Abstract
Context:
Renal dysfunction is associated with poor outcomes in critically ill children.
Objectives:
To evaluate the current evidence for criteria defining renal dysfunction in critically ill children and association with adverse outcomes. To develop contemporary consensus criteria for renal dysfunction in critically ill children.
Data Sources:
PubMed and EMBASE were searched from January 1992 to January 2020.
Study Selection:
Included studies evaluated critically ill children with renal dysfunction, performance characteristics of assessment tools for renal dysfunction, and outcomes related to mortality, functional status, or organ-specific or other patient-centered outcomes. Studies with adults or premature infants (≤36 weeks gestational age), animal studies, reviews, case series, and studies not published in English with inability to determine eligibility criteria were excluded.
Data Extraction:
Data were extracted from included studies into a standard data extraction form by task force members.
Results:
The systematic review supported the following criteria for renal dysfunction: 1) urine output <0.5mL/kg/h for ≥6 hours and serum creatinine increase of 1.5–1.9 times baseline or ≥0.3mg/dL, or 2) urine output <0.5mL/kg/h for ≥12 hours, or 3) serum creatinine increase ≥2 times baseline, or 4) estimated glomerular filtration rate <35mL/min/1.73m2, or 5) initiation of renal replacement therapy, or 6) fluid overload ≥20%. Data also support criteria for persistent renal dysfunction and for high risk of renal dysfunction.
Limitations:
All included studies were observational and many were retrospective.
Conclusions:
We present consensus criteria for renal dysfunction in critically ill children, and for persistent renal dysfunction and risk for renal dysfunction.
Table of Contents Summary:
This manuscript reports a systematic review on renal dysfunction scoring tools and proposes evidence-based criteria for renal dysfunction in critically ill children.
INTRODUCTION
Renal dysfunction occurs commonly in critically ill children admitted to the pediatric intensive care unit (ICU) with an incidence of 25%.1–3 The hallmark of renal dysfunction is a reduced ability to clear waste, regulate electrolytes, and maintain fluid homeostasis. Traditionally, renal dysfunction has been defined based upon increased serum creatinine (SCr), oliguria, and the receipt of renal replacement therapy (RRT). Currently, consensus criteria for acute kidney injury (AKI) developed by the Kidney Diseases: Improving Global Outcomes (KDIGO) group represents the gold standard to define AKI.4
Renal dysfunction is common in the setting of the multi-organ dysfunction syndrome (MODS) and is independently associated with poorer short- and long-term outcomes.2,5–7 As a result, accurately characterizing renal dysfunction in children with MODS is critical. To this end, after completing a systematic review of the literature, the Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Renal Organ Dysfunction task force created a set of definitional criteria. These criteria are built upon the KDIGO AKI criteria, underscoring the importance of SCr and oliguria in identifying renal dysfunction.4 The PODIUM definition adds to KDIGO by incorporating total body fluid overload (FO); FO may occur in the absence of overt AKI and substantial literature supports the association of FO and poor outcomes.8–12 Additionally, FO has a dilutional effect on SCr which may mask a SCr rise, so inclusion of FO captures silent episodes of renal injury. While the definition proposed herein is operationally dichotomous, we offer recommendations regarding criteria for renal organ dysfunction persistence and risk, and tools for defining baseline SCr.
METHODS
The PODIUM collaborative sought to develop evidence-based criteria for organ dysfunction in critically ill children. The present manuscript reports on the systematic review on renal dysfunction scoring tools performed as part of PODIUM, provides a critical evaluation of the available literature, proposes evidence-based criteria for renal dysfunction in critically ill children, as well as recommendations for future research listed in the Data Supplement. The PODIUM Executive Summary details Population, Interventions, Comparators, and Outcomes (PICO) questions, search strategies, study inclusion and exclusion criteria, and processes for risk of bias assessment, data abstraction and synthesis, and for drafting and developing agreement for criteria indicating renal dysfunction.13
RESULTS
Systematic Review
Of 6007 unique citations published between 1992 and 2020 identified, 192 met the inclusion/exclusion criteria, as shown in the PRISMA flowchart (Fig. 1), data tables (Supplemental Tables 1 and 2), and risk of bias assessment summary (Supplemental Fig. 1). Seventy-seven studies were performed in a pediatric cardiac ICU population, while 103 were performed in non-cardiac, mixed cardiac and non-cardiac, or pediatric ICU populations of unknown composition. The remaining 12 studies were performed in newborn units or mixed inpatient settings that included pediatric ICU patients.
