Summary
We investigated the awareness, perceived usefulness, and use of genetic testing among Hispanic and Latino individuals. Annual follow-up surveys for the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) from 2019 to April 2020 assessed participants’ level of awareness and use of genetic tests to determine disease risks, likelihood of passing disease to children, disease treatment, or drug selection. They also were asked to rate the usefulness of the tests for managing a person’s health on a 1 (not at all useful) to 10 (extremely useful) scale. There were 5,769 HCHS/SOL participants who completed at least one survey question. Of the target population, 55.2% was aware of at least one type of genetic test. Awareness varied between HCHS/SOL enrollment sites and was higher among individuals who had higher educational attainment and had higher incomes. Only 3.3% of the target population reported receiving one or more of the tests described. HCHS/SOL individuals rated the usefulness as 8.4, on average, with lower scores observed among U.S.-born individuals compared to individuals born outside the United States, with differences by HCHS/SOL enrollment sites. In conclusion, while awareness of genetic testing among Hispanic and Latino individuals varies by location, education, and income, perceptions about its usefulness are high while experiences with testing are rare. Results identify groups and locations that may benefit from greater outreach about the capabilities of genetic testing and precision medicine.
Key words: genetic testing, ELSI; Hispanic or Latino; awareness; survey; perceived usefulness; population-based study
Hispanic and Latino individuals who are unaware of genetic testing may not benefit from genomic medicine improvement. Our survey of the HCHS/SOL participants showed low use of genetic tests despite positive usefulness perceptions, and found awareness of genetic testing varied by location, education, and income.
Introduction
The roles of genomic testing in all aspects of medicine are expanding rapidly. Already, genomic testing is accelerating diagnoses and identifying individuals with genetic predispositions for highly actionable conditions.1,2,3,4 Pharmacogenomic (PGx) testing allows healthcare providers to tailor treatment decisions and medication dosing according to individuals’ genomic profiles.5,6,7 Preconception and prenatal genomic testing assists potential parents with reproductive decisions by informing them about their carrier status for genetic disorders.8,9,10 These examples, among others, demonstrate the increasing usefulness and breadth of genomic testing applications in healthcare.
Analyses of genomic testing show uneven uptake between racial and ethnic groups, including Hispanic and Latino populations.11,12,13 Many factors contribute to these disparities, including access to services, how often healthcare providers recommend testing, and logistical barriers. Awareness about genomic tests may be particularly important. Racial and ethnic minority populations typically report lower awareness and knowledge about genetic testing.11,12 Hispanic and Latino populations merit special attention. Comprising more than 18% of the population, Hispanic and Latino populations are the largest racial and ethnic minority group in the United States. Numerous studies report that awareness and use of genetic testing among U.S. Hispanics and Latinos is low compared with non-Hispanics/Latinos.14,15,16,17,18,19,20,21 In contrast, a survey conducted in 2017 showed no statistical differences by race and ethnicity in terms of knowledge about genetic testing. Studies that address characteristics associated with genetic testing awareness among Hispanic and Latino individuals are lacking.
Attitudes toward genomic testing, including perceived usefulness, may also influence the use of genetic tests. Perceived usefulness is a key component of numerous health behavior theories, including the health belief model, protection motivation theory, and adaptations of self-regulation theory to address genetic service use22,23,24 and has consistently been shown to influence genomic testing uptake.25,26 A number of studies suggest that, while most communities have positive attitudes toward genomic testing,15 racial and ethnic minority communities tend to perceive less usefulness to genomic testing than non-Hispanic White populations. Limited research has examined factors that may influence the perceived usefulness of genomic testing specifically among Hispanics and Latinos.
We addressed this lack of data by surveying participants of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).27 We expanded the annual follow-up survey administered by this large multi-center epidemiologic study in Hispanic and Latino populations to gather data about participants’ awareness and use of different genetic tests. The goal of our study was to provide population-based insights about factors that may influence awareness, perceived usefulness, and genomic testing uptake.
Materials and methods
The HCHS/SOL
The HCHS/SOL is a population-based longitudinal cohort study established to study risk and protective factors in cardiovascular disease development among Hispanic and Latino individuals in the United States. Details of the HCHS/SOL study design and cohort have been reported previously.27 Briefly, the study follows 16,415 Hispanic and Latino participants aged 18–74 years recruited in field centers from four metropolitan areas: The Bronx, New York; Miami, Florida; Chicago, Illinois; and San Diego, California. Individuals were recruited via probability sampling from pre-defined census block units chosen to provide diversity with respect to socioeconomic status as well as national origin or Hispanic or Latino background.28 To recruit Spanish- or English-speaking individuals into the HCHS/SOL who self-identify as having a Hispanic or Latino background, research staff asked potential participants the following question during screening: “Do you consider yourself to be Hispanic or Latino?” If the question was not clear to the potential participant, the interviewer clarified with the statement, “We consider Hispanic/Latino individuals to be people from Latin America, South American, Central American, and the Caribbean.” A baseline clinic visit took place from 2008 to 2011, and individuals participated in a second clinic visit 6 years later, on average. Participants were asked during their interview to self-identify with specific Hispanic or Latino background, “Which of the following best describes your Hispanic/Latino heritage?”, with potential responses being Dominican or Dominican descent, Central American or Central American descent, Cuban or Cuban descent, Mexican or Mexican descent, Puerto Rican or Puerto Rican descent, South American or South American descent, more than one heritage, or other. Thus, individuals are identified with the following Hispanic or Latino backgrounds: Mexican, Central American, Cuban, Dominican, Puerto-Rican, South American, and other or multiple backgrounds. All study participants provided written informed consent at their recruitment center during their clinical exam. The informed consent form included the statement “I (agree/do not agree) to allow HCHS/SOL staff to contact me once a year to ask questions about my health and where I live.” All enrolled individuals agreed to this condition and were asked to participate in a series of annual follow-up (AFU) phone calls. Protocols were approved by the institutional review boards at each institution of enrollment.
