethi PM, Romeo AA, et al. Strategies to decolonize the shoulder of Cutibacterium acnes: a review of the literature. J Shoulder Elbow Surg 2020; 29: 660–666. [DOI] [PubMed] [Google Scholar]
- 22.De Brún C. Finding the evidence: a key step in the information production process, https://www.england.nhs.uk/wp-content/uploads/2017/02/tis-guide-finding-the-evidence-07nov.pdf (2013, accessed 15 March 2020).
- 23.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Critical Appraisal Skills Programme. CASP appraisal checklists, https://casp-uk.net/casp-tools-checklists/ (2018, accessed 15 March 2020).
- 25.Hernandez P, Sager B, Fa A, et al. Bactericidal efficacy of hydrogen peroxide on Cutibacterium acnes. Bone Joint Res 2019; 8: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson A, White H, Bath-Hextall F, et al. A mixed-methods approach to systematic reviews. Int J Evid Based Healthc 2015; 13: 121–131. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers PN, Beck L, Stertz I, et al. Hydrogen peroxide skin preparation reduces Cutibacterium acnes in shoulder arthroplasty: a prospective, blinded, controlled trial. J Shoulder Elbow Surg 2019; 28: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JE, Whitson AJ, Woodhead BM, et al. Randomized controlled trial of chlorhexidine wash versus benzoyl peroxide soap for home surgical preparation: neither is effective in removing Cutibacterium from the skin of shoulder arthroplasty patients. Int Orthop 2020; 44: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 29.Koh CK, Marsh JP, Drinković D, et al. Propionibacterium acnes in primary shoulder arthroplasty: rates of colonization, patient risk factors, and efficacy of perioperative prophylaxis. J Shoulder Elbow Surg 2016; 25: 846–852. [DOI] [PubMed] [Google Scholar]
- 30.Matsen FA, Whitson AJ, Hsu JE. While home chlorhexidine washes prior to shoulder surgery lower skin loads of most bacteria, they are not effective against Cutibacterium (Propionibacterium). Int Orthop 2020; 44: 531–534. [DOI] [PubMed] [Google Scholar]
- 31.Dizay HH, Lau DG, Nottage WM. Benzoyl peroxide and clindamycin topical skin preparation decreases Propionibacterium acnes colonization in shoulder arthroscopy. J Shoulder Elbow Surg 2017; 26: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 32.Sabetta JR, Rana VP, Vadasdi KB, et al. Efficacy of topical benzoyl peroxide on the reduction of Propionibacterium acnes during shoulder surgery. J Shoulder Elbow Surg 2015; 24: 995–1004. [DOI] [PubMed] [Google Scholar]
- 33.Stull JD, Nicholson TA, Davis DE, et al. Addition of 3% hydrogen peroxide to standard skin preparation reduces Cutibacterium acnes positive culture rate in shoulder surgery: a prospective randomized controlled trial. J Shoulder Elbow Surg 2019; 29: 212–216. [DOI] [PubMed] [Google Scholar]
- 34.Yamakado K. Propionibacterium acnes suture contamination in arthroscopic rotator cuff repair: a prospective randomized study. Arthroscopy 2018; 34: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 35.Blonna D, Allizond V, Bellato E, et al. Single versus double skin preparation for infection prevention in proximal humeral fracture surgery. Biomed Res Int 2018; 2018: 8509527–8509527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolakowski L, Lai JK, Duvall GT, et al. Neer Award 2018: benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elbow Surg 2018; 27: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 37.Duvall G, Kaveeshwar S, Sood A, et al. Benzoyl peroxide use transiently decreases Cutibacterium acnes load on the shoulder. J Shoulder Elbow Surg 2020; 29: 794–798. [DOI] [PubMed] [Google Scholar]
- 38.Hancock DS, Rupasinghe SL, Elkinson I, et al. Benzoyl peroxide + chlorhexidine versus chlorhexidine alone skin preparation to reduce Propionibacterium acnes: a randomized controlled trial. ANZ J Surg 2018; 88: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 39.Heckmann N, Sivasundaram L, Heidari KS, et al. Propionibacterium acnes persists despite various skin preparation techniques. Arthroscopy 2018; 34: 1786–1789. [DOI] [PubMed] [Google Scholar]
- 40.Heckmann N, Heidari KS, Jalali O, et al. Cutibacterium acnes persists despite topical clindamycin and benzoyl peroxide. J Shoulder Elbow Surg 2019; 28: 2279–2283. [DOI] [PubMed] [Google Scholar]
- 41.Scheer VM, Bergman Jungestrom M, Lerm M, et al. Topical benzoyl peroxide application on the shoulder reduces Propionibacterium acnes: a randomized study. J Shoulder Elbow Surg 2018; 27: 957–961. [DOI] [PubMed] [Google Scholar]
- 42.van Diek FM, Pruijn N, Spijkers KM, et al. The presence of Cutibacterium acnes on the skin of the shoulder after the use of benzoyl peroxide: a placebo-controlled, double-blinded, randomized trial. J Shoulder Elbow Surg 2020; 29: 768–774. [DOI] [PubMed] [Google Scholar]