Figure 1.
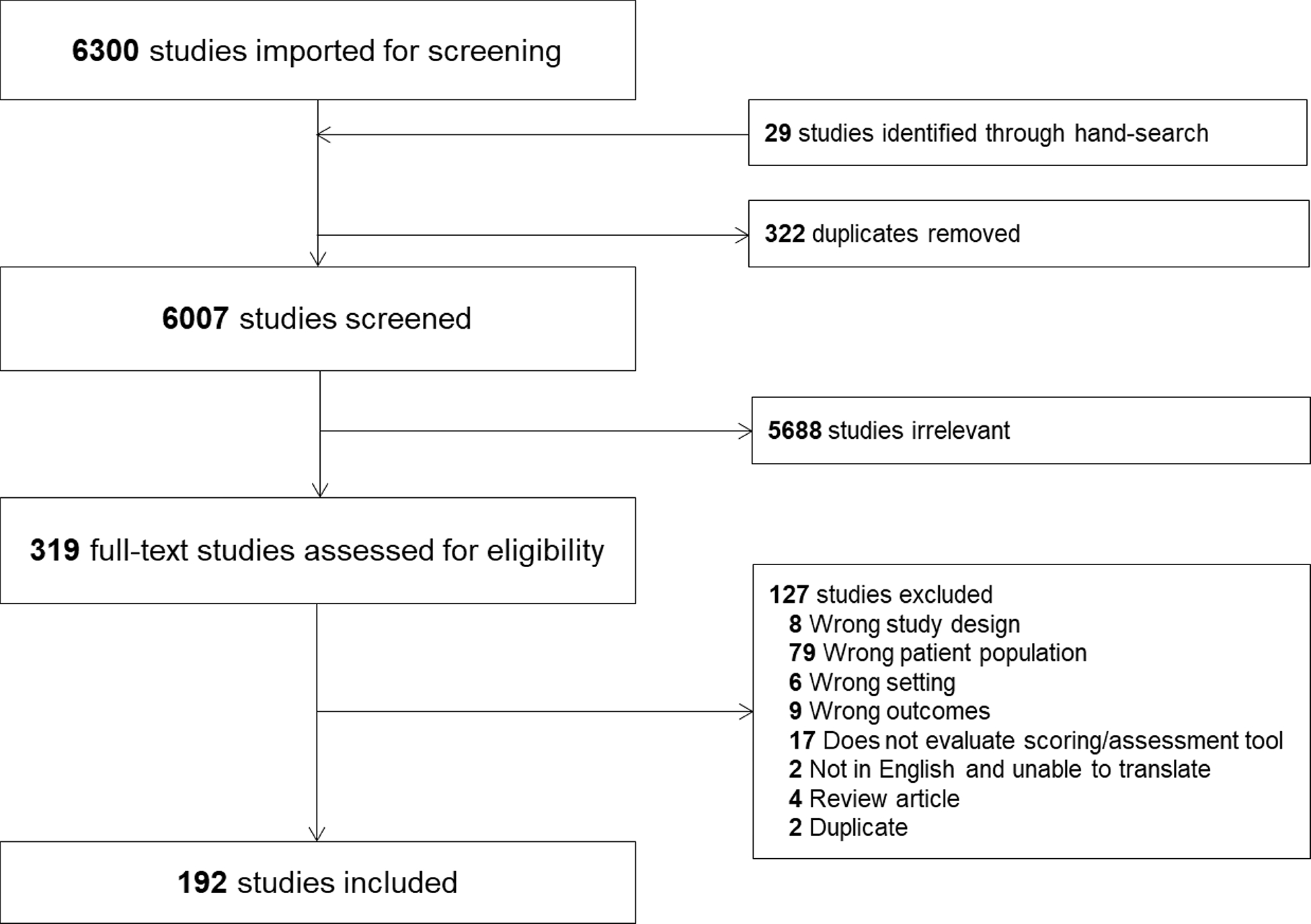
Study flow diagram according to the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols recommendations.