Measures of genetic testing awareness, use, and perceived benefits
Between April 2019 and April 2020, the HCHS/SOL AFU survey included items about genetic testing awareness, use, and perceived benefits (Figures S1 and S2). Awareness questions were adapted from items in the 2017 National Cancer Institute’s Health Information National Trends Survey (HINTS),29 asking respondents, “Have you heard of a genetic test to determine” each of the following: (1) “the risk or likelihood of getting a particular disease,” (2) “the likelihood of passing an inherited disease to your children,” (3) “to determine how a disease should be treated after diagnosis,” and (4) “to determine which drug(s) may or may not work for an individual.” Survey administrators classified responses as yes, no, or refuse to answer, with instructions to classify don’t know and not sure responses as refusals. Don’t know/not sure was omitted as a formal response option to mirror the design of the HINTS survey and facilitate data comparisons. All individuals who answered an item were asked, “If offered to you, would you be interested in receiving this kind of test?” with responses classified as yes, no, not sure/it depends, and refuse to answer. Individuals who responded yes about hearing about a type of genetic test were also asked if they had ever been offered such a test, and, if so, whether they had received the test. Response options on these types of questions were classified as yes, no, don’t know, and refuse to answer. All survey participants were also asked to rate the usefulness they perceived in genetic testing. The question was presented as, “On a scale of 1–10, where 1 is ‘not at all’ and 10 is ‘extremely,’ how useful do you think genetic testing is for managing a person’s health?”, with response options ranging from 1 (not at all useful) to 10 (extremely useful). This item was adapted from a pilot clinical trial of genomic sequencing.30
The survey was administered in either English or Spanish, depending on participant preference. Survey items are presented in the Appendix.
Covariates
We extracted fixed and time varying covariates from the data collected at the most recent HCHS/SOL examination available for each individual. Covariates included age at the time of survey completion (categorized as 25–40, 41–60, or ≥61 years), gender, study center (Bronx, Chicago, Miami, or San Diego), self-reported Hispanic or Latino background (Dominican, Mexican, Central American, Cuban, Puerto Rican, South American, or more than one/other), educational attainment (less than high school degree, high school degree, associate, bachelor, or vocational degree, or masters, doctoral or professional degree), household income level (<$10,000, $10,001–20,000, $20,001–40,000, $40,001–75,000, or >$75,000), current health insurance status, number of doctor visits within the prior year at the baseline visit (categorized to no visits, 1–2 visits, or ≥3), employment status (retired/not currently employed, not retired but not currently employed, employed part time [≤35 h a week], or employed full time [>35 h a week]), nativity (born in the 50 U.S. states or not), and marital status (single, married or living with a partner, separated, divorced or widowed). Marital status was available only from the baseline exam and second AFU year (7–9 years before the genetic testing survey items were administered). Although a question about self-identified race was administered to HCHS/SOL participants, it was not used in the analyses because the majority of HCHS/SOL participants refused to answer the question or reported other. In computation of the inverse probability weights (described below) we also used sampling strata as a covariate.
Computation of inverse probability weights for population-representative estimates
To obtain estimates that were applicable to the target population represented by the HCHS/SOL, we applied inverse probability weighting (IPW) to respondents’ data. Weights were estimated as , where was the probability of a person from the target population participating in HCHS/SOL, was the probability of an HCHS/SOL subject participating in the relevant AFU survey, and was the probability of AFU survey participants responding with a yes or no response to at least one of the genetic testing awareness items (e.g., if an individual was classified as refuse to answer to all four testing awareness questions, they were considered as non-respondent). We estimated and using logistic regression. Logistic regression used all covariates described above. For time-varying covariates, we used the measured values from visit 1 to compute , and, when possible, values from visit 2 to compute . Missing data in these models were imputed via fully conditional specification using the mice R package (version 3.13.0) with five iterations to create each of five imputed datasets. For each imputed dataset, we obtained estimates of and , and then averaged them. The final estimates and are based on these averages, e.g., where is the inverse of the logistic function and is the average of the estimates across the five imputed datasets. While we used imputation to generate IPW weights, in association analyses when adjusting to covariates, we performed a complete case analysis. In secondary analyses, we also computed the IPW for participation in the AFU as .
Data analyses
All analyses, including computations of percentages and association analyses, accounted for the stratified sampling strategy and clustering of HCHS/SOL participants by applying functions from the survey R package (version 4.0).
Primary analyses of genetic test awareness used a composite measure, where individuals were assigned value of 1 (aware) if they reported awareness of any of the four types of genetic tests described and 0 (unaware) if they were not aware of any test and answered at least one item about genetic test awareness. Individuals with no data on any genetic test awareness items because of refusals and missing data were considered non-respondent and omitted from analyses (although they were taken into account in the IPW computation). A similar approach was used to classify whether participants were interested in these genetic tests, as well as to classify whether they had ever been offered or used them (individuals who were unaware of these tests were classified as never being offered them, and individuals who were never offered these tests were classified as never using them, in agreement with the survey’s skip pattern). Participants who were classified as don’t know/not sure on items about being offered or using genetic were included in analyses as not being offered or using those tests.
We identified demographic and healthcare-related factors associated with genetic testing awareness using logistic regression. We identified demographic and healthcare-related factors associated with perceived usefulness using Poisson regression, because of the highly skewed distribution of the responses. We used contrasts to compare awareness and perceived usefulness of genetic tests between extreme levels of variables associated with awareness and perceived usefulness.
Because of the small numbers of participants reporting being offered or using genetic tests, we performed unadjusted analyses reporting overall numbers of individuals and percentages for these outcomes, without performing association analysis. Findings were reported as statistically significant if p values were less than 0.01.
In secondary analyses, we compared the characteristics of participants who participated in the AFU but did not respond to the genetic testing survey to those who responded (unadjusted analysis), and repeated (fully adjusted) regression analyses of genetic testing awareness and perceived usefulness stratified by gender and by HCHS/SOL study center. Also, in supplemental analyses, we repeated the main analyses stratified by study center, because there could be systematic differences between regions with respect to genetic testing.
Results
Of 16,415 HCHS/SOL visit 1 participants, 9,408 participated in the study’s 2019–2020 AFU phone survey and 5,769 completed at least one item about genetic testing for an overall participation rate of 35.1% (unweighted), or 32.6% of the target population (weighted estimate). Individuals who participated in the AFU but did not complete any genetic testing awareness items tended to be less educated, had lower household incomes, were more likely to be single and retired, and were more likely to be enrolled at the Bronx site and less likely to be enrolled at the Chicago and San Diego sites than individuals who completed at least one awareness item (all p < 0.001) (Table S1). Characteristics of HCHS/SOL participants who were analyzed and the target population their represent are summarized in Table 1. Visualization of missingness patterns in the dataset across all HCHS/SOL individuals, participants in the AFU, and genetic testing questionnaire responders are provided in the dedicated GitHub repository. Overall, the highest missingness was in the income level variable, followed by reported doctor visits.
Table 1.