- 43.MacLean SBM, Phadnis J, Ling CM, et al. Application of dermal chlorhexidine antisepsis is ineffective at reducing Proprionibacterium acnes colonization in shoulder surgery. Shoulder Elbow 2018; 11: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phadnis J, Gordon D, Krishnan J, et al. Frequent isolation of Propionibacterium acnes from the shoulder dermis despite skin preparation and prophylactic antibiotics. J Shoulder Elbow Surg 2016; 25: 304–310. [DOI] [PubMed] [Google Scholar]
- 45.Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother 2009; 10: 2555–2562. [DOI] [PubMed] [Google Scholar]
- 46.Del Rosso J. Benzoyl peroxide cleansers for the treatment of acne vulgaris: status report on available data. Cutis 2008; 82: 336–342. [PubMed] [Google Scholar]
- 47.Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis 2007; 79: 9–25. [PubMed] [Google Scholar]
- 48.Kircik LH. The role of benzoyl peroxide in the new treatment paradigm for acne. J Drugs Dermatol 2013; 12: s73–s76. [PubMed] [Google Scholar]
- 49.Oprica C, Nord CE. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect 2005; 11: 204–213. [DOI] [PubMed] [Google Scholar]
- 50.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999; 12: 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray MR, Saltzman MD, Gryzlo SM, et al. Efficacy of preoperative home use of 2% chlorhexidine gluconate cloth before shoulder surgery. J Shoulder Elbow Surg 2011; 20: 928–933. [DOI] [PubMed] [Google Scholar]
- 52.Hyslop PA, Hinshaw DB, Scraufstatter IU, et al. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic Biol Med 1995; 19: 31–37. [DOI] [PubMed] [Google Scholar]
- 53.Murphy EC, Friedman AJ. Hydrogen peroxide and cutaneous biology: translational applications, benefits, and risks. J Am Acad Dermatol 2019; 81: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 54.Matsen FA, 3rd, Butler-Wu S, Carofino BC, et al. Origin of Propionibacterium in surgical wounds and evidence-based approach for culturing Propionibacterium from surgical sites. J Bone Joint Surg Am 2013; 95: e1811–e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sethi PM, Sabetta JR, Stuek SJ, et al. Presence of Propionibacterium acnes in primary shoulder arthroscopy: results of aspiration and tissue cultures. J Shoulder Elbow Surg 2015; 24: 796–803. [DOI] [PubMed] [Google Scholar]
- 56.Eck CF, Neumann JA, Limpisvasti O, et al. Lack of level I evidence on how to prevent infection after elective shoulder surgery. Knee Surg Sports Traumatol Arthrosc 2018; 26: 2465–2480. [DOI] [PubMed] [Google Scholar]
- 57.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg 2010; 19: 303–307. [DOI] [PubMed] [Google Scholar]
- 58.Levy O, Iyer S, Atoun E, et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013; 22: 505–511. [DOI] [PubMed] [Google Scholar]
- 59.Sperling JW, Cofield RH, Torchia ME, et al. Infection after shoulder instability surgery. Clin Orthop Relat Res 2003; 414: 61–64. [DOI] [PubMed] [Google Scholar]
- 60.Bokshan SL, Ramirez Gomez J, Chapin KC, et al. Reduced time to positive Cutibacterium acnes culture utilizing a novel incubation technique: a retrospective cohort study. J Shoulder Elbow Arthroplasty 2019; 3: 1–6. doi.org/10.1177/2471549219840823. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu JE, Neradilek MB, Russ SM, et al. Preoperative skin cultures are predictive of Propionibacterium load in deep cultures obtained at revision shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 765–770. [DOI] [PubMed] [Google Scholar]
- 62.MacNiven I, Hsu JE, Neradilek MB, et al. Preoperative skin-surface cultures can help to predict the presence of Propionibacterium in shoulder arthroplasty wounds. JB JS Open Access 2018; 3: e0052–e0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsen FA, 3rd, Russ SM, Bertelsen A, et al. Propionibacterium can be isolated from deep cultures obtained at primary arthroplasty despite intravenous antimicrobial prophylaxis. J Shoulder Elbow Surg 2015; 24: 844–847. [DOI] [PubMed] [Google Scholar]
- 64.Namdari S, Nicholson T, Parvizi J, et al. Preoperative doxycycline does not decolonize Propionibacterium acnes from the skin of the shoulder: a randomized controlled trial. J Shoulder Elbow Surg 2017; 26: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 65.Furustrand Tafin U, Aubin GG, Eich G, et al. Occurrence and new mutations involved in rifampicin-resistant Propionibacterium acnes strains isolated from biofilm or device-related infections. Anaerobe 2015; 34: 116–119. [DOI] [PubMed] [Google Scholar]
- 66.Sardana K, Gupta T, Garg VK, et al. Antibiotic resistance to Propionobacterium acnes: worldwide scenario, diagnosis and management. Expert Rev Anti Infect Ther 2015; 13: 883–896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-2-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-3-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow
Supplemental material, sj-pdf-4-sel-10.1177_17585732211032523 for Evaluation of the effectiveness of skin preparation methods for the reduction of Cutibacterium acnes (formerly Propionibacterium acnes) in shoulder surgery: a systematic review by Maria Sagkrioti, Stephen Glass and Georgios Arealis in Shoulder & Elbow