Thirty-eight studies evaluated existing AKI scoring systems [KDIGO, RIFLE (Risk Injury Failure Loss of kidney function End-stage kidney disease), pRIFLE (pediatric-modified RIFLE), AKIN (Acute Kidney Injury Network),]. Twenty-four studies evaluated FO. Eleven studies reported on scoring systems to predict renal dysfunction. A variety of studies used clinical tests, such as furosemide responsiveness, hemodynamic measures, or other novel scores predicting renal dysfunction or outcomes. Seventy-seven studies reported on biomarkers that may measure or predict renal dysfunction.
Criteria for Renal Organ Dysfunction and Rationale
MODS-associated renal dysfunction is defined when a critically ill child meets any ONE of the criteria listed in Table 1.
Table 1.
PODIUM: Criteria for Renal Organ Dysfunction in Pediatric Critical Illness
| Organ system | Criterion for organ dysfunction | Suggested thresholds | Conditions | Severity |
|---|---|---|---|---|
| Renal | Urine outputa | <0.5mL/kg/h for ≥6 hours | Concomitant serum creatinine increase 1.5–1.9 times baselineb OR ≥0.3mg/dL (≥26.5 μmol/L) increase | Not graded |
| <0.5mL/kg/h for ≥12 hours | None | Not graded | ||
| Renal | Serum creatinine | Increase 1.5–1.9 times baselineb OR ≥0.3mg/dL (≥26.5 μmol/L) increase | Concomitant urine outputa <0.5mL/kg/h for ≥6 hours | Not graded |
| Increase ≥2 times baselineb | None | Not graded | ||
| Renal | eGFRc | decrease to <35mL/min/1.73m2 | Excludes neonates <30 days of age | Not graded |
| Renal | Initiation of RRTd | NA | Initiation of RRT for any reason other than toxic ingestion or hyperammonemia | Not graded |
| Renal | Fluid overloade | 20% | Measured starting 48 hours after ICUf admission | Not graded |
Consider ruling out obstructive uropathy in the setting of low urine output
Use the lowest serum creatinine value available in the 3 months prior to admission as the baseline serum creatinine. If a prior serum creatinine is unavailable, baseline creatinine should be extrapolated from a normal eGFR for age and an appropriate estimating equation. In many critically ill children, heights are unavailable, making a height-independent equation preferential. Table 4 provides estimated baseline creatinine values based on a height-independent equation and normal reference eGFR for age. These creatinine values are derived from a healthy pediatric population30 and have been validated in critically ill children28.
eGFR: estimated glomerular filtration rate
RRT: renal replacement therapy
Fluid overload (FO) can be calculated using intake and output or weight. For weight-based determination, For ins/outs based determination, Use of weight-based formula for fluid overload is preferential if weight data are available.