Demographic characteristics, reported as unweighted n and weighted %, representative of the HCHS/SOL target population
| Total N | Site |
|||||
|---|---|---|---|---|---|---|
| Overall |
Bronx |
Chicago |
Miami |
San Diego |
% Missing |
|
| 5,769 | 1.030 | 1.950 | 1.065 | 1.724 | ||
| Age, years | 0 | |||||
| 25–40 | 1,000 (30.7) | 198 (33.4) | 377 (37.6) | 137 (21.6) | 288 (32.9) | |
| 41–60 | 2,674 (43.1) | 442 (40.6) | 916 (42.7) | 508 (45.5) | 808 (43.6) | |
| 61 or older | 2,095 (26.2) | 390 (26.0) | 657 (19.7) | 420 (32.9) | 628 (23.5) | |
| Gender | 0 | |||||
| Female | 3,701 (52.4) | 679 (54.5) | 1,163 (48.1) | 713 (52.9) | 1,146 (52.1) | |
| Male | 2,068 (47.6) | 351 (45.5) | 787 (51.9) | 352 (47.1) | 578 (47.9) | |
| Education | 0.4 | |||||
| Less than high school degree | 2,004 (31.6) | 381 (39.8) | 894 (42.9) | 203 (20.6) | 526 (27.1) | |
| High school degree | 1,458 (27.4) | 258 (25.6) | 494 (30.0) | 282 (28.4) | 424 (26.8) | |
| Associate, bachelor, or vocational degree | 2,078 (37.5) | 345 (31.8) | 516 (25.1) | 521 (46.1) | 696 (42.2) | |
| Masters, doctoral, professional degree | 206 (3.5) | 41 (2.8) | 38 (1.9) | 57 (4.8) | 70 (3.9) | |
| Household income | 2.5 | |||||
| Less than $10,000 | 613 (10.9) | 188 (16.5) | 150 (6.8) | 135 (11.4) | 140 (6.9) | |
| $10,001–$20,000 | 1,532 (27.3) | 316 (33.4) | 497 (24.1) | 334 (30.0) | 385 (19.6) | |
| $20,001–$40,000 | 1,999 (33.7) | 261 (28.3) | 757 (38.6) | 360 (36.1) | 621 (34.5) | |
| $40,001–$75,000 | 1,039 (18.6) | 146 (15.0) | 369 (22.3) | 150 (15.6) | 374 (23.4) | |
| More than $75,000 | 441 (9.6) | 81 (6.9) | 130 (8.2) | 56 (6.9) | 174 (15.7) | |
| Health insurance status | 0.3 | |||||
| Uninsured | 1,686 (31.6) | 121 (17.4) | 738 (43.9) | 395 (38.5) | 432 (32.4) | |
| Has health insurance | 4,068 (68.4) | 899 (82.6) | 1,212 (56.1) | 669 (61.5) | 1,288 (67.6) | |
| Doctor visit in last 12 months | 1.3 | |||||
| No | 1,521 (32.2) | 133 (20.0) | 531 (33.4) | 441 (44.6) | 416 (31.2) | |
| Yes, one or two times | 1,820 (29.9) | 309 (32.4) | 635 (31.4) | 276 (22.8) | 600 (33.7) | |
| Yes, at least three times | 2,352 (38.0) | 544 (47.6) | 778 (35.3) | 332 (32.7) | 698 (35.1) | |
| Hispanic or Latino background | 0.3 | |||||
| Dominican | 413 (9.8) | 380 (31.9) | 15 (0.5) | 17 (1.3) | 1 (0.1) | |
| Mexican | 2,753 (38.3) | 46 (10.3) | 1,093 (62.3) | 8 (0.9) | 1,606 (92.7) | |
| Central American | 575 (6.9) | 56 (4.2) | 226 (7.2) | 271 (15.2) | 22 (0.8) | |
| Cuban | 593 (19.4) | 8 (1.2) | 17 (1.5) | 565 (67.8) | 3 (0.4) | |
| Puerto Rican | 820 (16.8) | 442 (43.7) | 342 (18.5) | 19 (2.3) | 17 (1.5) | |
| South American | 424 (5.1) | 44 (3.9) | 201 (6.9) | 157 (9.5) | 22 (0.8) | |
| More than one/other | 172 (3.7) | 48 (4.8) | 53 (3.1) | 26 (2.9) | 45 (3.6) | |
| Language preference | 0 | |||||
| Spanish | 4,731 (74.7) | 679 (58.8) | 1,676 (79.8) | 1,030 (94.3) | 1,346 (68.8) | |
| English | 1,038 (25.3) | 351 (41.2) | 274 (20.2) | 35 (5.7) | 378 (31.2) | |
| Marital status | 0.1 | |||||
| Single | 1,234 (30.3) | 363 (42.4) | 336 (25.1) | 234 (26.6) | 301 (23.8) | |
| Married or living with partner | 3,288 (52.3) | 421 (40.6) | 1,265 (61.7) | 532 (49.7) | 1,070 (62.0) | |
| Separated, divorced or widowed | 1,240 (17.4) | 244 (17.1) | 349 (13.2) | 298 (23.7) | 349 (14.2) | |
| Employment status | 0.3 | |||||
| Retired/not currently employed | 2,362 (39.3) | 507 (46.8) | 714 (30.3) | 427 (40.6) | 714 (36.0) | |
| Employed part time (≤35 h/week) | 1,318 (21.2) | 210 (18.9) | 443 (22.8) | 251 (20.8) | 414 (22.8) | |
| Employed full time (>35 h/week) | 2,071 (39.5) | 298 (34.3) | 793 (46.9) | 387 (38.6) | 593 (41.3) | |
| Nativity | 0.3 | |||||
| Not U.S. born | 4,835 (77.2) | 786 (70.1) | 1,684 (77.7) | ,1017 (92.2) | 1,348 (69.5) | |
| U.S. born | 918 (22.8) | 239 (29.9) | 263 (22.3) | 46 (7.8) | 370 (30.5) | |
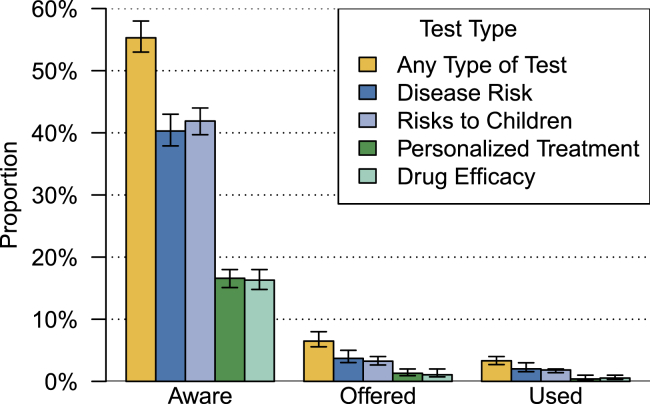
Overall, 2,891 survey responders, or 55.3% (weighted percentage) of individuals in the target population reported awareness of at least one of the four types of genetic tests. These included tests to determine risks for particular diseases, tests to determine the likelihood of passing inherited risks to children, tests to inform disease treatment, and tests to determine medication selection. Individuals were most likely to report awareness of genetic tests to determine risks of getting disease (2,089 participants, target population weighted percentage 40.3%) and the likelihood of passing disease to children (2,059 participants, weighted percentage 42.1%), and least likely to know about genetic tests providing information about how diseases should be treated (874 participants, weighted percentage 16.6%) or determining medication selection (885 individuals, weighted percentage 16.4%). Few individuals reported ever being offered or receiving the types of genetic tests described in the survey (Figure 1). Only 362 respondents (weighted percentage 6.5%) reported being offered any of the four types of tests described, and 190 respondents (weighted percentage 3.3%) reported receiving at least one of the tests described. When restricted to individuals who reported an awareness of at least one type of genetic test, analyses showed that 11.7% of the target population (362 respondents) reported being offered at least one of the tests described, while 6.0% of the target population (190 respondents) reported receiving at least one of the tests (Figure S3). Findings were consistent in analyses that were stratified by gender (Figure S4).
Figure 1.
Percentages of the HCHS/SOL target population who report awareness of specific types of genetic tests. Proportions and 95% confidence intervals, represented as bars in the figure, were computed in a weighted analysis to be representative of the HCHS/SOL target population. Proportions were multiplied by 100 to obtain percentages.
Few demographic or medical care factors that were examined by logistic regression analyses were associated with overall awareness of genetic testing (Table 2). Some of the greatest differences were observed between HCHS/SOL field centers (Figure S5). Awareness was greatest among individuals enrolled at the Miami site (weighted percentage 67.2%), and lowest among individuals enrolled at the Chicago site (weighted percentage 36.2%) (odds ratio [OR], 3.39; 95% confidence interval [CI], 2.44–4.71, p < 0.001). Income was also associated with genetic testing awareness: 73.3% (weighted percentage) of individuals with household incomes of more than $75,000 reported awareness, compared with 46.3% (weighted percentage) with incomes of less than $10,000 (OR, 2.53; 95% CI, 1.60–3.99; p < 0.001). Finally, we observed differences by educational attainment: 66.2% of individuals with associate, bachelor, or vocational degree reported awareness, compared with 45.0% of individuals with less than a high school degree (OR, 1.65; 95% CI, 1.33–2.05; p < 0.001).
Table 2.
Summary of logistic regression models that examine associations between participant characteristics and awareness of at least one of the four types of genetic testing (n = 5,768)
| Term | N aware in stratum | Weighted proportion | OR (95% CI) |
|---|---|---|---|
| Age, years (ref: 40 and younger) | 538 | 0.58 | |
| 41–60 | 1,355 | 0.55 | 1.02 (0.82–1.27) |
| 61 or older | 998 | 0.52 | 0.95 (0.70–1.28) |
| Gender (ref: female) | 1,901 | 0.56 | |
| Male | 990 | 0.55 | 0.88 (0.73–1.06) |
| Education, years (ref: less than high school degree) | 798 | 0.45 | |
| High school degree | 690 | 0.51 | 1.04 (0.83–1.30) |
| Associate, bachelor, or vocational degree | 1,258 | 0.66 | 1.65 (1.33–2.05)∗∗ |
| Masters, doctoral, professional degree | 137 | 0.65 | 1.48 (0.88–2.50) |
| Center (ref: Bronx) | 540 | 0.52 | |
| Chicago | 697 | 0.36 | 0.54 (0.39–0.75)∗∗ |
| Miami | 692 | 0.67 | 1.80 (1.25–2.60)∗ |
| San Diego | 962 | 0.58 | 1.17 (0.78–1.76) |
| Income (ref: Less than $10,000) | 282 | 0.46 | |
| $10,001–$20,000 | 711 | 0.51 | 1.14 (0.84–1.55) |
| $20,001–$40,000 | 986 | 0.53 | 1.24 (0.91–1.69) |
| $40,001–$75,000 | 558 | 0.62 | 1.77 (1.24–2.52)∗ |
| More than $75,000 | 290 | 0.73 | 2.53 (1.60–3.99)∗∗ |
| Health Insurance Status (ref: uninsured) | 790 | 0.53 | |
| Has health insurance | 2,093 | 0.56 | 1.02 (0.81–1.27) |
| Doctor Visit in Last 12 Months (ref: none) | 762 | 0.56 | |
| Yes, one or two times | 925 | 0.55 | 0.98 (0.77–1.24) |
| At least three times | 1,165 | 0.55 | 1.00 (0.77–1.29) |
| Hispanic or Latino Background (ref: Dominican) | 222 | 0.51 | |
| Mexican | 300 | 0.57 | 0.98 (0.63–1.54) |
| Central American | 392 | 0.68 | 1.07 (0.67–1.73) |
| Cuban | 1,269 | 0.51 | 1.21 (0.74–1.97) |
| Puerto Rican | 381 | 0.53 | 0.98 (0.67–1.43) |
| South American | 207 | 0.71 | 0.95 (0.60–1.51) |
| More than one/other | 112 | 0.51 | 1.47 (0.80–2.72) |
| Language Preference (ref: Spanish) | 2,273 | 0.53 | |
| English | 618 | 0.62 | 1.18 (0.91–1.54) |
| Marital Status (ref: Single) | 673 | 0.6 | |
| Married or living with partner | 1,627 | 0.55 | 0.84 (0.68–1.05) |
| Separated, divorced or widowed | 586 | 0.49 | 0.69 (0.52–0.93) |
| Employment Status (ref: Retired/not currently employed) | 1,128 | 0.53 | |
| Employed part time (≤35 h/week) | 694 | 0.57 | 1.08 (0.84–1.39) |
| Employed full time (>35 h/week) | 1,058 | 0.57 | 0.97 (0.77–1.21) |
| Nativity (ref: Not U.S. born) | 2,339 | 0.53 | |
| U.S. born | 545 | 0.62 | 1.14 (0.86–1.52) |
Analyses included all covariates jointly, and were weighted to generate estimates representative of the HCHS/SOL target population. N aware in stratum further provide the raw number of individuals aware of at least one type of test, and their proportion in the strata, weighted to represent the HCHS/SOL target population, is provide in the column weighted proportion.
∗p < 0.01.
∗∗p < 0.001.
Stratified analyses showed that some of the above-mentioned patterns were stronger at specific sites (Table S2). Differences by educational attainment only emerged for individuals enrolled at the Chicago site, while differences by income only for individuals enrolled at the San Diego site. Differences by gender were not observed overall, and gender-specific analyses (Table S3) generally showed similar differences by educational attainment. Differences by site were driven mainly by men, while differences by income were driven mainly by women.