ICU: intensive care unit
Rationale
1). Serum creatinine, urine output, and renal replacement therapy
Following the derivation of the RIFLE criteria,14 there have been three iterations of consensus criteria used to define AKI: the AKIN criteria,15 the pRIFLE criteria,16 and the KDIGO criteria.4 All utilize a combination of changes in SCr or creatinine clearance and urine output (UOP) to describe AKI thresholds ranging from “Risk” or Stage I to “Failure” or Stage 3. We reviewed 8 studies (n=19,382 children) that assessed the association between AKIN-defined Stage 2/3 AKI and mortality, length of stay (LOS), and duration of mechanical ventilation in mixed, cardiac, and non-cardiac ICU populations (Supplemental Table 3). All but one consistently reported increased odds of poorer outcomes in children with AKI with greater risk in higher AKI severity. Of the 20 studies (n=31,754) that used pRIFLE to define AKI, all found increased odds of mortality, LOS, and/or duration of mechanical ventilation; this association was strongest with “Injury” or “Failure” staged disease (Supplemental Table 3). Finally, there were 15 pediatric studies (n=37,837) investigating KDIGO-defined AKI with similar findings (Supplemental Table 3). The largest study, which examined 14,795 children from a single center over 5 years, assessed the association between mortality and AKI across all three sets of criteria. Regardless of the criteria used, incremental increases in the likelihood ratios for mortality at each stage were similar,3 supporting the consistency and validity of all three definitions. As KDIGO incorporates all prior definitions, is the most recent iteration, and is applicable to adults and children, we used these criteria as the basis of our renal dysfunction definition.4
It is important to note that data are inconsistent regarding the association between Stage 1 AKI and poorer outcomes. Thus, we believe that MODS-associated renal dysfunction should primarily be defined as meeting either the SCr or UOP criteria for KDIGO Stage 2 AKI. However, studies have found prognostically worse outcomes in children who met both SCr and UOP Stage 1 criteria rather than meeting either in isolation 5. As a result, we believe those who meet both Stage 1 SCr and UOP thresholds should be considered to have MODS-associated renal dysfunction.
2). Fluid overload
Data strongly support the physiologic relationship between FO and impaired renal function. Preservation of euvolemia is a primary renal function, and an inability to maintain fluid homeostasis indicates renal dysfunction. Epidemiological data has shown that the development of significant FO can pre-date meeting diagnostic criteria for AKI and delay the timely diagnosis of AKI, suggesting that FO may be an early biomarker of renal dysfunction. The interplay between FO and renal dysfunction is complex and most likely bidirectional, as both can lead to and exacerbate the other. Development of significant FO can further impair renal function by inhibiting renal perfusion (high venous pressure, interstitial edema, intraabdominal hypertension). While the complex relationship between FO and AKI warrants further study, mounting evidence exists on the independent association of FO with clinical outcomes.
We reviewed 24 pediatric studies (n= 3,632, Supplemental Table 4) that found consistent associations between positive net fluid balance and poor outcomes (oxygenation indices, duration of mechanical ventilation, LOS, mortality). FO, even in the absence of AKI, has been independently associated with morbidity and mortality in children.9,17–20 The incorporation of FO into renal scoring systems further supports its inclusion.21
Despite the strength of the aforementioned associations, the threshold for defining pathologic FO and the timing of assessment continue to be debated. Studies have found that even 5% FO (equivalent to 50 ml per kg) is associated with poorer outcomes,9,22 and that each 1% increase in fluid balance increases the odds of death incrementally by 3–8%.11,12,17,23 We recommend a conservative threshold of 20% FO as a criterion for renal dysfunction with the caveat that future data may support lower thresholds. We suggest that the reference weight for determining percent FO (Supplemental Table 4) should be the ICU admission weight as pre-admission weights are unavailable in many patients.
3). Timing:
To ensure complete and comparable data, the above criteria are to be evaluated every 24-hours beginning at ICU admission, with the exception of FO. FO should be measured as the cumulative fluid balance from admission to 48 hours after ICU admission and for every 24-hour period after that. Adjudication of the impact of cumulative FO should begin 48 hours after ICU admission as net fluid balance positive is to be expected during resuscitation, but inability to start toward diuresis beyond the initial resuscitative phase should be considered pathologic. The adjudication of timing and threshold of FO, proper delineation of epochs of fluid balance 24, and the impact on the ICU course are important areas of future research.