Analyses about specific genetic tests showed similar trends (Table S4). Differences by education were observed on each of the items asking about awareness of specific tests (OR between more than high school degree vs less than high school degree ≥1.70; p < 0.001 for all tests). Differences by income were observed for knowledge of tests for disease risk and risks to children (OR between >$75,000 vs <$10,000 ≥ 1.84; p < 0.01 for both tests). Differences in awareness by study site were observed only in analyses of genetic testing for risks of getting disease (OR of individuals at Miami vs Bronx site, 3.02; 95% CI, 2.11–4.31; p < 0.001). Finally, 11.6% of individuals who were separated, divorced, or widowed reported awareness of drug efficacy genetic tests, compared to 17.5% of individuals who were married or living with a partner (OR, 0.66; 95% CI, 0.48–0.89; p = 0.007). We did not run models to examine factors associated with being offered or using specific tests because the numbers of participants were small, but unadjusted, weighted percentages are summarized in Figure 1.
Overall, participants rated the usefulness of genetic testing for managing a person’s health 8.4 (standard error = 0.05), on average, on a 1 (not at all useful) to 10 (extremely useful) scale. Few demographic factors were associated with usefulness scores in Poisson regression models (Table 3). Analyses showed that individuals enrolled at the Miami site rated the usefulness of genetic testing 7% higher than participants enrolled at the Bronx site, on average (95% CI, 2%–12% higher; p = 0.004). Conversely, mean perceived usefulness scores were 6% lower among U.S.-born individuals than individuals born outside the United States (95% CI, 4%–9% lower; p < 0.001). Site-specific analyses (Table S5) were generally consistent with these findings, although some differences by Hispanic or Latino background were observed. No demographic factors were associated with perceived usefulness in gender-specific analyses (Table S6).
Table 3.
Summary of a Poisson regression model that examines associations between participant characteristics and the perceived usefulness of genetic testing, rated on a 1 (not at all useful) to 10 (extremely useful) scale
| Term | Estimate (95% CI) |
|---|---|
| Age, years (ref: 40 and younger) | |
| 41–60 | 1.03 (1.00–1.06) |
| 61 or older | 0.98 (0.95–1.02) |
| Male gender (ref: female) | 0.98 (0.96–1.00) |
| Education, years (ref: less than high school degree) | |
| High school degree | 1.01 (0.98–1.04) |
| Associate, bachelor, or vocational degree | 1.03 (1.00–1.06) |
| Masters, doctoral, professional degree | 0.97 (0.92–1.02) |
| Center (ref: Bronx) | |
| Chicago | 1.01 (0.96–1.06) |
| Miami | 1.07 (1.02–1.12)∗ |
| San Diego | 1.03 (0.97–1.09) |
| Income (ref: Less than $10,000) | |
| $10,001–$20,000 | 1.01 (0.96–1.06) |
| $20,001–$40,000 | 1.01 (0.96–1.06) |
| $40,001–$75,000 | 1.03 (0.98–1.08) |
| More than $75,000 | 0.98 (0.93–1.04) |
| Has health insurance status (ref: uninsured) | 1.00 (0.98–1.02) |
| Doctor visit (ref: none) | |
| Yes, one or two times | 1.00 (0.98–1.03) |
| More than three times | 1.01 (0.98–1.03) |
| Hispanic or Latino background (ref: Dominican) | |
| Mexican | 0.99 (0.93–1.06) |
| Central American | 1.00 (0.95–1.05) |
| Cuban | 1.03 (0.97–1.09) |
| Puerto Rican | 1.00 (0.95–1.05) |
| South American | 0.99 (0.94–1.05) |
| More than one/other | 1.00 (0.94–1.07) |
| English language preference (ref: Spanish) | 0.95 (0.92–0.98)∗ |
| Marital status (ref: single) | |
| Separated, divorced or widowed | 1.02 (0.98–1.05) |
| Married or living with partner | 1.02 (0.99–1.06) |
| Employment status (ref: retired/not currently employed) | |
| Employed part time (≤35 h/week) | 1.02 (1.00–1.05) |
| Employed full time (>35 h/week) | 1.00 (0.97–1.02) |
| U.S. born (ref: Not U.S. born) | 0.96 (0.93–0.99)∗ |
Estimates represent exponentiated coefficients, and show the relative increase in perceived efficacy scores relative to the reference category. Analyses included all covariates jointly, and were weighted to generate estimates valid for the HCHS/SOL target population.
∗p < 0.01.
∗∗p < 0.001.
Discussion
We leveraged one of the largest, population-based cohort studies of U.S. Hispanic and Latino individuals to provide novel insights about demographic factors associated with genetic testing awareness in this growing population. Our findings showed that approximately 55% of this population was aware of genetic tests for health. This proportion was only slightly higher than findings from the 2017 HINTS, where just under one-half of Hispanic and Latino respondents and 57% of respondents overall reported awareness of health-related genetic testing.31 Similar to prior work that included all population groups, awareness of PGx applications, including tailored treatment, was much lower than awareness about personal disease risks or risks to children in our population.31 The rates that individuals reported being offered genetic testing were low, and only 3% reported ever using these tests.
Our findings highlight concerns about the potential for important segments of Hispanic and Latino populations to miss opportunities to benefit from improvements in genetic testing. Tremendous advances have occurred in medical genetics in recent years. These include the refinement of approaches to assess genetic risk, such as polygenic risk predictions,32,33 evolutions in the use of sequencing for expanded carrier screening,8,9 successes in tumor sequencing to guide cancer treatments, and continued development of best practices for PGx applications.6,34 The potential for genomics to revolutionize population health will not be realized without substantial efforts to improve how often Hispanic and Latino individuals are offered genetic services and facilitating access to testing and counseling.35,36 HCHS/SOL’s enrollment of more than 16,000 Hispanic and Latino individuals to date provides an instructive model for other cohort studies to consider to address awareness limitations in populations that are traditionally under-represented in biomedical research. Our study found that few Hispanic and Latino participants had even been offered services, a finding that has been observed in clinical settings.13 Efforts need to be improved to ensure Hispanic and Latino individuals who meet the criteria for a genetics referral are informed of such services and their potential benefits. Such efforts may need to be complemented with policies that address access barriers if we hope to achieve improvements in clinical settings.
A number of differences observed in prior work were not observed in our analyses. Specifically, differences in Hispanic and Latino populations by gender, Hispanic or Latino background, and birth location reported from the 2005 HINTS data were not statistically significant in our analyses. Only a few demographic factors were associated with genetic testing awareness in our study population. Specifically, we observed differences in awareness between centers of enrollment and by income and education. A greater awareness of genetic testing among individuals with higher educational status and income was consistent with previous findings.37,38 Geographic differences were unexpected and large. We found the least awareness among Hispanic and Latino individuals at the Chicago site and the greatest awareness among Hispanic and Latino individuals at Miami, findings that that persisted after adjustments for demographic characteristics such as socioeconomic status and language preferences. In contrast, prior reports of HINTS data had shown the greatest awareness in 2005 among respondents from the Midwest and the lowest awareness among respondents from the South, although regional differences in HINTS data were not observed in 2010 data.37,39 It is possible that the differences we observed reflect current differences by location, such as differences in the way genetic testing services are advertised by city and target audience or the way genetic testing is regulated. Lower awareness about direct-to-consumer tests have also been observed previously among individuals living in rural areas compared with urban areas,40 suggesting that the geographic breadth of enrollment at particular HCHS/SOL centers may have also affected awareness. Regardless, the large variation by geography likely reflects the wide diversity of experiences and attitudes within the Hispanic and Latino community.41,42 Education and outreach efforts that are targeted to the beliefs, needs, and concerns of Hispanic and Latino residents of specific communities are likely to yield the greatest improvements in awareness of genetic services.
Our study also showed that very few Hispanic and Latino participants remembered being offered or receiving genetic testing. HINTS data across populations from 2017 showed that 12% of respondents overall reporting ever having undergone a genetic test.31 In contrast, only 3% of respondents in our study reported ever receiving a genetic test. Differences in survey administration may explain some of these differences, as the 2017 HINTS instrument asked about a more expansive set of tests, including ancestry testing, paternity testing, and DNA fingerprinting.31 Additional work may be beneficial to provide insight about potential disparities by ethnicity in genetic testing use.
Our work also showed relatively high perceptions among Hispanic and Latino participants about the usefulness of genetic testing. Interestingly, we observed greater perceived usefulness of genetic testing among individuals who were not U.S. born, which is contrasts with prior work with Hispanic and Latinos populations, which has found a lower awareness among individuals of lower acculturation and those who have resided in the United States for shorter times.39,43 Studies have also found that Hispanic and Latino individuals with lower acculturation cite more barriers and perceived harms from genetic testing as well. Moreover, in previous findings from HCHS/SOL, we found that individuals with lower acculturation (not born within the 50 U.S. states, prefer Spanish over English) refused to share their genetic data with for-profit organizations at higher rates compared with those with higher acculturation.42 Our findings also highlight challenges to addressing existing disparities in the epidemiological evidence about genomics44,45,46,47,48 and efforts to enrich the participation of Hispanic and Latino individuals in precision medicine initiatives such as the All of Us Research Program.38,49 It is possible that attitudes about genetic testing differ in the countries of birth of our survey respondents; more positive attitudes and greater rates of perceived usefulness have been reported in studies conducted outside the United States, although such studies were not conducted in countries with primarily Hispanic and Latino populations.50,51,52
The strengths of our study include the large sample size of an understudied population and post hoc adjustment to provide population-based estimates. Our analytic sample not only focused on Hispanics and Latino individuals, but also included large numbers of respondents with lower educational attainment and income. Limitations to our analyses included moderate completion rates for the genetic awareness questions and the use of brief questions that provide limited insight about the barriers to awareness and test use or why participants felt genetic testing was useful or not useful. We did not study psychosocial and cultural factors, such as religiosity, medical distrust, and acculturation, which may vary within the Hispanic and Latino population and may be associated with outcomes of interest, which remains a topic of future work. Participants were enrolled at four centers with limited representation from rural areas. Our descriptions of genetic tests were adapted from items used previously in the national HINTS instrument and do not reflect the range of health-related applications that currently exist. Furthermore, response rates to the HCHS/SOL AFU survey were moderate, and numerous individuals who started the survey did not provide data for the genetic testing section. Don’t know responses were analyzed as no throughout, and the findings may underestimate the proportion of respondents with genetic test awareness, as well as the proportion of respondents who had been offered and used genetic tests.
Nevertheless, our work adds weight to evidence that use of genetic testing is limited in Hispanic and Latino individuals, although attitudes toward these applications are largely positive. These findings highlight the need to tailor genetic testing education and outreach to account for the wide variation in perception, needs, and potentially, believes, among Hispanic and Latino individuals. Education and outreach that accounts for the large diversity of Hispanic and Latino communities are likely to yield the greatest improvements in genetic test awareness and better ensure all individuals are able to capitalize on improvements in genomic medicine.
Data availability
HCHS/SOL data are available via a data use agreement with the HCHS/SOL Data Coordinating Center. See https://sites.cscc.unc.edu/hchs/ for study procedures. HCHS/SOL data are also available on the National Heart Lung and Blood Institute’s BioLINCC (Biologic Specimen and Data Repository Information Coordinating Center) repository under accession number HLB01141422a.
Code availability
Code is publicly available on the GitHub repository: https://github.com/tamartsi/Genetic_testing_awareness_SOL.
Ethics declaration
The HCHS/SOL was approved by the institutional review boards at each field center, where all participants gave written informed consent during their clinical exam, and by the Non-Biomedical IRB at the University of North Carolina at Chapel Hill, to the HCHS/SOL Data Coordinating Center. The informed consent form included the statement “I (agree/do not agree) to allow HCHS/SOL staff to contact me once a year to ask questions about my health and where I live.”
Acknowledgments
The authors thank the staff and participants of HCHS/SOL for their important contributions. The investigators website is: http://www.cscc.unc.edu/hchs/. The authors acknowledge the valuable contributions of Dr. Gerardo Heiss, a pillar of the community, a PI of the HCHS/SOL, and a contributor to this study. Dr. Heiss passed away in June 2022. The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following institutes, centers, and offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and the NIH Institution-Office of Dietary Supplements. K.D.C. was supported by NIH grant K01-HG009173.
Author contributions
Conceptualization: K.D.C., L.N.G., C.L.B.Z., and T.S.; data curation: M.Z. and T.S.; formal analysis: M.Z. and T.S.; funding acquisition: R.I., R.K., T.S. C.I., and M.L.D.; investigation: R.I. and R.K.; methodology: K.D.C., C.L.B.Z, R.I., R.K., and T.S.; project administration: resources: R.C.G., R.I., R.K., and T.S.; supervision: R.I., R.K., and T.S.; validation: T.S.; visualization: M.G. and T.S.; writing – original draft: K.D.C., L.N.G., M.Z., and T.S.; writing – review and editing: K.D.C., L.N.G., K.P., T.S., C.L.B.Z, M.A., C.I., M.L.D., G.A.T., and G.H.
Declaration of interests
K.D.C. was supported by a research grant from Sanford Health. R.C.G. has received compensation for advising the following companies: AIA, Embryome, Genome Web, Genomic Life, Grail, Humanity, OptumLabs, Plumcare, Verily; and is co-founder of Genome Medical, Inc. All other authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2022.100160.