Determination of Baseline Creatinine
Determination of baseline SCr is imperative when defining renal function. Prior studies have found variation in AKI incidence depending on the baseline determination method used, emphasizing the importance of standardization.25
The ideal baseline SCr would be one measured prior to critical illness. In practice, SCr measurements are frequently unavailable in pediatric patients.26,27 When such measurements are available, the most common practice is to use the lowest value from the 3 months prior to admission as a baseline.16,26 As a general principle, the first SCr obtained during critical illness should not be used as the baseline as renal dysfunction is often present on admission.25,27
When a pre-admission SCr is not available, one must be estimated. An approach often used is to back-calculate a baseline SCr using the Schwartz formula assuming a “normal” estimated glomerular filtration rate (eGFR) of 120 mL/min/1.73 m2.25,28 This approach presents two potential issues: (1) the heights required for the Schwartz equation are commonly not available,27 and (2) the “normal” pediatric GFR has age-dependent variation, especially in the first 2 years if life.29 Another approach, which addresses these issues, is the use of height independent, age- and gender-based norms.29,30 Normal age- and gender-based SCr values are provided in Table 2;31 we recommend using these values as a proxy baseline SCr when prior measurements are not available as the standard approach.
Table 2. Determination of Baseline Serum Creatinine.
If pre-admission serum creatinine (SCr) measurements are available for a patient, use the lowest SCr in the 3 months prior to admission as baseline. If a prior SCr is unavailable, a baseline SCr based on age and gender norms may be used. The SCr values in the table were derived from healthy children and have been validated in critically ill pediatric patients.28,29
| Age | Reference eGFRa,28 | Baseline SCrb Boys | Baseline SCrb Girls |
|---|---|---|---|
| < 1 month | 45 | 0.57 | 0.62 |
| 1–2 months | 55 | 0.43 | 0.46 |
| 3–5 months | 70 | 0.35 | 0.37 |
| 6–11 months | 85 | 0.31 | 0.32 |
| 12–17 months | 90 | 0.32 | 0.32 |
| 18–23 months | 100 | 0.31 | 0.31 |
| 2–4 years | 120 | 0.31 | 0.30 |
| 5–7 years | 120 | 0.37 | 0.37 |
| 8–11 years | 120 | 0.46 | 0.46 |
| 12–18 years | 120 | 0.65 | 0.58 |
eGFR: estimated glomerular filtration rate
SCr: serum creatinine
Persistent Renal Dysfunction
The best available data demonstrate that non-transient renal dysfunction carries additional outcome risk. Thus, we propose a subcategory of MODS-associated renal dysfunction: persistent renal dysfunction. Persistent renal dysfunction should be defined in patients meeting any one of five criteria for >48 hours:
UOP <0.5mL/kg/h
Increase in SCr of ≥2 times baseline
Decrease in eGFR to <35ml/min/1.73m2 (eGFR criterion excludes neonates <30 days of age)
Use of RRT for any reason other than toxic ingestion or hyperammonemia
20% FO
Rationale
Renal dysfunction criteria are inherently time-dependent, with stratified severity phenotypes that are associated with outcomes. In addition to severity strata, renal dysfunction can be broken down into time courses: transient, in which a patient regains baseline renal function within 48 hours, and persistent, in which a patient demonstrates renal dysfunction past 48 hours.32
In an effort to harmonize these concepts, the Acute Disease Quality Initiative (ADQI) 16 Workgroup defined persistent renal dysfunction lasting > 48 hours. In our literature review, persistent renal dysfunction carries higher risk for poorer outcomes including increased RRT, LOS, and mortality when compared with transient dysfunction.16,21,33–39
Classifying a separate phenotype of persistent renal dysfunction allows evaluation of a distinct cohort of patients whose renal dysfunction does not respond to initial resuscitation alone. Early identification of persistent renal dysfunction may allow for tailored therapeutic strategies for potential intervention, both in clinical trials and quality improvement efforts. Defining this cohort of patients signals to practicing clinicians the importance of reassessing the patients’ risk factors for additional organ dysfunction and ongoing kidney disease. Finally, given the multifactorial pathophysiology of renal dysfunction, it also allows the clinician to reevaluate the consequences of renal dysfunction and the therapies being utilized to treat the systemic disease as renal function changes.