Web resources
GitHub page with code and results used in this manuscript: https://github.com/tamartsi/Genetic_testing_awareness_SOL
Hispanic Community Health Study/Study of Latinos website: https://sites.cscc.unc.edu/hchs/
National Human Genome Research Institute. Genome Statute and Legislation Database: https://www.genome.gov/about-genomics/policy-issues/Genome-Statute-Legislation-Database
U.S. Census Bureau: QuickFacts. https://www.census.gov/quickfacts/fact/table/US
Supplemental information
References
- 1.Esplin E.D., Oei L., Snyder M.P. Personalized sequencing and the future of medicine: discovery, diagnosis and defeat of disease. Pharmacogenomics. 2014;15:1771–1790. doi: 10.2217/pgs.14.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg J.S., Agrawal P.B., Bailey D.B., Jr., Beggs A.H., Brenner S.E., Brower A.M., Cakici J.A., Ceyhan-Birsoy O., Chan K., Chen F., et al. Newborn sequencing in genomic medicine and public health. Pediatrics. 2017;139:e20162252. doi: 10.1542/peds.2016-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amendola L.M., Dorschner M.O., Robertson P.D., Salama J.S., Hart R., Shirts B.H., Murray M.L., Tokita M.J., Gallego C.J., Kim D.S., et al. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res. 2015;25:305–315. doi: 10.1101/gr.183483.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia S.S., Adelman K., Bale S.J., Chung W.K., Eng C., Evans J.P., Herman G.E., Hufnagel S.B., Klein T.E., Korf B.R., et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017;19:249–255. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell P.H., Wadhwa N., Danahey K., Borden B.A., Lee S.M., Hall J.P., Klammer C., Hussain S., Siegler M., Sorrentino M.J., et al. Pharmacogenomics-based point-of-care clinical decision support significantly alters drug prescribing. Clin. Pharmacol. Ther. 2017;102:859–869. doi: 10.1002/cpt.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roden D.M., McLeod H.L., Relling M.V., Williams M.S., Mensah G.A., Peterson J.F., Van Driest S.L. Lancet. 2019;394:521–532. doi: 10.1016/S0140-6736(19)31276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relling M.V., Evans W.E. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauchamp K.A., Muzzey D., Wong K.K., Hogan G.J., Karimi K., Candille S.I., Mehta N., Mar-Heyming R., Kaseniit K.E., Kang H.P., et al. Systematic design and comparison of expanded carrier screening panels. Genet. Med. 2018;20:55–63. doi: 10.1038/gim.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft S.A., Duenas D., Wilfond B.S., Goddard K.A.B. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet. Med. 2019;21:790–797. doi: 10.1038/s41436-018-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monaghan K.G., Leach N.T., Pekarek D., Prasad P., Rose N.C., ACMG Professional Practice and Guidelines Committee The use of fetal exome sequencing in prenatal diagnosis: a points to consider document of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2020;22:675–680. doi: 10.1038/s41436-019-0731-7. [DOI] [PubMed] [Google Scholar]
- 11.Suther S., Kiros G.E. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet. Med. 2009;11:655–662. doi: 10.1097/GIM.0b013e3181ab22aa. [DOI] [PubMed] [Google Scholar]
- 12.Canedo J.R., Miller S.T., Myers H.F., Sanderson M. Racial and ethnic differences in knowledge and attitudes about genetic testing in the US: systematic review. J. Genet. Couns. 2019;28:587–601. doi: 10.1002/jgc4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cragun D., Weidner A., Kechik J., Pal T. Genetic testing across young Hispanic and non-Hispanic white breast cancer survivors: facilitators, barriers, and awareness of the Genetic Information Nondiscrimination Act. Genet. Test. Mol. Biomarkers. 2019;23:75–83. doi: 10.1089/gtmb.2018.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agurs-Collins T., Ferrer R., Ottenbacher A., Waters E.A., O'Connell M.E., Hamilton J.G. Public awareness of direct-to-consumer genetic tests: findings from the 2013 U.S. Health Information National Trends Survey. J. Cancer Educ. 2015;30:799–807. doi: 10.1007/s13187-014-0784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hann K.E.J., Freeman M., Fraser L., Waller J., Sanderson S.C., Rahman B., Side L., Gessler S., Lanceley A., PROMISE study team Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Publ. Health. 2017;17:503. doi: 10.1186/s12889-017-4375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragun D., Weidner A., Lewis C., Bonner D., Kim J., Vadaparampil S.T., Pal T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123:2497–2505. doi: 10.1002/cncr.30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields A.E., Burke W., Levy D.E. Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genet. Med. 2008;10:404–414. doi: 10.1097/GIM.0b013e3181770184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall M.J., Reid J.E., Burbidge L.A., Pruss D., Deffenbaugh A.M., Frye C., Wenstrup R.J., Ward B.E., Scholl T.A., Noll W.W. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaikh T., Handorf E.A., Meyer J.E., Hall M.J., Esnaola N.F. Mismatch repair deficiency testing in patients with colorectal cancer and nonadherence to testing guidelines in young adults. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Correa M., Pérez-Mayoral J., Dutil J., Echenique M., Mosquera R., Rivera-Román K., Umpierre S., Rodriguez-Quilichini S., Gonzalez-Pons M., Olivera M.I., Pardo S., Puerto Rico Clinical Cancer Genetics Consortia Hereditary cancer syndromes in Latino populations: genetic characterization and surveillance guidelines. Hered. Cancer Clin. Pract. 2017;15:3. doi: 10.1186/s13053-017-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagsi R., Griffith K.A., Kurian A.W., Morrow M., Hamilton A.S., Graff J.J., Katz S.J., Hawley S.T. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J. Clin. Oncol. 2015;33:1584–1591. doi: 10.1200/JCO.2014.58.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstock I.M., Strecher V.J., Becker M.H. Social learning theory and the health belief model. Health Educ. Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh S. Illness representations, self-regulation, and genetic counseling: a theoretical review. J. Genet. Couns. 2006;15:325–337. doi: 10.1007/s10897-006-9044-5. [DOI] [PubMed] [Google Scholar]
- 24.Rogers R.W. In: Social Psychophysiology. Cacioppo J.T., Petty R.E., editors. Guilford Press; 1983. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation; pp. 153–176. [Google Scholar]
- 25.Christensen K.D., Roberts J.S., Zikmund-Fisher B.J., Kardia S.L., McBride C.M., Linnenbringer E., Green R.C., REVEAL Study Group Associations between self-referral and health behavior responses to genetic risk information. Genome Med. 2015;7:10. doi: 10.1186/s13073-014-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeny K., Ghane A., Legg A.M., Huynh H.P., Andrews S.E. Predictors of genetic testing decisions: a systematic review and critique of the literature. J. Genet. Couns. 2014;23:263–288. doi: 10.1007/s10897-014-9712-9. [DOI] [PubMed] [Google Scholar]
- 27.Sorlie P.D., Avilés-Santa L.M., Wassertheil-Smoller S., Kaplan R.C., Daviglus M.L., Giachello A.L., Schneiderman N., Raij L., Talavera G., Allison M., et al. Design and implementation of the hispanic community health study/study of Latinos. Ann. Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavange L.M., Kalsbeek W.D., Sorlie P.D., Avilés-Santa L.M., Kaplan R.C., Barnhart J., Liu K., Giachello A., Lee D.J., Ryan J., et al. Sample design and cohort selection in the hispanic community health study/study of Latinos. Ann. Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson D.E., Kreps G.L., Hesse B.W., Croyle R.T., Willis G., Arora N.K., Rimer B.K., Viswanath K.V., Weinstein N., Alden S. The health information national trends survey (HINTS): development, design, and dissemination. J. Health Commun. 2004;9:443–460. doi: 10.1080/10810730490504233. discussion 481-444. [DOI] [PubMed] [Google Scholar]
- 30.Vassy J.L., Christensen K.D., Slashinski M.J., Lautenbach D.M., Raghavan S., Robinson J.O., Blumenthal-Barby J., Feuerman L.Z., Lehmann L.S., Murray M.F., et al. ‘Someday it will be the norm’: physician perspectives on the utility of genome sequencing for patient care in the MedSeq Project. Per. Med. 2015;12:23–32. doi: 10.2217/PME.14.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krakow M., Ratcliff C.L., Hesse B.W., Greenberg-Worisek A.J. Assessing genetic literacy awareness and knowledge gaps in the US population: results from the Health Information National Trends Survey. Public Health Genomics. 2017;20:343–348. doi: 10.1159/000489117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavaddat N., Michailidou K., Dennis J., Lush M., Fachal L., Lee A., Tyrer J.P., Chen T.H., Wang Q., Bolla M.K., et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am. J. Hum. Genet. 2019;104:21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan P., Young R., Stitziel N.O., Padmanabhan S., Baber U., Mehran R., Sartori S., Fuster V., Reilly D.F., Butterworth A., et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135:2091–2101. doi: 10.1161/CIRCULATIONAHA.116.024436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caraballo P.J., Sutton J.A., Giri J., Wright J.A., Nicholson W.T., Kullo I.J., Parkulo M.A., Bielinski S.J., Moyer A.M. Integrating pharmacogenomics into the electronic health record by implementing genomic indicators. J. Am. Med. Inform. Assoc. 2020;27:154–158. doi: 10.1093/jamia/ocz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurle B., Citrin T., Jenkins J.F., Kaphingst K.A., Lamb N., Roseman J.E., Bonham V.L. What does it mean to be genomically literate?: national human genome research Institute meeting report. Genet. Med. 2013;15:658–663. doi: 10.1038/gim.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins F.S., Varmus H. A new initiative on precision medicine. N. Engl. J. Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mai P.L., Vadaparampil S.T., Breen N., McNeel T.S., Wideroff L., Graubard B.I. Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 national health interview surveys. Am. J. Prev. Med. 2014;46:440–448. doi: 10.1016/j.amepre.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagán J.A., Su D., Li L., Armstrong K., Asch D.A. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am. J. Prev. Med. 2009;37:524–530. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Heck J.E., Franco R., Jurkowski J.M., Sheinfeld Gorin S. Awareness of genetic testing for cancer among United States Hispanics: the role of acculturation. Community Genet. 2008;11:36–42. doi: 10.1159/000111638. [DOI] [PubMed] [Google Scholar]
- 40.Salloum R.G., George T.J., Silver N., Markham M.J., Hall J.M., Guo Y., Bian J., Shenkman E.A. Rural-urban and racial-ethnic differences in awareness of direct-to-consumer genetic testing. BMC Publ. Health. 2018;18:277. doi: 10.1186/s12889-018-5190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González Burchard E., Borrell L.N., Choudhry S., Naqvi M., Tsai H.J., Rodriguez-Santana J.R., Chapela R., Rogers S.D., Mei R., Rodriguez-Cintron W., et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am. J. Public Health. 2005;95:2161–2168. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez S., Strizich G., Isasi C.R., Hua S., Comas B., Sofer T., Thyagarajan B., Perreira K.M., Talavera G.A., Daviglus M.L., et al. Consent for use of genetic data among US hispanics/latinos: results from the hispanic community health study/study of Latinos. Ethn. Dis. 2021;31:547–558. doi: 10.18865/ed.31.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton J.G., Shuk E., Arniella G., González C.J., Gold G.S., Gany F., Robson M.E., Hay J.L. Genetic testing awareness and attitudes among Latinos: exploring shared perceptions and gender-based differences. Public Health Genomics. 2016;19:34–46. doi: 10.1159/000441552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landry L.G., Ali N., Williams D.R., Rehm H.L., Bonham V.L. Lack of diversity in genomic databases Is a barrier to translating precision medicine research into practice. Health Aff. 2018;37:780–785. doi: 10.1377/hlthaff.2017.1595. [DOI] [PubMed] [Google Scholar]
- 45.Kim I.E., Jr., Sarkar I.N. Racial representation disparity of population-level genomic sequencing efforts. Stud. Health Technol. Inform. 2019;264:974–978. doi: 10.3233/SHTI190369. [DOI] [PubMed] [Google Scholar]
- 46.Roberts M.C., Mensah G.A., Khoury M.J. Leveraging implementation science to address health disparities in genomic medicine: examples from the field. Ethn. Dis. 2019;29:187–192. doi: 10.18865/ed.29.S1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin A.R., Kanai M., Kamatani Y., Okada Y., Neale B.M., Daly M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spratt D.E., Chan T., Waldron L., Speers C., Feng F.Y., Ogunwobi O.O., Osborne J.R. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2016;2:1070–1074. doi: 10.1001/jamaoncol.2016.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The All of Us Research Program Investigators The “all of us” research Program. N. Engl. J. Med. 2019;381:668–676. doi: 10.1056/NEJMsr1809937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arafah A., AlJawadi M.H., Aldheefi M., Rehman M.U. Attitude and awareness of public towards genetic testing in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2021;28:255–261. doi: 10.1016/j.sjbs.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altaany Z., Khabour O.F., Al-Taani G. Knowledge, beliefs, and attitudes concerning genetic testing among young Jordanians. J. Multidiscip. Healthc. 2019;12:1043–1048. doi: 10.2147/JMDH.S233614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson T. Zenodo; 2020. SIENNA D2.5: Public Views on Genetics, Genomics and Gene Editing in 11 EU and Non-eu Countries. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
HCHS/SOL data are available via a data use agreement with the HCHS/SOL Data Coordinating Center. See https://sites.cscc.unc.edu/hchs/ for study procedures. HCHS/SOL data are also available on the National Heart Lung and Blood Institute’s BioLINCC (Biologic Specimen and Data Repository Information Coordinating Center) repository under accession number HLB01141422a.