Determining Risk for Renal Dysfunction
Some patients are at higher risk for developing renal dysfunction. At the present time, widely available diagnostic tests continue to lack sensitivity for early stage or subtle injury, making the determination of ‘risk’ crucial for clinicians adjudicating multi-organ injury. Systematic and objective criteria are required for the assessment of patients at risk for renal dysfunction. While not part of the consensus criteria for renal organ dysfunction, three discrete metrics can be used to identify the at-risk patient, and patients meeting any one of these should be considered at risk of developing renal dysfunction:
UOP <0.5mL/kg/h for ≥6 hours in a single ICU day
Increase in SCr of 1.5 to 1.99 times baseline (or an absolute increase of ≥0.3mg/dL (26.5 umol/L)
15% FO
Although the first two of these metrics fulfill the KDIGO definition of Stage 1 AKI, there are few data in any population demonstrating adverse outcomes associated with this stage. However, incipient AKI can be progressive and evidence suggests even subtle changes may represent a separate risk tier. As a result, we feel that these patients should be categorized as at risk for developing MODS-associated renal dysfunction. The third metric for identifying patients “at-risk” is drawn from the renal angina index (RAI), which utilizes a FO of 15% to define higher AKI risk. The RAI is a score measured 12 hours into the ICU course which has been used to identify patients at highest risk for developing severe AKI after three ICU days.21 Once at-risk patients are identified, a systematic daily evaluation of kidney function is recommended. A multimodal approach, combining markers of filtration, tubular function (urine output or response to diuresis), assessment of FO, and exposure to nephrotoxins may be useful for associative predictions with patient outcome.40
CONCLUSIONS
Renal dysfunction is common in critically ill children and negatively impacts ICU outcomes. After a systematic review of 192 published manuscripts via a modified Delphi process, we present criteria for MODS-associated renal dysfunction in critically ill children that include measures of SCr, UOP, RRT, and FO, and provide criteria for persistence of and risk for renal dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the contributions to the early portion of the project, including formulation of literature search terms, title and abstract review, and full text data extraction, by Dr. Michael Zappitelli.
Funding/Support:
The Russell Raphaely Endowed Chair for Critical Care Medicine at the Children’s Hospital of Philadelphia contributed to funding for publication costs for this manuscript. Dr. Fitzgerald is supported by NIH NIDDK K23DK119463.
Abbreviations:
- ADQI
Acute Disease Quality Initiative
- AKI
acute kidney injury
- AKIN
Acute Kidney Injury Network
- eGFR
estimated glomerular filtration rate
- FO
fluid overload
- ICU
intensive care unit
- KDIGO
Kidney Diseases: Improving Global Outcomes
- LOS
length of stay
- MODS
multi-organ dysfunction syndrome
- PICO
population, interventions, comparators, and outcomes
- PODIUM
Pediatric Organ Dysfunction Information Update Mandate
- RAI
renal angina index
- RIFLE
Risk Injury Failure Loss End-stage
- pRIFLE
pediatric-modified RIFLE
- RRT
renal replacement therapy
- SCr
serum creatinine
- UOP
urine output
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this article to disclose.
The guidelines/recommendations in this article are not American Academy of Pediatrics policy, and publication herein does not imply endorsement.
REFERENCES
- 1.Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M. Acute Kidney Injury in Critically Ill Children and Subsequent Chronic Kidney Disease. Can J Kidney Health Dis. 2019;6:2054358119880188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Section 2: AKI Definition. Kidney Int Suppl (2011). 2012;2(1):19–36.25018918 [Google Scholar]
- 5.Kaddourah A, Basu RK, Goldstein SL, Sutherland SM, Assessment of Worldwide Acute Kidney Injury RAaEI. Oliguria and Acute Kidney Injury in Critically Ill Children: Implications for Diagnosis and Outcomes. Pediatr Crit Care Med. 2019;20(4):332–339. [DOI] [PubMed] [Google Scholar]
- 6.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530. [DOI] [PubMed] [Google Scholar]
- 7.Uber AM, Sutherland SM. Acute kidney injury in hospitalized children: consequences and outcomes. Pediatr Nephrol. 2020;35(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13(3):253–258. [DOI] [PubMed] [Google Scholar]
- 9.Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med. 2014;15(2):131–138. [DOI] [PubMed] [Google Scholar]
- 10.Kwiatkowski DM, Krawczeski CD. Acute kidney injury and fluid overload in infants and children after cardiac surgery. Pediatr Nephrol. 2017;32(9):1509–1517. [DOI] [PubMed] [Google Scholar]
- 11.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. [DOI] [PubMed] [Google Scholar]
- 13.Bembea MM, Agus M, Akcan-Arikan A, et al. Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Contemporary Organ Dysfunction Criteria: Executive Summary. Pediatrics. 2022;149(1 Suppl 1):S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8(6):509–514. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. [DOI] [PubMed] [Google Scholar]
- 17.Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–1776. [DOI] [PubMed] [Google Scholar]
- 19.Sinitsky L, Walls D, Nadel S, Inwald DP. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16(3):205–209. [DOI] [PubMed] [Google Scholar]
- 20.Valentine SL, Sapru A, Higgerson RA, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40(10):2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Wang J, Bai Z, et al. Early fluid overload is associated with acute kidney injury and PICU mortality in critically ill children. Eur J Pediatr. 2016;175(1):39–48. [DOI] [PubMed] [Google Scholar]
- 23.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37(7):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. [DOI] [PubMed] [Google Scholar]
- 25.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Pinto LN, Goldstein SL, Schneider JB, Khemani RG. Association Between Progression and Improvement of Acute Kidney Injury and Mortality in Critically Ill Children. Pediatr Crit Care Med. 2015;16(8):703–710. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessey E, Ali R, Dorais M, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol. 2017;32(10):1953–1962. [DOI] [PubMed] [Google Scholar]
- 30.Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29(5):1082–1091. [DOI] [PubMed] [Google Scholar]
- 31.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. Kidney Int Suppl. 2012;2(1):1–38. [Google Scholar]
- 32.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. [DOI] [PubMed] [Google Scholar]
- 33.Basu RK, Kaddourah A, Goldstein SL, Investigators AS. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health. 2018;2(2):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gawadia J, Mishra K, Kumar M, Saikia D. Prediction of Severe Acute Kidney Injury using Renal Angina Index in a Pediatric Intensive Care Unit. Indian Pediatr. 2019;56(8):647–652. [PubMed] [Google Scholar]
- 35.Menon S, Goldstein SL, Mottes T, et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31(4):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sethi SK, Raghunathan V, Shah S, et al. Fluid Overload and Renal Angina Index at Admission Are Associated With Worse Outcomes in Critically Ill Children. Front Pediatr. 2018;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanski N, Menon S, Goldstein SL, Basu RK. Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care. 2019;53:1–7. [DOI] [PubMed] [Google Scholar]
- 38.Wilder NS, Yu S, Donohue JE, Goldberg CS, Blatt NB. Fluid Overload Is Associated With Late Poor Outcomes in Neonates Following Cardiac Surgery. Pediatr Crit Care Med. 2016;17(5):420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong HR, Cvijanovich NZ, Anas N, et al. A Multibiomarker-Based Model for Estimating the Risk of Septic Acute Kidney Injury. Crit Care Med. 2015;43(8):1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akcan-Arikan A, Gebhard DJ, Arnold MA, Loftis LL, Kennedy CE. Fluid Overload and Kidney Injury Score: A Multidimensional Real-Time Assessment of Renal Disease Burden in the Critically Ill Patient. Pediatr Crit Care Med. 2017;18(6):524–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


